Abstract
Purpose
To evaluate the potential of drug-eluting bead (DEB)-transcatheter arterial chemoembolization (TACE) as a treatment option for patients with refractory to conventional lipiodol-based TACE (c-TACE) especially with decreased liver function.
Patients and Methods
We retrospectively evaluated the treatment results of DEB-TACE for 89 HCC nodules in 27 patients with c-TACE refractory according to liver function.
Results
Although overall survival was significantly better in Child–Pugh A patients than in Child–Pugh B patients (median survival time, MST: 561 vs 347 days, p=0.031), progression-free survival was almost similar in both patients between Child–Pugh A and B (MST: 79 vs 87 days, p=0.534). Regarding antitumor response, the objective response rate (ORR) and disease-control rate (DCR) were 5.3/12.5% and 52.7/87.5% in Child–Pugh A/B, respectively. In each 89 HCC nodules, ORR and DCR were almost similar between Child–Pugh A and B (ORR, 20.3 vs 13.3%; DCR, 77.0 vs 73.3%, respectively). Adverse events of DEB-TACE were well-tolerated, and liver function was reserved during DEB-TACE procedures.
Conclusion
DEB-TACE could be a therapeutic option for advanced HCC patients with c-TACE refractory and decreased liver function.
Keywords: TACE-refractory, drug-eluting bead, post-embolization syndrome, microsphere, tyrosine kinase inhibitor
Introduction
Hepatocellular carcinoma (HCC) is the third most frequent cause of cancer-related deaths worldwide and has a high disease burden especially in Asia.1 Patents with HCC are frequently found with advanced stage of disease, few patients can receive curative therapies such as surgical resection.1 Therefore palliative but effective therapies that have survival benefits for patients with HCC who are unable to be curatively treated are required.
Transcatheter arterial chemoembolization (TACE) is a standard treatment for patients with multiple HCC with Barcelona Clinic Liver Cancer study group (BCLC)-B stage.2,3 TACE showed survival benefits for unresectable HCC in randomized controlled trials4,5 and prospective large cohort studies.6,7 In TACE procedures, HCC nodules are embolized through feeding hepatic artery with embolic agents and anticancer agents, inducing antitumor effects by anticancer effects and ischemic effects. Chemoembolization with drug-eluting bead (DEB)-TACE is technically similar to conventional lipiodol-based TACE (c-TACE), providing similar therapeutic benefits compared with c-TACE as shown in prospective randomized studies8,9 and meta-analyses.10–12 DEB-TACE is made from uniform particles and can induce permanent embolization and long-sustaining local concentration of anticancer drugs although c-TACE has a transient embolic effect.13 In addition with those chemoembolic effects, DEB-TACE is shown to be less harmful and to induce mild postembolization syndrome compared with that with c-TACE as shown in randomized trials and meta-analyses,8–10,14 although it is debatable as shown in other meta-analyses.11,12 From those observations, DEB-TACE is expected as the therapeutic modality for patients with huge HCC or decreased liver function.
However, repeated TACE procedure for patients with HCC was shown to worsen the prognosis of HCC patients with refractory to TACE compared with those in patients who were switched from TACE to the therapy with sorafenib.15–17 Furthermore, repeated TACE was shown to worsen hepatic reserve function and patients’ prognosis especially in those with TACE-refractory.18 Systemic chemotherapy with tyrosine kinase inhibitors (TKIs) such as sorafenib or lenvatinib has been developed for the treatment of advanced HCC.19,20 However TKIs can be applied to patients with good hepatic reserve function test such as Child–Pugh A. Therefore therapeutic modalities for multiple advanced HCC with TACE-refractory and decreased liver function are desired. In this study, we retrospectively evaluated the treatment results of DEB-TACE for patients with c-TACE refractory according to liver function to explore therapeutic options for patients with advanced HCC.
Patients and Methods
Patients Selection
Between January 2015 and December 2019, 296 patients underwent TACE at our department. Of those patients, 27 consecutive patients with HCC who could not be eligible for curative resection or local curative treatment and refractory to c-TACE were enrolled in this study. As the definition of TACE refractory, we defined it according to the Japanese Society of Hepatology Consensus Guidelines.21 Briefly, refractoriness to TACE is defined as more than two consecutive ineffective responses of treated tumors with viable lesions >50% or more than two consecutive progressive increases in total tumor count. Furthermore, continuous elevation of tumor marker levels and new emergence of vascular invasion or extrahepatic spread after TACE are also considered as TACE refractory.17 In our department, c-TACE with miriplatine hydrate (Miripla®, Dainippon Sumitomo Pharma Co., Ltd, Tokyo, Japan) is used as the first chemoembolic agent in TACE procedures. For a group of patients with decreased renal function, c-TACE with epirubicin hydrochloride (Epirubicin®, Nippon Kayaku Co., Ltd, Tokyo, Japan) is considered as an alternative. Regarding c-TACE procedures, lipiodol emulsion with those anticancer agents were injected into super-selected tumor-feeding artery, followed by an injection of 1 mm gelatin sponges (Gelpart®, Nippon Kayaku Co., Ltd) and Furthermore, DEB-TACE is considered also as the first TACE procedures for patients with huge HCC, decreased liver function or decreased performance status. To evaluate hepatic reserve function, modified albumin-bilirubin (mALBI) grade, which is shown to be a more accurate marker of hepatic reserve function and predictive value,22,23 was also assessed in addition to Child–Pugh classification. Furthermore, we evaluated HCC status of the liver with “up-to-seven (UT7) criteria”24 because intrahepatic tumor factors such as size and number of tumor are known to be associated with effectiveness of TACE and patients’ survival after TACE.25,26
Written informed consent was obtained from all participants before treatment, and this study was approved by our institutional ethics committee (Ethics Committee, University of Toyama, Approved Number: 25-31). This study was conducted in accordance with the Declaration of Helsinki.
DEB-TACE Procedure
Angiography was performed by inserting a 3-Fr catheter through the femoral artery. The tip of the microcatheter was superselected into the tumor-feeding branches. After identification of the tumor-feeding artery, DEB-TACE was performed. The method for loading with anticancer agents was prepared as previously described. As an embolic agent, microsphere with 50–100 µm (HepaSphere®, Nippon Kayaku Co., Ltd) was used. As anticancer agents, epirubicin hydrochloride or arterial cisplatin (IA-call®, Nippon Kayaku Co., Ltd), were used according to patient's condition. DEB-TACE procedure was repeated every 8–12 weeks if residual viable tumor was evident and it could be associated with good prognosis.
Tumor Response and Toxicity Assessment
Tumor response was evaluated by dynamic CT or MRI conducted every 8–12 weeks using the modified Response Evaluation Criteria in Solid Tumors (mRECIST).27 A complete response (CR) was defined as the disappearance of any arterial enhancement in the target tumor, a partial response (PR) was defined as over 30% decrease in the sum of the diameters of viable lesions, progressive disease (PD) was defined as over 20% increase in the sum of the diameters of viable lesions, and a stable disease (SD) was defined as any cases with nonPR or nonPD. An objective response rate (ORR) was defined as the percentage of patients achieving either CR or PR, and disease control rate (DCR) as the percentage of patients achieving CR, PR, or SD.27 The best tumor response among each examination was documented. Assessment of adverse events (AEs) found during treatment was evaluated based on National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0.
Statistical Analyses
Statistical analyses were performed using SPSS version 19.0 (IBM Corporation, Armonk, NY, USA). Categorical variables were evaluated using the chi-squared test or Fisher’s exact test, as appropriate. Continuous variables were analyzed by using the Mann–Whitney U-test. Progression-free survival (PFS) and overall survival (OS) after the first DEB-TACE procedures were analyzed using the Kaplan–Meier method, and compared by log-rank tests. Univariate and multivariate analyses were performed using the Cox proportional hazards model. A p-value <0.05 was considered statistically significant.
Results
Patients
Patient's characteristics are shown in Table 1. Twenty-seven patients were included in this study. All patients were refractory to c-TACE according to the criteria of TACE-refractory. Median age was 76 years and 21 cases were male. Etiology of 10 cases were hepatitis C virus infection and 12 cases were nonviral causes. Performance status was preserved in most patients, except for a patient with past history of cerebral hemorrhage. Nineteen patients were Child–Pugh A and the rest, eight cases, were Child–Pugh B. Most patients were included in mALBI grade 2 (2a, seven case; 2b, 11 cases), which is known to be associated with TACE-refractory and intolerable to repeated c-TACE.18 Twenty-three patients were BCLC-B and four were BCLC-C. Median maximum size, median number of HCC were 2.5 cm and four, respectively, and beyond UT7 cases were found in 15 cases. All cases were refractory to TACE and median number of prior c-TACE was two (range: 1–8).
Table 1.
Characteristics of Patients
| Factors | Median (Range) or Number |
|---|---|
| Age | 76 (38–88) |
| Male/female | 21/6 |
| Etiology (HCV/HBV/NBNC) | 10/5/12 |
| ECOG-PS | 1 (0–3) |
| BCLC stage (B/C) | 23/4 |
| Child–Pugh A/B | 19/8 |
| Platelet (×104/µL) | 11.5 (5.7–47.9) |
| Serum creatinine (mg/dL) | 0.77 (0.47–1.30) |
| Serum albumin (g/dL) | 3.3 (2.2–4.2) |
| Serum total bilirubin (mg/dL) | 0.7 (0.4–2.0) |
| Prothrombin activity (INR) | 1.03 (0.95–1.32) |
| mALBI grade (1/2a/2b/3) | 5/7/11/4 |
| Alpha fetoprotein (ng/mL) | 50.4 (2.4–35768) |
| Tumor max size (cm) | 2.5 (1–10) |
| Tumor number | 4 (1–13) |
| Up-to-seven (in/out) | 12/15 |
| Prior c-TACE number | 2 (1–8) |
Abbreviations: HCV, hepatitis C virus; HBV, hepatitis B virus; NBNC, nonHBV nonHCV; ECOG-PS, Eastern Cooperative Oncology Group—Performance status; BCLC, Barcelona Clinic Liver Cancer; INR, international normalized ratio; mALBI, modified albumin-bilirubin; c-TACE, conventional transcatheter arterial chemoembolization.
Procedure of DEB-TACE
Thirteen cases were treated with epirubicin hydrochloride (median: 25 mg; range: 3.5–50 mg), and 14 cases were treated with arterial cisplatin (median: 11.5 mg; range: 4–80 mg). DEB-TACE was performed for HCC cases with c-TACE refractory until tumor progression (median: 2 sessions, range: 1–7 sessions). Median time intervals between DEB-TACE procedures was twp months (range: 1–8 months). In the present cohort, 13 cases with Child–Pugh A and two cases with Child–Pugh B had been treated with sorafenib around DEB-TACE procedures.
Clinical Course After DEB-TACE
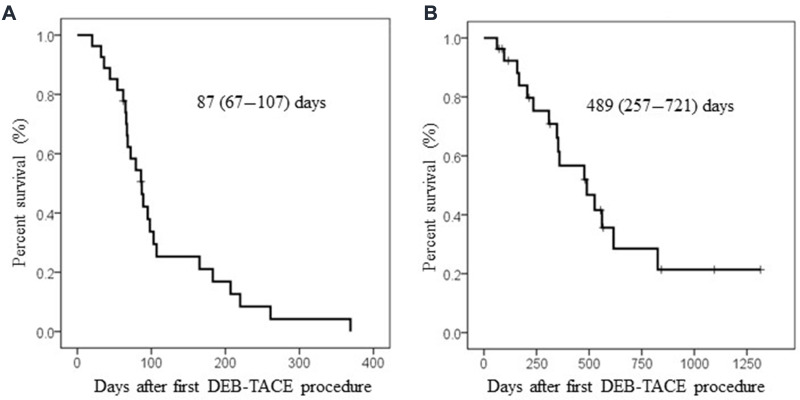
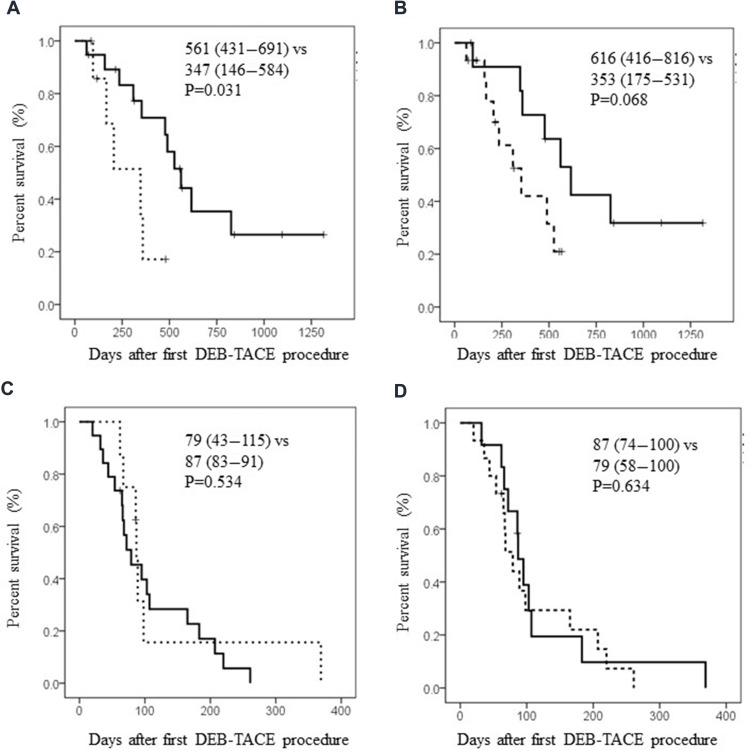
In all cohort, PFS and OS after DEB-TACE were 87 (67–107) and 489 (257–721) days, respectively (Figure 1A and B). When we divided patients according to liver function, patients’ OS was significantly better in Child–Pugh A patients than in Child–Pugh B patients (Figure 2A, MST: 561 (431–691) vs 347 (146–584) days, p=0.031). Furthermore, better tendency of OS was also found in patients with UT7-in criteria (Figure 2B). Interestingly, patients’ PFS was almost similar in both patients between Child A and B or UT7 in and out, Figure 2C, MST: 79 (43–115) vs 87 (83–91) days, p=0.534 and Figure 2D, MST: 87 (74–100) vs 79 (58–100) days, p=0.634. Regarding OS and PFS in patients with or without sorafenib administration, survival benefits could not be shown in sorafenib administration, OS; MST: 235 (9–554) vs 615 (231–1000), p=0.165, PFS: 67 (62–72) vs 107 (77–137), p=0.03, respectively. These results indicate that antitumor effects of DEB-TACE in c-TACE refractory patients is independent of liver reserve function, intrahepatic tumor progression or sorafenib administration.
Figure 1.
Kaplan–Meier analyses after DEB-TACE in overall cohort. Median survival time (95%CI) was shown in the column. (A) Progression-free survival after DEB-TACE. (B) Overall survival after DEB-TACE.
Figure 2.
Kaplan–Meier analyses after DEB-TACE. Median survival time (95%CI) was shown in the column. (A) Overall survival after DEB-TACE according to liver reserve function. Solid line represents survival curve in patients with Child–Pugh A. Dotted line represents that with Child–Pugh B. (B) Overall survival after DEB-TACE according to up-to-seven (UT7) criteria. Solid line represents survival curve in patients with UT7 in. Dotted line represents that with UT7 out. (C) Progression-free survival after DEB-TACE according to liver reserve function. Solid line represents survival curve in patients with Child–Pugh A. Dotted line represents that with Child–Pugh B. (D) Progression-free survival after DEB-TACE according to UT7 criteria. Solid line represents survival curve in patients within UT7. Dotted line represents that without UT7.
Antitumor Response of DEB-TACE
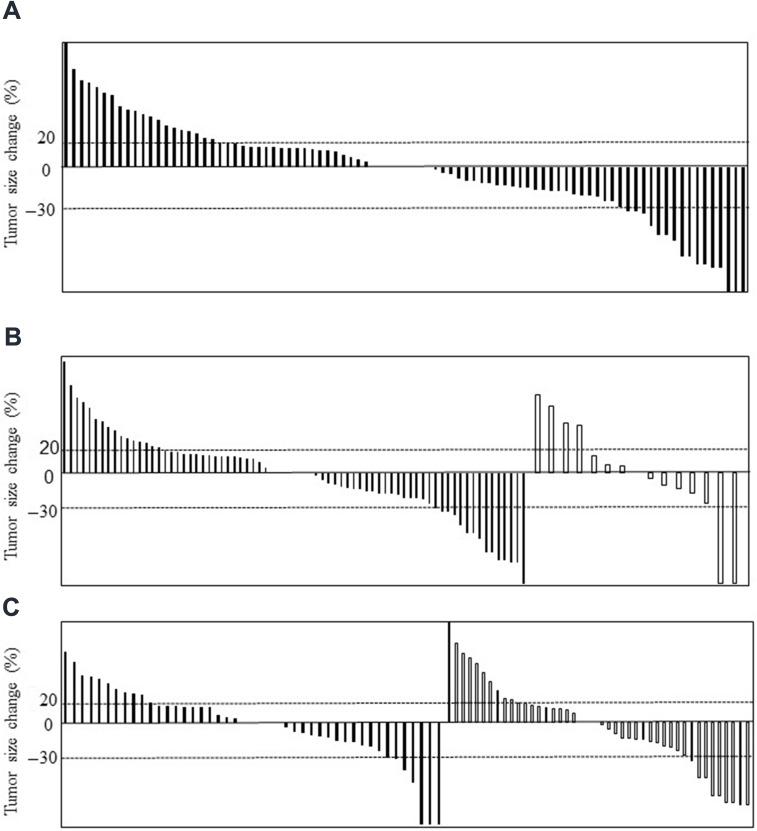
Next, we evaluated antitumor response of DEB-TACE according to mRESIST criteria (Table 2). In overall cohort, objective response was found in 2/27 cases (ORR, 7.4%) and disease control was found in 17/27 cases (DCR, 63.0%). According to liver reserve function, ORR and DCR were 5.3/12.5% and 52.7/87.5% in Child–Pugh A/B, respectively. In addition, regarding with intrahepatic tumor progression, ORR and DCR were 0/16.7% and 66.7/68.4% in UT7 in/out, respectively. For detail evaluation, we evaluated antitumor response according to mALBI criteria (Table 3). ORR and DCR were 0/0/9.1/25.0% and 40.0/57.1/72.7/75.0% in mALBI 1/2a/2b/3, respectively. Regarding with sorafenib administration, 0/15.4% and 57.1/69.2% (9/13) in patients with/without sorafenib administration. Furthermore, we evaluated antitumor response of DEB-TACE in each HCC nodule. Overall, in 89 HCC nodules, ORR was 17/89 nodules (19.1%), and DCR was 68/89 nodules (76.4%) (Figure 3A). According to liver reserve function, ORR and DCR were almost similar between Child–Pugh A and B, ORR: 15/74 nodules (20.3%); DCR: 57/74 nodules (77.0%) and ORR: 2/15 nodules (13.3%); DCR: 11/15 nodules (73.3%), respectively (Figure 3B). Regarding with UT7, ORR, and DCR were 7/45 (15.6%) and 34/45 (75.6%), 10/44 (22.7%) and 34/44 (77.3%) in UT7 in or out, respectively (Figure 3C). Findings strongly suggest that antitumor effects of DEB-TACE in c-TACE refractory patients could be acquired independent of liver reserve function or intrahepatic tumor progression.
Table 2.
Antitumor Best Response of DEB-TACE
| Antitumor Response | Number of Cases (Percent) | ||||
|---|---|---|---|---|---|
| Overall, n=27 | Child–Pugh | Up-to-7 | |||
| Child–Pugh A, n=19 | Child–Pugh B, n=8 | Up-to-7 In, n=15 | Up-to-7 Out, n=12 | ||
| CR | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| PR | 2 (7.4) | 1 (5.3) | 1 (12.5) | 0 (0) | 2 (16.7) |
| SD | 15 (55.6) | 9 (47.4) | 6 (75.0) | 10 (66.7) | 5 (41.7) |
| PD | 10 (37.0) | 9 (47.4) | 1 (12.5) | 5 (33.3) | 5 (41.7) |
Abbreviations: DEB-TACE, drug-eluting beads-transcatheter arterial chemoembolization; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Table 3.
Antitumor Best Response According to mALBI Grade
| Antitumor Response | Number of Cases (Percent) | |||
|---|---|---|---|---|
| mALBI 1 n=5 | mALBI 2a n=7 | mALBI 2b n=11 | mALBI 3 n=4 | |
| CR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| PR | 0 (0) | 0 (0) | 1 (9.1) | 1 (25.0) |
| SD | 2 (40.0) | 4 (57.1) | 7 (63.6) | 2 (50.0) |
| PD | 3 (60.0) | 3 (42.9) | 3 (27.3) | 1 (25.0) |
Abbreviations: mALBI, modified albumin-bilirubin; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Figure 3.
Forrest plot analysis for tumor size change in each HCC nodules after DEB-TACE according to mRESIST criteria. (A) Overall nodules. (B) According to liver reserve function. Solid bars represent change of HCC nodules in patients with Child–Pugh A. Blank bars represent of those with Child–Pugh B. (C) According to UT7 criteria. Solid bars represent change of HCC nodules in patients within UT7. Blank bars represent of those without UT7.
Adverse Events and Hepatic Function During DEB-TACE
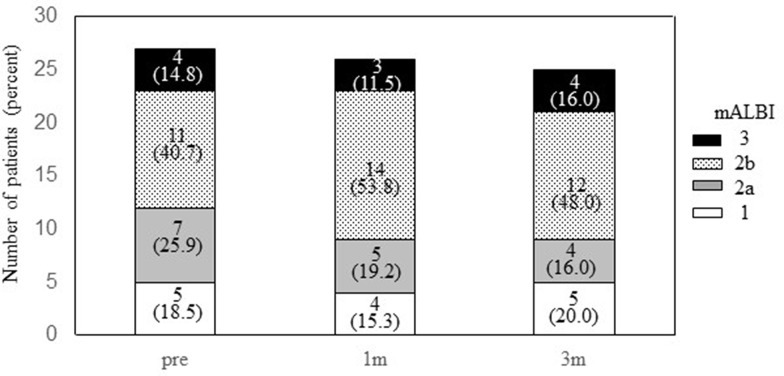
During DEB-TACE procedures, serious AEs above CTCAE grade 3 were not found. Most frequent AE (total 6/27. 22.2%) was elevation of serum aspartate aminotransferase (CTCAE grade 2, 3 cases; grade 1, 3 cases). Another frequent AE was fever, CTCAE grade 1, n=5 (18.5%). Serum alanine aminotransferase elevation was found in three cases: grade 2, one case, (3.7%); grade 1, two cases, (7.4%). Grade 1 bilirubinemia was found in two cases (7.4%). Grade 1 appetite loss was found in two cases (7.4%), and grade 1 thrombocytopenia was occurred in one case (3.7%). Such AEs were similar in patients with or without sorafenib administration in the present study. According to mALBI grade, hepatic reserve function was almost reserved during three months after DEB-TACE (mALBI 1+2a/2b+3, 12/15 at pre-DEB-TACE; 9/17 at one month after DEB-TACE; 9/16 at three months after DEB-TACE) (Figure 4). Thus, DEB-TACE is well-tolerated for patients with refractory to c-TACE, and hepatic reserve function is reserved during the procedures.
Figure 4.
Changes of hepatic reserve function after DEB-TACE. Y-axis represents the number of patients. X-axis represents the timing of assessment. “Pre” means the mALBI at just before DEB-TACE procedure. “1m” and “3m” mean one month and three months after DEB-TACE, respectively. White column represents mALBI 1, and gray, dotted and black represent mALBI 2a, 2b, and 3, respectively. Numbers and those with parenthesis in each column represent number of cases and the percentage.
Discussion
In present study, we showed that DEB-TACE was effective for antitumor response in HCC patients with refractory to c-TACE independent of liver reserve function and intrahepatic tumor progression. Administration of TKIs is a recommended treatment for advanced HCC with TACE refractory. Sorafenib is the first tyrosine kinase inhibitor (TKI) showing a survival benefit and had been a standard treatment for advanced HCC with BCLC-C stage for a decade.19 Recently, lenvatinib, a multikinase inhibitor, has shown noninferiority in survival benefit with sorafenib and has been available in the treatment of advanced HCC.20 Lenvatinib might show better antitumor effects compared with those of sorafenib and those are superior than those of TACE, especially in multiple HCC with BCLC-B stage.20,28 Such good antitumor effect of these TKIs might change therapeutic strategy for advanced HCC. However treatment with TKIs are not recommended for patients with decreased liver reserve function. Present results support that DEB-TACE could be a therapeutic option for advanced HCC with c-TACE refractory and decreased liver reserve function.
Regarding antitumor response of DEB-TACE for c-TACE refractory, our study showed that ORR and DCR were 7.4 and 63.0% in overall cohort and 19.1 and 76.4% in each HCC nodule, and OS was 16.3 months in overall cohort. In previous randomized phase 3 trials for patients naïve to TACE, DCR was reported to be approximately 70%,8,9 consistent with the present result. Furthermore, in these previous randomized trials, antitumor effect of DEB-TACE was similar between Child–Pugh A and B.8,9 These findings strongly suggest antitumor response of DEB-TACE is independent of liver reserve function. Limited study has been found for antitumor response of DEB-TACE for c-TACE refractory. A pilot study with 10 HCC patients showed that DEB-TACE using DC-beads was effective in HCC patients with c-TACE refractory, especially when tumors were small and showed a delayed enhancement pattern.29 Another study showed that DEB-TACE was effective and safe independent of times of previous c-TACE.30 Furthermore, a recent study with HCC patients (including patients with Child–Pugh A, 85.7% and Child–Pugh B, 14.3%) showed that DEB-TACE was effective in HCC patients with multiple c-TACE treatments history compared with continuous c-TACE treatments.31 In this study, a large nodule (more than 7 cm) and advanced BCLC stage (C/D) were independent poor prognostic factors.31 In the present study, although antitumor effect was independent of UT7 criteria, tumor size was relatively small (median 2.5 cm). Findings of previous studies and our present study suggests that relatively small and noninfiltrative HCC might be a good candidate for DEB-TACE, especially applying for patients with c-TACE refractory.
As for adverse events during DEB-TACE, serious AEs were not found in the present study. In previous randomized studies, AEs were similar or decreased in DEB-TACE than c-TACE.8,9 Especially postembolic syndrome, a major AE after TACE, was reduced in DEB-TACE procedures.8,9 In systematic reviews, AEs of DEB-TACE were similar with those of c-TACE, including postembolic syndrome.10–12 In our study, in addition to tolerable AEs, hepatic reserve function was preserved during DEB-TACE procedures. These findings might be helpful in applying DEB-TACE for HCC patients with c-TACE refractory and decreased liver function. In a recent study, DEB-TACE could achieve good tumor responses but had a risk of hepatotoxicity within liver transplant candidates.32 Careful consideration should be required for patients with marginal hepatic reserve function.
Our study has several limitations. First, a retrospective design and limited number of patients weaken the power of study. Especially antitumor effect for HCC patients with both c-TACE refractory and decreased liver reserve function should be evaluated in future. Second, some influence of TKI might be considered in the present study. In the present cohort, sorafenib had been administered to 13 patients with Child–Pugh A (13/19, 68.4%) and two patients with Child–Pugh B (2/8, 25.0%). Some unrecognized AEs might be increased by sorafenib administration, especially in patients with Child–Pugh B. In addition, some advantages in combination with sorafenib and TACE might be found because its synergistic effects were shown in recent studies.33,34 However its synergistic effects were mainly the prolongation of PFS or DCR, and they could not be shown in the present study, suggesting the effect of sorafenib was limited. Third, procedures of TACE are hard to standardize. The treatment outcome of TACE is known to be largely correlated with the technique of TACE. The level of hepatic arterial embolization has been shown to be associated with the prognosis in HCC patients treated with TACE.35 In present study, we performed super-selective DEB-TACE in each HCC nodules. Super-selective TACE technique is fundamental technique to acquire effective therapeutic effects. Furthermore some effects with embolic agents and eluting anticancer drugs might also be considered. In previous studies, the differences in embolic agents and eluted anticancer agents, in addition to TACE techniques, might be found. DEB-TACE with DC bead and doxorubicin was the most frequent procedure in previously published works.8–13 In the present study, microsphere (HepaSphere®) was used as an embolic agent. An in vitro study comparing various DEBs including HepaSphere® and DC bead, the size of DEB after anticancer loading and time in drug elution were shown to be different between each DEB.36 In an in vivo model, HepaSphere® was shown to induce higher local concentration of anticancer drug compared with DC bead.37 Furthermore, we could not show the differences of antitumor effect between epirubicin and arterial cisplatin in the present study. Anticancer effects with different DEBs or eluting anticancer drugs for c-TACE refractory should be confirmed in further studies.
In conclusion, DEB-TACE could be a therapeutic option for advanced HCC patients with c-TACE refractory. Especially relatively small (<3 cm) HCC nodules might be a good target of super-selective DEB-TACE even if for patients with refractory to c-TACE. Therapeutic options for advanced HCC with large, infiltrative, and decreased liver reserve function should be explored in future studies.
Acknowledgments
We thank medical staff of our department, including the residents and nurses, for their help with patient care.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi: 10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. American association for the study of liver D. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elshaarawy O, Gomaa A, Omar H, et al. Intermediate stage hepatocellular carcinoma: a summary review. J Hepatocell Carcinoma. 2019;6:105–117. doi: 10.2147/JHC.S168682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi: 10.1053/jhep.2002.33156 [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X [DOI] [PubMed] [Google Scholar]
- 6.Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131(2):461–469. doi: 10.1053/j.gastro.2006.05.021 [DOI] [PubMed] [Google Scholar]
- 7.Takayasu K, Arii S, Kudo M, et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol. 2012;56(4):886–892. doi: 10.1016/j.jhep.2011.10.021 [DOI] [PubMed] [Google Scholar]
- 8.Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. doi: 10.1007/s00270-009-9711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111(2):255–264. doi: 10.1038/bjc.2014.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie ZB, Wang XB, Peng YC, et al. Systematic review comparing the safety and efficacy of conventional and drug-eluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol Res. 2015;45(2):190–200. doi: 10.1111/hepr.12450 [DOI] [PubMed] [Google Scholar]
- 11.Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta-analysis. Dig Liver Dis. 2016;48:571–577. doi: 10.1016/j.dld.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 12.Chen P, Yuan P, Chen B, et al. Evaluation of drug-eluting beads versus conventional transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41(1):75–85. doi: 10.1016/j.clinre.2016.05.013 [DOI] [PubMed] [Google Scholar]
- 13.Liu YS, Ou MC, Tsai YS, et al. Transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol-doxorubicin versus doxorubicin-loaded beads for the treatment of hepatocellular carcinoma. Korean J Radiol. 2015;16(1):125–132. doi: 10.3348/kjr.2015.16.1.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang YJ, Lee BC, Kim JK, et al. Conventional versus small doxorubicin-eluting bead transcatheter arterial chemoembolization for treating barcelona clinic liver cancer stage 0/A hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2020;43:55–64. doi: 10.1007/s00270-019-02349-9 [DOI] [PubMed] [Google Scholar]
- 15.Ogasawara S, Chiba T, Ooka Y, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87:330–341. doi: 10.1159/000365993 [DOI] [PubMed] [Google Scholar]
- 16.Arizumi T, Ueshima K, Chishina H, et al. Validation of the criteria of transcatheter arterial chemoembolization failure or refractoriness in patients with advanced hepatocellular carcinoma proposed by the LCSGJ. Oncology. 2014;87(Suppl s1):32–36. doi: 10.1159/000368143 [DOI] [PubMed] [Google Scholar]
- 17.Arizumi T, Ueshima K, Minami T, et al. Effectiveness of sorafenib in patients with Transcatheter Arterial Chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015;4(4):253–262. doi: 10.1159/000367743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiraoka A, Kumada T, Kudo M, et al. Hepatic function during repeated TACE procedures and prognosis after introducing sorafenib in patients with unresectable hepatocellular carcinoma: multicenter analysis. Dig Dis. 2017;35(6):602–610. doi: 10.1159/000480256 [DOI] [PubMed] [Google Scholar]
- 19.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 20.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 21.Kudo M, Matsui O, Izumi N, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer. 2014;3(3–4):458–468. doi: 10.1159/000343875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiraoka A, Michitaka K, Kumada T, et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer. 2017;6(4):325–336. doi: 10.1159/000479984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiraoka A, Kumada T, Tsuji K, et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: a multicenter analysis. Liver Cancer. 2019;8(2):121–129. doi: 10.1159/000488778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35–43. doi: 10.1016/S1470-2045(08)70284-5 [DOI] [PubMed] [Google Scholar]
- 25.Kudo M, Arizumi T, Ueshima K, et al. Subclassification of BCLC B stage hepatocellular carcinoma and treatment strategies: proposal of modified Bolondi’s subclassification (kinki criteria). Dig Dis. 2015;33(6):751–758. doi: 10.1159/000439290 [DOI] [PubMed] [Google Scholar]
- 26.Arizumi T, Minami T, Chishina H, et al. Impact of tumor factors on survival in patients with hepatocellular carcinoma classified based on kinki criteria stage B2. Dig Dis. 2017;35(6):583–588. doi: 10.1159/000480186 [DOI] [PubMed] [Google Scholar]
- 27.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(01):52–60. doi: 10.1055/s-0030-1247132 [DOI] [PubMed] [Google Scholar]
- 28.Kudo M. Lenvatinib may drastically change the treatment landscape of hepatocellular carcinoma. Liver Cancer. 2018;7(1):1–19. doi: 10.1159/000487148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song DS, Choi JY, Yoo SH, et al. DC bead transarterial chemoembolization is effective in hepatocellular carcinoma refractory to conventional transarteral chemoembolization: a pilot study. Gut Liver. 2013;7(1):89–95. doi: 10.5009/gnl.2013.7.1.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Zhou J, Zhu DD, et al. CalliSpheres (R) drug-eluting beads (DEB) transarterial chemoembolization (TACE) is equally efficient and safe in liver cancer patients with different times of previous conventional TACE treatments: a result from CTILC study. Clin Transl Oncol. 2018. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Wu F, Duan M, et al. Drug-eluting bead transarterial chemoembolization (TACE) vs conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history: a comparison of efficacy and safety. Medicine (Baltimore). 2019;98(21):e15314. doi: 10.1097/MD.0000000000015314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fidelman N, Johanson C, Kohi MP, et al. Prospective Phase II trial of drug-eluting bead chemoembolization for liver transplant candidates with hepatocellular carcinoma and marginal hepatic reserve. J Hepatocell Carcinoma. 2019;6:93–103. doi: 10.2147/JHC.S206979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tajiri K, Futsukaichi Y, Kobayashi S, et al. Efficacy of on-demand intrahepatic arterial therapy in combination with sorafenib for advanced hepatocellular carcinoma. Onco Targets Ther. 2019;12:2205–2214. doi: 10.2147/OTT.S191741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamakado K, Miyayama S, Hirota S, et al. Hepatic arterial embolization for unresectable hepatocellular carcinomas: do technical factors affect prognosis? Jpn J Radiol. 2012;30(7):560–566. doi: 10.1007/s11604-012-0088-1 [DOI] [PubMed] [Google Scholar]
- 36.Guiu B, Hincapie G, Thompson L, et al. An in vitro evaluation of four types of drug-eluting embolics loaded with idarubicin. J Vasc Interv Radiol. 2019;30(8):1303–1309. doi: 10.1016/j.jvir.2018.12.022 [DOI] [PubMed] [Google Scholar]
- 37.Namur J, Pascale F, Maeda N, et al. Safety and efficacy compared between irinotecan-loaded microspheres HepaSphere and DC bead in a model of VX2 liver metastases in the rabbit. J Vasc Interv Radiol. 2015;26(7):1067–1075 e3. doi: 10.1016/j.jvir.2015.03.014 [DOI] [PubMed] [Google Scholar]