Abstract
COVID-19 has manifested with ventricular dysfunction and cardiac arrhythmias, most commonly atrial fibrillation (AFib), in adults. However, very few pediatric patients with acute COVID-19 have had cardiac involvement. AFib, an exceedingly rare arrhythmia in otherwise healthy children, has not been reported in children with COVID-19. We report a 15 year-old girl with acute COVID-19, fulminant myocarditis and AFib.
Keywords: COVID-19, Fulminant myocarditis, Left ventricular dysfunction, Hypotension, Atrial Fibrillation
Case report
A 15 year-old, previously healthy African-American girl presented with headache, vomiting, and fatigue to an outside emergency room where she was noted to be febrile (102.8 F), tachycardic (150 bpm) and hypotensive (70/40 mmHg). She was found to be COVID-19 positive (PCR assay) and received 2 l of normal saline via boluses before she was transported to our pediatric intensive care unit where an echocardiogram showed severe left ventricular dysfunction (SF ~ 20%) without any atrial or ventricular dilation (Video 1). No patent foramen ovale or intracardiac thrombus was noted. Given the ongoing hypotension and poor ventricular function, therapy was initiated with milrinone (0.5 μg/kg/min) and epinephrine (0.03 μg/kg/min). Serum pro-NT BNP, inflammatory markers and high sensitivity troponin concentration were all markedly elevated (Supplement 1). She was initially treated with intravenous immune globulin (1 g/kg), intravenous methylprednisolone 60 mg every 12 h and subcutaneous low molecular weight heparin (Supplement 1). Epinephrine infusion was discontinued within 12 h. Milrinone (0.7 μg/kg/min) was continued until her ventricular function normalized (day 4). Hypotension recurred 24 h post-admission, and immunomodulatory therapy was therefore intensified via addition of the IL-1 receptor antagonist, anakinra (100 mg subcutanous every 12 h). She did not require any respiratory support though her chest radiograph showed minimal vascular engorgement. Atrial fibrillation with rapid ventricular conduction (ventricular rate 140 s to 190 s) developed 27 h after admission and she was successfully cardioverted (50 J, synchronized) (Fig. 1 ). Post-cardioversion, an amiodarone infusion was initiated and 12 h later, transitioned to an oral regimen. Our patient denied a history of drug or alcohol abuse and the family history was negative for channelopathy, cardiomyopathy or early onset AFib. Her serum electrolytes were within the normal range at the time of onset of AFib (Potassium = 3.7 mmol/L, Calcium 9.1 mg/dL and Magnesium 2.2 mg/dL) and her oxygen saturation measured normal during the duration of her hospital stay. Her electrocardiogram in sinus rhythm was normal without any evidence of pre-excitation.
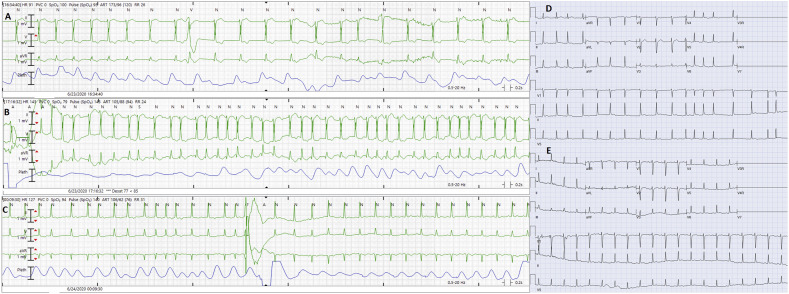
Fig. 1.
The onset of atrial fibrillation is shown in panel A (after 8th QRS complex from the start of the strip). The patient soon develops rapid ventricular response during atrial fibrillation (Panel B). Panel C shows termination of atrial fibrillation following delivery of 50 J synchronized shock. Panel D shows 12 lead electrocardiogram during atrial fibrillation. No pre-excitation is noted on 12‑lead electrocardiogram during sinus rhythm following successful cardioversion (Panel E).
A cytokine panel (ARUP Laboratories, Salt Lake City, UT, USA) obtained on our patient prior to initiation of immunomodulatory therapy revealed elevated plasma concentrations of soluble interleukin (IL) 2 Receptor (soluble) (2670 pg/mL; range: 175.3 to 858.2 pg/mL) and IL-10 (5.4 pg/mL; normal ≤2.8 pg/mL). Plasma IL-2, IL-12, TNF-α, INF-γ, IL-4, IL-5, IL-13, IL-17, IL-1β, IL-6 and IL-8 concentrations measured within the normal range.
Following initiation of amiodarone, she has done well without any recurrence. Her cardiac and inflammatory biomarkers continue to trend downwards (Supplement 1). COVID-19 antibody test (Roche Elecsys Anti-SARS-CoV-2 antibody test, Roche labs, Basel, Switzerland) was obtained on day 2 of admission and was positive. COVID-19 PCR assay (Cepheid GeneXpert system, Sunnyvale, California, USA) was repeated on post-admission days 8 and 9 and was negative.
Discussion
While ventricular dysfunction and the whole gamut of arrhythmias have been reported in adults with acute COVID-19 [1], pediatric patients have either been asymptomatic or have mostly manifested with mild respiratory illness [2]. Rare pediatric patients with COVID-19 have presented with ventricular dysfunction [3]; however, arrhythmias such as AFib, which are exceedingly rare in otherwise healthy children, have not been reported in children with acute COVID-19 [[4], [5], [6], [7], [8], [9]].
A broad range of electrocardiographic abnormalities and arrhythmias such as T wave alterations, ST segment elevation, SI, QIII, TIII pattern, high degree atrioventricular block, atrial tachycardia, atrial flutter, ventricular tachycardia or fibrillation and pulseless electrical activity have been reported in adults with COVID-19 [10]. The pediatric data however remains somewhat limited. In a recently published single center pediatric series (n = 36), nonsustained ventricular tachycardia (n = 5) and sustained atrial tachycardia (n = 1) were noted in approximately 17% of children with acute COVID-19. Of note, all of these arrhythmias self-resolved. However, half of the children in this series were started on an anti-arrhythmic therapy. The electrocardiographic parameters like corrected QT interval were no different between children with and without cardiac arrhythmias [4]. One of these patients was on high dose epinephrine and nor-epinephrine infusions and the other patient had dyselectrolytemia (hypokalemia, hypomagnesemia and hypocalcemia). In addition, abnormal echocardiographic findings such as left ventricular dysfunction, left ventricular dilation and pericardial effusion were noted in two- thirds of the patients.
AFib in young patients (<18 yrs) is exceedingly rare and is usually associated with alcohol or drug abuse [5], an inherited channelopathy such as Brugada syndrome, long QT syndrome, short QT syndrome or a cardiomyopathy [6,7]. Familial AFib has also been reported in the pediatric population [8]. Children with pre-excitation are also predisposed to AFib which can conduct rapidly to the ventricles [11]. Although children with fulminant myocarditis are predisposed to conduction abnormalities and potentially lethal arrhythmias, AFib has not previously been reported in this population [12,13].
Hypothesized mechanisms of arrhythmias in patients with COVID-19 include downregulation of myocardial and pulmonary ACE2 pathways, leading to myocardial inflammation, lung edema, and acute respiratory failure. Additionally, cytokine storm triggered by an imbalanced response by type 1 and 2 T-helper cells, interferon-mediated immunopathological events, respiratory dysfunction and hypoxemia are also thought to play a role in damaging the myocardium [14]. While elevated plasma soluble IL-2 receptor concentrations which were noted in our patient are known to predispose to atrial fibrillation [15], elevated serum IL-10 concentrations have been shown to be protective in both animal models and humans [16,17]. Additional factors which might have contributed to the genesis of AFib in our patient include severe left ventricular dysfunction induced acute hemodynamic perturbations, leading to an elevation atrial pressure and use of inotropic agents such as milrinone. Short term intravenous infusion (48 h) of milrinone (0.5 μg/kg/min) has been associated with a higher incidence of new onset atrial fibrillation in adults with NYHA class III and IV heart failure (mean left ventricular ejection fraction 23%) [18]. Milrinone use has also been associated with postoperative AFib in both children and adults with heart disease [19,20]. However, except for children with congenital heart disease, the data on association between the use of milrinone and atrial arrhythmias remains limited in the pediatric population.
To the best of our knowledge, this is the first report of AFib in a child with COVID-19 and fulminant myocarditis. These findings are of importance as AFib, especially with rapid ventricular conduction, can lead to acute decompensation in children with marginal ventricular function and can also enhance the risk of thromboembolism to which patients with acute COVID-19 are predisposed. The optimal strategy for anticoagulation in patients with acute COVID-19 and sustained atrial arrhythmias is not known. However, given the heightened risk of thromboembolism with COVID-19, we have maintained our patient on low dose aspiring (81 mg daily) and low molecular weight heparin despite a low CHADS2-VASc score of 1 (female gender). The optimal duration of anticoagulation for these patients has not been systematically evaluated and therefore remains unknown.
Funding
None.
Declaration of Competing Interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jelectrocard.2020.10.004.
Appendix A. The following are the supplementary data related to this article
Supplementary material 1
Supplementary video 1
References
- 1.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review [published online ahead of print, 2020 Mar 27] JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 2.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oberweis M.L., Codreanu A., Boehm W., Olivier D., Pierron C., Tsobo C., et al. Pediatric life-threatening coronavirus disease 2019 with myocarditis. Pediatr Infect Dis J. 2020;39:e147–e149. doi: 10.1097/INF.0000000000002744. [DOI] [PubMed] [Google Scholar]
- 4.Samuel S., Friedman R.A., Sharma C., Ganigara M., Mitchell E., Schleien C., et al. Incidence of arrhythmias and electrocardiographic abnormalities in symptomatic pediatric patients with PCR positive SARS-CoV-2 infection including drug induced changes in the corrected QT interval (QTc) Heart Rhythm. 2020 Jul 1;S1547–5271(20):30632–30639. doi: 10.1016/j.hrthm.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramphul K., Joynauth J. Cardiac arrhythmias among teenagers using Cannabis in the United States. Am J Cardiol. 2019;124:1966. doi: 10.1016/j.amjcard.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Platonov P.G., McNitt S., Polonsky B., Rosero S.Z., Zareba W. Atrial fibrillation in long QT syndrome by genotype. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gourraud J.B., Khairy P., Abadir S., Tadros R., Cadrin-Tourigny J., Macle L., et al. Atrial fibrillation in young patients. Expert Rev Cardiovasc Ther. 2018;16:489–500. doi: 10.1080/14779072.2018.1490644. [DOI] [PubMed] [Google Scholar]
- 8.Bartos D.C., Anderson J.B., Bastiaenen R., Johnson J.N., Gollob M.H., Tester D.J., et al. A KCNQ1 mutation causes a high penetrance for familial atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:562–569. doi: 10.1111/jce.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohli U., Lodha R. Cardiac involvement in children with COVID-19. Indian Pediatr. 2020 Aug;7 doi: 10.1007/s13312-020-1998-0. 32769232 S097475591600222. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haseeb S., Gul E.E., Çinier G., Bazoukis G., Alvarez-Garcia J., Garcia-Zamora S., et al. International Society of Electrocardiology Young Community (ISE-YC). Value of electrocardiography in coronavirus disease 2019 (COVID-19) J Electrocardiol. 2020;62:39–45. doi: 10.1016/j.jelectrocard.2020.08.007. 32805546 Epub ahead of print. PMC7409871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etheridge S.P., Escudero C.A., Blaufox A.D., Law I.H., Dechert-Crooks B.E., Stephenson E.A., et al. Life-threatening event risk in children with Wolff-Parkinson-white syndrome: a multicenter international study. JACC Clin Electrophysiol. 2018;4:433–444. doi: 10.1016/j.jacep.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Ichikawa R., Sumitomo N., Komori A., Abe Y., Nakamura T., Fukuhara J., et al. The follow-up evaluation of electrocardiogram and arrhythmias in children with fulminant myocarditis. Circ J. 2011;75:932–938. doi: 10.1253/circj.cj-10-0918. [DOI] [PubMed] [Google Scholar]
- 13.Lin K.M., Li M.H., Hsieh K.S., Kuo H.C., Cheng M.C., Sheu J.J., et al. Impact of extracorporeal membrane oxygenation on acute fulminant myocarditis-related hemodynamic compromise arrhythmia in children. Pediatr Neonatol. 2016;57:480–487. doi: 10.1016/j.pedneo.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Gennaro L., Brunetti N.D., Montrone D., De Rosa F., Cuculo A., Di Biase M. Inflammatory activation and carbohydrate antigen-125 levels in subjects with atrial fibrillation. Eur J Clin Investig. 2020;42:371–375. doi: 10.1111/j.1365-2362.2011.02592.x. [DOI] [PubMed] [Google Scholar]
- 16.Kondo H., Takahashi N., Gotoh K., Fukui A., Saito S., Aoki K., et al. Splenectomy exacerbates atrial inflammatory fibrosis and vulnerability to atrial fibrillation induced by pressure overload in rats: possible role of spleen-derived interleukin-10. Heart Rhythm. 2016;13:241–250. doi: 10.1016/j.hrthm.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Kondo H., Abe I., Gotoh K., Fukui A., Takanari H., Ishii Y., et al. Interleukin 10 treatment ameliorates high-fat diet-induced inflammatory atrial remodeling and fibrillation. Circ Arrhythm Electrophysiol. 2018;11 doi: 10.1161/CIRCEP.117.006040. [DOI] [PubMed] [Google Scholar]
- 18.Cuffe M.S., Califf R.M., Adams K.F., Jr., Benza R., Bourge R., Colucci W.S., et al. Outcomes of a prospective trial of intravenous milrinone for exacerbations of chronic heart failure (OPTIME-CHF) investigators. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. 11911756 [DOI] [PubMed] [Google Scholar]
- 19.Smith A.H., Owen J., Borgman K.Y., Fish F.A., Kannankeril P.J. Relation of milrinone after surgery for congenital heart disease to significant postoperative tachyarrhythmias. Am J Cardiol. 2011;108:1620–1624. doi: 10.1016/j.amjcard.2011.07.023. 21890079 Epub 2011 Sep 3. PMC3941037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleming G.A., Murray K.T., Yu C., Byrne J.G., Greelish J.P., Petracek M.R., et al. Milrinone use is associated with postoperative atrial fibrillation after cardiac surgery. Circulation. 2008;118:1619–1625. doi: 10.1161/CIRCULATIONAHA.108.790162. Epub 2008 Sep 29. PMID: 18824641; PMCID: PMC2770257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary video 1