Abstract
Helicanthes elasticus (Desv.) Danser is a common type of mistletoes of Indian origin. In Indian traditional and folklore medicines the plant is claimed to possess a range of medicinal values such as immunomodulator, anti-diabetic and anti-microbial properties. However, there is no experimental proof for its therapeutic claim. The aqueous and alcoholic extracts of H. elastica were evaluated for its immuno-modulatory effect on antibody formation against sheep red blood cells and on cell mediated immunity of immunological paw edema model. Ethanolic and aqueous extracts have shown dose dependent elevation in the antibody titer value in comparison to control group at 14th and 21st day of sensitization (**p < 0.01). There is a mild to moderate elevation were observed in the immunological paw edema at highest dose (400 mg/kg) during 21st day after sensitization. The histopathological observation shows that there is an increase in the white pulp of spleen and increased cellularity and formation of distinct germinal cells in lymph node. H. elasticus extracts possess marked antibody formation propensity without significant modification on cell mediated immunity.
Keywords: Antibody titer, Cell mediated immunity, Germinal cells, Helicanthes elasticus, Sheep red blood cells, White pulp
1. Introduction
The mammalian immune system is a multifaceted interface of cells and molecules having wide array of factors for providing protection against challenge by pathogenic factors. Ontogenesis of immune cells is performed by central lymphoid organs, bone marrow and thymus while, the peripheral lymphoid organs orchestrate the immune response with the help of spleen, lymph nodes and mucosal lymphoid tissue (Agarwal et al., 2010). The immune system is influenced by several factors such as diet, pharmaceuticals, physical and mental stress, hormonal variations etc. The total health of a living being is based on a complex fine tuning of immune functions (Patwardhan et al., 1991). Immunomodulatory agents normalize or modulate pathophysiological processes being specifically stimulatory or suppressive (Wagner and Proksch, 1985). Complex balance between regulatory and effector cells effect correct function and any imbalance can lead to disease conditions (Sehar et al., 2008).
Mistletoes like Viscum album are important group of plants possessing a range of important pharmacological activities like immunomodulatory, cytotoxicity (anticancer), hypotensive, anti-diabetic and anti-microbial properties (Arndt, 2004). Helicanthes elasticus commonly known as Indian mango mistletoe, is a parasitic shrub found widely growing on mango trees in southern India. It is known as Bandaka in Sanskrit and Pulluruvi in Tamil. Ligaria cuneifolia (Argentine mistletoe), a morphologically and chemically close relative of H. elasticus is a substitute for Viscum album (Fernandez et al., 2004). Though some are mentioned in Indian systems of medicine such as Ayurveda, Siddha and folklore medicines, they have not been exposed to thorough experimental studies to a noteworthy level. Augmentation of humoral as well as cell mediated immunity there by elevating immunological status by fermented extract of H. elasticus was reported. Extracts of H. elasticus growing on Mangifera indica and Citrus maxima (host trees) was cytotoxic and controlled solid and ascites tumours in mice (Mary, 1995). The H. elasticus extracts showed antimicrobial (Sunil Kumar et al., 2014), antioxidant (Joshi et al., 2018), anti-hyperglycemic (Rajesh and Rajasekhar, 2015), and diuretic (Jadhav et al., 2010) activities. The plant extracts were found to possess no toxicity and it is proved to show hepatoprotective activity (Sunil Kumar et al., 2016).
With this background information ethanolic and aqueous extracts of the whole plant of H. elasticus were evaluated for immunological changes by employing two experimental protocols. The first protocol is for assessing activity of the test extracts on antibody development against sheep red blood cells (SRBC) and the second protocol is for assessing the effect of test extracts on cell mediated immunity by their modulatory effect on immunological oedema.
2. Materials and methods
2.1. Preparation of test extracts
Whole plants of H. elasticus growing specifically on Mangifera indica, which is supposed to be the most preferred host, was collected, shade dried, powdered and extracted. Cold percolated aqueous extract (HEAq) (yield 12% w/w) was concentrated and freeze to obtain dark brown gummy material and the ethanolic (95%) extract (HEAl) (yield 19% w/w) was dried over a water bath to obtain dark brown gummy extract.
2.2. Experimental animals
Rats of either sex (Wistar albino) weighing180–200 g body weight procured from Pharmacology Laboratory of Central Research Institute for Siddha was used after getting consent from the animal ethical committee (Grant No: 79/PHARMA/SCRI/2010). Exposure of 12 h day and night cycles with temperature (22 ± 2 °C), humidity (50–60%), Amrut brand rat pellet and water given ad libitum were maintained throughout experiment. Acclimatized rats at standard laboratory condition for 14 days of the experimentation were fed with extract dose of 200 and 400 mg/kg body weight (Sunil Kumar et al., 2016). Each group had 6 animals.
2.3. Antigen preparation
Blood was taken from sheep under aseptic condition and added to Alsever’s solution which contains 2.05 g dextrose (2.05 g); sodium citrate (0.8 g); NaCl (0.4 g) and citric acid (0.05 g). The collected SRBCs were washed thrice with normal saline by centrifugation until supernatant become colourless. The number of SRBC’s were adjusted to 1x 108 after counting in Neubauers chamber and used for immunization to induce antibody formation.
2.4. Effect on humoral antibody formation
Effect of antibody formation by SRBC in rats fed with HEAq and HEAl was studied as described by Vaghasia et al. (2010). Group I received 0.5% CMC at a dose 5 ml/kg body weight served as SRBC control group. Group II, III, IV and V administered with test drugs HEAq and HEAl at 200 and 400 mg/kg body weight respectively for 21 days. On 7th and 14th day, rats from groups I to V were immunized and challenged respectively, using 0.1 ml of 20% SRBC in normal saline by intra-peritoneal route. On 14th and 21st day retro-orbital plexus blood was withdrawn under anesthesia (ether) from all antigen sensitized and challenged rats respectively. Serum was obtained by centrifugation using normal saline, SRBC count was adjusted to 0.1%. All the wells of micro titer plate was added with 20 μl followed by addition of 20 μl of serum to the first well and mixing. Subsequently 20 μl diluted serum was pipette out from the first well and added to the next well to get two fold dilutions of the antibodies present in the serum of 24th well. The antibody concentration of any of the dilution was half of the previous dilution. 20 μl of 0.1% of SRBC were added to all the wells and the plates were incubated in at 37 °C for 1 h and examined visually for hemagglutination. The dilution giving highest hemagglutination (antibody titer) was expressed in terms of log 2 values and the value for different groups was analyzed for statistical significance. The antibody titer attained on day 14 after immunization (given on 7th day) and on day 21 after challenge (14th day) with SRBCs was taken as primary and secondary humoral immune response respectively. Rats were sacrificed under deep ether anesthesia; the organs such as spleen and lymph node were removed and stored in 10% formalin for histopathological study.
2.5. Delayed hypersensitivity response
On the 21st day, 0.03 ml of 20% SRBC and normal saline was injected in sub plantar region of right and left hind paw respectively. The foot pad reaction was assessed at 3 and 24 h after sensitization was measured using digital plethysmograph (difference in right and left hind paw volume) for the calculation of cellular immune response.
3. Results
3.1. Effect of HEAq and HEAl on immunological paw edema
Effect of test extracts on immunological edema is shown in Table 1. Injection of edema eliciting antigen lead to formation of marked paw edema at 3rd hour in the control group. No such edema formation could be observed in the left paw in which no eliciting agent was injected. At 3rd hour the right hand edema formation was found to be comparatively less in test extracts administered group which was statistically not significant. The 24 h edema formation in lower dose (200 mg/kg) of test extract was found insignificant compared to control group. Though moderate increase was observed at higher (400 mg/kg) dose test extract in comparison to the control group the difference was insignificant.
Table 1.
Effect of Helicanthes elasticus whole plant extracts on anti-body titer in SRBC sensitized Wistar Alnino rats.
| Groups | 14th day | 21st day |
|---|---|---|
| Control | 1.85 ± 0.23 | 4.39 ± 0.23 |
| HEAq 200 | 4.28 ± 0.55** | 7.74 ± 0.49** |
| HEAq 400 | 4.15 ± 0.59* | 8.09 ± 0.61** |
| HEAl 200 | 4.05 ± 0.55* | 8.78 ± 0.39** |
| HEAl 400 | 4.16 ± 0.40* | 9.94 ± 0.73** |
Data: Mean ± SEM *P < 0.05, **p < 0.01 and (Tuckey-Kramer test) in comparison to control.
3.2. Effect of HEAq and HEAl on humoral antibody formation
The data related to the effect of test extracts on anti-body formation against SRBC can be found to in Table 2. A moderate anti-body titer elevation was observed on both 14th and 21st day of administration in control group. Statistically significant elevation in anti-body log2 titer was found in test extracts administered group when compared to control group. In HEAq 200 mg/kg administered group the anti-body titer increase was found to be higher by 131% on 14th day (p < 0.01) and by 76% on 21st day of sensitization in comparison to the anti-body titer recorded in control group. In HEAq 400 mg/kg administered group the anti-body titer increase was found to be higher by 124% on 14th day (p < 0.05) and by 84% (p < 0.01) on 21st day of sensitization in comparison to the anti-body titre recorded in control group. In HEAl 200 mg/kg treated group the anti-body titre increase was found to be higher by 119% on 14th day (p < 0.05) and by 100% (p < 0.01) on 21st day of sensitization in comparison to the anti-body titer recorded in control group. In HEAl 400 mg/kg administered group the anti-body titer increase was found to be higher by 125% on 14th day (p < 0.05) and by 126% (p < 0.01) on 21st day of sensitization in comparison to the anti-body titer recorded in control group. There was insignificant difference between the response observed in HEAq and HEAl treated groups.
Table 2.
Effect of Helicanthes elasticus whole plant extracts on percentage increase in right paw volume of Wistar Albino rats at various time intervals after the injection of the antigen.
| Groups | 3rd h | 24th h |
|---|---|---|
| Control | 56.75 ± 11.93 | 20.18 ± 06.18 |
| HEAq 200 | 30.58 ± 07.03 | 21.11 ± 05.11 |
| HEAq 400 | 42.23 ± 10.49 | 37.13 ± 09.91 |
| HEAl 200 | 41.28 ± 09.52 | 21.11 ± 05.11 |
| HEAl 400 | 16.62 ± 19.25 | 38.88 ± 04.32 |
3.3. Histopathology of spleen
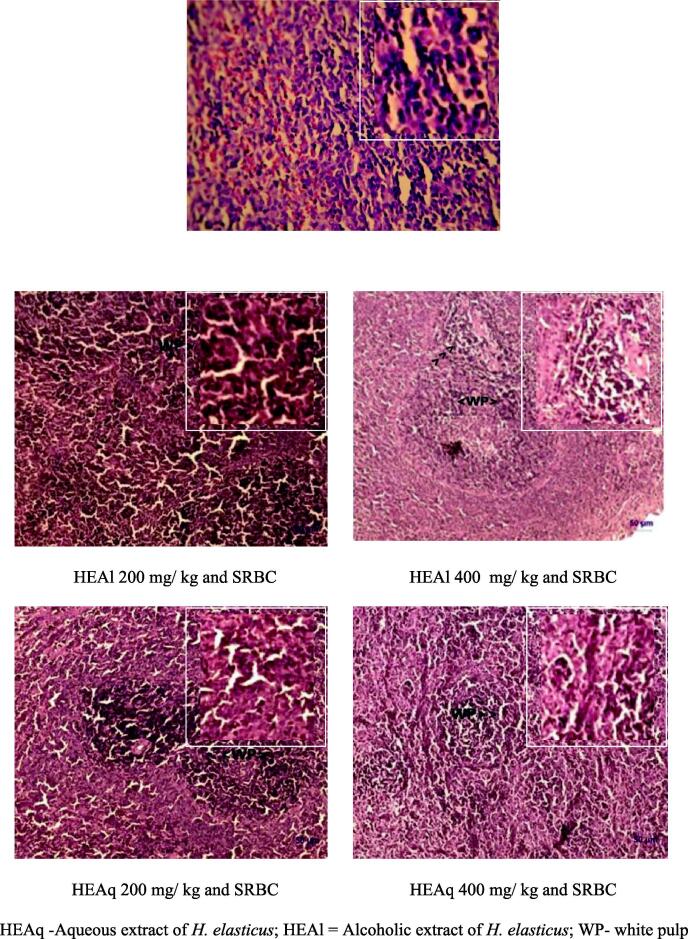
Spleen tissue of SRBC control group rats exhibited normal structure. In test extracts treated SRBC sensitized rats, features of stimulation in the form of increased proportion of white pulp and general increased cellularity was observed (Fig. 1).
Fig. 1.
Histopathology of rat spleen in humoral immunity model by administration of extracts of Helicanthes elasticus.
3.4. Histopathology of lymph node
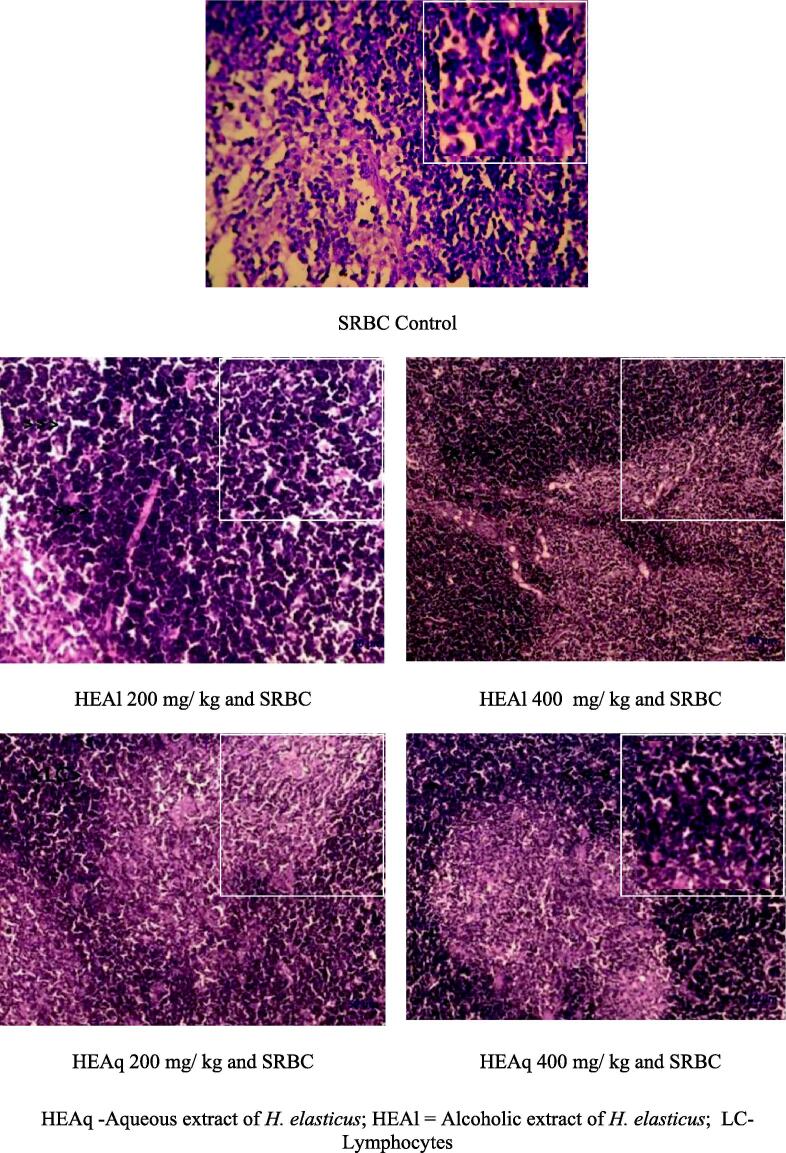
Lymph node tissue of SRBC control group rats exhibited normal structure. In test extracts treated SRBC injected rats, features of increased activity in the form of increased cellularity and formation of distinct germinal center was observed (Fig. 2).
Fig. 2.
Histopathology of rat lymph node in humoral immunity model by administration of extracts of Helicanthes elasticus.
4. Discussion
Humoral anti-body response is mediated by antibody produced by B-lymphocytes. The influence of the test extracts was assessed for immunomodulation activity by noting their effect on antibody formation against SRBC. The results obtained indicate that the test extracts possess antibody formation stimulation propensity without significantly modifying cell mediated immunity evaluated through studying the effect on immunological edema.
Careful analysis of the results clearly shows that both the test extracts increase the antibody formation against SRBC at both the dose level studied. It seems both of the test extracts contain common single or group of active principles that contain anti-body stimulation property. Chemical analysis of the extracts shows presence of phenolic compounds (Sunil Kumar et al., 2015). Modulation of immune functions by polyphenols reveals a variety of mechanistic bases that include both down and up-regulation. Polyphenols have been reported to produce both immunosuppression and immunostimulation (Alejandro et al., 2013). Proanthocyanidins, polymeric polyphenols from grape, green-tea polyphenols, epigallocatechingallate, quercetin, resveratrol, catechin-polysaccharide complex in green tea extract are some of the important examples of polyphenols possessing immunomodulation activity (Vinardell and Montserrat, 2008). It would be interesting to characterize the polyphenol fractions in the test extracts to delineate their structure and ascertain their effect on immunity.
Effect on innate immunity- like activation of macrophage activity, activation of NK function, and increase in the activity of other phagocytosing cells were not studied in this research. Considering that the test extracts possess significant anti-body formation stimulation effect it would be interesting to ascertain what would be their effect on the above parameters. The results would indicate whether the extracts produce immune stimulation by a general mechanism of action or by specific modulation of formation and activity of some of the critical cytokines involved in the observed effect.
A SRBC shot get diffused in the extra vascular space and penetrate the lymph node through lymphatic channels. During a primary response, IgM is secreted, often followed by a switch to increased proportion of Ig (Goldsby et al., 2003, Dale and Formanj, 1989). The amount of secondary antibody response to the antigen is increased in terms of antibody production. Effect of test extracts was assessed for both primary and secondary responses - both of them were found to be significantly increased.
The observed immunopotentiation involving anti-body formation is likely to involve influence on specific cytokines. Thus, it is clear that cytokines play important role in regulating different aspects of immune responses. Another aspect which is required to be taken into consideration is that stimulation of multiple receptors may exhibit synergistic effect in cytokine production (Jacqueline and Bryony, 2001).
The active principles present in the test extracts seems to influence the Th-2 pathway to enhance the anti-body formation without modulating the Th1 pathway which is involved in the expression of cell mediated immunity pathway. Normally the drug which stimulates anti-body formation is likely to suppress CMI by stimulating formation of IL4 while down regulating formation of IL12 which is involved in the activation of CMI pathway. The active principles in the test extracts seems to act in a complex manner which do not significantly interfere with the formation and activity of IL12 while stimulating the formation of IL4 which is involved in activation of Th2 pathway responsible for anti-body formation. However, the exact mechanism can be elucidated only through detailed specific studies involving measurement of different types of cytokines in both in vivo and in vitro conditions.
Analysis of the data showed that the test extracts, as explained above, do not influence cell mediated immunity (CMI) to significant extent. CMI involves effectors mechanism initiated by T lymphocytes and their products (lymphokines) (Lele, 2001). It seems that the test extracts do not modulate the above processes to significant extent.
The plant is a rich source of various kind of phytochemical moieties. Important among them are gallic acid, ethyl gallate, phytosterols, triterpenoids and other phenolics. Review of the literature shows that some of these components have immunomodulatory activity. Yadav et al. (2012) have shown in their study involving QSAR docking studies with respect to immunomodulation activity that the gallic acid derivatives have strong binding affinity for the INF-α-2, IL-6 and IL-4. The authors have suggested on the basis of their analysis one of the derivatives G-7 exhibits marked immunomodulatory activity which is similar to that of levamisole. In another study by Takatoshi et al. (1999), gallate were found to inhibit cytokine induced activation of Nuclear Translocation Factor (NF-kB) which plays important role in inflammation of immunological origin. Ethyl and methyl gallates have been shown to remarkably suppress the secretion of IL-4, IL-5, Th-2 cytokines. The other gallic acid derivatives were found to suppress the secretion of both both IL-4 and IFN-ƛ. The authors of this study (Kei et al., 2001) have concluded that ethyl gallate as the most selective inhibitor of Th2 cytokines among the studied derivatives. Phytosterols are also known to modulate immune function- β-sitosterol and one of its glycosides has been reported to target specific T-helper lymphocytes (Th1 and Th2) helping to normalize their function (Patrick and Lamprecht, 1999). It was also observed that they also normalize DHEA-cortisol ratio. From the above it becomes clear that the plant contains potent immunomodulatory active principles. Their exact role in combination form to enhance the anti-body response to SRBC remains to be elucidated. It can be suggested that the combination may favor up-regulation of formation of cytokines which favor Th2 dependent immune reactions which are important for the anti-body formation.
5. Conclusion
Helicanthes elasticus (Desv) Danser has shown marked immune modulatory activity against SRBC induced antibody formation. Repeated administration of alcoholic and aqueous extract of Helicanthes elasticus significantly increased antibody formation at both dose levels. We could observe only mild to moderate elevation in the immunological paw edema at higher dose of Helicanthes elasticus extract during 21st day after sensitization. Histopathological examination of spleen tissues revealed that there was marked increase in the white pulp of mass and in lymph node there was increased cellularity and formation of distinct germinal cells. Helicanthes elasticus holds potential immunomodulatory activity especially humoral immunity without significant modification in cell mediated immunity.
Acknowledgement
Authors are highly grateful to the Director General, CCRS, Chennai for providing facilities. Help rendered by Manjula, Indu and Ann are thankfully acknowledged. The authors acknowledge King Saud University, Riyadh, Saudi Arabia, for funding this work through Researchers Supporting Project number (RSP-2020/11).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
K.N. Sunil Kumar, Email: kn.sunil@gov.in.
Hak-Jae Kim, Email: hak3962@sch.ac.kr.
References
- Agarwal S.S., Saurabh C.K., Gokul S.T. Studies on immunomodulatory activity of Capparis zeylanica leaf extracts. Int. J. Pharm. Sci. Nanotech. 2010;3:887–892. [Google Scholar]
- Alejandro C., Nicolás S., Luis A.S., Dulcineia S.P.A. Modulation of immune function by polyphenols: possible contribution of epigenetic factors. Nutrients. 2013;5:2314–2332. doi: 10.3390/nu5072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt, B., 2004. Biological and pharmacological properties of Viscum album L. - from tissue flask to man. In: Mistletoe, The genus Viscum. Arndt Büssing (Ed.). The Netherlands, The Gordon and Breach Publishing Group, pp. 123–82.
- Dale M.M., Formanj C. second ed. Blackwell Scientific Publication; Oxford: 1989. Text-book of Immunopharmacology. [Google Scholar]
- Fernández, T.B., Varela, B.G., Taira, C.A., Ricco, R.A., Gurni, A.A., Hajos, S.E., Alvarez, E.M.C., Wagner, M.L., 2004. Mistletoes from Argentina – Ligaria cuneifolia var. cuneifolia as a substitute for the European mistletoe (Viscum album L.). In: Mistletoe, The Genus Viscum. Arndt Büssing (ed). The Netherlands, The Gordon and Breach Publishing Group, 61–72.
- Goldsby, R.A., Kindt, T.J., Osborne, B.A., Kuby, J., 2003. Immunology, fifth ed. W. H. Freeman and Co, New York, p. 1–25.
- Jacqueline P., Bryony C. An overview of the immune system. The Lancet. 2001;357:1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- Jadhav N., Patil C.R., Chaudhari K.B., Wagh J.P., Surana S.J., Jadhav R.B. Diuretic and natriuretic activity of two mistletoe species in rats. Pharmacogn. Res. 2010;2(1):50–57. doi: 10.4103/0974-8490.60576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi A.A., Wankhade P.P., Patil S.M., Umbare R.P. Phytochemical analysis, chemical characterization and antioxidant activity of Helicanthus elastica Desr. Ind. Amer. J. Pharm. Sci. 2018;05(02):1159–1173. [Google Scholar]
- Kei K., Shozo Y., Susumu K., Satoshi T. Effect of gallic acid derivatives on secretion of Th1 cytokines and Th2 cytokines from anti CD3-stimulated spleen cells. Yakugaku Zasshi. 2001;121:451–457. doi: 10.1248/yakushi.121.451. [DOI] [PubMed] [Google Scholar]
- Lele, R.D., 2001. Ayurveda and modern medicine. IInd ed, Mumbai, Bharatiya Vidya Bhavan, p. 475.
- Mary, K.T., 1995. Anticancer activity of medicinal plants. Ph.D. Thesis. Amala Cancer Research Centre, Mahatma Gandhi University, Thrissur.
- Patrick J.D.B., Lamprecht J.H. Plant sterols and sterolins: a review of their immune-modulating properties. Altern. Med. Rev. 1999;4:170–177. [PubMed] [Google Scholar]
- Patwardhan B., Kalbag D., Patki P.S., Nagasampangi B.A. Search of immunomodulatory agents – a review. Indian Drugs. 1991;28:249–254. [Google Scholar]
- Sehar I., Kaul A., Bani S., Pal H.C., Saxena A.K. Immune up-regulatory response of a non-caloric natural sweetener, stevioside. Chem.-Biol. Interact.. 2008;173:115–121. doi: 10.1016/j.cbi.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Sunil Kumar K.N., Rajakrishnan R., Thomas J., Aadinaath R.G. Hepatoprotective effect of Helicanthes elasticus. Bangladesh J. Pharmacol. 2016;11:525–530. [Google Scholar]
- Sunil Kumar K.N., Saraswathy A., Amerjothy S., Balakrishna K. Phytochemical examination of compounds from mango mistletoe – Helicanthes elasticus (Desr.) Danser. Indian J. Chem.-B. 2015;54(B):924–929. [Google Scholar]
- Sunil Kumar K.N., Saraswathy A., Amerjothy S., Ravishankar B. Antimicrobial potential of Helicanthes elasticus (Desr.) Danser growing on mango (Mango mistletoe) J. Trad. Complem. Med. 2014;4:258–262. doi: 10.4103/2225-4110.126183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatoshi M., Noriaki K., Tadashi H., Yusuke S., Yoshinori N., Ichiro T., Toru K. Gallates inhibit cytokine-induced nuclear translocation of NF-κB and expression of leukocyte adhesion molecules in vascular endothelial cells. Arterioscler., Thromb. Vasc. Biol. 1999;19:1412–1420. doi: 10.1161/01.atv.19.6.1412. [DOI] [PubMed] [Google Scholar]
- Vaghasiya J., Datani M., Nandkumar K., Malaviya S., Jivani N. Comparative evaluation of alcoholic and aqueous extracts of Ocimum sanctum for immunomodulatory activity. Int. J. Pharmaceut. Biol. Res. 2010;1:25–29. [Google Scholar]
- Vinardell M.P., Montserrat M. Immunomodulatory effects of polyphenols. Ele. J. Envir. Agric. Food Chem. 2008;7:3356–3362. [Google Scholar]
- Wagner H., Proksch A. Vol. 1. Academic Press; London: 1985. Immunomodulatory drugs of fungi and higher plants; pp. 113–153. (Economic and Medicinal Plant Research). [Google Scholar]
- Yadav D.K., Khan F., Negi A.S. Pharmacophore modelling, molecular docking, QSAR, and in silico ADMET studies of gallic acid derivatives for immunomodulatory activity. J. Mol. Model. 2012;18:2513–2525. doi: 10.1007/s00894-011-1265-3. [DOI] [PubMed] [Google Scholar]