Abstract
E. coli is an Enterobacteriaceae that could develop resistance to various antibiotics and become a multi-drug resistant (MDR) bacterium. Options for treating MDR E. coli are limited and the pipeline is somewhat dry when it comes to antibiotics for MDR bacteria, so we aimed to explore more options to help in treating MDR E. coli. The purpose of this study is to examine the synergistic effect of a liposomal formulations of co-encapsulated azithromycin and N-acetylcysteine against E. coli. Liposomal azithromycin (LA) and liposomal azithromycin/N-acetylcysteine (LAN) were compared to free azithromycin. A broth dilution was used to measure the MIC and MBC of both formulations. The biofilm reduction activity, thermal stability measurements, stability studies, and cell toxicity analysis were performed. LA and LAN effectively reduced the MIC of E. coli SA10 strain, to 3 μg/ml and 2.5 μg/ml respectively. LAN at 1 × MIC recorded a 93.22% effectiveness in reducing an E. coli SA10 biofilm. The LA and LAN formulations were also structurally stable to 212 ± 2 °C and 198 ± 3 °C, respectively. In biological conditions, the formulations were largely stable in PBS conditions; however, they illustrated limited stability in sputum and plasma. We conclude that the formulation presented could be a promising therapy for E. coli resistance circumstances, providing the stability conditions have been enhanced.
Keywords: Liposomes, Nanoparticle, Azithromycin, N-acetylcysteine, E. coli, Multi-drug resistance bacteria
1. Introduction
E. coli is a gram-negative coliform bacterium, generally found in the lower intestine of warm-blooded organisms and in fecal matter. E. coli is the leading cause of food and water borne diarrhoea in humans; however increased resistance to antibiotics has been reported globally (Kibret and Abera, 2011). Multi-drug resistant (MDR) strains of E. coli, can be developed through multiple mechanisms of resistance one of them is called pumping, which is effective against several types of antibiotics and known as cross-resistance (Blanco et al., 2016) or when a single bacterium is equipped with resistance genes. The different resistance genes, which are often carried on plasmids, makes it resistant to different antibiotics (Nikaido, 2009). Other types of MDR mechanisms are the mutation of a bacterial cell target site, and the modification of the cell wall protein, which result in low cell permeability causing the inhibition of drug uptake (Singh et al., 2014). MDR bacteria are increasingly problematic. According to Rai et al., infections due to MDR bacteria are more difficult to cure and require broad-spectrum antibiotics, which are toxic, expensive, and less effective (Rai et al., 2012). Moreover, the shortage of new antibiotics that overcome the aforementioned mechanisms of resistance has made it more difficult to treat such infections. The World Health Organization flagged E. coli as one of the most widespread bacteria with acute resistance to antibiotics (WHO, 2018).
Azithromycin (AZM) and N-acetylcysteine (NAC) are very effective in their current clinical applications. Azithromycin is a macrolide, and function through inhibiting protein synthesis in bacterial cells (Imamura et al., 2005). Essentially, azithromycin stops bacterial growth by impeding the synthesis of protein by trussing reversibly to 50S ribosomal subunits of susceptible microbes. Azithromycin is also categorized as a dicationic macrolide antibiotic with the ability to permeate the outer membrane of bacteria (Imamura et al., 2005). The ability of azithromycin to permeate the outer membrane vesicles (OMV) is important, as OMV’s are a type of bacterial self-defence.
N-acetylcysteine is a mucolytic agent used to dissolve mucus linings, and to prevent the formation of a biofilm. NAC also has the ability to reform the structure of proteins by interfering with the disulfide bonds of the proteins, thus altering protein ligand bonding and lowering the secretion and viscosity of mucus (El-Feky et al., 2009). The National Cancer Institute in the United States (US) confirmed that NAC liquefies mucus by weakening the disulfide bonds (NCI Thesaurus). NAC can be used as a powerful modulator of antibiotic activity (Goswami and Jawali, 2010).
Nanoparticles play an important role in drug delivery due to their unique physicochemical characteristics. Among other nanoparticles, liposomal delivery is advantageous due to the high cell penetration and target accuracy associated with this delivery (Bozzuto and Molinari, 2015).
Liposomes are effective as a drug delivery system as reported by Bozzuto and Molinari (Bozzuto and Molinari, 2015). Generally, liposomal drug delivery is highly beneficial compared to free oral and parenteral antibiotics, which are less effective due to limitations of the local drug concentration, increasing antibiotic-resistant strains, and the inability of the drug to reach the targeted site (Rukavina et al., 2018). Solleti et al. found that liposomal encapsulation of azithromycin resulted in an increased bactericidal activity against bacteria compared with free azithromycin (Solleti et al., 2015). Similarly, Mugabe et al. found that liposomal antibiotics are more effective than the free drug (Mugabe et al., 2006). Liposomes are an important factor in pharmacological therapy as they enhance the drug’s effectiveness and reduce the chances of extreme side-effects in the patient due to the intake of a high concentration of the drug.
We hypothesize that it may be possible to achieve a synergistic effect of combining azithromycin and NAC against E. coli. Azithromycin has the ability to stop bacterial growth, permeate the outer membrane, and kill bacteria. NAC add the ability to dissolve biofilms, a protection mechanism of bacteria, and to improve the immune system’s responses in various cases. Creating a combined liposomal formulation that can deliver both agents at the same time to the infection site may result in an improved outcome for reducing the antibiotic resistant dilemma. To expand the range of the antimicrobial spectrum of azithromycin, in the current study, we assessed the synergistic effect of liposome formulations of co-encapsulated azithromycin and N-acetylcysteine (LAN) against E. coli.
2. Materials and methods
Chemicals: Azithromycin was obtained from Sigma-Aldrich. N-acetyl cysteine DSPC(1,2-distearoyl-sn-glycero-3-phosphocholine), DOPE(1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine), DPPC(1,2-dipalmitoyl-sn-glycero-3-phosphocholine) and Cholesterol were purchased from UFC BIOTECH (USA, Amherst, NY, USA). MTT assay kit was purchased from (Abcam, UK). Normal human fibroblast cell line (Hs27 ATCC-CRL-1634) were purchased from (ATCC, USA).
Bacteria strains: Clinical Escherichia coli SA057, Clinical Escherichia coli SA10 and staphylococcus aureus ATCC 25922.
2.1. Preparation of liposomal drug formulations
For the liposomal azithromycin (LA) preparation, DSPC(1,2-distearoyl-sn-glycero-3-phosphocholine), DOPE(1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine), and Cholesterol lipids were dissolved with a (2:1) Methanol/Ethanol in a molar ratio 2:4:1 respectively and 1 mg of dissolved azithromycin was added to the lipids. A lipid film was formed by using a rotary evaporator system (BUCHI rotavapor R-300, USA) and 1 mL of PBS was added to the lipid film and mixed by hand shaking to form liposomal vesicles. The samples were sonicated (Ultrasonic Processor UPS 125, India) for 5 min (10 sec pulse ON and 2 sec pulse OFF). Sonication power adjusted up to 60% of amplitude. The size of un-lyophilized formulations was measured, and the size distribution analyzed by a high performance two angle particle sizer (MALVERN ZetaSizer Nano ZSP system, UK) by using dynamic light scattering. Unencapsulated drug was washed three times using a centrifuge at 20,000 rpm (HERMLE Z36 HK Centrifuge, Germany). The collected pellets were frozen at − 80 °C, and exposed for lyophilization by using the Freeze-Dryer system (Alpha 3–4 LSCbasic, Germany) for 48 h. Samples were stored as powder in a cool and dry place until required.
To reform the liposomal formulation, the lyophilized samples were gradually rehydrated. Basically, 10% of the final aqueous volume was added to the lyophilized samples and incubated for 30 min at 40 °C in a water bath; the same step was repeated until the final volume is reached.
Similarly, liposomal N-acetylcysteine was prepared by following the same steps as for the liposomal azithromycin preparation but using different lipids and ratios. The DPPC(1,2-dipalmitoyl-sn-glycero-3-phosphocholine), and Cholesterol had a molar ratio of 3:7 respectively. After a lipid film was formed, 50 mM of NAC was added. Finally, the liposomal formulations (liposomal azithromycin and liposomal N-acetylcysteine) were combined in a molar ratio (1:1) and mixed to obtain the Azithromycin and N-acetylcysteine liposomal nanoparticles (LAN).
The quantification of Azithromycin was performed using an ultra high-performance liquid chromatography - tandem mass spectrometer (UHPLC-MS/MS). The UHPLC system consisted of LPG-300RS quaternary rapid separation pump with integrated degasser, WPS-300TRS autosampler, TCC-300RS Column compartment and Xcalibur™ software (ThermoFisher Scientific). All samples were centrifuged and filtered through 0.22 μm filters before analysis (centrifugation and filtration used to maximize samples purity for HPLC samples injection processing). Separation was done with a Thermo Scientific™ Syncronis™ C18 column (100 × 2.1 mm, 3 μm particle size). The oven temperature was maintained at 40 °C and the mobile phase was LC/MS grade, water plus 0.1% formic acid (A) and methanol plus 0.1% formic acid (B). A linear gradient program was used at a flow rate of 0.300 mL/min: 0.0–2.0 min 2% (B), 2.0–4.0 min from 2% to 98% (B), 4.0–6.0 min from 98% (B), 6.0–7.0 min from 98% to 2% (B) and finally 7.0–10.0 min 2% (B). The identification and quantification of Azithromycin was done on a triple quadrupole mass spectrometer (TSQ Altis, ThermoFisher Scientific). The mass spectrometer was operated in the positive ion electrospray ionization (ES + ). The ion for azithromycin was analysed in a selected ion monitoring (SIM) scanning mode (748.6–749.4) at a retention time (Rt) of 6.64 min. A standard calibration curve (R2 = 0.9991) was created using different concentrations of Azithromycin and analysis was performed in triplicate. A Microbiological assay was also performed for results confirmation.
2.2. The minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) of liposomal formulations
The MICs and MBCs of the liposomal formulations against the clinical bacterial isolates EC SA057 and EC SA10 were determined with the broth dilution method. The standard bacterial strain S. aureus ATCC 29213 was used as a control to validate the procedure. Firstly, 96-well plates were filled with 100 μL of sterilized broth. Secondly, 100 μL of liposomal formulations were separately added in each first well of the rows and followed by a two-fold serial dilution with a starting concentration of 431 μg/ml for both liposomal formulations. Next 100 μL of adjusted bacteria at 1 × 103 of 0.5 McFarland standard were added to each well. The MICs were determined by selecting the visualized growth inhibition after plates were incubated for 24 h at 37 °C.
For the MBC, 50 μL of the MICs, the higher concentrations and the positive controls, were streak-plated on Mueller-Hinton agar plates. The MBCs were determined by selecting complete growth eradication of the Agar Petri Dishes after they were incubated for 24 h at 37 °C.
2.3. Biofilm reduction activity
EC SA057 and EC SA10 isolates were inoculated on fresh culture plates for 24 h at 37 °C with shaking. Adjusted bacteria at 1 × 103 of 0.5 McFarland standard were diluted (1:100) into fresh media (107 UFC/mL) and 100 μL of bacteria were transferred to each well of the 96-well plate. The plate was incubated for 72 h at 37 °C in a shaking incubator, at 75 rpm. In the first 24 h, the planktonic cells were washed twice with sterile dH2O, followed by an addition of 100 μL broth. After 48 h incubation, the media was exchanged with fresh media. After 72 of incubation, the planktonic cells were washed twice with sterile dH2O followed by the addition of 125 μL of the MIC, pre-MIC concentration of each liposomal formulation. The positive control (untreated biofilm), and negative control (10% added bleach into biofilm) were also examined. The plate was incubated for 24 h at 37 °C.
After the incubation period, 125 μL of 0.1% crystal violet was added into each well and incubated for 15 min at room temperature. The stain was washed twice with sterile dH2O, and allowed to dry. The biofilm was treated with 200 μL of 99% ethanol and set to solubilize for 15 min. The OD was measured at 570 nm in triplicates using a spectrophotometer plate reader (Spectramax plus 384, USA), and the average was recorded.
2.4. Liposomal nanoparticles thermal stability measurements
All liposomal formulations were subjected to thermal analysis by using a PerkinElmer Thermogravimetric Analyzer (TGA 4000), USA. The initial temperature was 20.00 °C, and the final temperature 500 °C. The sample was heated from 20 °C to 100 °C at 5.00 °C/min and held for 5.0 min at 100 °C. Then heated from 100 °C to 500 °C at 5.00 °C/min and finally cooled from 500 °C to 30.00 °C at 5.00 °C/min.
2.5. Liposomal nanoparticle stability studies
The stability studies of the liposomal formulations were assessed in four biological and storage conditions: PBS at 4 °C, PBS at 37 °C as well as plasma and sputum, and 100 μL of liposomal formulation was added to 100 μL of each condition, separately. For the PBS at 4 °C, samples were incubated in a refrigerator at 4 °C. The samples containing PBS at 37 °C, plasma, and sputum were incubated in a shaking incubator (Maxq 420 hp, USA) at 100 rpm. At each time interval (0, 1, 6, 9, 12, 18, 24, 48 h), samples were collected and centrifuged at 20000 rpm for 30 min to wash the released drug. The supernatant was assembled and analyzed with a UHPLC-MS/MS. Each sample was done in triplicate and the average was recorded.
2.6. Cell toxicity analysis
The MTT assay is an effective colorimetric method for determining in vitro cytotoxicity and cellular viability by measuring mitochondrial activity in cells. Basically, normal (Hs27 ATCC-CRL-1634) cell line viability was maintained in DMEM supplemented with 10% FBS without antibiotics at sub-confluency 75%, the cells were seeded in 96-well plates at a density of 5 × 103 cells/well for the MTT assay to measure the toxicity profile of the formulas in human cells. The MTT assay was used according to manufacturer’s instructions. Normal cells were cultured without antibiotics for 24 h at 37 °C. After incubation, the media was changed, and pre-prepared serial-diluted liposomal formulation done as follows (in 96-well plate, each well was filled with 100 μL of media without antibiotic. Then, 100 μL of liposomal formulation was added to the first well of each row followed by a two-fold serial dilution) was added. The controls included untreated cells in media alone (as a positive control), cells treated with H2O2 (as a negative control), empty liposomes (to account for any effect of the liposomes on cell viability). The plates were incubated for 24 h at 37 °C. On the second day, the media was discarded from the cell culture and MTT buffers were added according to the instructor’s protocol. The plate was incubated for 4 h at 37 °C in the dark, and measurements were obtained by spectrophotometry at 590 nm. The negative control (cells treated with H2O2) were also used as a blank, which were subtracted from each value, and cellular viability was expressed as a percentage. The same process was done for each formula including LA, and LAN.
2.7. Data analysis
We have used GraphPad software to plot our figures, we have used NOVA analysis to compare the three groups and measure P value. A (P < .05) was considered significant. All experiments were done in triplicates.
3. Results
Liposomal nanoparticle characterization:
3.1. Size and polydispersity index
The average size of the free liposome was 496 ± 36.2, with a 0.2 polydispersity index. The LA had a size of 484.5 ± 34.7 nm and the polydispersity index was 0.4, indicating the uniformity of size distribution. The liposomal LAN shows a slight reduction and homogeneity in size with an average size of 451.6 ± 21.16 nm and a 0.4 polydispersity index (Table 1).
Table 1.
Size and polydispersity (PDI) of liposomal nanoparticles using a ZetaSizer.
| Liposomal Formula | Size (nm) | PDI |
|---|---|---|
| Free Liposome | 496 ± 36.2 | 0.2 |
| LA | 484.5 ± 34.7 | 0.4 |
| LAN | 451.6 ± 21.1 | 0.4 |
3.2. Antimicrobial profile of LA and LAN formulations compared with the free form of azithromycin
Table 2 shows the MIC and MBC of the liposomal formulations. The free liposome was used as a control showing the growth of bacteria when tested on E. Coli SA057 and E. Coli SA10, indicating that it had no effect on the bacteria. On the E. Coli SA057, the MIC of the LA was 0.38 μg/ml, and the MBC was 0.75 μg/ml. With E. Coli SA057, the MIC of the LAN was 0.62 μg/ml, and the MBC was 1.25 μg/ml. However, with the E. Coli SA10 strain, the LA recorded a reasonable enhancement in the MIC and MBC values (3 μg/ml, and 6 μg/ml, respectively). Similarly, with the addition of NAC to the liposomal formulation, the MIC and MBC of the LAN was reduced to 2.50 μg/ml and 5 μg/ml, respectively.
Table 2.
MIC and MBC of Free AZM, liposomal azithromycin (LA), and liposomal azithromycin/NAC (LAN); *: was used according to ICSL as reference strains to validate MIC/MBC experiments; –**: bacteria were undetectable.
| Free AZM | LA | LAN | ||||
|---|---|---|---|---|---|---|
| (μg/ml) | ||||||
| Microorganism | MIC | MBC | MIC | MBC | MIC | MBC |
| S. aureus ATTC 29213* | 1 | 0.5 | –** | –** | –** | –** |
| E. coli SA057 | 8 | 16 | 0.38 | 0.75 | 0.62 | 1.25 |
| E. coli SA10 | 16 | 32 | 3 | 6 | 2.50 | 5 |
3.3. LA & LAN formulations activity to reduce biofilm formed by E. coli strains
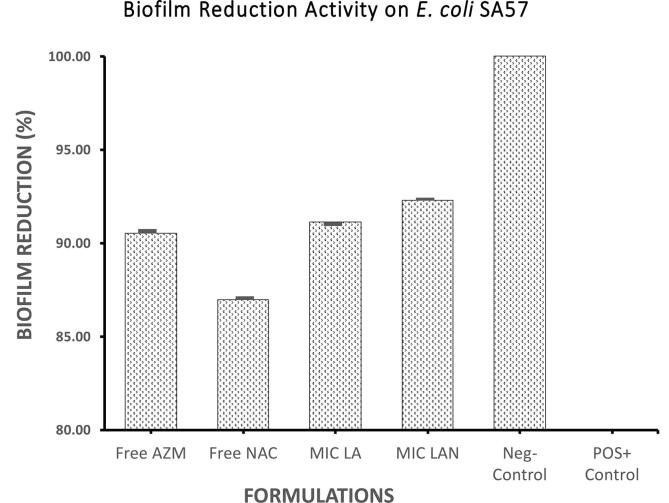
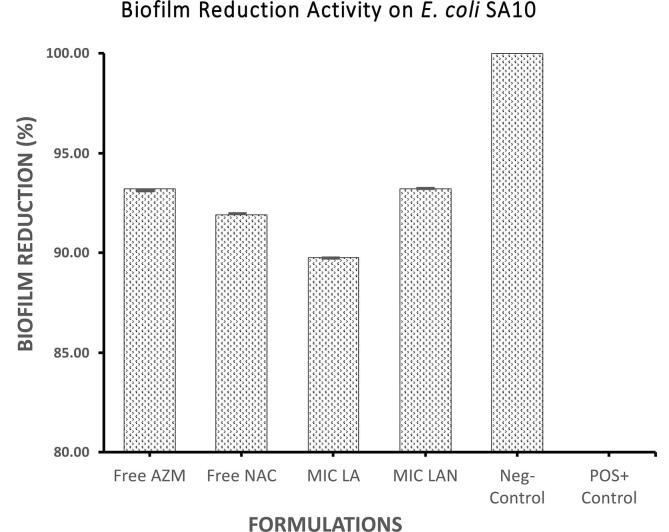
Fig. 1 illustrate the effectiveness of the liposomal formulations against the biofilm formed by E. coli SA057. Effectively, LA and LAN, at their respective MIC’s, reduced biofilm by 91.1% and 92.3% respectively; which show an improved reduction compared to AZM (Fig. 1). In contrast, the liposomal formulations appeared less affective against the biofilm formed by E. coli SA10 compared to the free AZM activity (93.2%). The LA formulation reduced the biofilm by 89.7% (Fig. 2). A higher effect was recorded by the LAN (93.2%) against a biofilm formed by E. coli SA10 (Fig. 2).
Fig. 1.
Biofilm reduction (in percentage) for free AZM, free NAC, liposomal azithromycin, and liposomal azithromycin + liposomal N-acetylcysteine against E. coli SA57.
Fig. 2.
Biofilm reduction (in percentage) for free AZM, free NAC, liposomal azithromycin, and liposomal azithromycin + liposomal N-acetylcysteine against E. coli SA10.
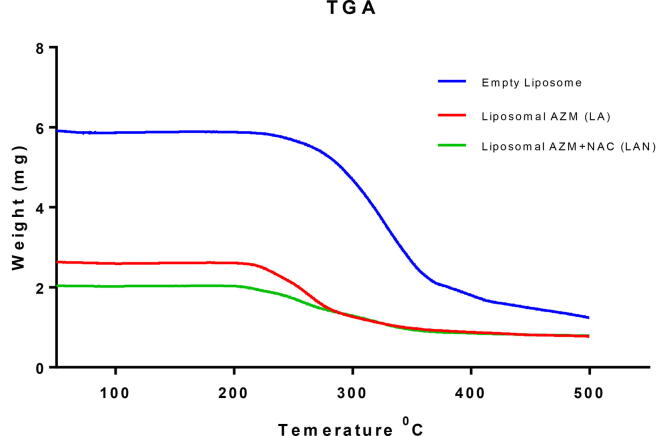
3.4. Thermogravimetric analysis of liposomal formulations
The thermal study for the liposomal formulations indicated a fairly stable formula construction at a transitional temperature of 200 °C. The temperature shifting of the thermal stability for the free liposomal formula was analyzed as a parameter for the formulas’ stability. The free liposomal formulation remained intact until 221 °C (melting point) (Fig. 3). The LA formulation also kept its structure consistent until reaching the melting point of 212 ± 2 °C. The LAN formulation similarly kept its structure consistent until reaching the melting point at 198 ± 3 °C (Fig. 3). The temperature changes observed in all the formulations are strongly suggestive of the successful encapsulation of AZM and NAC dependently.
Fig. 3.
Thermogravimetric analysis (TGA) of empty liposome, LA, and LAN formulations.
3.5. Liposomal drug formulation stability with biological conditions
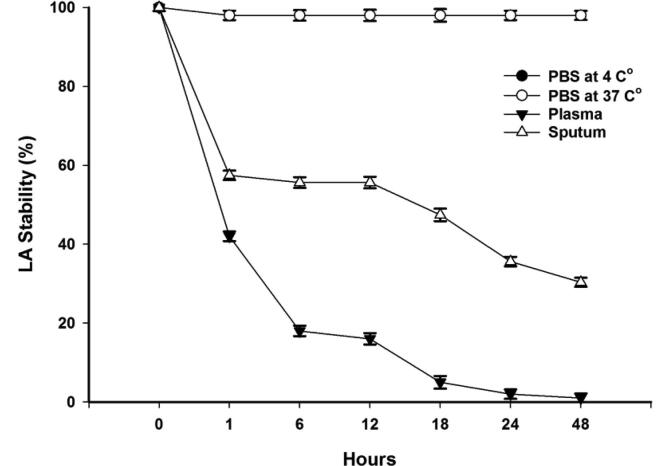
3.5.1. Liposomal azithromycin
The LA remained stable in biological storage at a temperature of 37 °C as well as at the storage temperature at 4 °C in PBS conditions. The LA maintained an average stability of 98% in both conditions. However, with the biological condition of sputum, the formulation kept 55.65% of the azithromycin for the first 12 h and then gradually started to lose the encapsulated azithromycin until only 30.33% remained inside the liposomal formulation after 48 h. The LA was unstable in the plasma condition where it lost 58% of its azithromycin content in the first hour and lost all of the azithromycin in 48 h. Fig. 4 shows the average stability of LA in storage and biological conditions.
Fig. 4.
Stability of LA under storage and biological conditions over a period of 48 h.
3.5.2. Liposomal azithromycin/N-acetylcysteine
The LAN formulation is stable (99% stability) in both biological storage temperatures (Fig. 5). However, the LAN had a very weak stability in the sputum condition, and was completely unstable in the plasma condition. The LAN retained only 29.38% of its azithromycin for the first 12 h with the percentage reducing to only 15.02% of encapsulated azithromycin at 48 h (Fig. 5). Notably, the LAN formulation was capable to retain 5.53% of the azithromycin for the first 12 h in a plasma condition but lost it completely in 48 h (Fig. 5).
Fig. 5.
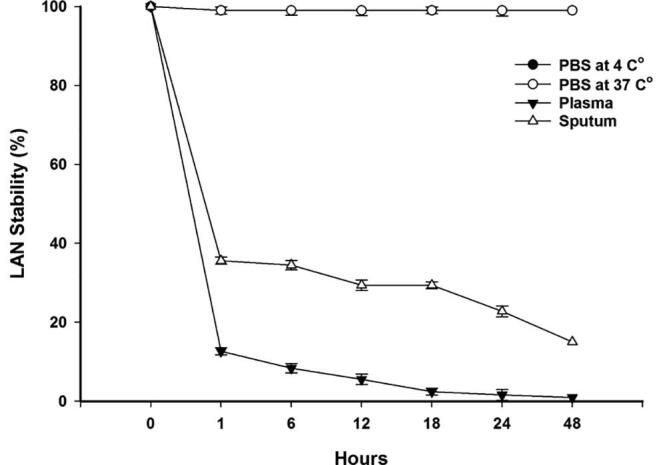
Shows the average stability of LAN under storage, and biological conditions.
3.5.3. Formulations toxicity toward human cells
The LAN formulation illustrated very low toxicity toward human cell Hs27 ATCC-CRL-1634 as presented cell viability within 24 h exposure time even at high concentration of 500 µg/ml. In fact, starting from as low as 1 ug/mL concentration up to 500 µg/ml concentration, the LAN formulations shows the least toxicity effects on human cell line, where 81% of the cells held out at the highest concentration. In addition, free AZM and LA formulations were also illustrated acceptable toxicity profile toward human cell Hs27 ATCC-CRL-1634 at high concentration of 500 µg/ml. Toxicity study clearly indicated the encapsulating both azithromycin and NAC in liposomal formulations enhanced cell tolerances toward these drug agents (Fig. 6).
Fig. 6.
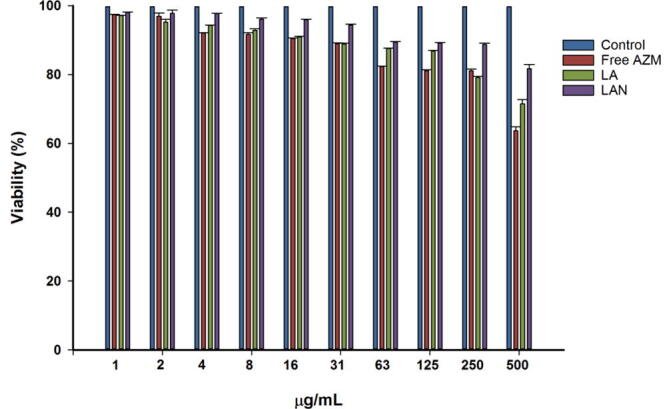
Shows the percentage of cell viability after the exposure to free AZM, LA, and LAN.
4. Discussion
Nanoparticles have unique characteristics making them suitable for drug delivery. Size is a defining aspect of a nanoparticle. According to Laouini et al., “the average size and size distribution of liposomes are important parameters especially when the liposomes are intended for therapeutic use by inhalation or parenteral route” (Laouini et al., 2012). The size and polydispersity index of the liposome nanoparticles can vary, which could be due to the encapsulated drug, the liposomal formulation preparation methods, and the temperature at which the liposomal nanoparticles were measured. In this study, all the prepared liposomal nanoparticles were in a similar size range. The liposomal azithromycin and liposomal N-acetylcysteine formulations registered slightly elevated polydispersity values compared to the free liposome formulation. Nevertheless, the final formulation falls within the acceptable limit for particle size dispersion. Danaei et al. cite that lipid carriers should have a polydispersity index of 0.3 and below to be considered homogenous and acceptable, but highlight the lack of definitive criteria to determine the viability of a liposomal formulation in the “Guidance for Industry,” a publication by the Food and Drug Administration (FDA) (Danaei et al., 2018). In a recent study conducted to measure the effect of particle size on liposomal-drug uptake, the authors reported that “the systemic absorption of griseofulvin was lower when the drug was given in the form of larger liposomes, compared to the administration of the control preparation with moderate-size liposomes” (Ong et al., 2016). It is accepted that smaller, homogenously sized nanoparticles are generally better as they are appropriate for inhalation and parenteral delivery and more easily absorbed in the body.
The MIC refers to the lowest concentration of the antibiotic preventing the observable growth of bacteria and the MBC refers to the lowest concentration of an antibiotic required to kill bacteria. Analyzing the MIC and MBC of the liposomal formulations, the LA formulation was very effective (MIC 0.38 μg/ml and MBC 0.75 μg/ml) against E. coli (SA057). In addition, against E. coli SA10, the MIC and MBC was 3 μg/ml and 6 μg/ml respectively. The results illustrate a five to three-fold reduction. Interestingly, when the LAN formulation was applied against the same strains, the antibacterial efficacy of LAN against E. coli was selectively different compared with LA, possibly due to the activity of NAC, which positively modulated the activity of azithromycin against E. coli SA10. The low MIC and MBC achieved are indicators of the viability of LAN against MDR E. coli.
N-acetylcysteine is a useful modulator of the bactericidal activity of azithromycin. Though azithromycin permeates OMV’s, it is not as effective against biofilms. According to Gillis and Iglewski, azithromycin can retard biofilm formation, but the bacteria quickly resist and form a stronger biofilm (Gillis and Iglewski, 2004). As a result, azithromycin tends to be ineffective against biofilm-forming bacteria. Blasi et al. reported that NAC is capable of disrupting developing and mature biofilms (Blasi et al., 2016). Concentrations of LA and LAN equivalent to MIC showed contrasting levels of impact in reducing biofilm formation. LAN had significant success in disrupting biofilm formation compared to LA. Nevertheless, both formulations achieved a high biofilm reduction percentage against E. coli SA057. Consistently, LA and LAN reduced the biofilm formed by E. coli SA10 by 89.75% and 93.22%, respectively. The significant effect of LA on biofilm observed can be explained by the documented ability of liposomal nanoparticles particles to adhere to mucus linings, which improve their uptake. The results obtained are consistent with literature related to liposomes. Rukavina and Vanic cite a study by the Omri group where liposomal azithromycin significantly retarded the growth of bacteria within a biofilm (Rukavina and Vanić, 2016). While NAC is typically more active against biofilms than azithromycin, the liposomal formulation enables the latter to permeate the biofilm and act on bacteria. Evidently, liposomal azithromycin and liposomal NAC have the capacity to produce a synergistic effect against bacterial strains.
Thermal stability is an important factor in the integrity of a liposomal particle. Roy et al. confirmed that temperature affects the degree of hydrolysis, and therefore the shelf life, of a liposome (Roy et al., 2016). The ideal liposomal particle should have a high stability over a broad temperature range. In this study, all liposomal nanoparticles, LA and LAN, were observed to have a relatively high thermal stability, 212 ± 2 °C and 198 ± 3 °C respectively. Good thermal stability is also important because it contributes to maintaining the integrity of the drug loaded in the liposome (Roy et al., 2016). The observed temperature range, within which LAN and LA are stable, is appropriate for maintaining a significant shelf life and the integrity of the drug. Temperature also affects liposomes in terms of size as liposomes increase in size when heated to temperatures above the transition temperature (Roy et al., 2016). The observation is supported by Wu et al. (2014). As mentioned earlier in this section, a particle’s size is known to affect its absorption in the system. Specifically, the particle size is an important consideration for liposomal nanoparticles since it affects the encapsulation efficiency, the drug release, distribution within the body, the ability of the particle to adhere to mucus linings, and the cells’ ability to incorporate it (Danaei et al., 2018). A liposomal particle that is generally thermally unstable will significantly reduce the efficacy of the encapsulated drug as it may reduce the formulation’s shelf life and its therapeutic effectiveness. In this study, LA and LAN formulations were remarkably stable at the biological temperature however the stability profile differed when they were exposed to plasma and sputum conditions. Biological conditions such as the plasma assay are used to determine the formulation’s vulnerability to enzymes. Several studies support our finding of the instability in a plasma condition. However, the instability of liposomes in plasma can be avoided through modulation of the lipid composition (Rukavina et al., 2018).
Stability tests are vital in drug testing since they predict the behaviour of the formula in different conditions. Storage conditions are simulated to determine the optimum storage parameters that ensure the drug’s stability. The drug’s stability under different biological conditions is crucial to its efficacy since instability can lead to premature release of the drug. Notably, the sustained stability of the formulations at an increased temperature of 37 °C is consistent with the results of the thermal stability test. According to Solleti et al., the observed stability at high temperatures can be attributed to the use of cholesterol during the preparation of the liposomal formulations (Solleti et al., 2015). Cholesterol reduces the bilayer permeability of the lipid nanoparticle, which in turn increases drug retention. The drug’s stability is a potential indicator of high efficiency since it is unlikely to be affected by the biological conditions during delivery.
The toxicity of a drug on living cells is an important tool to measure the risk value when the drug is used in humans. Lila and Ishida write that an increasingly large body of work supports the use of liposomal drug delivery due to the method’s low toxicity to the system compared to the free drug (Abu Lila and Ishida, 2017). Indeed, Sercombe et al. reported that liposomes has the ability to stabilize encapsulated drugs, enhance their uptake, improve their targeted distribution in the body and minimizes the toxic effects of the drugs (Sercombe et al., 2015). According to Daraee et al., an advantage of liposomal drug delivery is the reduction in the drug’s toxicity (Daraee et al., 2016). The tendency of liposomes to release various drugs gradually could explain the reduced cytotoxicity in the final formulation. The slow release of drugs from liposomes could also explain the generally higher viability of liposomal azithromycin compared to free azithromycin. The encapsulation of a drug in a liposome interferes with the drug’s molecular distribution, which can reduce its toxicity (Daraee et al., 2016). Our findings support and are aligned with existing literature.
5. Conclusion
The liposomal formulations of azithromycin seem to have be in vitro good therapeutic option against resistant E. coli clinical strains. Notably, the synergistic effect of lipid formulation azithromycin and NAC is thought to be due to the encapsulation of azithromycin and NAC in liposomal formulations. Biofilm study, LAN formulation generally achieved better results than the LA formulation. However, both were capable to reduce biofilm and thus is mimicking the in vivo conditions were bacteria can be intruders in host. The A recommendation of the study is to optimize stability of the liposomal formulation within plasma and sputum biological conditions and then test it in an animal model.
Funding
This work was funded by two students grants from King Abdullah International Medical Research Center (KAIMRC), Ministry of National Guard Health Affairs (MNG-HA), Riyadh, Saudi Arabia (Grants No. SP18/252/R)/(SP18/245/R). The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.“
CRediT authorship contribution statement
Shokran A. Aljihani: Investigation, Writing - original draft. Zeyad Alehaideb: Investigation, Writing - original draft. Reem E. Alarfaj: Investigation. Majed F. Alghoribi: Methodology. Maaged A. Akiel: Validation. Thamer H. Alenazi: Validation, Visualization. Ahmed J. Al-Fahad: Writing - review & editing. Saad M. Al Tamimi: Investigation. Turki M. Albakr: Project administration, Investigation. Abdulrahman Alshehri: Investigation. Saad M. Alyahya: Investigation. Alaa Eldeen B. Yassin: Funding acquisition, Writing - review & editing. Majed A. Halwani: Writing - original draft, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Authors appreciate and thank Dr. Susanna Wright for her support with the English editing of this manuscript.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Zeyad Alehaideb, Email: alehaidebze1@ngha.med.sa.
Maaged A. Akiel, Email: akielma@mymail.vcu.edu.
Saad M. Al Tamimi, Email: AlTamimi253@ksau-hs.edu.sa.
Turki M. Albakr, Email: albakar401@ksau-hs.edu.sa.
Abdulrahman Alshehri, Email: alshehri395@ksau-hs.edu.sa.
Saad M. Alyahya, Email: alyahya315@ksau-hs.edu.sa.
Alaa Eldeen B. Yassin, Email: yassina@ksau-hs.edu.sa.
Majed A. Halwani, Email: halawanima@ngha.med.sa.
References
- Abu Lila A.S., Ishida T. Liposomal delivery systems: design optimization and current applications. Biol. Pharm. Bull. 2017;40:1–10. doi: 10.1248/bpb.b16-00624. [DOI] [PubMed] [Google Scholar]
- Blanco P. Bacterial multidrug efflux pumps: much more than antibiotic resistance determinants. Microorganisms. 2016;4:14. doi: 10.3390/microorganisms4010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi F. The effect of N -acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Respir. Med. 2016;117:190–197. doi: 10.1016/j.rmed.2016.06.015. [DOI] [PubMed] [Google Scholar]
- Bozzuto G., Molinari A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015;10:975–999. doi: 10.2147/IJN.S68861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:57. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daraee H., Etemadi A., Kouhi M., Alimirzalu S., Akbarzadeh A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016;44:381–391. doi: 10.3109/21691401.2014.953633. [DOI] [PubMed] [Google Scholar]
- El-Feky M.A. Effect of ciprofloxacin and N-acetylcysteine on bacterial adherence and biofilm formation on ureteral stent surfaces. Pol. J. Microbiol. 2009;58:261–267. [PubMed] [Google Scholar]
- Gillis R.J., Iglewski B.H. Azithromycin retards Pseudomonas aeruginosa biofilm formation. J. Clin. Microbiol. 2004;42:5842–5845. doi: 10.1128/JCM.42.12.5842-5845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami M., Jawali N. N-acetylcysteine-mediated modulation of bacterial antibiotic susceptibility. Antimicrob. Agents Chemother. 2010;54:3529–3530. doi: 10.1128/AAC.00710-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y. Azithromycin exhibits bactericidal effects on Pseudomonas aeruginosa through interaction with the outer membrane. Antimicrob. Agents Chemother. 2005;49:1377–1380. doi: 10.1128/AAC.49.4.1377-1380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibret M., Abera B. Antimicrobial susceptibility patterns of E. coli from clinical sources in northeast Ethiopia. Afr. Health Sci. 2011;11(Suppl 1):S40–45. doi: 10.4314/ahs.v11i3.70069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouini A. Preparation, characterization and applications of liposomes: state of the art. J. Coll. Sci. Biotechnol. 2012;1:147–168. [Google Scholar]
- Mugabe C., Halwani M., Azghani A.O., Lafrenie R.M., Omri A. Mechanism of enhanced activity of liposome-entrapped aminoglycosides against resistant strains of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006;50:2016–2022. doi: 10.1128/AAC.01547-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCI Thesaurus. https://ncit.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI%20Thesaurus&code=C200.
- Nikaido H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009;78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S., Ming L., Lee K., Yuen K. Influence of the encapsulation efficiency and size of liposome on the oral bioavailability of griseofulvin-loaded liposomes. Pharmaceutics. 2016;8:25. doi: 10.3390/pharmaceutics8030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M.K., Deshmukh S.D., Ingle A.P., Gade A.K. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria: Activity of silver nanoparticles against MDR bacteria. J. Appl. Microbiol. 2012;112:841–852. doi: 10.1111/j.1365-2672.2012.05253.x. [DOI] [PubMed] [Google Scholar]
- Roy B. Influence of lipid composition, pH, and temperature on physicochemical properties of liposomes with curcumin as model drug. J. Oleo Sci. 2016;65:399–411. doi: 10.5650/jos.ess15229. [DOI] [PubMed] [Google Scholar]
- Rukavina Z., Vanić Ž. Current trends in development of liposomes for targeting bacterial biofilms. Pharmaceutics. 2016;8:18. doi: 10.3390/pharmaceutics8020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukavina Z., Šegvić Klarić M., Filipović-Grčić J., Lovrić J., Vanić Ž. Azithromycin-loaded liposomes for enhanced topical treatment of methicillin-resistant Staphyloccocus aureus (MRSA) infections. Int. J. Pharm. 2018;553:109–119. doi: 10.1016/j.ijpharm.2018.10.024. [DOI] [PubMed] [Google Scholar]
- Sercombe L. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015;6 doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Smitha M.S., Singh S.P. The role of nanotechnology in combating multi-drug resistant bacteria. J. Nanosci. Nanotech. 2014;14:4745–4756. doi: 10.1166/jnn.2014.9527. [DOI] [PubMed] [Google Scholar]
- Solleti V.S., Alhariri M., Halwani M., Omri A. Antimicrobial properties of liposomal azithromycin for Pseudomonas infections in cystic fibrosis patients. J. Antimicrob. Chemother. 2015;70:784–796. doi: 10.1093/jac/dku452. [DOI] [PubMed] [Google Scholar]
- WHO Data Show Antimicrobial Resistance Growing Among Common Bacteria in Both High-, Low-Income Countries, Jan 30 & 2018. The Henry J. Kaiser Family Foundation. https://www.kff.org/news-summary/who-data-show-antimicrobial-resistance-growing-among-common-bacteria-in-both-high-low-income-countries/.
- Wu H.-L., Sheng Y.-J., Tsao H.-K. Phase behaviors and membrane properties of model liposomes: Temperature effect. J. Chem. Phys. 2014;141 doi: 10.1063/1.4896382. [DOI] [PubMed] [Google Scholar]