Abstract
The objective of this study was to characterize an endophytic fungi producing-bioactive compound from the aquatic plant, Nelumbo nucifera. All parts of such plant were cleaned with surface sterilization technique and cultured on potato dextrose agar to isolate endophytic fungi. The identification was characterized by morphological and molecular technique. Fungal isolates were screened to discover antimicrobial activities by disc diffusion method against Methicillin-resistant Staphylococcus aureus DMST20651 (MRSA). MIC and MBC for those crude fungal extracts were determined. Finally, the chemical profile of crude extract was determined by gas chromatography and mass spectrometry. Six endophytic fungi were isolated from the surface-satirized parts of N. nucifera. Based on disc diffusion assay, the highest antibacterial activity against MRSA was isolate ST9.1 identified as Aspergillus cejpii. Results demonstrated that the ethyl acetate extraction had more active fractions with MIC of 2.5 mg/ml and MBC concentration of 50.0 mg/ml. The crude extracts were developed to identify the chemical constituents by gas chromatography and mass spectrometry. The major component of crude extract of endophytic fungi was 5-(1H-Indol-3-yl)-4,5-dihydro-[1,2,4]triazin-3-ylamine (C11H11N5). Thus, the plant could be used in the treatment of infectious diseases caused by bacterial pathogen.
Keywords: Antimicrobial activity, N. nucifera, Methicillin-resistant S. aureus, Endophytic fungi
1. Introduction
In recent years, the increasing antimicrobial resistance called superbug has driven a critical need to develop a novel antimicrobial agents (Spellberg et al., 2008). Multi-drug resistant strains do not occur only through nosocomial infection but also in the public condition (Talbot et al., 2006), especially resistant sexually transmitted infectious bacteria (Unemo and Jensen, 2017, Shaskolskiy et al., 2016), Escherichia coli, Klebsiella pneumonia, Haemophilus sp., vancomycin-resistnat Enterococcus (VRE), Methicillin-resistant Staphylococcus aureus (MRSA), as well as opportunistic pathogens (Giamarellou, 2006). The antibiotic resistant crisis can mainly be caused by the overuse of broad spectrum antibiotics. In addition, drug use in livestock and agriculture also directly affects environmental microorganisms (Gootz, 2010, Chang et al., 2015, Amador et al., 2019, Coyne et al., 2019). However, several novel antibiotic agents that are best suited for use in the hospital environment have been developed to combat both Gram-positive and Gram-negative bacteria, especially screening the active bio-molecule with the potential in pharmacological properties and drug safety, with a return to traditional remedies medicines.
Endophytic fungi, ubiquitously found in the internal tissue of all plant species, have been reported as the producers of important biologically bioactive molecules (Spellberg et al., 2008, Vasundhara et al., 2016, Manganyi et al., 2019). Generally, they generate in plant tissues without inciting disease symptoms as called symbiotic. They are capable of synthesizing bioactive compounds that can be used by plants for the defense against pathogen and/or stimulating plant growth, and other agents have been proven useful for novel drug discovery process (Talbot et al., 2006). However, the endophytes contained in different plants may have unique biosynthetic capabilities, leading to the production of unique metabolites with varied biological activities. Aquatic plants or hydrophytes could highly adapt to their environment with unique morphological and physiological features, and these features are likely to make them unique habitats for the growth of potentially distinctive endophytic fungi (Uzma et al., 2018). However, the study of endophytic fungi of aquatic plants or their bioactive metabolites in Thailand have not been investigated earlier.
Hence, this paper describes the isolation and identification including the screening of endophytic fungi-producing bioactive agents of the aquatic plant, Nelumbo nucifera against Methicillin-resistant S. aureus DMST20651 (MRSA).
2. Materials and methods
2.1. Preparation of plant materials
A healthy aquatic plant, Nelumbo nucifera, was collected from Rajamangala University of Technology Thanyaburi, Pathumthani, Thailand. All parts of such plant including root, seed, petal, leaf vein, leaf intervein, pollen, stolon, stem and receptacle were washed with tap water for 10 min to remove dust and debris. They were, then, cut into small pieces with a dimension of 1 cm × 1 cm each. To sterilize the surface, all parts were sequentially soaked in 70% ethanol for 1 min, 3% sodium hypochlorite for 1 min, and 70% ethanol for 1 min, respectively. Then, they were rinsed in sterile-distilled water and further dried with sterilized tissue paper. All plant segments were placed on potato dextrose agar medium (PDA) supplemented with streptomycin (100 µg/ml). The agar plates were incubated at 30 °C for 7 days in the dark condition. The pure fungal strains were obtained and morphologically differentiated, and the spores were identified by using Lacto Phenol Cotton blue staining (Spellberg et al., 2008, Talbot et al., 2006, Unemo and Jensen, 2017).
2.2. Identification of fungal endophytes
Initially, the morphological characteristics (color, colony surface, colony margin, pattern, and pigment exuded) of the fungal isolated were examined. The identification of the fungal isolate was mainly performed using molecular characterization by analyzing the DNA sequence of the ITS region of the ribosomal RNA gene. The fungal strain was cultured in PDA at a room temperature for 3 days. Mycelium of fungus was scraped and the isolation of genomic DNA from the mycelia of fungal isolate was carried out according to the published protocol as previously reported (Dissanayake et al., 2016). The ITS region of the isolated genomic DNA was amplified by polymerase chain reaction (PCR) using the forward primer ITS1 and the reverse primer ITS4 under the conditions. The amplified DNA was commercially sequenced and BLAST was analyzed (National Center for Biotechnology Information, NCBI). When the top three matching BLAST hits were from the same species and were ≥ 98% similar to the query sequence, this species name was assigned to the selected isolate (Zhou et al., 2018).
2.3. Preliminary screening of bioactive compounds
Each fungal isolate was cultured in 10 ml of potato dextrose broth (PDB) at 30 °C for 14 days. Mycelium of the fungus was filtered and tested against Methicillin-resistant S. aureus DMST20651 (MRSA). 10 mg/ml streptomycin was used as standard in agar disc diffusion assay and PDB was used as a negative control. Bioassay was conducted in triplicate. After a 24 h-incubation, the mean diameter of the inhibition zones was recorded and a standard error was calculated. Further, the most potent of the fungal strain was selected and identified.
2.4. Fungal growth of endophytic fungi
To determine the fungal growth rate, all fungal endophytes isolates were grown on potato dextrose agar (PDA) and incubated at 30 °C for 21 days. The fungal growth rate was measured by the diameter of mycelial growth on the agar plates (mm) every 3 days (Basu et al., 2015).
2.5. Production time of bioactive compounds
After previous screening of antimicrobial activity, a positive fungal isolate was selected to study the secretion of bioactive compounds. Briefly, three pieces of fungal colony were grown into 50 ml of potato dextrose broth (PDB) medium in 100 ml Erlenmeyer flask and incubated for 30 days at 30 °C for bioactive compound production. The culture broth of fermentation was taken off and transferred into a micro-centrifuge tube on day 1, 3, 5, 7 14, 21 and 28 for antibacterial determination by disc diffusion assay.
2.6. Submerged fermentation of endophytic fungi
The fungal strain with the best antimicrobial activity was grown into 500 ml potato dextrose broth (PDB) medium (potato: 200 g/L; dextrose: 20 g/L; pH 6.0) in 1000 ml Erlenmeyer flask and incubated for 30 days at 30 °C for bioactive compound production. After incubation, the culture was filtrated using filtrate paper Whatman No. 1 and kept at 4 °C for solvent extraction.
2.7. Extraction of bioactive compounds of endophytic fungi
The culture filtrate was extracted twice with the equal volume of hexane, ethyl acetate, and methanol using fungal separation. Each solvent phase was collected and condensed using the rotary vacuum evaporator. The dry crude extract metabolite was obtained at 4 °C, kept at − 20◦C and 2% DMSO, respectively, for use in the future (Palanichamy et al., 2018a).
2.8. Antimicrobial determination
2.8.1. Preparation of inoculums
The bacterial pathogen, Methicillin-resistant S. aureus DMST20651 (MRSA), was obtained from the National Institute of Health, Thailand. Tested bacteria was inoculated in nutrient broth and incubated for 16–18 h at 37 °C in aerobic condition. The suspension was adjusted to 0.5 McFarland turbidity standard corresponding to 107–108 CFU/ml.
2.8.2. Antimicrobial assay
The disc diffusion method was used to determine the antibacterial activity of the endophytic fungi extracts by using nutrient agar (NA). A fresh bacterial suspension adjusted to 107–108 CFU/ml was spread on NA plates with sterile cotton swab. Disc diffusion assay was completed by adding tested crude extract in 2% DMSO at an initial concentration of 100 mg/ml onto a 6 mm paper disc. The culture plates were then incubated at 37 °C for 24 h in an incubator. The experiment was carried out in triplicate and the diameter in millimeters (mm) of the inhibition zone was recorded. The results were represented as mean ± sd (Tamijothi et al., 2011).
2.9. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
To assess the MIC and MBC of endophytic fungi extracts, the serial two-flow dilution was carried out with broth dilution assay. The absence of bacterial growth with the lowest concentration was interpreted as the MIC. The MBC was determined by sub-culturing which showed no bacterial growth on agar plates after incubated at 37 °C for 24 h in an incubator. The lowest concentration that did not show bacterial growth was defined as the MBC value. The experiment was conducted in triplicate and the mean diameter in millimeters (mm) of the zone of inhibition was recorded. Erythromycin and 2% dimethyl sulfoxide (DMSO) solution were used as a positive control and a negative control, respectively. All experiments were performed in triplicate. The results were represented as mean ± sd (Chen et al., 2016).
2.10. Gas chromatography-mass spectrometry analysis
GC–MS analysis tests for bioactive compounds of endophytic fungi crude extract composition analysis were performed on a Agilent 7890 GC system instrument equipped with HP-5MS (5% diphenyl 95% dimethylpolysiloxane) column (30 m × 0.25 mm, 0.25 µm) and interfaced to a 5975C inert XL MSD with Triple-Axis Detector. An injection volume of 2 µL was employed (split ration of 10:1) at an injector temperature of 250 °C. The column temperature was increased from 60 to 250 °C with a rate of 5 °C /min. The outlet temperature was 280 °C. Mass spectra were taken at 70 Da. Ms transfer line temperature was 250 °C. The components of the extract were identified by the comparison of fragmentation patterns in mass spectra with those stored on the spectrometer database and reported in the literature. The relative percentage of individual components was calculated from the GC peak areas (Valgas et al., 2007).
2.11. Identification of bioactive constituents
Interpretation on Mass-spectrum GC–MS was carried out by using the database of National institute Standard and Technology (NIST) with more than 62,000 patterns. The spectrum of the unknown components was compared with the spectrum of known components stored in the NIST library. The name, molecular formula, weight and chemical structure of the components of the test materials were ascertained (Carbonero, et al., 2012).
2.12. Time kill kinetics
Time-kill assay was analyzed using the MIC values, the MRSA suspension were adjusted a bacterial serial ten-fold dilutions (10−3 and 10−4 from 1.5 × 108 cfu/mL) and tested antimicrobial activity with concentration of MIC values of 1MIC, 2MIC and 3 MIC of fungal extracts including EtOAc (E), intracellular (I) and intra + extracellular extracts (IE). Streptomycin at concentration of 40 mg/mL was used as a positive control. The 80 µL of each treatment was put into each well containing 100 µL nutrient broth supplemented with 20 µL of bacterial suspension and incubated at 37 °C for 24 h. All samples were collected at 3, 6, 9, 18 and 24 h. After that, 100 µL of collected samples were cultured on nutrient agar and incubated at 37 °C for 24 h. A number of viable bacterial cell was determined.
2.13. Statistical analysis
All data of the experiments were statistically analyzed by mean ± sd for groups (n = 3).
3. Results and discussion
3.1. Isolation of endophytic fungi from Nelumbo nucifera
Six endophytic fungi were isolated from several parts of Nelumbo nucifera (Fig. 1): root, seed, petal, leaf veins, leaf interveins, pollen, stolon, stem, and receptacle. The result showed that endophytic fungi were more prevalent in the root of N. nucifera (3/6 or 50.0%) than other parts. Similarly to the report from the researcher in Sri Lanka, eight out of twenty endophytic fungi were isolated from leaves of Sri Lankan aquatic plant Nymphaea nouchali. All fungal isolates were screened to test the anti-bacterial activity (Dissanayake et al., 2016).
Fig. 1.
The lotus (Nelumbo nucifera) crop at Pathum Thani Province, Thailand: (A) the lotus field and (B) lotus flower.
3.2. Preliminary screening of endophytic fungi from Nelumbo nucifera
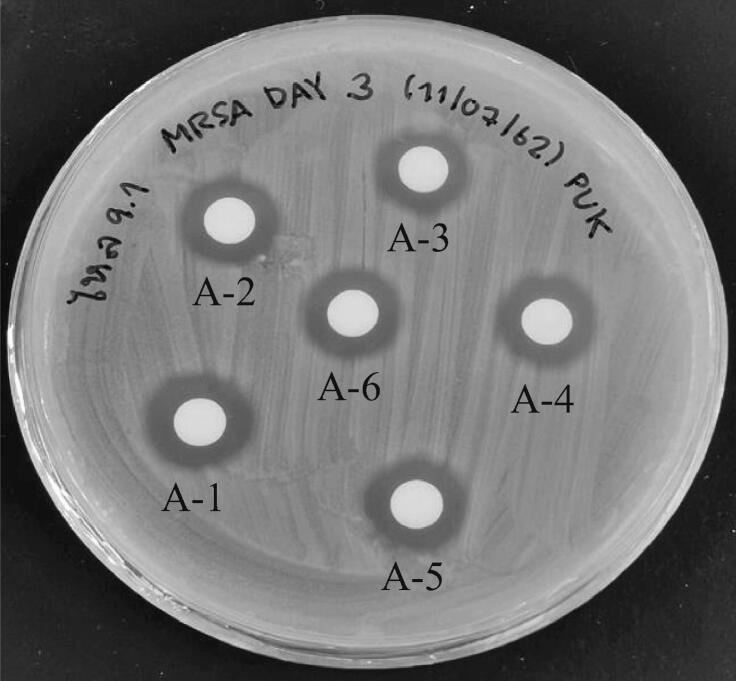
For the primary screening of bioactive compound production, cultured cell-free broth of all fungal strains were tested against Methicillin-resistant S. aureus DMST20651 (MRSA) by agar diffusion assay. It was found that one out of six endophytic fungi, named ST9.1 isolated from the stolon was strongly potential against Methicillin-resistant S. aureus DMST20651 (MRSA) (Fig. 2) indicating that the cell-free broth displayed extra-cellular bio-active molecule.
Fig. 2.
Extracellular activity of bioactive molecules of fungal cell-free broth isolate ST9.1 against Staphylococcus aureus DMST20651 (MRSA) under submerge fermentation at 30 °C for three days (A1-6).
3.3. Identification of endophytic fungi isolate ST9.1
Fungal endophytic fungi isolate ST9.1 was characterized based on molecular identification and its ITS1 and ITS4 sequences were submitted in Genbank. Identified isolates ST9.1 belongs to Aspergillus cejpii with a similarity of 99.57%. When compared to the results of the research from Sri Lanka (Dissanayake et al., 2016), which isolated fungal endophyte from Sri Lankan aquatic plant (Nymphaea nouchali), the best endophytic fungus, Chaetomium globosum produced chaetoglobosin A and C, with an antimicrobial activity against S. aureus (ATCC 25923) and Bacillus cereus (ATCC 11778), Pseudomonas aeruginosa (ATCC 9027) and Escherichia coli (ATCC 35218).
3.4. Production time of bioactive compounds
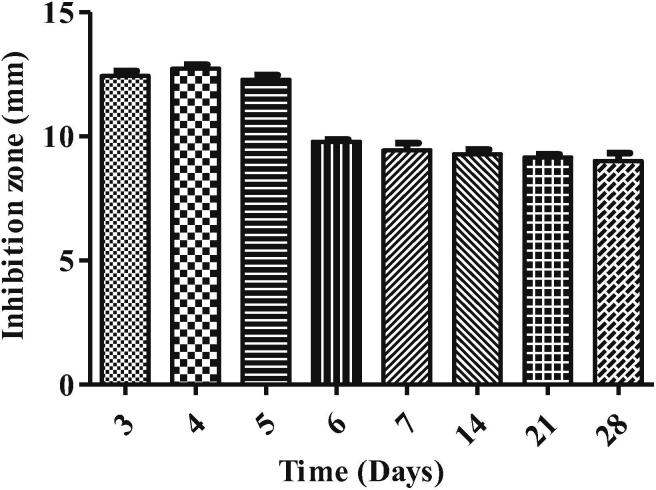
For bioactive compound production, the fungal endophyte isolate ST9.1 was selected and grown on PDB and incubated at 30 °C for 3, 5, 7, 14, 21, and 28 days before the antibacterial activity test. Results showed that the highest antibacterial activity of the fungal endophytes isolate ST9.1 was performed on day 4 with an inhibition zone of 12.67 ± 0.47 mm. The activity was highest on early fermentation suggested that such metabolism could lead to an increase metabolic products (Adebo and Medina-Meza, 2020) and the diversity of metabolites synthesis were produced by various metabolic pathway (Palanichamy et al., 2018b). Noticeably, when the incubation time was increased, the function of bioactive molecule decreased (Fig. 3). These results suggest the possibility that several compounds may also inhibits the specific biosynthesis pathway (Pant et al., 2015).
Fig. 3.
The production time of bioactive molecule of fungal endophyte isolate ST9.1.
3.5. Fungal growth rate
In this study, agar medium, PDA, was used to assess the growth characteristic of mycelial growth of fungal endophyte because the solid-state medium might be more realistic to study the growth characteristics of fungal species (Singh and Kumar, 2012). Fig. 4 shows the results of the mycelial growth rate of all endophytic fungi. For each fungal isolate, the vegetative mycelial growth was determined by the colony diameter of radial mycelial growth. The radial growth of the colonies of isolates PT1.1 (from petal), ST9.1 (from stolon), and RT1.1 (from root) showed a more rapid production in agar media when compared with fungal isolates RE8.1, IT1.1, and RE3.1 in week 3, indicating that nutritionally-rich media could promote abundant mycelial density and fungal growth rate (Singh and Kumar, 2012).
Fig. 4.
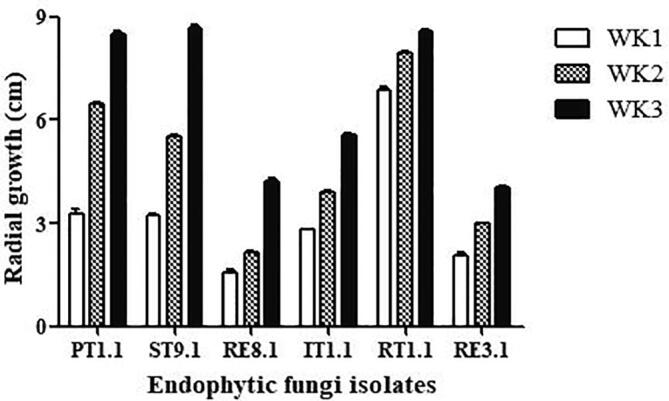
The mycelial growth rate of endophytic fungi.
3.6. Antibacterial activities
Antimicrobial activity results of endophytic fungal isolate ST9.1 were determined by paper disc diffusion methods in Table 1. Results of disc diffusion method in terms of size of inhibition zone (mm) for the extracts were compared against microorganisms studied, Methicillin-resistant S. aureus (MRSA). The highest antibacterial activity was determined against S. aureus (MRSA) in a clearing zone of 11.33 ± 0.29 mm when extracted with ethyl acetate (EtOAc-En fraction) whereas hexane extract (Hex-En fraction), butanol extract (Bu-En fraction), and methanol extract (Me-En fraction) displayed no activity. In the previous study, endophytic fungal extracts from Nymphaea nouchali (Sri Lankan aquatic plant) have been reported as having antimicrobial activity against Gram-positive bacteria, S. aureus (ATCC 25923) and Bacillus cereus (ATCC 11778) identified as Chaetomium globosum (Dissanayake et al., 2016). There are many reports of antimicrobial compounds produced by endophytic fungi active against plant and human pathogens (Chareprrasert et al., 2006).
Table 1.
Bacterial activities of the crude extracts of fungal endophyte isolate ST9.1.
| Extracts | Inhibition zone (mm) of MRSA |
|---|---|
| Hex-En (Hexane) | – |
| EtOAc-En (Ethyl acetate) | 11.33 ± 0.29 |
| Bu-En (Buthanol) | – |
| Me-En (Methanol) | – |
| Negative controla | – |
| Positive controlb | 19.00 ± 0.29 |
Note:anegative control (2% DMSO); bpositive control (streptomycin; 10 mg/ml); statistical analysis as mean ± sd.
3.7. Bacteriostatic and bactericidal effect
With a minimum inhibitory concentration (MIC) and a minimum bactericidal concentration (MBC) against Methicillin-resistant S. aureus (MRSA), a lower MIC was observed in Table 2. The MIC test indicated that the extract of endophytic fungi isolate ST9.1 exhibited minimal values of MIC (2.50 mg/ml) against Methicillin-resistant S. aureus (MRSA). With a minimal bactericidal concentration, MBC at the concentration of 50.0 mg/ml was discovered.
Table 2.
The minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of fungal endophyte isolate ST9.1 used to inhibit Methicillin-resistant S. aureus (MRSA).
| Bacterial strain | Concentration (mg/ml) |
|
|---|---|---|
| MICa | MBCb | |
| MRSA | 2.5 | 50 |
Note:aMIC: the minimum inhibitory concentration; bMBC: the minimum bactericidal concentration (mean ± SD).
3.8. Gas Chromatography-Mass spectrometry analysis of crude endophytic fungi isolate ST9.1 extract
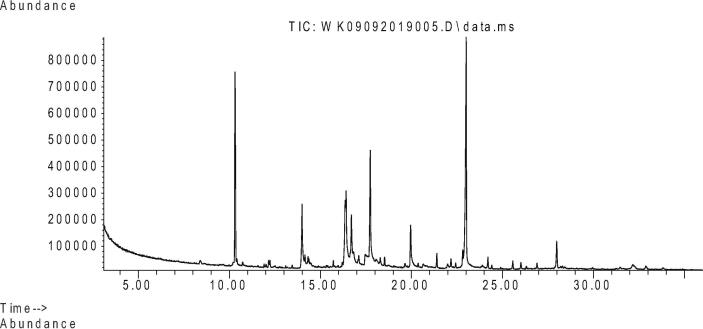
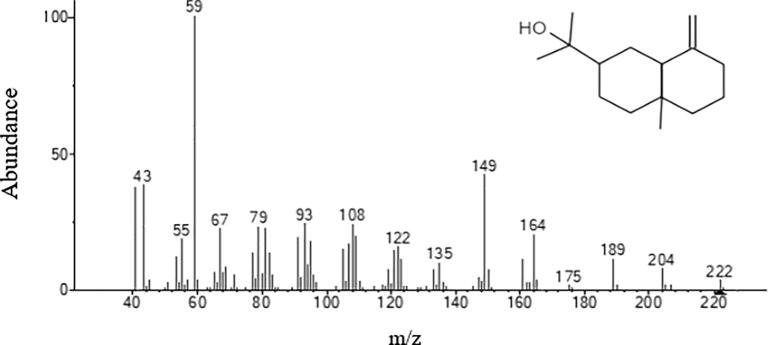
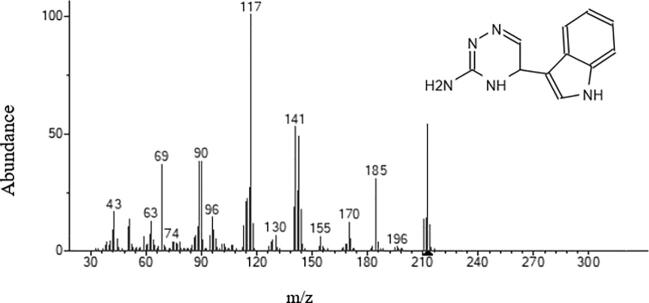
Fungal strain ST9.1 showed the most promising antimicrobial activities and was selected for bioassay-guided fractionations to isolate and characterize the active compounds. The active principles with their retention time (RT), molecular formula, molecular weight (MW), concentration (Peak area %) and the chemical structure were analyzed. Table 3 shows the components present in the ethyl acetate extract as identified by GC–MS. The chromatogram of endophytic fungi extracts which promoted a high peak area was shown in Fig. 5. 2-Naphthalenemethanol (RT10.309) was shown in Fig. 6, Fig. 5-(1H-Indol-3-yl)-4,5-dihydro-[1,2,4] triazin-3-ylamine (RT 23.009) was shown in Fig. 7. However, these compounds derivatives like amino acid conjugated antimicrobial drugs was prepared by the previously reported for their antimicrobial activities (Saundane et al., 2013).
Table 3.
Characterized chemical constituents of fungal endophyte isolate ST 9.1.
| Compounds | RTa | MWb | MFc |
|---|---|---|---|
| 2-Naphthalenemethanol | 10.309 | 222 | C15H26O |
| n-Hexadecanoic acid | 13.989 | 256 | C16H32O2 |
| 9,12-Octadecadienoic acid (Z,Z) | 16.415 | 280 | C18H32O2 |
| Oleic Acid | 16.701 | 282 | C18H34O2 |
| Pyrazino[1,2-a]indole-1,4-dione, 2,3-dihydro-2-methyl-3-methylene- | 17.743 | 226 | C13H10N2O2 |
| n-Octanoic acid,methyl(tetramethylene)silyl ester | 19.956 | 242 | C13H26O2Si |
| Nonadecane | 21.395 | 268 | C19H40 |
| 5-(1H-Indol-3-yl)-4,5-dihydro-[1,2,4] triazin-3-ylamine | 23.009 | 213 | C11H11N5 |
| Diisooctyl phthalate | 22.17 | 390 | C24H38O4 |
| 9,12,15-Octadecatrienoic acid, 2-[(trimethylsilyl)oxy]-1-[[(trimethylsilyl) oxy]methyl]ethyl ester, (Z,Z,Z)- | 22.428 | 496 | C27H52O4Si2 |
| 1-Ethyl-1-heptyloxy-1-silacyclohexane | 21.985 | 242 | C14H30OS |
| Hydroxylamine, O-decyl- | 24.199 | 173 | C10H23NO |
| 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl- | 26.016 | 222 | C15H26O |
| Dehydroergosterol 3,5-dinitrobenzoate | 27.98 | 588 | C35H44N2O6 |
Note:aRT: retention time (min); bMW: molecular weight; cMF: molecular formula.
Fig. 5.
GC chromatogram of fungal endophytic extracts with ethyl acetate (EtOAc-En).
Fig. 6.
Mass spectrum of 2-Naphthalenemethanol.
Fig. 7.
Mass spectrum of 5-(1H-Indol-3-yl)-4,5-dihydro-[1,2,4] triazin-3-ylamine.
3.9. Time kill kinetics assay
The time kill assay is widely used to examine the ability of an antimicrobial compounds against various pathogens. These assay was subjected over 24 h with the MRSA being revealed to MIC (2.5 mg/mL), 2MIC (5 mg/mL) and 3MIC (7.5 mg/mL) of fungal endophyte extracts. From the results, a graph was plotted between the logarithmic number of CFU/mL and incubation time (Table 4). At 3MIC concentration, the fungal extracts isolate ST9.1 greatly exhibited MRSA at 3 h and gradually rise up to the 24 h when compared with the MIC, 2MIC and the control, respectively (Fig. 8, Fig. 9, Fig. 10). From the results were exposed that the effect of fungal endophyte extracts at different concentrations and incubation times was bacteriostatic.
Table 4.
Time kill kinetics of fungal endophyte isolate ST9.1 extracts with various different concentration.
| Samples | Concentration | Logrithmic CFU/mL (h) | ||||
|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 18 | 24 | ||
| Negative control | – | 6.58 | 6.60 | 7.81 | 7.81 | 7.81 |
| Streptomycin | 40 mg/mL | 4.40 | 4.40 | 4.40 | 4.40 | 4.40 |
| EtOAC (E) | MICa | 7.29 | 5.77 | 5.35 | 5.04 | 4.40 |
| 2MICb | 7.27 | 5.50 | 5.30 | 4.54 | 4.40 | |
| 3MICc | 5.42 | 5.40 | 5.23 | 4.52 | 4.40 | |
| Intracellular (I) | MICa | 7.24 | 5.47 | 5.30 | 4.48 | 4.40 |
| 2MICb | 5.73 | 5.39 | 5.16 | 4.40 | 4.40 | |
| 3MICc | 5.45 | 5.37 | 5.09 | 4.40 | 4.40 | |
| Intra + Extracellular (IE) | MICa | 7.31 | 6.48 | 7.34 | 7.81 | 7.81 |
| 2MICb | 7.07 | 5.47 | 5.35 | 7.19 | 7.24 | |
| 3MICc | 5.45 | 5.42 | 5.41 | 5.35 | 5.28 | |
Note:aMIC (2.5 mg/mL), b2MIC (5 mg/mL) and c3MIC (7.5 mg/mL).
Fig. 8.
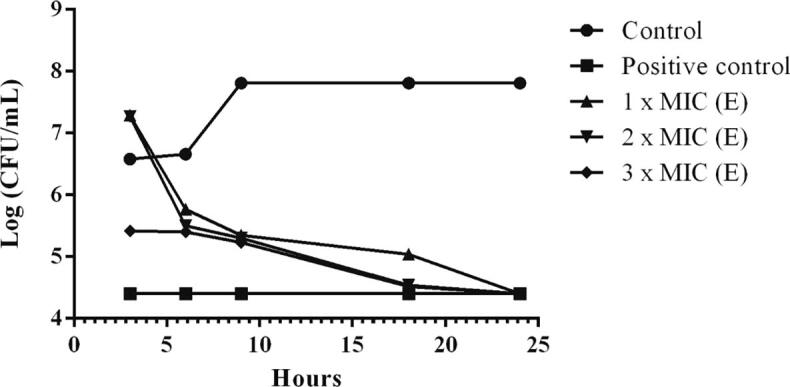
The time kill kinetics of ethyl acetate extracts (E) of isolate RT9.1 against MRSA. Control indicates cells without treatment while positive control indicates treated cells with 40 mg/mL of streptomycin.
Fig. 9.
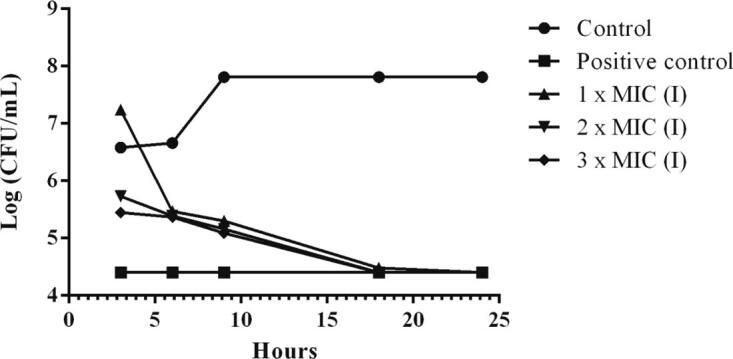
The time kill kinetics of intracellular extracts (I) of isolate RT9.1 against MRSA. Control indicates cells without treatment while positive control indicates treated cells with 40 mg/mL of streptomycin.
Fig. 10.
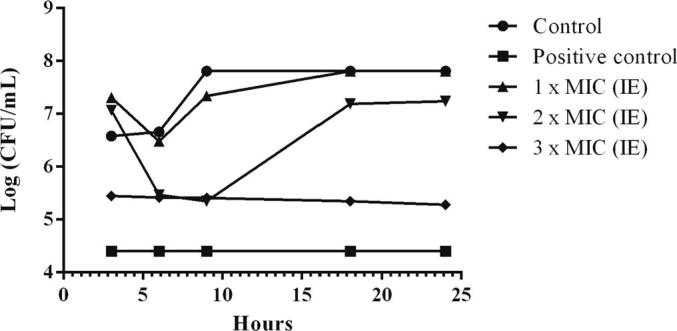
The time kill kinetics of intra and extracellular extracts (IE) of isolate RT9.1 against MRSA. Control indicates cells without treatment while positive control indicates treated cells with 40 mg/mL of streptomycin.
4. Conclusion
From the above study, the results revealed that the endophytic fungi isolate ST9.1 isolated from the lotus stolon was Aspergillus cejpii. The fungal ethyl acetate extracts showed antibacterial activity against Methicillin-resistant S. aureus with MIC and MBC of 2.5 mg/ml and 50.00 mg/ml, respectively. Such results also characterized the chemical profile of extracts by GC–MS and displayed major bioactive compounds as 5-(1H-Indol-3-yl)-4,5-dihydro-[1,2,4]triazin-3-ylamine(C11H11N5). Finally, the fungal extracts isolate ST9.1 greatly exhibited MRSA at concentration of 3MIC for 3 h and gradually rise up to the 24 h when compared with the MIC, 2MIC and control. However, in order to use the fungal endophyte in medicine, more detailed studies need to be conducted.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Rajamangala University of Technology Thanyaburi and Thai Traditional Medicine College, RMUTT for all facilities. This research would not have been completed without the financial support from the National Research Council of Thailand (Grant no. IRF62A1207).
Footnotes
Peer review under responsibility of King Saud University.
References
- Adebo O.A., Medina-Meza I.G. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules. 2020;25(4):1–19. doi: 10.3390/molecules25040927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador P., Fernandes R., Rrudencio C., Duarte I. Prevalence of antibiotic resistance genes in multidrug-resistant Enterobacteriaceae on Portuguese livestock manure. Antibiotics. 2019;8(23):1–23. doi: 10.3390/antibiotics8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Bose C., Ojha N., Das N., Das J., Pal M., Khurana S. Evolution of bacterial and fungal growth media. Bioinformation. 2015;11(4):182–184. doi: 10.6026/97320630011182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonero E.R., Ruthes C., Freitas C.S., Utrilla P., Gálvez J., Silva E.V., Sassaki L., Gorin P.A.J., Iacomini M. Chemical and biological properties of a highly branched β-edible mushroom Pleurotus sajor-caju. Carbohydr. Polym. 2012;90:814–819. doi: 10.1016/j.carbpol.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Chang Q., Wang W., Yochay G.R., Lipsitch M., Hanage W.P. Antibiotics in agriculture and the risk to human health: how worried should we be? Evol. Appl. 2015;8(3):240–247. doi: 10.1111/eva.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareprrasert S., Piapukiew J., Thienhirun S., Whalley A., Sihanonth P. Endophytic fungi of teak leaves Tectona grandis L. and rain tree leaves Samanea saman Meer. World J. Microbiol. Biotechnol. 2006;22:481–486. [Google Scholar]
- Chen H., Tian T., Miao H., Zhao Y.Y. Traditional uses, fermentation, phytochemistry and pharmacology of Phellinus linteus: A review. Fitoterapia. 2016;113:6–26. doi: 10.1016/j.fitote.2016.06.009. [DOI] [PubMed] [Google Scholar]
- Coyne L., Arief R., Benigno C., Giang V.N., Huong L.Q., Jeamsripong S., Kalpravidh W., McGrane J., Padungtod P., Patrick I., Schoonman L., Setyawan E., Sukarno A.H., Srisamran J., Ngoc P.T., Rushton J. Characterizing antimicrobial use in the livestock sector in three south East Asian countries (Indonesia, Thailand and Vietnam) Antibiotics. 2019;8(33):1–25. doi: 10.3390/antibiotics8010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake R.K., Ratnaweera P.B., Williams D.E., Wijayarathne D.W., Wijesundera R.L.C., Andersen R.J., Silva E.D. Antimicrobial activities of endophytic fungi of the Sri Lankan aquatic plant Nymphaea nouchali and chaetoglobosin A and C, procuede by the endophytic fungus Chaetomium globosum. Mycology. 2016;7(1):1–8. doi: 10.1080/21501203.2015.1136708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellou H. Treatment options for multidrug-resistant bacteria. Exp. Rev. Anti. Infect. Ther. 2006;4:601–608. doi: 10.1586/14787210.4.4.601. [DOI] [PubMed] [Google Scholar]
- Gootz T.D. The global problem of antibiotic resistance. Rcrit. Rev. Immunol. 2010;30:79–93. doi: 10.1615/critrevimmunol.v30.i1.60. [DOI] [PubMed] [Google Scholar]
- Manganyi M.C., Regnier T., Tchatchouang C.D.K., Bezuidenhout C.C., Ateba C.N. Antibacterial activity of endophytic fungi isolated from Sceletium tortuosum L. (Kougoed) Ann. Microbiol. 2019;69(6):659–663. [Google Scholar]
- Palanichamy P., Krishnamoorthy G., Kannan S., Marudhamuthu M. Bioactive potential of secondary metabolites derived from medicinal plant endophytes. Egypt J. Basic Appl. Sci. 2018;5:303–312. [Google Scholar]
- Palanichamy P., Krisnamoorthy G., Kannan S., Marudhamuthu M. Bioactive potential of secondary metabolites derived from medicinal plant endophytes. Egypt. J. Basic Appl. Sci. 2018;5:303–312. [Google Scholar]
- Pant B.D., Pant P., Erban A., Huhman D., Kopka J., Scheible W.R. Identification of primary and secondary metabolites with phosphorus status-dependent abundance in Arabidopsis, and of the transcription factor PHR1 as a major regulator of metabolic changes during phosphorus limitation. Plant, Cell Environ. 2015;38:172–187. doi: 10.1111/pce.12378. [DOI] [PubMed] [Google Scholar]
- Saundane A.R., Verma V.A., Katkar V.T. Synthesis and antimicrobial and antioxidant activities of Some New 5-(2-Methyl-1H-indol-3-yl)-1,3,4-oxadiazol-2-amine derivatives. J. Chem. 2013;213:1–9. [Google Scholar]
- Shaskolskiy B., Dementieva E., Leinsoo A., Runina A., Vorobyev D., Plakhova X., Kubanov A., Deryabin D., Gryadunov D. Drug Resistance Mechanisms in Bacteria Causing Sexually Transmitted Diseases and Associated with Vaginosis. Front. Microbiol. 2016;79:1–14. doi: 10.3389/fmicb.2016.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh G., Kumar P. Antibacterial potential of alkaloids of Withania somnifera L. and Euphorbia hirta L. Int. J. Pharm. Pharm. Sci. 2012;4:78–81. [Google Scholar]
- Spellberg B.R., Guidos D., Gilbert J., Bradly H.W., Boucher W.M., Scheld J.G., Bartlett J.E., Jr. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the infectious diseases society of America. Clin. Infect. Dis. 2008;46:155–164. doi: 10.1086/524891. [DOI] [PubMed] [Google Scholar]
- Talbot G.H., Bradley J., Edwards J.E., Gilbert D.J., Scheld M., Bartlet J.G. Bad bugs need drugs: and update on the development pipeline from the antimicrobial availability task force of the infectious diseases society of America. Clin. Infect. Dis. 2006;42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- Tamijothi E., Ravichandiran V., Chandrasekhar N., Suba V. Pharmagognostic and preliminary phytochemical screening of leaves of Tecomaria capensis. Asian J. Plant Sci. Res. 2011;1:34–40. [Google Scholar]
- Unemo M., Jensen J.S. Antimicrobial-resistant sexually transmitted infections: gohorrhoea and Mycoplasma genitalium. Nat. Rev Urol. 2017;14:139–152. doi: 10.1038/nrurol.2016.268. [DOI] [PubMed] [Google Scholar]
- Uzma F., Mohan C.D., Hashem A., Konappa N.M., Rangappa S., Kamath P.V., Singh B.P., Mudili V., Cupta V.K., Siddaiah C.N., Chawdappa S., Alqarawi A.A., Allah E.F.A. Endophytic fungi-Alternative sources of cytotoxic compounds: A review. Front Pharmacol. 2018;9(309):1–37. doi: 10.3389/fphar.2018.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valgas C., Souza S.M., Smânia E.F.A., Smânia A.J. Screening methods to determine antibacterial activity of natural products. Bra. J. Microbiol. 2007;38:369–379. [Google Scholar]
- Vasundhara M., Kumar A., Reddy M.S. Molecular approaches to screen bioactive compounds from endophytic fungi. Front. Microbiol. 2016;7:1–12. doi: 10.3389/fmicb.2016.01774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Diao X., Wang Tao, Chen G., Lin Q., Yang X., Xu J. Phylogenetic diversity and antioxidant activities of culturable fungal endophytes associated with the mangrove species Rhizophora stylosa and R. mucronata in the South China sea. PLoS One. 2018;13(6):1–18. doi: 10.1371/journal.pone.0197359. [DOI] [PMC free article] [PubMed] [Google Scholar]