Abstract
Aim
The incidence of a diabetic foot ulcer (DFU) is increasing over the previous decade with an increasing prevalence of diabetes mellitus (DM). Despite the increasing incidence of DFU, there is limited information about the problem in Ethiopia. Hence, this study aimed to investigate the incidence of DFU and its predictors among newly diagnosed DM patients who were on follow-up at Felege Hiwot Referral Hospital.
Methods
Institution-based retrospective follow-up study was conducted at Felege Hiwot Referral Hospital among newly diagnosed DM patients from January 1, 2009, to December 31, 2018. A simple random sampling method was used to select 401 study participants from a total of 723 eligible population. Data was entered using Epi-Data version 3.1 and exported to STATA version 14 for analysis. The incidence rate was estimated using person-years of observation and Nelson–Aalen cumulative hazard function, showing the cumulative probability of diabetic foot ulcer, was done. The best model (Gompertz) was selected using the AIC and log-likelihood method. Hazard ratio (HR) with its 95% confidence interval was computed and variables having a p-value less than 0.05 in the multivariable model were considered to be significantly associated with DFU.
Results
A total of 387 patients were followed retrospectively for a median follow-up time of 95 months. Out of all, 66 (17.05%) patients developed DFU with an incidence rate of 4 cases per 100 person-years of observation. Diabetic nephropathy (adjusted hazard ratio (AHR) = 2.37, 95% CI: 1.33–54.24), diabetic retinopathy (AHR = 5.56, 95% CI: 2.64–11.74), and increased body mass index (AHR = 1.13, 95% CI: 1.01–1.27) were found to increase the hazard of DFU.
Conclusion
The incidence of DFU was relatively high. Diabetic nephropathy, diabetic retinopathy, and body mass index were its significant predictors. Therefore, close monitoring of patients with co-morbidities and increased body mass index should be considered to reduce DFU.
Keywords: incidence, diabetic foot ulcer, Ethiopia
Introduction
Diabetes mellitus (DM) is a combination of different metabolic disorders characterized by the presence of hyperglycemia due to impairment of insulin secretion, defective insulin action or both.1,2 Diabetes is a major public health problem that is approaching epidemic proportions globally and is one of the largest global health emergencies of the 21st century.3 The prevalence of both type 1 and type 2 diabetes is increasing and in 2019, 463 million adults were living with diabetes. In Africa, by 2019, around 19 million adult populations were estimated to have diabetes and it is expected that by 2045 it will be around 47 million (showing an alarming increase by 143%). Similarly, in Ethiopia, an estimated 1,699,400 adults were living with DM.4
Diabetes mellitus is characterized by multiple long-term complications that affect almost every system in the body.5 It is associated with increased rates of several microvascular complications such as nephropathy, retinopathy, and neuropathy, and macrovascular complications such as atherosclerosis and stroke.4–7
Diabetic foot ulcer (DFU), which often results in lower extremity amputations, is one of the most common complications of DM.8 Exactly where DFU fits into microvascular or macrovascular is not always clear and these diabetic individuals mostly have neuropathy and/or peripheral arterial disease. This might imply that it is both a macro and microvascular illness.9 However, none of the above complications are more devastating than those involving the foot and the incidence of diabetic foot complications are increasing often due to negligence by both patients and physicians.10–13
Diabetic foot ulcer has significant health and socioeconomic problems holding adverse effects on the quality of life of the patients and imposing a heavy economic burden on the patient and their family.14 Diabetic foot problems account for more hospital admission than any other long term complications of diabetes and are responsible for nearly 50% of all-diabetes-related hospital bed days.15 The lifetime risk of a patient with diabetes developing a DFU is 25%, and up to 85% of all lower-limb amputations in diabetes are preceded by foot ulcers.13,16 Diabetic foot ulcer affects not only the quality of life and physiological welfare but also premortal events and following major leg amputations due to the ulcer, mortality ranges from 24.6% within 5 years and 45.4% within 10 years.17–21 It is also estimated that 24.4% of the total health care expenditure among the diabetic population is related to foot complications.22
The pooled worldwide prevalence of DFU in people diagnosed with diabetes mellitus is 6.3%, of which most (13%) is in North America, 7.2% in Africa and the lowest (3%) in Europe.23 In different sub-Saharan African countries, the prevalence of DFU ranges from 3.4% to 18.1%24–27 and studies conducted in different parts of Ethiopia showed that the prevalence of DFU ranges from 12 to 17.86%.28–31
Studies indicated that DFU is affected by socio-demographic factors, clinical factors, and comorbidities. Among socio-demographic factors male sex,32,33 being in the older age group,34–36 and rural residency28,31 are related to a higher risk of DFU. Of clinically related factors, duration of DM,28,37 increased body mass index (BMI),31,37 increased hemoglobin A1c (HgbA1c),33,38 higher low-density lipoprotein (LDL), higher triglyceride level, and lower high-density lipoprotein (HDL) level39–42 are associated with increased risk for vascular complication including DFU. Comorbidities like retinopathy,23,43 nephropathy,23,37,41,44 and neuropathy31,45–48 are also associated with an increased risk of having DFU.
The development of DFU and amputation is preventable if recognized early through adequate glycemic control, modification of risk factors and educating the patient about self-care practice. In Ethiopia, there is a scarcity of information about the incidence of DFU and its predictors and up to our knowledge this study is the first retrospective follow-up study in Ethiopia. Therefore, this study estimated the incidence of DFU and its predictors in Bahirdar Felege Hiwot Referral Hospital. This study could provide information for health care workers, policymakers and other governmental and non-governmental organizations to increase efforts on prevention and risk reduction on DFU and prevent amputation and other complications of DFU.
Methods
Study Design and Setting
Institution-based retrospective follow-up study was conducted among diabetic patients in Felege Hiwot Referral Hospital, from January 1, 2009, to December 31, 2018. Felege Hiwot Referral Hospital is found in Bahir Dar, which is the capital city of Amhara regional state located at 565 km from Addis Ababa, Northwest Ethiopia. It is a tertiary and referral hospital with 400 beds capacity and around 15 adult outpatient departments (OPD) serving over 7 million people from the surrounding area. The OPD serves around 900 patients per day. The hospital provides obstetric, pediatric, internal medicine, ophthalmology, gynecologic, otorhinolaryngology (ENT) and orthopedic surgery services. Around 21,218 people had a chronic follow-up in this hospital and among these 6567 were DM patients. For this study, a total of 723 DM patients were eligible for our study (the remaining patients were transferred out to other facilities for follow-up after their diagnosis).
Population, Sample Size Determination, and Sampling Procedure
All DM patients who were newly diagnosed from January 1, 2009, to December 31, 2018, and had follow-up at Felege Hiwot Referral Hospital were included in the study. However, patients who had DFU at diagnosis, as well as patients whose date of treatment initiation and/or date of development of the outcome was not recorded, were excluded from the study.
The sample size for the first objective was determined using the log-rank method by taking the incidence of DFU to be 5.3% from the study conducted in Iran49 and it was found to be 306. Moreover, the sample size for the second objective was determined using the Schoenfeld formula50 by using the estimates of the study from Ghana37 and the final sample size was found to be 401 after adding 10% non-response rate. After identifying patients who fulfill the inclusion criteria, the sampling frame was prepared by collecting the medical registration numbers of DM patients from the registration book. Then the study participants were selected by using a computer-generated simple random sampling technique from 723 eligible DM patients.
Variables of the Study
The outcome variable/event of this study was DFU, found documented in the patient’s follow-up card. The outcome variable (DFU) was diagnosed by the clinical decision of the physician and it was defined by almost all of the physicians as non-traumatic lesions of the skin (partial or full thickness) on the foot of a person who has diabetes mellitus. Physicians in our setup used the definition/classification of DFU developed by the International Working Group on the Diabetic Foot (IWGDF) and they classified the DFU according to Wagner’s classification.51,52 Participants who were lost from the follow-up, died before the end of the study period and did not experience DFU by the end of the study were considered as censored. The independent variables were classified into three subsections as; baseline sociodemographic variables (age, sex, residence, religion, and educational status, and occupation), baseline clinical variables (duration of DM, HDL level, LDL level, triglyceride level, BMI, HgbA1c, and type of DM), and co-morbidities at baseline (diabetic neuropathy, diabetic nephropathy, and diabetic retinopathy). The normal levels of LDL, HDL, and triglyceride were defined as < 100 mg/dl, >40 mg/dl, and <150 mg/dl, respectively.53 In addition, the co-morbidities were defined based on the assessment of the physician.
Baseline and Follow-Up Data Collection
Baseline independent variables were selected using different literatures and patients without DFU initially were followed until the development of DFU or until censored (death, lost to follow-up or the end of the study period). To diagnose the baseline independent variables, we just used information recorded by physicians and to diagnose whether a patient had DFU or not we also used recorded information. Regarding the frequency of follow-up visits, every patient with DM had a follow-up every month.
During the first follow-up visit, in the clinical setup where we collected the data (in Felege Hiwot Referral Hospital), every diabetic patient is evaluated for baseline characteristics such as lipid profiles, organ function tests, and assessment of any of the complications/comorbidities related to DM, like DFU. In addition, sociodemographic characteristics of patients such as sex, age, BMI, place of residence, occupation, educational status, and others were collected/recorded at the start or during the first follow-up visit. The clinical characteristics such as different diagnostic investigations (organ function tests, lipid profiles, HgbA1c) and screening of co-morbidities and complications such as DFU continue in the follow-up visit of each patient every month. At each follow-up visit, routine screening and evaluation of neuropathic and vascular involvement of the lower extremities and careful feet inspection were performed and DFU was diagnosed on the basis of clinical signs and symptoms such as the presence of drainage on the socks of the person, redness and swelling in the leg, odor, fever, and other signs and symptoms of inflammation if the ulcer had progressed significantly and become superinfected.
Data Quality Management
Before collecting our data, the data extraction checklist was prepared in English and pre-tested to gather relevant data from the medical records. Health workers who work at chronic OPD (two BSC nurses and one health officer) were assigned as data collectors and a medical doctor was assigned as a supervisor. Then data on important variables were collected as per the data extraction sheet by reviewing the chart of the patient. In addition, we used the electronic database during difficulty while reviewing the chart. The quality of data was ensured through the training of the data collectors and the supervisor. Close supervision was also done during the data collection and data were checked for completeness and consistency by the supervisor and principal investigator on a daily base.
Data Processing and Analysis
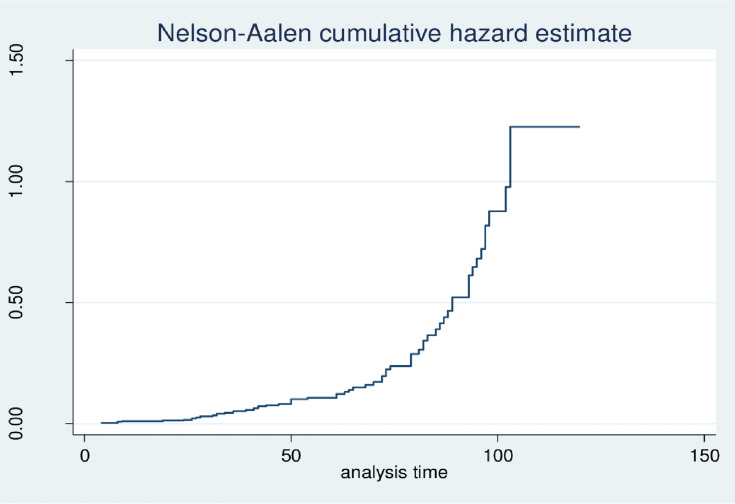
Data were entered into Epi Data version 3.1 and exported to STATA version 14 statistical software for further analysis. After the data was edited and cleaned, coding was performed to make the variables suitable for analysis. The continuous variables were described in terms of median and Inter Quartile Range (IQR) were as the categorical variables were described using frequency and percentages. The incidence rate of DFU was calculated for the entire cohort by dividing the total number of cases to the total person-years of observation. Nelson–Aalen cumulative curve was used to show the cumulative probability of DFU (Figure 1). Interacting of each covariate with time (Additional file 1) and Schoenfeld residual test (both global and detail) (Additional file 2) was used to test proportional hazard assumption. Model comparison was carried out using Akaike information criteria (AIC) and likelihood method. Then the parametric survival model with Gompertz baseline hazard function was found to be the best model. Model fitness was also checked by using the Cox-snell residual and the hazard ratio (HR) with its 95% confidence interval was computed to show the strength of association. Variables having a p-value <0.20 from the bi-variable analysis were fitted into the multivariable model and those with p-value <0.05 on the multivariable model were declared to be statistical significance predictors of DFU.
Figure 1.
Showed the Nelson–Aalen cumulative curve showing the cumulative probability of DFU among DM patients at Felege Hiwot referral hospital was increasing.
Result
Socio-Demographic Characteristics of Respondents
From the total 401 DM patient records of 14 were excluded because of incomplete records on important clinical factors. The median age of participants was 46 (IQR= 35–58) years and more than half, 233 (60.2%) of patients were male. Regarding the residence, 241 (62.3%) of participants were urban dwellers. Besides, 183 (47.3%) of the participants had secondary and above education (Table 1).
Table 1.
Socio-Demographic Characteristics of DM Patients on Follow-Up at Felege Hiwot Referral Hospital, from January 1, 2009, to December 31, 2018
| Characteristics | Frequency | Percent (%) |
|---|---|---|
| Sex | ||
| Female | 154 | 39.8 |
| Male | 233 | 60.2 |
| Religion | ||
| Orthodox | 292 | 75.5 |
| Muslim | 81 | 20.9 |
| Protestant | 14 | 3.6 |
| Residence | ||
| Urban | 241 | 62.3 |
| Rural | 146 | 37.8 |
| Educational status | ||
| No education | 119 | 30.7 |
| Primary school | 85 | 22.0 |
| Secondary and above | 183 | 47.3 |
| Occupation | ||
| Unemployed | 32 | 8.3 |
| Government employed | 100 | 25.8 |
| Private Job | 78 | 20.2 |
| Farmer | 108 | 27.9 |
| Student | 41 | 10.6 |
| Housewife | 28 | 7.2 |
Baseline Clinical Factors and Co-Morbidities
In this study, the median HgbA1c was 11% (IQR=9.08–12.22) and the median BMI was 22.06 (IQR=19.72–24.57) Kg/m2. Of the total participants, 187 (48.3%) had LDL level ≥ 100mg/dl whereas 265 (68.48%) had triglyceride level <150 mg/dl. Regarding co-morbidities, 80 (20.7%) had retinopathy, and 56 (14.5%) had nephropathy at baseline (Table 2).
Table 2.
Baseline Clinical and Comorbidity Information of DM Patients on Follow-Up at Felege Hiwot Referral Hospital, from January 1, 2009, to December 31, 2018
| Variables | Frequency | Percentage |
|---|---|---|
| Duration of DM | ||
| <5 year | 256 | 66.15 |
| ≥5 year | 131 | 33.85 |
| Type of DM | ||
| Type 1 | 131 | 33.85 |
| Type 2 | 256 | 66.15 |
| HDL level (mg/dl) | ||
| ≤40 | 178 | 45.99 |
| >40 | 209 | 54.01 |
| Triglyceride level (mg/dl) | ||
| <150 | 265 | 68.48 |
| ≥150 | 122 | 31.52 |
| LDL level (mg/dl) | ||
| <100 | 200 | 51.68 |
| ≥100 | 187 | 48.32 |
| Retinopathy | ||
| Yes | 80 | 20.67 |
| No | 307 | 79.33 |
| Nephropathy | ||
| Yes | 56 | 14.47 |
| No | 331 | 85.53 |
Incidence of DFU
A total of 66, 17.05% (95% CI: 13.61–21.15) newly diagnosed patients who were free from DFU at the start of follow-up developed DFU during the follow-up. The patients were followed for a minimum of 4 months and a maximum of 120 months with the median survival time of 95 months. Based on this the total person-time of observation was found to be 1657.08 person-years. The overall incidence rate of DFU was found to be 4.00 (95% CI; 3.13–5.05) per 100 person-years.
Predictors of DFU
Table 3 showed model comparison methods and the Gompertz regression model with the highest log-likelihood = −89.38487 and the lowest AIC = 200.7697 was found to be the best model.
Table 3.
Summary of Model Comparison by AIC, BIC and Log-Likelihood Method
| Model | Df | Log-Likelihood | AIC | BIC |
|---|---|---|---|---|
| Cox PH | 9 | −256.8541 | 531.7081 | 567.334 |
| Exponential | 10 | −118.0876 | 256.1751 | 295.7594 |
| Gompertz | 11 | −89.38487 | 200.7697 | 244.3124 |
| Weibull | 11 | −94.56383 | 211.1277 | 244.3124 |
| Lognormal | 11 | −107.2141 | 236.4281 | 279.9708 |
| Log logistic | 11 | −97.63152 | 217.263 | 260.8057 |
Abbreviations: Df, the degree of freedom; AIC, Akaike information criteria; BIC, Bayesian information criteria.
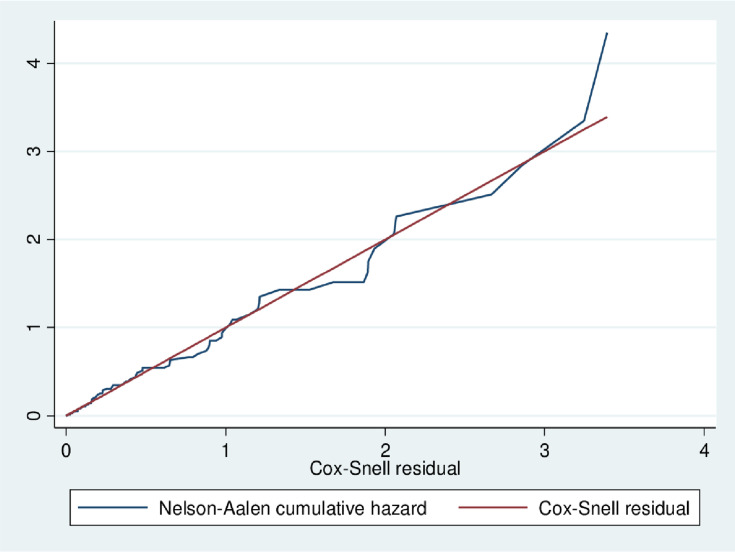
Besides, Nelson–Aalen’s cumulative hazard function against the Cox-snell residual is close to a straight line through the origin of the Gompertz model when compared to other models. This suggests that the Gompertz model provided the best fit for the data (Table 3 and Figure 2).
Figure 2.
The Nelson–Aalen cumulative hazard function and Cox-snell residuals obtained by fitting the Gompertz model for DM patients at Felege Hiwot Referral Hospital, January 1, 2009, to December 31, 2018.
Based on the bivariable analysis at a p-value of 0.2, variables namely sex, religion, residence, occupation, duration of DM, HDL level, and LDL level were excluded from the final multivariable analysis. On the multivariable analysis, retinopathy, nephropathy, and BMI were found to be significant predictors of DFU. In this study, for a unit increase in baseline BMI, the hazard of DFU was increased by 13% (AHR = 1.13, 95% CI: 1.01–1.27). Similarly, having retinopathy, and nephropathy at baseline increases the hazard of DFU by 5.56 (AHR = 5.56, 95% CI: 2.64–11.74), and 2.37 (AHR = 2.37, 95% CI: 1.33–4.24) times, respectively as compared to their counterparts (Table 4).
Table 4.
Multivariable Gompertz Regression Analysis for Predictors of DFU Among DM Patients at Felege Hiwot Referral Hospital, from January 1, 2009, to December 31, 2018
| Variables | Survival Status | CHR (95% CI) | AHR (95%CL) | |
|---|---|---|---|---|
| Event | Censored | |||
| Age(years) | Median =46 | 1.03(1.02–1.05) | 0.99(0.96–1.01) | |
| Educational status | ||||
| No education | 31 | 88 | 1 | 1 |
| Primary education | 16 | 69 | 0.89(0.48–1.61) | 0.92(0.43–1.95) |
| Secondary and above | 19 | 164 | 0.49(0.27–0.86) | 0.48(0.17–1.35) |
| BMI (Kg/m*2) | Mean=22.3 | 1.34(1.23–1.64) | 1.13(1.01–1.27)* | |
| Type of DM | ||||
| Type 1 | 10 | 121 | 1 | 1 |
| Type 2 | 56 | 200 | 2.40(1.22–4.71) | 1.01(0.44–2.07) |
| Retinopathy | ||||
| No | 12 | 295 | 1 | 1 |
| Yes | 54 | 26 | 9.90(5.28–18.55) | 5.56(2.64–11.74)** |
| Nephropathy | ||||
| No | 23 | 308 | 1 | 1 |
| Yes | 43 | 13 | 5.98(3.57–10.04) | 2.37(1.33–4.24)* |
| HgbA1c (%) | Mean=10.9% | 1.00(0.80–1.03) | 1.07(0.99–1.15) | |
| Triglyceride (mg/dl) | ||||
| <150 | 31 | 234 | 1 | 1 |
| ≥150 | 35 | 87 | 1.78(1.10–2.89) | 1.46(0.86–2.49) |
Notes: **p-value <0.001, *p-value <0.05.
Abbreviations: CHR, crude hazard ratio; AHR, adjusted hazard ratio.
Discussion
This study investigated the incidence and predictors of DFU among DM patients at Felege Hiwot referral Hospital. In this study of 387 individual DM patients, 17.05% of study participants had a DFU. This finding is in line with studies done in Ethiopia.28,29 But it was higher than the studies conducted in Japan,54 England55 and Iran.33 This difference might be because of the difference in the denominator population and the study area because all the compared studies were population-based but the current study was institution-based. Also, diabetic care in these countries and other developed countries might be well organized than low- and middle-income countries like Ethiopia. Besides, in low and middle-income countries many factors such as fragmentation of health care services, limited resource allocation, inadequate training among health-care professionals and low health literacy among DM patients contribute to high DFU.56 Regarding incidence rate of DFU, in this study, the incidence rate was 4 per 100 person-year which means in 100 DM patients there will be 4 DFU patients per year or if we follow 100 persons with DM for one year four patients will experience or develop the case [DFU]. This finding was comparable with the study done in Washington which found the incidence rate of 5 per 100-person year.38
Diabetic nephropathy was independently associated with an increased hazard of DFU in this study. This is consistent with a cross-sectional study in Thailand,41 a retrospective longitudinal study done in Ghana,37 and systematic-review and meta-analysis done globally.23 This might be because of peripheral neuropathy and vascular insufficiency are more common in patient with diabetic nephropathy, which in turn results in ischemic ulceration or foot ulcer.57 Moreover, this presence of vascular insufficiency, which is common in a patient with nephropathy, significantly increases the risk of chronic inflammation, malnutrition, fluid retention, rennin-angiotensin system alterations, and ischemic ulcerations that eventually ends up with foot ulcer.58,59
The hazard of developing DFU among diabetic patients with retinopathy was higher than DM patients without diabetic retinopathy. A similar association was found with a cross-sectional study done in Brazil43 and systemic review and meta-analysis done globally.23 This might be due to diabetic patients with diabetic retinopathy had reduced visual activities, so it is difficult to give foot care activities such as examining their feet daily and practicing good foot hygiene and these increases the risk of DFU.60
Diabetes mellitus patients with increasing BMI had an increased hazard of DFU. This is in line with the study done in Ethiopia31 and Ghana.37 This might be due to an increase in BMI is associated with the incensement of obesity. This obesity increases atherosclerosis and in turn decreases blood supply to lower extremities and this makes the environment suitable for the growth of bacteria and this might result in the DFU.
In this study, the association of age and DFU was not significant. However, previous cross-sectional studies conducted in Thailand,35 India,36 and Saudi Arabia34 showed older age to be significantly associated with DFU. This difference might be due to the current study considers only newly diagnosed diabetes patients and the majority 61% of the participants were younger than 50 years but in the former study, for example in Saudi Arabia,34 the majority 66% of patients were older than 50 years.
Since this is a follow-up study it has the advantage of showing temporal relationships. However, because of the secondary nature of the data and in turn the quality of records, some potentially important variables like self-care practices and major traditional risk factors for DFU such as foot deformity were not studied. Also, we used baseline variables and there may be a change of these variables at any time during follow-up.
The clinical, as well as the public health importance of this study, is providing information for health care providers and patients about factors that are associated with the risk of diabetic foot ulcer and to act on them to minimize the risk and increase their effort on prevention of having the problem and to prevent complication as well as to reduce economic losses associated to DFU.
Conclusion
The incidence of DFU among DM patients was relatively high. It was confirmed that DFU among DM patients was determined by multiple influential factors. Significant predictors for DFU were; diabetic nephropathy, diabetic retinopathy, and increase BMI. So, greater attention to diabetic patients with co-morbidities especially, diabetic nephropathy, and diabetic retinopathy, as well as patients with higher BMI, could decrease the incidence of DFU and its complications.
Acknowledgments
We would like to thank the University of Gondar for setting this chance and providing the program as well as all necessary services. Our thanks go to Felege Hiwot Referral Hospital administrations, data collectors, supervisors and our colleagues for sharing their valuable ideas and experiences.
Funding Statement
No funding was received from any organization.
Abbreviations
AHR, Adjusted Hazard Ratio; AIC, Akaike Information Criteria; BIC, Bayesian Information Criteria; BMI, Body Mass Index; CHR, Crude Hazard Ratio; DFU, Diabetic Foot Ulcer; DM, Diabetes Mellitus; HDL, High Density Lipoprotein; IDF, International Diabetic Federation; LDL, Low Density Lipoprotein; OPD, Outpatient Department.
Data Sharing Statement
All result-based data are available within the manuscript and supporting information. The dataset used for this analysis can be provided after a reasonable request of the corresponding author.
Ethics Approval and Informed Consent
This study was conducted in accordance with the Declaration of Helsinki. Since this study used an analysis of secondary data from patient charts, we received a waiver for informed consent. Data was collected after ethical clearance was obtained from the Institutional Review Committee of the University of Gondar institute of public health. For the sake of privacy and confidentiality no personal identifiers (names, address and any private information) was not collected. Data was anonyms and handled confidentially during all phases of research activities.
Consent for Publication
Not applicable.
Author Contributions
All authors were involved in the conception, study design, execution, acquisition of data, analysis and interpretation, drafting the manuscript, revising or critically reviewing the manuscript, gave final approval of the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement 1):S81–S90. doi: 10.2337/dc14-S081 [DOI] [PubMed] [Google Scholar]
- 2.Baynest HW. Classification, pathophysiology, diagnosis and management of diabetes mellitus. J Diabetes Metab. 2015;06(5). doi: 10.4172/2155-6156.1000541 [DOI] [Google Scholar]
- 3.Tabish SA. Is diabetes becoming the biggest epidemic of the twenty-first century? Int J Health Sci. 2007;1(2):V. [PMC free article] [PubMed] [Google Scholar]
- 4.International Diabetes federation. Idf Diabetes Atlas. 9th IDF; 2019. [Google Scholar]
- 5.Leontis LM, Hess-Fischl A. Type 2 diabetes complications; how to prevent short- and long-term complications; 2019. Available from: https://www.endocrineweb.com/conditions/type-2-diabetes/type-2-diabetes-complications.
- 6.Fowler MJ. Microvascular and Macrovascular Complications of Diabetes. Clin Diabetes. 2008;26(2):77–82. doi: 10.2337/diaclin.26.2.77 [DOI] [Google Scholar]
- 7.Safaa M, Hassanein ZME-S. Predicted complications factors among patients with diabetes mellitus: suggested nursing map of care. J Nurs Health Sci. 2019;8(6):49–62. [Google Scholar]
- 8.Boulton AJM. Diabetic Foot Complications. Eur Endocrinol. 2006;2006(1):51–52. doi: 10.17925/EE.2006.00.01.51 [DOI] [Google Scholar]
- 9.Margolis DJ, Hofstad O, Feldman HI. Association between renal failure and foot ulcer or lower-extremity amputation in patients with diabetes. Diabetes Care. 2008;31(7):1331–1336. doi: 10.2337/dc07-2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun DI, Kim S, Kim J, et al. Epidemiology and burden of diabetic foot ulcer and peripheral arterial disease in Korea. J Clin Med. 2019;8(5):748. doi: 10.3390/jcm8050748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain AK. A new classification of diabetic foot complications: a simple and effective teaching tool. J Diab Foot Comp. 2012;4(1):1–5. [Google Scholar]
- 12.Abbas Z, Archibald L. The diabetic foot in subSaharan Africa: a new management paradigm. Diabetic Foot J. 2007;10(3):128–133. [Google Scholar]
- 13.Okonofua FE, Odimegwu C, Ajabor H, Daru PH, Johnson A. Assessing the prevalence and determinants of unwanted pregnancy and induced abortion in Nigeria. Stud Fam Plann. 1999;30(1):67–77. doi: 10.1111/j.1728-4465.1999.00067.x [DOI] [PubMed] [Google Scholar]
- 14.Kumhar M, Dara N, Saini T. Foot wear and footcare knowledge—an independent risk factor for diabetic foot in Indian diabetics. Ind Med Gaz. 2014;148(1):25–28. [Google Scholar]
- 15.Agwu E, Dafiewhare EO, Ekanem PE. Possible diabetic-foot complications in Sub-Saharan Africa. Global Pers Diabetic Foot Ulcer. 2010;2007:3–15. [Google Scholar]
- 16.Boulton AJ. The diabetic foot. Medicine. 2015;43(1):33–37. doi: 10.1016/j.mpmed.2014.10.006 [DOI] [Google Scholar]
- 17.Jeyaraman K, Berhane T, Hamilton M, Chandra AP, Falhammar H. Mortality in patients with diabetic foot ulcer: a retrospective study of 513 cases from a single Centre in the Northern Territory of Australia. BMC Endocr Disord. 2019;19(1):1. doi: 10.1186/s12902-018-0327-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sothornwit J, Srisawasdi G, Suwannakin A, Sriwijitkamol A. Decreased health-related quality of life in patients with diabetic foot problems. Diabetes Metab Syndr Obes. 2018;11:35. doi: 10.2147/DMSO.S154304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rezaie W, Lusendi F, Doggen K, Matricali G, Nobels F. Health-related quality of life in patients with diabetic foot ulceration: study protocol for adaptation and validation of patient-reported outcome measurements (PROMs) in Dutch-speaking patients. BMJ Open. 2019;9(12):1–7. doi: 10.1136/bmjopen-2019-034491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alrub AA, Hyassat D, Khader YS, Bani-Mustafa R, Younes N, Ajlouni K. Factors associated with health-related quality of life among Jordanian patients with diabetic foot ulcer. J Diabetes Res. 2019;2019:1–8. doi: 10.1155/2019/4706720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wukich DK, Raspovic KM. Assessing health-related quality of life in patients with diabetic foot disease: why is it important and how can we improve? The 2017 Roger E. Pecoraro award lecture. Diabetes Care. 2018;41(3):391–397. doi: 10.2337/dci17-0029 [DOI] [PubMed] [Google Scholar]
- 22.Sargen MR, Hoffstad O, Margolis DJ. Geographic variation in medicare spending and mortality for diabetic patients with foot ulcers and amputations. J Diabetes Complications. 2013;27(2):128–133. doi: 10.1016/j.jdiacomp.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med. 2017;49(2):106–116. doi: 10.1080/07853890.2016.1231932 [DOI] [PubMed] [Google Scholar]
- 24.Almobarak AO, Awadalla H, Osman M, Ahmed MH. Prevalence of diabetic foot ulceration and associated risk factors: an old and still major public health problem in Khartoum, Sudan? Ann Transl Med. 2017;5(17):340. doi: 10.21037/atm.2017.07.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiwanga FS, Njelekela MA. Diabetic foot: prevalence, knowledge, and foot self-care practices among diabetic patients in Dar es Salaam, Tanzania–a cross-sectional study. J Foot Ankle Res. 2015;8(1):20. doi: 10.1186/s13047-015-0080-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyamu PN, Otieno CF, Amayo EO, McLigeyo SO. Risk factors and prevalence of diabetic foot ulcers at Kenyatta National Hospital, Nairobi.I. East Afr Med J. 2003;80(1):36–43. [DOI] [PubMed] [Google Scholar]
- 27.Ugwu E, Adeleye O, Gezawa I, Okpe I, Enamino M, Ezeani I. Burden of diabetic foot ulcer in Nigeria: current evidence from the multicenter evaluation of diabetic foot ulcer in Nigeria. World J Diabetes. 2019;10(3):200–211. doi: 10.4239/wjd.v10.i3.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deribe B, Woldemichael K, Nemera G. Prevalence and factors influencing diabetic foot ulcer among diabetic patients attending Arbaminch Hospital, South Ethiopia. J Diabetes Metab. 2014;5(1):1–7. doi: 10.4172/2155-6156.1000322 [DOI] [Google Scholar]
- 29.Bekele F, Fekadu G, Dugassa KB. Incidence of diabetic foot ulcer among diabetes mellitus patients admitted to Nekemte Referral Hospital, Western Ethiopia: prospective observational study. Endocrinol Metabol Syndr. 2019;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gebrekirstos K, Gebrekiros S, Fantahun A. Prevalence and factors associated with diabetic foot ulcer among adult patients in ayder referral hospital diabetic clinic mekelle, North Ethiopia, 2013. J Diabetes Metab. 2015;6(579):2. [Google Scholar]
- 31.Mariam TG, Alemayehu A, Tesfaye E, et al. Prevalence of diabetic foot ulcer and associated factors among adult diabetic patients who attend the diabetic follow-up clinic at the university of gondar referral hospital, North West Ethiopia, 2016: institutional-based cross-sectional study. J Diabetes Res. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakri FG, Allan AH, Khader YS, Younes NA, Ajlouni KM. Prevalence of diabetic foot ulcer and its associated risk factors among diabetic patients in Jordan. J Med. 2012;46(2):118–125. [Google Scholar]
- 33.Yazdanpanah L, Shahbazian H, Nazari I, et al. Incidence and risk factors of diabetic foot ulcer: a population-based diabetic foot cohort (ADFC study)-two-year follow-up study. Int J Endocrinol. 2018;2018:1–9. doi: 10.1155/2018/7631659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fawzy MS, Alshammari MA, Alruwaili AA, et al. Factors associated with diabetic foot among type 2 diabetes in Northern area of Saudi Arabia: a descriptive study. BMC Res Notes. 2019;12(1):51. doi: 10.1186/s13104-019-4088-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarinnapakorn V, Sunthorntepwarakul T, Deerochanawong C, Niramitmahapanya S, Napartivaumnuay N. Prevalence of diabetic foot ulcers and risk classifications in type 2 diabetes mellitus patients at Rajavithi Hospital. J Med Assoc Thai. 2016;99(2):S99–S105. [PubMed] [Google Scholar]
- 36.Shahi SK, Kumar A, Kumar S, Singh SK, Gupta SK, Singh T. Prevalence of diabetic foot ulcer and associated risk factors in diabetic patients from North India. J Diab Foot Comp. 2012;4(3):83–91. [Google Scholar]
- 37.Sarfo-Kantanka O, Kyei I, Mbanya JC, Owusu-Ansah M. Diabetes-related foot disorders among adult Ghanaians. Diabet Foot Ankle. 2018;9(1):1511678. doi: 10.1080/2000625X.2018.1511678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyko EJ, Ahroni JH, Cohen V, Nelson KM, Heagerty PJ. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care. 2006;29(6):1202–1207. doi: 10.2337/dc05-2031 [DOI] [PubMed] [Google Scholar]
- 39.Bjornstad P, Hwang DJ, Lee KM, et al. Association between diabetic foot ulcer and diabetic retinopathy. PLoS One. 2017;12(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Y, Wang X, Xia L, et al. A cohort study of diabetic patients and diabetic foot ulceration patients in China. Wound Repair Regener. 2015;23(2):222–230. doi: 10.1111/wrr.12263 [DOI] [PubMed] [Google Scholar]
- 41.Veerasak STS, Chaicharn D, Sathit N, Navaporn N. Prevalence of diabetic foot ulcers and risk classifications in type 2 diabetes mellitus patients at Rajavithi Hospital. J Med Assoc Thai. 2016;99:S99–S105. [PubMed] [Google Scholar]
- 42.Wolde HF, Atsedeweyen A, Jember A, et al. Predictors of vascular complications among type 2 diabetes mellitus patients at University of Gondar Referral Hospital: a retrospective follow-up study. BMC Endocr Disord. 2018;18(1):52. doi: 10.1186/s12902-018-0280-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva J, Haddad M, Rossaneis MA, Vannuchi MTO, Marcon SS. Factors associated with foot ulceration of people with diabetes mellitus living in rural areas. Rev Gaucha Enferm. 2017;38(3). [DOI] [PubMed] [Google Scholar]
- 44.Naemi R, Chockalingam N, Lutale JK, Abbas ZG. Predicting the risk of future diabetic foot ulcer occurrence: a prospective cohort study of patients with diabetes in Tanzania. BMJ Open Diabetes Res Care. 2020;8(1):e001122. doi: 10.1136/bmjdrc-2019-001122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reiber GE, Vileikyte L, Boyko E, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999;22(1):157–162. doi: 10.2337/diacare.22.1.157 [DOI] [PubMed] [Google Scholar]
- 46.Hameed S, Azmat SK, Bilgrami M, Ishaque M. Determining the factors associated with “Unmet need for family planning”: a cross-sectional survey in 49 districts of Pakistan. Pakistan J Public Health. 2011;1:21–26. [Google Scholar]
- 47.Frykberg RG, Lavery LA, Pham H, Harvey C, Harkless L, Veves A. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care. 1998;21:1714–1719. doi: 10.2337/diacare.21.10.1714 [DOI] [PubMed] [Google Scholar]
- 48.Naemi R, Chockalingam N, Lutale JK, Abbas ZG. Can a combination of lifestyle and clinical characteristics explain the presence of foot ulcer in patients with diabetes? J Diabetes Complications. 2019;33(6):437–444. doi: 10.1016/j.jdiacomp.2019.02.006 [DOI] [PubMed] [Google Scholar]
- 49.Yazdanpanah L, Shahbazian H, Nazari I, et al. Risk factors associated with diabetic foot ulcer-free survival in patients with diabetes. Diabetes Metab Syndr. 2018;12(6):1039–1043. doi: 10.1016/j.dsx.2018.06.020 [DOI] [PubMed] [Google Scholar]
- 50.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39(2):499–503. doi: 10.2307/2531021 [DOI] [PubMed] [Google Scholar]
- 51.Wagner FW. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1981;2(2):64–122. doi: 10.1177/107110078100200202 [DOI] [PubMed] [Google Scholar]
- 52.Apelqvist J, Bakker K, van Houtum WH, Nabuurs-Franssen MH, Schaper NC. International consensus and practical guidelines on the management and the prevention of the diabetic foot. Int Working Group Diabetic Foot Diabetes Metab Res Rev. 2000;16(S1):S84–92. [DOI] [PubMed] [Google Scholar]
- 53.Ogbera AO, Fasanmade OA, Chinenye S, Akinlade A. Characterization of lipid parameters in diabetes mellitus–a Nigerian report. Int Arch Med. 2009;2(1):19. doi: 10.1186/1755-7682-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iwase M, Fujii H, Nakamura U, et al. Incidence of diabetic foot ulcer in Japanese patients with type 2 diabetes mellitus: the Fukuoka diabetes registry. Diabetes Res Clin Pract. 2018;137:183–189. doi: 10.1016/j.diabres.2018.01.020 [DOI] [PubMed] [Google Scholar]
- 55.Abbott C, Carrington A, Ashe H, et al. The North‐West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community‐based patient cohort. Diabetic Med. 2002;19(5):377–384. doi: 10.1046/j.1464-5491.2002.00698.x [DOI] [PubMed] [Google Scholar]
- 56.Guell C, Unwin N. Barriers to diabetic foot care in a developing country with a high incidence of diabetes related amputations: an exploratory qualitative interview study. BMC Health Serv Res. 2015;15(1):377. doi: 10.1186/s12913-015-1043-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaminski M, Frescos N, Tucker S. Prevalence of risk factors for foot ulceration in patients with end‐stage renal disease on haemodialysis. Intern Med J. 2012;42(6):e120–e8. doi: 10.1111/j.1445-5994.2011.02605.x [DOI] [PubMed] [Google Scholar]
- 58.MacFarlane D. Chronic renal failure and its effect upon the lower limb. Br J Podiatr. 2002;5:105–110. [Google Scholar]
- 59.de Vinuesa SG, Ortega M, Martinez P, Goicoechea M, Campdera FG, Luño J. Subclinical peripheral arterial disease in patients with chronic kidney disease: prevalence and related risk factors. Kidney Int. 2005;67:S44–S7. doi: 10.1111/j.1523-1755.2005.09310.x [DOI] [PubMed] [Google Scholar]
- 60.Iraj B, Khorvash F, Ebneshahidi A, Askari G. Prevention of diabetic foot ulcer. Int J Prev Med. 2013;4(3):373–376. [PMC free article] [PubMed] [Google Scholar]