Abstract
The present study focused on the evaluation of phytochemical properties, essential mineral elements, and heavy metals contained in raw propolis produced by stingless bees Geniotrigona thoracica, Heterotrigona itama, and Tetrigona binghami found in the same ecological conditions and environment in Brunei Darussalam. The results indicated that propolis of the three stingless bee species mainly consisted of lipids (45.60–47.86%) and very low carbohydrate (0.17–0.48%) and protein contents (0.18–1.18%). The propolis was rich in mineral elements, thus good sources of minerals, while they contained low concentrations of all heavy metals. Propolis of the different bee species could be distinguished based on their mineral compositions. The vibrational and absorption spectra suggested that propolis contains π-conjugated aliphatic and aromatic compounds as well as aromatic acids having amine, ester, carbonyl, alkyl, and hydroxyl functional groups which might be attributed to the presence of phenolic and flavonoid compounds. The antioxidant capacity of the propolis, based on radical scavenging activity of their ethanol extract, was in line with their total phenolic content. The ethanol extract of the propolis also showed antimicrobial activities against four bacterial strains (Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa). The propolis showed slightly higher antibacterial activity against Gram-positive (B. subtilis and S. aureus) bacteria, indicating that the antimicrobial active compounds could be associated with flavonoids, which were quantified to be approximately comparable in all the propolis.
Keywords: Stingless bee propolis, Phytochemicals, Mineral elements, Antioxidant, Antibacterial
1. Introduction
Stingless bees (Hymenoptera; Apidae) with more than 500 species within the tribe Meliponini are found in tropical dry and humid forests and some subtropical regions throughout the world (Michener, 2013). The stingless bees can be classified into 2 genera, namely, the Melipona and the Trigona. They are ecologically active and play an important role in the forest ecosystem, and they have been the focus of sociality, phylogeny, and colony evolutionary study (Rasmussen and Camargo, 2008, Lichtenberg et al., 2010, Rasmussen et al., 2017). The stingless bees are also of interest, owing to their honey, wax, and propolis (Ajibola et al., 2012).
Stingless bee honey is consumed and utilized in health care products since ancient time to the modern era (Meo et al., 2017), due to its important biologically active compounds, such as polyphenols, carotenoids, minerals, proteins, free amino acids, and vitamins (Alvarez-Suarez et al., 2013). Stingless beeswax which is mainly comprised by fats is utilized in cosmetics, including body lotions, facial creams, and lip balms, as well as in pharmaceutical and health products and coating materials for tablets and capsules. Besides, beeswax is also applied in the manufacture of textiles, polishes, and candles. Stingless bee propolis is a resinous adhesive, composed mainly by plant resins, pollen, and bud excretions, which are collected from various trees and utilized by stingless bees to coat, seal, and protect their hives from predators as well as to prevent them from bacteria, viruses, or parasites (Castaldo and Capasso, 2002, Massaro et al., 2014, Bankova et al., 2014). Propolis has been found to have antiseptic effects (Marcucci, 1995, Righi et al., 2011, Kakino et al., 2012), and it has long been traditionally used as medicines and remedies (Carvalho et al., 2015). Several studies have revealed that propolis has antimicrobial (Velikova et al., 2000, Barrientos et al., 2013), antioxidant (Sawaya, 2009, Guimarães et al., 2012, Campos et al., 2014), anti-inflammatory (Barbarić et al., 2011, Cavendish et al., 2015), and antifungal properties (Viuda-Martos et al., 2008). The biological activities of propolis have been attributed to its flavonoid, phenolic, diterpenic acid, and aromatic acid contents (Coneac et al., 2008), and the chemical composition of the stingless bee honey, wax, and propolis varied depending on the bee species, botanical origin, environmental, and storage conditions (Gheldof et al., 2002, Alvarez-Suarez et al., 2013, Biluca et al., 2016, Lim et al., 2019).
Different countries and regions may have their own specific and unique stingless bee species, which adapted to the local ecosystem. In Borneo, including Brunei, Kalimantan, Sabah, and Sarawak, for instance, there are at least 50 species of stingless bees, and their genetic and behavioral characteristics have been well documented (Rasmussen and Cameron, 2007, Silvestre et al., 2008). Among them, the stingless bee species Geniotrigona thoracica, Heterotrigona itama, and Tetrigona binghami (Fig. 1) are commonly domesticated, because their log hives can be found and collected from natural forests, and they can be easily cultivated and maintained in a suburban area. A number of studies focusing on the physicochemical property, biological activity against Gram-positive (Mycobacterium smegmatis, Bacillus subtilis, and Staphylococcus aureus) and Gram-negative (Escherichia coli and Pseudomonas aeruginosa) bacteria, and toxicity of stingless bee propolis from Borneo have been scarcely reported, compared with those from Brazil (Sforcin et al., 2000), Bulgaria (Bankova et al., 2014), India (Kasote et al., 2019), Indonesia (Trusheva et al., 2011), Malaysia (Ibrahim et al., 2016), Mexico (Guzmán-Gutiérrez et al., 2018), Thailand (Sanpa et al., 2015), Turkey (Keskin et al., 2001), and Vietnam (Georgieva et al., 2019). Based on the literature, the different species, botanical origins, and surrounding environment are generally pointed out to be the reasons for the different phytochemical properties and biological activities of propolis. To the best of our knowledge, reports on a comparative study of phytochemical properties, chemical compositions, and biological activities of propolis from different species of stingless bees are limited (Silici and Kutluca, 2005, Przybyłek and Karpiński, 2019). Driven by the importance of obtaining as much information and comparative properties of the stingless bee propolis as possible, in this study, the phytochemical properties and mineral contents in propolis of three stingless bees, G. thoracica, H itama, and T. binghami, found in the same ecological conditions and environment in Brunei Darussalam were investigated. The antioxidant and antibacterial activities of the ethanol extract of the propolis were also evaluated.
Fig. 1.
The images of stingless bees (A) Geniotrigona thoracica, (B) Heterotrigona itama, and (C) Tetrigona binghami along with their respective propolis.
2. Materials and methods
2.1. Chemicals, reagents, and standard solutions
All chemicals and reagents were of analytical grade and were used without further purification. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical, ascorbic acid, boric acid, gallic acid, D-glucose, phenol, hydrochloric acid (HCl), nitric acid (HNO3), sodium carbonate (Na2SO4), aluminum chloride (AlCl3), sulfuric acid (H2SO4), and sodium hydroxide (NaOH) were purchased from Sigma-Aldrich (USA), while ethanol (96%) and multielement standard solution (ICP TraceCERT) were purchased from Merck (Darmstadt-Germany). Ultrapure water was used throughout the sample preparations and measurements. Standard solutions of each gallic acid, ascorbic acid, and quercetin were prepared in ethanol.
2.2. Propolis samples
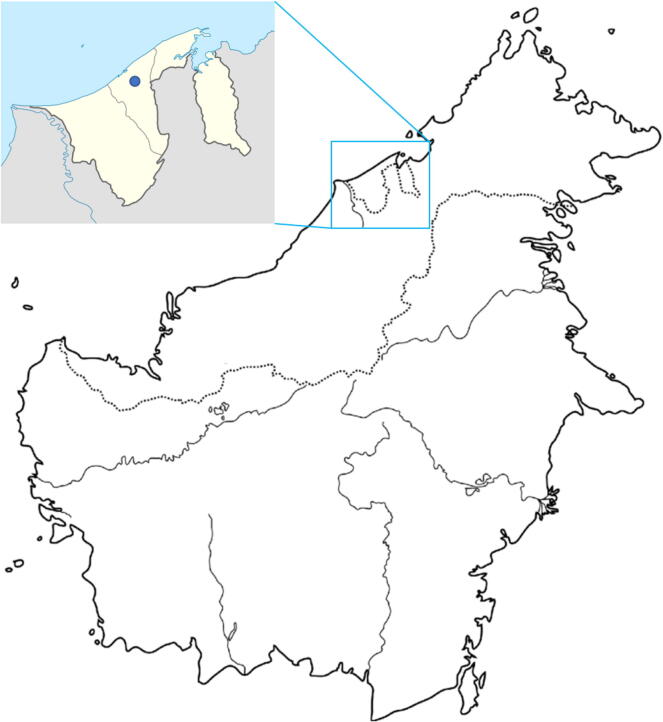
The propolis of three stingless bee species, G. thoracica, H. itama, and T. binghami, were collected from Tasbee Meliponiculture Farm in Tutong District, Brunei Darussalam (Fig. 2). It is noteworthy to mention that this stingless bee farm is located in a suburban area far from high industrial or agricultural activities. The propolis was scrapped from their beehives during the nectar flow season in July-September 2019. The propolis collection did not disrupt any endangered or protected species.
Fig. 2.
Borneo island, indicating Brunei map and the location of Tasbee Meliponiculture Farm (locality; latitude 4°49′02.0″N, longitude 114°45′48.2″E) where the propolis of stingless bees G. thoracica, H. itama, and T. binghami was collected.
Ethanolic extract of the propolis was obtained by macerating the dried raw propolis in 96% ethanol (Abdullah et al., 2019). The suspension was agitated at 150 rpm at 37 °C for 18 h. The suspension was then passed through vacuum filtration to remove particulates, and the supernatant was rotary evaporated until approximately half solvent volume reduction, followed by oven drying at 40 °C.
2.3. Analytical characterizations
The phytochemical properties, including total carbohydrates, lipids, and crude proteins of propolis of stingless bees, G. thoracica, H. itama, and T. binghami, were determined using the phenol–sulfuric acid, the acid hydrolysis and semi-continuous solvent extraction, and Kjeldahl method, respectively, while the mineral contents in the propolis were analyzed using inductively coupled plasma-optical emission spectrometry (ICP-OES) analyzer (iCAP 7200, Thermo Fisher Scientific) with the detection limit being 0.1 μg kg−1.
The phenol-sulfuric acid method was performed according to the procedures reported by Albalasmeh et al. (2013) with some modifications. In this method, D-glucose was used as a standard, where 1 mL of six different concentrations of D-glucose (0, 20, 40, 60, 80, and 100 mg L–1) was mixed with 50 µL of 80% phenol in water, followed by addition 5 mL of 97% H2SO4. The solution mixture was left to cool at room temperature. The same procedure was repeated for homogenous suspensions of raw propolis (approximately 100 mg L–1), which were prepared by soaking the raw propolis in distilled water, followed by sonication. Once cooled, the absorbance of the solution was measured at 490 nm using a UV-Vis spectrophotometer (Shimadzu UV-1900, Japan). The analysis was performed in duplicates. A linear regression plot of absorbance as a function of the concentration of D-glucose was constructed and it was used as a calibration curve to calculate the total carbohydrates contained in the raw propolis, where the percentage of total carbohydrates was calculated as the ratio between the concentration of D-glucose and mass of raw propolis.
The acid hydrolysis and semi-continuous solvent extraction were carried out according to the procedure described by Nielsen (2010). Here, 0.1 g of the dried raw propolis was weighed in a glass capsule fitted with filter paper. The capsule was then placed in the boiling stand before subjected to acid hydrolysis. The hydrolysis beaker (SoxCapTM, Sweden) was filled up with 800 mL of 3 M HCl. The boiling stand along with the capsule was lowered into the beaker, and the solution was allowed to boil gently for 1 h. The capsule was then transferred to a drying stand and left to dry overnight in a convection oven at 60 °C. The dried sample was then transferred into a paper thimble and it was subjected to extraction using Soxhlet (SoxtecTM, Sweden) with petroleum ether as extraction solvent. The extracted sample was allowed to dry in an oven at 100 °C for 30 min, and it was put in a desiccator before weighing. The percentage of lipids was then calculated based on the ratio between the extracted lipids and mass of raw propolis.
The protein analysis is divided into three stages; e.g. digestion, distillation, and titration, according to the procedures reported by Bradstreet, 1965, Owusu-Apenten, 2002 with some modifications. Here, 0.1 g of the raw propolis was first subjected to digestion with H2SO4 and Kjeldahl tablet (Gerhard Kjeldatherm®, Germany) at 200 °C for 1 h. The solutions were then allowed to cool and filtered, followed by neutralization using NaOH and then distillation using 1% boric acid solution. The protein content was calculated based on the spent borate ions, which was determined by titration.
The mineral elements, including aluminium (Al), calcium (Ca), cadmium (Cd), cobalt (Co), chromium (Cr), copper (Cu), iron (Fe), potassium (K), magnesium (Mg), manganese (Mn), sodium (Na), nickel (Ni), lead (Pb), zinc (Zn), and arsenic (As), were determined according to the procedure described by González-Martín et al. (2015) with some modifications. Here, 1 g of the dried raw propolis was ground and then digested using concentrated HNO3 followed by 2.5 mL of 37% HCl. The sample was cooled to room temperature and was diluted with ultrapure water. The calibration was performed with ICP-multielement standard solutions in the range of 10–200 μg kg−1.
2.4. Scanning electron microscopy, UV-vis absorption and Fourier-transform infrared spectroscopy (FTIR) spectroscopy
The morphological characteristics of the propolis were investigated using a scanning electron microscope (SEM; JEOL JSM-6490LA, Japan) operating at 50 kV. The SEM images with a magnification of 700 × were recorded. The absorption spectra of ethanolic extract of propolis of the different stingless bees were measured using UV-Vis spectrophotometer (Shimadzu UV-1900, Japan). The spectra were recorded in the range of 200–500 nm. The vibrational spectra of the propolis were analyzed using FTIR spectrophotometer (Shimadzu Prestige-21, Japan). Approximately 2 mg of the raw propolis was added into 200 mg pre-heated KBr powder. The mixture was pressurized using the SPECAC hydraulic pellet press, and the pellet was then subjected to the measurement. The vibrational spectrum was recorded in the range of 400–4000 cm−1 with a resolution of 2 cm−1.
2.5. Antioxidant assays
The antioxidant capacity of ethanolic extract of propolis of the different stingless bees was determined using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay according to reported procedures (Moreira et al., 2008). In this measurement, solutions with various concentrations of the ethanolic extract were prepared, and 0.5 mL of each solution was then mixed with 3.5 mL of DPPH solution (50 mg L–1) in ethanol. The mixture was vigorously vortexed and then kept at room temperature for 30 min in the dark. The decrease of DPPH radical was monitored by measuring the absorbance of the mixture at 517 nm using an ELISA microplate reader (Biobase EL-10A, China) with ethanol acting as a blank. The IC50 was defined as the concentration of the ethanolic extract of propolis to scavenge 50% initial DPPH radical, as reflected by a 50% reduction of absorbance. Based on the IC50 values of the propolis and ascorbic acid as the standard, the total antioxidant capacity (TAC) of the propolis was calculated. The TAC value was then expressed as milligrams (mg) ascorbic acid equivalent (AAE) per gram (g) of propolis.
2.6. Total phenolic and flavonoid contents
The presences of phenols and flavonoids in ethanolic extract of propolis were analyzed using colorimetric methods. The total phenolic contents (TPC) were determined using the Folin-Ciocalteau analysis as adapted by Singleton et al., 1999, Kumazawa et al., 2002 with some modifications. Three different concentrations of propolis in ethanol (10, 50, and 100 mg L–1) were prepared. A 100 µL of the solutions were mixed with equivolume of 7.5% Na2CO3 in a 96 well plate. A 50 µL of Folin-Ciocalteau reagent was added into the mixture, which was then incubated in the dark for 30 min at room temperature. The absorbance of the mixture was measured at 760 nm. A similar procedure was carried out for ten different concentrations of gallic acid (0–100 mg L–1). A linear regression plot of absorbance as a function of the concentration of gallic acid was used as a calibration curve to calculate the TPC contained in the raw propolis. The results were expressed as mg gallic acid equivalent (GAE) per gram (g) of propolis.
The total flavonoid content (TFC) of propolis was investigated using the spectrophotometric method based on flavonoid-AlCl3 complexation as described by Franchin et al. (2012) with modifications. A 100 μL each solution of propolis in ethanol (10, 50, and 100 mg L–1) was mixed with an equivolume of 2% AlCl3 solution in a 96 well plate and was left to incubate for 30 min at room temperature. The absorbance of the mixture was then measured at 420 nm. The absorbance of quercetin solutions (0–100 mg L–1) was recorded, plotted as a function of the concentration, and used as a calibration curve. The TFC of the raw propolis was expressed as mg quercetin equivalent (QE) per gram (g) of propolis.
2.7. Antibacterial analysis
The antibacterial activity of ethanolic extract of propolis of the three different stingless bee species, G. thoracica, H itama, and T. binghami, were evaluated using the disc diffusion method (Bauer et al., 1966). The ethanolic extract of propolis was suspended in water, thereby the concentration of the suspension was 2 g L–1. Sterile filter paper discs (Whatman No. 1; 6 mm in diameter) were fully soaked into the suspension, and they were then allowed to dry at ambient condition. The dried discs containing the propolis particles were then placed in Petri dishes containing Mueller-Hinton agar (Bio-Rad) which were inoculated with 100 µL of the diluted bacterial culture. The bacteria were two Gram-positive bacterial strains (Staphylococcus aureus ATCC-29213 and Bacillus subtilis ATCC-11774) and two Gram-negative bacterial strains (Escherichia coli ATCC-11775 and Pseudomonas aeruginosa ATCC-27853), which were cultured separately for 24 h at 37 °C in Nutrient Broth (Merck) and then diluted 10 fold, equivalent to 0.5 McFarland standard. Similarly, discs containing streptomycin or rifampicin at 2 g L–1 were used as positive controls (reference standards) and discs containing water were used as a negative control. The Petri dishes were then placed in an incubator at 37 °C for 24 h before the diameter of inhibition zone as defined by the bacterial growth inhibition was measured. The results were expressed as average diameter ± standard deviation of 3 to 4 replicates for the propolis samples and of 8 to 9 replicates for the controls.
2.8. Data analysis
All measurements have been performed at least in triplicates, and all of the data were carefully analyzed. Certain data were checked for normal distribution using Shapiro-Wilk test and statistical analysis was carried out using an unpaired t-test to compare the significant difference between two means at a significance level of p < 0.05. The data were presented as the mean values along with the standard deviation.
3. Results and discussion
3.1. Surface morphology of propolis
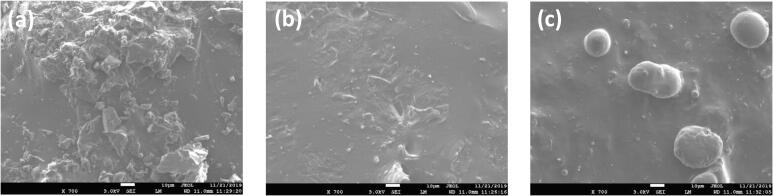
Surface morphology of the raw propolis of stingless bee species G. thoracica, H. itama, and T. binghami was assessed using SEM imaging. As shown in Fig. 3(A)–(C), SEM images indicated that the propolis has different surface morphologies. The surface of H. itama propolis tends to be smooth, and that of G. thoracica propolis contains some spherical grain size textures embedded on the surface. In contrast, the surface of T. binghami propolis is rough, suggesting that the propolis contained the agglomeration of irregularly shaped particles. This finding may imply different nature of raw materials, compositions, surface properties, and structures of propolis of the different stingless bee species.
Fig. 3.
SEM images of the raw propolis of stingless bee (a) G. thoracica, (b) H. itama, and (c) T. binghami. The scale bar represents 5 μm size.
3.2. Phytochemical properties
The phytochemical properties, particularly different nutritional parameters, including lipid, protein, and carbohydrate content of the raw propolis of stingless bee species (G. thoracica, H itama, and T. binghami) are summarized in Table 1. The total lipids, proteins, and carbohydrates of the propolis varied in the range of 45.60–47.86%, 0.18–1.18%, and 0.17–0.48%, respectively. This highlights that lipids were the major component of the propolis irrespective of their different stingless bee species, and they can be considered to be originated from plant waxes and resin (Bonvehí et al., 1994, Kalogeropoulos et al., 2009, Nedji and Loucif-Ayad, 2014). Within the uncertainty, the lipid content of the propolis of three stingless bee species in this study is comparable with each other. It is, however, noteworthy that the lipid content of the stingless bee propolis was 3–5 fold higher compared with those of the Apis melifera honeybees (8.19–15.61%) (Devequi-Nunes et al., 2018). The higher lipid content makes the propolis of stingless bees to be more water-resistant compared with honeybee hives. The significant difference in the lipid content may be due to the different floral sources used by the stingless bees and honeybees to build their propolis. In particular, the propolis of stingless bees is made of a mixture of beeswax and resins collected from a variety of plant parts with the addition of the mandibular secretion (dos Santos et al., 2009, Simone-Finstrom and Spivak, 2010). This water-resistant cerumen is used as a storage pot for honey which has a water content of 14.74 g/100 g (Shamsudin et al., 2019) and as a depository for mummified intruders to keep the sterile environment in the hive. On the other hand, honeybee hive is made of a mixture of beeswax and resins without the mandibular secretion, and it is used as an internal layer and a sealer surrounding the hexagon-shaped nest combs.
Table 1.
Phytochemical parameters of G. thoracica, H. itama, and T. binghami propolis.
| Parameters |
Stingless bee propolis |
||
|---|---|---|---|
| G. thoracica | H. itama | T. binghami | |
| Total Lipids (%) | 47.86 ± 1.61 | 45.60 ± 1.45 | 46.82 ± 1.85 |
| Crude Proteins (%) | 1.18 ± 0.04 | 0.18 ± 0.02 | 0.22 ± 0.01 |
| Total Carbohydrates (%) | 0.17 ± 0.01 | 0.43 ± 0.03 | 0.24 ± 0.01 |
The crude protein of G. thoracica propolis was found to be approximately 5 times higher than those of H. itama and T. binghami propolis. On the other hand, the total carbohydrate of H. itama was twice higher than those of G. thoracica and T. binghami propolis. These slight variations of nutritional compositions might depend on the flowering season and the availability of plants surrounding the stingless bee hives. It is important to highlight that, all the raw propolis of stingless bees, regardless of the species, shows the same trend, i.e. low contents of total crude fiber, carbohydrate, and crude protein being <1% of the dry weight of propolis (Abdullah et al., 2019). It can be postulated that the low fiber, carbohydrate, and protein contents are the key important factor to prevent the propolis from fermentation process (Alibardi and Cossu, 2016), which shortens the shelf life of the propolis and influences the phytochemical properties of its honey (Temaru et al., 2007, Massaro et al., 2011).
3.3. Mineral contents
Mineral elements detected in the raw propolis of stingless bee in this study were Al, Ca, Cu, Fe, K, Mg, Mn, Na, and Zn. As summarized in Table 2, for all propolis of the three different stingless bee species, the greatest composition of mineral elements per gram propolis was found to be K. This finding is generally in agreement with those reported by González-Martín et al. (2015) on propolis collected from different geographical origins in Chile and Spain, and the high content of this macro element has also been found in stingless bee propolis obtained from China and USA (Gong et al., 2012).
Table 2.
Mineral element and heavy metal mean values (μg g−1) of G. thoracica, H. itama, and T. binghami propolis.
| Elements | Stingless bee propolis |
||
|---|---|---|---|
| G. thoracica | H. itama | T. binghami | |
| Al | 7.92 | 33.03 | 48.59 |
| Ca | 150.06 | 18.11 | 550.05 |
| Cd | --- | 0.599 | 0.664 |
| Co | 0.149 | 0.438 | 0.774 |
| Cr | 0.884 | 2.74 | 2.08 |
| Cu | 24.25 | 14.58 | 10.85 |
| Fe | 28.37 | 19.84 | 69.64 |
| K | 402.14 | 974.24 | 728.50 |
| Mg | 92.16 | 357.99 | 378.23 |
| Mn | 6.98 | 34.49 | 106.03 |
| Na | 7.64 | 273.26 | 48.06 |
| Ni | 0.102 | 2.20 | 2.10 |
| Pb | 0.195 | 0.75 | 1.00 |
| Zn | 2.97 | 0.24 | – |
| As | 0.716 | 4.45 | 4.45 |
In general, the main mineral elements in propolis are K, Mg, Ca, Mn, Na, and Al, suggesting that the propolis are very rich with respect to these mineral elements. However, the sequence going from the greatest to the smallest amount of these mineral elements depends on the stingless bee species as well as the geographical origin (Popov et al., 2017), demonstrating that the minerals in propolis are originated from pollens collected by stingless bees from various flowering plants in the surrounding area of their hives (Campos et al., 2008, Dagaroglu, 2004, De-Melo et al., 2015). This notion is also supported by the fact that K, Mg, and Ca were the most metal elements found in bee pollen collected from Turkey (Altunatmaz et al., 2017), Brazil (Campos et al., 2008, Morgano et al., 2012), Serbia (Kostic et al., 2015), and Chile (González-Martín et al., 2015), although bee pollens were also very rich with respect to P, Fe, and Zn. In contrast, the mineral elements present in stingless bee honey are K, Zn, P, Ca, Na, Mg, S, Cu, Fe, and Mn (Rao et al., 2016). This highlights the different minerals contained in the stingless bee propolis, pollen, and honey (Dagaroglu, 2004, De-Melo et al., 2015).
It is noteworthy that Ca content in T. binghami propolis (550 μg g−1) is much higher than those in G. thoracica (150.1 μg g−1) and H. itama (18.1 μg g−1). Since the three stingless bee species are found at the same location, implying equivalent geological formations, the high Ca content suggested that T. binghami propolis could belong to geopropolis and the stingless bee acquired the minerals from Ca-rich soil. An extremely high Ca content has also been reported in propolis of stingless bees classified as geopropolis found in South Spain (Bonvehí and Orantes-Bermejo, 2013), and the ethnobiological and ecological perspectives of the stingless bees have been reported (Reyes-González et al., 2014). Thus, further detailed studies to interrelate propolis, biology, classification, and behavior of the stingless bee species in this study are pursued in the future.
3.4. Heavy metal contents
As summarized in Table 2, the ICP analysis also showed that the stingless bees G. thoracica, H. itama, and T. binghami propolis also contains traceable heavy metals, including cadmium (Cd; 0.599–0.664 μg g−1), cobalt (Co; 0.149–0.774 μg g−1), chrome (Cr; 0.884–2.74 μg g−1), nickel (Ni; 0.1–2.20 μg g−1), lead (Pb; 0.195–1.00 μg g−1), and arsenic (As; 0.716–4.45 μg g−1). All the heavy metal elements were detected at low concentrations, two to three orders lower than the mineral contents. Irrespective of the stingless bee species in this study, Cd content in their propolis was much higher as compared to those observed in propolis found in Macedonia (0.012–0.038 μg g−1) (Popov et al., 2017) and in Poland (0.012–0.055 μg g−1) (Formicki et al., 2013). In addition, Pb content in the propolis was also much higher compared to those in propolis found in Macedonia (0.033–0.041 μg g−1) (Popov et al., 2017), but they tended to be lower than those in propolis found in Poland (0.89–2.94 μg g−1) (Formicki et al., 2013). Considering that stingless bees may collect waxes, resin, nectar, and minerals from the plants and soil over a distance of sub-kilometer from their hives (Przybyłowski and Wilczyńska, 2001), the low contents of heavy metals in the propolis reflect low contaminations of heavy metals in plants, soil, and atmosphere in the surrounding area where the propolis was collected. This finding also supported the notion that the heavy metal contents can be used to monitor environmental contamination (Gilbert and Lisk, 1978, Uren et al., 1998), where high contents of heavy metals in bee products have been attributed to the beehives being located in densely populated areas with high industrial or agricultural activities. Nevertheless, the major pathways of the presence of heavy metals in propolis might be figured out by surveying the plants and soil surrounding the stingless beehives from which the bees collect resins, buds, pollen, nectar, and minerals.
3.5. Vibrational and absorption spectroscopies of propolis
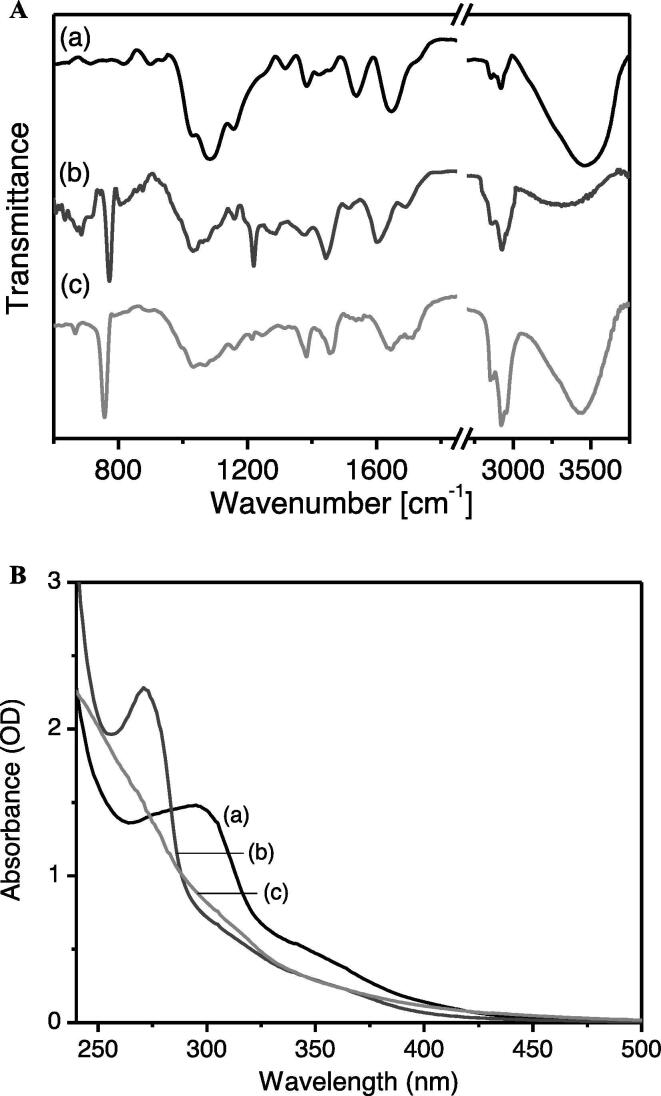
FTIR spectroscopy has been applied to identify functional groups corresponding to the chemical bonds of constituents present in the propolis which inevitably correlate to the chemical components potent in the propolis of stingless bees G. thoracica, H. itama, and T. binghami. As shown in Fig. 4(A), propolis of the three stingless bee species show different vibrational spectra to some extent, which can be attributed to their distinct compositions of compounds. Table 3 summarizes the vibrational bands and their tentative vibrational assignments. Despite the different FTIR spectral pattern, in general, the spectral bands of all the propolis can be assigned to the stretching, bending, wagging, and out-of-plane vibrations of O—H, N—H, C—H, C—O, C—N, C—C, C C, C O, C—H of alcohols, phenols, carboxylic acids, alkenes, and aromatic rings, suggesting that all the raw propolis contains alkyl and aromatic compounds having amine, ester, carbonyl, alkyl, and hydroxyl functional groups. This may further indicate the presences of aromatic acids, terpenes, flavonoids and phenolic acids in the raw propolis. In particular, the total phenolics and flavonoids in propolis were quantified further using chemical assay, as described in the following section.
Fig. 4.
(A) The FTIR of raw propolis and (B) UV-Vis absorption spectra of ethanolic extract of propolis of (a) G. thoracica, (b) H. itama, and (c) T. binghami.
Table 3.
Vibrational peaks and assignments of FTIR spectra of G. thoracica, H. itama, and T. binghami propolis.
| Stingless bee propolis |
Vibrational modes | ||
|---|---|---|---|
| H. itama | G. thoracica | T. binghami | |
| 3312(b) | 3466(b) | 3425(b) | νO-H of alcohols, phenols and carboxylic acids |
| 2926(s) | 2918(s) | 2920 | νN-H of amines |
| 2853(s) | 2851(s) | 2851 | νCH3, νCH2 and νCH of alkenes |
| 1693(b) | 1721(l) | 1710 | νC = O of carbonyl |
| 1603(b) | 1646(s) | 1644 | νC = C of conjugated aromatic rings |
| 1515(w) | 1538(s) | 1545(b) | δN-H of amines; νC-C aromatic ring |
| 1443(s) | 1430(b) | 1459(s) | δCH of aromatic rings |
| 1379(s) | 1383(s) | 1382 | νC-O of carboxylic acids |
| --- | 1315(s) | 1315(w) | νC-O alcohols; ωCH2 of alkenes |
| 1283(w) | 1243(w) | 1244 | νC-O phenols |
| 1221(s) | --- | 1213 | νC-O of phenols |
| 1160(w) | 1157(s) | 1160 | νC-N of amines |
| 1073(w) | 1082(s) | 1069 | νC-C aromatic rings |
| 1033(b) | 1029(s) | 1032 | νC-O of esters |
| 813(w) | 817 | --- | γCH2 of alkenes |
| 771(s) | --- | 757 | γC-H of alkenes |
| 717 | 714 | --- | γC-H of alkenes |
| 687 | --- | 667 | γO-H of alcohols and phenols |
The absorption spectra of ethanolic extract of stingless bees G. thoracica, H. itama, and T. binghami propolis are shown in Fig. 4(B). The absorption spectra exhibited elastic Rayleigh scattering, suggesting that the ethanolic extract of propolis forms colloidal suspensions. The G. thoracica propolis clearly showed an absorption maximum at 293 nm and a shoulder at 350 nm, H. itama propolis exhibited a peak at 272 nm and a shoulder at 310 nm respectively, and T. binghami propolis showed a low-intensity peak at 270 and a slight shoulder at 308 nm. The absorption peaks can be assigned to the presences of π-conjugated aliphatic and aromatic compounds as well as aromatic acids in the propolis. However, the chemical structures and compositions in the propolis of stingless bees G. thoracica, H itama, and T. binghami in this study will be precisely quantified in detail using chromatographic techniques in the future. Nevertheless, it might be interesting to recall that, as it has been documented in the literature, in general, the chemical compounds in various stingless bee propolis are mainly the phenolic and flavonoid compounds, including tocopherol, quercetin, vanilic, caffeic acid, ferulic acid, coumaric acid, benzoic acid, cinnamic acid, pinobanksin 5-methyl ether, apigenin, kaempferol, pinobanksin, cinnamylideneacetic acid, chrysin, pinocembrin, galangin, pinobanksin 3-acetate, phenethyl caffeate, cinnamyl caffeate, and tectochrysin (Marcucci and Bankova, 1999, Medić-Šarić et al., 2004, Coneac et al., 2008, Bonamigo et al., 2017).
3.6. Antioxidant capacity
The antioxidant capacity of the raw propolis was represented by DPPH radical scavenging activity (RSA) of their ethanol extract. The IC50 value along with total phenolic content (TPC) and total flavonoid content (TFC) are summarized in Table 4. The IC50 values were 570.2, 76.5, and 1975.0 mg L–1 for G. thoracica, H. itama, and T. binghami propolis particles, respectively, revealing their relative amounts of antioxidants. By comparing the IC50 value of ethanolic extract of the propolis with that of ascorbic acid (24.3 ± 0.1 mg L–1) as the standard, the total antioxidant capacity (TAC) of the raw propolis of G. thoracica, H. itama, and T. binghami was estimated to be 42.5, 317.6, and 12.3 mg AAE g−1, respectively. Among the propolis in this study, H. itama shows the highest TAC, equivalent to 7- and 25-fold higher than that of G. thoracica and T. binghami, respectively. A similar trend of TAC of propolis of the three different stingless bee species G. thoracica, H. itama, and T. binghami found in Malaysia has also been reported by Awang et al. (2018).
Table 4.
The IC50, the total antioxidant capacity (TAC), total phenolic content (TPC), and total flavonoid content (TFC) of ethanolic extract of G. thoracica, H. itama, and T. binghami propolis.
| Parameters | Stingless bee propolis |
||
|---|---|---|---|
| G. thoracica | H. itama | T. binghami | |
| IC50 (mg L-1) | 570.2 ± 6.1 | 76.5 ± 1.3 | 1975 ± 22.5 |
| TAC (mg AAE g−1) | 42.5 ± 0.5 | 317.6 ± 5.4 | 12.3 ± 0.3 |
| TPC (mg GAE/g propolis) | 2192.7 ± 12.3 | 2391.0 ± 16.1 | 2151.9 ± 12.1 |
| TFC (mg QE/g propolis) | 299.4 ± 2.6 | 275.2 ± 3.5 | 275.9 ± 2.1 |
| Note: IC50 of ascorbic acid 24.3 mg L-1 | |||
The variations in the TAC values might be attributed to the different types and contents of phenolic and flavonoid compounds in the raw propolis, as each phenolic and flavonoid compound has different RSAs (Aljadi and Kamaruddin, 2004, Kucuk et al., 2007). This revealed that the considerable amount of phenolic compounds contained in propolis of stingless bees can be attributed to its high antioxidant capacity, as it has been pointed out by Araujo et al. (2016). In other words, the RSA of the propolis is strongly determined by the TPC, rather than the TFC. It further suggested that among the phenolics, flavonoids, terpenes, and aromatic acids contained in propolis (Coneac et al., 2008, Bonamigo et al., 2017), regardless the synergetic effects, the phenolic compounds play an important role in the antioxidant activity (Sousa et al., 2008, Lima et al., 2009).
3.7. Antimicrobial activity
In order to evaluate the potential of propolis of the stingless bees G. thoracica, H. itama, and T. binghami as an antimicrobial agent, they were screened against two Gram-positive (B. subtilis and S. aureus) and two Gram-negative bacteria (E. coli and P. aeruginosa). The inhibitory activities of ethanol extract of the propolis were assessed by disc diffusion assay which is considered as a qualitative screening method. However, comparison of the inhibition zones would likely suggest that larger inhibition zone has a better antibacterial activity compared to smaller inhibition zone. Ultrapure water which has no inhibition was used as a negative control, and two different antibiotics (rifampicin and streptomycin) were used as positive controls. The inhibition zone for the different propolis and the two standard antibiotics was determined (Table 5), under the same experimental conditions.
Table 5.
Antibacterial activities of ethanolic extract of G. thoracica, H. itama, and T. binghami propolis along with the two standard antibiotics, rifampicin and streptomycin, for comparison. Negative control (water) did not show any inhibition zone as expected. R and S denote significant difference (p < 0.05) between the inhibition zones of the propolis and that of rifampicin (RIF) and streptomycin (STR), respectively.
| Bacterial strain | Inhibition zone (mm) |
||||
|---|---|---|---|---|---|
| G. thoracica propolis | H. itama propolis | T. binghami propolis | Antibiotics |
||
| RIF | STR | ||||
| B. subtilis | 10.8 ± 1.0 | 13.0 ± 4.6 | 11.0 ± 2.7 | 14.0 ± 4.4 | 14.0 ± 4.1 |
| S. aureus | 7.8 ± 0.5S | 9.7 ± 4.6S | 7.0 ± 0.8RS | 12.9 ± 4.6 | 16.8 ± 4.2 |
| E. coli | 11.3 ± 1.0 | 10.8 ± 1.0 | 10.0 ± 4.4 | 13.8 ± 4.6 | 12.4 ± 4.0 |
| P. aeruginosa | 9.0 ± 1.2RS | 9.8 ± 1.3S | 8.8 ± 1.3S | 14.0 ± 5.1 | 14.8 ± 2.6 |
The present result indicated that all of the ethanol extracts of propolis showed distinct antibacterial strengths against the four bacterial strains, suggesting that the propolis have strong antibacterial activities to some extent against B. subtilis, S. aureus, and P. aeruginosa, as reported by Kujumgiev et al., 1999, Moreno et al., 1999, and Sforcin et al. (2000). This consideration was supported by the inhibition zone of the ethanol extract of propolis which was not significantly different compared to the standard antibiotic, rifampicin. However, when compared to another antibiotic, streptomycin, the propolis, in general, has significantly lower inhibitory activities against S. aureus and P. aeruginosa but the activities were mostly found to be comparable against B. subtilis and E. coli. This bacterial strain-dependent antibacterial activity reflects the variations of bioactive compounds contained in propolis of the different stingless bee species. Although the propolis in a few of the tests against Gram-positive and Gram-negative bacteria showed less activities than any of the two standard antibiotics, nevertheless, as natural products, they are potentially applicable for various biomedical applications (Tosi et al., 1996, Sforcin and Bankova, 2011).
The understanding of the antimicrobial activity of the propolis particles is important. In the literature, the antimicrobial activity of propolis is related to the phenolic and flavonoid compounds with various polarities and their synergetic effect. Although the exact mechanism of the antibacterial activities is still unknown (Santos et al., 2002), it may be attributed to polar and lipophilic phenolic and flavonoid compounds. In particular, those compounds having electronegative carbonyl, amine, imine, sulfide, thiol, methoxyl, and hydroxyl groups are highly polar and lipophilic and could be responsible for the contact with bacterial cells and induce structural damage to the cell wall and membrane, leading to the leakage of cellular contents and cell death (Cushnie et al., 2003, Cushnie and Lamb, 2005, Kim and Chung, 2011, Sanpa et al., 2015, Echeverría et al., 2017), followed by aggregation (Pohjala and Tammela, 2012).
The aforementioned slight higher sensitivity of the Gram-positive bacteria compared with Gram-negative bacteria indicates the important role of flavonoids, rather than phenolic compounds, in the antibacterial activities of the propolis, since flavonoids such as galangin and quercetin derivatives have minimum inhibitory concentrations as low as sub-ppm to ppm levels against the Gram-positive bacteria. This notion is supported by the similar trends of inhibition zones of the bacterial strains (within their experimental errors) (Table 5) and the TFC values (Table 4) which reflect the total amount of flavonoids (regardless their chemical structures) in the propolis. It should also be noted that the important role of flavonoids contained in stingless bee propolis has also been observed for other propolis against two Gram-positive bacteria, B. cereus and S. aureus (Nedji and Loucif-Ayad, 2014).
4. Conclusions
In the present study, phytochemical properties, the major mineral elements, and heavy metal contents of raw propolis of stingless bees Geniotrigona thoracica, Heterotrigona itama, and Tetrigona binghami found in the same environment and ecological conditions in Brunei Darussalam were quantitatively analyzed. The results indicated that total lipids, total proteins, total carbohydrates of the propolis varied in the range of 45.60–47.86%, 0.18–1.18%, and 0.18–1.18%, highlighting that the major component of the propolis was plant waxes and resin, irrespective of different stingless bee species. Based on the mineral contents, the main of mineral elements in the propolis of three different stingless bee species are K, Mg, Ca, Mn, Na, and Al with K being the greatest. Variation of mineral compositions, e.g. the sequence going from the greatest to smallest amount in the propolis, depends on the stingless bee species, highlighting that the mineral compositions can be used as an indicator to discriminate among the stingless bee propolis. Low concentrations of heavy metals, including Cd, Co, Cr, Ni, Pb, and As, were traced within two to three orders lower than the mineral contents, and the toxic heavy metals are much below the maximum permissible limit. The vibrational and absorption spectral analyses demonstrated the presences of aromatic compounds such as aromatic acids, terpenes, flavonoids and phenolic acids having amine, ester, carbonyl, alkyl, and hydroxyl functional groups. Those compounds contained in the propolis are considered to be bioactive. Irrespective of the stingless bee species, for instance, the antioxidant capacity of the propolis can be attributed to phenolic compounds as represented by TPC. All the propolis also showed distinct antibacterial activities against two Gram-positive (B. subtilis and S. aureus) and two Gram-negative (E. coli and P. aeruginosa) bacteria with the inhibition zone depending on the bacterial strain. This further reflected the variations of antimicrobial active compounds contained in propolis of the different bee species. The antimicrobial active compounds could be associated with flavonoid compounds as represented by TFC. Overall, with the qualitative phytochemical properties, rich in mineral elements and low heavy metal contents, and with the strong antioxidant capacity and antimicrobial activities against Gram-positive and Gram-negative bacteria, as natural products, the propolis of three different stingless bee species found in Brunei Darussalam showed strong potential for various biomedical applications.
Author Contributions
NAA, NZ, and SNZZ performed experiments; AU, HT and FH conceived this research, designed experiments and interpreted the data; AU wrote the paper. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdullah N.A., Ja'afar F., Yasin H.M., Taha H., Petalcorin M.I.R., Mamit M.H., Kusrini E., Usman A. Physicochemical analyses, antioxidant, antibacterial, and toxicity of propolis particles produced by stingless bee Heterotrigona itama found in Brunei Darussalam. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajibola A., Chamunorwa J.P., Erlwanger K.H. Nutraceutical values of natural honey and its contribution to human health and wealth. Nutr. Metab. (Lond.) 2012;9:61. doi: 10.1186/1743-7075-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albalasmeh A.A., Berhe A.A., Ghezzehei T.A. A new method for rapid determination of carbohydrate and total carbon concentrations using UV spectrophotometry. Carbohydr. Polym. 2013;97:253–261. doi: 10.1016/j.carbpol.2013.04.072. [DOI] [PubMed] [Google Scholar]
- Alibardi L., Cossu C. Effects of carbohydrate, protein and lipid content of organic waste on hydrogen production and fermentation products. Waste Manag. 2016;47:69–77. doi: 10.1016/j.wasman.2015.07.049. [DOI] [PubMed] [Google Scholar]
- Aljadi A.M., Kamaruddin M.Y. Evaluation of the phenolic contents and antioxidant capacities of two Malaysian floral honeys. Food Chem. 2004;85:513–518. [Google Scholar]
- Altunatmaz S., Tarhan D., Aksu F., Barutçu U.B., Or M.E. Mineral element and heavy metal (cadmium, lead and arsenic) levels of bee pollen in Turkey. Food Sci. Technol, Campinas. 2017;37:136–141. [Google Scholar]
- Alvarez-Suarez J.M., Giampieri F., Battino M. Honey as a source of dietary antioxidants: structures, bioavailability and evidence of protective effects against human chronic diseases. Curr. Med. Chem. 2013;20:621–638. doi: 10.2174/092986713804999358. [DOI] [PubMed] [Google Scholar]
- Araujo, K.S.d.S., dos Santos Junior, J.F., Sato, M.O., Finco, F.D.B.A., Soares, I.M., Barbosa, R.d.S., Ascêncio, S.D., Mariano, S.M.B., 2016. Physicochemical properties and antioxidant capacity of propolis of stingless bees (Meliponinae) and Apisfrom two regions of Tocantins. Brazil. Acta Amazon. 46, 61–68.
- Awang N., Ali N., Abd Majid F.A., Hamzah S., Abd Razak S.B. Total flavonoids and phenolic contents of sticky and hard propolis from 10 species of Indo-Malayan stingless bees. Malaysian J. Anal. Sci. 2018;22:877–884. [Google Scholar]
- Bankova V., Popova M., Trusheva B. Propolis volatile compounds: chemical diversity and biological activity: A review. Chem. Cent. J. 2014;8:1–8. doi: 10.1186/1752-153X-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarić M., Mišković K., Bojić M., Lončar M.B., Smolčić-Bubalo A., Debeljak Z., Medić-Šarić M. Chemical composition of the ethanolic propolis extracts and its effect on HeLa cells. J. Ethnopharmacol. 2011;135:772–778. doi: 10.1016/j.jep.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Barrientos L., Herrera C.L., Montenegro G., Ortega X., Veloz J., Alvear M., Cuevas A., Saavedra N., Salazar L.A. Chemical and botanical characterization of Chilean propolis and biological activity on cariogenic bacteria Streptococcus mutans and Streptococcus sobrinus. Braz. J. Microbiol. 2013;44:577–585. doi: 10.1590/S1517-83822013000200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A.W., Kirby W.M.M., Serris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- Biluca F.C., Braghini F., Gonzaga L.V., Costa A.C.O., Fett R. Physicochemical profiles, minerals and bioactive compounds of stingless bee honey (Meliponinae) J. Food Compos. Anal. 2016;50:61–69. [Google Scholar]
- Bonamigo, T., Campos, J.F., Alfredo, T.M., Balestieri, J.B.P., Cardoso, C.A.L., Paredes-Gamero, E.J., de Picoli Souza, K., dos Santos, E.L., 2017. Antioxidant, cytotoxic, and toxic activities of propolis from two native bees in Brazil: Scaptotrigona depilis and Melipona quadrifasciata anthidioides. Oxid. Med. Cell. Longev. 2017, Article ID 1038153. [DOI] [PMC free article] [PubMed]
- Bonvehí J.S., Coil F.V., Jordà R.E. The composition, active components and bacteriostatic activity of propolis in dietetics. J. Am. Oil Chem. Soc. 1994;71:529–532. [Google Scholar]
- Bonvehí J.S., Orantes-Bermejo F.J.O. The element content of propolis collected from different areas of South Spain. Environ. Monit. Assess. 2013;185:6035–6047. doi: 10.1007/s10661-012-3004-3. [DOI] [PubMed] [Google Scholar]
- Bradstreet R.B. Academic Press; New York and London: 1965. The Kjeldahl method for organic nitrogen. [Google Scholar]
- Campos M.G.R., Bogdanov S., Almeida-Muradian L.B., Szczesna T., Mancebo Y., Frigerio C., Ferreira F. Pollen composition and standardisation of analytical methods. J. Apic. Res. 2008;47:154–161. [Google Scholar]
- Campos J.F., dos Santos U.P., Macorini L.F.B., Melo A.M.M.F., Balestieri J.B.P., Paredes-Gamero E.J., Cardoso C.A.L., Souza K.L., Santos E.L. Antimicrobial, antioxidant and cytotoxic activities of propolis from Melipona orbignyi (Hymenoptera, Apidae) Food Chem. Toxicol. 2014;65:374–380. doi: 10.1016/j.fct.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Carvalho, R., Silva-Carvalho, R., Baltazar, F., Almeida-Aguiar, C., 2015. Propolis : A complex natural product with a plethora of biological activities that can be explored for drug development. Evid. Based Complement. Alternat. Med. 29, Article ID 206439. [DOI] [PMC free article] [PubMed]
- Castaldo S., Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2002;73:S1–S6. doi: 10.1016/s0367-326x(02)00185-5. [DOI] [PubMed] [Google Scholar]
- Cavendish, R.L., Santos, J.d.S., Neto, R.B., Paixão, A.O., Oliveira, J.V., de Araujo, E.D., Berretta, E., Silva, A.A., Thomazzi, S.M., Cardoso, J.C., Gomes, M.Z., 2015. Antinociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents. J. Ethnopharmacol. 173, 127–133. [DOI] [PubMed]
- Coneac G., Gafitanu E., Hadaruga D.I., Hadaruga N.G., Pinzaru I.A., Bandur G., Ursica L., Paunescu V., Gruia A. Flavonoid contents of propolis from the west side of Romania and correlation with the antioxidant activity. Chem. Bull. Politehnica Univ. (Timisoara) 2008;53:1–2. [Google Scholar]
- Cushnie T.P.T., Hamilton V.E.S., Lamb A.J. Assessment of the antibacterial activity of selected flavonoids and consideration of discrepancies between previous reports. Microbiol. Res. 2003;158:281–289. doi: 10.1078/0944-5013-00206. [DOI] [PubMed] [Google Scholar]
- Cushnie T.P.T., Lamb A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents. 2005;26:343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagaroglu M. Second Ed. Doga Arıcılık Tic; Tekirdağ: 2004. Modern beekeeping techniques. [Google Scholar]
- De-Melo A.A.M., Estevinho M.L.M.F., Almeida-Muradian L.B. A diagnosis of the microbiological quality of dehydrated bee-pollen produced in Brazil. Lett. Appl. Microbiol. 2015;61:477–483. doi: 10.1111/lam.12480. [DOI] [PubMed] [Google Scholar]
- Devequi-Nunes D., Machado B.A.S., de Abreu Barreto G., Silva J.R., da Silva D.F., da Rocha J.L.C., Brandão H.N., Borges V.M., Umsza-Guez M.A. Chemical characterization and biological activity of six different extracts of propolis through conventional methods and supercritical extraction. PLoS One. 2018;13 doi: 10.1371/journal.pone.0207676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos C.G., Megiolaro F.L., Serrao J.E., Blochtein B. Morphology of the head salivary and intramandibular glands of the stingless bee Plebeia emerina (Hymenoptera: Meliponini) workers associated with propolis. Ann. Entomol. Soc. Am. 2009;102:137–143. [Google Scholar]
- Echeverría J., Opazo J., Mendoza L., Urzúa A., Wilkens M. Structure-activity and lipophilicity relationships of selected antibacterial natural flavones and flavanones of Chilean flora. Molecules. 2017;22:608. doi: 10.3390/molecules22040608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchin M., da Cunha M.G., Denny C., Napimoga M.H., Cunha T.M., Koo H., de Alencar S.M., Ikegaki M., Rosalen P.L. Geopropolis from Melipona scutellaris decreases the mechanical inflammatory hypernociception by inhibiting the production of IL-1β and TNF-α. J. Ethnopharmacol. 2012;143:709–715. doi: 10.1016/j.jep.2012.07.040. [DOI] [PubMed] [Google Scholar]
- Formicki G., Greń A., Stawarz R., Zyśk B., Gał A. Metal content in honey, propolis, wax, and bee pollen and implications for metal pollution monitoring. Pol. J. Environ. Stud. 2013;22:99–106. [Google Scholar]
- Georgieva K., Popova M., Dimitrova L., Trusheva B., Thanh L.N., Phuong D.T.L., Lien N.T.P., Najdenski H., Bankova V. Phytochemical analysis of Vietnamese propolis produced by the stingless bee Lisotrigona cacciae. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheldof N., Wang X.H., Engeseth N.J. Identification and quantification of antioxidant components of honeys from various floral sources. J. Agric. Food Chem. 2002;50:5870–5877. doi: 10.1021/jf0256135. [DOI] [PubMed] [Google Scholar]
- Gilbert M.D., Lisk D.J. Honey as an environmental indicator of radionuclide contamination. Bul. Environ. Contam. Toxicol. 1978;19:32–34. doi: 10.1007/BF01685763. [DOI] [PubMed] [Google Scholar]
- Gong S., Luo L., Gong W., Gao Y., Xie M. Multivariate analyses of element concentrations revealed the groupings of propolis from different regions in China. Food Chem. 2012;134:583–588. [Google Scholar]
- González-Martín M.I., Escuredo O., Revilla I., Vivar-Quintana A.M., Coello M.C., Riocerezo C.P., Moncada C.W. Determination of the mineral composition and toxic element contents of propolis by near infrared spectroscopy. Sensors (Basel) 2015;15:27854–27868. doi: 10.3390/s151127854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães N.S.S., Mello J.C., Paiva J.S., Bueno P.C., Berretta A.A., Torquato R.J., Nantes I.L., Rodrigues T. Baccharis dracunculifolia, the main source of green propolis, exhibits potent antioxidant activity and prevents oxidative mitochondrial damage. Food Chem. Toxicol. 2012;50:1091–1097. doi: 10.1016/j.fct.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Guzmán-Gutiérrez S.L., Nieto-Camacho A., Castillo-Arellano J.I., Huerta-Salazar E., Hernández-Pasteur G., Silva-Miranda M., Argüello-Nájera O., Sepúlveda-Robles O., Espitia C.I., Reyes-Chilpa R. Mexican propolis: A source of antioxidants and anti-inflammatory compounds, and isolation of a novel chalcone and ε-caprolactone derivative. Molecules. 2018;23:334. doi: 10.3390/molecules23020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim N., Zakaria A.J., Ismail Z., Mohd K.S. Antibacterial and phenolic content of propolis produced by two Malaysian stingless bees, Heterotrigona itama and Geniotrigona thoracica. Int. J. Pharmacogn. Phytochem. Res. 2016;8:156–161. [Google Scholar]
- Kakino M., Izuta H., Tsuruma K., Araki Y., Shimazawa M., Ichihara K., Hara H. Laxative effects and mechanism of action of Brazilian green propolis. BMC Complement. Alternat. Med. 2012;12:192. doi: 10.1186/1472-6882-12-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalogeropoulos N., Konteles S.J., Troullidou E., Mourtzinos I., Karathanos V.T. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009;116:452–461. [Google Scholar]
- Kasote D.M., Pawar M.V., Gundu S.S., Bhatia R., Nandre V.S., Jagtap S.D., Mahajan S.G., Kulkarni M.V. Chemical profiling, antioxidant, and antimicrobial activities of Indian stingless bees propolis samples. J. Apic. Res. 2019;58:617–625. [Google Scholar]
- Keskin N., Hazir S., Baser K.H.C., Kürkçüoglu M. Antibacterial activity and chemical composition of Turkish propolis. Z. Naturforsch. 2001;56c:1112–1115. doi: 10.1515/znc-2001-11-1231. [DOI] [PubMed] [Google Scholar]
- Kim Y.H., Chung H.J. The effects of Korean propolis against foodborne pathogens and transmission electron microscopic examination. New Biotechnol. 2011;28:713–718. doi: 10.1016/j.nbt.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Kostic A.Z., Pesic M.B., Mosic M.D., Dojcinovic B.P., Natic M.M., Trifkovic J.D. The mineral content of bee pollen from Serbia. Arh. Hig. Rada Toksiko. 2015;66:251–258. doi: 10.1515/aiht-2015-66-2630. [DOI] [PubMed] [Google Scholar]
- Kucuk M., Kolaylı S., Karaoğlu Ş., Ulusoy E., Baltacı C., Candan F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007;100:526–534. [Google Scholar]
- Kujumgiev A., Tsvetkova I., Serkedjieva Y., Bankova V., Christov R., Popov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J. Ethnopharmacol. 1999;64:235–240. doi: 10.1016/s0378-8741(98)00131-7. [DOI] [PubMed] [Google Scholar]
- Kumazawa S., Taniguchi M., Suzuki Y., Shimura M., Kwon M.S., Nakayama T. Antioxidant activity of polyphenols in carob pods. J. Agric. Food Chem. 2002;50:373–377. doi: 10.1021/jf010938r. [DOI] [PubMed] [Google Scholar]
- Lichtenberg E.M., Imperatriz-Fonseca V.L., Nieh J.C. Behavioral suites mediate group-level foraging dynamics in communities of tropical stingless bees. Insectes Soc. 2010;57:105–113. doi: 10.1007/s00040-009-0055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D.C.C., Abu Bakar M.F., Majid M. Nutritional composition of stingless bee honey from different botanical origins. IOP Conf. Series: Earth Environ. Sci. 2019;269 [Google Scholar]
- Lima B., Tapia A., Luna L., Fabani M.P., Schmeda-Hirschmann G., Podio N.S., Wunderlin D.A., Feresin G.E. Main flavonoids, DPPH activity, and metal content allow determination of the geographical origin of propolis from the province of San Juan (Argentina) J. Agric. Food Chem. 2009;57:2691–2698. doi: 10.1021/jf803866t. [DOI] [PubMed] [Google Scholar]
- Marcucci M.C. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83–99. [Google Scholar]
- Marcucci M.C., Bankova V. Chemical composition, plant origin and biological activity of Brazilian propolis. Cur. Top. Phytochem. 1999;2:115–123. [Google Scholar]
- Massaro F.C., Brooks P.R., Wallace H.M., Russell F.D. Cerumen of Australian stingless bees (Tetragonula carbonaria): gas chromatography-mass spectrometry fingerprints and potential anti-inflammatory properties. Naturwissenschaften. 2011;98:329–337. doi: 10.1007/s00114-011-0770-7. [DOI] [PubMed] [Google Scholar]
- Massaro C.F., Shelley D., Heard T.A., Brooks P. In vitro antibacterial phenolic extracts from ‘sugarbag’ pot-honeys of Australian stingless bees (Tetragonula carbonaria) J. Agric. Food Chem. 2014;62:12209–12217. doi: 10.1021/jf5051848. [DOI] [PubMed] [Google Scholar]
- Medić-Šarić M., Jasprica I., Mornar A., Smolcic-Bubalo A., Golja P. Quantitative analysis of flavonoids and phenolic acids in propolis by two dimensional thin layer chromatography. JPC J. Planar Chromat – Modern TLC. 2004;17:459–464. [Google Scholar]
- Meo S.A., Al-Asiri S.A., Mahesar A.L., Ansari M.J. Role of honey in modern medicine. Saudi J. Biol. Sci. 2017;24:975–978. doi: 10.1016/j.sjbs.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener C.D. In: Pot-honey: A Legacy of Stingless Bees. Vit P., Pedro S.R.M., Roubik D., editors. Springer; New York: 2013. The Meliponini; pp. 3–17. [Google Scholar]
- Moreira L., Dias D.L., Pereira J.A., Estevinho L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem. Toxicol. 2008;46:3482–3485. doi: 10.1016/j.fct.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Moreno M.I.N., Isla M.I., Cudmani N.G., Vattuone M.A., Sampietro A.R. Screening of antibacterial activity of Amaicha del Valle (Tucumán, Argentina) propolis. J. Ethnopharmacol. 1999;68:97–102. doi: 10.1016/s0378-8741(99)00051-3. [DOI] [PubMed] [Google Scholar]
- Morgano M.A., Martin M.C.T., Rabonato L.C., Milani R.F., Yatsuyanagi K., Rodriguez-Amaya D.B. A comprehensive investigation of the mineral composition of Brazillian bee pollen: geographic and seasonal variations and contribution to human diet. J. Braz. Chem. Soc. 2012;23:727–736. [Google Scholar]
- Nedji N., Loucif-Ayad W. Antimicrobial activity of Algerian propolis in foodborne pathogens and its quantitative chemical composition. Asian Pac. J. Trop. Dis. 2014;4:433–437. [Google Scholar]
- Nielsen S.S. Springer. West Lafayette; Indiana, USA: 2010. Food Analysis, Fourth Edition, Dietary Fibre; pp. 165–168. [Google Scholar]
- Owusu-Apenten R. First ed. CRC Press; New York, USA: 2002. Food Protein Analysis Quantitative Effects on Processing. [Google Scholar]
- Pohjala L., Tammela P. Aggregating behavior of phenolic compounds — A source of false bioassay results? Molecules. 2012;17:10774–10790. doi: 10.3390/molecules170910774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov B.B., Hristova V.K., Presilski S., Shariati M.A., Najman S. Assessment of heavy metals in propolis and soil from the Pelagonia Region, Republic of Macedonia. Maced. J. Chem. Chem. Eng. 2017;36:23–33. [Google Scholar]
- Przybyłek I., Karpiński T.M. Antibacterial properties of propolis. Molecules. 2019;24:2047. doi: 10.3390/molecules24112047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyłowski P., Wilczyńska A. Honey as an environmental marker. Food Chem. 2001;74:289–291. [Google Scholar]
- Rao P.V., Krishnan K.T., Salleh N., Gan S.H. Biological and therapeutic effects of honey produced by honey bees and stingless bees: A comparative review. Rev. Bras. Farmacogn. 2016;26:657–664. [Google Scholar]
- Rasmussen C., Cameron S.A. A molecular phylogeny of the old world stingless bees (Hymenoptera: Apidae: Meliponini) and the non-monophyly of the large genus trigona. Syst. Entomol. 2007;32:26–39. [Google Scholar]
- Rasmussen C., Camargo J.M.F. A molecular phylogeny and the evolution of nest architecture and behavior in Trigona s.s. (Hymenoptera: Apidae: Meliponini) Apidologie. 2008;39:102–118. [Google Scholar]
- Rasmussen C., Thomas J.C., Engel M.S. A new genus of eastern hemisphere stingless bees (Hymenoptera: Apidae), with a key to the supraspecific groups of Indomalayan and Australasian Meliponini. Am. Mus. Novit. 2017;3888:1–33. [Google Scholar]
- Reyes-González, A., Camou-Guerrero, A., Reyes-Salas, O., Argueta, A., Casas, A., 2014. Diversity, local knowledge and use of stingless bees (Apidae: meliponini) in the municipality of Nocupétaro, Michoacan, Mexico. J. Ethnobiol. Ethnomed. 10, Article No. 47. [DOI] [PMC free article] [PubMed]
- Righi A.A., Alves T.R., Negri G., Marques L.M., Breyer H., Salatino A. Brazilian red propolis: Unreported substances, antioxidant and antimicrobial activities. J. Sci. Food Agric. 2011;91:2363–2370. doi: 10.1002/jsfa.4468. [DOI] [PubMed] [Google Scholar]
- Sanpa S., Popova M., Bankova V., Tunkasiri T., Eitssayeam S., Chantawannakul P. Antibacterial compounds from propolis of Tetragonula laeviceps and Tetrigona melanoleuca (Hymenoptera: Apidae) from Thailand. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos F.A., Bastos E.M., Uzeda M., Carvalho M.A., Farias L.M., Moreira E.S., Braga F.C. Antibacterial activity of Brazilian propolis and fractions against oral anaerobic bacteria. J. Ethnopharmacol. 2002;80:1–7. doi: 10.1016/s0378-8741(02)00003-x. [DOI] [PubMed] [Google Scholar]
- Sawaya A.C.H.F. Composition and antioxidant activity of propolis from three species of Scaptotrigona stingless bees. J. ApiProd. ApiMed. Sci. 2009;1:37–42. [Google Scholar]
- Sforcin J.M., Fernandes A., Jr., Lopes C.A.M., Bankova V., Funari S.R.C. Seasonal effect on Brazilian propolis antibacterial activity. J. Ethnopharmacol. 2000;73:243–249. doi: 10.1016/s0378-8741(00)00320-2. [DOI] [PubMed] [Google Scholar]
- Sforcin J.M., Bankova V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011;133:253–260. doi: 10.1016/j.jep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- Silici S., Kutluca S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J. Ethnopharmacol. 2005;99:69–73. doi: 10.1016/j.jep.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Silvestre D., Dowton M., Arias M.C. The mitochondrial genome of the stingless bee Melipona bicolor (Hymenoptera, Apidae, Meliponini): Sequence, gene organization and a unique tRNA translocation event conserved across the tribe Meliponini. Genet. Mol. Biol. 2008;31:451–460. [Google Scholar]
- Simone-Finstrom M., Spivak M. Propolis and bee health: The natural history and significance of resin use by honey bees. Apidologie. 2010;41:295–311. [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- Shamsudin S., Selamat J., Sanny M., Abd Razak S.B., Jambari N.N., Mian Z., Khatib A. Influence of origins and bee species on physicochemical, antioxidant properties and botanical discrimination of stingless bee honey. Int. J. Food Prop. 2019;22:239–264. [Google Scholar]
- Sousa A., Ferreira I.C.F.R., Barros L., Bento A., Pereira J.A. Effect of solvent and extraction temperatures on the antioxidant potential of traditional stoned table olives ‘alcaparras’. LWT-Food Sci Technol. 2008;41:739–745. [Google Scholar]
- Temaru E., Shimura S., Amano K., Karasawa T. Antibacterial activity of honey from stingless honeybees (Hymenoptera; Apidae; Meliponinae) Polish J. Microbiol. 2007;56:281–285. [PubMed] [Google Scholar]
- Tosi B., Domini A., Romagnoli C., Bruni A. Antimicrobial activity of some commercial extracts of propolis prepared with different solvents. Phytother. Res. 1996;10:335–336. [Google Scholar]
- Trusheva B., Popova M., Koendhori E.B., Tsvetkova I., Naydenski C., Bankova V. Indonesian propolis: Chemical composition, biological activity and botanical origin. Nat. Prod. Res. 2011;25:606–613. doi: 10.1080/14786419.2010.488235. [DOI] [PubMed] [Google Scholar]
- Uren A., Şerifoğlu A., Sarikahya Y. Distribution of elements in honey and effect of a thermoelectric power plant on the element contents. Food Chem. 1998;61:185–190. [Google Scholar]
- Velikova M., Bankova V., Tsvetkova I., Kujumgiev A., Marcucci M.C. Antibacterial entkaurene from Brazilian propolis of native stingless bees. Fitoterapia. 2000;71:693–696. doi: 10.1016/s0367-326x(00)00213-6. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M., Ruiz-Navajas Y., Fernández-López J., Pérez-Álvarez J.A. Functional properties of honey, propolis, and royal jelly. J. Food Sci. 2008;73:R117–R124. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]