Abstract
Metabolic syndrome (MetS) and osteoporosis are two medical problems plaguing the ageing populations worldwide. Though seemingly distinctive to each other, metabolic derangements are shown to influence bone health. This review summarises the relationship between MetS and bone health derived from epidemiological studies and explains the mechanistic basis of this relationship. The discourse focuses on the link between MetS and bone mineral density, quantitative sonometric indices, geometry and fracture risk in humans. The interesting sex-specific trend in the relationship, probably due to factors related to body composition and hormonal status, is discussed. Mechanistically, each component of MetS affects the bone distinctly, forming a complex interacting network influencing the skeleton. Lastly, the effects of MetS management, such as pharmacotherapies, exercise and bariatric surgery, on bone, are presented. This review aims to highlight the significant relationship between MetS and bone, and proper management of MetS with the skeletal system in mind could prevent cardiovascular and bone complications.
Keywords: bone mineral density, diabetes, dyslipidemia, fracture, hypertension, obesity
Introduction
The skeleton is a dynamic system responsive to internal and external stimuli to provide support to the body and regulate mineral homeostasis. Deterioration of skeletal mass and microstructure will result in osteoporosis and increase the risk of fragility fracture.1 Apart from primary causes, such as sex hormone deficiency and advanced age, metabolic derangements are significant secondary risk factors of skeletal deterioration.2 Meta-analyses reported that diabetes mellitus increases the total [relative risk (RR) 1.32; 95% confidence interval (CI) 1.17–1.48] and hip fracture risk (RR 1.77; 95% CI 1.56–2.02),3 and obesity increases the upper arm [hazard ratio (HR) 1.60, 95% CI 1.42–1.80] and all osteoporotic fracture risk (HR: 1.16; 95% CI 1.09–1.23) after adjustment for bone mineral density (BMD).4 The increasing aged, obese and diabetic populations worldwide highlight the burden of fracture secondary to these conditions.5,6
The definition of metabolic syndrome (MetS) has evolved with time (Table 1), but it pivots on central obesity, hypertension (HPT), hyperglycaemia and dyslipidaemia. MetS alters the mechanical loading, hormonal and biochemical profile of the body, thereby producing complex effects on bone health. While increased mechanical loading and production of certain hormones are protective against osteoporosis, the proinflammatory and pro-oxidative body environment might be detrimental to bone health.7 Thus, the overall impact of MetS on bone health depends on the sum of these interactions. However, the relationship between MetS and bone health reported in epidemiological studies is heterogeneous. Although some studies reported a positive association between MetS and BMD, others reported a negative relationship.8,9 A potential sex difference in the relationship between MetS and bone is also observed.10–12
Table 1.
Definition of MetS
| Definition | Central Obesity | Insulin Resistance | Abnormality in Lipid Metabolism | Hypertension | |
|---|---|---|---|---|---|
| World Health Organization152 (Additional criteria: microalbuminuria) |
Waist/hip ratio: Men > 0.90 Women > 0.85 BMI > 30 kg/m2 |
FBG ≥ 110 mg/dL (6.1 mmol/L) | TG ≥ 150 mg/dL (1.7 mmol/L) | HDL-c Men < 35 mg/dL (0.9 mmol/L) Women < 39 mg/dL (1.0 mmol/L) |
Systolic ≥ 160 and/or diastolic ≥ 90 mm Hg |
| NCEP-ATP III153 | Waist circumference: Men > 120 cm Women > 88 cm |
FBG ≥ 110 mg/dL (6.1 mmol/L) | TG ≥ 150 mg/dL (1.7 mmol/L) | HDL-c Men < 40 mg/dL (1.0 mmol/L) Women < 50 mg/dL (1.3 mmol/L) |
Systolic≥ 130 and/or diastolic ≥ 85 mm Hg |
| International Diabetes Federation154 | Waist circumference: Follow population specific cut-off values |
FBG ≥ 100 mg/dL (5.6 mmol/L) | TG ≥ 150 mg/dL (1.7 mmol/L) or taking drugs to lower TG |
HDL-c Men < 40 mg/dL (1.0 mmol/L) Women < 50 mg/dL (1.3 mmol/L) or taking drugs to improve HDL level |
Systolic≥ 130 and/or diastolic ≥ 85 mm Hg or taking drugs to lower blood pressure |
| AHA/NHLBI33 | Waist circumference: Men > 102 cm Women > 88 cm |
FBG ≥ 100 mg/dL (5.6 mmol/L) or taking drugs to lower FBG |
TG ≥ 150 mg/dL (1.7 mmol/L) or taking drugs to lower TG |
HDL-c Men < 40 mg/dL (1.0 mmol/L) Women < 50 mg/dL (1.3 mmol/L) or taking drugs to improve HDL level |
Systolic≥ 130 and/or diastolic ≥ 85 mm Hg or taking drugs to lower blood pressure |
| Joint Interim Statement30 | Waist circumference: Follow population specific cut-off values |
FBG ≥ 110 mg/dL (6.1 mmol/L) or taking drugs to lower FBG |
TG ≥ 150 mg/dL (1.7 mmol/L) or taking drugs to lower TG |
HDL-c Men < 40 mg/dL (1.0 mmol/L) Women < 50 mg/dL (1.3 mmol/L) or taking drugs to improve HDL level |
Systolic≥ 130 and/or diastolic ≥ 85 mm Hg or taking drugs to lower blood pressure |
Abbreviations: AHA/NHLBI, The American Heart Association and the National Heart, Lung, and Blood Institute; FBG, fasting blood glucose; HDL-c, high-density lipoprotein cholesterol; NCEP-ATP III, National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults III; TG, triglycerides.
This review aims to summarise the relationship between MetS and bone, primarily from epidemiological studies collecting BMD and bone fracture data. According to the World Health Organization, BMD measured by dual-energy X-ray absorptiometry is the standard in defining osteoporosis.13 Studies using alternative bone health assessment methods, such as quantitative ultrasonometry and peripheral quantitative computed tomography, were also discussed. The mechanistic basis of this relationship is discussed in the second part of this review. The review will help the readers to understand that influence of MetS on bone health, beyond its well-recognized impacts on cardiovascular diseases.
Relationship Between MetS and Bone Mineral Density
Positive Association Between MetS and Bone Mineral Density
A retrospective study by Park et al (n=399, women aged 59 ± 6.7 years) suggested a positive association between MetS on BMD. Age-adjusted femoral neck BMD was higher in post-menopausal Korean women with MetS compared to those without MetS, but their lumbar spine BMDs were similar.14 The difference in the strength of association between the two skeletal sites could be attributed by weight-bearing capacity, in which femoral neck endures more loading compared to lumbar spine.15
Multiple cross-sectional studies reported a positive relationship between MetS and BMD.16–19 The Third National Health and Nutrition Examination Survey (NHANES III) (n=8197, ≥20 years) reported a higher femoral neck BMD among subjects with MetS compared to subjects without MetS. Adjustment for BMI attenuated the difference in BMD between the two groups.17 However, this study only measured one skeletal site. The Rancho Bernardo Study (417 Caucasian men and 671 Caucasian women aged 38 to 97 years) reported that subjects with MetS had higher total hip BMD compared to non-MetS subjects. Men with MetS in the study also had a higher femoral neck BMD compared to men without MetS. However, BMI adjustment reversed the relationship between MetS and BMD.18 Additionally, a positive relationship between MetS and lumbar spine T-score was found among subjects from Saudi Arabia (1578 subjects, >35 years) with high BMI (BMI > 25 kg/m2 for 87.3% women and 79.4% men recruited).19
More conclusive evidence on the positive influence of MetS on BMD comes from a three-year longitudinal study by Kim et al (n=1128). In this study, Korean post-menopausal women with MetS experienced 31.1%, 26.7%, 21.7% and 12.0% less BMD loss at the lumbar spine, trochanter, total hip and femoral neck compared to non-MetS women, respectively. The rate of bone loss also decreased with an increasing number of MetS components.20 It should be noted the subjects were recruited from a health screening centre, who could be more health-conscious and have an upper socioeconomic background. Thus, they might not represent the general population.
In a meta-analysis by Xue et al (11 studies, n=13,122), lumbar spine and femoral neck BMD values unadjusted for BMI was higher in MetS subjects compared to non-MetS subjects. The association between MetS and femoral neck BMD was more prominent in the Caucasians than Asians. The unadjusted BMD was used because the adjustment could distort the relationship between BMD and MetS.8 Multiple studies suggested that the positive association between MetS and BMD was driven by mechanical loading reflected through BMI.17,18 This observation is evident when BMI-adjustment attenuates or reverses the association between MetS and BMD. However, a high BMI could reflect either large body size or high adiposity, whereas waist circumference is a better measure of adiposity.21 At its existing cut-offs, BMI is also an imprecise method to measure obesity in the elderly.22 In lieu of this opinion, a study among Korean post-menopausal women (n=3058) aged >50 years old classified the subjects into waist circumference (WC) obesity (>80 cm) and BMI obesity (>25 kg/m2). The study found that the prevalence of osteoporosis was lower in subjects classified as having BMI obesity with or without WC obesity.23 On the other hand, the prevalence of osteoporosis was higher among non-obese subjects and subjects with WC obesity only. Thus, BMI adjustment can delineate the effects of mechanical loading in the relationship between MetS and BMD.
Overall, the positive association between bone health and MetS may be mediated by body size reflected by BMD.
Negative Association Between MetS and Bone Mineral Density
Multiple studies among the East Asian populations demonstrated a negative relationship between MetS and BMD. A retrospective study by Chen et al reported that both MetS and the associated non-alcoholic fatty liver disease were positively associated with osteoporosis among Chinese post-menopausal women (n=938).24
The negative relationship was further consolidated in cross-sectional studies. Hwang and Choi reported that the lumbar spine BMD adjusted for age, weight and height decreased with increasing MetS components in Korean adult women (n=2548).25 Similarly, body weight-adjusted femoral neck BMD was lower in Korean men (n=1780, 55.7 ± 8.1 years) and post-menopausal women (n=1109, 57.1 ± 6.7 years) with MetS compared to subjects without MetS in the study by Kim et al.26 In a study by Jeon et al, pre- (n=1234) and post-menopausal Korean women (n=931) with MetS showed a lower lumbar spine BMD after multiple adjustments, including weight. The post-menopausal women with MetS also showed a lower femoral neck BMD.27 The authors postulate that the effects of MetS on BMD are more significant among post-menopausal women because they are not protected by oestrogen.
The studies in this section considered only women. Since apparent differences in body composition and hormonal profile exist between men and women, it would be interesting to explore if sex influences the relationship between MetS and BMD.
Sex-Specific Relationship MetS and Bone Mineral Density
Several sex-specific trends on the association between MetS and BMD have been observed. Two retrospective studies among Taiwanese population showed protective effects of MetS on skeletal health of men but not in women >50 years. In these studies, men with MetS had a higher BMD at the lumbar spine and the hip28 and were less likely to have osteoporosis compared to non-MetS men.29 The relationship between MetS and BMD was negative in women was negative28 or negligible.29 Multiple adjustments for confounders attenuated the relationship between MetS and bone health in both sexes.29 Thus, the overall effects of MetS on bone are weak and inconsistent in both studies.28,29
Two studies on the Caucasian populations showed that MetS protects bone health in women but not in men.10,12 In the Camargo Cohort Study (n=1508, >50 years), women with MetS showed higher age-adjusted BMD at the femoral neck, lumbar spine and total hip compared to women without MetS. Again, adjustment for BMI attenuated most differences, except for the total hip.12 The presence of MetS did not influence BMD of men in the same study.12 In the Berlin Aging Study II (n=1402, 68±4 years), the relationship between MetS and lumbar spine and total hip BMD was positive among women, but not significant among men.10
A series of studies in Korean population showed that MetS was a negative predictor of BMD in men but not in women. The Korean National Health and Nutrition Examination Survey (KNHANES, n=3207) reported that BMD of the femoral neck, total hip and lumbar spine between subjects with MetS (defined by Joint Interim Statement)30 and those without MetS was similar after multiple adjustments, including BMI. However, men with MetS had higher odds of suffering from suboptimal bone health (T-score < −1).31 Re-analysis using National Cholesterol Education Program Adult Treatment Panel III definition32 showed that adjusted BMD of the lumbar spine and femoral neck was consistently lower in young (<45 years) and older men with MetS compared to subjects without MetS (n=2989). However, pre- and post-menopausal women with MetS showed similar BMD values.11 Using the criteria of American Heart Association/National Heart, Lung, and Blood Institute,33 Korean men (n=6659, aged 43.62±0.3 years) with MetS showed lower total hip and femoral neck BMD compared to non-MetS counterparts after multiple adjustments, including BMI. Again, the difference was not observed in pre- (n=4547, aged 35.15±0.19 years) and post-menopausal women (n=3279, aged 63.17±0.26 years).34
A meta-analysis by Zhou et al (9 studies, n=18,380) showed that subjects without MetS had a higher lumbar spine and femoral neck BMD. The negative effects of MetS were more prominent in men compared to women.9
Overall, there is an apparent sex difference in the relationship between MetS and bone health. When sex difference is studied, men seem to be more susceptible to the negative effects of MetS on bone. Some researchers postulate that at any given BMI, men had higher visceral fat compared to women, so they are more susceptible to the adverse effects of MetS.34
Relationship Between MetS and Quantitative Ultrasound
Quantitative ultrasound is another method of assessing individual bone health.35 Two of the basic quantitative ultrasound indices (QUIs) are speed of sound, which reflects the distance travelled by the ultrasound across time (m/s), and broadband ultrasound attenuation, which refers to the slope between ultrasound signal attenuation due to absorption by cortical bone or scattering by trabecular bone in relationship with its frequency (dB/MHz).36 Other composite QUIs can be derived from these two basic indices, but they differ between devices. In general, higher values of QUIs indicate better bone health.35 QUI has been shown to correlate with bone mass and microarchitecture,37,38 and it can predict fracture risk.39 QUIs also showed a high diagnostic accuracy of osteoporosis with modified cut-offs.40,41
In the PREMED study (124 men aged 55 −80 years and 127 women, aged 60–80 years), BUA and QUI were higher in subjects with MetS compared to those without after adjustment for sex. BUA was also higher in subjects with diabetes compared to normal subjects.42 In the study of Cvijetic et al (n=211 drawn from voter registry, aged 77.9±4.5 years), QUI was significantly lower in men with MetS but higher in women with MetS compared to their non-MetS counterparts. In the multivariate regression model, adjustment for BMI weakened the relationship between MetS and QUI in women but not in men. Further adjustment for glucose level diminished the relationship between MetS and QUI in men.43
In the Camargo Cohort Study, women with MetS (n=788, aged 63±9 years) showed higher age-adjusted QUI compared to those without MetS but the BMI-adjusted QUI values were similar between the two groups. MetS was not associated with QUI in all models for men (n=421 aged 65±9 years old) in the study.44 Similarly, Chin et al showed reported that MetS was not significantly associated with the calcaneal speed of sound among 303 Malaysian men of Chinese and Malay ethnicity aged >40 years old.45
Overall, the effects of MetS on QUI may be similar to BMD, which might be mediated by mechanical loading reflected by BMI.
Relationship Between MetS and Bone Geometry
Evidence on the association between MetS and bone geometry in human is somewhat limited. The Rotterdam Study showed that MetS was associated with lower bone width and cortical bucking ratio, indicative of bone instability, in women (n=2040, aged 72.38 ±6.81 years) but not in men (n=1510, aged 72.04 ±6.51 years).46
Table 2 summarises the relationship between MetS and bone health, including BMD, QUI and bone geometry.
Table 2.
Summary of the Relationship Between MetS and Bone Health
| Study | Study Design | Subjects Characteristics | Definition of MetS | Summary of Bone Health Assessment |
|---|---|---|---|---|
| Xue et al (2012)8 | Meta-analysis of 9 studies (1 outlier study excluded) | 11 studies included for review (10 for LS, 7 for FN BMD), involving 13,122 subjects, 2779 were MetS. | NCEP-ATPIII | MetS was associated with increased spine BMD [weighted mean difference (WMD) 0.03; 95% CI 0.01~0.04] not femoral BMD (WMD 0.01; 95% CI −0.01~0.03) |
| Zhou et al (2013)9 | Meta-analysis of 9 studies published before 2013 | Studies that compared BMD between subjects with or without MetS | NCEP-ATPIII | Subjects without MS had higher BMD at: Femoral neck: WMD 0.02 (95% CI 0.01~0.03) Lumbar spine: WMD 0.01 (95% CI 0.00~0.03) Sub-analysis: covariate-adjusted lumbar spine BMD significantly higher in men only (WMD 0.02, 95% CI 0.01~0.03). |
| von Muhlen et al (2007)18 | Rancho Bernardo Study, a longitudinal cohort study with mean follow-up of 2 years. | Community-dwelling ambulatory men (417) and women (671), all Caucasian in southern California, aged 38 to 97 years. | NCEP-ATPIII | MetS reduced the femoral neck BMD of in men after BMI-adjustment (0.737; SEM 0.013 g/cm2 in MetS vs 0.769; SEM 0.007 g/cm2 in non-MetS). Positive predictors of BMD in women (age-adjusted): TG for total hip (β 0.104; p 0.004) and spine (β 0.107; p=0.006) WC for total hip (β 0.293; p <0.001), femoral neck (β 0.225; p<0.001) and spine (β 0.267; p<0.001) FBG for femoral neck (β 0.093; p=0.01) Positive predictors of BMD in men (age-adjusted): HDL for total hip (β 0.093; p=0.01) WC for total hip (β 0.368; p<0.001), femoral neck (β 0.287; p <0.001) and spine (β 0.347; p<0.001) FBG for spine (β −0.136; p=0.006) |
| Kinjo et al (2007)17 | NHANES III: A cross-sectional study | 8197 Americans > 20 years. Mean age for MetS = 56.7±16.7 years; non-MetS = 44.2±8.7 years. | NCEP-ATPIII | Femoral neck BMD increased in subjects with MetS (0.86; 95% CI 0.85~0.86 g/cm2) compared to subjects without MetS (0.80; 95% CI 0.80~0.80 g/cm2) (p<0.001). BMI-adjustment attenuated the difference (p>0.05). Abdominal obesity increased femoral neck BMD (0.86; 95% CI 0.85~0.86 g/cm2 vs 0.78; 95% CI 0.78~0.79 g/cm2 for those without abdominal obesity) |
| Park et al (2010)14 | Cross-sectional study | 399 post-menopausal Korean women (mean age 59.4, SD=6.7 years) who went to a medical hospital for check-up | NCEP-ATPIII | Femoral neck BMD was higher in women with MetS (0.82; SD 0.12 g/cm2) compared to those without MetS (0.85; SD 0.09 g/cm2) in age-adjusted model (p=0.011). Further adjustment with BMI attenuated the difference (p>0.05). |
| Kim et al (2010)155 | Cross-sectional study | 1780 men > 40 years (55.7 ± 8.1 years) and 1108 post-menopausal women (57.1 ± 6.7 years) attending a health promotion centre. | IDF and AHA/NHLBI | Men with MetS had lower BMD adjusted for all covariates: AHA/NHLBI criteria: MetS 0.813, SD 0.008 g/cm2; non-MetS 0.839, SD 0.004 g/cm2; p=0.002 IDF criteria: MetS 0.766, SD 0.012 g/cm2; non-MetS 0.839, SD 0.003 g/cm2; p<0.001 Postmenopausal women with MetS had lower BMD adjusted for all covariates: AHA/NHLBI criteria: MetS 0.684, SD 0.008 g/cm2; non-MetS 0.721, SD 0.004 g/cm2; p<0.001 IDF criteria: MetS 0.675, SD 0.011 g/cm2; non-MetS 0.719, SD 0.004 g/cm2; p<0.001 MetS components associated with BMD: Men: WC (β −0.250, p<0.001) Women: WC (β −0.138, p<0.001), TG (β −0.097, p=0.001) |
| Hwang and Choi (2010)25 | Cross-sectional study | 2548 women aged 18 years attending a health promotion centre | NCEP-ATPIII | Women without MetS (0.898, SEM 0.007 g/cm2) had higher vertebral BMD compared to those with (0.914, SEM 0.003 g/cm2) (p=0.031). Women without abdominal obesity (0.922, SEM 0.004 g/cm2) had higher vertebral BMD compared to those without (0.894, SEM 0.005 g/cm2) (p<0.001). Women without hypertriglyceridemia (0.916, SEM 0.003 g/cm2) had higher vertebral BMD compared to those without (0.895, SEM 0.006 g/cm2) (p=0.002). |
| Hernández et al (2010)12 | Camargo Cohort Study: A cross-sectional Study (2006–2009) | 1508 subjects (495 men and 1013 women) > 50 years, > 90% Caucasians from Cantabria, Spain | NCEP-ATPIII | In women, age-adjusted BMD values were higher in those with MetS: Lumbar spine - MetS: 0.942, SEM 0.008 g/cm2; non-MetS: 0.909, SEM 0.005 g/cm2; p=0.001 Femoral neck - MetS: 0.755, SEM 0.007 g/cm2; non-MetS: 0.709, SEM 0.004 g/cm2; p<0.001 Total hip - MetS: 0.886, SEM 0.007 g/cm2; non-MetS: 0.831, SEM 0.004 g/cm2; p=0.001 BMI adjustment attenuated differences at all site (p>0.05) In women, components of MetS associated with BMD were: Lumbar spine – WC (mean difference 0.110, p= 0.005) and HPT (mean difference 0.204, p< 0.001) Femoral neck - WC (mean difference 0.123, p= 0.001) and HPT (mean difference 0.277, p<0.001) Total hip - WC (mean difference 0.133, p<0.001) and HPT (mean difference 0.354, p<0.001) |
| Hernández et al (2011)44 | Camargo Cohort Study: A cross-sectional study$ | 1209 adults (421 men aged 65±9 (50–92 years) and 788 women 63±9 (44–88 years)) visiting a primary care centre | JIS | Women with MetS had higher quantitative ultrasound indices adjusted for age compared to women without: SOS: 1545(2.2) vs 1538(1.5), p=0.01 BUA: 68(1.3) vs 63(0.8), p=0.006 QUI: 91(1.4) vs 85(0.9), p=0.008 Further adjustment for BMI attenuated the difference (p>0.05) In women, WC was associated with all quantitative ultrasound indices SOS: β 0.127, p=0.002 BUA: β 0.164, p< 0.0001 QUI: β 0.354, p< 0.0001 MetS did not influence quantitative ultrasound indices in men. |
| Bullo et al (2011)42 | The NUTERA-PREDIMED Cohort study: A large, parallel-group, randomized, controlled study$ | 251 subjects with 124 men and 127 women, aged between 55–80 years (men) or 60–80 years (women) from Spain | NCEP-ATPIII | Subjects with MetS (74.35, SEM 1.83) had higher BUA than those without (79.04, SEM 1.36) (p=0.045). Other quantitative ultrasound indices were similar between the two groups (p>0.05). Subjects with type II diabetes mellitus (79.41, SEM 1.43) had higher BUA than those without (74.73, SEM 1.62) (p=0.032). Other quantitative ultrasound indices were similar between the two groups (p>0.05). All comparison adjusted to sex only. |
| Cvijetic et al (2011)43 | Cross-sectional study$ | 211 men and women, mean age 77.9±4.5 years, randomly selected from voter registry. | IDF | Men with MetS (94.0, SD 23.7) had lower QUI compared to men without (108.5, SD 13.9) (p=0.007), even after BMI-adjustment. Women with MetS (81.7, SD 23.7) had higher QUI compared to women without (69.5, SD 13.9) (p=0.01). After BMI adjustment, the difference was not significant. Diabetes was associated with QUI (β=0.44, p=0.005) in men. Obesity (β=0.45, p=0.035) and WC (β=0.57, p=0.027) was associated with QUI in women. |
| Jeon et al (2011)27 | Cross-sectional study | 2265 women (1234 premenopausal and 931 postmenopausal) > 45 years visiting the Health Promotion Center | NHLBI | Premenopausal women Women without MetS had higher BMD compared to those with MetS after adjusting for all covariates. Lumbar spine: 1.154, SEM 0.004 vs 1.110, SEM 0.017 (MetS); p=0.014 Femoral neck: 0.903, SEM 0.003 vs 0.885, SEM 0.013 (MetS); p=0.177 Predictor of lumbar spine BMD: SBP (β −0.122, p<0.001) Predictor of femoral neck BMD: DBP (β −0.112, p<0.001) and WC (β 0.079, p=0.007) Post-menopausal women Women without MetS had higher BMD compared to those with MetS after adjusting for all covariates. Lumbar spine: 0.147, SEM 0.005 vs 1.111, SEM 0.014 (MetS); p=0.017 Femoral neck: 0.896, SEM 0.004 vs 0.870, SEM 0.010 (MetS); p=0.016 Predictor of lumbar spine BMD: DBP (β −0.080, p=0.014) Predictor of femoral neck BMD: DBP (β −0.097, p=0.002) and CRP (β −0.064, p=0.001) |
| Kim et al (2013)34 | KNHANES: A cross-sectional study | 14,485 adults (6659 men and 7826 women) from South Korea. | NHLBI | Men with MetS had lower BMD compared to those without MetS after adjusting for all covariates including BMI. Total hip: 0.962, SEM 0.004 (MetS) vs 0.971, SEM 0.003; p=0.030 Femoral neck: 0.801, SEM 0.004 (MetS) vs 0.814, SEM; 0.003 p<0.001 MetS did not influence the BMD of women. Predictors of lumbar spine BMD: Linear regression - Men: TG (β −70.91, p<0.001) Premenopausal women: HDL-c (β −6.08, p=0.002) Postmenopausal women: None Logistic regression - Men: Obesity (OR 1.393, 95% CI 1.330~1.458), abdominal obesity (OR 1.339, 95% CI 1.271~1.410), diabetes mellitus (OR 1.103, 95% CI 1.017~1.196), high TG (OR 0.927, 95% CI 0.865~0.994) Premenopausal women: Obesity (OR 1.696, 95% CI 1.572~1.830), abdominal obesity (OR 1.606, 95% CI 1.504~1.714), LDL-c (OR 1.145, 95% CI 1.071~1.224) Postmenopausal women: Obesity (OR 1.512, 95% CI 1.394~1.640), abdominal obesity (OR 1.349, 95% CI 1.241~1.467), HPT (OR 1.133, 95% CI 1.029~1.246) Predictors of total hip BMD (components of MetS): Linear regression - Men: WC (β −2.41, p=0.007), insulin (β −4.58, p<0.001), HOMA-IR (β −1.11, p<0.001), TG (β −78.88, p<0.001) Premenopausal women: insulin (β −1.74, p=0.009), HOMA-IR (β −0.37, p=0.034) Postmenopausal women: None Logistic regression - Men: Obesity (OR 1.734, 95% CI 1.628~1.847), abdominal obesity (OR 1.471, 95% CI 1.378~1.569), high TG (OR 0.922, 95% CI 0.854~0.994) Premenopausal women: Obesity (OR 2.214, 95% CI 2.033~2.412), abdominal obesity (OR 1.971, 95% CI 1.833~2.119), LDL-c (OR 1.086, 95% CI 1.007~1.171), HDL-c (OR 1.230, 95% CI 1.033~1.465) Postmenopausal women: Obesity (OR 1.850, 95% CI 1.665~2.056), abdominal obesity (OR 1.651, 95% CI 1.487~1.833) Predictors of femoral neck BMD (components of MetS): Linear regression - Men: DBP (β −4.50, p=0.001), insulin (β −4.16, p<0.001), HOMA-IR (β −1.09, p<0.001), triglycerides (β −115.25, p<0.001) Premenopausal women: WC (β 2.13 p=0.007), HOMA-IR (β −0.33, p=0.036) Logistic regression – Men: Obesity (OR 1.559, 95% CI 1.467~1.657), abdominal obesity (OR 1.438, 95% CI 1.347~1.534), hypertension (OR 0.937, 95% CI 0.879~0.998) high triglyceride (OR 0.862, 95% CI 0.798~0.932) and MetS (OR 0.899, 95% CI 0.840~0.962) Premenopausal women: Obesity (OR 1.939, 95% CI 1.776~2.117), abdominal obesity (OR 1.802, 95% CI 1.677~1.936), high triglycerides (OR 1.244, 95% CI 1.040~1.488) Postmenopausal women: Obesity (OR 1.751, 95% CI 1.558~1.968), abdominal obesity (OR 1.606, 95% CI 1.426~1.809) |
| Kim et al (2013)31 | KNHANES: A cross-sectional study | 3207 South Koreans, mean age for men = 48.4 years, premenopausal women = 36.5 years and postmenopausal women = 64.8 years. | JIS | MetS was associated with low bone mass (osteopenia+osteoporosis) in multiple adjusted models: Overall subjects: OR 1.38, 95% CI 1.07~1.79 Men: OR 1.49, 95% CI 1.04~2.14 MetS was not associated with low bone mass Men with hyperglycemia had higher total hip BMD (mean difference compared to normal men 0.018, SEM 0.007, p<0.05) Men with high TG had lower femoral neck BMD (mean difference compared to normal men −0.018, SEM 0.007, p<0.01) Women with high TG had higher BMD at all site Lumbar spine: mean difference 0.021, SEM 0.010, p<0.05 Femoral neck: mean difference 0.016, SEM 0.007, p<0.05 Total hip: mean difference 0.019, SEM 0.008, p<0.05 |
| Kim et al (2013)11 | KNHANES cross-sectional study | 2989 South Koreans > 20 years. | NCEP-ATPIII | Men with MetS had higher BMD in fully adjusted models: NCEP <45 years Femoral neck 0.876, SD 0.004 (MetS) vs 0.843, SD 0.014, p<0.05 Spine 1.000, SD 0.004 (MetS) vs 0.979, SD 0.013, p=0.147 ≥45 years Femoral neck 0.773, SD 0.004 (MetS) vs 0.746, SD 0.008, p=0.01 Spine 0.955, SD 0.006 (MetS) vs 0.927, SD 0.011, p=0.05 IDF <45 years Femoral neck 0.877, SD 0.004 (MetS) vs 0.826, SD 0.016, p=0.01 Spine 1.001, SD 0.004 (MetS) vs 0.963, SD 0.016 p=0.05 ≥45 years Femoral neck 0.772, SD 0.004 (MetS) vs 0.745, SD 0.010, p=0.05 Spine 0.95, SD 0.005 (MetS) vs 0.919, SD 0.013, p=0.05 Similar differences in BMD were not observed in women. Predictors of femoral neck BMD: Men < 45 years: WC (β −0.007, p<0.001), SBP (β 0.001, p<0.05), DBP (β −0.002, p<0.01), TG (β −0.001, p<0.05) Men ≥ 45 years: WC (β −0.004, p=0.001), DBP (β −0.001, p=0.05) Premenopausal women: TG (β −0.001, p<0.05) Postmenopausal women: WC (β −0.002, p<0.05) Predictors of spine BMD: Men < 45 years: WC (β −0.005, p<0.001), SBP (β 0.001, p<0.01), DBP (β −0.002, p<0.01), TG (β −0.001 p<0.05) Men ≥ 45 years: WC (β −0.005, p<0.01), SBP (β 0.001, p<0.01), DBP (β −0.002 p<0.01) Premenopausal women: WC (β −0.001, p<0.05), SBP (β 0.001, p<0.05), DBP (β −0.001 p<0.05), HDL-c (β −0.001, p<0.05) Postmenopausal women: WC (β −0.002, p<0.05) |
| Kim et al (2013)20 | 3-year retrospective longitudinal study, follow-up period 3 years | 1218 post-menopausal women | NCEP-ATPIII | More decline in BMD in women with MetS (Bone site: Annualized BMD % change (95% CI) of MetS vs No MetS, p-value) Total femur: −1.118 (−1.195~−1.041) vs −0.895 (−1.045~−0.745), p=0.011 Femur neck: −1.078 (−1.157~−0.998) vs −0.904 (−1.059~−0.749), p=0.048 Trochanter: −1.503 (−1.629~−1.377) vs −1.128 (−1.373~−0.882), p=0.009 Lumbar spine: −0.877 (−0.990~−0.765) vs −0.619 (−0.838~−0.399), p=0.043 Predictors of % change of BMD: Total femur: WC β 0.105, p=0.025; TG β 0.076, p=0.006 Trochanter: WC β 0.135, p=0.005; TG β 0.085, p=0.003 |
| El Maghraoui et al (2014)53 | Cross-sectional study | 270 post-menopausal women with mean age 61.0 years ± 7.8 (range 50 to 90) from Rabat area, Morocco. | NCEP-ATPIII | Metabolic syndrome was associated with osteoporosis (OR 0.291, 95% CI 0.130~0.651) |
| Muka et al (2015)46 | The Rotterdam Study: A cross-sectional study with prospective follow up (mean follow-up 6.7 years) for fracture risk | 2040 women (mean age 72.38 ±6.81 years) and 1510 men (72.04 ± 6.51 years) from Dutch | JIS | MetS was associated with: Bone width: Women (β −0.054, 95% CI −0.091~-0.018), men (β −0.029 95% CI −0.068~0.010) Buckling ratio: Women (β −0.81, 95% CI −1.34~-0.27), NS for men. Longitudinal bone loss was associated with MetS in women (β 0.028, 95% CI 0.012~0.043) in fully adjusted model, NS for men. Predictors for femoral neck BMD: Women: hyperglycemia (β=0.016, p=0.01), HDL-c (β=0.013, p=0.01) Men: hyperglycemia (β=0.022, p=0.004), WC (β=−0.030, p=0.004) |
| Eckstein et al (2016)10 | Berlin Aging Study II: A cross-sectional study | 1402 subjects (51.1% women) aged 68 ± 4 years | JIS | In women, MetS was associated positively with BMD in the fully adjusted model: Spine: β 0.369 p=0.005 Hip: β 0.202 p=0.028 NS for femoral BMD In men, MetS was not associated with BMD of any site in the fully adjusted model. In men with MetS, BMD was associated with trunk fat: Hip: β –0.039, p=0.004 Femur: β –0.042, p=0.002 In men with or without MetS, BMD was associated with WC: Spine - No MetS: β –0.089, p=0.024; MetS: β –0.099, p=0.043 Hip - No MetS: β –0.075, p=0.001; MetS: β –0.054, p=0.043 Femur - No MetS: β –0.079, p=0.001; MetS: β –0.091, p=0.001 |
| Lin et al (2018)29 | Cross-sectional study | 2007 subjects (1045 men) aged ≤ 50 years (average 58.9 years) who went to a preventive examination agency in urban Taiwan | Definition of Bureau of Health Promotion, Taiwan | MetS was not significant associated with BMD in men: BMI< 24 kg/m2: 0.579, 95% CI 0.213~1.575 BMI≥ 24 kg/m2: 0.986, 95% CI 0.390~2.496 MetS was not significant associated with BMD in men: BMI< 24 kg/m2: 0.579, 95% CI 0.213~1.575 BMI≥ 24 kg/m2: 0.986, 95% CI 0.390~2.496 None of the MetS components was associated with BMD in both men and women. |
| Loke et al (2018)28 | Retrospective cross-sectional study | 1162 subjects (59.5% men) from a hospital in southern Taiwan with mean age 59.9 ± 7.3 years | NCEP-ATPIII | MetS was associated with decreased BMD in age-adjusted model (osteoporosis+osteopenia): Men: OR 0.35, 95% CI 0.18~0.68 Women: OR 2.24, 95% CI 1.06~4.69 In men, decreased BMD was associated with: Central obesity: OR 1.06, 95% CI 1.03~1.10 Hyperglycemia: OR 0.99, 95% CI 0.99~1.00 In women, no significant relationship between decreased BMD and MetS components. |
| Chen et al (2018)24 | Cross-sectional study | 938 post-menopausal women from Eastern China | Diabetes Society of Chinese Medical Association | MetS (OR 2.132, 95% CI 1.166~3.900) NAFLD (OR 1.945, 95% CI 1.162~3.253) or both conditions together (OR 5.632, 95% CI 3.281~9.666) predicted higher risk of osteoporosis. |
| Wani et al (2019)19 | Cross-sectional study | 1587 subjects (84.7% women), aged > 35 years from hospitals around Riyadh city, Saudi Arabia. | NCEP-ATPIII | Men: 3rd tertile T-score predicted high risk of MetS (OR 2.12, 95% CI 1.1~4.6) 3rd tertile T-score predicted high risk of central obesity (OR 3.07, 95% CI 1.2~7.5) Women: 2nd tertile T-score (OR 1.46, 95% CI 1.1~2.0) and 3rd tertile (OR 1.47, 95% CI 1.1~2.0) T-score predicted high risk of MetS. 2nd tertile T-score (OR 1.83, 95% CI 1.3~2.6) and 3rd tertile (OR 1.97, 95% CI 1.4~2.8) T-score predicted high risk of MetS. |
| Kim and Kim (2019)23 | KNHANES cross-sectional study (2008–2011) | 3058 Korean post-menopausal women ≥ 50 years. The subjects were classified as having WC obesity (≥80 cm), BMI obesity (>25 kg/m2) or both. | NCEP-ATPIII | Compared to non-obese subjects, the risk of osteoporosis: Reduced in BMI-defined obesity: OR 0.42, 95% CI 0.18~0.99 Reduced in BMI or WC-defined obesity: OR 0.66, 95% CI 0.44~1.00 |
| Chin et al (2020)16 | Cross-sectional study | 400 Malaysians aged ≥ 40 years (52.5% women) in Klang Valley | Joint interim statement | Before BMI-adjustment Lumbar spine: β 0.128, 95% CI 0.022~0.035 Femoral hip: β 0.165, 95% CI: 0.081~0.249 Osteoporosis risk: OR 0.545, 95% CI 0.221–1.346 After BMI-adjustment Lumbar spine: β 0.009, 95% CI −0.097~0.115 Femoral hip: β 0.006, 95% CI −0.079~0.091 Osteoporosis risk: OR 0.907, 95% CI 0.186~4.411 |
Notes: All the studies performed the BMD measurement via dual-energy X-ray absorptiometry, except for42–44 (labeled with$), which used quantitative ultrasonometry.
Abbreviations: β, standard regression coefficient; AHA/NHLBI, American Heart Association/National Heart, Lung, and Blood Institute; BMD, bone mineral density; BMI, body mass index; BUA, broadband ultrasound attenuation; CI, confidence interval; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL-c, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; HPT, hypertension; IDF, International Diabetes Federation; LDL-c, low-density lipoprotein cholesterol; MetS, metabolic syndrome; NAFLD, non-alcoholic fatty liver disease; NCEP-ATP–III, National Cholesterol Education Program Adult Treatment Panel III; NS, not significant; OR, odds ratio; QUI, quantitative ultrasound index; SBP, systolic blood pressure; SD, standard deviation; SEM, standard error of mean; SOS, speed of sound; TG, triglyceride; WC, waist circumference; WMD, weighted mean difference.
Relationship Between MetS and Bone Fracture
Another indicator of osteoporosis is fragility fracture since most patients are not aware of their bone health status until a fracture occurs. Each component of MetS exerts distinct influence on fracture risk. For instance, increased mechanical loading and estradiol synthesis due to obesity could preserve bone mass and prevent fracture.47,48 Type 2 diabetes is associated with increased BMD but higher fracture risk.49,50 Variation in lipid profile is also associated with fracture risk.51
Several studies reported reduced fracture risk among MetS subjects compared to non-MetS subjects.52–54 In the Tromsø Study (n=26,991, 25–98 years), subjects with MetS showed decreased non-vertebral fracture (NVF) risk compared to non-MetS subjects. Particularly, NVF risk decreased in men with increased blood pressure and in women with increased BMI.52
A sex-specific trend in the relationship between MetS and fracture risk was also observed. In a cross-sectional study by Yu et al (n=897 men and 1792 women; ≥40 years), MetS was associated with increased risk of any fracture in women (22.8% in MetS vs 16.3% in non-MetS women) and the inverse happened in men.55 In the MINOS study, men with MetS showed a reduced incidence of vertebral fractures (VF) and peripheral fractures compared to men without MetS.56 The Rotterdam study found a reduced risk of any fracture and NVF among men with an increasing number of MetS components, but a lack of significant relationship among women.46 A Chinese study (2814 males and 4675 females, ≥40 years) also showed that women with MetS experienced an increased risk of any fractures compared to non-MetS women.57 Other studies among the Chinese population also obtained similar findings.58,59
In contrast to the studies mentioned earlier, the Camargo cohort study found no significant difference in the prevalence of VF and NVF between MetS and non-MetS subjects of either sex.44 Other smaller studies, such as PREMED also discovered a lack of association between MetS and fracture risk.42
Overall, the majority of the studies found a significant association between MetS and fracture risk except for a few smaller studies. Women with MetS might have elevated fracture risk, whereas MetS might protect men from fracture. Could these sex discrepancies be attributed to the fact that women with higher fat mass than men suffer from the negative effects of MetS on bone, while men benefit from increased mechanical loading? This question worth further investigation.
Table 3 summarises the relationship between MetS and fracture risk.
Table 3.
The Relationship Between MetS and Fracture Risk
| Study | Study Design | Subjects Characteristics | Definition of MetS | Bone Fracture Risk/Incidence |
|---|---|---|---|---|
| Ahmed et al (2006)52 | The 4th Tromsø study: A population-based cohort study (January 1994- December 2000) | 26,991 participants with 12,780 men and 14,211 women, aged 25–98 years old from Norway | NCEP-ATPIII | MetS reduced fracture risk in men (RR 0.71, 95% CI 0.51–0.99) and women (RR 0.66, 95% CI 0.53–0.82) Women with MetS: lower fracture risk in HPT (RR 0.89, 95% CI 0.8–0.99) Women with MetS: lower fracture with increased BMI (RR 0.91, 95% CI 0.84–0.98) or reduced HDL (RR 1.12, 95% CI 1.05–1.21) |
| von Muhlen et al (2007)18 | Rancho Bernardo Study, a longitudinal cohort study with mean follow-up of 2 years. | Community-dwelling ambulatory men (417) and women (671), all Caucasian in southern California, aged 38 to 97 years. | NCEP-ATPIII | Women with MetS: increased NVF (OR 3.76, 95% CI 1.27–11.13) NS for men. |
| Hernández et al (2010)12 | The Camargo Cohort Study: A cross-sectional study (February 2006-August 2009) | 1508 subjects from the Camargo, Spain with 495 men and 1013 women, aged >50 years | NCEP-ATPIII | MetS was not associated with fracture in men (OR 0.98, 95% CI 0.76–1.27) or women (OR 0.93, 95% CI; 0.79–1.10) |
| Hernández et al (2011)44 | The Camargo Cohort Study: A cross-sectional study (2006–2009) | 1209 adults (421 men aged 65±9 (50–92 years) and 788 women 63±9 (44–88 years)) visiting a primary care centre. | JIS | MetS was not associated with fracture in men and women (OR not disclosed) |
| Szulc et al (2010)56 | The MINOS study: A prospective cohort study (10-years from 1995–1996) | 762 men from France who aged 50–80 years old | NCEP-ATPIII | Men with MetS: reduced any spine and peripheral fracture (OR 0.33, 95% CI 0.15–0.76) |
| Bullo et al (2011)42 | The NUTERA-PREDIMED Cohort study: A large, parallel-group, randomized, controlled study | 251 subjects with 124 men and 127 women, aged between 55–80 years (men) or 60–80 years (women) from Spain | NCEP-ATPIII | T2DM: increased all fracture risk (OR 0.393, 95% CI 0.167–0.965) MetS: not associated with all fracture risk (OR 0.523, 95% CI 0.214–1.274) |
| El Maghraoui et al (2014)53 | Cross-sectional study (June 2012-March 2013) | 270 Caucasian post-menopausal women from the Rabat, Morocco who aged 50–90 years old | NCEP-ATPIII | MetS: not associated with prevalence of VF (22.1% without VF, 23.8% grade 1 VF, 25.0% with grade 2–3 VF, p>0.05). |
| Sun et al (2014)57 | A community-based cross-sectional study (June 2011 - December 2011) | 7489 Chinese adults from the Gulou, Nanjing, China with 2814 males and 4675 females aged > 40 years | IDF | Women with MetS: increased fragile fractures incidence (8% vs 5.3%, p = 0.034). |
| Lee et al (2014)54 | A longitudinal study with an average of 3 years follow-up period | 16,078 men from South Korea were recruited who aged >50 years | NCEP-ATPIII | MetS: reduced fracture risk (HR 0.662, 95% CI 0.445–0.986). After BMI-adjustment, the association was NS (HR 0.737, 95% CI 0.479–1.136) |
| Wang et al (2014)59 | A population-based cross-sectional study of 6 months (May 2011 - November 2011) | 9930 Chinese adults from the Chongming District, Shanghai, China who aged ≥40 years | NCEP-ATPIII | Women with MetS: increased osteoporotic fractures after adjusting for all confounders (OR 1.20, 95% CI 1.11–1.53) |
| Muka et al (2015)46 | The Rotterdam Study: A prospective population-based cohort (2 years) | 2040 women and 1510 men from the Netherlands, aged >55 years old | IDF, AHA/NHLBI, World Heart Federation, International Atherosclerosis Society and International Association for the Study of Obesity | Increased MetS components were associated with reduced risk of: any fracture (HR 0.82, 95% CI 0.70–0.9964) VF (HR 0.81, 95% 0.64–1.03) NVF (HR 0.75, 95% 0.66–0.96) in adjusted model in men. NS for women. |
| Qin et al (2016)58 | The REACTION study: a 1-year cross-sectional study (2011–2012) | 9930 Chinese adults from China who aged >40 years old | NCEP-ATPIII | Women with MetS: increased osteoporotic fracture risk (OR 1.22, 95% CI 1.12–1.54). |
| Yu et al (2017)55 | A cross-sectional study of 17 months (March 2014 - August 2015) | 2689 participants from Taiwan with 897 men and 1792 women aged >40 years | NCEP-ATPIII | Women with MetS: increased fracture risk (OR 0.304, 95% CI 0.165–0.559) Men with MS: NS association (OR 1.126, 95% CI 0.273–4.650) |
Abbreviations: AHA/NHLBI, American Heart Association/National Heart, Lung, and Blood Institute; BMI, body mass index; CI, confidence interval; HPT, hypertension; HR, hazard ratio; IDF, International Diabetes Federation; MetS, metabolic syndrome; NCEP-ATP–III, National Cholesterol Education Program Adult Treatment Panel III; NS, not significant; NVF, non-vertebral fracture; OP, osteoporosis; OR, odds ratio; RR, relative risk; VF, vertebral fracture.
Mechanistic Basis of the Relationship Between MetS and Bone
The effects of MetS on BMD and fracture risk are underlaid by altered cellular and mechanical properties of the skeletal system. Each MetS components exert a distinct effect on bone, and the sum determines the overall effects of MetS on BMD and fracture risk.
The Effects of MetS on Osteoblastogenesis and Osteoclastogenesis
Bone remodelling is a process of bone repair consisting of bone formation by osteoblasts and bone resorption by osteoclasts. Various endogenous and exogenous factors influence the differentiation of osteoblasts and osteoclasts from their corresponding progenitors of mesenchymal and haematopoietic origins. Hormones, nutrients, drugs and mechanical loading are some of the factors influencing bone remodelling. The early event of osteoblastogenesis requires the presence of Wnt and bone morphogenetic protein-2 (BMP-2) to direct the commitment of mesenchymal stem cells (MSCs) into pre-osteoblastic fate along with induction of other osteoblast-specific transcription factors, such as Runt-related transcription factor 2 (Runx-2) and Osterix (OSX) to promote osteoblastogenesis.60 Alkaline phosphatase (ALP) is highly expressed in committed pre-osteoblasts, thus represent an early marker of osteoblast phenotype. Subsequently, active and mature osteoblasts secrete other bone matrix proteins including collagen I (COL1), osteocalcin (OCN), osteopontin (OPN), osteonectin and bone sialoprotein (BSP) associated with mineralization and calcification.61 Osteoblasts regulate the differentiation of osteoclasts through receptor activator of nuclear factor kappa-Β (RANK) ligand (RANKL) and osteoprotegerin (OPG). RANKL binds to the RANK receptor on osteoclast progenitors and stimulates their differentiation, whereas OPG is a decoy receptor of RANKL which prevents its binding to RANK.62,63 The binding of RANKL to RANK recruits tumour necrosis factor receptor-associated factor 6 (TRAF6) and activates nuclear factor-kappa B (NFκB) and Fos proto-oncogene (c-Fos). The latter interacts with Jun proto-oncogene (c-Jun) and forms activator protein 1 (AP-1) complex, which cooperates with NF-κB to trigger nuclear factor of activated T-cells cytoplasmic 1 (NFATc1) for osteoclast differentiation and function.61 Bone formation and bone resorption markers are the by-products of osteoblast and osteoclast activities, respectively. They serve as an assessment tool complementary to BMD, which measure bone remodelling rate and ultimately predict fracture risk.64
Omental adipose tissue-derived MSCs isolated from MetS subjects expressed lower ALP and osteonectin.65 The Camargo Cohort Study reported lower propeptide of type 1 collagen (P1NP) and C-terminal telopeptide of type I collagen (CTX) in individuals with MetS as compared to healthy controls in either sex.12 A population-based study of health in Pomerania also found that higher bone turnover markers (OCN, P1NP and CTX) were associated with lower odds for MetS or T2DM among 2671 adult men and women.66 The MINOS study demonstrated lower OCN, CTX and higher deoxypyridinoline (DPD) in 762 older men with MetS.56 The association between MetS and OPG was inconsistent whereby some studies reported no correlation,67,68 while some studies indicated positive associations.69,70 In short, the accumulating evidence from human studies demonstrated heterogeneous outcomes on the association between MetS and bone markers, thus the net effect of MetS on bone needs to be resolved.
The Effects of MetS on Osteocyte-Driven Bone Remodelling
Osteocytes, the terminally differentiated osteoblasts entombed by the organic matrix, are the mechanosensor of the skeletal system playing a significant role in coordinating the bone remodelling process.71 Osteocytes also secrete regulators of bone remodelling similar to osteoblasts (OPG and RANKL), Wnt pathway [sclerostin (SOST) and Dickkopf-related protein 1 (DKK1)] and phosphate homeostasis [fibroblast growth factor-23 (FGF-23), phosphate-regulating neutral endopeptidase (Phex) and dentin matrix protein (DMP1)].72 The interplay between MetS and Wnt signalling has been proposed based on several considerations: (a) Increased level of β-catenin upon activation of the canonical Wnt pathway decreased peroxisome proliferator-activated receptor-gamma (PPAR-γ) and CCAAT-enhancer-binding protein alpha (C/EBPα) transcription factors essential for adipocyte gene expressions, (b) Glycogen synthase kinase 3β (GSK3β) has an important role in regulating blood glucose whereby its sustained activation-induced apoptosis of islet β cells, reduction of insulin secretion and inhibition of glycogen synthase, and (c) low-density lipoprotein receptor-related protein 6 (LRP6) forms a complex with low-density lipoprotein receptor (LDLR) and clathrin to facilitate low-density lipoprotein (LDL) clearance.73–75 The activation of Wnt/β-catenin results in the expression of direct downstream Wnt target genes, Runx-2, thus promoting osteogenesis.76 Wnt antagonists (eg SOST and DKK1) bind to LRP5/6 and antagonize the Wnt signalling, favouring bone resorption and bone loss.77 Recent report delineated the levels of SOST and DKK-1 were significantly increased in a MetS-induced bone loss animal model, suggesting the involvement of osteocyte-mediated Wnt inhibition during MetS.78
On the other hand, a previous study reported an inverse relationship between MetS and the level of phosphate. Patients with a higher number of MetS components had a lower circulating phosphate level.79 FGF-23 acts as a regulator for phosphate homeostasis by increasing phosphate excretion and reducing phosphate reabsorption.80 FGF-23 also inhibits 1α-hydroxylase, an enzyme essential for the conversion of inactive 25-hydroxyvitamin D [25(OH)D] to 1.25-dihydroxy vitamin D [1,25(OH)2D], suggesting its pivotal role in bone metabolism.81 In MetS animals induced by a high-carbohydrate high-fat diet, the level of FGF-23 in bone was increased, subsequently contributing to bone loss.78 In an epidemiological study, serum FGF-23 was higher in elderly with MetS and was associated with increased TG, BMI, waist circumference, trunk and body fat, as well as reduced HDL-c.82 Patients with impaired glucose tolerance also showed higher circulating FGF-23 level compared to normal control.83 Based on this evidence, the reduction of phosphate level in MetS might be explained by the increase in FGF-23 that suppressed phosphate reabsorption and enhanced phosphate excretion. The synergistic action of FGF-23 on phosphate and vitamin D homeostasis postulated the possible association between MetS and bone loss.
However, the link between MetS on bone through the osteocyte-driven bone remodelling in humans is yet to be investigated extensively. Thus, the findings obtained from in vivo study await further validation from human studies.
The Effects of Individual MetS Components on Bone
Each component of MetS affects bone metabolism. The role of adiposity on bone health is complex. Adiposity increases body mass and exerts mechanical loading on the bone, thereby stimulating bone accrual.84 Aromatase enzymes in the abdominal subcutaneous and visceral adipose tissue synthesize oestrogens which are protective of bone health.85,86 On the other hand, adipose tissue is also a major source of proinflammatory enzymes, such as interleukin (IL)-1 (IL-1), IL-6 and tumour necrosis factor-alpha (TNF-α).87 In particular, visceral fat was shown to be a significant source of IL-1β, IL-6 and IL-15 in obese men.88 These cytokines encourage the formation of osteoclasts and bone resorption activities while decreasing the formation of osteoblasts and bone formation.89 Adipose tissue also sequesters lipid-soluble hormones, such as testosterone and vitamin D essential in maintaining bone health, making them unavailable to bone tissue.90,91 A study in the Chinese population demonstrated that visceral fat area, an index of visceral adiposity, correlated negatively with serum 25-hydroxyvitamin D3 level.92 Apart from that, visceral adipose tissue also secretes adipokines, such as leptin and adiponectin.93 Leptin regulates energy metabolism by controlling satiety.93 Leptin has both anabolic and catabolic effects on the skeleton. Leptin exerts anabolic effects on osteoblasts, and it inhibits osteoclast formation by acting through RANKL/OPG pathway. At the same time, leptin exerts catabolic actions on the bone by acting on the sympathetic nervous system and increasing catecholamine and neuropeptide Y (NPY) secretion.94 Overall, a higher leptin level is associated with higher BMD and bone mineral content according to a meta-analysis, especially post-menopausal women.95 Adiponectin improves the insulin sensitivity of liver and muscle,93 but its level is inversely associated with BMD in humans,96 although preclinical studies reveal an anabolic effect, through inhibition of SOST and modulation of RANKL/OPG pathway.97
Type 2 diabetes mellitus (T2DM) is associated with increased BMD and increased fracture risk.49,98 Individuals with MetS suffer from impaired glucose tolerance and are predisposed to T2DM.99 Chronic hyperglycaemia will trigger the formation of advanced glycation end products (AGEs) when carbonyl group of a reducing sugar reacts with the amino group of macromolecules. The resultant Amadori products are unstable and will undergo subsequent reactions to form AGEs.100 Intracellular AGEs induce apoptosis of osteoblasts through endoplasmic reticulum stress.101 AGEs possess a biphasic effect on osteoclasts, whereby early exposure to AGEs inhibit osteoclasts differentiation and bone resorption, whereas late-stage exposure increases osteoclast fusion, podosome number and bone resorption.102 High glucose and AGEs increase SOST level production by osteocytes, thereby inhibiting bone formation. RANKL production by osteocytes is suppressed by AGEs, thereby inhibiting bone resorption.103 In combination, the effects caused low bone turnover and affect bone material properties. Human studies showed that skin AGEs were negatively correlated with bone material strength index regardless of diabetic status.104,105 In the Baltimore Longitudinal Study of Aging, both impaired glucose tolerance and diabetes status were negatively associated with hip geometry parameters and hip bending strength in women. This highlights a progressive bone impairment which starts even in prediabetic state.106 Besides, diabetes is also associated with bone marrow adiposity. Since adipocyte and osteoblast share the same mesenchymal progenitor, increased adipocyte differentiation decreases osteoblast differentiation, thereby limiting the osteoblast available for bone turnover.107 Insulin resistance is linked to high circulating insulin level, which exerts anabolic effects on the skeleton through direct and indirect actions on osteoblasts.108 All these effects precipitate altered bone geometry, such as increased intracortical porosity but increased trabecular bone density due to ineffective load distribution, as well as reduced bone strength observed in T2DM patients.109 Impaired glucose tolerance, when progresses to T2DM, has other indirect effects on fracture risk. Patients with diabetes have a higher risk of sarcopenia,110 which could increase their risk of falls and fractures.111
HPT due to high circulating level of sodium ion prevents the reabsorption of calcium, thereby increasing calcium excretion. Subsequently, parathyroid hormone (PTH) production will increase and lead to bone resorption.112 Mutations in thiazide‐sensitive sodium-chloride co‐transporter (NCCT) responsible in sodium resorption at the distal convoluted tubule have also been postulated as a genetic link between HPT and bone health. Inactivating mutation of this transporter, as in the case of Gitelman’s syndrome, leads to excessive sodium excretion but high BMD phenotype. Heterozygote of mutated NCCT may also confer protection to the bone but the prevalence of this genotype is not clear.113
A diet rich in fat will increase the free fatty acid (FA) in the blood. Oxidized FA can activate PPAR-γ,114 which subsequently induces the differentiation of adipocytes in the bone marrow and suppresses differentiation of osteoblasts.115 Oxidized LDL impairs osteoblast differentiation and mineralization process.116 Accumulation of oxidized LDL can induce apoptosis of osteoblasts by lysosomal membrane damage.117 Oxidized LDL can increase the production of RANKL of osteoblasts without corresponding changes in OPG level and stimulate the differentiation of osteoclasts.118 On the other hand, oxidized LDL can directly suppress the formation of osteoclasts and the fusion of lysosomes to the ruffled border, thereby affecting their bone resorption activity, via scavenger receptor-A.119,120 Mevalonate pathway responsible for the synthesis of cholesterol and isoprenoids is the drug target for dyslipidaemia treatment. Isoprenoids generated from the mevalonate pathway are involved in the prenylation of GTPases, such as Rho, Ras and Rac. Suppression of GTPase prenylation though mevalonate is known to suppress osteoclast differentiation, function and survival.121,122
HDL can prevent oxidized LDL-induced apoptosis of osteoblast by preserving lysosome integrity.123 Paraoxonase 1 associated with HDL has been shown to prevent oxidation of LDL and lipid peroxidation products,124 thereby contributing to the protection of HDL on the bone. Apart from cholesterol transport, HDL also suppresses proinflammatory cytokine synthesis from macrophages,125 which would otherwise promote the formation of osteoclasts over osteoblasts, leading to bone loss.
The Role of Inflammation and Oxidative Stress in Linking MetS and Bone
Chronic low-grade inflammation and induction of oxidative stress are the hallmark features in the pathogenesis of MetS. Proinflammatory cytokines and free radical species are known to modulate the bone remodelling process, favouring bone loss.126–128 As aforementioned, visceral adipose tissue has a major role in for the activation of the inflammatory mechanism via the release of cytokines, adipokines and chemokines. In osteoblast, the mechanistic pathways involved under inflammatory condition include the activation of NF-κB, suppressor of mothers against decapentaplegic (SMAD) ubiquitylation regulatory factor (SMURF) 1 and SMURF2, as well as inhibition of mitogen-activated protein kinase (MAPK) activities. These signalling events inhibit osteoblast-specific gene transcription.129 Additionally, the interaction of M-CSF, RANKL, TNF-α, IL-6 and monocyte chemoattractant protein-1 (MCP-1) with their respective receptors signals through the activation of NF-κB, MAPK and Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathways, leading to the massive upregulation of downstream signalling in osteoclasts129,130 and cytokine production.131
The increased production of reactive oxygen species (ROS) during MetS is triggered by the excessive macronutrient intake such as fat and carbohydrate in the oxidative phosphorylation process. Hyperglycaemia can activate a metabolic pathway involving diacylglycerol/protein kinase C/NADPH-oxidase, which leads to the generation of free oxygen radicals.132 The depletion of antioxidant capacity in the body to counterbalance the synthesis of ROS results in oxidative stress. Inflammation is also a contributor to oxidative stress and vice versa. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, xanthine oxidase, myeloperoxidase, lipoxygenase and cyclooxygenase are catalytic enzymes for the generation of ROS that sensitive to inflammatory conditions.133 Inversely, oxidative stress induces an inflammatory response through the activation of NF-κB transcription factor.134 Excessive oxidative stress promotes osteoclast differentiation, osteoblast and osteocyte apoptosis but suppresses osteoclast apoptosis and osteoblast differentiation, thereby affecting bone homeostasis.135,136
A meta-analysis showed that MetS is associated with increased proinflammatory cytokines (IL-6 and TNF-α), adipokines (leptin) and prooxidants (oxidized LDL and uric acid), as well as decreased anti-inflammatory cytokine (IL-10) and antioxidant (paraoxonase 1).137 Overview from a cross-sectional survey consisting of 10,475 participants revealed that the level of inflammatory mediator [C-reactive protein (CRP)] was inversely correlated with total BMD in both men and women.138 Another study also demonstrated higher levels of cytokines [interferon alpha 2 (IFNα2), interferon-gamma (IFN-γ), interleukin-12p70 (IL-12p70) and interleukin-33 (IL-33)] and chemokines (MCP-1) in osteoporotic post-menopausal women.139 Treating rheumatoid arthritis and ankylosing patients with anti-TNF-α therapy for a year were found to slow down generalized bone loss with decreased DKK-1, cathepsin K (CTSK) and increased P1NP.140 Taken together, the available evidence reiterated that inflammation and oxidative stress are simultaneously found in MetS and bone loss.
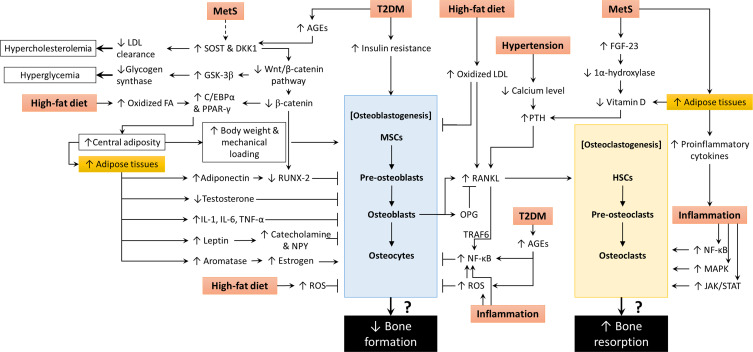
The mechanistic basis of the relationship between MetS, osteoblastogenesis and osteoclastogenesis is summarized in Figure 1.
Figure 1.
The molecular action of MetS and its components on osteoblastogenesis and osteoclastogenesis.
Abbreviations: ↑, increase or upregulate; ↓, decrease or downregulate; → (solid line), promote or induce; (dotted line), yet to be validated; ┬, inhibit or prevent; ?, unknown outcome; AGEs, advanced glycation end products; C/EBPα, CCAAT-enhancer-binding protein alpha; DKK1, Dickkopf-related protein 1; FA, fatty acid; FGF-23, fibroblast growth factor-23; GSK-3β, glycogen synthase kinase 3β; HSCs, hematopoietic stem cells; IL-1, interleukin-1; IL-6, interleukin-6; JAK/STAT, Janus kinase/signal transducer and activator of transcription; LDL, low-density lipoprotein; MAPK, mitogen-activated protein kinase; MetS, metabolic syndrome; MSCs, mesenchymal stem cells; NF-κB, nuclear factor kappa-B; NPY, neuropeptide Y; OPG, osteoprotegerin; PPARγ, peroxisome proliferator-activated receptor-gamma; PTH, parathyroid hormone; RANKL, receptor activator of nuclear factor kappa-Β ligand; ROS, reactive oxygen species; RUNX-2, Runt-related transcription factor 2; SOST, sclerostin; T2DM, type 2 diabetes mellitus; TNF-α, tumour necrosis factor-alpha; TRAF6, tumour necrosis factor receptor-associated factor 6.
Effects of MetS Treatment on Bone
Management of MetS using pharmacological agents targeting individual components are commonly implemented. The improvements of glycaemic status, lipid profile and blood pressure of the patients will lead to reduced inflammation and oxidative status of the patients and benefit their bone health. These drugs also exert other pleiotropic effects directly on the skeleton. For example, statins are known to suppress the mevalonate pathway by inhibiting 3-hydroxy-3-methyl-glutaryl-CoA reductase, thereby reducing prenylation of GTPases, which favours bone formation. The different classes of anti-diabetic agents possess distinct actions on bone remodelling. Biguanides, insulin, sulfonylureas, glucagon-like peptide-1 and dipeptidyl peptidase-4 inhibitors promote osteoblast differentiation, while sodium-glucose co-transporter 2 inhibitors and thiazolidinedione enhance bone loss.141 Anti-diabetic agents are often associated with hypoglycaemia, which contributes to increased fracture risk.142 A meta-analysis shows that sulfonylurea increased fracture risk by 14%, which was a rate similar to thiazolidinedione, lower than insulin but higher than metformin.143 The use of thiazide diuretics and beta-blockers are associated with a small benefit in fracture risk reduction.144 Therefore, an understanding of the baseline bone health status of the patients may help to minimize skeletal adverse effects of the drugs.
Weight reduction through diet modification and physical exercise is recommended to patients with MetS and abdominal obesity as per the recommendation of the American Heart Association/National Heart, Lung, and Blood Institute.33 Since mechanical loading is important in maintaining bone health, excess weight reduction might be harmful to the bone. A meta-analysis of randomized controlled trials showed that hip BMD was significantly reduced by weight loss after 4 months, and spine BMD reduction was significant after 13 months. In comparison, weight loss by exercise did not induce BMD reduction.145 Other researchers suggest that the changes in BMD due to weight loss are exaggerated due to variation in the surrounding soft tissue, and the new BMD should be ratioed to the new bodyweight.146 The effects of high impact exercise could offset the loss of mechanical loading by exercise.147 Resistant training could also enhance lean mass and reduce fat mass, which could be beneficial to bone health.147
Bariatric surgery is recommended for morbidly obese patients. A meta-analysis showed that bariatric surgery might be associated with increased PTH, bone turnover and reduced circulating calcium level and BMD.148 Another meta-analysis showed that bariatric surgery increased the risk of total and non-vertebral fractures, especially of the upper arms.149 Comparing two forms of bariatric surgery, circulating PTH is higher and 25(OH)D is lower in patients undergoing gastric bypass compared to sleeve gastrectomy.150 Deficiency of other nutrients may occur, such as protein, folate, vitamin B6, B12 and trace elements.151 Therefore, ensuring sufficient intake of calcium, vitamin D, protein and other micronutrients, as well as exercise, are important steps to counteract the adverse effects of bariatric surgery to bone.151
Conclusion
The relationship between MetS and BMD is complex. After adjusting for the effects of mechanical loading exerted by BMI, the association seems to be negligible or negative. In addition, the relationship may be mediated by sex. The proinflammatory, pro-oxidative and pro-calciuric body environment may contribute to the negative effects of MetS on bone. Improving the metabolic profile of the patients though medications could potentially alleviate the negative effects on BMD. However, excessive weight loss due to MetS management could be detrimental to the bone, and exercises can balance it. In particular, the combination of calorie restriction and exercise can promote a reduction in fat mass while retaining lean and bone mass. Thus, proper management of MetS can benefit not only the cardiovascular system but also the skeletal system.
Acknowledgments
The researchers thank Universiti Kebangsaan Malaysia for providing Fundamental Research Grant (FF-2018-405).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Edwards MH, Dennison EM, Aihie Sayer A, Fielding R, Cooper C. Osteoporosis and sarcopenia in older age. Bone. 2015;80:126–130. doi: 10.1016/j.bone.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aspray TJ, Hill TR. Osteoporosis and the ageing skeleton In: Harris JR, Korolchuk VI, editors. Biochemistry and Cell Biology of Ageing: Part II Clinical Science. Singapore: Springer Singapore; 2019:453–476. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Ba Y, Xing Q, Du J-L. Diabetes mellitus and the risk of fractures at specific sites: a meta-analysis. BMJ Open. 2019;9(1):e024067. doi: 10.1136/bmjopen-2018-024067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson H, Kanis JA, Odén A, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2014;29(1):223–233. doi: 10.1002/jbmr.2017 [DOI] [PubMed] [Google Scholar]
- 5.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 6.Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–298. doi: 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 7.Wong SK, Chin K-Y, Suhaimi F, Ahmad F, Ima-Nirwana S. The relationship between metabolic syndrome and osteoporosis: a review. Nutrients. 2016;8(6):347. doi: 10.3390/nu8060347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue P, Gao P, Li Y. The association between metabolic syndrome and bone mineral density: a meta-analysis. Endocrine. 2012;42(3):546–554. doi: 10.1007/s12020-012-9684-1 [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, Zhang Q, Yuan X, et al. Association between metabolic syndrome and osteoporosis: a meta-analysis. Bone. 2013;57(1):30–35. doi: 10.1016/j.bone.2013.07.013 [DOI] [PubMed] [Google Scholar]
- 10.Eckstein N, Buchmann N, Demuth I, et al. Association between metabolic syndrome and bone mineral density–data from the Berlin Aging Study II (BASE-II). Gerontology. 2016;62(3):337–344. doi: 10.1159/000434678 [DOI] [PubMed] [Google Scholar]
- 11.Kim T, Park S, Pak YS, Lee S, Lee EH. Association between metabolic syndrome and bone mineral density in Korea: the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV), 2008. J Bone Miner Metab. 2013;31(6):652–662. doi: 10.1007/s00774-013-0459-4 [DOI] [PubMed] [Google Scholar]
- 12.Hernández JL, Olmos JM, Pariente E, et al. Metabolic syndrome and bone metabolism: the Camargo Cohort study. Menopause. 2010;17(5):955–961. doi: 10.1097/gme.0b013e3181e39a15 [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Report of a World Health Organization Study Group. Geneva: World Health Organization; 1994. [PubMed] [Google Scholar]
- 14.Park KK, Kim SJ, Moon ES. Association between bone mineral density and metabolic syndrome in postmenopausal Korean women. Gynecol Obstet Invest. 2010;69(3):145–152. doi: 10.1159/000264665 [DOI] [PubMed] [Google Scholar]
- 15.Grimston SK, Willows ND, Hanley DA. Mechanical loading regime and its relationship to bone mineral density in children. Med Sci Sports Exerc. 1993;25(11):1203–1210. doi: 10.1249/00005768-199311000-00002 [DOI] [PubMed] [Google Scholar]
- 16.Chin K, Chan C, Subramaniam S, et al. Positive association between metabolic syndrome and bone mineral density among Malaysians. Int J Med Sci. 2020;17(16):2585–2593. doi: 10.7150/ijms.49030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinjo M, Setoguchi S, Solomon DH. Bone mineral density in adults with the metabolic syndrome: analysis in a population-based U.S. sample. J Clin Endocrinol Metab. 2007;92(11):4161–4164. doi: 10.1210/jc.2007-0757 [DOI] [PubMed] [Google Scholar]
- 18.von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos Int. 2007;18(10):1337–1344. doi: 10.1007/s00198-007-0385-1 [DOI] [PubMed] [Google Scholar]
- 19.Wani K, Yakout SM, Ansari MGA, et al. Metabolic syndrome in Arab adults with low bone mineral density. Nutrients. 2019;11(6):1405. doi: 10.3390/nu11061405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim BJ, Ahn SH, Bae SJ, et al. Association between metabolic syndrome and bone loss at various skeletal sites in postmenopausal women: a 3-year retrospective longitudinal study. Osteoporos Int. 2013;24(8):2243–2252. doi: 10.1007/s00198-013-2292-y [DOI] [PubMed] [Google Scholar]
- 21.Chan DC, Watts GF, Barrett PH, Burke V. Waist circumference, waist-to-hip ratio and body mass index as predictors of adipose tissue compartments in men. QJM. 2003;96(6):441–447. doi: 10.1093/qjmed/hcg069 [DOI] [PubMed] [Google Scholar]
- 22.Pasco JA, Holloway KL, Dobbins AG, Kotowicz MA, Williams LJ, Brennan SL. Body mass index and measures of body fat for defining obesity and underweight: a cross-sectional, population-based study. BMC Obes. 2014;1(1):9. doi: 10.1186/2052-9538-1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HY, Kim Y. Associations of obesity with osteoporosis and metabolic syndrome in Korean postmenopausal women: a cross-sectional study using national survey data. Arch Osteoporos. 2019;14(1):64. doi: 10.1007/s11657-019-0615-0 [DOI] [PubMed] [Google Scholar]
- 24.Chen DZ, Xu QM, Wu XX, et al. The combined effect of nonalcoholic fatty liver disease and metabolic syndrome on osteoporosis in postmenopausal females in Eastern China. Int J Endocrinol. 2018;2018:2314769. doi: 10.1155/2018/2314769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang DK, Choi HJ. The relationship between low bone mass and metabolic syndrome in Korean women. Osteoporos Int. 2010;21(3):425–431. doi: 10.1007/s00198-009-0990-2 [DOI] [PubMed] [Google Scholar]
- 26.Kim KC, Shin DH, Lee SY, Im JA, Lee DC. Relation between obesity and bone mineral density and vertebral fractures in Korean postmenopausal women. Yonsei Med J. 2010;51(6):857–863. doi: 10.3349/ymj.2010.51.6.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon YK, Lee JG, Kim SS, et al. Association between bone mineral density and metabolic syndrome in pre- and postmenopausal women. Endocr J. 2011;58(2):87–93. doi: 10.1507/endocrj.K10E-297 [DOI] [PubMed] [Google Scholar]
- 28.Loke SS, Chang HW, Li WC. Association between metabolic syndrome and bone mineral density in a Taiwanese elderly population. J Bone Miner Metab. 2018;36(2):200–208. [DOI] [PubMed] [Google Scholar]
- 29.Lin HH, Huang CY, Hwang LC. Association between metabolic syndrome and osteoporosis in Taiwanese middle-aged and elderly participants. Arch Osteoporos. 2018;13(1):48. doi: 10.1007/s11657-018-0467-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. [DOI] [PubMed] [Google Scholar]
- 31.Kim H, Oh HJ, Choi H, Choi WH, Lim SK, Kim JG. The association between bone mineral density and metabolic syndrome: a Korean population-based study. J Bone Miner Metab. 2013;31(5):571–578. doi: 10.1007/s00774-013-0446-9 [DOI] [PubMed] [Google Scholar]
- 32.National Cholesterol Education Program (NCEP) Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. doi: 10.1161/circ.106.25.3143 [DOI] [PubMed] [Google Scholar]
- 33.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 34.Kim YH, Cho KH, Choi YS, et al. Low bone mineral density is associated with metabolic syndrome in South Korean men but not in women: the 2008–2010 Korean National Health and Nutrition Examination Survey. Arch Osteoporos. 2013;8:142. doi: 10.1007/s11657-013-0142-3 [DOI] [PubMed] [Google Scholar]
- 35.Chin KY, Ima-Nirwana S. Calcaneal quantitative ultrasound as a determinant of bone health status: what properties of bone does it reflect? Int J Med Sci. 2013;10(12):1778–1783. doi: 10.7150/ijms.6765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oo WM, Naganathan V, Bo MT, Hunter DJ. Clinical utilities of quantitative ultrasound in osteoporosis associated with inflammatory rheumatic diseases. Quant Imaging Med Surg. 2018;8(1):100–113. doi: 10.21037/qims.2018.02.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin Y-X, Lin W, Mittra E, et al. Prediction of trabecular bone qualitative properties using scanning quantitative ultrasound. Acta Astronaut. 2013;92(1):79–88. doi: 10.1016/j.actaastro.2012.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langton CM, Langton DK. Comparison of bone mineral density and quantitative ultrasound of the calcaneus: site-matched correlation and discrimination of axial BMD status. Br J Radiol. 2000;73(865):31–35. doi: 10.1259/bjr.73.865.10721317 [DOI] [PubMed] [Google Scholar]
- 39.Moayyeri A, Adams JE, Adler RA, et al. Quantitative ultrasound of the heel and fracture risk assessment: an updated meta-analysis. Osteoporos Int. 2012;23(1):143–153. doi: 10.1007/s00198-011-1817-5 [DOI] [PubMed] [Google Scholar]
- 40.Nayak S, Olkin I, Liu H, et al. Meta-analysis: accuracy of quantitative ultrasound for identifying patients with osteoporosis. Ann Intern Med. 2006;144(11):832–841. doi: 10.7326/0003-4819-144-11-200606060-00009 [DOI] [PubMed] [Google Scholar]
- 41.Subramaniam S, Chan CY, Soelaiman IN, et al. The performance of a calcaneal quantitative ultrasound device, CM-200, in stratifying osteoporosis risk among Malaysian population aged 40 years and above. Diagnostics. 2020;10(4):178. doi: 10.3390/diagnostics10040178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bulló M, Garcia-Aloy M, Basora J, Covas MI, Salas-Salvado J. Bone quantitative ultrasound measurements in relation to the metabolic syndrome and type 2 diabetes mellitus in a cohort of elderly subjects at high risk of cardiovascular disease from the PREDIMED study. J Nutr Health Aging. 2011;15(10):939–944. doi: 10.1007/s12603-011-0046-0 [DOI] [PubMed] [Google Scholar]
- 43.Cvijetic S, Pavlovic M, Pasalic D, Dodig S. Ultrasound bone measurement in an older population with metabolic syndrome. Aging Clin Exp Res. 2011;23(1):29–34. doi: 10.1007/BF03324950 [DOI] [PubMed] [Google Scholar]
- 44.Hernández JL, Olmos JM, de Juan J, et al. Heel quantitative ultrasound parameters in subjects with the metabolic syndrome: the Camargo Cohort Study. Maturitas. 2011;69(2):162–167. doi: 10.1016/j.maturitas.2011.02.017 [DOI] [PubMed] [Google Scholar]
- 45.Chin KY, Ima-Nirwana S, Mohamed IN, et al. The association between bone health indicated by calcaneal quantitative ultrasound and metabolic syndrome in Malaysian men. J Diabetes Metab Disord. 2015;14:9. doi: 10.1186/s40200-015-0136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muka T, Trajanoska K, Kiefte-de Jong JC, et al. The association between metabolic syndrome, bone mineral density, hip bone geometry and fracture risk: the Rotterdam study. PLoS One. 2015;10(6):e0129116. doi: 10.1371/journal.pone.0129116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45(3):S116–124. doi: 10.1067/mjd.2001.117432 [DOI] [PubMed] [Google Scholar]
- 48.Migliaccio S, Greco EA, Fornari R, Donini LM, Lenzi A. Is obesity in women protective against osteoporosis? Diabetes Metab Syndr Obes. 2011;4:273–282. doi: 10.2147/DMSO.S11920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma L, Oei L, Jiang L, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–332. doi: 10.1007/s10654-012-9674-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider AL, Williams EK, Brancati FL, Blecker S, Coresh J, Selvin E. Diabetes and risk of fracture-related hospitalization: the atherosclerosis risk in communities study. Diabetes Care. 2013;36(5):1153–1158. doi: 10.2337/dc12-1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, Dai J, Zhong W, Hu C, Lu S, Chai Y. Association between serum cholesterol level and osteoporotic fractures. Front Endocrinol (Lausanne). 2018;9:30. doi: 10.3389/fendo.2018.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahmed LA, Schirmer H, Berntsen GK, Fønnebø V, Joakimsen RM. Features of the metabolic syndrome and the risk of non-vertebral fractures: the Tromsø study. Osteoporos Int. 2006;17(3):426–432. doi: 10.1007/s00198-005-0003-z [DOI] [PubMed] [Google Scholar]
- 53.El Maghraoui A, Rezqi A, El Mrahi S, Sadni S, Ghozlani I, Mounach A. Osteoporosis, vertebral fractures and metabolic syndrome in postmenopausal women. BMC Endocr Disord. 2014;14:93. doi: 10.1186/1472-6823-14-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SH, Baek S, Ahn SH, et al. Association between metabolic syndrome and incident fractures in Korean men: a 3-year follow-up observational study using national health insurance claims data. J Clin Endocrinol Metab. 2014;99(5):1615–1622. doi: 10.1210/jc.2013-3608 [DOI] [PubMed] [Google Scholar]
- 55.Yu CY, Chen FP, Chen LW, Kuo SF, Chien RN. Association between metabolic syndrome and bone fracture risk: a community-based study using a fracture risk assessment tool. Medicine (Baltimore). 2017;96(50):e9180. doi: 10.1097/MD.0000000000009180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szulc P, Varennes A, Delmas PD, Goudable J, Chapurlat R. Men with metabolic syndrome have lower bone mineral density but lower fracture risk–the MINOS study. J Bone Miner Res. 2010;25(6):1446–1454. doi: 10.1002/jbmr.13 [DOI] [PubMed] [Google Scholar]
- 57.Sun M, Cao M, Fu Q, et al. Association of calcaneal quantitative ultrasound parameters with metabolic syndrome in middle-aged and elderly Chinese: a large population-based cross-sectional study. BMC Endocr Disord. 2014;14:14. doi: 10.1186/1472-6823-14-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qin L, Yang Z, Zhang W, et al. Metabolic syndrome and osteoporotic fracture: a population-based study in China. BMC Endocr Disord. 2016;16(1):27. doi: 10.1186/s12902-016-0106-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang D, Liu N, Gao Y, Li P, Tian M. Association between metabolic syndrome and osteoporotic fracture in middle-aged and elderly Chinese peoples. Cell Biochem Biophys. 2014;70(2):1297–1303. doi: 10.1007/s12013-014-0054-x [DOI] [PubMed] [Google Scholar]
- 60.Marcellini S, Henriquez JP, Bertin A. Control of osteogenesis by the canonical Wnt and BMP pathways in vivo: cooperation and antagonism between the canonical Wnt and BMP pathways as cells differentiate from osteochondroprogenitors to osteoblasts and osteocytes. Bioessays. 2012;34(11):953–962. doi: 10.1002/bies.201200061 [DOI] [PubMed] [Google Scholar]
- 61.Rucci N. Molecular biology of bone remodelling. Clin Cases Miner Bone Metab. 2008;5(1):49–56. [PMC free article] [PubMed] [Google Scholar]
- 62.Siddiqui JA, Partridge NC. Physiological bone remodeling: systemic regulation and growth factor involvement. Physiology (Bethesda, Md). 2016;31(3):233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ono T, Hayashi M, Sasaki F, Nakashima T. RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen. 2020;40:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shetty S, Kapoor N, Bondu JD, Thomas N, Paul TV. Bone turnover markers: emerging tool in the management of osteoporosis. Indian J Endocrinol Metab. 2016;20(6):846–852. doi: 10.4103/2230-8210.192914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oliva-Olivera W, Leiva Gea A, Lhamyani S, et al. Differences in the osteogenic differentiation capacity of omental adipose-derived stem cells in obese patients with and without metabolic syndrome. Endocrinology. 2015;156(12):4492–4501. doi: 10.1210/en.2015-1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lerchbaum E, Schwetz V, Nauck M, Völzke H, Wallaschofski H, Hannemann A. Lower bone turnover markers in metabolic syndrome and diabetes: the population-based Study of Health in Pomerania. Nutr Metab Cardiovasc Dis. 2015;25(5):458–463. doi: 10.1016/j.numecd.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 67.Gannagé-Yared MH, Fares F, Semaan M, Khalife S, Jambart S. Circulating osteoprotegerin is correlated with lipid profile, insulin sensitivity, adiponectin and sex steroids in an ageing male population. Clin Endocrinol (Oxf). 2006;64(6):652–658. doi: 10.1111/j.1365-2265.2006.02522.x [DOI] [PubMed] [Google Scholar]
- 68.Nabipour I, Kalantarhormozi M, Larijani B, Assadi M, Sanjdideh Z. Osteoprotegerin in relation to type 2 diabetes mellitus and the metabolic syndrome in postmenopausal women. Metabolism. 2010;59(5):742–747. doi: 10.1016/j.metabol.2009.09.019 [DOI] [PubMed] [Google Scholar]
- 69.Pérez de Ciriza C, Moreno M, Restituto P, et al. Circulating osteoprotegerin is increased in the metabolic syndrome and associates with subclinical atherosclerosis and coronary arterial calcification. Clin Biochem. 2014;47(18):272–278. doi: 10.1016/j.clinbiochem.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 70.Bernardi S, Fabris B, Thomas M, et al. Osteoprotegerin increases in metabolic syndrome and promotes adipose tissue proinflammatory changes. Mol Cell Endocrinol. 2014;394(1–2):13–20. doi: 10.1016/j.mce.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 71.Bonewald LF. The role of the osteocyte in bone and nonbone disease. Endocrinol Metab Clin North Am. 2017;46(1):1–18. doi: 10.1016/j.ecl.2016.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int. 2014;94(1):25–34. doi: 10.1007/s00223-013-9774-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ali A, Ali A, Ahmad W, et al. Deciphering the role of WNT signaling in metabolic syndrome-linked Alzheimer’s disease. Mol Neurobiol. 2020;57(1):302–314. [DOI] [PubMed] [Google Scholar]
- 74.Abou Ziki MD, Mani A. The interplay of canonical and noncanonical Wnt signaling in metabolic syndrome. Nutr Res. 2019;70:18–25. doi: 10.1016/j.nutres.2018.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Embi N, Rylatt DB, Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980;107(2):519–527. doi: 10.1111/j.1432-1033.1980.tb06059.x [DOI] [PubMed] [Google Scholar]
- 76.Gaur T, Lengner CJ, Hovhannisyan H, et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280(39):33132–33140. doi: 10.1074/jbc.M500608200 [DOI] [PubMed] [Google Scholar]
- 77.Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37. doi: 10.1016/j.bone.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong SK, Chin KY, Ima-Nirwana S. The effects of tocotrienol on bone peptides in a rat model of osteoporosis induced by metabolic syndrome: the possible communication between bone cells. Int J Environ Res Public Health. 2019;16(18):3313. doi: 10.3390/ijerph16183313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stoian M, Stoica V. The role of disturbances of phosphate metabolism in metabolic syndrome. Maedica. 2014;9(3):255–260. [PMC free article] [PubMed] [Google Scholar]
- 80.Jüppner H. Phosphate and FGF-23. Kidney Int Suppl. 2011;79(121):S24–S27. doi: 10.1038/ki.2011.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perwad F, Zhang MY, Tenenhouse HS, Portale AA. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293(5):F1577–1583. doi: 10.1152/ajprenal.00463.2006 [DOI] [PubMed] [Google Scholar]
- 82.Mirza MAI, Alsiö J, Hammarstedt A, et al. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler Thromb Vasc Biol. 2011;31(1):219–227. doi: 10.1161/ATVBAHA.110.214619 [DOI] [PubMed] [Google Scholar]
- 83.Gateva A, Assyov Y, Tsakova A, Kamenov ZJH. metabolisme mrH-uSHe. Prediabetes is characterized by higher FGF23 levels and higher prevalence of vitamin D deficiency compared to normal glucose tolerance subjects. Horm Metab Res. 2019;51(2):106–111. doi: 10.1055/a-0813-3164 [DOI] [PubMed] [Google Scholar]
- 84.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30. doi: 10.1186/1749-799X-6-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Purohit A, Reed MJ. Regulation of estrogen synthesis in postmenopausal women. Steroids. 2002;67(12):979–983. doi: 10.1016/S0039-128X(02)00046-6 [DOI] [PubMed] [Google Scholar]
- 86.Hetemäki N, Savolainen-Peltonen H, Tikkanen MJ, et al. Estrogen metabolism in abdominal subcutaneous and visceral adipose tissue in postmenopausal women. J Clin Endocrinol Metab. 2017;102(12):4588–4595. doi: 10.1210/jc.2017-01474 [DOI] [PubMed] [Google Scholar]
- 87.Nishimura S, Manabe I, Nagai R. Adipose tissue inflammation in obesity and metabolic syndrome. Discov Med. 2009;8(41):55–60. [PubMed] [Google Scholar]
- 88.Jonas MI, Kurylowicz A, Bartoszewicz Z, et al. Interleukins 6 and 15 levels are higher in subcutaneous adipose tissue, but obesity is associated with their increased content in visceral fat depots. Int J Mol Sci. 2015;16(10):25817–25830. doi: 10.3390/ijms161025817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agidigbi TS, Kim C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ROS-mediated osteoclast diseases. Int J Mol Sci. 2019;20(14):3576. doi: 10.3390/ijms20143576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Migliaccio S, Di Nisio A, Mele C, et al. Obesity and hypovitaminosis D: causality or casualty? Int J Obes Suppl. 2019;9(1):20–31. doi: 10.1038/s41367-019-0010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Di Nisio A, Sabovic I, De Toni L, et al. Testosterone is sequestered in dysfunctional adipose tissue, modifying androgen-responsive genes. Int J Obes (Lond). 2020;44(7):1617–1625. doi: 10.1038/s41366-020-0568-9 [DOI] [PubMed] [Google Scholar]
- 92.Zhang M, Li P, Zhu Y, et al. Higher visceral fat area increases the risk of vitamin D insufficiency and deficiency in Chinese adults. Nutr Metab (Lond). 2015;12:50. doi: 10.1186/s12986-015-0046-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khan M, Joseph F. Adipose tissue and adipokines: the association with and application of adipokines in obesity. Scientifica. 2014;2014:328592. doi: 10.1155/2014/328592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reid IR, Baldock PA, Cornish J. Effects of leptin on the skeleton. Endocr Rev. 2018;39(6):938–959. doi: 10.1210/er.2017-00226 [DOI] [PubMed] [Google Scholar]
- 95.Liu K, Liu P, Liu R, Wu X, Cai M. Relationship between serum leptin levels and bone mineral density: a systematic review and meta-analysis. Clin Chim Acta. 2015;444:260–263. doi: 10.1016/j.cca.2015.02.040 [DOI] [PubMed] [Google Scholar]
- 96.Biver E, Salliot C, Combescure C, et al. Influence of adipokines and ghrelin on bone mineral density and fracture risk: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(9):2703–2713. doi: 10.1210/jc.2011-0047 [DOI] [PubMed] [Google Scholar]
- 97.Pal China S, Sanyal S, Chattopadhyay N. Adiponectin signaling and its role in bone metabolism. Cytokine. 2018;112:116–131. doi: 10.1016/j.cyto.2018.06.012 [DOI] [PubMed] [Google Scholar]
- 98.Jia P, Bao L, Chen H, et al. Risk of low-energy fracture in type 2 diabetes patients: a meta-analysis of observational studies. Osteoporos Int. 2017;28(11):3113–3121. [DOI] [PubMed] [Google Scholar]
- 99.Dragsbæk K, Neergaard JS, Laursen JM, et al. Metabolic syndrome and subsequent risk of type 2 diabetes and cardiovascular disease in elderly women: challenging the current definition. Medicine. 2016;95(36):e4806–e4806. doi: 10.1097/MD.0000000000004806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194–222. doi: 10.3390/biom5010194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suzuki R, Fujiwara Y, Saito M, et al. Intracellular accumulation of advanced glycation end products induces osteoblast apoptosis via endoplasmic reticulum stress. J Bone Miner Res. 2020. doi: 10.1002/jbmr.4053 [DOI] [PubMed] [Google Scholar]
- 102.Li Z, Li C, Zhou Y, et al. Advanced glycation end products biphasically modulate bone resorption in osteoclast-like cells. Am J Physiol Endocrinol Metab. 2016;310(5):E355–366. doi: 10.1152/ajpendo.00309.2015 [DOI] [PubMed] [Google Scholar]
- 103.Tanaka K, Yamaguchi T, Kanazawa I, Sugimoto T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun. 2015;461(2):193–199. doi: 10.1016/j.bbrc.2015.02.091 [DOI] [PubMed] [Google Scholar]
- 104.Samakkarnthai P, Sfeir JG, Atkinson EJ, et al. SUN-LB68 advanced glycation endproducts are associated with worse bone material strength in older adults with and without type 2 diabetes. J Endocrine Soc. 2020;4(Supplement_1). doi: 10.1210/jendso/bvaa046.2045. [DOI] [Google Scholar]
- 105.Samakkarnthai P, Sfeir JG, Atkinson EJ, et al. Determinants of bone material strength and cortical porosity in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2020;105(10). doi: 10.1210/clinem/dgaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moseley KF, Chia CW, Simonsick EM, Egan JM, Ferrucci L, Sellmeyer DE. Sex-specific differences in progressive glucose intolerance and hip geometry: the Baltimore longitudinal study of aging. Osteoporos Int. 2015;26(5):1555–1562. doi: 10.1007/s00198-015-3027-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim TY, Schafer AL. Diabetes and Bone Marrow Adiposity. Curr Osteoporos Rep. 2016;14(6):337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang N, Jiang H, Bai Y, et al. The molecular mechanism study of insulin on proliferation and differentiation of osteoblasts under high glucose conditions. Cell Biochem Funct. 2019;37(5):385–394. doi: 10.1002/cbf.3415 [DOI] [PubMed] [Google Scholar]
- 109.Burghardt AJ, Issever AS, Schwartz AV, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95(11):5045–5055. doi: 10.1210/jc.2010-0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Trierweiler H, Kisielewicz G, Hoffmann Jonasson T, Rasmussen Petterle R, Aguiar Moreira C, Zeghbi Cochenski Borba V. Sarcopenia: a chronic complication of type 2 diabetes mellitus. Diabetol Metab Syndr. 2018;10(1):25. doi: 10.1186/s13098-018-0326-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yeung SSY, Reijnierse EM, Pham VK, et al. Sarcopenia and its association with falls and fractures in older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2019;10(3):485–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Caudarella R, Vescini F, Rizzoli E, Francucci CM. Salt intake, hypertension, and osteoporosis. J Endocrinol Invest. 2009;32(4Suppl):15–20. [PubMed] [Google Scholar]
- 113.Cruz DN. The renal tubular Na-Cl co-transporter (NCCT): a potential genetic link between blood pressure and bone density? Nephrol Dial Transplant. 2001;16(4):691–694. doi: 10.1093/ndt/16.4.691-a [DOI] [PubMed] [Google Scholar]
- 114.Itoh T, Fairall L, Amin K, et al. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol. 2008;15(9):924–931. doi: 10.1038/nsmb.1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhuang H, Zhang X, Zhu C, et al. Molecular mechanisms of PPAR-γ governing MSC osteogenic and adipogenic differentiation. Curr Stem Cell Res Ther. 2016;11(3):255–264. doi: 10.2174/1574888X10666150531173309 [DOI] [PubMed] [Google Scholar]
- 116.Mazière C, Savitsky V, Galmiche A, Gomila C, Massy Z, Mazière JC. Oxidized low density lipoprotein inhibits phosphate signaling and phosphate-induced mineralization in osteoblasts. Involvement of oxidative stress. Biochim Biophys Acta. 2010;1802(11):1013–1019. doi: 10.1016/j.bbadis.2010.07.010 [DOI] [PubMed] [Google Scholar]
- 117.Brodeur MR, Brissette L, Falstrault L, Ouellet P, Moreau R. Influence of oxidized low-density lipoproteins (LDL) on the viability of osteoblastic cells. Free Radic Biol Med. 2008;44(4):506–517. doi: 10.1016/j.freeradbiomed.2007.08.030 [DOI] [PubMed] [Google Scholar]
- 118.Mazière C, Salle V, Gomila C, Mazière JC. Oxidized low density lipoprotein enhanced RANKL expression in human osteoblast-like cells. Involvement of ERK, NFkappaB and NFAT. Biochim Biophys Acta. 2013;1832(10):1756–1764. doi: 10.1016/j.bbadis.2013.05.033 [DOI] [PubMed] [Google Scholar]
- 119.Dawodu D, Patecki M, Dumler I, Haller H, Kiyan Y. oxLDL inhibits differentiation of mesenchymal stem cells into osteoblasts via the CD36 mediated suppression of Wnt signaling pathway. Mol Biol Rep. 2019;46(3):3487–3496. doi: 10.1007/s11033-019-04735-5 [DOI] [PubMed] [Google Scholar]
- 120.Dawodu D, Patecki M, Hegermann J, Dumler I, Haller H, Kiyan Y. oxLDL inhibits differentiation and functional activity of osteoclasts via scavenger receptor-A mediated autophagy and cathepsin K secretion. Sci Rep. 2018;8(1):11604. doi: 10.1038/s41598-018-29963-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weivoda MM, Oursler MJ. The roles of small GTPases in osteoclast biology. Curr Res. 2014;3:1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wan Hasan WN, Chin KY, Jolly JJ, Abd Ghafar N, Soelaiman IN. Identifying potential therapeutics for osteoporosis by exploiting the relationship between mevalonate pathway and bone metabolism. Endocr Metab Immune Disord Drug Targets. 2018;18(5):450–457. doi: 10.2174/1871530318666180423122409 [DOI] [PubMed] [Google Scholar]
- 123.Brodeur MR, Brissette L, Falstrault L, Moreau R. HDL3 reduces the association and modulates the metabolism of oxidized LDL by osteoblastic cells: a protection against cell death. J Cell Biochem. 2008;105(6):1374–1385. doi: 10.1002/jcb.21938 [DOI] [PubMed] [Google Scholar]
- 124.Eren E, Ellidag HY, Aydin O, Yılmaz N. HDL-associated paraoxonase 1 as a bridge between postmenopausal osteoporosis and cardiovascular disease. Chonnam Med J. 2014;50(3):75–81. doi: 10.4068/cmj.2014.50.3.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Inoue M, Niki M, Ozeki Y, et al. High-density lipoprotein suppresses tumor necrosis factor alpha production by mycobacteria-infected human macrophages. Sci Rep. 2018;8(1):6736. doi: 10.1038/s41598-018-24233-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Singh A, Mehdi AA, Srivastava RN, Verma NS. Immunoregulation of bone remodelling. Int J Crit Illn Inj Sci. 2012;2(2):75–81. doi: 10.4103/2229-5151.97271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang X, Chen B, Sun J, et al. Iron-induced oxidative stress stimulates osteoclast differentiation via NF-κB signaling pathway in mouse model. Metabolism. 2018;83:167–176. doi: 10.1016/j.metabol.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 128.Zhao B. TNF and bone remodeling. Curr Osteoporos Rep. 2017;15(3):126–134. doi: 10.1007/s11914-017-0358-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11(3):234–250. [DOI] [PubMed] [Google Scholar]
- 130.Boyce BF, Xiu Y, Li J, Xing L, Yao Z. NF-κB-mediated regulation of osteoclastogenesis. Endocrinol Metab. 2015;30(1):35–44. doi: 10.3803/EnM.2015.30.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Krum SA, Chang J, Miranda-Carboni G, Wang C-Y. Novel functions for NFκB: inhibition of bone formation. Nat Rev Rheumatol. 2010;6(10):607–611. doi: 10.1038/nrrheum.2010.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9(2):119. doi: 10.1038/s41419-017-0135-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vona R, Gambardella L, Cittadini C, Straface E, Pietraforte D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxid Med Cell Longev. 2019;2019:8267234. doi: 10.1155/2019/8267234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Biswas SK. Does the Interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016;2016:5698931. doi: 10.1155/2016/5698931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Domazetovic V, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT. Oxidative stress in bone remodeling: role of antioxidants. Clin Cases Miner Bone Metab. 2017;14(2):209–216. doi: 10.11138/ccmbm/2017.14.1.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang B, Xie Q-Y, Quan Y, Pan X-M, Liao D-F. Reactive oxygen species induce cell death via Akt signaling in rat osteoblast-like cell line ROS 17/2.8. Toxicol Ind Health. 2015;31(12):1236–1242. doi: 10.1177/0748233713491801 [DOI] [PubMed] [Google Scholar]
- 137.Srikanthan K, Feyh A, Visweshwar H, Shapiro JI, Sodhi K. Systematic review of metabolic syndrome biomarkers: a panel for early detection, management, and risk stratification in the West Virginian population. Int J Med Sci. 2016;13(1):25–38. doi: 10.7150/ijms.13800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Pablo P, Cooper MS, Buckley CD. Association between bone mineral density and C-reactive protein in a large population-based sample. Arthritis Rheum. 2012;64(8):2624–2631. doi: 10.1002/art.34474 [DOI] [PubMed] [Google Scholar]
- 139.Ilesanmi-Oyelere BL, Schollum L, Kuhn-Sherlock B, et al. Inflammatory markers and bone health in postmenopausal women: a cross-sectional overview. Immun Ageing. 2019;16:15. doi: 10.1186/s12979-019-0155-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gulyás K, Horváth Á, Végh E, et al. Effects of 1-year anti-TNF-α therapies on bone mineral density and bone biomarkers in rheumatoid arthritis and ankylosing spondylitis. Clin Rheumatol. 2020;39(1):167–175. doi: 10.1007/s10067-019-04771-3 [DOI] [PubMed] [Google Scholar]
- 141.Adil M, Khan RA, Kalam A, et al. Effect of anti-diabetic drugs on bone metabolism: evidence from preclinical and clinical studies. Pharmacol Rep. 2017;69(6):1328–1340. doi: 10.1016/j.pharep.2017.05.008 [DOI] [PubMed] [Google Scholar]
- 142.Hung YC, Lin CC, Chen HJ, et al. Severe hypoglycemia and hip fracture in patients with type 2 diabetes: a nationwide population-based cohort study. Osteoporos Int. 2017;28(7):2053–2060. doi: 10.1007/s00198-017-4021-4 [DOI] [PubMed] [Google Scholar]
- 143.Zhang Z, Cao Y, Tao Y, et al. Sulfonylurea and fracture risk in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes Res Clin Pract. 2020;159:107990. doi: 10.1016/j.diabres.2019.107990 [DOI] [PubMed] [Google Scholar]
- 144.Wiens M, Etminan M, Gill SS, Takkouche B. Effects of antihypertensive drug treatments on fracture outcomes: a meta-analysis of observational studies. J Intern Med. 2006;260(4):350–362. doi: 10.1111/j.1365-2796.2006.01695.x [DOI] [PubMed] [Google Scholar]
- 145.Soltani S, Hunter GR, Kazemi A, Shab-Bidar S. The effects of weight loss approaches on bone mineral density in adults: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2016;27(9):2655–2671. [DOI] [PubMed] [Google Scholar]
- 146.Seimon RV, Wild-Taylor AL, Keating SE, et al. Effect of weight loss via severe vs moderate energy restriction on lean mass and body composition among postmenopausal women with obesity: the TEMPO diet randomized clinical trial. JAMA Netw Open. 2019;2(10):e1913733–e1913733. doi: 10.1001/jamanetworkopen.2019.13733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hunter GR, Plaisance EP, Fisher G. Weight loss and bone mineral density. Curr Opin Endocrinol Diabetes Obes. 2014;21(5):358–362. doi: 10.1097/MED.0000000000000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu C, Wu D, Zhang JF, et al. Changes in bone metabolism in morbidly obese patients after bariatric surgery: a meta-analysis. Obes Surg. 2016;26(1):91–97. doi: 10.1007/s11695-015-1724-5 [DOI] [PubMed] [Google Scholar]
- 149.Zhang Q, Chen Y, Li J, et al. A meta-analysis of the effects of bariatric surgery on fracture risk. Obes Rev. 2018;19(5):728–736. doi: 10.1111/obr.12665 [DOI] [PubMed] [Google Scholar]
- 150.Tian Z, Fan XT, Li SZ, Zhai T, Dong J. Changes in bone metabolism after sleeve gastrectomy versus gastric bypass: a meta-analysis. Obes Surg. 2020;30(1):77–86. doi: 10.1007/s11695-019-04119-5 [DOI] [PubMed] [Google Scholar]
- 151.Ben-Porat T, Elazary R, Sherf-Dagan S, et al. Bone health following bariatric surgery: implications for management strategies to attenuate bone loss. Adv Nutr. 2018;9(2):114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Med. 1998;15(7):539–553. doi: [DOI] [PubMed] [Google Scholar]
- 153.Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 154.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet. 2005;366(9491):1059–1062. doi: 10.1016/S0140-6736(05)67402-8 [DOI] [PubMed] [Google Scholar]
- 155.Kim HY, Choe JW, Kim HK, et al. Negative association between metabolic syndrome and bone mineral density in Koreans, especially in men. Calcif Tissue Int. 2010;86(5):350–358. doi: 10.1007/s00223-010-9347-2 [DOI] [PubMed] [Google Scholar]