Abstract
Global climate change and increased population caused significant depletion of freshwater especially in arid and semi-arid regions including Saudi Arabia. Saline water magnetization before irrigation may help in alleviating the adverse effects of salinity on plants. The current study aimed to examine the potential beneficial effects of water magnetization and soil amendments on growth, productivity, and survival of Calendula officinalis L. plants. Three types of water (tap water “control”, well water, and magnetized well water) and two types of soil amendments (Fe2SO4 and peat moss) were examined. Our results showed that irrigating C. officinalis plants with saline well water (WW) adversely affected growth and flowering as compared to tap water (TW). However, plants irrigated with magnetized water (MW) showed significant enhancement in all the studied vegetative and flowering growth parameters as compared to those irrigated with WW. Furthermore, mineral contents and survival of C. officinalis plants irrigated with MW were higher than those irrigated with TW. Irrigation with MW significantly reduced levels of NA+ and Cl− ions in leaves of C. officinalis plants indicating the role of magnetization in alleviating harmful effects of salinity. The current study showed that water magnetization enhanced water quality and increased plant’s ability to absorb water and nutrients. Further studies are needed to examine the possibility of irrigating food crops with magnetized water.
Keywords: Ferrous sulfate, Peat moss, Salinity, Well water, Flowering
1. Introduction
Water scarcity is considered among the most important consequences of global climate change. In arid and semi-arid areas e.g. Saudi Arabia, more pressure has been put on fresh-water resources because of increased demand and limited resources. Agricultural sector in Saudi Arabia is the dominant user of freshwater using around 88% of the total amount of water utilized for different purposes (Gabr et al., 2020). Therefore, finding alternative sources for irrigation water could significantly help in alleviating pressure on limited fresh-water resources. Saline well water is one of the alternative options for irrigating crops especially ornamental plants. However, application of high saline water has several adverse effects on soil properties and plant growth and productivity. Some studies claimed that using magnetized water in irrigation enhance soil properties and reduce salinity and drought stresses on plants (Kney and Parsons, 2006, Mostafazadeh-Fard et al., 2011). Water is magnetized via passing through magnetic field converting the present salts into inactive state and therefore reducing adverse effects of salinity on plant growth (Teixeira da Silva and Dobránszki, 2014). Furthermore, magnetic water enhances soil properties via washing salts from the soil rhizosphere (Maheshwari and Grewal, 2009). Magnetization enhances water properties via increasing ability to penetrate root cells, reducing water viscosity, inter-attraction of molecules, and breaking hydrogen bonds which promote water absorption in roots (Abdul-Qados and Hozayn, 2010).
Soil organic and inorganic amendments could be used to reduce the negative effects of salts’ accumulation in soil resulted from irrigation with saline water. Organic amendments provide soil with organic matter that aids in moisture retention, soil aeration and porosity, and microorganisms activity (Goyal et al., 1999). Peat moss and ferrous oxide (Fe2SO4) is considered among the most important organic and inorganic soil amendments, respectively.
Calendula officinalis L. (Asteraceae) is an annual ornamental herbaceous plant produced mainly for cut flowers. However, it is used intensely in street and garden landscaping. C. officinalis is also considered as a medicinal plant used for different therapeutical purposes (Chaparzadeh et al., 2004). The yellow pigment extracted from C. officinalis flowers is used as food coloring additive. Flower decoction is utilized to treat cold, digestive system, and kidneys, and contains antioxidants that stimulate the immune system (Edwards et al., 2015). C. officinalis is considered as mild-tolerate to salinity stress as it could grow normally under salinity levels reaches 4–5 dS m−1 (Chaparzadeh et al., 2004). Therefore, the current study aimed to examine the effects of irrigation with magnetized saline well water and addition of soil organic and/or inorganic amendments on growth and productivity of Calendula officinalis L. plants.
2. Materials and methods
2.1. Plant material and study site
The current study was conducted in the nursery of Sustainability and Environmental Development Department, King Saud University, Riyadh, Saudi Arabia. Seeds of C. officinalis L. cv. Bon-Bon Orange were obtained from Muller Bloemzaden BV, Lisse, The Netherlands. Purchased seeds were germinated in 454-hole germination trays filled with peat moss only inside a plastic greenhouse with 11 h of light at 24/20 °C day/night temperature and 70% relative humidity. Seeds were left to grow for 30 days and then all seedlings were transferred into 10-cm plastic pots and grown in the plastic greenhouse under the same conditions but with 16 h light period.
For the permanent cultivation of C. officinalis plants, the soil was replaced in the cultivation area with sandy soil to a depth of 50 cm. The experiment was designed following the split-plot design with two factors namely irrigation water type (tap water “control”, magnetized well water, and well water) and soil amendments (without addition “control”, peat moss, ferrous oxide “Fe2SO4”, and peat moss + Fe2SO4). Therefore, cultivation area was divided into 12 different groups (plots) and each group was assigned a treatment randomly. Each treatment group was replicated 3 times in three different cultivation lines each of 1.6 m in length and 2 m in width. The final area of each experimental unit contains 3 replicates was 3.2 m2. Peat moss were added to the assigned plots at a rate of 3:1 (v: v) sand: peat moss while iron was added in the form of Fe2SO4 at a rate of 100 g per experimental unit to get final concentration of 20.5% Fe2+. Fe2SO4 was dissolved in 10 L of water and added as a solution to the designated units after emergence of the third leaf in C. officinalis plants. Plants were fertilized two times; the first directly after cultivation and the second after two months using Sangral NPK 20:20:20 (SQM Europe NV, Belgium).
Plants were irrigated with designated type of water via drip irrigation system equipped with three different 5000 L tanks: one for tap water (TW) and the other two for well water (WW). WW were magnetized using Model 8000 GMX Magnetic Fluid Conditioner (GMX Corporation, California, USA) with three units each with magnetic power of 1500 Gauss. Magnetization were performed for the whole irrigation duration (10 min) of designated plots with the magnetized well water (MW). In the drip irrigation system, installed drippers had a flow of 8 L h−1 at 2 bar pressure. Each experimental unit had three drip irrigation lines (25 mm diameter) with length of 1.6 m. Each line had 4 drippers (one for each cultivated plant) with 40 cm spaces in between. The spaces between each line and the other were 40 cm also.
2.2. Soil analysis
Random samples at two different depths (0–30 and 30–50 cm) were collected from the cultivation soil used in the study to examine the physical and chemical characteristics of the soil. The methods described by Black (1965) was followed to examine soil texture. Moreover, soil pH, EC, cation exchange capacity (CEC), and contents of organic matter (OM), CaCO3, N, P, and K were determined (Page, 1982).
2.3. Irrigation water analysis
Samples of different irrigation water types (TW, MW, and WW) were collected and analyzed to determine their pH, EC, sodium adsorption ratio (SAR), and dissolved cations (K+, Ca2+, Mg2+, and Na+) and anions (Cl−, SO42−, CO32−, and HCO3−).
2.4. Measured parameters
2.4.1. Growth
At the end of the experiment (flowering stage) after 90 days of cultivation in the permanent location, 6 plants were collected from each treatment and used for further measurement of the studied parameters. To examine variations in vegetative growth among different treatments, plant height (cm), number of leaves per plant, number of branches per plant, and stem diameter (cm) were determined. Moreover, leaf area (cm2) per plant was measured using LI-3000C Portable Leaf Area Meter (LI-COR Biosciences GmbH, Homburg, Germany). Shoot dry mass (SDM) in grams was measured after air-drying of plants in the oven at 70 °C for 72 h.
2.4.2. Flowering
Changes in productivity of C. officinalis plants were measured via calculating days to flowering starting from cultivation date and number of flowers per plant under different treatments. Furthermore, flower dry mass (FDM) under different treatments was measured by weighting all flowers for each plant after air-drying in oven at 70 °C for 72 h.
2.4.3. Survival percentage
Survival percentage under each treatment was measured as the percentage of survived plants to the end of the experiment out of total cultivated plants in each treatment.
2.4.4. Mineral contents
At the end of the experiment, dried plant material was grounded into fine powder and used for estimation of mineral content. Contents of N, P, K, Ca, Mg, Fe, Na, and Cl were measured (AOAC, 1990) in each plant sample collected from different treatments. Analysis was replicated 3 times for each treatment.
3. Statistical analysis
Two-way analysis of variance (ANOVA) was applied to examine the variation in the studied parameters resulted from irrigation water type, application of soil amendments, and their interaction using SAS v9.4 software. Values were reported as the mean of 3 replicates. Moreover, differences between means were identified using least significant difference (LSD) test (P ≤ 0.05).
4. Results and discussion
4.1. Soil analysis
Analysis of soil texture showed that soil at different depths was characterized by sandy texture with more than 90% sand. Clay percentage ranged from 5% to 7% while silt represented 2% of the cultivation soil. EC of soil solution was roughly 1.5 dS m−1 at different depths (Table 1). Cultivation soil solution was neutral with pH values of 7.68 and 7.71 at depths of 0–30 and 30–50 cm, respectively. OM content was higher in surface layer of the soil (1.82%) than in lower layer (1.31%). Therefore, CEC of the upper layer (35.99 meq 100 g−1) was higher as compared to the lower layer (33.87 meq 100 g−1). Calcium carbonate content ranged from 2.13% to 2.39% at different depths. Cultivation soil characterized by high K (106 – 120 ppm) but low P levels (6 – 8 ppm). Nitrogen content in the soil ranged from 45.82 ppm in the upper layer to 57.21 ppm in the lower layer.
Table 1.
Physical and chemical characteristics of soil used for cultivation of Calendula officinalis L. plants.
| Soil characteristics | Soil depth |
|
|---|---|---|
| (0–30) | (30–50) | |
| A. Physical | ||
| Sand (%) | 90.32 | 92.32 |
| Silt (%) | 2.0 | 2.0 |
| Clay (%) | 7.68 | 5.68 |
| Soil texture | Sandy | Sandy |
| B. Chemical | ||
| EC (dS m−1)* | 1.42 | 1.57 |
| pH | 7.68 | 7.71 |
| OM (%) | 1.82 | 1.31 |
| CEC (meq 100 g−1) | 35.99 | 33.87 |
| CaCO3 (%) | 2.39 | 2.13 |
| N (ppm) | 45.82 | 57.21 |
| P (ppm) | 7.98 | 6.11 |
| K (ppm) | 120.14 | 106.73 |
EC: electrical conductivity; OM: organic matter; CEC: cation exchange capacity.
4.2. Water analysis
Chemical characteristics of different types of irrigation water used in the current study were examined. TW (control) characterized by the lowest salinity level with the lowest EC (0.74 dS m−1) and Na+ and Cl− contents (7.0 and 0.8 meq L−1, respectively). On the other hand, MW was less saline (EC 3.7 dS m−1, Na+ content 21.6 ppm, Cl− content 28.0 ppm) than WW (EC 4.0 dS m−1, Na+ content 22.1 ppm, Cl− content 28.0 ppm). Indeed, both MW and WW were characterized by significantly higher salinity as compared to TW (Table 2). Furthermore, SAR were higher in MW and WW as compared to TW. On the other hand, TW had the lowest K content. MW showed the highest HCO3− content while WW showed the highest SO42− content. All water types used for irrigation showed almost neutral pH, but WW was slightly alkaline (pH 7.9).
Table 2.
Chemical characteristics of different water types used for irrigating Calendula officinalis L. plants.
| Irrigation water | EC (dS m−1) | pH | SAR* | Dissolved cations and anions (meq L−1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cations |
Anions |
||||||||||
| Ca2+ | Mg2+ | Na+ | K+ | CO32− | HCO3− | Cl− | SO42− | ||||
| TW | 0.74 | 7.0 | 4.0 | 4.0 | 3.0 | 7.0 | 0.3 | – | 0.9 | 8.0 | 2.1 |
| MW | 3.7 | 7.2 | 7.2 | 5.0 | 3.8 | 21.6 | 3.8 | – | 1.3 | 28.0 | 2.8 |
| WW | 4.0 | 7.9 | 7.4 | 5.1 | 3.6 | 22.1 | 3.6 | – | 0.9 | 28.0 | 5.0 |
Sodium adsorption ratio.
4.3. Plant vegetative growth
Effects of different water irrigation types and/or addition of soil amendments were examined on vegetative growth of C. officinalis plants. The results showed that irrigation with saline water (MW and WW) reduced plant growth in terms of plant height, stem diameter, number of branches and leaves, leaf area, and SDM; however, plants irrigated with MW showed better growth than those irrigated with WW (Table 3). Significant enhancements observed in plant vegetative growth parameters resulted from irrigation with MW as compared to saline WW (Fig. 1a) could be attributed to the role of water magnetization in strengthening the properties of water by regulating charges and consequently changes in the properties of water when placing water molecules within a magnetic field resulting in the dissociation of hydrogen bonds between molecules (Hilal and Hilal, 2000a, Hachicha et al., 2018). On the other hand, adverse effects of irrigation with WW may be due to obstructing the absorption of some essential nutrients resulted from the presence of salt ions in irrigation water or the permeability of these elements to the tissues of the plant, which in turn leads to the occurrence of ionic toxicity and metabolic changes causing reduction in most of the vegetative growth characteristics of plants (Hilal and Hilal, 2000b). Ionic toxicity further disrupts enzymes, ruptures the plasma membrane, and thus slows main metabolic processes e.g. photosynthesis, respiration, and protein synthesis (Ferrante et al., 2011). Moreover, irrigation water salinity causes damage to plant cell walls by saline tension and an increase in cell wall thickness (Orcutt and Nilsen, 2000). It was reported that saline tension reduces plant hormones that participate in the cellular division stage, which negatively affects plant height (Munns, 2002, Kozminska et al., 2017).
Table 3.
Effects of irrigation water type and different soil additives on growth of Calendula officinalis L. plants.
| Factors | Plant height (cm) | Stem diameter (cm) | No. of branches | No. of leaves | Leaf area (cm2) | SDM (g) | |
|---|---|---|---|---|---|---|---|
| A. Single factors | |||||||
| 1. Irrigation water type | |||||||
| TW | 17.96 | 2.09 | 12.14 | 116.3 | 985.1 | 38.37 | |
| MW | 16.88 | 1.88 | 10.69 | 104.5 | 862.6 | 36.81 | |
| WW | 14.49 | 1.47 | 8.54 | 86.6 | 702.7 | 36.12 | |
| LSD (P ≤ 0.05) | 0.07 | 0.03 | 0.07 | 0.13 | 2.72 | 0.11 | |
| 2. Soil additives | |||||||
| Cont. | 14.88 | 1.52 | 8.40 | 99.6 | 793.2 | 35.80 | |
| PM | 16.18 | 1.72 | 9.33 | 101.2 | 826.2 | 37.07 | |
| Fe2SO4 | 16.69 | 1.89 | 11.05 | 102.5 | 847.1 | 37.27 | |
| PM + Fe2SO4 | 18.02 | 2.13 | 13.04 | 106.5 | 934.0 | 38.26 | |
| LSD (P ≤ 0.05) | 0.82 | 0.56 | 1.09 | 1.58 | 21.89 | 1.22 | |
| B. Interaction | |||||||
| TW | Cont. | 16.23 | 1.82 | 9.76 | 113.2 | 919.2 | 36.98 |
| PM | 17.67 | 1.95 | 10.83 | 114.9 | 957.4 | 38.97 | |
| Fe2SO4 | 18.23 | 2.16 | 12.83 | 116.4 | 981.7 | 38.42 | |
| PM + Fe2SO4 | 19.70 | 2.43 | 15.15 | 121.0 | 1082.3 | 39.12 | |
| MW | Cont. | 15.30 | 1.56 | 8.59 | 101.6 | 804.9 | 35.59 |
| PM | 16.60 | 1.78 | 9.3 | 103.2 | 838.4 | 36.41 | |
| Fe2SO4 | 17.10 | 1.96 | 11.29 | 104.6 | 859.6 | 36.79 | |
| PM + Fe2SO4 | 18.51 | 2.23 | 13.33 | 108.6 | 947.8 | 38.45 | |
| WW | Cont. | 13.10 | 1.17 | 6.87 | 84.2 | 655.6 | 34.83 |
| PM | 14.27 | 1.43 | 7.62 | 85.5 | 682.9 | 35.83 | |
| Fe2SO4 | 14.73 | 1.56 | 9.02 | 86.6 | 700.2 | 36.61 | |
| PM + Fe2SO4 | 15.87 | 1.72 | 10.65 | 90.0 | 772.1 | 38.26 | |
| LSD (P ≤ 0.05) | 0.06 | 0.07 | 0.11 | 0.15 | 2.26 | 0.09 | |
TW: tap water, MW: magnetized water; WW: well water; PM: peat moss; SDM: shoot dry matter; LSD: least significant difference.
Fig. 1.
Morphological responses of Calendula officinalis L. plants to (a) irrigation with different water types and (b) addition of different soil amendments. TW: tap water, MW: magnetized water; WW: well water; C: control; PM: peat moss.
Addition of soil amendments significantly enhanced all the vegetative growth studied parameters regardless the type of water used for irrigation (Fig. 1b). The results showed that addition of Fe2SO4 significantly enhanced C. officinalis growth as compared to addition of peat moss or cultivating plants in sandy soils without amendments. Furthermore, plants cultivated under the combined addition of peat moss and Fe2SO4 showed the highest vegetative growth parameters among all other treatments (Table 3). Improvement of vegetative growth after addition of soil amendments may be due to their positive roles in improving the physical, chemical, and biological characteristics of the soil, as they contain many essential nutrients for plants (Halvin et al., 2005). Abd Elrahman et al. (2012) found that addition of Fe2SO4 increases the solubility of CaCO3 and its replacement of Na+ and thus reduces the percentage of sodium exchanged in the soil.
Analysis of variance revealed that interaction between irrigation water type and soil amendments affected all vegetative growth parameters (P ≤ 0.05). Plants irrigated with MW in soils with peat moss and Fe2SO4 showed higher plant height, stem diameter, and number of branches among all studied treatments except those plants irrigated with TW (control) with addition of peat moss and Fe2SO4 (Table 3). Under all irrigation water types, addition of soil amendments significantly enhanced different plant vegetative growth parameters with addition of both peat moss and Fe2SO4 resulted in the highest enhancement. It was observed that the growth of plants irrigated with MW without any addition of soil amendments was better than those irrigated with untreated WW in all cases even with the addition of peat moss and Fe2SO4 together. Reductions in number of leaves, leaf area, and dry matter in plants irrigated with WW as compared to those irrigated with MW or TW could be attributed to osmotic and toxic effects of Na+, the imbalance between nutrients within plant tissues, and the high osmotic pressure resulted from reduced amount of water (Grieve et al., 2006).
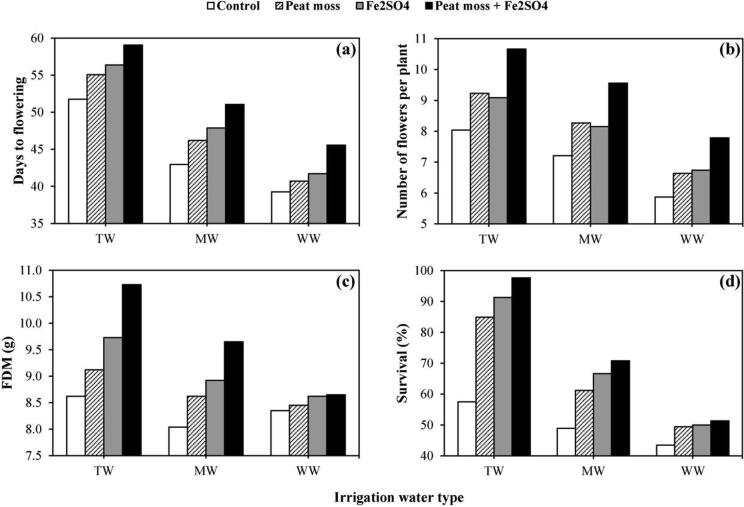
4.4. Plant flowering and survival
Irrigating C. officinalis plants with MW or WW reduced productivity in terms of number of flowers and FDM as compared to plants irrigated with TW; however, irrigation with MW showed less reduction than irrigation with WW. On the other hand, plants irrigated with TW showed delayed flowering (55.56 days to flowering) as compared to other plants irrigated with MW (47.02 days to flowering) and WW (41.82 days to flowering). Irrigation with MW and addition of both peat moss and Fe2SO4 significantly increased days to flowering (~51 days) to be comparable to control plats irrigated with TW without addition of soil amendments (Fig. 2a). The obtained results showed that addition of soil amendments (peat moss and/or Fe2SO4) significantly enhanced number of flowers and FDM under all irrigation water types (Fig. 2b–c). Peat moss plays pivot roles in increasing soil’s OM content and cation exchange capacity and thereby enhancing soil’s structure and texture leading to improvements in the ability to maintain water and nutrient availability (Eyras et al., 2008). Furthermore, plants irrigated with MW combined with addition of both soil amendments showed higher number of flowers than any other plants except those irrigated with TW with addition of both amendments. Similarly, FDM produced by plants irrigated with MW with addition of both soil amendments was higher than any other plants except those irrigated with TW with addition of peat moss only or with Fe2SO4. The beneficial effects of irrigation with MW as compared to WW may be due to the role of magnetization in increasing plant growth via enhancing water absorption (Abdul-Qados and Hozayn, 2010). Moreover, MW is characterized by high solubility which increased availability and uptake of nutrients (Hilal and Hilal, 2000b).
Fig. 2.
Effects of irrigation water type and different soil amendments on flower productivity and survival percentage of Calendula officinalis L. plants. TW: tap water, MW: magnetized water; WW: well water.
As shown in Fig. 2d, regardless of soil amendments, plants irrigated with TW showed the highest survival percentage (97.6%) followed by those irrigated with MW (70.8%). Plants irrigated with WW showed the lowest survival percentage (51.34%). Lower survival rates of plants irrigated with WW could be due to reduced vegetative growth resulted from inhibition of growth promoting hormones e.g. cytokinins (Younis et al., 1987). Moreover, the increased osmotic pressure in plants resulted from saline effects of WW adversely affects absorption of water and nutrients necessary for different biological processes e.g. photosynthesis (Prado et al., 2000). Increased survival rates of plants irrigated with MW compared to WW may be due to the positive effect of magnetization in reducing the size of water molecules, which eases the permeability of water and nutrients that stimulates plant growth and increase survival rate of plants (Noran et al., 1996). On the other hand, addition of soil amendments significantly enhanced plant survival percentages regardless of irrigation water type. Survival percentages of plants irrigated with MW with addition of both types of soil amendments was higher than that of plants irrigated with TW without addition of any soil amendments (Fig. 2d). Moreover, plants irrigated with saline WW showed higher percentage after addition of any soil amendment or both of them as compared to plants irrigated with MW without any addition.
4.5. Mineral contents
Irrigating C. officinalis plants with WW adversely affected the nutritional status in terms of N, P, K, Ca, Mg, and Fe as compared to irrigation with TW. However, irrigation with MW enhanced the plants’ contents of these nutrients as compared to irrigation with saline WW (Table 4). Increased dissolved salts in the soil reduces osmotic potential of the soil solution (becomes more negative) and thus increases the difference between the water potentials of soil solution and plant root solution leading to reduction of water and nutrient absorption (Boland, 2006). The weakened growth of roots in saline soils could be one of the reasons behind reduction in plant's content of different nutrients (Flowers and Colmer, 2008, Elhindi et al., 2017). Addition of peat moss and Fe2SO4 enhanced plant contents of N, P, and Fe as compared to other treatments of soil amendments. However, plants grown in soils with Fe2SO4 only or peat moss and Fe2SO4 showed similar (P ≤ 0.05) K, Ca, and Mg contents which were higher than contents of plants grown in soils without amendments or with peat moss only. In general, plants irrigated with TW in soils with peat moss and Fe2SO4 additions showed the highest N, K, Ca, Mg, and Fe contents among all treatments followed by those irrigated with MW in soils with peat moss and Fe2SO4 additions. There was no significant difference in K content in plants irrigated with TW or MW in case of adding peat moss and Fe2SO4 as soil amendments (Table 4). Adding soil amendments improves soil properties by increasing permeability, water movement, porosity, moisture retaining, and cation exchange capacity (Selim et al., 2009, Elhindi, 2012).
Table 4.
Effects of irrigation water type and different soil additives on macro and micronutrient contents of Calendula officinalis L. plants.
| Factors | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | Na (%) | Cl (%) | Fe (ppm) | |
|---|---|---|---|---|---|---|---|---|---|
| A. Single factors | |||||||||
| 1. Irrigation water type | |||||||||
| TW | 3.83 | 0.49 | 3.12 | 0.89 | 3.62 | 0.36 | 0.52 | 248.9 | |
| MW | 3.65 | 0.46 | 2.94 | 0.66 | 2.67 | 0.41 | 0.58 | 240.9 | |
| WW | 3.74 | 0.34 | 2.23 | 0.57 | 2.14 | 0.57 | 0.82 | 196.9 | |
| LSD (P ≤ 0.05) | 0.09 | 0.01 | 0.22 | 0.05 | 0.17 | 0.03 | 0.04 | 0.19 | |
| 2. Soil additives | |||||||||
| Cont. | 2.23 | 0.27 | 1.59 | 0.53 | 2.24 | 0.56 | 0.81 | 225.6 | |
| PM | 3.03 | 0.38 | 2.48 | 0.64 | 2.59 | 0.48 | 0.66 | 228.3 | |
| Fe2SO4 | 3.71 | 0.47 | 3.20 | 0.76 | 3.15 | 0.40 | 0.59 | 229.7 | |
| PM + Fe2SO4 | 4.65 | 0.59 | 3.77 | 0.89 | 3.25 | 0.33 | 0.49 | 232.1 | |
| LSD (P ≤ 0.05) | 0.89 | 0.08 | 0.57 | 0.12 | 0.54 | 0.08 | 0.11 | 1.67 | |
| B. Interaction | |||||||||
| TW | Cont. | 2.51 | 0.31 | 1.88 | 0.71 | 2.53 | 0.49 | 0.71 | 245.3 |
| PM | 3.41 | 0.43 | 2.92 | 0.86 | 3.31 | 0.39 | 0.56 | 248.2 | |
| Fe2SO4 | 4.17 | 0.54 | 3.63 | 0.96 | 4.31 | 0.31 | 0.46 | 249.7 | |
| PM + Fe2SO4 | 5.23 | 0.66 | 4.05 | 1.03 | 4.33 | 0.23 | 0.34 | 252.4 | |
| MW | Cont. | 2.39 | 0.29 | 1.72 | 0.51 | 2.14 | 0.54 | 0.78 | 237.3 |
| PM | 3.25 | 0.41 | 2.78 | 0.55 | 2.11 | 0.44 | 0.54 | 240.3 | |
| Fe2SO4 | 3.98 | 0.52 | 3.42 | 0.71 | 3.11 | 0.35 | 0.51 | 241.7 | |
| PM + Fe2SO4 | 4.99 | 0.63 | 3.82 | 0.86 | 3.32 | 0.32 | 0.47 | 244.3 | |
| WW | Cont. | 1.79 | 0.22 | 1.18 | 0.37 | 2.06 | 0.66 | 0.95 | 194.1 |
| PM | 2.42 | 0.31 | 1.73 | 0.51 | 2.35 | 0.61 | 0.88 | 196.5 | |
| Fe2SO4 | 3.04 | 0.36 | 2.56 | 0.61 | 2.02 | 0.55 | 0.79 | 197.6 | |
| PM + Fe2SO4 | 3.72 | 0.47 | 3.43 | 0.77 | 2.11 | 0.45 | 0.65 | 199.7 | |
| LSD (P ≤ 0.05) | 0.11 | 0.07 | 0.17 | 0.06 | 0.07 | 0.04 | 0.07 | 0.09 | |
TW: tap water, MW: magnetized water; WW: well water; PM: peat moss; LSD: least significant difference.
Plants irrigated with WW showed higher contents of Na and Cl as compared to those irrigated with TW or MW (Table 4). Furthermore, addition of peat moss and/or Fe2SO4 significantly reduced contents of Na and Cl in C. officinalis plants regardless of irrigation water type. Irrigation with TW with addition of Fe2SO4 either only or with peat moss resulted in the lowest contents of Na and CL in C. officinalis plants. Similarly, addition of peat moss and Fe2SO4 decreased contents of Na and Cl in plants irrigated with MW as compared to those irrigated with TW without soil amendments or with peat moss only. The beneficial effects of water magnetization could be mainly attributed to the reduction in surface tension of MW that improves the ability of roots to absorb water and nutrients, and thus improves different biosynthesis processes (Elhindi et al., 2017).
5. Conclusions
Climate change and increased population led to increased pressure on freshwater resources in arid and semi-arid areas such as Saudi Arabia. Therefore, finding alternative water resources for irrigation of ornamental crops attracts more research efforts. The results obtained in the current study showed that irrigating C. officinalis plants with high saline WW negatively affected vegetative and flowering growth parameters in addition to reducing contents of essential nutrients leading to lower survival rates of these plants. However, magnetization of WW before irrigation aids in recovering plant growth, productivity, and survival rate to levels comparable to those of plants irrigated with TW (control). Further studies to examine the potential of using MW in irrigation of food crops are recommended.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no. (RG-1436-020).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd Elrahman S.H., Mostafa M.A.M., Taha T.A., Elsharawy M.A.O., Eid M.A. Effect of different amendments on soil chemical characteristics, grain yield and elemental content of wheat plants grown on salt-affected soil irrigated with low quality water. Ann. Agric. Sci. 2012;57:175–182. doi: 10.1016/j.aoas.2012.09.001. [DOI] [Google Scholar]
- Abdul-Qados A., Hozayn M. Response of growth, yield, yield components and some chemical constituents of flax for irrigation with magnetized and tap water. World Appl. Sci. J. 2010;8:630–634. [Google Scholar]
- Black C. Method of soil analysis part 2. Chem. Microbiol. Prop. 1965;9:1387–1388. [Google Scholar]
- Boland A.-M. Management of saline and/or recycled water for irrigated horticulture. Acta Hortic. 2006;792:123–134. [Google Scholar]
- Chaparzadeh N., D'Amico M.L., Khavari-Nejad R.-A., Izzo R., Navari-Izzo F. Antioxidative responses of Calendula officinalis under salinity conditions. Plant Physiol. Biochem. 2004;42:695–701. doi: 10.1016/j.plaphy.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Edwards S.E., da Costa Rocha I., Williamson E.M., Heinrich M. John Wiley & Sons; 2015. Phytopharmacy: An evidence-based guide to herbal medicinal products. [Google Scholar]
- Elhindi K.M. Evaluation of composted green waste fertigation through surface and subsurface drip irrigation systems on pot marigold plants (Calendula officinalis L.) grown on sandy soil. Aust. J. Crop Sci. 2012;6:1249–1259. [Google Scholar]
- Elhindi K.M., Al-Amri S.M., Abdel-Salam E.M., Al-Suhaibani N.A. Effectiveness of salicylic acid in mitigating salt-induced adverse effects on different physio-biochemical attributes in sweet basil (Ocimum basilicum L.) J. Plant Nutr. 2017;40:908–919. doi: 10.1080/01904167.2016.1270311. [DOI] [Google Scholar]
- Eyras M.C., Defossé G.E., Dellatorre F. Seaweed compost as an amendment for horticultural soils in Patagonia, Argentina. Compost. Sci. Util. 2008;16:119–124. doi: 10.1080/1065657X.2008.10702366. [DOI] [Google Scholar]
- Ferrante A., Trivellini A., Malorgio F., Carmassi G., Vernieri P., Serra G. Effect of seawater aerosol on leaves of six plant species potentially useful for ornamental purposes in coastal areas. Sci. Hortic. 2011;128:332–341. doi: 10.1016/j.scienta.2011.01.008. [DOI] [Google Scholar]
- Flowers T.J., Colmer T.D. Salinity tolerance in halophytes. New Phytol. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- Gabr S.S., Farg E.F., Habeebullah T.M., Arafat S.M. Irrigation water consumption and its impact on the groundwater aquifer of Wadi Uranah, Makkah, Saudi Arabia using remote sensing techniques. The Egyptian Journal of Remote Sensing and Space. Science. 2020 doi: 10.1016/j.ejrs.2018.10.001. [DOI] [Google Scholar]
- Goyal S., Chander K., Mundra M.C., Kapoor K.K. Influence of inorganic fertilizers and organic amendments on soil organic matter and soil microbial properties under tropical conditions. Biol. Fertil. Soils. 1999;29:196–200. doi: 10.1007/s003740050544. [DOI] [Google Scholar]
- Grieve, C.M., Poss, J.A., Amrhein, C., 2006. Response of Matthiola incana to Irrigation with Saline Wastewaters. HortScience HortSci 41, 119–123. 10.21273/HORTSCI.41.1.119
- Hachicha M., Kahlaoui B., Khamassi N., Misle E., Jouzdan O. Effect of electromagnetic treatment of saline water on soil and crops. J. Saudi Soc. Agric. Sci. 2018;17:154–162. doi: 10.1016/j.jssas.2016.03.003. [DOI] [Google Scholar]
- Halvin J.L., Beaton J., Tisdale S., Nelson W. Pretice Hall; NJ: 2005. Soil fertility and fertilizers: an introduction to nutrient management. [Google Scholar]
- Hilal M.H., Hilal M.M. Application of magnetic technologies in desert agriculture. I- Seed germination and seedling emergence of some crops in a saline calcareous soil, Egypt. J. Soil Sci. 2000;40:413–422. [Google Scholar]
- Hilal M.H., Hilal M.M. Application of magnetic technologies in desert agriculture. II- Effect of magnetic treatments of irrigation water on salt distribution in olive and citrus fields and induced changes of ionic balance in soil and plant, Egypt. J. Soil Sci. 2000;40:423–435. [Google Scholar]
- Kney A.D., Parsons S.A. A spectrophotometer-based study of magnetic water treatment: Assessment of ionic vs. surface mechanisms. Water Res. 2006;40:517–524. doi: 10.1016/j.watres.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Kozminska A., Al Hassan M., Kumar D., Oprica L., Martinelli F., Grigore M.N., Vicente O., Boscaiu M. Characterizing the effects of salt stress in Calendula officinalis L. J. Appl. Bot. Food Qual. 2017;90:323–329. [Google Scholar]
- Maheshwari B.L., Grewal H.S. Magnetic treatment of irrigation water: Its effects on vegetable crop yield and water productivity. Agric. Water Manage. 2009;96:1229–1236. doi: 10.1016/j.agwat.2009.03.016. [DOI] [Google Scholar]
- Mostafazadeh-Fard B., Khoshravesh M., Mousavi S.-F., Kiani A.-R. Effects of Magnetized Water and Irrigation Water Salinity on Soil Moisture Distribution in Trickle Irrigation. J. Irrig. Drain. Eng. 2011;137:398–402. doi: 10.1061/(ASCE)IR.1943-4774.0000304. [DOI] [Google Scholar]
- Munns R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Noran R., Shani U., Lin I. The Effect of Irrigation With Magnetically Treated Water on The Translocation of Minerals in The Soil. Magn. Electrical Separation. 1996;7 doi: 10.1155/1996/46596. [DOI] [Google Scholar]
- Orcutt D.M., Nilsen E.T. John Wiley & Sons; NJ: 2000. Physiology of plants under stress: Soil and biotic factors. [Google Scholar]
- Page A.L. Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties. American Society of Agronomy; Wisconsin, USA: 1982. [Google Scholar]
- Prado F.E., Boero C., Gallardo M., González J.A. Effect of NaCl on germination, growth, and soluble sugar content in Chenopodium quinoa Willd. seeds. Botanical Bull. Academia Sinica. 2000;41:27–34. [Google Scholar]
- Selim E., Mosa A., El-Ghamry A. Evaluation of humic substances fertigation through surface and subsurface drip irrigation systems on potato grown under Egyptian sandy soil conditions. Agric. Water Manage. 2009;96:1218–1222. [Google Scholar]
- Teixeira da Silva J., Dobránszki J. Impact of magnetic water on plant growth. Environ. Exp. Biol. 2014;12:137–142. [Google Scholar]
- Younis M.E., Hasaneen M.N.A., Nemet-Alla M.M. Plant Growth, Metabolism and Adaptation in Relation to Stress Conditions IV. Effects of Salinity on Certain Factors Associated with the Germination of Three Different Seeds High in Fats. Ann. Bot. 1987;60:337–344. doi: 10.1093/oxfordjournals.aob.a087453. [DOI] [Google Scholar]