Abstract
The study, which covers the period between 2014 and 2018, was carried out in the city of Naberezhnye Chelny, Republic of Tatarstan, Russia. The aim of the study was to examine the biochemical response of maple trees growing in the anthropogenic environments. Leaf samples from 600 trees (Acer platanoides L. and Acer negundo L.) were collected at monthly intervals from June through August. Sampling was performed early in the morning (11 a.m.) in the middle of the month. The study offers statistical data on the tannin content, determined via permanganometry; the ascorbic acid concentration, found via titration with 2.6-dichlorophenolindophenol; the ascorbate oxidase activity determined by absorbance at 265 nm; and the polyphenol oxidase activity, found by the spectrophotometric method. Relatively higher ascorbate oxidase activity was detected in August among ash-leaved Acer platanoides L. and Acer negundo L. in areas with strong anthropogenic impact. Due to increased air pollution, maple trees were found to exhibit an increase of polyphenol oxidase activities. The condensed tannin content in Norway maple trees dropped over time: by 1.24 in July (avenue); by 0.94 (buffer area) and 0.76 (avenue) in August. The condensed tannin content in the ash-leaved maple trees also decreased: by 0.69 (buffer area) and 0.22 (avenue) in July; by 0.37 (buffer area) and 0.61(avenue) in August.
Keywords: Ascorbate oxidase activity, Ascorbic acid, Norway maple (Acer platanoides L.), Polyphenol oxidase activity, Sah-leaved maple (Acer negundo L.), Tannin, Urban trees
1. Introduction
The most important mechanism of plant resistance in anthropogenic conditions is the antioxidant defense system, the biochemical basis of which consists of low molecular weight and high molecular weight substances with antioxidant properties. These substances play an important role in redox reactions and facilitate neutralization of reactive oxygen species. Among them are tannins (Shah et al., 2015, Alici and Arabaci, 2016), polyphenol oxidase, ascorbic acid (Hafez and Gharib, 2016, Liang et al., 2017) and ascorbate oxidase (Anahita et al., 2015, Akram et al., 2017, Fox et al., 2018).
Polyphenol oxidases in combination with phenolic substrates are involved in plant respiration at the intermediate stages of hydrogen transfer. The activity of polyphenol oxidase (PPO) increases in damaged tissues. Ascorbate oxidase (AAO) participates in the neutralization of reactive oxygen species, protecting the plant organism and preventing the occurrence of oxidative stress (Kraj, 2016, Bukharina et al., 2018, Tran et al., 2018). Tree species continuously exchange different gaseous pollutants in and out of the foliar system and are very sensitive to gaseous pollutants. Hence, they serve as bioindicators. Due to air pollution, the foliage surface undergoes various structural and functional changes (Sen et al., 2017). Ascorbic acid is crucial in redox homeostasis. It has been suggested that its content in trees indicates the degree of environmental pollution (Bilska et al., 2019). Studies on different plant species confirmed a decline of ascorbic acid in the leaves during the vegetation period (Mitrofanova et al., 2016, Skrynetska et al., 2019). Tannins, which belong to the group of phenolic compounds, are an essential substance involved in the protection of woody plants from both abiotic and biotic stresses. Among other functions, they take part in biological oxidation processes, interact with proteins, and serve as growth regulators (Kuzmin et al., 2017).
Particular attention is paid to the parallel activities of non-enzymatic and enzymatic systems. Enzymatic antioxidants exhibit high specificity in action, while non-enzymatic antioxidants normally are not specific for the oxidant (Bela et al., 2015). For example, a study on the antioxidant properties of deciduous shrubs Cassia alata L. explores the functioning of the enzymatic system and the photosynthetic apparatus in different abiotic environments. It was found that in the occurrence of abiotic stress, the activity of catalase, ascorbate peroxidase and glutathione reductase increases. The role of antioxidant enzymes, proline, abscisic acid, phenolics and the ascorbic acid in poplar and green tea plants was defined. These plant species showed a specific response to abiotic stress, manifested in the increase of the enzymatic antioxidant activity and a decrease of proline and abscisic acid contents (Garcia et al., 2016, Kloseiko, 2016).
Studies provide no information about the behavior of binary non-enzymatic and enzymatic systems of antioxidant defense. The quantitative research on changes in the components of the antioxidant defense system is, however, available. This work aims to identify the biochemical characteristics of the Norway maple (Acer platanoides L.) and the ash-leaved maple (Acer negundo L.) growing in the urban areas. The choice of trees stemmed from the fact that Norway maple and ash-leaved maple tree species were widespread across the city. The lifespan of these trees is up to 200 years. They are fast-growing species that are frost-resistant, wind-resistant, and undemanding to the soil type. While Acer platanoides was native to the Republic of Tatarstan, Acer negundo was introduced to the city landscape. The hypothesis of the study is that Acer platanoides and Acer negundo growing in green areas and sanitary protection zones around industrial facilities (buffer areas) have a specific biochemical response to the growing conditions.
2. Materials and methods
The study, which covers the period between 2014 and 2018, was carried out in the city of Naberezhnye Chelny, Republic of Tatarstan, Russia (55°43′31.4″N/52°24′40.3″E). The research objects were the Norway maple (Acer platanoides L.) and the ash-leaved maple (Acer negundo L.) growing in the buffer areas around the foundry and forging facilities, Kamaz PJSC, and along the avenues (Mira Avenue, Kazansliy Avenue). The control population consisted of trees from the Chelninskoye forest district and a park in the city of Naberezhnye Chelny.
Naberezhnye Chelny is a large industrial city of the Republic of Tatarstan. Naberezhnye Chelny along with other cities such as Nizhnekamsk, Mendeleevsk, and Elabuga belongs to the Kama economic cluster. The city is characterized by a high level of air pollution. Production volumes in this region continuously grow and the number of vehicles increases causing an adverse impact on environment and human health.
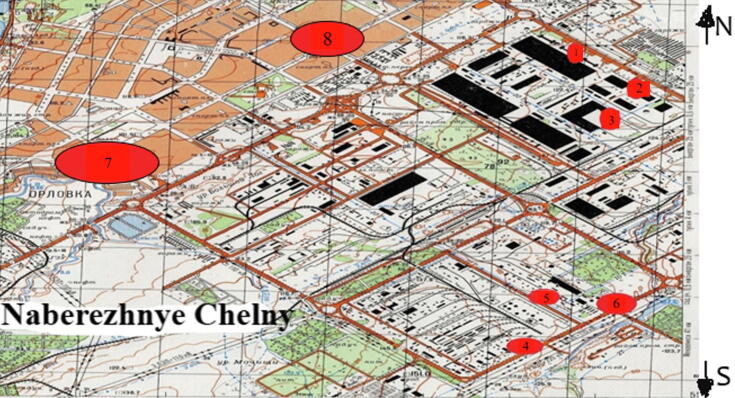
For this study, three pilot plots with geometrically different landscape-driven configurations (triangle and square) were established via area sampling. As vertices of geometrically shaped plots were interconnected within a continuous network, the edge length of plots varied in the range from 100 m to 1–2 km depending on the vegetation density. Sampling areas were plotted on a topographic map or aerial photograph prior to finding them in the field. All plots were created using a compass and a tape measure. The geometry-based approach to area sampling is suitable for plains where the moisture profile is uniform and where vehicles can move freely. The advantages of this method are representativeness of the sample and convenient data processing. The plot size was 0.25 ha. The tree sampling procedure involved selecting mid-reproductive trees in good ontogenetic state (at least 10 per plot), which were then exposed to biochemical analysis (Fig. 1).
Fig. 1.
Map of Naberezhnye Chelny showing the location of pilot plots (scale 1: 50.000): 1, 2, 3 −buffer areas around forging facilities; 4, 5, 6−buffer areas around foundry facilities; 7−green zone along Kazansliy Avenue; 8−green zone along Mira Avenue.
The level of air pollution around the woody plants was estimated by measuring the total air pollution index (API). The Integrated Air Pollution Index is used to rank single districts and cities in terms of their pollution. It is a relative indicator associated with the concentration of the hazardous substance in the air within the territory under study, its maximum allowable concentration (MAC), and the amount of pollutants. The integrated API index is calculated using data from stationary observations. The calculation was guided by the following principles: risk to the human health depends on the harmful substance; the risk of health damage grows with the MAC value.
The level of air pollution from one substance was generally expressed through the partial API index, calculated by the formula:
where: C is the mean substance concentration; MAC is the daily average maximum allowable concentration; and ‘Cd’ is a dimensionless constant to convert the pollutant harmfulness degree to that of sulfur dioxide.
The API value of 12.6 found for the period from 2013 to 2018 suggested a high level of air pollution in the city.
Shade foliage samples were collected on the southern side of the trees from the annual vegetative shoots during the period of active vegetation (months of June, July, and August). The sampling side of threes lining the avenues was facing the road. All research samples were collected within the same day.
The content of condensed tannins in the foliage samples was determined in laboratory conditions via permanganometry. The ascorboric acid was determined via titration with 2,6-dichlorophenolindophenol (DCPIP), which exhibits decolorization in the presence of ascorbic acid. When all the ascorbic acid in the solution was used up, the solution became pink due to the lack of electrons that could reduce the colored DCPIP compound.
AAO activities were estimated by absorbance (also known as optical density) at 265 nm. PPO activities were determined spectrophotometrically by recording absorbance at 420 nm. A 200-mg portion of leaf material was ground in liquid nitrogen and resuspended in phosphate buffer (pH 7.4, 67 mM). To rule out interaction of endogenous phenolics with proteins and enzymes, homogenization was performed with the addition of polyamide at a constant low above zero temperature of 4 °C. The homogenate was centrifuged at 15.000 g for 20 min using a laboratory centrifuge OPN-8 (Dastan, Bishkek, Kyrgyzstan).
Each sample was exposed to triplicate assays. The contents of ascorbic acid and tannins in leaves were determined for the period between 2015 and 201. PPO and AAO activities were measured on samples collected in 2014 and 2015. Data processing was carried out by methods of descriptive statistics using Statistica 10.0.
3. Results
The biochemical response of Acer platanoides and Acer negundo to anthropogenic stress was assessed by measuring the concentration of low molecular weight and high molecular weight substances in their leaves (Table 1). As it turned out, differences in AAO activity between both tree species growing in different areas are not significant. Significant differences were found in the behavior of ascorbate oxidase over the period from June to July, regardless of the sampling site. The June concentrations of ascorbic acid in the leaves of both tree species growing along avenues (160.1 and 60.5) and in buffer areas (115.8 and 30.8) was significantly higher as compared to the API value. In July, the content of ascorbic acid dropped in Acer negundo and continued to be higher that API in Acer platanoides. A significant decrease in the ascorbic acid was detected in samples from each sampling site.
Table 1.
Summer statistics on the ascorbic acid concentration and ascorbate oxidase activity of Norway and ash-leaved maples.
| Sampling Site | Ascorbate oxidase activity, unit of activity |
Ascorbic acid concentration, mg per 100 g of leaves |
||||
|---|---|---|---|---|---|---|
| June | July | August | June | July | August | |
| Norway maple | ||||||
| Control | 2.44 ± 0.27 1.83…3.04 |
4.15 ± 0.06 4.01…4.29 |
3.82 ± 0.34 3.09…4.56 |
195.3 ± 8.7 157.7…232.5 |
164.1 ± 5.8 139.2…189.1 |
146.4 ± 2.1 137.4…155.5 |
| Buffer Area | 2.58 ± 0.31 1.90…3.28 |
4.13 ± 0.20 3.68…4.58 |
3.90 ± 0.27 3.31…4.49 |
311.1 ± 16.4 240.4…381.9 |
207.4 ± 19.5 123.3…291.4 |
184.9 ± 5.7 160.4…209.5 |
| Avenue | 3.18 ± 0.26 2.61…3.76 |
4.55 ± 0.24 4.01…5.09 |
3.96 ± 0.24 3.43…4.50 |
357.2 ± 19.1 275.2…439.1 |
186.1 ± 9.7 144.4…227.7 |
146.8 ± 13.2 90.1…203.5 |
| Ash-leaved maple | ||||||
| Control | 2.73 ± 0.36 1.93…3.52 |
4.28 ± 0.06 4.15…4.41 |
3.69 ± 0.30 3.04…4.34 |
318.4 ± 9.0 305.3…331.4 |
257.2 ± 6.1 241.5…272.9 |
177.1 ± 7.1 158.9…195.3 |
| Buffer Area | 2.76 ± 0.36 1.96…3.56 |
4.47 ± 0.11 4.23…4.71 |
4.60 ± 0.08 4.42…4.77 |
349.2 ± 8.9 336.3…372.2 |
252.9 ± 9.3 229.0…276.8 |
185.4 ± 13.8 149.9…220.9 |
| Avenue | 3.25 ± 0.28 2.64…3.87 |
4.73 ± 0.20 4.30…5.16 |
4.62 ± 0.06 4.48…4.76 |
378.9 ± 10.9 351.7…407.2 |
203.7 ± 6.2 187.8…219.6 |
164.9 ± 6.3 148.7…181.1 |
This suggests that both tree species, Acer platanoides and Acer negundo, exhibit an increase of AAO activity in July, followed by its decrease in August, regardless of their place of growth. Trees growing in buffer areas and along the avenues demonstrated a decrease in the content of ascorbic acid throughout the active vegetation phase.
Results from the analysis of tannin concentrations revealed that A. platanoides trees lining the avenues have had significantly lower contents of condensed tannins in their leaves as compared to controls: in June - by 0.28; in July - by 1.24; in August - by 0.78 mg per g−1 of dry weight (Table 2).
Table 2.
Summer statistics on the tannin concentrations and polyphenol oxidase activity of Norway and ash-leaved maples.
| Sampling Site | Polyphenol oxidase activity, unit of activity |
Condensed tannin concentration, mg per g−1 of dry weight |
||||
|---|---|---|---|---|---|---|
| June | July | August | June | July | August | |
| Norway maple | ||||||
| Control | 1.41 ± 0.06 1.28…1.53 |
3.59 ± 0.20 3.15…4.03 |
2.48 ± 0.08 2.30…2.66 |
4.46 ± 0.04 4.30…4.62 |
6.59 ± 0.11 6.11…7.07 |
8.10 ± 0.09 7.70…8.50 |
| Buffer Area | 1.50 ± 0.05 1.39…1.61 |
3.96 ± 0.26 3.39…4.53 |
4.24 ± 0.16 3.88…4.61 |
4.86 ± 0.04 4.66…5.06 |
5.84 ± 0.08 5.51…6.17 |
7.16 ± 0.09 6.79…7.53 |
| Avenue | 1.85 ± 0.16 1.50…2.20 |
4.49 ± 0.27 3.89…5.09 |
4.84 ± 0.27 4.69…4.99 |
4.18 ± 0.01 4.12…4.24 |
5.35 ± 0.03 5.20…5.50 |
7.34 ± 0.08 6.98…7.69 |
| Ash-leaved maple | ||||||
| Control | 1.22 ± 0.09 0.97…1.42 |
3.86 ± 0.12 3.57…4.12 |
3.41 ± 0.19 2.92…3.88 |
3.95 ± 0.07 3.78…4.13 |
5.24 ± 0.06 5.27…5.40 |
7.19 ± 0.03 7.11…7.27 |
| Buffer Area | 1.23 ± 0.08 0.96…1.44 |
4.27 ± 0.14 3.91…4.61 |
5.09 ± 0.12 4.77…5.37 |
3.84 ± 0.04 3.72…3.96 |
4.55 ± 0.07 4.37…4.73 |
6.82 ± 0.06 6.66…6.97 |
| Avenue | 1.65 ± 0.19 1.18…2.11 |
4.71 ± 0.17 4.27…5.14 |
5.28 ± 0.15 4.91…5.63 |
3.68 ± 0.08 3.48…3.87 |
5.02 ± 0.08 4.82…5.22 |
6.58 ± 0.07 6.39…6.76 |
A. negundo trees growing in buffer areas and along the avenues demonstrated a similar trend. The contents of condensed tannin in their leaves higher compared to controls: in July by 0.69 and 0.22; in August by 0.37 and 0.61 mg per g−1 of dry weight, respectively. A. platanoides trees in the buffer areas had more condensed tannins in their leaves than the controls in June (by 0.40 mg per g−1 of dry weight) and less in July and August (by 0.75 and 0.94 mg per g−1 of dry weight, respectively). This suggests resulting that condensed tannins are involved in adaptation to anthropogenic pollution. By the beginning of August, a significant increase of tannins in the leaves of both maple tree species was detected across the sampling sites.
Polyphenol oxidase is an enzyme involved in the synthesis of tannins. A comparative study of PPO activities in the maple leaves (Table 2) revealed a significant elevation in both tree species in July and August as compared to controls, regardless of the sampling site. The most significant differences were detected in August samples collected along the avenues, where the anthropogenic load was stronger. The findings were 2.99 units of PPO activity in A. platanoides samples and 1.87 units of PPO activity in A. negundo samples.
4. Discussion
This study confirms that plants exposed to air pollution have an antioxidant defense system with interconnected non-enzymatic and enzymatic components.
In samples from the anthropogenic sites under study, the following compounds were detected to exceed the maximum allowable concentration: benzo[a]pyrene, formaldehyde, phenolics, carbon oxides, and nitrogen. Samples from the buffer areas contained elevated carbon monoxide, nitrogen oxide, sulfur dioxide, formaldehyde, phenol, and benzo[a]pyrene that exceeded the annual average MAC. Along the avenues, the concentrations of carbon monoxide, formaldehyde, phenol, and benzo[a]pyrene were found to be beyond and benzo[a]pyrene.
The key defensive compounds of plants are phenolic substances and ascorbic acid, the most common antioxidants involved in photosynthesis, respiration, growth, and stress tolerance. The role of antioxidant defense systems has been shown on different plant species.
For example, it was found that rowan (Sorbus aucuparia L.) and gree ash trees (Fraxinus lanceolata Borkh) sensitive to air pollutants exhibited a decrease in PPO activities, while species with variable tolerance (Aesculus hippocastanum L., Acer negundo L., Populus simonii Carriere) demonstrated a reaction that was rather ambiguous (Prysedskyj, 2017). A significant increase of enzyme activity was detected among trees in the park in July, followed by a significant decrease in August. Although July readings showed a decrease, the enzyme activity continued to be significantly higher than that in June. In the industrial zones and streets, the enzyme activity was found to be higher in July and August. The study of PPO activities revealed August elevations in both buffer areas and along avenues.
Previously, studies did not examine the tannin content, while role in plant development is not fully understood, and the PPO activity of trees in an urban setting. This study revealed an upward trend in the concentration of tannins in maple leaves, which suggests their active role in adaptation to anthropogenic conditions.
Ascorbic acid is among the well-studied substances with antioxidant properties but the associated data are contradictory. For instance, it was recorded that the content of ascorbic acid in pine needles exposed to pollution from various industries was around 30% lower than in samples from areas less susceptible to industrial pollution (Michalec et al., 2015). Other studies, on the contrary, provide evidence on the increase in its content due to the anthropogenic stress. For example, the Indian scientists from have conducted a study to identify tolerant plant species. They regarded the content of ascorbic acid as a tolerance criterion (Manjunath and Reddy, 2019). This study revealed a higher content of ascorbic acid in the leaves exposed to anthropogenic stress as compared to controls, which exhibited a downward trend during the vegetation period. This suggests that plant species under consideration consumed the ascorbic acid to neutralize the effect of air pollution.
Overall, there was a strong negative correlation between ascorbic acid content and enzyme activity in leaves of all control plants (r = -0.89, P = 0.001, n = 100). Ascorbic acid content and active enzyme count in samples from buffer areas (r = 0.78, P = 0.001, n = 100) and streets (r = 0.81, P = 0.001, n = 100) had a strong positive correlation, by contrast. Tannin content and polyphenol oxidase activity were found to have a strong positive correlation too (r = 0.94, P = 0.001, n = 300).
5. Conclusions
Throughout the period of active vegetation, representatives of the genus Maple growing in areas with variable anthropogenic load demonstrated an increase in the AAO activities (P 〈10−5). The minimum contents of ascorbic acid were detected in August samples collected in areas of strong anthropogenic impact. Thus, the higher the anthropogenic load, the more ascorbic acid the Norway and ash-leaved maples produce at the initial stages of vegetation. In August, its content will fall due to intense ascorbic acid consumption (P 〈10−5).
The increase of PPO activity in samples from the industrial zones and roadsides was detected, accompanied by a drop in the content of tannins. This indicates an involvement of tannins in the defensive reactions of maple plants.
Funding Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Petr Kuzmin, Email: kuzminpe@rambler.ru.
Irina Bukharina, Email: bukharinair@rambler.ru.
Ajgul Kuzmina, Email: kuzminaajg@rambler.ru.
References
- Akram N.A., Shafiq F., Ashraf M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant. Sci. 2017;8:613. doi: 10.3389/fpls.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alici E.H., Arabaci G. Determination of SOD, POD, PPO and cat enzyme activities in Rumex obtusifolius L. Annu. Res. Rev. Biol. 2016;11(3):1–7. doi: 10.9734/ARRB/2016/29809. [DOI] [Google Scholar]
- Anahita A., Asmah R., Fauziah O. Evaluation of total phenolic content, total antioxidant activity, and antioxidant vitamin composition of pomegranate seed and juice. Int. Food Res. J. 2015;22(3):1212–1217. [Google Scholar]
- Bela K., Horváth E., Gallé Á., Szabados L., Tari I., Csiszár J. Plant glutathione peroxidases: Emerging role of the antioxidant enzymes in plant development and stress responses. J. Plant Physiol. 2015;176:192–201. doi: 10.1016/j.jplph.2014.12.014. [DOI] [PubMed] [Google Scholar]
- Bilska K., Wojciechowska N., Alipour S., Kalemba E.M. Ascorbic Acid – The Little-Known Antioxidant in Woody Plants. Antioxidants. 2019;8:645. doi: 10.3390/antiox8120645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukharina I.L., Kuzmina A.M., Kuzmin P.A. Dynamics of copper enzymes activity in tree leaves under conditions of large industrial centre (Middle Volga Region) Plant Res. 2018;54(2):280–289. [Google Scholar]
- Fox H., Doron-Faigenboim A., Kelly G., Bourstein R., Attia Z., Zhou J., Moshe Y., Moshelion M., David-Schwartz R. Transcriptome analysis of Pinus halepensis under drought stress and during recovery. Tree Physiol. 2018;38:423–441. doi: 10.1093/treephys/tpx137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia D.E., Glasser W.G., Pizzi A., Paczkowski S.P., Laborie M.P. Modification of condensed tannins: from polyphenol chemistry to materials engineering. New J. Chem. 2016;40(1):36–49. doi: 10.1039/C5NJ02131F. [DOI] [Google Scholar]
- Hafez E.M., Gharib H.S. Effect of exogenous application of ascorbic acid on physiological and biochemical characteristics of wheat under water stress. Int. J. Plant Prod. 2016;10:579–596. [Google Scholar]
- Kloseiko J. Cupric ferricyanide reaction in solution for determination of reducing properties of plant antioxidants. Food Anal. Methods. 2016;9:164–177. doi: 10.1007/s12161-015-0177-8. [DOI] [Google Scholar]
- Kraj W. Reactive oxygen species and antioxidant levels as the factors of autumn senescence in phonological forms of beech (Fagus sylvatica L) Acta Phys. Plant. 2016;38:32. doi: 10.1007/s11738-015-2052-z. [DOI] [Google Scholar]
- Kuzmin P.A., Bukharina I.L., Kuzmina A.M. Influence of urban environment on content and activity indicators of copper-containing enzymes in the leaves of Betula Pendula. J. Fundam. Appl. Sci. 2017;9(1):1472–1479. [Google Scholar]
- Liang D., Zhu T., Ni Z., Lin L., Tang Y., Wang Z., Wang X., Wang J., Lv X., Xia H. Ascorbic acid metabolism during sweet cherry (Prunus avium) fruit development. PLoS ONE. 2017;12(2) doi: 10.1371/journal.pone.0172818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunath B.T., Reddy J. Comparative evaluation of air pollution tolerance of plants from polluted and non-polluted regions of Bengaluru. J. Appl. Biol. Biotechnol. 2019;7:63–68. doi: 10.7324/JABB.2019.70312. [DOI] [Google Scholar]
- Michalec K., Barszcz A., Wasik R. Influence of industrial pollution on the content of essential oils and vitamin C in twigs and needles of Scots. Sylwan. 2015;159:516–522. [Google Scholar]
- Mitrofanova O.V., Grebennikova O.A., Paliy A.E., Brailko V.A., Mitrofanova I.V. Biochemical and physiological features of regenerants in some lavender and lavandin cultivars in vitro. Plant Cell Biotech. Molec. Biol. 2016;17(7–8):335–341. [Google Scholar]
- Prysedskyj Y. Influence of air pollution by compounds of fluorine, sulphur and nitrogen on changes of peroxidase and polyphenol oxidase activity in the leaves of trees and bushes. Biosyst. Divers. 2017;25(3):216–221. doi: 10.15421/011733. [DOI] [Google Scholar]
- Sen A., Khan I., Kundu D., Das K., Datta J.K. Ecophysiological evaluation of tree species for biomonitoring of air quality and identification of air pollution-tolerant species. Environ. Monit. Assess. 2017;189:262. doi: 10.1007/s10661-017-5955-x. [DOI] [PubMed] [Google Scholar]
- Shah A., Singh T., Vijayvergia R. In vitro antioxidant properties and total phenolic and flavonoid contents of Rumex vesicaius L. Int. J. Pharm. Pharmaceut. Sci. 2015;7:81–84. [Google Scholar]
- Skrynetska I., Karcz J., Barczyk G., Kandziora-Ciupa M., Ciepał R., Nadgórska-Socha A. Using Plantago major and Plantago lanceolata in environmental pollution research in an urban area of Southern Poland. Environ. Sci. Poll. Res. 2019;26:23359–23371. doi: 10.1007/s11356-019-05535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T.H., Mayzlish Gati E., Eshel A., Winters G. Germination, physiological and biochemical responses of acacia seedlings (Acacia raddiana and Acacia tortilis) to petroleum contaminated soils. Environ. Pollut. 2018;234:642–655. doi: 10.1016/j.envpol.2017.11.067. [DOI] [PubMed] [Google Scholar]