Abstract
This study was aimed to analyze the anti-cancer activity of silver nanoparticles (AgNPs) synthesized using aqueous plant extracts from the rhizome of Curcuma longa and Zingiber officinale. Synergistic aqueous extract of rhizome of C. longa and Z. officinale was used to green synthesis of AgNPs. Characterization of AgNPs was performed using UV–visible spectroscopy, FTIR, X-ray diffraction, TEM, and SEM analyses. Anti-cancer activity of AgNPs against human colon carcinoma (HT-29) cells was tested using MTT assay. UV–Visible spectroscopy analysis indicated the surface plasmon resonance (SPR) sharp peak at 350–430 nm wavelength that corresponds to the production of AgNPs. FTIR analysis reveals that existence of carboxyl (—C O) and amine (N—H) functional groups in the AgNPs. The X-ray diffraction analysis confirms four spectral peaks at 111, 200, 220, and 311. SEM analysis showed that AgNPs are in a spherical shape with a size of 42–61 nm and TEM analysis showed particle size are ranged between 20–51 nm. Anti-cancer study reveals that AgNPs had shown cytotoxicity against HT-29 cells at the concentrations ranged from 25 to 500 μg/mL and IC50 at 150.8 µg/mL. This study concludes that AgNPs synthesized using rhizome of Z. officinale and C. longa possesses potential anti-cancer activity.
Keywords: Silver nanoparticles, Characterizations, Curcuma longa, Zingiber officinale, Anti-colon cancer activity
1. Introduction
Medicinal plants are being used for human health care management in traditional medicine for centuries. High demand in the products from medicinal plants increased the commercial production and formulation of various herbal-based cosmetics and nutritional supplements (Ekor. 2014). Zingiberaceae family has around 52 plant genera and more than 1300 plant species, and also, many of these plant products were in use for various human health benefits (Kress et al., 2002). Some of the Zingiberaceae plants were reported for their therapeutic usage (Basak et al., 2010). Among those, Curcuma longa and Zingiber officinale are the most important medicinal plants, and they have possessed a variety of medicinal values. C. longa is an aromatic plant, commonly known as turmeric. In Ayurveda and Siddha, the vegetal root of C. longa has been used for the production of various pharmaceutical formulations for the different ailments, including wounds, acne, common cold, parasitic infections, urinary tract infection, and liver diseases (Chainani-Wu, 2003). This plant also possesses various important medicinal properties like antioxidant, anti-inflammatory, antibacterial, anti-human immunodeficiency virus properties (Araújo and Leon, 2011, Boaz et al., 2011). Polyphenol curcumin is a vital phytocompound responsible for the pharmacodynamics action of C. longa (Zhang et al., 2012, Sharma et al., 2005). Mainly, it has potential anti-cancer property against liver, pancreatic, colon, cervical, lung, brain, breast, and bone cancers (Maheshwari et al., 2006, Bar-Sela et al., 2010).
Another most important medicinal plant is Z. officinale, generally called as Ginger. The rhizome of this plant is widely used as a nutraceutical and in the formulation of folk medicine. In Ayurvedic and Chinese traditional systems of medicines, Z. officinale has being used for treating indigestion, arthritis, rheumatism, fever, and microbial infections (Darvesh et al., 2012). Asgingerols, zingerone, shogaols, paradols, and gingerdiols are the major phytochemical constituents that have been reported for its antioxidant, anti-inflammatory, antihyperglycemic, immunomodulatory, anti-cancer, and cardioprotective properties (Shehzad et al., 2013, Scalbert et al., 2005).
Medicinal plants based synthesis of nanoparticles has various biological benefits since they have no toxic chemicals and biological compounds as capping agents (Garima et al., 2011). Plant-based synthesized AgNPs are having strong antimicrobial activity and they are widely used as an ingredient in the pharmaceutical industry for preparation of human health care medicines (Manach et al., 2004, Ali et al., 2008). Biological synthesis of NPs is an environmental friendly method than chemical, electrochemical (Nicoll and Henein, 2009), radiation (Li et al., 2012), photochemical methods (Rhode et al., 2007). The biological and medicinal properties of NPs are mostly determined by various physical properties like particle size, structure, and crystallinity composition. AgNPs are interacting with cells and regulate active and passive cellular responses. AgNPs also cause DNA damage and chromosomal aberrations at a low concentration without toxicity, especially no genotoxicity effects on human cells (Zhang et al., 2016a, Zhang et al., 2016b, Gurunathan et al., 2018). Since the usage of AgNPs as a drug carrier in cancer treatment has recently gained considerable attention (Brahmbhatt et al., 2013). In this study, AgNPs synthesized using synergistic aqueous extracts of the rhizome of Z. officinale and C. longa were used for analyzing in vitro anti-cancer activity against human colon carcinoma (HT-29) cells.
2. Materials and methods
2.1. Collection of plant and preparation of extracts
The rhizome of Z. officinale and C. longa was collected from the region of Tiruvallore District, Tamil Nadu, and India. Soil and other surface contaminants present on fresh rhizome were removed using tap water followed by distilled water. Further, the rhizome was air-dried and macerated to make a fine powder. Followed by 5 g of mixed rhizome powder of both the Z. officinale and C. longa was added into 250 mL of distilled water and boiled for 30 min. After cooling to room temperature, the extract was centrifuged at 5000 rpm, and filtered using Whatman number-1 filter paper (20–24 μm). Filtered extract was further used for green synthesis of AgNPs.
2.2. Green synthesis of AgNPs using rhizome extracts
The prepared extract was utilized for the synthesis of AgNPs. 20 mL of 1 M AgNO3 solution was added into 80 mL of rhizome extract. Then extract along with optimized AgNPs was incubated until the colorless solution turns into brownish color, which reveals the reduction of Ag+ into Ag0 nanoparticle. The visible color change was observed at a 1:9 ratio.
2.3. Characterization of AgNPs
UV spectrum analysis was estimated using UV-1800 spectrophotometer (Shimadzu, Japan) with the wavelength range from 200 to 800 nm. AgNPs were further characterized using FTIR to identify the functional groups, dynamic light scattering (DLS) particle size analyzer, XRD for elemental composition, TEM, and SEM for identification of morphology and size of biosynthesized AgNPs (Karuppiah et al., 2014).
2.4. In-vitro cytotoxic activity of AgNPs by MTT assay
The cytotoxicity activity of AgNPs was performed using MTT assay (Kleemann, 1993) against HT29 cells. In this method, 200 µL HT29 cells, roughly 1 × 104 cells/well, were seeded into 96 well plates and permitted to achieve confluence cell growth. After cells were completely attached to the well, culture media was discarded. Further, 100 µL of various concentrations such as 25, 50, 100, 250, and 500 µg/mL of AgNPs were added and incubated for 24 h and followed for 48 and 72 h. After that, freshly prepared MTT [5 mg/mL of phosphate buffer solution] was added and incubated at 37 °C for 4–6 h.
3. Results and discussion
3.1. Visual analysis of AgNPs
Several studies evaluated the green synthesis of AgNPs by different medicinal plant aqueous extracts for increasing various biological applications (Li et al., 2007). It was observed that NPs synthesis was initiated once the synergistic aqueous extracts of Z. officinale and C. longa added in the 1 mM AgNO3 solution. The color of AgNPs and Z. officinale and C. longa aqueous solutions was progressively modified from yellow to brownish color which reveals the AgNPs formation. The color transformation during the synthesis of AgNPs is related to the excitation effect of surface plasmon resonance (Mittal et al., 2014, Balaraman et al., 2020). The current study investigation reveals that AgNPs were synthesized using rhizome extracts and the phytocompounds in the extracts of Z. officinale and C. longa have potential for acting as a reducing agent.
3.2. UV–visible spectroscopy analysis
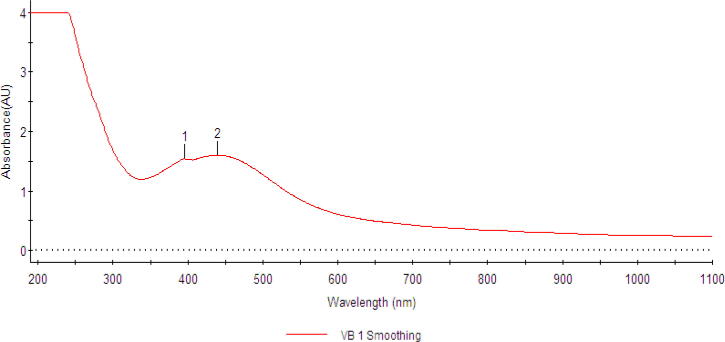
UV–visible spectroscopy was used to observe the AgNPs formation by the reduction of Ag ions through the exposure of plant extracts (Cittrarasu et al., 2019). UV–Visible spectroscopy indicated the surface plasmon resonance (SPR) sharp peak at 350–430 nm wavelength, which corresponds to the AgNPs production. AgNPs were absorbed radiation at 400 nm wavelength due to the transition of electrons. Colloidal AgNPs exhibit absorption wavelength at 390–420 nm (Naik et al., 2002). The SPR peak explained that NPS synthesized were poly-dispersed environmental particles (Firdhouse & Lalitha, 2015). AgNPs synthesis from various medicinal plants such as Ocimum tenuiflorum, Centella asiatica, and Clonorchis sinensis extracts and observed the absorbance at 420 nm using UV-spectrophotometer (Chung et al., 2016). In a study, maximum absorbance was reported at 440 nm in the NPs synthesis using Plumbago zeylanica and 400 nm in the Catharanthus roseus (Nayak et al., 2016). In our study, the spectrum analysis peak data [Fig 1, Fig 2] showing that the silver synthesized product is only AgNPs. In a study, AgNPs were effectively synthesized using C. longa extract, then Z. officinale even at higher concentrations, and NPs are stable (Majeed et al., 2016).
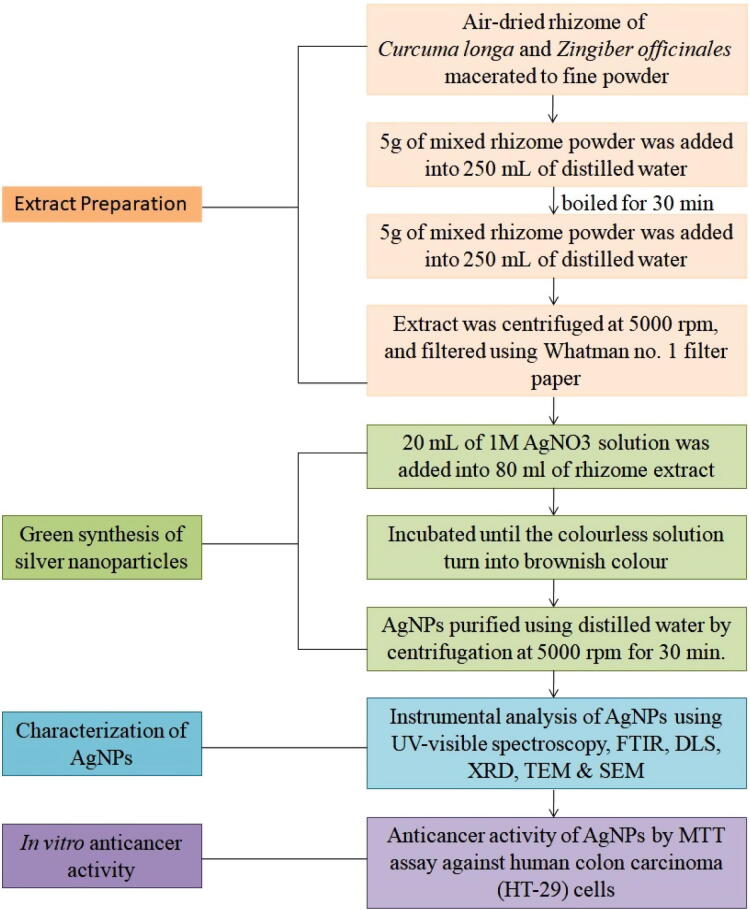
Fig 1.
Flow chart of green synthesis of silver nanoparticles and anti-cancer study.
Fig 2.
UV–Vis absorption spectra of green synthesized AgNPs.
3.3. FTIR analysis
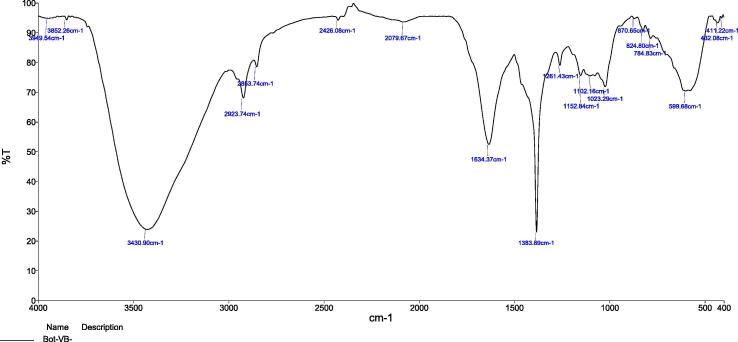
FTIR analyzed data of AgNPs and Z. officinale, and C. longa rhizome extracts are shown in [Fig 1, Fig 3]. FTIR characterization was used to examine the possible functional molecules, FTIR spectrum of the Z. officinale and C. longa rhizome extracts showed a major absorption peak at 1387 cm−1 in the synthesis of AgNPs (Logeswari et al., 2015). A report revealed that the presence of absorption peak at 1055 cm−1, which may have been attributed to vibration of (—C O) and amine (N—H) groups (Jyoti et al., 2020, Kalaimurugan et al., 2019). The absorption peak appeared at 1459 cm−1 was specific for the vibration of proteins being a stabilizing agent through free amine groups or cysteine groups (Pirtarighat et al., 2019). In another study, FTIR spectra results identified the presence of amide groups in the fruit shell extract, and they were found to be involved in the reduction of silver ions to AgNPs. Additionally, plant phytocompounds perform double role functions (Gomathi et al., 2020).
Fig 3.
FTIR spectra of green synthesized AgNPs.
3.4. Dynamic light scattering (DLS) studies
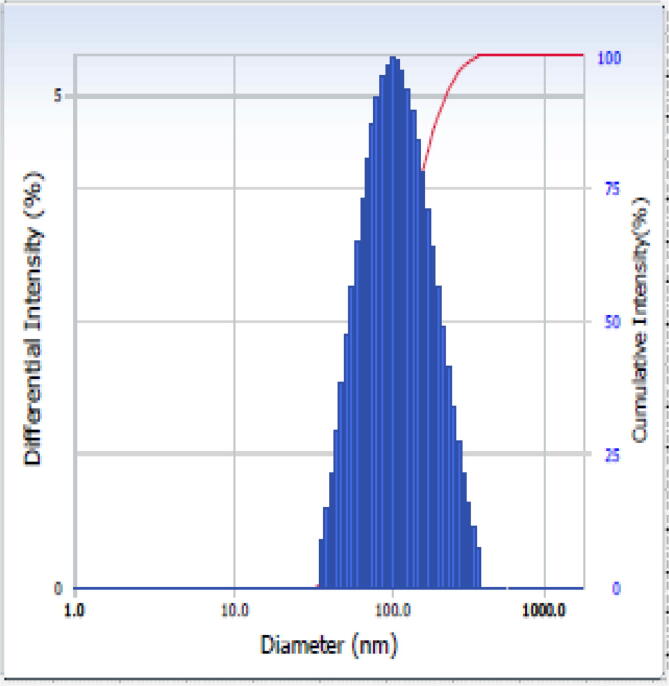
DLS technique makes use of particle size analysis of colloidal solution upon irradiating with the light source. The measurement condition was maintained at temperature 25 °C. From the DLS histogram [Fig 1, Fig 4], the average particle size is estimated to be 76.4 nm with a polydispersity index 0.325 and diffusion coefficient 6.438 x108 cm2/s, respectively.
Fig 4.
Dynamic Light Scattering (DLS) plot for green synthesized AgNPs.
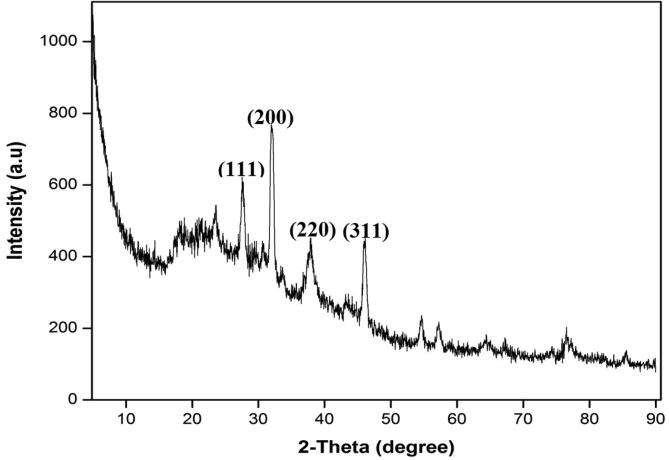
3.5. XRD analysis
Vetrivel et al. (2019) synthesized crystalline AgNPs through green synthesis using Ceropegia bulbosa Roxb root tuber extract and observed XRD diffraction peaks at the 111, 200, 220, and 311 planes, which correspond to the AgNPs. Synthesized AgNPs were also confirmed for their antibacterial and larvicidal activity. In this study, the X-ray diffraction (XRD) patterns of the dried synergistic powder sample of green synthesized AgNPs had shown distinct four diffraction peaks at 2θ angles of 38°, 44°, 64°, and 77°, which can be endorsed to the reflections from lattice planes indexed to the (1 1 1), (2 0 0), (2 2 0), and (3 1 1) planes, reflected the crystal structures [Fig 1, Fig 5] (Ali et al., 2016, Paulkumar et al., 2014, Shankar et al., 2004) of AgNPs.
Fig 5.
XRD spectrum of green synthesized silver nanoparticles.
3.6. SEM analysis
SEM image provides morphological characteristics and size measurement of synthesized AgNPs [Fig 1, Fig 6] as reported by Rautela et al., (2019). The SEM size was examined the range of 41.91to 60.91 nm. A study reported that AgNPs synthesized using Z. officinale extracts were in spherical shape and size of 30–50 nm (Zhang et al., 2016a, Zhang et al., 2016b). These size variations might be the presence of biomolecules from the rhizome extracts, which were the caping surface of AgNPs. It was observed that AgNPs have a uniform crystalline structure and relatively spherical. Accumulation of NPs was induced by solvent evaporation during the sample production. In a study, SEM analysis of NPs synthesized using C. longa possess that the synthesized nanoparticle metals are spherical in shape throughout in the colloidal solution (Aswathy and Philip, 2012).
Fig 6.
SEM images of green synthesized AgNPs.
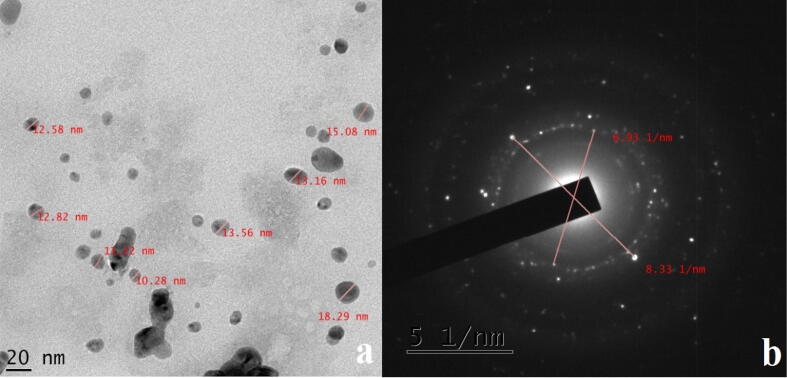
3.7. TEM study
TEM microscopic analysis was used to determine the morphology and size distribution of biosynthesized AgNPs. The particle shape of the AgNPs is spherical, with ranged from 20 to 51 nm [Fig 1, Fig 2, Fig 3, Fig 4, Fig 5, Fig 6, Fig 7]. Consuming the Z. officinale and C. longa extracts during the time lack the decrease of reducing agents, which lead to different size of AgNPs (Chandran et al., 2006, Dubey et al., 2010). In a study, microbial synthesis of AgNPs was carried out using Pseudomonas fluorescens YPS3 and observed the size of NPs as 26 nm. In our study, TEM analysis showed the smallest size of AgNPs synthesized was 20 nm (Kalaimurugan et al., 2019).
Fig 7.
(a) TEM image of green synthesized AgNPs, and its SAED pattern (b) of green synthesized AgNPs.
3.8. In-vitro anticancer activity of AgNPs using MTT assay
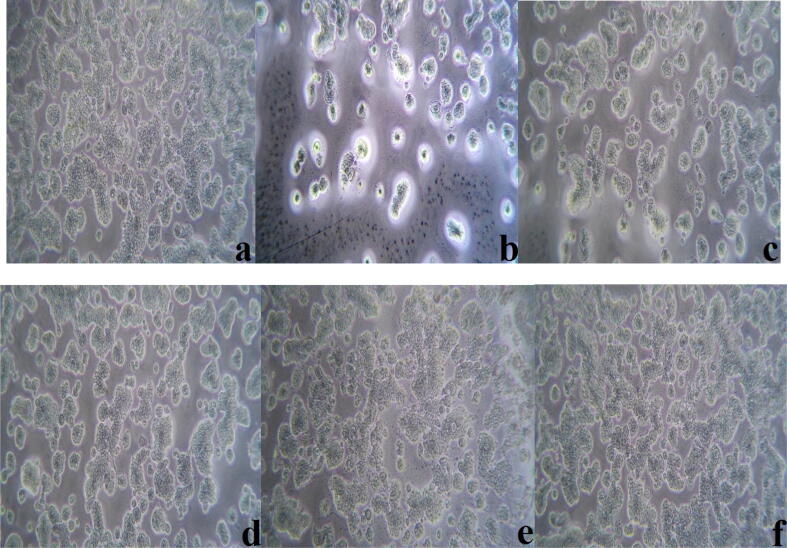
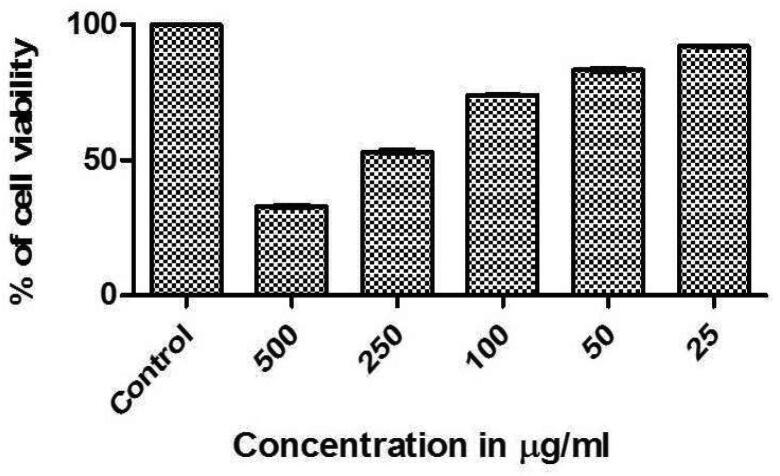
Colorectal cancer is the main primary colon cancer and it is the third most common cancer worldwide (Yin et al., 2003). In this present study, the cytotoxicity of green synthesized AgNPs was analyzed using MTT assay and found that AgNPs showed anti-cancer activity against colon cancer (HT29) cells at the concentration of 25–500 μg/mL. It was noted that cell viability was deceased on the increased concentration of AgNPs [Fig 1, Fig 8]. AgNPs required concentration to reduce the cell viability of HT29 cells to 50% (IC50) of the preliminary population was 150.8 µg/mL. The results showed dose-dependent cytotoxicity of human colon carcinoma cells after 24 h of AgNPs treatment since it was tested at various concentrations such as 25, 50, 100, 250, and 500 μg/mL [Fig 1, Fig 9]. Previous studies in 2013 reported that a natural polyphenol compound found in C. longa inhibits the proliferation of various tumor cells, including breast cancer (Ponarulselvam et al., 2012). This statement supports the finding from the present study for anti-colon cancer activity of AgNPs synthesized using rhizome extracts of C. longa and Z. officinale. Other than medicinal plants, seaweed also studied for AgNPs synthesis with various biological applications. In a study by Balaraman et al. (2020), who synthesized AgNPs from seaweed Sargassum myriocystum aqueous extract and confirmed its anti-cancer activity.
Fig 8.
Anticancer activity of Zingibe officinale and Curcuma longa silver nanoparticles (a) control, (b)500 µg/mL, (c) 250 µg/mL, (d) 100 µg/mL, (e) 50 µg/mL and (f) 25 µg/mL.
Fig 9.
In vitro cytotoxicity effects of green synthesized AgNPs.
4. Conclusion
In this study, synergistic aqueous extracts of rhizome of C. longa and Z. officinale have been used for the green synthesis of AgNPs. The spectral vibration of carboxyl (—C O) and amine (N—H) groups in the Z. officinale and C. longa extracts might be involved in the synthesis of AgNPs. SEM results revealed that AgNPs are in a spherical shape with the size ranging from 42 to 61 nm, and from 20 to 51 nm by TEM analysis. MTT assay reveals that AgNPs had shown good anti-cancer activity against HT29 cells with the IC50 value of 150.8 µg/mL. Based on the study findings, it is concluded that AgNPs synthesized using rhizome of Z. officinale and C. longa have potential anti-cancer properties.
Declaration of Competing Interest
None declared.
Acknowledgement
This study was supported by Rajiv Gandhi National Fellowship (RGNF-2013-14-ST-TAM-48307), University Grants Commission, and New Delhi, India. Authors also extend their appreciation to The Researchers Supporting Project (RSP-2020/20), King Saud University, Riyadh, Saudi Arabia. This research was also supported by the Research Center for Environmental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan from “The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project” by the Ministry of Education (MOE) in Taiwan.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Vinoth Kumar Ponnusamy, Email: kumar@kmu.edu.tw.
P. Agastian, Email: agastian@loyolacollege.edu.
References
- Ali B.H., Blunden G., Tanira M.O., Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food. Chem. Toxicol. 2008;46(2):409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Ali M., Kim B., Belfield K.D., Norman D., Brennan M., Ali G.S. Green synthesis and characterization of silver nanoparticles using Artemisia absinthium aqueous extract - a comprehensive study. Mater. Sci. Eng. C, Mater. Biol. Appl. 2016;58(1):359–365. doi: 10.1016/j.msec.2015.08.045. [DOI] [PubMed] [Google Scholar]
- Araújo C.C., Leon L.L. Biological activities of Curcuma longa L. Mem. Inst. Oswaldo. Cruz. 2011;96(5):723–728. doi: 10.1590/S0074-02762001000500026. [DOI] [PubMed] [Google Scholar]
- Aswathy S., Philip D. Green synthesis of gold nanoparticles using Trigonella foenum–graecum and its size–dependent catalytic activity. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2012;97(1):1–5. doi: 10.1016/j.saa.2012.05.083. [DOI] [PubMed] [Google Scholar]
- Balaraman, P., Balasubramanian, B., Kaliannan, D., Durai, M., Kamyab, H., Park, S., Chelliapan, S., Lee, C.T., Maluventhen, V., Maruthupandian, A., 2020. Phyco‑synthesis of Silver Nanoparticles Mediated from Marine Algae Sargassum myriocystum and Its Potential Biological and Environmental Applications. Waste Biomass Valorization. May 1. 10.1007/s12649-020-01083-5.
- Bar-Sela G., Epelbaum R., Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr. Med. Chem. 2010;17(3):190–197. doi: 10.2174/092986710790149738. [DOI] [PubMed] [Google Scholar]
- Basak S., Sarma G.C., Rangan L. Ethnomedical uses of Zingiberaceous plants of Northeast India. J. Ethnopharmacol. 2010;132(1):286–296. doi: 10.1016/j.jep.2010.08.032. [DOI] [PubMed] [Google Scholar]
- Boaz M., Leibovitz E., Dayan Y.B., Wainstein J. Functional foods in the treatment of type 2 diabetes: olive leaf extract, turmeric and fenugreek, a qualitative review. Funct. Foods. Health. Dis. 2011;1(11):472–481. doi: 10.31989/ffhd.v1i11.114. [DOI] [Google Scholar]
- Brahmbhatt M., Gundala S.R., Asif G., Shamsi S.A., Aneja R. Ginger phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation. Nutr. Cancer. 2013;65(2):263–372. doi: 10.1080/01635581.2013.749925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) J. Altern. Complement. Med. 2003;9(1):161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- Chandran S.P., Chaudhary M., Pasricha R., Ahmad A., Sastry M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006;22(2):577–583. doi: 10.1021/bp0501423. [DOI] [PubMed] [Google Scholar]
- Chung I.M., Park I., Seung-Hyun K., Thiruvengadam M., Rajakumar G. Plant-mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications. Nanoscale. Res. Lett. 2016;11(1):40. doi: 10.1186/s11671-016-1257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cittrarasu V., Balasubramanian B., Kaliannan D., Park S., Maluventhan V., Kaul T., Liu W.C., Arumugam M. Biological mediated Ag nanoparticles from Barleria longiflora for antimicrobial activity and photocatalytic degradation using methylene blue. Artif. Cell. Nanomed. B. 2019;47(1):2424–2430. doi: 10.1080/21691401.2019.1626407. [DOI] [PubMed] [Google Scholar]
- Darvesh A.S., Aggarwal B.B., Bishayee A. Curcumin and liver cancer: a review. Curr. Pharm. Biotechnol. 2012;13(1):218–228. doi: 10.2174/138920112798868791. [DOI] [PubMed] [Google Scholar]
- Dubey S.P., Lahtinen M., Sarkka H., Sillanpaa M. Bioprospective of Sorbus aucuparia leaf extract in development of silver and gold nanocolloids. Colloids Surf. B. Biointerfaces. 2010;80(1):26–33. doi: 10.1016/j.colsurfb.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;10:4–177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firdhouse, M.J., Lalitha, P., 2015. Biosynthesis of silver nanoparticles and its applications. J. Nanotechnol. (1) 10.1155/2015/829526.
- Garima S., Riju B., Kunal K., Ashish R.S., Rajendra P.S. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J Nanopart Res. 2011;13(7):2981–2988. doi: 10.1007/s11051-010-0193-y. [DOI] [Google Scholar]
- Gomathi A.C., Xavier Rajarathinam S.R., Mohammed Sadiq A., Rajeshkumar S. Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J. Drug. Deliv. Sci. Tec. 2020;55(2020) doi: 10.1016/j.jddst.2019.101376. [DOI] [Google Scholar]
- Gurunathan S., Qasim M., Park C., Yoo H., Kim J.H., Hong K. Cytotoxic potential and molecular pathway analysis of silver nanoparticles in human colon cancer cells HCT116. Int. J. Mol. Sci. 2018;19(8):22–69. doi: 10.3390/ijms19082269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyoti K., Singh A., Fekete G., Singh T. Cytotoxic and radiosensitizing potential of silver nanoparticles against HepG-2 cells prepared by biosynthetic route using Picrasma quassioides leaf extract. J. Drug. Deliv. Sci. Tec. 2020;55(2020):101–479. doi: 10.1016/j.jddst.2019.101479. [DOI] [Google Scholar]
- Kalaimurugan D., Vivekanandhan P., Sivasankar P., Durairaj K., Senthilkumar P., Shivakumar M.S., Venkatesan S. Larvicidal activity of silver nanoparticles synthesized by Pseudomonas fluorescens YPS3 isolated from the Eastern Ghats of India. J. CLUST. SCI. 2019;30(1):225–233. doi: 10.1007/s10876-018-1478-z. [DOI] [Google Scholar]
- Karuppiah C., Palanisamy S., Chen S., Emmanuel R., Ali M.A., Muthukrishnan P., Prakash P., AlHemaid F.M.A. Green biosynthesis of silver nanoparticles and nanomolar detection of pnitrophenol. J. Solid. State. Electro. chem. 2014;18(1):1847–1854. doi: 10.1007/s10008-014-2425-z. [DOI] [Google Scholar]
- Kleemann W. Random-field induced antiferromagnetic, ferroelectric and structural domain states. Inter. J. Modern. Phy. B. 1993;7(13):24–69. doi: 10.1142/S0217979293002912. [DOI] [Google Scholar]
- Kress W.J., Prince L.M., Williams K.J. The phylogeny and a new classification of the gingers (Zingiberaceae): evidence from molecular data. Am. J. Bot. 2002;89(10):1682–1696. doi: 10.3732/ajb.89.10.1682. [DOI] [PubMed] [Google Scholar]
- Li S., Shen Y., Xie A., Yu X., Qiu L., Zhang L., Zhang Q. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green. Chem. 2007;9(8):852–858. doi: 10.1039/B615357G. [DOI] [Google Scholar]
- Li Y., Tran V.H., Duke C.C., Roufogalis B.D. Preventive and protective properties of Zingiber officinale (ginger) in diabetes mellitus, diabetic complications, and associated lipid and other metabolic disorders: a brief review. Evid. Based. Complement. Altern. Med. 2012;2012(1):516–870. doi: 10.1155/2012/516870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logeswari P., Silambarasan S., Abraham J. Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi. Chem. Soc. 2015;19(3):311–317. doi: 10.1016/j.jscs.2012.04.007. [DOI] [Google Scholar]
- Maheshwari R.K., Singh A.K., Gaddipati J., Srimal R.C. Multiple biological activities of curcumin: a short review. Life. Sci. 2006;78(18):2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Majeed A., Ullah W., Anwar A.W., Shuaib A., Ilyas U., Khalid P., Mustafa G., Junaid M., Faheem B., Ali S. Cost-effective biosynthesis of silver nanoparticles using different organs of plants and their antimicrobial applications: a review. Mater. Technol. 2016;33(5):1–8. doi: 10.1080/10667857.2015.1108065. [DOI] [Google Scholar]
- Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nut. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Mittal A.K., Bhaumik J., Kumar S., Banerjee U.C. Biosynthesis of silver nanoparticles: Elucidation of prospective mechanism and therapeutic potential. J. Colloid. Interface. Sci. 2014;415(1):39–47. doi: 10.1016/j.jcis.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Naik R.R., Stringer S.J., Agarwal G., Jones S., Stone M.O. Biomimetic synthesis and patterning of silver nanoparticles. Nature. Mater. 2002;1(3):169–172. doi: 10.1038/nmat758. [DOI] [PubMed] [Google Scholar]
- Nayak D., Ashe S., Rauta P.R., Kumari M., Nayak B. Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C. 2016;58(1):44–52. doi: 10.1016/j.msec.2015.08.022. [DOI] [PubMed] [Google Scholar]
- Nicoll R., Henein M.Y. Ginger (Zingiber officinale Roscoe): a hot remedy for cardiovascular disease? Int. J. Cardiol. 2009;131(3):408–409. doi: 10.1016/j.ijcard.2007.07.107. [DOI] [PubMed] [Google Scholar]
- Paulkumar K., Gnanajobitha G., Vanaja M., Rajeshkumar S., Malarkodi C., Pandian K., Annadurai G. Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens. Sci. World. J. 2014;2014(10):1–9. doi: 10.1155/2014/829894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirtarighat S., Ghannadnia M., Baghshahi S. Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nano. Struct. Chem. 2019;9(1):1–9. doi: 10.1007/s40097-018-0291-4. [DOI] [Google Scholar]
- Ponarulselvam S., Panneerselvam C., Murugan K., Aarthi N., Kalimuthu K., Thangamani S. Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian. Pac. J. Trop. Bio. 2012;2(7):574–580. doi: 10.1016/S2221-1691(12)60100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautela A., Rani J., Debnath M. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019;10(1):5. doi: 10.1186/s40543-018-0163-z. [DOI] [Google Scholar]
- Rhode J., Fogoros S., Zick S., Wahl H., Griffith K.A., Huang J., Liu J.R. Ginger inhibits cell growth and modulates angiogenic factors in ovarian cancer cells. BMC. Complement. Altern. Med. 2007;7(1):44. doi: 10.1186/1472-6882-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalbert A., Johnson I.T., Saltmarsh M. Polyphenols: antioxidants and beyond. Am. J. Clin. Nutr. 2005;81(1):215–217. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- Shankar S.S., Rai A., Ahmad A., Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid. Interface. Sci. 2004;275(2):496–502. doi: 10.1016/j.jcis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Sharma R.A., Gescher A.J., Steward W.P. Curcumin: the story so far. Eur. J. Cancer. 2005;41(13):1955–1968. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Shehzad A., Lee J., Lee Y.S. Curcumin in various cancers. BioFactors. 2013;39(1):56–68. doi: 10.1002/biof.1068. [DOI] [PubMed] [Google Scholar]
- Vetrivel C., Balamuralikrishnan B., Durairaj K., Sungkwon P., Velmurugan P., Ragavendran C., Sigamani S., Maruthupandian A. Fabrication and characterization of noble crystalline silver nanoparticles from Ceropegia bulbosa Roxb root tuber extract for antibacterial, larvicidal and histopathology applications. Nanosci. Nanotechnol. 2019;11(1):11–21. doi: 10.1166/nnl.2019.2845. [DOI] [Google Scholar]
- Yin B., Ma H., Wang S., Chen S. Electrochemical Synthesis of Silver Nanoparticles under Protection of Poly (N-vinylpyrrolidone) J. Phy. Chem. B. 2003;107(34):88–98. doi: 10.1021/jp0349031. [DOI] [Google Scholar]
- Zhang X., Chibli H., Kong D., Nadeau J. Comparative cytotoxicity of gold–doxorubicin and InP–doxorubicin conjugates. Nanotechnology. 2012;23(27):103–275. doi: 10.1088/0957-4484/23/27/275103. [DOI] [PubMed] [Google Scholar]
- Zhang X.-F., Liu Z.-G., Shen W., Gurunathan S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016;17:15–34. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.F., Liu Z.G., Shen W., Gurunathan S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016;17(9):15–34. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- Haniadka R., Rajeev A.G., Palatty P.L., Arora R., Baliga M.S. Zingiber officinale (ginger) as an anti-emetic in cancer chemotherapy: a review. J. Altern. Complement. Med. 2012;18(5):440–444. doi: 10.1089/acm.2010.0737. [DOI] [PubMed] [Google Scholar]
- Khalili H., Shandiz S.A., Baghbani-Arani F. Anticancer properties of phyto-synthesized silver nanoparticles from medicinal plant Artemisia tschernieviana Besser aerial parts extract toward HT29 human colon adenocarcinoma cells. J. Clust. Sci. 2017;28(3):1617–1636. doi: 10.1007/s10876-017-1172-6. [DOI] [Google Scholar]
- Salunke G.R., Ghosh S., Santosh Kumar R., Khade S., Vashisth P., Kale T., Chopade S., Pruthi V., Kundu G., Bellare J.R., Chopade B.A. Rapid efficient synthesis and characterization of silver, gold, and bimetallic nanoparticles from the medicinal plant Plumbago zeylanica and their application in biofilm control. Int. J. Nanomed. 2014;9(1):2635–2653. doi: 10.2147/IJN.S59834. [DOI] [PMC free article] [PubMed] [Google Scholar]