Abstract
Salt stress is known to be momentous abiotic stress which treats agricultural lands and crop production throughout the world and effects the system of food security. The current study aims to investigate the effect of foliar application of 10 mg/l of zinc oxide (ZnO) as a bulk or as a green synthesized nanoparticle (ZnO-NPs) which were hexagonal and spherical in shape and at size 16–35 nm to alleviate the impact of salinity concentrations (0, 10, 25, 50, 75 and 100% SW) on Okra (Abelmoschus esculentus L. Moench cv. Hasawi) species. The results demonstrated a gradual decrease in the photosynthetic pigments (i.e., chlorophyll a and b with total chlorophylls and carotenoids) with the growth of salinity conc. However, the sea water levels between 0 and 75% will led to increase in proline, total soluble sugar and activity of the antioxidant enzymes i.e., superoxide dismutase (SOD) and catalase (CAT) and then decrease at 100% SW. The addition of bulk ZnO or ZnO-NPs enhances the contents of the photosynthetic pigments, activity of both SOD and CAT and then lowers the accumulation of proline and total soluble sugar when equated with controls. Plants treated with ZnO-NPs showed the greatest results when compared with other treatments. The results of current study showed ZnO-NPs as an appropriate eco-friendly and low-cost application for plant growth under salinity which has an ability to moderate the salt stress effect of plants.
Keywords: Okra, Sea-water, Salt-stress, ZnO-NPs, Foliar spray
1. Introduction
One of the important abiotic factors in salt-stress which severely affects plant growth and courses a serious problem in both arid and semi-arid lands that leads to lowering the crop yield (Hussain et al., 2019). Salinity affecting almost one-third of irrigated areas around the world and causes 50% of loss in global plant productivity (Zhao et al., 2020, Masilamani et al., 2020). Salt stress causes a serious danger to the global food security and agricultural sustainability (Farooq et al., 2015). Salinity has harm and negative effects on the metabolic processes in plants by an amalgamation of different origins, includes osmotic injuries caused by high solute accumulation and ion toxicity (Kamran et al., 2020, Egamberdieva et al., 2019). In most of the agricultural lands, there is a shortage in freshwater supplies. That makes the researchers study the effect of irrigates the crops by using seawater for promoting agriculture, food supplying and for saving the resources of freshwater especially with the increase of food demand with the increase of population (Fiaz et al., 2018). The salinity of seawater reaches 3.5%, the major dissolved salts in seawater include sodium, chloride, calcium, potassium and magnesium, encourage salt stress to plants (Hirt et al., 2020, Ayub et al., 2020). Nanotechnology is an interesting field of sciences which opens up a wide array of opportunities in many fields like medicine, pharmaceuticals, electronics, engineering and agriculture. It has enormous potential benefits and uses, and provides green and efficient alternatives for the management of plant productions. Nanotechnology has a possibility to help the plants appropriate to tolerate biotic and abiotic stress conditions, to reduce the environmental pollution, and prone in usage of applications of nanoparticles (NPs) in an agricultural field. Nanotechnology is an interesting field of sciences, which has a possibility to help the plants appropriate to tolerate stress conditions. To reduce the environmental pollution, and prone in usage of applications of nanoparticles (NPs) in an agricultural field. The size of NPs will be in between 1 and 100 nm; which influences the plant system for improving the plant growth and the potential of ROS scavenging. The green synthesized nanoparticles from plants are economical and environmentally friendly (Iqbal et al., 2020). The researchers have a growing interest in the metal oxide nanoparticles due to their size dependent features which enormously unlike from their bulk materials. Nanoparticles applications can help to minimize usage of toxic, harsh and expensive chemicals are commonly used in the ordinary processes of plant production (Maroufpour et al., 2020).
ZnO is known to be as zinc-oxide which is considered as one of the useful materials applied in fetchers; known as non-toxic, photo activity, inexpensive and long-term appearances (Lee and Moon, 2020, Sakir et al., 2020). ZnO is considered to be safe and secure when compared with other metal oxides (Kim et al., 2020). Approximately, 550 tons of NPs of ZnO is produced per annum throughout the globe for multiple usages (Bondarenko et al., 2013). Zinc oxide-nanoparticles (ZnO-NPs) have many benefits on the fertility of the soil, plant productions and zinc source, which is an important microelement for enhancing plant development and protection (Esper Neto et al., 2020, Rossi et al., 2019).
Okra (Abelmoschus esculentus L. Moench) is one of the heats loving plant in the field of vegetable crops and widely grownup in tropics, sub-tropics and temperature areas involves Africa, Asia and North-America (Benchasri, 2012, Shiri et al., 2020). It is an annually renewable crop, herbaceous, day-neutral, shrub-like dicotyledonous and erect plant that can grow from 1 to 3 m, it has alternate broad and polymorphous leaves. Okra flowers have five yellow or white petals with red or purple center. The entire plant organs are edible, and this plant is a powerhouse of valuable nutrients (Boateng et al., 2020). The present study aims to explore and comparing the roles of ZnO and ZnO-NPs to alleviate the negative effects of seawater concentrations on Okra plant.
2. Materials and methods
2.1. Used materials
In the present study, chemicals were supplied from Sigma-Aldrich, U.S.A and Loba Chemie Company, India. All glassware washed with distilled water and dried before use. Nanoparticles of ZnO (hexagonal and spherical in shape and at size 16 to 35 nm) were synthesized from the leaflets extract of (Phoenix dactylifera L. var. Khalas) collected from Al-Hasa city. (Bulk ZnO) and (ZnO NPs) powders were blended with deionized water. The seawater was diluted to six levels (0, 10, 25, 50, 75 and 100%). Seeds of Okra (Abelmoschus esculentus L. Moench var.Hasawi) supplied from Altuajri, K.S.A.
2.2. Method(s)
2.2.1. Experiment with pot
Sodium hypochlorite solution (4%) have been used to surface sterilization of the okra seeds for 1 min, after that the seeds rinsed with distilled water for two times, then germinated 5 seeds in every plastic pot with an equal amount of sand (2.5 kg) in each pot with the same spaces between the seeds at 25–28 °C in the greenhouse. Tap water was used for the irrigation of the seeds and then, after 15-days of planting, the seedlings were divided into three groups; every group includes three replicates for each treatment. All of the three groups were irrigated with seawater levels (0–100). The first group did not treat with any of ZnO types. The second group treated with the foliar application (10 mg/l) from (Bulk ZnO), while the third group treated with the foliar application of (10 mg/l) from (ZnO-NPs). After (15-days and 35-days) from sowing, the hand-held sprayer has been used to applying each of the foliar treatments. Separately, the seedlings were sprayed with until made the leaves completely humid then stopped prior the dripping point. A plastic film has been used to cover pots surfaces during the foliar applications treatments to block any touch with the soil. After 60 days from sowing, randomly picked up the seedlings from the pots of treated plants and control plants for analysis.
2.2.2. Extraction and determination of photosynthetic pigments
The leaf samples collected 60 days after germination and washed with distilled water. The photosynthetic pigments were extracted according to (Mittal et al., 2012) method by grinding 0.25 g (fresh weight) of the leaves with 5 mL of four-fifth (80%) acetone was used for 5-7mins at room temperature and centrifuged at 3000 rpm for 10 mins at 4 °C followed through UV/Visible spectrophotometer-[Spectro UV-2505] readings at wavelengths (663, 645 and 480 nm) to determine chlorophyll-a (Chl-a), chlorophyll-b (Chl-b) and carotenoids concentrations by using (Lichtenthaler, 1987) method as follows;
Chl-a (mg/g) = 12.25 (O. D663.2) − 2.79 (O. D646.8)
Chl-b (mg/g) = 21.50 (O. D646.8) − 5.10 (O. D663.2)
Total Chl (mg/g) = 7.15 (O. D663.2) + 18.71 (O. D646.8)
Carotenoids (mg/g) = [1000 (O. D470) − 1.82 Chla-85.02 Chlb]/198
Whereas, OD indicates optimal density.
2.2.3. Purpose of proline
Proline was determined using (Bates, 1973) method. Freshly collected leaves of 0.5 g were collected in 10 mL of 3% aqueous sulfosalicylic acid and filtered using the Whatman filter paper. Next, 2 mL of filtrate was taken in the test-tube and add 2 mL of ninhydrin solution along with 2 mL of glacial acetic-acid. The mixture was further heated for 1-hour at 95 °C and later on cool in an ice-bath. Later on, the mixture is extracted with 10 mL of Toluene as a chromophore. The air is strongly passed in the reaction mixture for couple of minutes to separate aqueous phase from the chromophore with an involvement of toluene and finally allowed to isolate colored phase to stand randomly 2-3mins at RT and its absorbance was defined as 520 nm using UV/Visible spectrophotometer.
2.2.4. Determination of complete soluble sugar
The estimation process for total sugar is done as per the (Yemm, 1954) method by extracting 100 mg from plant tissue in 10 mL of 80% ethanol, centrifuged at 2683g for 20mins at 20 °C. Supernatant was removed and the remaining residue was extracted with the repeating procedure. The obtained supernatant of 0.2 mL were evaporated in a test-tube until it dries in the water bath and cooled at 25 °C. One ml of distilled water is added to test tube and mixed softly. Next, then 4 mL of Anthrone reagent was added, mixed and incubated in water bath for 10mins at 100 °C and later on cool down with cold water. The optimal density was measured at 620 nm.
2.2.5. Extraction assay of antioxidant enzyme activities
Antioxidant enzymes are extracted were done as per the (Mukherjee, 1983) method by 0.5 g of fresh leaves in 10 mL of phosphate buffer (KH2PO4/K2HPO4) pH-7. Homogenization of centrifugation was done at 15,000g for 10mins at 4 °C. Thereafter, supernatant was stored at 4 °C for analyzing the activity of the antioxidant enzyme of both CAT and SOD.
2.2.5.1. Determination of SOD activity
SOD activity was measured with nitro-blue-tetrazolium (NBT) in lowered method (Chen and Wang, 2006). The reaction mixture of 3 mL consists of 50µ enzyme extract, 2.5 mL methionine (13 µM), 150µ of riboflavin (13 µM), 50µ of phosphate buffer (50 mM, pH-7.8) and 250µ of NBT (63 µM). The absorbance was recorded at 560 nm. Single unit of SOD activity was defined as the quantity of enzyme that causes the inhibition of 50% reduction of NBT.
2.2.5.2. Determination of CAT activity
CAT activity was measured as per (Chen et al., 2000) method. For this, 40 mL of the plant extracted mixed with 9.96 mL of the solution containing 0.016 mL of 30% H2O2 and 100 mL of PBS at pH-7 and then determined absorbance at 240 nm.
2.2.6. Statistical analysis
Statistical analysis was performed with both SPSS (version 20.0) (Khan et al., 2015) and Openepi softwares (Khan et al., 2014) which is used for Post Hoc L.S.D test (least significant difference). The results of the samples were shown as mean ± standard deviation and the experiment was carried out in triplicates. P value is considered as significant for <0.05 (Khan et al., 2019).
3. Results
The results presented in [Table 1] show that chlorophyll a and b in the control leaves decreased high significantly below the untreated ones except those treated with (10% SW) chlorophyll a decreased significantly. Applying (Bulk ZnO) increased significantly chlorophyll a at (0, 10 and 25% SW) and non-significantly at the higher seawater concentrations, while chlorophyll b increased non-significantly in all seawater concentrations except (10 and 25% SW) where the increases were significant. Applying (ZnO NPs) to the plants increased most of chlorophyll a and b high significant except those treated with (100% SW) the increases were non-significant in chlorophyll b and total chlorophylls. Data also show that the carotenoid contents in the control leaves decreased high significant at all seawater concentrations except (10% SW) where the decrease was only significant [Figure: 62]. Addition of (Bulk ZnO) and (ZnO-NPs) enlarged the total pigment contents in all seawater concentrations, but (ZnO NPs) gave higher values than (Bulk ZnO) especially in the lower seawater concentrations (10 and 25% SW).
Table 1.
Effect of different levels of seawater with the absence or presence of (Bulk ZnO) or (ZnO NPs) on photosynthetic pigments (mg/g) of (Abelmoschus esculentus) plants.
| Treatments | SW (%) | Chlorophyll (a) | Chlorophyll (b) | Total Chlorophyll | Carotenoid |
|---|---|---|---|---|---|
| Control | 0 | 11.67 | 7.28 | 18.95 | 1.02 |
| 10 | 8.74b | 4.38a | 13.12b | 0.67b | |
| 25 | 3.91a | 2.35a | 6.26a | 0.29a | |
| 50 | 2.86a | 1.61a | 4.47a | 0.17a | |
| 75 | 0.74a | 0.69a | 1.43a | 0.14a | |
| 100 | 0.37a | 0.249a | 0.62a | 0.0042a | |
| Bulk ZnO | 0 | 15.09b | 7.54c | 22.63b | 1.52c |
| 10 | 10.50b | 6.78b | 17.28b | 1.26b | |
| 25 | 5.13b | 3.71b | 8.84b | 0.36c | |
| 50 | 3.45c | 1.82c | 5.27c | 0.20c | |
| 75 | 1.25c | 1.04c | 2.29c | 0.16c | |
| 100 | 0.40c | 0.28c | 0.68c | 0.0063c | |
| ZnO NPs | 0 | 16.07a | 9.69b | 25.76a | 2.03a |
| 10 | 11.99b | 9.25a | 21.24a | 1.59a | |
| 25 | 10.63a | 5.35a | 15.98a | 1.44a | |
| 50 | 6.17b | 5.27a | 11.44a | 1.17a | |
| 75 | 4.87a | 3.69a | 8.56a | 0.54b | |
| 100 | 1.86b | 1.50c | 3.36c | 0.44b | |
Significance of values at p<0.05, a= (highly significant), b= (significant), c= (not significant).
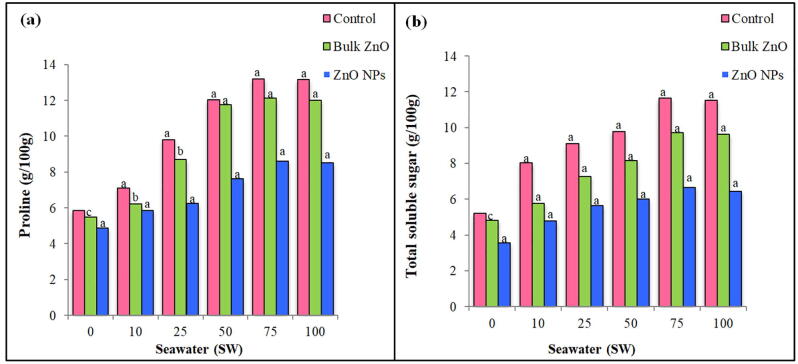
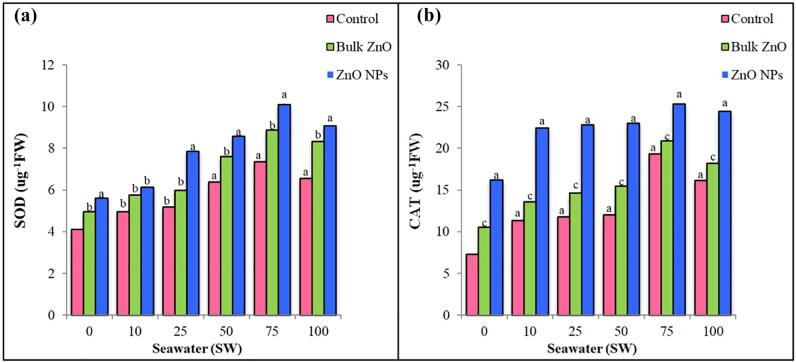
Data presented in [Fig. 1a] reveal that proline contents increased high significantly with the increase of seawater but decreased at (100% SW). Addition of (bulk ZnO) decreased non-significantly the proline contents at (0% SW), significantly at (10 and 25 %SW) and high significantly at (50, 75 and 100 %SW) as compared to their respective controls. Addition of (ZnO NPs) decreased high significantly these contents in all seawater concentrations. The proline values in plants treated with (ZnO NPs) are less than (bulk ZnO) and their respective controls. Total soluble sugar show high significant increases when these plants treated with different seawater concentrations [Fig. 1b]. These contents increased gradually as seawater concentrations increased then decreased at (100 %SW). Applying either (Bulk ZnO) or (ZnO NPs) reduced high significantly soluble sugar contents in all plants grow under seawater concentrations. The reduction was more in plants treated with (ZnO NPs) as compared to their corresponding controls. Gradual increases in the activity of superoxide dismutase (SOD) ware detected in all treated and untreated plants as sweater concentrations increased. The application of (bulk ZnO) or (ZnO NPs) increased the (SOD) activity as seawater concentrations increased, however the results of either (ZnO) applications were higher than their corresponding controls [Fig. 2a].
Fig. 1.
Effect of different levels of seawater with the absence or presence of (Bulk ZnO) or (ZnO NPs) on (a) proline content and (b) total soluble sugar content of (Abelmoschus esculentus) plants. Significance of values at p<0.05, a= (highly significant), b= (significant), c= (not significant).
Fig. 2.
Effect of different levels of seawater with the absence or presence of (Bulk ZnO) or (ZnO NPs) on (a) SOD activity, (b) CAT activity of (Abelmoschus esculentus) plants. Significance of values at p<0.05, a= (highly significant), b= (significant), c= (not significant).
Data shows that the (CAT) activity increased high significantly as seawater concentrations increased to reach its maximum at (75 %SW), then decreased at (100 %SW). Addition of (bulk ZnO) gave lower values in (CAT) activities than (ZnO NPs). Generally, the increase was high significantly in the plant treated with (ZnO NPs) relative to their corresponding controls. However, both (ZnO) applications gave results higher than their respective control [Fig. 2b].
4. Discussion
Photosynthesis is the most important process in green plants transform solar energy into chemical energy in organic compounds form synthesized by fixation of atmospheric (CO2). Salt stress causes a significant decrease in photosynthesis (Tang et al., 2015). Salt is mixed up in the chloroplast causing toxic effect on photosynthetic components as well as processes (Munns, 2008). The reduction in chlorophyll conc under salinity stress might be relate with membrane deterioration (Silveira and Carvalho, 2016). The decrease in photosynthetic rates in plants under salinity stress basically occurs due to the lowers in water potential (Parihar et al., 2015). Salt stresses causes modifications in both the size as well as number of chloroplasts and limit the starch contents, damage in chloroplast membranes and injury in grana and thylakoids (Acosta-Motos et al., 2017). Our findings demonstrate under the salt stress ZnO-NPs treated with enhance the contents of chlorophyll a, b, total chlorophyll and carotenoid compared to ZnO bulk treating of plants controls. The prior study by (Venkatachalam et al., 2017) revealed the similar results on cotton (Gossypium hirsutum L.) plant.
Nanoparticles has ability to develop functioning of efficiency of chemical energy and production of photosynthetic systems (Singh et al., 2015, Govorov, 2007). Zn is an important for the formation of protochlorophyllide and has a positive impact on chloroplast development and it helps in repairs the photosystem II (Hänsch and Mendel, 2009, Salama et al., 2019). An increase in carotenoid content may related with response of plant inhibition and oxidative damage (Mohsenzadeh, 2017).
The compatible solutes total soluble sugars and free from amino-acid proline are the common most and efficient role in osmoregulation under stress condition (Munns, 2008, Di Martino et al., 2003, Murakeözy et al., 2003). These compatible solutes can decrease the potential of osmotic cell can reach to the supply level of high turgor efficiency which makes plant more ability to maximize storage reserves to support metabolism of process under the stressed conditions and maintaining plant growth (Hurry et al., 1995, Cha-um et al., 2004). Accumulation of proline is due to increased protein demolition and conversion to amino acids, including proline (Ashraf and Foolad, 2007) or due to the proline activity biosynthesis enzyme or due to lowering of proline oxidation (Ahmad, 2005). Soluble sugars are extremely sensitive to abiotic stresses and helpful in turgor in keeping and enhancing the membrane stabilization by acting as reactive oxygen species (ROS) scavengers (Hossain et al., 2013). The results of this work demonstrated as ZnO bulk and ZnO-NPs led to decrease in accumulation of proline and total soluble sugars in the stressful plants compared to control. The lowest content of proline and total soluble sugars are in stressful plants that were treated with NPs from ZnO. Our obtained study results confirmed the data by (Li et al., 2017) on citrus (citrus reticulata) plant.
Major mechanisms to ROS-scavenging in plants include CAT and SOD which are anti-oxidant enzymes that supply protection and defense to plant cells from the oxidative stress of reactive free radicals (El-Beltagi et al., 2017, Mittler, 2002). Rising the antioxidant enzyme activities and developing antioxidant metabolism is one of the crucial ways to improve salinity tolerance in plants (Mao and Xu, 2004). In the present work, our findings confirm for both the applications of ZnO improved activities of SOD and CAT in okra plants under salinity stress compared with the control plants. ZnO-NPs application gives the highest contents of both antioxidant enzymes in the experimental plants and these results are in agreement with the findings of (Rizwan et al., 2019) on maize (Zea mays L.). Metal NPs have an inhibition effect on ROS formation by acting as antioxidants and also by improving the antioxidant activity of the plant cells (Ye et al., 2019). Increased content of the Zn by the application of ZnO-NPs substantially improves the SOD expression in plant leaves and more effective to minimize the oxidative stress (Pavithra et al., 2017). The growth in CAT activity in plants which have been treated with ZnO-NPs suggests NPs can effectively modify the enzyme activity.
5. Conclusion
Salt stress caused harmful effects on plant development. The present experiment showed that both of ZnO treatments increased the salinity tolerance in Okra plants. And proved the efficiency of the foliar application of the green synthesized (ZnO-NPs) in alleviation the negative effects of seawater on the plants compared with the application of their bulks counterparts. (ZnO-NPs) treatment extremely increased the contents of photosynthetic pigments and activity of the antioxidant enzymes, and reduced proline and total soluble sugar contents in the stressful plants compared to the other treatments. Overall, the data suggest that the nanoparticles of ZnO can be a beneficial environmentally friendly application to enhance the plant's toleration and the antioxidant activity to diminish the damages caused by salinity stress. The exposure of plants to (ZnO-NPs) applications could open a new path of studies in the agriculture field.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Acosta-Motos, J.R., Ortuño, M.F., Bernal-Vicente, A., Diaz-Vivancos, P., Sanchez-Blanco, M.J., Hernandez, JAJA, 2017. Plant responses to salt stress: adaptive mechanisms. 7(1), 18.
- Ahmad, P., Jhon, RJAoA, 2005. Science S: Effect of salt stress on growth and biochemical parameters of Pisum sativum L. (Einfluss von Salzstress auf Wachstum und biochemische Parameter von Pisum sativum L.). 51(6), 665–672.
- Ashraf, M., Foolad, MRJE, 2007. botany e: Roles of glycine betaine and proline in improving plant abiotic stress resistance. 59(2), 206–216.
- Ayub M.A., Ahmad H.R., Ali M., Rizwan M., Ali S., ur Rehman M.Z., Waris A.A. Plant Life Under Changing Environment. Elsevier; 2020. Salinity and its tolerance strategies in plants; pp. 47–76. [Google Scholar]
- Bates, L.S., Waldren, R.P., Teare, IJP, 1973. Soil: Rapid determination of free proline for water-stress studies. 39(1), 205–207.
- Benchasri, SJRip, 2012. Okra (Abelmoschus esculentus (L.) Moench) as a valuable vegetable of the world. 49(1), 105–112.
- Boateng, E.F., Nasiru, M.M., Agyemang, MJAFSJ, 2020. Meat: valuable animal-derived nutritional food. A review. 9–19.
- Bondarenko, O., Juganson, K., Ivask, A., Kasemets, K., Mortimer, M., Kahru AJAot, 2013. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. 87(7), 1181–1200. [DOI] [PMC free article] [PubMed]
- Cha-um, S., Kirdmanee, C., Supaibulwatana, KJSA, 2004. Biochemical and physiological responses of Thai jasmine rice (Oryza sativa L. ssp. indica cv. KDML105) to salt stress. 30, 247–253.
- Chen, J., Wang, XJHEP, 2006. Beijing: Plant physiology experimental guide. 24(25), 55–56.
- Chen, Y., Cao, X., Lu, Y., Wang, XJBoec, 2000. Toxicology: Effects of rare earth metal ions and their EDTA complexes on antioxidant enzymes of fish liver. 65(3), 357–365. [DOI] [PubMed]
- Di Martino, C., Delfine, S., Pizzuto, R., Loreto, F., Fuggi, AJNp, 2003. Free amino acids and glycine betaine in leaf osmoregulation of spinach responding to increasing salt stress. 158(3), 455–463. [DOI] [PubMed]
- Egamberdieva, D., Wirth, S., Bellingrath-Kimura, S.D., Mishra, J., Arora, N.K.J.F.i.M., 2019. Salt-tolerant plant growth promoting Rhizobacteria for enhancing crop productivity of saline soils. 10. [DOI] [PMC free article] [PubMed]
- El-Beltagi, H.S., Ahmed, S.H., Namich, A.A.M., Abdel-Sattar, RRJFEB, 2017. Effect of salicylic acid and potassium citrate on cotton plant under salt stress. 26, 1091–1100.
- Esper Neto, M., Britt, D.W., Lara, L.M., Cartwright, A., dos Santos, R.F., Inoue, T.T., Batista, MAJA, 2020. Initial development of corn seedlings after seed priming with nanoscale synthetic zinc oxide. 10(2), 307.
- Farooq, M., Hussain, M., Wakeel, A., Siddique, K.H.J.A.f.S.D., 2015. Salt stress in maize: effects, resistance mechanisms, and management. A review. 35(2), 461-481.
- Fiaz, S., Noor, M.A., Aldosri, FOJJotSSoAS, 2018. Achieving food security in the Kingdom of Saudi Arabia through innovation: Potential role of agricultural extension. 17(4), 365–375.
- Govorov, A.O., Carmeli, IJNl, 2007. Hybrid structures composed of photosynthetic system and metal nanoparticles: plasmon enhancement effect. 7(3), 620–625. [DOI] [PubMed]
- Hänsch R., Mendel R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl) Curr. Opinion Plant Biol. 2009;12(3):259–266. doi: 10.1016/j.pbi.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Hirt, H., De Zelicourt, A., Saad, M., 2020. Compositions and methods for increasing salt tolerance in plants. In: US Patent App. 16/733,068.
- Hossain, M.A., Mostofa, M.G., Fujita, MJMPB, 2013. Cross protection by cold-shock to salinity and drought stress-induced oxidative stress in mustard (Brassica campestris L.) seedlings. 4.
- Hurry, V.M., Strand, A., Tobiaeson, M., Gardestrom, P., Oquist, GJPP, 1995. Cold hardening of spring and winter wheat and rape results in differential effects on growth, carbon metabolism, and carbohydrate content. 109(2), 697–706. [DOI] [PMC free article] [PubMed]
- Hussain, S., Shaukat, M., Ashraf, M., Zhu, C., Jin, Q., Zhang, J., 2019. Salinity stress in arid and semi-arid climates: Effects and management in field crops. In: Climate Change and Agriculture. IntechOpen https://www.intechopen.com/books/climate-change-and-agriculture/salinity-stress-in-arid-and-semi-arid-climates-effects-and-management-in-field-crops.
- Iqbal M.S., Singh A.K., Singh S.P., Ansari M.I. Nanomaterials and Environmental Biotechnology. Springer; 2020. Nanoparticles and plant interaction with respect to stress response; pp. 1–15. [Google Scholar]
- Kamran, M., Parveen, A., Ahmar, S., Malik, Z., Hussain, S., Chattha, M.S., Saleem, M.H., Adil, M., Heidari, P., Chen, J.-T.J.I.J.o.M.S., 2020. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. 21(1), 148. [DOI] [PMC free article] [PubMed]
- Khan, I.A., Movva, S., Shaik, N.A., Chava, S., Jahan, P., Mukkavali, K.K., Kamineni, V., Hasan, Q., Rao, PJMG, 2014. Investigation of Calpain 10 (rs2975760) gene polymorphism in Asian Indians with gestational diabetes mellitus. 2, 299–306. [DOI] [PMC free article] [PubMed]
- Khan, I.A., Jahan, P., Hasan, Q., Rao, PJJoHS, 2015. Relationship between PTEN and gestational diabetes in Asian Indians womens. 3(3), 184.
- Khan, I.A., Jahan, P., Hasan, Q., Rao, PJD, 2019. Research MSC, Reviews: Genetic confirmation of T2DM meta-analysis variants studied in gestational diabetes mellitus in an Indian population. 13(1), 688–694. [DOI] [PubMed]
- Kim, I., Viswanathan, K., Kasi, G., Thanakkasaranee, S., Sadeghi, K., Seo, JJFRI, 2020. ZnO nanostructures in active antibacterial food packaging: preparation methods, antimicrobial mechanisms, safety issues, future prospects, and challenges. 1–29.
- Lee Y.-C., Moon J.-Y. Introduction to Bionanotechnology. Springer; 2020. Bionanotechnology in agriculture, food, cosmetic and cosmeceutical; pp. 199–217. [Google Scholar]
- Li, J., Hu, J., Xiao, L., Gan, Q., Wang, YJW, 2017. Air, Pollution S: Physiological effects and fluorescence labeling of magnetic iron oxide nanoparticles on citrus (citrus reticulata) seedlings. 228(1),52.
- Lichtenthaler, H., 1987. Chlorophylls and caroteniods pigments of photosynthetic biomembranes in Methods in Enzymology vol. 148, 183-350. In.: Academic Press Orlando FI, USA.
- Mao, G., Xu, X., Xu, ZJCJoE-A, 2004. Advances in physiological and biochemical research of salt tolerance in plant. 12(1), 43–46.
- Maroufpour N., Mousavi M., Abbasi M., Ghorbanpour M. Biogenic Nano-Particles and their Use in Agro-ecosystems. Springer; 2020. Biogenic nanoparticles as novel sustainable approach for plant protection; pp. 161–172. [Google Scholar]
- Masilamani, P., Arulmozhiselvan, K., Alagesan, A.J.J.o.A., 2020. Science N: Prospects of biodrainage to mitigate problems of waterlogging and soil salinity in context of India-A review. pp. 229–243.
- Mittal, S., Kumari, N., Sharma, VJPP, 2012. Biochemistry: Differential response of salt stress on Brassica juncea: photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. 54, 17–26. [DOI] [PubMed]
- Mittler, RJTips, 2002. Oxidative stress, antioxidants and stress tolerance. 7(9), 405–410. [DOI] [PubMed]
- Mohsenzadeh, S., Moosavian, SSJAJoPS, 2017. Zinc sulphate and nano-zinc oxide effects on some physiological parameters of Rosmarinus officinalis. 8(11), 2635–2649.
- Mukherjee, S., Choudhuri, MJPp, 1983. Implications of water stress‐induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. 58(2), 166–170.
- Munns, R., 2008. Tester MJARPB: Mechanisms of salinity tolerance. 59, 651–681. [DOI] [PubMed]
- Murakeözy, É.P., Nagy, Z., Duhazé, C., Bouchereau, A., Tuba, ZJJopp, 2003. Seasonal changes in the levels of compatible osmolytes in three halophytic species of inland saline vegetation in Hungary. 160(4), 395–401. [DOI] [PubMed]
- Parihar, P., Singh, S., Singh, R., Singh, V.P., Prasad, SMJES, 2015. Research P: Effect of salinity stress on plants and its tolerance strategies: a review. 22(6), 4056–4075. [DOI] [PubMed]
- Pavithra, G., Reddy, B.R., Salimath, M., Geetha, K., Shankar, AJIjopp, 2017. Zinc oxide nano particles increases Zn uptake, translocation in rice with positive effect on growth, yield and moisture stress tolerance. 22(3), 287–294.
- Rizwan, M., Ali, S., ur Rehman, M.Z., Adrees, M., Arshad, M., Qayyum, M.F., Ali, L., Hussain, A., Chatha, S.A.S., Imran, MJEP, 2019. Alleviation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. 248, 358–367. [DOI] [PubMed]
- Rossi, L., Fedenia, L.N., Sharifan, H., Ma, X., Lombardini, LJPp, 2019. Biochemistry: Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. 135, 160–166. [DOI] [PubMed]
- Sakir, M., Salem, S., Sanduvac, S.T., Sahmetlioglu, E., Sarp, G., Onses, M.S., Yilmaz, E.J.C., 2020. Physicochemical SA, Aspects E: Photocatalytic green fabrication of Au nanoparticles on ZnO nanorods modified membrane as flexible and photocatalytic active reusable SERS substrates. 585, 124088.
- Salama D.M., Osman S.A., Abd El-Aziz M.E., Abd Elwahed M.S.A., Shaaban E.A. Effect of zinc oxide nanoparticles on the growth, genomic DNA, production and the quality of common dry bean (Phaseolus vulgaris) Biocatal. Agric. Biotechnol. 2019;18:101083. [Google Scholar]
- Shiri, T., Gaurav, S.S., Singh, S., Kumar, A., Sharma, S., Teja, S. Health benefits of nutritive value and qualitative symptoms in Okra (Abelmoschus Esculentus): A.
- Silveira, J.A., Carvalho, FEJJop, 2016. Proteomics, photosynthesis and salt resistance in crops: An integrative view. 143, 24–35. [DOI] [PubMed]
- Singh, A., Singh, N., Hussain, I., Singh, H., Singh, SJIJPSI, 2015. Plant-nanoparticle interaction: an approach to improve agricultural practices and plant productivity. 4(8), 25–40.
- Tang, X., Mu, X., Shao, H., Wang, H., Brestic, MJCrib, 2015. Global plant-responding mechanisms to salt stress: physiological and molecular levels and implications in biotechnology. 35(4), 425–437. [DOI] [PubMed]
- Venkatachalam P., Priyanka N., Manikandan K., Ganeshbabu I., Indiraarulselvi P., Geetha N., Muralikrishna K., Bhattacharya R.C., Tiwari M., Sharma N., Sahi S.V. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.) Plant Physiol. Biochem. 2017;110:118–127. doi: 10.1016/j.plaphy.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Ye, Y., Medina-Velo, I.A., Cota-Ruiz, K., Moreno-Olivas, F., Gardea-Torresdey, JLJE, 2019. safety e: Can abiotic stresses in plants be alleviated by manganese nanoparticles or compounds? 184, 109671. [DOI] [PubMed]
- Yemm, E., Willis, AJBj, 1954. The estimation of carbohydrates in plant extracts by anthrone. 57(3), 508. [DOI] [PMC free article] [PubMed]
- Zhao, C., Zhang, H., Song, C., Zhu, J.-K., Shabala, S.J.T.I., 2020. Mechanisms of plant responses and adaptation to soil salinity. 1(1):100017. [DOI] [PMC free article] [PubMed]