Abstract
In this study, eighteen sites at Jeddah coastal area, Red Sea, have been assessed for water quality status, depending on nutrients, metals, Chlorophyll-a (Chl-a) and physical variables during 2018 and 2019. The investigated parameters of the Water Quality Index (WQI) are temperature, pH, salinity, dissolved oxygen (DO), DO saturation, oxidizable organic matter (OOM), suspended particulate matter (SPM), Chl-a, ammonium, nitrite, nitrate, total nitrogen, reactive phosphate, total phosphorus, silicate, Zn, Fe, Mn Cu, Cd, Pb, and Ni. The results revealed that the pH values were slightly alkaline with a range of 7.85–8.20. The results of other parameters were as follow: salinity (36.95–42.61PSU), DO (5.22–6.67 mg/L), OOM (0.40–1.23 mg/L), SPM (12.39–21.5 mg/L), Chl-a (0.10–0.83 µg/L). The range of nutrients (μM) were 0.07–0.22, 0.45–1.47, 9.62–18.64, 23.31–57.65, 0.05–0.15, 0.55–2.78 and 2.54–5.51 for NH4/N, NO2/N, NO3/N, TN, PO4/P, TP and SiO4/Si, respectively. Cluster analysis was used to classify the stations studied. From the current study, five clusters were found, indicating the need to perform cluster analysis in the water quality assessment process to confirm the durability and consistency of the data discovered in the current application.
Keywords: Nutrient Salts, Metals, Contaminants, Seawater, Jeddah, Saudi Arabia
1. Introduction
The Red Sea is about 1930 km long and 280 km wide. The surface of the Red Sea is about 437,000 square kilometers, with an average depth of 490 m. The Red Sea is famous for its marine life and coral. The number of invertebrates on the Red Sea is higher than 1000 species, with 200 soft and hard coral reefs (Hassan et al., 2002, Fahmy et al., 2016). Seawater quality has a major concern, with safety and accessibility, throughout the world. Water contaminated with excess chemicals causes a highly risk on health. In recent times high levels of contaminants and waste elements at the global level are being thrown into the marine environment.
The problem of the disposal of trace metals waste has many obvious effects on marine environment such as increased levels of waste in water, sediments and organisms, reduced productivity (Shriadah et al., 2004). Thereby increasing human exposure to many harmful environmental problems. The economic impacts on the fishing and tourist trades have been related to the degree of environmental conditions deterioration. Due to metals toxic effect and their bioaccumulation, the metals demonstrate a significant and serious contaminant source that is associated with human activities in marine environments (Abohassan, 2013). Due to the increase of anthropogenic, agricultural activities, nitrogen and phosphorus inputs, nutrient contaminants (nitrate, phosphate and silicate) have adverse effects on human health due to the eutrophication widespread problem (De Jonge et al., 2002). Nitrogen and phosphorus are also generated from human and industrial wastes (Palaniappan et al., 2012).
Signs of eutrophication in surface waters show an increase in primary product rates to an abnormal level. This result in a significant increase in the growth of vascular plants, the occurrence of blooming phenomenon, and a severe decrease in the concentration of dissolved oxygen in aquatic systems, which affects the water environment (Palaniappan et al., 2012, Bhagowati and Ahamad, 2018). The effects of anthropogenic activities on the marine conditions of shorelines have been investigated in some Jeddah sites (Badr et al., 2009, Abu-Zied et al., 2013, Abu-Zied et al., 2016, Al-Mur et al., 2017, Al-Mur, 2019a, Al-Mur, 2019b). The main objectives of this study are to monitor and assess physical and chemical characteristics as well as trace elements contamination and eutrophication status of seawater along the Red Sea coast at Jeddah City, Saudi Arabia.
2. Material and methods
The area of study is in the middle region of the Red Sea between longitude 38° 90′00″ − 39° 10′00″ E and latitude 21° 20′00″ − 22°00′00″ N (Fig. 1). This area has been characterized by a rare rainfall and extreme evaporation with tropical to subtropical climate (Chen et al., 2016). The north to north–northwest wind is usually occurred throughout the year (Omar, 2013).
Fig. 1.

Sampling location of the studied eighteen sites (indicated by red dots) on the Jeddah coastal area, Red Sea.
2.1. Sampling and chemical analysis
Samples were collected from different sites such as Sharm Obhur, Jeddah Sea Port, Salman Bay, Southern Corniche, Downtown, and Al-Khumrah industrial area. Seawater samples were collected from eighteen sites, at each station and during each sampling period seawater was collected in duplicate, stored in 0.5-liter polyethylene containers in the icebox and analyzed in vitro. Different analytical tools such as salinity, temperature, pH as well as the oxygen content (DO) were determined at each site by using CTD (YSI-6000). The oxidizable organic matter (OOM) was performed using KMnO4 (Calberg, 1972).
Total suspended matter (TSM) was separated by filtration through a 0.45 μm GF/C filter paper from seawater samples. The filters containing the trapped particles were then washed and dried at 65 °C for 48 h until the weight was constant. The difference in weight before and after filtration is measured in mg/L (IOC, 1983). Water samples for nutrient salts were separated after sampling collection by using GF/C filters. The samples were remained frozen until analyzed by using calorimetric techniques (Grasshoff, 1976). According to APHA (1998), Nitrate (NO3/N) was analyzed using reduction column and color reagent (sulphanilamide and N-(1-naphthyl)- ethylene diamine dihydrochloride).
Ammonia in water (NH4+/N) was fixed in the field without filtration (indophenol blue colorimetry method). Total nitrogen (TN) and total phosphorus (TP) were analyzed in the non-filtered water samples by the method described by Valderrama (1981). Nutrient salts were analyzed by double beam UV/V spectrophotometer (Shimadzu UV-150-02). Chlorophyll-a in water samples was extracted with 90% acetone and analyzed spectrophotometrically and its concentration was calculated using the SCORE-UNESCO equation (Jeffrey and Humphrey, 1975).
Heavy metals analysis in seawater was performed by Martin method (1972) whereas trace metals were extracted and analyzed. Water sample (750 ml) was filtered through membrane filter of 0.45 μM. The pH of water sample was adjusted (4–5) with dilute HCl (Boniforti et al., 1984, Martin, 1972). Trace metals were extracted using APDC-MIBK as a solvent extraction to chelate the metals. Ammonium pyrolidine thiocarbamate (APDC) used to perform complexation with the extracted metals into methyl isobutyl ketone (MIBK). Heavy metals contaminants (Zn, Fe, Mn, Cu, Cd, Pb and Ni) in the final extracts were determined using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) (Perkin Elmer, NexION 300D).
NASS-5 reference material (from NRC- Canada) was used as a quality control sample. The used glassware was cleaned by detergent and soaked in 10% HNO3. A batch of synthetic seawater samples were analyzed by eight measurements. The detection limit was 0.055, 0.010, 0.010, 0.009, 0.04,0.01 and 0.02 μgL−1 for Fe, Zn, Mn, Pb, Cu, Cd and Ni, respectively. The data precision was designated as a coefficient of variation (CV). CV with a value of 10% was determined by three replicate analyses of one sample. For accuracy measurement, natural seawater (750 ml) was spiked using NASS-5 reference material. The metals spiked recovery values were 92%, 93%, 95%, 90%, and 94% for Cu, Pb, Mn, Fe, and Zn, respectively. The data analysis of the metals is demonstrated in Table 1.
Table 1.
Min, max and average ± SD of the hydrographic parameters, nutrient salts and Chl-a concentrations in the coastal surface water, Red Sea, Jeddah, during March 2018–2019.
| Parameters | Mar-18 | Mar-19 | Parameters | Mar-18 | Mar-19 |
|---|---|---|---|---|---|
| Temp.(°C) | 22.91–23.05 | 21.99–23.21 | TN(μM) | 23.31–49.72 | 27.22–57.65 |
| 22.99 ± 0.04 | 22.33 ± 0.31 | 40.01 ± 7.99 | 46.10 ± 9.47 | ||
| pH | 7.96–8.20 | 7.85–8.16 | PO4(μM) | 0.07–0.13 | 0.05–0.15 |
| 8.07 ± 0.07 | 8.04 ± 0.09 | 0.10 ± 0.02 | 0.10 ± 0.03 | ||
| Salinity | 37.21–42.45 | 36.95–42.61 | TP(μM) | 0.55–2.78 | 0.74–2.58 |
| 40.59 ± 1.62 | 40.60 ± 1.77 | 1.67 ± 0.74 | 1.84 ± 0.62 | ||
| DO (mg/L) | 5.22–6.55 | 5.86–6.67 | SiO4(μM) | 2.54–5.51 | 2.58–5.42 |
| 6.01 ± 0.4 | 6.31 ± 0.27 | 4.10 ± 0.91 | 3.85 ± 0.97 | ||
| DO(Sat.%) | 75.9–102 | 86.2–101.1 | Fe (µg/L) | 19.33–42.12 | 12.5–68.58 |
| 88.07 ± 6.58 | 92.43 ± 4.57 | 29.33 ± 7.21 | 30.81 ± 18.74 | ||
| OOM (mg/L) | 0.51–1.14 | 0.40–1.23 | Mn (µg/L) | 0.81–2.76 | 1.18–4.63 |
| 0.88 ± 0.21 | 0.91 ± 0.27 | 1.56 ± 0.56 | 2.46 ± 1.16 | ||
| SPM (mg/L) | 12.39–21.2 | 13.1–21.5 | Zn (µg/L) | 11.20–33.60 | 13.32–35.70 |
| 16.33 ± 3.12 | 17.24 ± 3.15 | 22.02 ± 8.00 | 19.52 ± 6.83 | ||
| Chl-a(µg/L) | 0.10–0.78 | 0.15–0.83 | Cu (µg/L) | 0.92–2.90 | 1.55–5.38 |
| 0.42 ± 0.21 | 0.45 ± 0.21 | 1.67 ± 0.60 | 3.15 ± 1.14 | ||
| NH4 (μM) | 0.07–0.20 | 0.08–0.22 | Pb (µg/L) | 0.84–1.93 | 1.14–2.34 |
| 0.12 ± 0.13 | 0.16 ± 0.20 | 1.36 ± 0.30 | 1.67 ± 0.36 | ||
| NO2(μM) | 0.45–1.25 | 0.75–1.47 | Cd (µg/L) | 0.11–0.30 | 0.15–0.84 |
| 0.89 ± 0.27 | 1.06 ± 0.21 | 0.18 ± 0.05 | 0.52 ± 0.22 | ||
| NO3(μM) | 9.62–16.53 | 8.71–18.64 | Ni (µg/L) | 1.15–2.14 | 1.24–1.91 |
| 13.87 ± 2.34 | 14.52 ± 2.95 | 1.47 ± 0.35 | 1.60 ± 0.21 |
2.2. Statistical analyses
Statistical and correlation analysis for the samples were carried out using SPSS version 23, to explore the relationship between hydrographic, nutrient salts and trace metals in surface seawater. The range and average concentration of the variables were calculated during the period of study for18 sample sites at fixed statistical significance level (p = 0.05 and p = 0.01).
The data structures were explained in various studies by multivariate statistical techniques such as cluster analysis (CA) and principal component analysis (PCA) (Vialle, et al., 2011). The nearest neighbor program, which is called SPSS-23, was applied for the data structures analysis. Correlative and cluster analysis were applied for a sample quantitative analysis of 22 variables (temperature, pH, salinity, dissolved oxygen (DO), DO saturation, oxidizable organic matter (OOM), suspended particulate matter (SPM), Chl-a, ammonium, nitrite, Nitrate , total nitrogen, reactive phosphate, total phosphorus, silicate, and heavy metals concentration).
The TRIX index was calculated using formula applied by Vollenweider et al., 1998, Peng, 2015.
TRIX = Log10(Chl-a * [%DO] * DIN * SRP + 1.5) / 1.2
This method relies on four important basic variables associated with productivity such as chlorophyll, oxygen and dissolved inorganic nitrogen) DIN), whereas, DIN = NO3/N + NO2/N + NH4/N(. The absolute value of the oxygen saturation deviation (DO%) is calculated as [100- %DIN].
3. Results and discussion
3.1. Physicochemical characteristics
Water temperature has a vital role in the metabolism of aquatic ecosystems. When the water temperature rises, the toxicity of heavy elements becomes more dangerous for aquatic life. The variations of spatial and temporal surface water temperature values (°C) are shown in Fig. 2. The temperature values ranged between 22.91 and 23.05 °C during March 2018 and from 21.99 to 23.21 °C during March 2019 with an average value of 22.99 ± 0.04 in 2018 and 22.33 ± 0.31 °C in 2019. Slight variation was recorded during the seasons of study period.
Fig. 2.
Spatiotemporal variation of the physicochemical parameters, DOM and Chl-a values in the study area.
The salinity changes were noticed due to mixing of fresh and marine water. The coastal water salinity of Jeddah can be affected mostly by the wastewater from the urbanized surrounding area. The spatiotemporal surface salinities are presented in Fig. 2, the average salinity values showed limited changes with an average of 40.60 ± 1.77 PSU. The minimum salinity (around 37.00 PSU) was found at St. 6 and the maximum (42.00 PSU) was found at stations 11 and 17. The higher salinity results can be attributed to occurrence of high temperature and absence of river discharges into the Red Sea.
The pH of seawater is an important parameter for the biological activities in the marine environment. It is a reflection of the state of pollution and productivity. The pH values of natural seawater are around 8.2. The pH values explained by the regional and temporal distribution as shown in Fig. 2. It was found that the spatial changes of the pH values of the study area were undetectable within the studied sites during the years 2018 and 2019, where the pH data of the present period has shown that all sites showed no trends in the magnitude of pH readings (Fig. 2). The pH ranged between 7.96 and 8.20 (8.07 ± 0.07) during the year 2018 and 7.85–8.16 (8.04 ± 0.09) during the year 2019 (Table 1).
There is an increase in phytoplankton and photosynthesis processes, which results in a higher pH value and an increase in the dissolved oxygen concentration (Sawidis and Bellos, 2005, Abu-Zied et al., 2013). This was confirmed from the positive relationship exists in the pH values of water with dissolved oxygen (r = 0.515, n = 18, p < 0.005), where the two parameters were used as good indicator for the production level.
One of the most important variables in the marine environment is the dissolved oxygen (DO), which is one of the factors that determines the different water masses and the role of distribution of domestic production and consumption to a large extent. In Jeddah coastal area, the absolute DO values varied between 5.22 mg /l at St. 6 during 2018 and 6.67 mg /l at St. 14 during 2019. The regional distribution of the DO showed anomalous behavior (Fig. 2). The average concentrations during 2018 and 2019 were 6.01 ± 0.40 and 6.31 ± 0.27 mg/l, respectively (Table 1).
Based on temporal variations, the DO content revealed slight variations. The high values in surface water can be attributed to the exchange of O2 from the air to the surface water as well as to algal photosynthesis (Abu-Zied et al., 2013). On another hand, the DO lower values of surface water could be related to marine life respiration, biochemical reactions and organic matter decomposition.
The oxidizable organic matter (OOM) may cause eutrophication in the water, where a sudden bloom of algae occurs due to the presence of high amount of limiting nutrients. The degradation of organic wastes by aerobic microbes produces carbon dioxide as a byproduct. Then, carbonic acid is produced as a result of the dissolution of CO2 into the water, resulting in an increase in the acidity of the water and the environment becomes not suitable for the growth of some fishes due to development of low pH which has an adverse effect on the solubility of heavy metals. An acidic environment helps the growth of some organisms that are dangerous to humans and where they have the ability to live in such conditions with pH<7.
In the present work OOM fluctuated between a minimum of 0.40 mg/l at St.2 and a maximum of 1. 23 mg/l at St.14 during 2019 (Fig. 2). The average concentrations during 2018 and 2019 were 0.88 ± 0.21 mg/l and 0.91 ± 0.27 mg/l, respectively. The results of total suspended matter (TSM) are shown in Table 1. It ranged between 12.39 mg/l at St. 1 during 2018 and 21.50 mg/l at St. 12 during 2019, with averages of 16.33 ± 3.12 and 17.24 ± 3.15 mg/l during 2018 and 2019, respectively. The TSM concentrations reported in this work were very close to the previous study in the Red Sea coast (Fahmy et al., 2016, Heba et al., 2004). The TSM ranged from 1.08 to 38.11 mg/l, while it showed lower concentrations than those reported by Heba et al. (2004) (TSM ranged from 10 to 440 mg/l).
Chlorophyll-a (Chl-a) is one of the main pigments that can be used to measure phytoplankton biomass (Carlson, 1977). In general, there are high concentrations of the Chl-a in waters containing high level of nutrients originated from urban runoff, fertilizers, sewage treatment plants. In the present work, the Chl-a varied between 0.10 μg/l (St.1) and 0.78 μg/l (St.17) with an average of 0.42 ± 0.21 μg/l during 2018 and ranged from 0.15 μg/l (St.2) and 0.83 μg/l (St. 17) with average of 0.45 ± 0.21 μg/l during 2019 (Table 1 and Fig. 3). These results are different from the work stated by Faragallah et al. (2009), reporting values of 0.41 μg/l and 78.68 μg/l with an average of 13.57 μg/l. The Chl-a concentrations showed lower values at different sites in comparison to the rest of the Red Sea coastal area of Egypt, where they ranged from 0.24 to 2.46 μg/l (Guerguess et al.,2009), but they are similar to those reported by Eladawy (2017), reporting Chl-a values of 0.1–1.0 mg L-1.
Fig. 3.
Spatiotemporal variation of NH4, NO2, PO4, TP, NO3, TN and SiO4 values in the study area.
3.2. Nutrient salts
Ammonium is the main nitrogenous product resulting from the decomposition of organic material containing nitrogen, and this is done by microbial degradation. It is also an important release product from invertebrates and vertebrates. Also, ammonia is one of the inorganic forms of nitrogen and is preferred for aquatic plants via absorption (Faragallah et al., 2009). A high ammonium concentration could lead to a high or even higher phytoplankton productivity if its cells utilizes NH4+/N rather than NO3– /N (Dugdale et al., 2007). In the present study; NH4+/N fluctuated between 0.07 μM at St.8 and 0.20 μM at St.15 during 2018, while it was ranged from 0.08 μM at St. 8 to 0.22 μM at St. 11 during 2019 (Fig. 3 and Table 1). The values of NH4+/N in this study ranged from 0.12 ± 0.13 to 0.16 ± 0.20 μM, displaying no clear regional variations. Generally, these values differed slightly from those observed by Fahmy et al. (2016) of 0.23–4.03 μM and Faragallah et al. (2009) of 2.24 μM.
Nitrite (NO2–) concentration fluctuated between 0.45 μM at St.1 and 1.25 μM at St.16 during 2018, while it was ranged from 0.75 μM at St. 6 to 1.47 μM at St. 2 during 2019 (Table 1 and Fig. 3). The average of NO2– concentration during the study period ranged from 0.89 ± 0.0.27 to 1.06 ± 0.21 μM during 2018 and 2019, respectively. The results of NO2– concentration during 2018–2019 were in the range values obtained by Fahmy et al. (2016) of 0.02–0.72 μM.
The nitrate ions (NO3–) were very stable, showing the most common inorganic nitrogen compound in the oxygenated seawaters of the present study. The NO3– concentration during the period of investigation was fluctuated between 8.71 μM at St.3 and 18.64 μM at St.11 during 2019 with an average value of 13.87 ± 2.34 μM and 14.52 ± 2.95 μM during 2018 and 2019, respectively (Table 1 and Fig. 3). These values were higher than those recorded by Fahmy et al. (2016) of 0. 17–6.93 μM, while they were lower than those recorded by Madkour and Dar (2007) of 0.42–72.02 μM. Total nitrogen (TN) varied between 23.31 μM (St.1) during March 2018, but it showed the highest value of 57.65 μM (St.5) during March 2019. Its mean values were 40.01 ± 7.99 and 46.10 ± 9.47 μM during 2018 and 2019, respectively (Table 1 and Fig. 3).
The dissolved inorganic nitrogen (DIN) represents the sum of NO2–+NO3–+NH4+ which is assimilated by aquatic organisms. The results showed that the average NO3–/N concentration (15.97 μM) represents 91.37% of the total dissolved inorganic nitrogen (17.36 μM). The average value of NO2–/N concentration was 0.15 μM, representing 0.87% of the DIN. It is clear that from the average percentage values of different N species relative to DIN, the NO3-N is the most abundant forms, while NO2/N and NH4/N occurred as secondary constituents.
The present data showed that the abundance of inorganic nitrogen forms followed this order: nitrate ≫ ammonium ~ nitrite. The absorption of these inorganic nitrogen forms by phytoplankton can be demonstrated by the correlation between the variables such as nitrate and ammonium (r = 0.487, ρ ≤ 0.05). Significant positive correlations between Chl-a and NO3-N, NH4-N and TN (r = 0.592, 0.494 and 0.866 at p ≤ 0.05 &p ≤ 0.01) were recorded (Table 3).
Table 3.
Pearson correlation coefficients between the physicochemical parameters, nutrient salts and metals of the seawater of Jeddah coastal area.
| Temp. | pH | Salinity | DO | DOM | SPM | Chl-a | NH4 | NO2 | NO3 | TN | PO4 | TP | SiO4 | Fe | Mn | Zn | Cu | Pb | Cd | Ni | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temp. | 1 | ||||||||||||||||||||
| pH | 0.151 | 1 | |||||||||||||||||||
| Salinity | 0.165 | 0.044 | 1 | ||||||||||||||||||
| DO | 0.020 | −0.315 | 0.499* | 1 | |||||||||||||||||
| OOM | 0.089 | 0.639** | 0.098 | −0.056 | 1 | ||||||||||||||||
| SPM | 0.184 | 0.258 | 0.637** | 0.536* | 0.502* | 1 | |||||||||||||||
| Chl-a | 0.009 | 0.622** | 0.434 | 0.073 | 0.750** | 0.706** | 1 | ||||||||||||||
| NH4 | −0.006 | 0.201 | 0.006 | −0.196 | 0.348 | 0.201 | 0.494* | 1 | |||||||||||||
| NO2 | 0.005 | −0.575* | 0.074 | 0.386 | −0.325 | −0.200 | −0.448 | −0.151 | 1 | ||||||||||||
| NO3 | 0.059 | 0.423 | 0.393 | 0.177 | 0.619** | 0.618** | 0.592** | 0.487* | −0.210 | 1 | |||||||||||
| TN | 0.149 | 0.677** | 0.326 | 0.080 | 0.837** | 0.663** | 0.866** | 0.438 | −0.519* | 0.711** | 1 | ||||||||||
| PO4 | 0.021 | 0.245 | 0.136 | −0.209 | 0.085 | 0.098 | 0.331 | 0.649** | −0.340 | 0.158 | 0.309 | 1 | |||||||||
| TP | 0.001 | 0.563* | 0.611** | 0.211 | 0.744** | 0.803** | 0.863** | 0.267 | −0.414 | 0.669** | 0.861** | 0.176 | 1 | ||||||||
| SiO4 | −0.016 | 0.299 | 0.495* | 0.467 | 0.434 | 0.778** | 0.572* | −0.032 | −0.140 | 0.503* | 0.496* | −0.252 | 0.636** | 1 | |||||||
| Fe | −0.043 | 0.402 | −0.456 | −0.584* | 0.308 | −0.322 | 0.052 | 0.060 | −0.533* | −0.109 | 0.197 | 0.281 | 0.085 | −0.484* | 1 | ||||||
| Mn | 0.160 | −0.425 | −0.286 | −0.146 | −0.231 | −0.402 | −0.338 | −0.385 | 0.442 | −0.605** | −0.461 | −0.351 | −0.435 | −0.373 | 0.105 | 1 | |||||
| Zn | 0.228 | −0.352 | 0.326 | 0.353 | −0.007 | 0.331 | −0.519 | 0.028 | 0.545* | −0.112 | −0.193 | −0.315 | 0.021 | 0.414 | 0.649** | 0.238 | 1 | ||||
| Cu | −0.005 | 0.063 | −0.737** | −0.338 | −0.043 | −0.515* | −0.602 | −0.043 | −0.049 | −0.255 | −0.189 | −0.169 | −0.448 | −0.543* | 0.562 | 0.245 | −0.379 | 1 | |||
| Pb | −0.187 | −0.267 | −0.375 | −0.247 | −0.254 | −0.364 | −0.366 | 0.123 | 0.128 | −0.584* | −0.438 | 0.286 | −0.348 | −0.621** | 0.402 | 0.270 | 0.036 | 0.299 | 1 | ||
| Cd | 0.224 | 0.198 | 0.184 | 0.248 | 0.382 | 0.461 | 0.377 | 0.214 | −0.438 | 0.263 | 0.490* | 0.288 | 0.485* | 0.049 | 0.363 | −0.188 | −0.229 | 0.059 | 0.100 | 1 | |
| Ni | −0.118 | −0.120 | 0.080 | 0.006 | −0.187 | −0.070 | −0.236 | −0.199 | 0.239 | −0.095 | −0.340 | −0.112 | −0.036 | −0.023 | 0.132 | 0.271 | 0.096 | -0.198 | 0.203 | 0.034 | 1 |
Correlation is significant at the 0.05 level.
Correlation is significant at the 0.01 level. No. of samples = 18
Reactive phosphate (PO4/P) is an essential nutrient which plays a major role in biological metabolism, photosynthesis and other biological processes in plants. The minimum PO4/P concentrations value was 0.07 μM at St.12 during 2018 and 0.05 μM at St.3 during 2019, while the maximum value was 0.13 and 0.15 μM at St.8 and St. 15 during the study period. The phosphate sustained relatively low concentrations in the present study with absolute values were between 0.05 and 0.15 μM, and an average value of 0.10 ± 0.02 μM during the study period (Table 1 and Fig. 3). Total phosphorus (TP) fluctuated between 0.55 μM (St.2) and 2.78 μM (St.7) during March 2018, while during March 2019, it ranged from 0.74 μM at St. 1 to 2.58 μM at St. 8. The TP average was 1.67 ± 0.74 μM and 1.84 ± 0.62 μM during 2018 and 2019 as shown in Table 1 and Fig. 3. The highest concentration of total phosphorus (TP) in seawater of the middle study area at St.’s (5–10) are very important factors to verify the pollution status on the coastal system because they are a reflection of the anthropogenic impact and the recreational use of some beaches and intensive human activities and the effluent inputs of waste, sewage and recycled processes in the coastal waters. The TP concentrations during the study period were low compared to the previous work of Fahmy (2003) who mentioned that the spatiotemporal distribution pattern of TP revealed a large variability in the Red Sea of Egypt with TP ranged from 0.49 to 2.13 μM.
Silicate (SiO4) is one of the major constituents in seawater. The heterogeneous distribution of silicate in the study area indicates that, the source of silicate is not allachthonus from the drains, but its main source is autochthonous through the production of biogenic silicate by diatoms, which could cause preservation in silicate content distribution (Verschuren et al., 1998). The concentration ranges and spatiotemporal average values of SiO4 were presented in Table 1 and Fig. 3.
The concentrations of SiO4 ranged between 2.54 μM at St.6 and 5.51 μM at St. 14 during March 2018, while the same range was recorded during March 2019, ranging from 2.58 μM at St. 5 to 5.42 μM at St.18. The average values were 4.10 ± 0.91 μM and 3.85 ± 0.97 μM during the study period (Table 1). These values are within the ranges and averages recorded by Madkour and Dar, 2007, Fahmy et al., 2016. Generally, the lowest SiO4 values and other nutrient salts were observed, due to low population density and pollution from nearby land dominated by hot, arid climate (Fahmy et al., 2016).
3.3. Distribution of trace metals
To determine the status and severity of dissolved elements in a specific region, the concentration of dissolved metals for each site is compared with the minimum concentrations of these soluble elements for water quality standards (WQC, 1972). A concentration of 50 µg/L for Fe, 20 µg/L for Mn and 10 µg/L for Cu, Pb and Cd, 7 µg/L for Ni, 20 µg/L for Zn, 2 µg/L for Mn, represents minimal risk of deleterious effect.
3.3.1. Iron and manganese
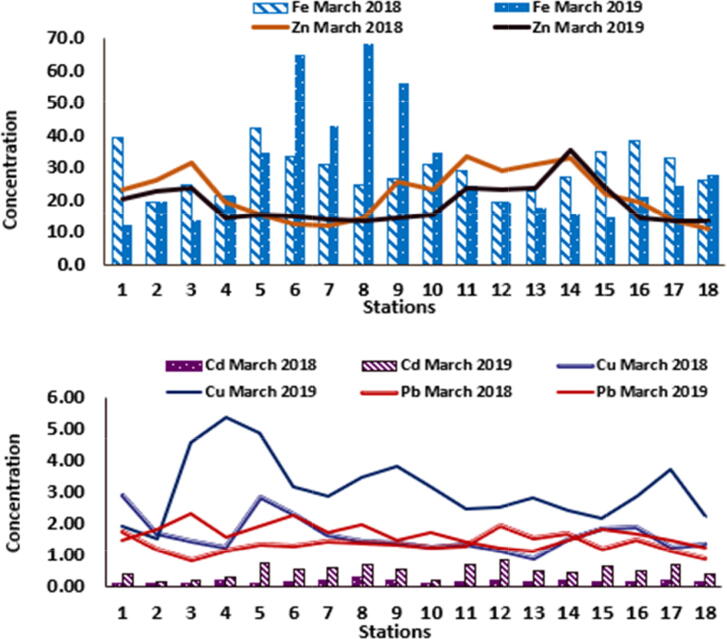
The spatiotemporal variation of dissolved iron and manganese contents along the coastal region of study is shown in Table 1 and Fig. 4, Fig. 5. The level of dissolved Fe fluctuated from 12.50 μg L−1 at St. 1 and 68.58 μg L−1 at St.8 (during 2019), the average concentration of Fe during the studied period was around 30.81 ± 18.74 μg L−1. The Mn concentrations in the area ranged from 0.81 μg L−1 at St.18 (during 2018) to 4.63 μg L−1 at St.2 (during 2019); the range of the average content of Mn was between 1.56 ± 0.56 μg L−1 and 2.46 ± 1.16 μg L−1 (Fig. 5). The contents of dissolved iron and manganese (Fig. 4, Fig. 5) showed a wide variability, the different between the high and low concentration of both iron and manganese were approximately two to five-folds.
Fig. 4.
Spatiotemporal distribution of Fe, Zn, Cd, Cu and Pb concentrations in the study area.
Fig. 5.
Spatiotemporal distribution of Ni and Mn concentrations in The surface water of the study area.
In general, the dissolved iron and manganese values revealed greater concentration in the Red Sea of the study region (increasing by fifteen- and four-times, respectively) than those recorded in the previous study (Martin and Whitfield 1983). They were lower than the smaller risk content for criteria of water quality; with values of 50 and 20 µg/L for iron and manganese, respectively (WQC, 1972) (Table 4). Weak negative correlations were recorded between salinity and both iron and manganese (r = − 0.456, −0.286, respectively), indicating a decrease in iron and manganese with the increase of saline water (Hariri and Abu-Zied, 2018). The lower concentration of both manganese and iron with the increase in salinity may be explained by the Fe+2 oxidation process to iron hydroxides.
Table 4.
Dissolved trace-metal concentrations (μg L−1) in the Jeddah coastal waters, Red Sea, compared with those reported in the literature.
| Location | Fe | Mn | Zn | Cu | Pb | Cd | Ni | References |
|---|---|---|---|---|---|---|---|---|
| Coastal waterJeddah Red Sea | 12.5–68.6 | 0.81–4.63 | 11.2–35.70 | 0.92–5.38 | 0.84–2.34 | 0.11–0.84 | 1.15–2.14 | Present study |
| Northern Red Sea and the Gulf of Aqaba | 0.56–4.4 | 0.06–0.21 | 0.13–1.17 | 0.07–0.29 | 0.02–0.68 | 0.02–0.78 | 0.05–0.52 | Shriadah et al., (2004) |
| Red Sea -Jeddah coast | – | – | 6–14 | 4–9 | 1.1–27 | – | – | Hamza and Amierh (1992) |
| Red Sea (Coast, Egypt) | 16.2 | 0.83 | – | 5.1 | – | – | – | Saad and Kandeel (1988) |
| Red Sea (Suez Gulf) | 39.4–40.78 | 2.30–2.53 | 14.8–16.5 | 4.34–6.93 | 4.16–4.66 | 0.40–0.67 | 1.31–1.66 | Abo-El-Khair et al., (2016) |
| Mediterranean Sea | 11.92–30.45 | 5.79–17.36 | 0.86–7.40 | 0.30–0.83 | 0.53–10.31 | – | 0.51–2.90 | Okbah and Nasr (2006) |
| Coastal water | 2.0 | 0.4 | 2.5 | 1.0 | 0.03 | – | – | Martin and Whitfield (1983) |
| Minimal Risk Conc. | 50.0 | 20.0 | 20.0 | 10.0 | 10.0 | – | 2.0 | WQC (1972) |
3.3.2. Copper and zinc
The spatiotemporal distribution of dissolved copper in the study area is shown in Fig. 4. During the period of study, the values of dissolved copper concentration fluctuated between a minimum of 0.92 μg L−1 at station 13 during 2018 and a maximum of 5.38 μg L−1 at station 4 during 2019. The annual average concentrations of dissolved copper were in the range of 1.67 ± 0.60 and 3.15 ± 1.14 μg L−1in 2018 and 2019, respectively (Table 1). Dissolved zinc values at the studied sites of the coastal region of Jeddah are shown in Fig. 4. Zinc concentration ranged from 11.20 μg L−1 at station 18 during 2018 and 35.70 μg L−1 at station 14 during 2019. The annual distribution of dissolved zinc varied from 22.02 ± 8.00 μg L−1 to 19.52 ± 6.83 μg L−1 during 2018 and 2019, respectively. Generally, there is a strong influence of biological factors on bioaccumulation of the metals. Zinc is naturally available and considered as one of the known contaminants in the residues of agricultural and food waste, pesticides, and anti-corrosion paints (Badr et al., 2009, Mortuza and Al-Misned, 2017).
In the present study, phytoplankton plays an important role in the distributing of dissolved minerals via absorption. A significant negative correlation between Ch1-a and both copper and zinc; r = -0.519 and −0.602 (respectively) were found confirming the role of Fe in scavenging the other metals. In the other hand, the solubility of copper and zinc is greatly affected by the solubility of Fe. The contents of dissolved copper and zinc of the coastal area revealed lower levels than the smaller risk content for criteria of water quality of 10 and 20 µg/L for copper and zinc, respectively (WQC, 1972) (Table 4).
3.3.3. Lead, cadmium, and nickel
The spatiotemporal variations of dissolved lead, cadmium, and nickel concentrations along the coastal area of study are shown in Table 1 and Fig. 4, Fig. 5. The absolute values of dissolved lead ranged from 0.84 μg L−1 at St. 3 (during 2018) and 2.34 μg L−1 at the same station (during 2019). The temporal average content of lead showed slightly increasing trend from 1.36 ± 0.30 μg L−1 in 2018 to 1.67 ± 0.36 μg L−1 during 2019. The minimum cadmium concentration during the period of study was 0.11 and 0.15 μg L−1 at St. 2, while the maximum content was 0.30 at St. 8 (during 2018) and 0.84 at St. 12 during 2019. The range of the average content of cadmium was between 0.18 ± 0.05 μg L−1 and 0.52 ± 0.22 μg L−1 (|Fig. 4).
The levels of dissolved nickel showed a slight variability with a low content at St.11 (1.15 μg L−1) and the high level (2.14 μg L−1) at St.8 during 2018 (|Fig. 5). The average concentration of nickel ranged from 1.47 ± 0.35 μg L−1 to 1.60 ± 0.21 μg L−1 during the study period. The difference between the high and low absolute values were approximately increased two folds. Generally, the levels of dissolved nickel are lower than the smaller risk content for the criteria of water quality 7 µg/L for nickel (WQC, 1972) (Table 4). Generally, the mean concentrations of iron in the two seasons exhibited slight differences, with no obvious seasonal variations. While the concentration of dissolved manganese, copper, lead and cadmium generally increased during 2019. The concentrations of zinc and Nickle showed no obvious temporal distribution patterns; this may indicate that the main sources of the dissolved metals are an integration of many playing factors (Fig. 4, Fig. 5).
3.4. Statistical analysis
3.4.1. N/P ratio
The readily bioavailable N and P for the growth of phytoplankton are mainly in forms of dissolved inorganic nitrogen (DIN) and PO4/P. The average data of DIN and PO4/P during the period of study (2018–2019) showed that the N/P ratios varied from 110 at St. 2 to 204 at St. 9 (Table 2) with an irregular distribution fluctuated between 110 and 143 (4 Stations) and from 143 to 176 (11 Stations), whereas the highest values of N/P ratio were observed at 3 stations (176–209). The N/P ratio in the present study is significantly higher than the assimilatory optimal N/P = 16/1 ratio reported by Redfield et al. (1963).
Table 2.
Average of N/P ratios and Trophic State Index of the coastal surface water, Red Sea, Jeddah, March 2018–2019.
| St.’s | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N/P | Aver. | 159 | 110 | 147 | 199 | 125 | 154 | 126 | 147 | 204 | 153 | 165 | 144 | 167 | 154 | 133 | 150 | 175 | 186 |
| TRIX | 2018 | 1.48 | 1.72 | 1.74 | 1.97 | 1.99 | 2.39 | 1.9 | 1.93 | 2.3 | 2.43 | 2.28 | 2.21 | 2.05 | 2.31 | 2.46 | 2.54 | 2.45 | 2.43 |
| 2019 | 1.7 | 1.97 | 1.75 | 1.95 | 2.33 | 2.28 | 2.2 | 2.06 | 2.34 | 2.37 | 2.42 | 2.32 | 2.25 | 2.17 | 2.5 | 2.41 | 2.57 | 2.37 |
According to the previous studies, nitrogen and phosphorus are the limiting factors for the growth of marine algae. Phosphorous is the limiting factor for marine algal growth when the ratio of phosphorous and nitrogen more than six. On the other hand, nitrogen is the limiting factor for the growth when the ratio is less than 4.5 (Chiaudani and Vighi, 1978). The ratios of phosphorous and nitrogen are in the range of 4.5 and 6 that considered the optimal assimilation ratio of nutrient approaches. The present data revealed extreme variation of N/P ratio along the Red Sea coast of Jeddah, particularly at areas exposed to land-based runoff.
3.4.2. Trophic index (TRIX)
Water quality index is an important tool to summarize the complex of water quality data. The ecological risks of nitrogen and phosphorus were assessed using the trophic index (TRIX) formulas calculated according to Vollenweider et al., (1998). The multimetric trophic index TRIX is another important acceptable method for evaluating coastal eutrophication. The TRIX collects major effect and outcome variables of eutrophication conditions including environmental disorder, biological response, and stress response (Primpas and Karydis, 2011).
The TRIX has been chosen as an assessment reference for coastal eutrophication in this study. It is a linear mixture of several variables related to primary production (Chl-a and O2), DIN (NO2–+NO3–+NH4+) and PO4 (Melaku et al., 2003). Five category scale were defined for water quality state (Moncheva et al., 2002) such as excellent (oligotrophic), very good (low trophic level with TRIX < 4), good (moderate trophic level with 4 < TRIX < 5), fair (high trophic level with 5 < TRIX < 6), and poor (very high trophic level with TRIX > 6), which revealed high nutrient levels, low transparency, and recurrent hypoxia/anoxia in bottom waters (Peng, 2015).
The TRIX model is used to express the state of prosperity and water quality for a wide range of components of elements, especially nitrogen and phosphorous, causing the state of prosperity if they are present in high concentrations from the surrounding areas. This model has been applied in many water bodies and on coastal marine waters in many seas in Europe. There are many regions where this model has been applied, for example: the Adriatic Sea, the Tyrrhenian Sea, the Black Sea, the eastern Mediterranean Sea and the Caspian Sea (Tugrul et al., 2011)
The eutrophication of seawater quality of the selected coastal area was assessed. The trophic index of the study period ranged from 1.48 at St.1 to 2.54 at St.16 during 2018, showing a slight variation than those calculated during 2019 which ranged between 1.70 at St.1 and 2.57 at St. 17. Generally, low trophic index (TRIX < 4) was recorded in the study area (Table 2 and Fig. 6) and the eutrophication range showed no ecological risk based on the investigated of the water quality status during 2018–2019.
Fig. 6.
Distribution of TRIX Values in the surface water of the study area.
3.4.3. Factor analysis
In general, the variables including nutrient salts and metals effect, and environmental hydrographic parameters were classified into two groups according to their correlation with biological response (Chl-a). The first group is located in the right part of the graph (Fig. 7); it includes salinity, SiO4, SPM, NO3, TN, TP, PH, OOM, NH4, PO4, Fe and Chl-a. The second one lie in the left part of the graph and includes the DO, NO2, Cu, Zn and Mn.
Fig. 7.
PCA of the physicochemical and metals variables.
3.4.4. Cluster analysis (CA)
In recent years, there have been an effective application of multivariate statistical methods such as cluster analysis (CA) and principal component analysis (PCA) in the assessment of surface water quality, evaluation of spatiotemporal variations in coastal seawater and in identifying pollution sources (Pekey et al., 2004, Ouyang et al., 2006, Abu-Zied et al., 2013). The CA is one of the common methods used in multivariate statistical analysis that it is used to classify the data into groups depending on their (dis-) similarity. It is used here to assess surface water quality (Shrestha and Kazama, 2007, Tokatli et al., 2014).
The CA was applied to the data set in respect of seawater quality during the period of study, using 22 variables and 18 different sites. This study showed the benefit of multivariate statistical techniques for assessing and interpreting large data sets for water quality, identifying sources of pollution, and obtaining better concepts of water quality. The variables that are measured using this type of analysis are classified into different categories, so that all similar variables are set together into one group. Then the clusters of the resulting bodies should reveal high internal and high external homogenization (between groups). These hierarchical groups are the most common approach, which shows similar relationships between samples, or the interconnections between variables and each other and this is usually illustrated in the form of a tree diagram.
In this study, cluster analysis was applied using physical and chemical analysis as well as Chl-a data of the coastal area, Jeddah, Red Sea. The dendrogram as shown in Fig. 8 revealed the association of all analyzed variables in five major associations. The first level of aggregation on the right side of the dendrogram revealed association between DO and metals (Zn, Ni, Cd, and Pb) as well as NO2. The second level of aggregation is established with the pair Chl-a-TN, SPM-TP, DOM-NO3 and Salinity-SiO4which aggregates with the first aggregation level. At the same time, the third clusters of aggregation on left side of the dendrogram forms a cluster with the pair of Fe-Cu and both of PO4 and NH4. Finally, the next stage of these clusters is associated with the third clusters of aggregation on left side of the dendrogram, it shows an association with the pair Temp.-Mn, and pH which at the same time forms the fourth clusters.
Fig. 8.
Dendrogram of the cluster analysis showing the groups of studied stations in the study area.
Also, in this study, CA was used to classify the selected stations of the coastal area, Jeddah, Red Sea. The dendrogram among stations showed five distinct groups (Fig. 9). The first group lie on left side of the dendrogram included stations located in the northern part (St.’s 1, 2 and 3), and the changes in water quality in them were mainly due to Salman Bay. The second group included three stations (St.’s 5, 6 and 7), which are located in the northern part; it is affected by the drainage wastewater from Sharm Obhur. The third group lie on left side of the dendrogram including St.’s 4, 8, 9 and 10, which are presented in the middle and northern parts of the study area where water quality in these stations is mainly affected by residential sources of pollutants from Jeddah port. The fourth group covers St.’s 11, 12, 13 and 14, situated in the southern part of the study area, on the right side of the dendrogram. It is noticeable that the water quality of this region is affected mainly by human activity at the Southern Corniche. The fifth group includes stations located in the southern part of the study area (St.’s 15, 16, 17 and 18). It can be said that differences between groups may be an indication of differences in pollution sources.
Fig. 9.
Dendrogram of the cluster analysis showing the groupings of physicochemical nutrient salts and metals parameters.
3.4.5. Correlation matrix
Pearson correlation analysis depends on the variability of two parameters. Correlation analysis was achieved to reveal the relationship between any pair of variables. A correlation analysis was carried out in order to reveal the relationship between nutrient salts, metals and hydrographic parameters (Table 3). There were several significant correlations between the studied parameters. High positive correlations were found between chlorophyll-a (Chl-a) and each of NH4, NO3, TN, TP and SiO4 (r = 0.494, 0.592, 0.866, 0.863 and 0.572, respectively at P ≤ 0.01 and 0.05, N = 18). Suspended particulate matter (SPM) showed a positive and significant correlation with Chl-a, NO3, TN, TP and SiO4 (r = 0.706, 0.618, 0.663, 0.803 and 0.778, respectively at P ≤ 0.01 and 0.05, N = 18).
The oxidizable organic matter (OOM) revealed significant positive correlation with SPM, Chl-a NO3, TN and TP (r = 0.502, 0.750, 0.619, 0.837 and 0.744, respectively at P ≤ 0.01 and 0.05, N = 18). The pH values are positively correlated with OOM (r = 0.639), Chl-a (0.622), TN (r = 0.677), TP (r = 0.563), while it is negatively correlated with NO2 (r = −0.575). Fe showed positive correlation with pH (r = 0.402 at P ≤ 0.05) and negative correlations with DO (r = -0.584), salinity (r = −0.456) and SPM (r = -0.322). Mn showed negative correlations with pH, salinity and SPM (r = −0.425, −0.286 and −0.402). Cu showed significant negative correlations with salinity (r = −0.737) and SPM (r = −0.515). Weak correlations between both Zn and Cd and other environmental parameters pH, salinity, DO, SPM and Chl a were observed. In general, the observed associations between dissolved elements and environmental variables are related to different behaviors, sources, and sinks of these metals.
4. Conclusions
The chlorophyll-a revealed high positive significantly correlation with both NH4 and SiO4 (r = 0.494, 0.572) at P ≤ 0.05) and with both of NO3, TN and TP, (r = 0.592, 0.866, 0.863 at P ≤ 0.01, respectively. Dissolved nitrogen, reactive phosphate and silicate concentrations were relatively low, and allowed classifying Jeddah Red Sea coastal water as oligotrophic to mesotrophic. From the average percentage values of different N forms relative to DIN, it can be indicated that NO3-N is the abundant forms, representing 91.37% from the total dissolved inorganic nitrogen (17.36 μM), while NO2-N and NH4-N are the least constituent in the studied seawater samples. The mean concentrations of dissolved Fe, Mn, Zn, Cu, Pb, Cd and Ni, in the coastal area of Jeddah, Red Sea were within the ranges of Minimal Risk Concentrations. This indicates that the seawater of Jeddah, Red Sea was not polluted by these metals.
Declaration of Competing Interest
I declare that this manuscript is original, has not been published before and is not currently being considered for publication elsewhere. I wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
The author gratefully acknowledges the Deanship of Scientific Research (DSR) for its technical and financial support. This work was supported by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant no. B: 11-155-1439.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abo-El-Khair E.M., Fattah L.M.A., Abdel-Halim A.M., AbdElnaby M.A., Fahmy M.A., Ahdy H.H., Hemeilly A., El-Soud A.A., Shreadah M.A. Assessment of the hydrochemical characteristics of the suez gulf coastal waters during 2011–2013. J. Environ. Protection. 2016;7:1497–1521. [Google Scholar]
- Abohassan R.A. Heavy metal pollution in avicennia marina mangrove systems on the red sea coast of Saudi Arabia. JKAU: Met., Env. & Arid Land Agric. Sci. 2013;24:35–53. [Google Scholar]
- Abu-Zied R.H., Basaham A.S., El Sayed M.A. Effect of municipal wastewaters on bottom sediment geochemistry and benthic foraminifera of two Red Sea coastal inlets, Jeddah Saudi Arabia. Environ. Earth Sci. 2013;68:451–469. [Google Scholar]
- Abu-Zied R.H., Al-Dubai T.A.M., Bantan R.A. Environmental conditions of shallow waters alongside the southern Corniche of Jeddah based on benthic foraminifera, physico-chemical parameters and heavy metals. J. Foramin. Res. 2016;46:149–170. [Google Scholar]
- Al-Mur B.A., Quicksall Andrew N., Ahmed M.A. Al-Ansari.: Spatial and temporal distribution of heavy metals in coastal core sediments from the Red Sea, Saudi Arabia. Oceanologia. 2017;122:456–463. [Google Scholar]
- Al-Mur B.A. Geochemical fractionation of heavy metals in sediments of the Red Sea, Saudi Arabia. Oceanologia. 2019;62:31–44. [Google Scholar]
- Al-Mur B.A. Assessing the ecological risks from hydrocarbons in the marine coastal sediments of Jeddah Red Sea. Environ. Monit. Assess. 2019;191:180. doi: 10.1007/s10661-019-7262-1. [DOI] [PubMed] [Google Scholar]
- APHA. Standard methods for the examination of water and wastewater (19th ed.). Washington, D.C.: American Public Health Association. (1998).
- Badr N.B.E., El-Fiky A.A., Mostafa A.R. Al-Mur B.A.: metal pollution records in core sediments of some Red Sea coastal areas, Kingdom of Saudi Arabia. Environ. Monit. Assess. 2009;155:509–526. doi: 10.1007/s10661-008-0452-x. [DOI] [PubMed] [Google Scholar]
- Bhagowati B., Ahamad K.U. A review on lake eutrophication dynamics and recent developments in lake modeling. Ecohydrol. Hydrobiol. 2018;3:002. [Google Scholar]
- Boniforti R., Ferraroli R., Frigileri P., Heltai D., Queirazza G. Intercomparison of five methods for the determination of trace metals in sea water. Anal. Chem. Acta. 1984;162:33. [Google Scholar]
- Calberg, S.R.: New Baltic Manual. International Council for the Exploration of the Sea. Comparative Research Report. Copenhagen. Series A, 29. (1972).
- Carlson R.E.A. Trophic state index for lakes. Limnol. Oceanog. 1977;22:363–369. [Google Scholar]
- Chen J., Wu H., Qian H. Groundwater nitrate contamination and associated health risk for the rural communities in an agricultural area of Ningxia, northwest China. Exposure Health. 2016;8:349–359. [Google Scholar]
- Chiaudani G., Vighi M. Metodologia standard di saggioalgale per lostudiodell acontaminazione delleacque marine. Quad. Instituto di RicercaSulleAcque (IRSA) 1978;39:120–130. [Google Scholar]
- De Jonge V.N., Elliott M., Orive E. Causes, historical development, effects and future challenges of a common environmental problem: eutrophication. Hydrobiologia. 2002;475:1–19. [Google Scholar]
- Dugdale R.C., Wilkerson F.P., Hogue V.E., Marchi A. The role of ammonium and nitrate in spring bloom development in San Francisco Bay. Estuar. Coast. Shelf Sci. 2007;73:17–29. [Google Scholar]
- Eladawy A. Characterization of the northern Red Sea's oceanic features with remote sensing data and outputs from a global circulation model. Oceanologia. 2017;59:213–237. [Google Scholar]
- Fahmy M. Water quality in the Red Sea coastal waters (Egypt): Analysis of spatial and temporal variability. Chem. Ecol. 2003;19:67–77. [Google Scholar]
- Fahmy M.A., Fattah L.M.A., Abdel-Halim A.M., Aly-Eldeen M.A., Abo-El-Khair E.M., Ahdy H.H., Hemeilly A., El-Soud A.A., Shreadah M.A. Evaluation of the quality for the egyptian red sea coastal waters during 2011–2013. J. Environ. Protect. 2016;7:1810–1834. [Google Scholar]
- Faragallah H.M., Tadros H.R.Z., Okbah M.A. Nutrient salts and chlorophyll-a during short term scale in the Eastern Harbour, Alexandria (Egypt) Egypt. J. Aquat. Res. 2009;35:243–250. [Google Scholar]
- Grasshoff.: K Methods of seawater analysis, Verlag ChemieWeikeim, NewYork. 22, 1103 (1976).
- Guerguess M.Sh., Mohamed A., Shreadah Mamdouh A., Fahmy E., Aboel-khair Ahmed M. Abdel-halim.: assessment of water quality in the Red Sea using in situ measurements and remote sensing data Egypt. J. Aquat. Res. 2009;35:1–13. [Google Scholar]
- Hamza A.G., Amierh T.A. Determination of Pb, Cd, Cu and Zn ions in Red Sea water along Jeddah coast by differential pulse anodic stripping voltammetry. J. Fac. Sci., UAE Univ. 1992;4:80–88. [Google Scholar]
- Hariri M.S.B., Abu-Zied R.H. Factors influencing heavy metal concentrations in the bottom sediments of the Al-Kharrar Lagoon and Salman Bay, eastern Red Sea coast Saudi Arabia. Arab. J. Geosci. 2018;11:495. [Google Scholar]
- Hassan, M.; Kotb, M.M.A.; Al-Sofyani, A.A.: Status of Coral Reefs in the Red Sea-Gulf of Aden. In: Wilkinson, C.R., Ed., Status of Coral Reefs of the World: GCRMN Report, Australian Institute of Marine Science, Townsville, Chapter. 2, 45-52 (2002).
- Heba H. M.A.; AL-Edresi H. T.; AL-Saad.; M. A. Abdelmoneim.: Background Levels of Heavy Metals in Dissolved, Particulate Phases of Water and Sediment of Al-Hodeidah Red Sea Coast of Yemen. JKAU Mar. Sci. 15, 53-71 (2004).
- Intergovernmental Oceanographic Commission (IOC) Chemical Methods for Use in Marin Environmental Monitoring. Manuals and Guides, UNESCO, 53. (1983).
- Jeffrey S.W., Humphrey G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochemie Physiologie der Pflanzen. 1975;167:191–194. [Google Scholar]
- Madkour H.A., Dar M.A. The anthropogenic effluents of the human activities on the Red Sea coast at Hurghada harbour (Case study) Egypt. J. Aqua. Res. 2007;33:43–58. [Google Scholar]
- Martin D.F. Marcel Dekker; New York: 1972. Atomic absorption spectroscopy. Marine Chemistry Analytical Boniforti Methods; p. 225. [Google Scholar]
- Martin T.M., Whitfield M. In: Trace Metals in Sea Water. Wong C.S., editor. Plenum Press; New York: 1983. p. 265. [Google Scholar]
- Melaku C.D., Solidoro C., Umgiesser G. Modelling the response of the Lagoon of Venice o variations in physical forcings. Ecol. Model. 2003;170:265–289. [Google Scholar]
- Moncheva S., Dontcheva V., Shtereva G., Kamburska L., Malej A., Gorinstein S. Application of eutrophication indices for assessment ofthe Bulgarian Black Sea coastal ecosystem ecological quality. Wat. Sci. Technol. 2002;46:19–28. [PubMed] [Google Scholar]
- Mortuza MG.; Al-Misned FA.: Environmental Contamination and Assessment of Heavy Metals in Water, Sediments and Shrimp of Red Sea Coast of Jizan, Saudi Arabia. J Aquat. Pollut. Toxicol, 1:1. I Med Pub Journals. (2017).
- Okbah M.A., Nasr S.M. Dissolved trace-metal concentrations along the Mediterranean Sea, to the north of the Nile Delta Region Egypt. Chem. Ecol. 2006;22:125–135. [Google Scholar]
- Omar H.H. Red sea water and biochemical composition of seaweeds at southern coast of jeddah Saudi Arabia. Life Sci. J. 2013;2013(10):1073–1080. [Google Scholar]
- Ouyang Y., Nkedi-Kizza P., Wu Q., Shinde D., Huang C. Assessment of seasonal variations in surface water quality. Water Res. 2006;40:3800–3810. doi: 10.1016/j.watres.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Palaniappan, M.; Gleick, P. H; Allen, L.; Cohen, M.J.; Christian-Smith, J.; and Smi, C.: Water Quality. In the World’s Water Volume 7 The Biennial Report on Freshwater Resources (ed, Gleick, P. H.), Island Press: 45-72 (2012).
- Pekey H., Karakas D., Bakog L.M. Source apportionment of trace metals in surface waters of a polluted stream using multivariate statistical analyses. Mar. Pollut. Bull. 2004;49:809–818. doi: 10.1016/j.marpolbul.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Peng S. The nutrient, total petroleum hydrocarbon and heavy metal contents in the seawater of Bohai Bay, China: Temporal–spatial variations, sources, pollution statuses, and ecological risks. Mar. Pollut. Bull. 2015;95:445–451. doi: 10.1016/j.marpolbul.2015.03.032. [DOI] [PubMed] [Google Scholar]
- Primpas I., Karydis M. Scaling the trophic index (TRIX) in oligotrophic marine environments. Environ. Monit. Assess. 2011;178:257–269. doi: 10.1007/s10661-010-1687-x. [DOI] [PubMed] [Google Scholar]
- Redfield, A.C.; Ketchum, B.H.; Richards, F.A.: The influence oforganisms on the composition of seawater. In the Sea, Ed. M.N. Hill. WileyInterscience, New York, 26- 79 (1963).
- Saad M.A.H., Kandeel M.M. Distribution of copper, iron and manganese in the coastal Red Sea Waters in front of Al-Ghardaqa. Proc. Indian Natn. Sci. Acad. 1988;54:642. [Google Scholar]
- Sawidis T., Bellos D. Chemical pollution monitoring of the River Pinions (Thessalian-Greece) J. Environ. Manage. 2005;76:282–292. doi: 10.1016/j.jenvman.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Shrestha S., Kazama F. Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin Japan. Environ. Modell. Software. 2007;22:464–475. [Google Scholar]
- Shriadah, M. A.; Okbah M. A.; EL-Deek M. S.: trace metals in the water columns of the red sea and the gulf of Aqaba, Egypt Water, Air, and Soil Pollution. 153, 115–124 (2004).
- Tokatli C., Çiçek A., Emiroğlu Ö., Arslan N., Köse E., Dayıoğlu H. Statistical approaches to evaluate the aquatic ecosystem qualities of a significant mining area: emet stream basin (Turkey) Environ. Earth Sci. 2014;71:2185–2197. [Google Scholar]
- Tugrul S., Uysal Z., Erdogan E., Yucel N. Kilikya Baseni (Kuzeydogu Akdeniz) sularinda otrofikasyon indicatoru parametrelerin (TP, DIN, Chl-a ve TRIX) degisimi. Ekoloji. 2011;20:33–41. [Google Scholar]
- Valderrama J.C. The simultaneous analysis of total nitrogen and totalphosphorus in natural waters. Mar. Chem. 1981;10:109–122. [Google Scholar]
- Verschuren D., Edgington D.N., Kling H.J., Johnsonl Th.C. silica depletion in lake victoria: sedimentary signals at offshore stations. J. Great Lakes Res. 1998;24:118–130. [Google Scholar]
- Vialle C., Sablayrolles C., Lovera M., Jacob S., Huau M.C., Montréjaud-Vignoles M. Monitoring of water quality from roof runoff: Interpretation using multivariate analysis. Water Res. 2011;45:3765–3775. doi: 10.1016/j.watres.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Vollenweider R.A., Giovanardi F., Montanari G., Rinaldi A. Characterization of the trophic conditions of marine coastal waters, withspecial reference to the NW Adriatic Sea: proposal for a trophic scale, turbidityand generalized water quality index. Environmetrics. 1998;9:329–357. [Google Scholar]
- WQC A Report of the Committee on Water Quality Criteria, p. 593, NAS, Washington, DC (1972).