Abstract
Background
Electronic health records are widely used in cardiovascular disease research. We appraised the validity of stroke, acute coronary syndrome and heart failure diagnoses in studies conducted using European electronic health records.
Methods
Using a prespecified strategy, we systematically searched seven databases from dates of inception to April 2019. Two reviewers independently completed study selection, followed by partial parallel data extraction and risk of bias assessment. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value estimates were narratively synthesized and heterogeneity between sensitivity and PPV estimates were assessed using I2.
Results
We identified 81 studies, of which 20 validated heart failure diagnoses, 31 validated acute coronary syndrome diagnoses with 29 specifically recording estimates for myocardial infarction, and 41 validated stroke diagnoses. Few studies reported specificity or negative predictive value estimates. Sensitivity was ≤66% in all but one heart failure study, ≥80% for 91% of myocardial infarction studies, and ≥70% for 73% of stroke studies. PPV was ≥80% in 74% of heart failure, 88% of myocardial infarction, and 70% of stroke studies. PPV by stroke subtype was variable, at ≥80% for 80% of ischaemic stroke but only 44% of haemorrhagic stroke. There was considerable heterogeneity (I2 >75%) between sensitivity and PPV estimates for all diagnoses.
Conclusion
Overall, European electronic health record stroke, acute coronary syndrome and heart failure diagnoses are accurate for use in research, although validity estimates for heart failure and individual stroke subtypes were lower. Where possible, researchers should validate data before use or carefully interpret the results of previous validation studies for their own study purposes.
Keywords: validation, myocardial infarction, heart failure, stroke; routinely collected health data
Introduction
Ischaemic heart disease and cerebrovascular disease have been the leading causes of death globally for more than 15 years.1 In Europe, cardiovascular disease (CVD) deaths and prevalence have decreased but remain substantial; in 2015 an estimated 85 million people had CVD including 11.3 million with new diagnoses.2
CVD determinants and outcomes research increasingly utilize electronic health records (EHRs). EHRs contain comprehensive longitudinal health data, extracted from primary and secondary care clinical systems, for large patient populations which provide cost-effective data for research. EHR data is mostly “structured” with diagnoses coded using, for example, the International Classification of Diseases (ICD) but can also be “unstructured” with anonymized free-text notes.3 EHR-based research predominantly uses structured data. As the primary purpose of EHR data collection is clinical, it is essential to consider the validity of the data’s use in research.
EHR use is widespread in Europe, where many countries have national healthcare systems, and several systematic reviews have previously explored the quality of specific European EHRs.4–7 Other systematic reviews8–12 have investigated the validity of CVD diagnoses in computerized health-related records, which included EHRs but mainly drew results from disparate claims-based systems. The previous reviews did not separate results for EHR and claims data, the quality of which may differ due to the differences in setup and collection rationale.
In our systematic review, we provide an up-to-date assessment of the validity of acute CVD diagnoses recorded in European EHRs. We defined acute CVD as heart failure (HF), acute coronary syndrome (ACS), and stroke. These high-burden conditions are key diagnoses commonly included in the composite endpoint of major adverse cardiovascular events (MACE) which is increasingly employed in both clinical trials and observational research studies.13 We investigated whether the validity of these diagnoses differed by subtype, definition, data source, reference standard, and study population.
Methods
Protocol and Registration
Our protocol was published in October 201914 following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocol guidelines (PROSPERO registration number CRD42019123898).
Eligibility Criteria
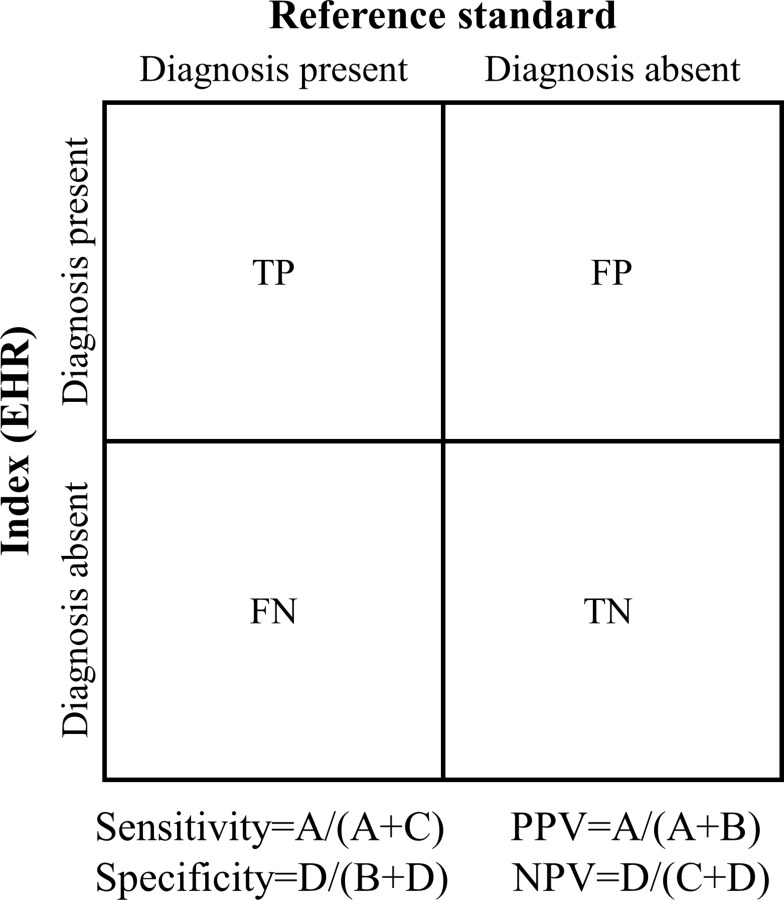
We included articles that validated diagnoses in patients aged ≥16 years captured in any European primary or secondary care EHR. We excluded claims-based databases, disease registries, vital registration systems, or locally held databases. Articles needed to validate clinical codes for the diagnoses of HF, ACS, or stroke (Table 1) against a suitable internal or external reference standard. HF is most frequently a chronic condition which can deteriorate with acute exacerbations. HF may also have an acute onset, for example after an MI. The European Society of Cardiology (ESC) defines acute HF as rapid onset or worsening of symptoms and/or signs of existing HF.15 ACS encompasses different clinical forms of myocardial ischaemia which includes myocardial infarction (MI) and unstable angina. The specific diagnosis of MI or unstable angina depends on symptoms, signs, biomarkers, and ECG and/or autopsy findings, with the definitions refined over time.16 The diagnosis of stroke includes subtypes ischaemic stroke, intracerebral haemorrhage (ICH), and subarachnoid haemorrhage (SAH).17 At least one validation estimate (Figure 1) or the raw data to calculate it was required.
Table 1.
Example Clinical Codes Included for Stroke, Acute Coronary Syndrome and Heart Failure Diagnosis Definitions
| Diagnosis | Subtype | ICD-10 | ICD-9 | ICPC |
|---|---|---|---|---|
| Acute coronary syndrome | Myocardial infarction | I21 | 410 | K75 |
| Unstable angina | I20.0 | |||
| Cardiac arrest | I46 | |||
| Other acute heart disease | I24 | 411 | ||
| Heart failure | I50 | 428 | K77 | |
| Stroke | Subarachnoid haemorrhage | I60 | 430 | K90 |
| Intracerebral haemorrhage | I61 | 431, 432 | ||
| Cerebral infarction | I63 | 433, 434 | ||
| Non-specific stroke | I64 | 436 |
Figure 1.
Illustration of validity estimates calculations.
Information Sources
We searched for eligible articles in five databases (Medline, Embase, Scopus, Web of Science, and Cochrane Library), two grey literature sources (OpenGrey and Ethos), and, where available, the bibliographies of EHR databases from the date of inception to April 2019 in any language.
Search Strategy
We searched medical subject heading terms and free-text (in the title and abstract) for the concepts of (1) CVD diagnoses, (2) EHRs, (3) Europe, and (4) validation. Search terms were developed for Medline and transcribed for the remaining databases (S1 Appendix). To identify any additional articles, we checked reference lists of eligible articles and relevant systematic reviews.
Study Selection and Data Collection
Two reviewers (J.A.D. and R.M.) independently screened the titles and abstracts of all retrieved articles, followed by the full-text of articles deemed eligible in the first stage. Our published protocol details the full data collection process.14 Briefly, we extracted data using a pre-defined template (S2 Appendix) which we piloted using dual extraction for three studies, followed by further parallel extraction for 20% of studies, and completed by a single reviewer (J.A.D.) for the remaining studies.
Risk of Bias in Individual Studies
We used a modified version of the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2)18 tool to assess bias (S3 Appendix). As with our data extraction, two authors (J.A.D. and R.M.) piloted the tool for three studies, then independently assessed risk in a further 10% of studies, with the process completed by a single reviewer (J.A.D.).
Synthesis of Results
We synthesized results with a narrative approach, grouping studies by acute CVD diagnosis (HF, ACS or stroke) and, where possible, subgroups of interest. Subgroups were; diagnosis type, definition, data source including diagnostic position and coding system, reference standard, and study population including time period, age and sex. For studies that reported validation estimates without confidence intervals (CIs), but included raw data, we calculated 95% CIs using the Wilson method for binomial proportions. We used the I2 statistic to assess heterogeneity between the sensitivity and positive predictive value (PPV) estimates, following the Cochrane thresholds.19 Heterogeneity assessment did not include specificity or negative predictive value (NPV), as few studies reported these measures. To investigate sources of heterogeneity, we compared I2 before and after removing studies at a high risk of bias and by the previously mentioned subgroups. We used the Stata metaprop command20 to calculate I2. Metaprop uses raw data rather than precalculated estimates; studies that reported sensitivity or PPV but not the data used to calculate were excluded from heterogeneity assessment.
Risk of Bias Across Studies
We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool for diagnostic accuracy systematic reviews21 to summarise cross-study quality. Evidence was categorised as “high”, “moderate”, “low” or “very low” quality. See S4 Appendix for the reasons we rated quality down or up.
Results
Studies Included
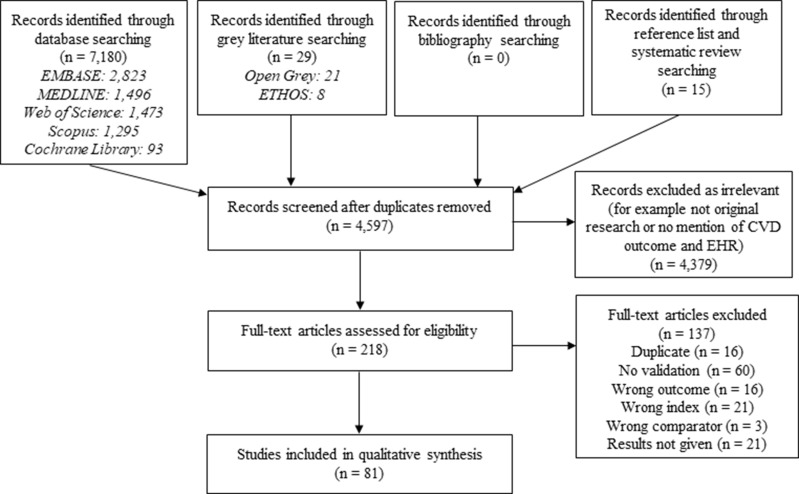
We identified 4595 studies, of which 218 were included in full-text review and 81 met eligibility criteria (Figure 2).
Figure 2.
Flow diagram of study selection.
Study characteristics are summarized in S1 Table, results are displayed in S2 Table, Figures 3–8 and S1–6 Figs, additional subgroup results are described in S5 Appendix, QUADAS-2 results are in S3 Table, and our GRADE assessment is detailed in S4 Table.
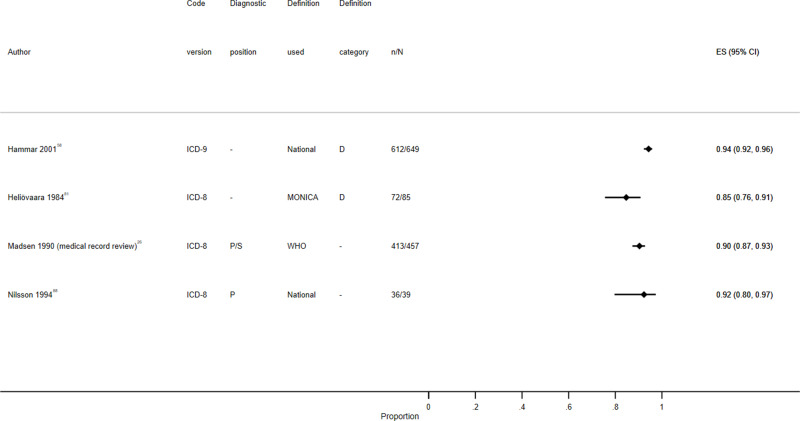
Figure 3.
Positive predictive value for heart failure diagnoses from studies which reported the number of records confirmed positive and the total number of records.
Abbreviations: D, definite; D & Pr, definite and probable; D, Pr & P, definite, probable and possible; P, primary; P/S, primary or secondary.
Figure 4.
Sensitivity for heart failure diagnoses from studies which reported the number of records confirmed positive and the total number of records.
Abbreviations: D & Pr, definite and probable; Pr & P, probable and possible; P, primary; P/S, primary or secondary.
Figure 5.
Positive predictive value for myocardial infarction diagnoses from studies which reported the number of records confirmed positive and the total number of records.
Abbreviations: D, definite; D & P, definite and possible; P, primary; P/S, primary or secondary; RS, reference standard.
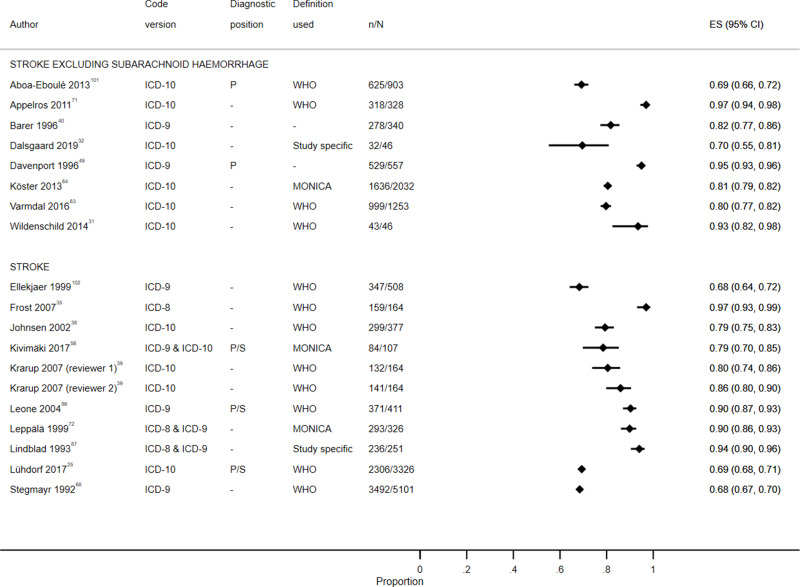
Figure 6.
Sensitivity for myocardial infarction diagnoses from studies which reported the number of records confirmed positive and the total number of records.
Abbreviations: D, definite; D & P, definite and possible; P, primary; P/S, primary or secondary; RS, reference standard.
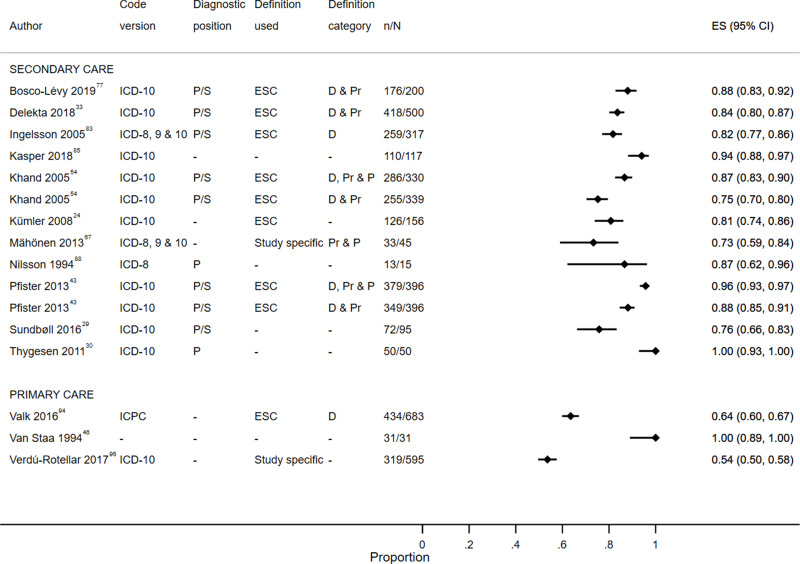
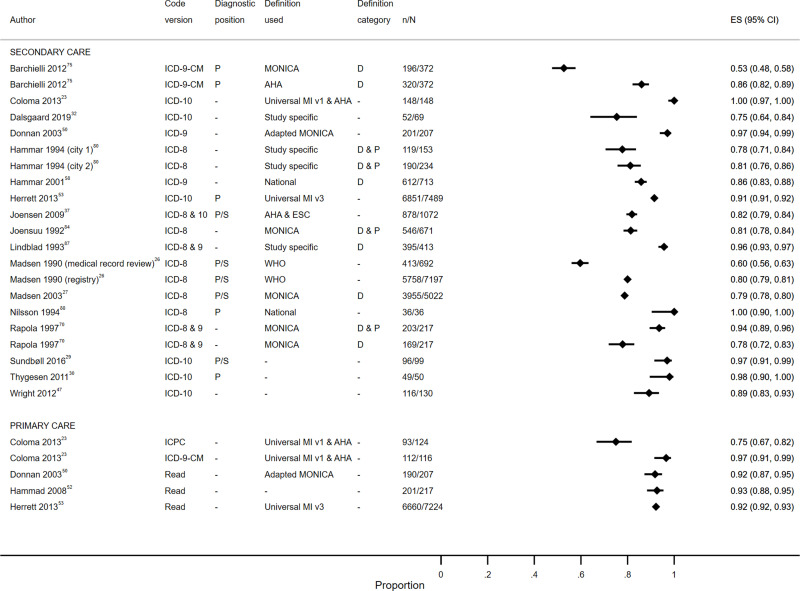
Figure 7.
Positive predictive value for stroke diagnoses recorded in secondary care EHRs from studies which reported the number of records confirmed positive and the total number of records.
Abbreviations: ES, effect size; P, primary; P/S, primary or secondary.
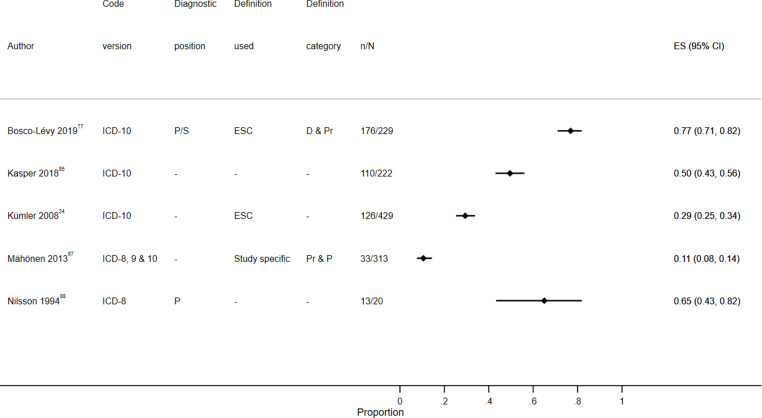
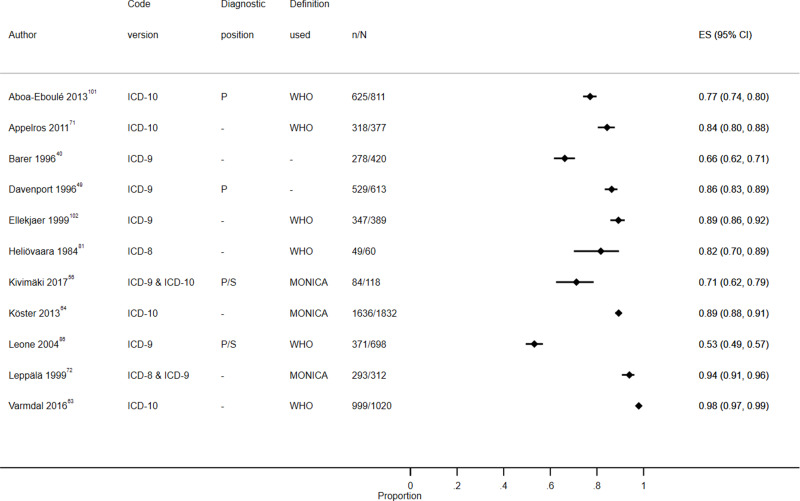
Figure 8.
Sensitivity for stroke diagnoses recorded in secondary care EHRs from studies which reported the number of records confirmed positive and the total number of records.
Abbreviations: ES, effect size; P, primary; P/S, primary or secondary.
Study Characteristics
The 81 included studies validated EHRs from 11 different countries, most frequently Denmark (18 studies)22–39 and the UK (17 studies).40–56 Validation was the primary aim of all but 10 studies.35,36,41,48,57–62 Fourteen studies26,27,31,63–73 validated a vital registration system or disease registry in addition to the EHR. The records validated included data from 1969–2015. Where ICD coding was validated this covered versions 8–10. Sixty studies used medical record review as a reference standard.22,23,25–39,42,43,45,46,49,50,54,55,57–63,69,72,74–96 Twenty studies validated HF,24,28–30,33,43,46,54,59,65,67,77,82,83,85,88,94–97 31 ACS22,23,26,27,29,30,32,34,37,42,46,47,50,52,53,58,65,68–70,75,76,80,81,84,87,88,91,98–100 and 41 stroke diagnoses.25,31,32,35,36,38–41,44,45,47–49,51,55–57,60–64,66,71–74,78,79,81,86,87,89–93,98,101,102
Study Quality
Study quality was high for 54 (67%) of studies,22–26,28,29,31–34,38,39,42–44,47,50,51,53,54,56,59,60,62–65,67–70,72,73,75–79,85–90,92–94,96,98–102 medium for 19 (24%) studies27,30,35–37,46,49,52,55,57,58,61,66,74,81–84,95 and low for eight (10%) of studies.40,41,45,48,71,80,91,97 Studies were overall at low risk of bias in patient selection (76 low, 3 unclear, 2 high), index test (71 low, 10 high), and flow and timing (78 low, 3 unclear) domains and higher risk in the reference standard domain (36 low, 28 unclear, 17 high). Generally, reference standard methods and definitions were poorly described, and on occasion the reference standard was not independent of the EHR. Risk of bias was also higher in studies which validated primary care EHRs. HF validation studies had high quality in 14 (70%) studies, medium in five (25%) and low in one (5%). For ACS validation, quality was high for 21 (68%), medium for eight (26%) and low for two (6%) studies. In stroke validation studies, quality was high for 26 (63%), medium for nine (22%) and low for six (15%) studies.
Heart Failure Study Characteristics
HF diagnoses were most extensively validated using EHR data from Denmark (five studies),24,28–30,33 the Netherlands (four studies),59,65,94,95 Sweden (three studies)82,83,88 and the UK (three studies).43,46,54 In addition, EHR data from Finland,67 France,77 Germany,85 Italy97 and Spain96 were validated in one study each. Fourteen studies validated secondary care EHRs24,28–30,33,43,54,59,65,67,77,83,85,88 and six studies validated primary care EHRs.46,82,94–97 Medical record review was used as the reference standard in all but three studies.24,65,97
Heart Failure Validation Results
Overall
From the main validation result reported by each of the studies; sensitivity (available from nine studies)24,46,65,67,77,82,85,88,95 was ≥50% in six studies46,77,82,85,88,95 but >66% (range 11–100%) in only one study,46 PPV (19 studies)24,28–30,33,43,46,54,59,65,67,77,83,85,88,94–97 was ≥80% (range 54–100%) in all but five studies,29,67,94,96,97 specificity (three studies)24,67,95 was ≥95% in all studies, and NPV (three studies)24,67,95 was ≥84% (range 84–96%) in all studies.
Diagnosis Type
In the three studies that reported results for first diagnosis, the PPV range was 76–88%.28,29,77 One study compared the PPV for all diagnoses (84%) to first diagnosis (80%),28 and another study found the same PPV for first diagnosis and recurrent diagnosis (both 76%).29
Definition
In seven of the eight studies24,28,33,43,54,77,83,94 which used the ESC definition,15 the PPV was ≥80%. The study94 with the lower PPV of 64% was the only one to validate a primary care EHR. Other studies used; both Framingham103 and Boston104 criteria (one study,59 PPV 80–81%), the American College of Cardiology (ACC)/American Heart Association (AHA) definition105 (one study,97 PPV 55%), or study-specific definitions (three studies,67,95,96 PPV 54–83%). An overview of the definitions used by the studies is presented in S6 Appendix.
Seven studies reported classification criteria; the PPV for definite HF ranged between 61–82%,33,43,54,77,83 including both definite and probable HF increased the PPV to 73–88%33,43,54,77,83,94 and the two studies which additionally included possible HF reported high PPV as 87%54 and 96%.43
Diagnostic Position
Six studies29,33,43,54,77,83 reported HF recorded in any diagnostic position (PPV 76–96%) and two studies30,88 only included primary position (PPV 87% and 100%). Three studies,33,77,83 which validated any position, also included breakdowns by primary (PPV 88–96%) and secondary (PPV 66–84%) positions.
Coding System
Twelve studies validated ICD-10,24,28–30,33,43,54,67,77,82,83,96 with all but one83 reporting results specifically for this version of ICD (PPV 78–99%). Six studies24,33,43,77,82,96 validated I50; two studies of primary care EHRs reported lower validity estimates (PPV 54%96 and sensitivity 66%)82 compared to four studies of secondary care EHRs (PPV 81–96%,24,33,43,77 and sensitivity 29%24 and 64%).77 Five studies included a broader range of ICD-10 codes, all of which differed. The estimates for ICD-10 codes were no higher than those for ICD-8 (PPV 87%),67,83,88 ICD-9 (PPV 79–97%),59,65,67,83 or combinations of the three ICD systems (PPV 73–82%).67,83 Two studies validated ICPC K77 in primary care EHRs (PPV 64%94 and 83%95).
Acute Coronary Syndrome Study Characteristics
Similar to HF, ACS diagnoses were most frequently validated using EHR data from Denmark (nine studies),22,23,26,27,29,30,32,34,37 followed by Finland (seven studies),68–70,81,84,99,100 the UK (six studies)42,46,47,50,52,53 and Sweden (4 studies).58,80,87,88 Two studies validated data in each of Italy,23,75 the Netherlands,23,65 and Spain,91,98 and a final study used data from France.76 Twenty-six of the studies validated a secondary care EHR,22,26,27,29,30,32,34,37,42,47,58,65,68–70,75,76,80,81,84,87,88,91,98–100 three studies validated both a primary and secondary care EHR23,50,53 and two studies validated a primary care EHR.46,52
Four studies22,37,68,76 presented overall ACS results, of which one study68 included an additional breakdown for MI and two studies37,76 included unstable angina and MI, one of which also included cardiac arrest.37 A further two studies29,65 did not report results for ACS overall but did include both unstable angina and MI. The remaining 25 studies solely validated MI diagnoses.23,26,27,30,32,34,42,46,47,50,52,53,58,69,70,75,80,81,84,87,88,91,98–100
Acute Coronary Syndrome Validation Results
Overall
For ACS, three studies33,37,76 reported one main PPV (range 66–87%), while results presented by Pajunen et al68 were broken down by age, sex and time period, with sensitivity of 66–87% and PPV of 63–86%.
Diagnosis Type
The PPV for unstable angina varied; with low values of 20%76 and 27.5%37 in two studies and higher values of 78%65 and 88%29 in the other two studies. Sensitivity was only reported by one study,65 at 53%. For MI, the main validation result for sensitivity (11 studies)26,27,34,42,46,50,58,65,81,88,98 was ≥80% in all but one study42 (range 56–97%), and six26,27,34,58,88,98 >90%. PPV (24 studies)23,26,27,29,30,32,34,37,42,46,47,50,52,53,58,65,70,75,76,80,84,87,88,98 was ≥80% (range 42–100%) in all but three studies27,32,34 with 1223,29,30,42,50,52,53,65,87,88,98 ≥90%. Three studies34,42,98 reported specificity (range 93–100%) and two34,98 included NPV (range 82–100%).
Four studies29,32,37,84 reported the PPV for first MI, with estimates of 75–97%, and one study29 also included recurrent MI with a PPV of 88% compared to 97% for first MI.
Definition
Varying MI definitions were used (S6 Appendix). Most frequently (nine studies)26,27,50,70,75,81,84,99,100 the World Health Organization (WHO) Monitoring trends and determinants in cardiovascular disease (MONICA) definition106 was used, with variable PPV estimates of 53–96% obtained. Two studies compared MONICA to another MI definition; one75 showed MONICA-defined definite MI had a substantially lower PPV than AHA/ESC-defined16 definite MI (53% vs 86%), while the other84 also showed a lower PPV for MONICA compared to “normal clinically defined MI” but with a smaller difference (81% vs 89%). One further study used the AHA/ESC definition37 (PPV 82%). The universal definition107 was used in a study23 which included EHR data from three countries, with PPVs of 75–100%. Three studies used the third universal definition,108 one76 of which combined it with the earlier universal definition (PPV 85%). In another53 PPVs of 92% with obtained for the primary and secondary care EHRs validated. The third34 validated MI diagnoses recorded for patients with drug-eluting coronary stents, the PPV was 42% for all admission and 73% for acute admissions.
Diagnostic Position
Of the 10 studies which reported the diagnostic position used to validate MI diagnoses, five26,27,29,34,68 used any diagnostic position (PPV 42–97%) and five30,75,76,88,98 primary position (PPV 53–100%). One study27 which validated any position (PPV 79%) also included a breakdown by primary position (PPV 80%) and another study29 included breakdowns by primary (PPV 99%) and secondary positions (PPV 80%).
Coding System
Ten studies validated ICD-10 coded MI, eight reported results specifically for ICD-10.23,29,30,32,34,47,53,76 Four studies validated ICD-10 I21 with PPV ≥85% (range 42–100%)23,29,34,76 in all but one.34 Two studies included I21-I23 and reported high PPVs of 92%53 and 98%;30 however, the latter study was small in size (50 patients). One study validated I21-I22 (PPV 89%)47 and another I21-I24 (PPV 75%).32 The estimates for ICD-10 codes were no higher than those for ICD-8 (PPV 79–100%),26,27,80,84,88 ICD-9 (86–100%),42,50,58,65,75,98 or combinations of three ICD systems (PPV 82–96%).37,87 Of the studies to validate data in primary care, one23 included IPCI K75 code (PPV 75%) and three50,52,53 validated Read coding in the UK (PPV 91–93%).
Reference Standard
The PPV for MI diagnoses varied between 53–100% when medical record review was the reference standard (20 studies)22,23,26,29,30,32,37,42,46,50,58,69,70,75,76,80,84,87,88,91 and 89–93% when a registry was used.26,27,53,68,98–100 One study34 used medical record review after comparing EHR and registry results (PPV 42%). Two studies used a GP questionnaire (PPV 89% and 93%),47,52 and one study used a local cardiology database (PPV 97%).65
Stroke Study Characteristics
Stroke diagnoses were most frequently validated in UK EHRs, with 10 studies conducted,40,41,44,45,47–49,51,55,56 followed by Denmark (seven studies),25,31,32,35,36,38,39 Sweden (5 studies)60,64,66,71,87 and Italy (4 studies).74,86,90,93 Data from Finland,72,73,81 France,78,79,101 Norway,63,89,102 and Spain62,91,98 were validated in three studies each. A further two studies validated EHR data from the Netherlands57,61 and one from the Czech Republic.92 All but three studies41,44,48 validated secondary care EHRs.
Twenty-eight studies presented validation estimates for overall stroke (including both ischaemic and haemorrhagic).25,31,32,35,38–41,44,45,48,49,56,60,63,64,66,71–73,81,86,87,91,92,98,101,102 Ischaemic stroke was assessed in 18 studies,25,32,38,39,47,57,62,72–74,78,79,86,90,92,93,101,102 in all but four studies62,74,79,90 this was done as a subgroup analysis after validating overall stroke. Similarly, haemorrhagic stroke was assessed by 21 studies; two reported results for overall haemorrhagic stroke32,51 with this the main focus of one study,51 17 studies reported results for ICH as a subgroup analysis25,38,39,47,51,55,57,72,73,78,86,87,89,92,93,101,102 and 18 studies reported results for SAH25,36,38,39,47,51,55,61,72,73,78,81,86,87,89,92,93,102 with this being the main result in two studies.36,61
Stroke Validation Results
Overall
For overall stroke, sensitivity (15 studies)31,40,45,49,56,63,64,71,73,81,86,91,98,101,102 was ≥80% (range 33–97%) in seven studies49,63,64,71,73,81,102 and ≥70% in 11 studies. PPV (27 studies)25,31,32,35,38–41,45,48,49,56,60,63,64,66,71–73,81,86,87,91,92,98,101,102 was ≥80% (range 20–97%) in 19 studies.31,35,39–41,45,48,49,60,63,64,71,72,81,86,87,92,98 Nine of the studies31,32,40,49,60,63,64,71,101 did not include codes to validate SAH, three of which had stated this in their inclusion criteria.40,71,101 Excluding these studies did not affect the sensitivity (53–89%) or PPV (68–97%). Specificity and NPV, reported by five studies, were 99–100%49,56,63,98 other than one study31 which obtained a specificity of 96% and NPV of 72%.
Diagnosis Type
Three studies56,64,101 included first and recurrent overall stroke with sensitivity from 71–89% and PPV 69–81%, while three studies32,71,73 also included only first stroke for which sensitivity was 85–89% and PPV 70–97%.
For ischaemic stroke, the main sensitivity reported (6 studies)74,79,81,86,90,102 was ≥66% in all but one86 study (range 37–82%). Fourteen studies25,32,38,47,57,62,72,74,78,79,86,90,92,102 included one main PPV of 66–96%. One study101 classified results separately for cardiac embolism, large artery atherosclerosis, lacunar infarct and ischaemic stroke of other aetiology. Sensitivity and PPV were highest in the cardiac embolism classification (83% and 87%, respectively) and lowest for other aetiology (67% and 35%, respectively). For ICH, the main sensitivity reported was 59–98% (4 studies)73,86,101,102 and main PPV 55–96% (15 studies).25,38,39,47,51,55,57,72,73,78,86,87,92,101,102 The sensitivity of SAH diagnoses was 35–92% (4 studies)73,81,86,102 and PPV was 42–96% (18 studies).25,36,38,39,47,51,55,61,72,73,78,81,86,87,89,92,93,102
Definition
Stroke was defined in 22 of the 41 studies, 1325,31,35,38,39,63,66,71,81,86,90,92,101,102 used the WHO definition109 (sensitivity 53–97%63,71,86,101,102 and PPV 68–97%),25,35,38,39,63,66,71,81,86,92,101,102 seven56,60,62,64,72,74,93 used MONICA110 (sensitivity 71–89%56,64 and PPV 79–92%),56,60,64,72 and two32,87 defined stroke specifically for their study (PPV 70% and 91%). The stroke definitions used are summarized in S6 Appendix.
Diagnostic Position
For overall stroke diagnoses recorded in any diagnostic positions, sensitivity ranged from 53–97%56,63,86 and PPV from 69–90%.25,56,63,86 In comparison, results only for primary position were 67–86% for sensitivity and 69–95% for PPV.49,63,73,98,101
Coding System
Thirteen studies validated ICD-10 (PPV 20–97%,31,32,38,39,45,47,55,60,63,64,71,78,92 sensitivity 76–97%).45,63,64,71,101 Four studies31,63,64,71 which excluded SAH from the stroke definition validated ICD-10 I61, I63 and I64 (sensitivity 89–97% and PPV 79–97%). Aboa-Eboule et al101 additionally included G46 in their definition (sensitivity 77% and PPV 69%) while Dalsgaard et al32 validated I61-I65 (PPV 70%). In comparison, Holmqvist et al60 only included I61 and I63, and obtained PPV estimates of 92% and 89% in people with and without rheumatoid arthritis, respectively. Three studies38,39,92 which included SAH in the stroke definition validated I60, I61, I63 and I64 (PPV 79–86%) and one45 additionally included I62 (PPV 96%). The estimates for ICD-10 codes were no higher than those for ICD-8 codes (sensitivity 82%),81 ICD-9 (PPV 20–95%,40,49,66,86,91,93,98,102 sensitivity 33–89%),40,49,86,91,98,102 or combinations of three ICD systems (PPV 79–97%,35,72,73,87 sensitivity 71–85%).73
Seven studies validated ICD-10 I63 for ischaemic stroke diagnosis (PPV 78–96%).25,32,38,47,78,79,92 One study73 used a broad (ICD-9433, 434, 436 and ICD-10 I63, I64) and narrow range of codes (ICD-9433, 434 and ICD-10 I63) to define ischaemic stroke, with similar sensitivity (82% vs 81%) and PPV (84% vs 83%). One other study74 reported results by ICD-9 codes 443*1 and 434*1 (PPV 86% and 90%, respectively). Six studies25,38,55,78,89,92 validated ICD-10 I61, with another two39,101 presumed to have also validated this code, for ICH (PPV 66–96%) and a further three studies86,93,102 validated ICD-9431 (PPV 71–78%). For SAH, eight studies25,38,39,47,55,78,89,92 validated ICD-10 I60 with PPV >90% in half of the studies (range 46–96%), four studies61,86,93,102 validated ICD-9430 (PPV 42–95%), one study81 validated ICD-8430 (PPV 85%) and two studies72,87 validated both versions for 430 (PPV 78–79%).
Reference Standard
In the 17 studies25,31,32,35,38,39,45,55,56,60,63,72,79,86,87,91,92 which used medical record review as the reference standard to validate overall stroke diagnoses, the PPV was ≥79% (range 20–97%) in all but four studies.25,31,32,91 A further eight studies used a registry reference standard (PPV 88–97%).40,64,66,71,73,98,101,102
Heterogeneity
We were able to assess the heterogeneity between the main PPV reported in; 14 studies with 16 estimates of HF (I2=97.0%), 18 studies with 26 estimates of MI (I2=98.5%), and 19 studies with 20 estimates of stroke (I2=97.9%) diagnoses. Additionally, we assessed heterogeneity between the main sensitivity for; six studies of HF (I2=98.6%), four of MI (I2=74.3%), and 11 of stroke (I2=98.8%) diagnoses. Heterogeneity between the estimates was considerable, at more than >95% in all cases other than sensitivity estimates for MI. Furthermore, heterogeneity remained considerable after removal of studies at a high risk of bias.
Overall Strength of Evidence
GRADE showed that cross-study quality was very low for all HF outcomes (sensitivity and PPV in secondary care EHRs and PPV in primary care EHRs), low for MI sensitivity and PPV in secondary care EHRs and moderate for PPV in primary care EHRs, and very low for stroke sensitivity in secondary care EHRs and PPV in primary care EHRs and moderate for PPV in secondary care EHRs.
Discussion
Summary of Findings
Our systematic review suggests that the sensitivity of coded data in European EHRs for HF diagnoses is low at ≤66% in all but one study. There was also wide variation in stroke sensitivity estimates, with only half of studies ≥80%, although three-quarters were ≥70%. The sensitivity of ACS was higher at ≥80% in the vast majority of studies. The majority of studies which validated ACS diagnosis did so specifically for MI.
The PPV of all diagnoses was ≥80% in the majority of studies; two-thirds for HF (nearly three-quarters for secondary care EHRs), nearly three-quarters for MI, and 70% of stroke validation studies. Where subtypes were validated, PPV was ≥80% for four-fifths of ischaemic stroke diagnoses but only 44% of ICH and SAH diagnoses.
The specificity and NPV were also high where available (three HF studies, three MI studies and five stroke studies). However, as most studies only included patients with the diagnosis of interest recorded in the EHR and reference standard, the results presented were mostly limited to sensitivity and PPV.
Both PPV and NPV are impacted by disease prevalence, with lower estimates for rare conditions.111 Our systematic review focused on Europe, drawing studies from 11 countries. Age-standardized prevalence of CVD in these countries is between 5000–6500 per 100,000, other than the Czech Republic (~8700 per 100,000) which only contributed one study.2 Therefore, prevalence differences should have limited impact on our comparison of validity estimates between geographies. The prevalence of CVD increases with age, but we did not find any systematic difference in results between studies with younger or older populations.
The low sensitivity of HF diagnoses we identified is consistent with a previous systematic review validating HF diagnoses in administrative data, which identified three European studies.11 Twelve more studies have since been published and included in our review. These more recent findings, however, do not suggest any improvement in the quality of data over time. This is perhaps unsurprising given the range of clinical aetiology and presentation. The high proportion of studies we found to have a PPV of <80% for stroke diagnoses appeared more substantial than in previous systematic reviews.9,12 We identified 15 new studies which were not included in these previous reviews.25,32,45,51,56,57,61–63,74,78,89,91,92,98 Our results for sensitivity and PPV of MI diagnoses are consistent with previous reviews,8,10 and identified five29,32,34,76,98 new MI validation studies with variable results.
There was substantial heterogeneity between the sensitivity and PPV estimates for all three acute CVD diagnoses. Heterogeneity was likely because studies differed in multiple ways; for example, even among studies which used medical record review as the reference standard, differences in study time period impacted upon the ICD version used. The heterogeneity caused by variable methods was highlighted in previous systematic reviews of atrial fibrillation and dementia diagnoses recorded in routine health data.112,113
Defining Diagnosis in the EHR
We were most interested in the results of ICD-10 validation, as this is the latest ICD coding system which is widely used in Europe and elsewhere. In McCormick et al’s10 review of MI diagnoses in administrative data, the authors noted a lack of ICD-10 validation with only three studies identified, whereas our review identified 10. Nevertheless, even within ICD-10, combinations of codes used, and therefore their validity, differed, which highlights the importance of tailoring codes to each research question. Codes are arguably even more important when using other, more complex coding systems such as Read codes, which are used in UK primary care data and can generate vast numbers of codes for every clinical condition.
Defining Diagnosis in the Reference Standard
There is no single recommended gold standard to determine the validity of EHR data.114 Nearly three-quarters (74%) of studies used medical records; more frequently for HF diagnoses (85%) than ACS (71%) or stroke (68%). This difference may be due to availability of MI and stroke registries, used in 26% and 22% of studies, respectively. No differences in the performance of the reference standard methods were discernable, probably due to heterogeneity.
Criteria to define CVD, especially MI, have been refined over time, driven by the development of more sensitive and specific biomarkers, and more precise imaging techniques.100 However, we did not identify any temporal trends in the accuracy of MI recording, again likely due to overall study heterogeneity.
When validating HF, which can vary in clinical aetiology and presentation, clarity on the criteria used to define, with explicit classification of acute and chronic HF along with ejection fraction would benefit understanding of results.
Comparing and Combining Data Sources
Only 14 (17%) studies validated primary care systems, more than half of which were in the UK. Using primary care EHRs may be beneficial for research into conditions such as HF which are frequently managed in primary care; in our study, 30% of HF EHR validation studies used primary care data, compared to 16% for ACS and 7% for stroke studies. For acute severe conditions resulting in hospitalization, secondary care records should be the most reliable data source. Where possible, the use of linked data to increase the ascertainment of acute CVD events should be considered.
Implications for Future Research
EHR-based research is a growing field – widely used in observational analyses and increasingly employed in trials.115 Researchers should consider the level of validity necessary for their own CVD outcome definition. When a composite outcome, such as MACE, is used researchers may need to address differing sensitivity in the individual components of the outcome. In studies which investigate CVD incidence, a sensitive definition is particularly important. For example, EHR data are being used for rapid COVID-19 pandemic analyses such as; the impact the virus has in those with CVD, CVD as an outcome after infection with the virus, and excess death estimates.116 It is important that these rapid analyses consider the validity of the data and definitions used. Conversely, in a pragmatic trial recruitment, a specific definition is likely more important than a sensitive one.
Strengths and Limitations
Our systematic review provides a comprehensive and up-to-date evaluation of the validity of acute CVD diagnoses in European EHRs, conducted without language or time restrictions using a broad search strategy. Two independent reviewers performed our study selection, and native speaking collaborators translated foreign language articles. Similar to other systematic reviews of validation studies, we repurposed the QUADAS-2 risk of bias tool developed for diagnostic test accuracy. Additionally, we followed the diagnostic test accuracy GRADE methodology to assess the overall evidence base.
Our work is not without limitations. Firstly, only one reviewer completed full data extraction and risk of bias assessment due to resource constraints, although a sample of 20% of studies had data dual extracted. Secondly, we limited our study to Europe, so theoretically our results are only generalizable to European countries. All previous systematic reviews8–12 on the validity of acute CVD diagnoses included both EHRs and claim-based systems, while most studies included in each of these reviews were from North America. From these existing reviews, it was unclear if the validity of EHRs differed to claims-based datasets, which reflect payments related to medical care given. Despite this, we obtained similar results to the previous reviews. Thirdly, our review focused on acute CVD events so excluded results from studies that validated broader diagnoses of ischaemic heart disease or cerebrovascular disease, which again limits generalizability to these specific conditions.
Recommendations
For ACS and stroke diagnoses, most sensitivity and PPV results were reasonably high, providing confidence in the use of European EHR data for research into these conditions. However, there was considerable heterogeneity between studies. Sensitivity for HF diagnoses was low, and our GRADE assessment found very low quality for all HF outcomes. For studies of HF, we strongly recommend either validating the definition or referring to existing validation studies to develop the case definition. New validation studies of HF diagnoses should report whether the diagnoses validated are for acute or chronic presentation and HF with reduced ejection fraction or preserved ejection fraction. These principles are also applicable to future ACS and stroke validation studies. Identifying specific stroke subtypes can be difficult; analysis of all stroke subtypes combined is preferable.
Conclusions
Our review on the accuracy of HF, ACS and stroke diagnoses in European EHRs should guide researchers in their selection of data sources and CVD definitions for epidemiological studies. Generally, the data assessed was of reasonable quality. However, it is difficult to summarize validity given the heterogeneity between studies. Where possible, researchers should validate data before use or carefully interpret the results of previous validation studies to consider the impact validity has on research findings. Additionally, the use of linked data will bolster quality.
Acknowledgments
We thank Hanne-Dorthe Emborg and Elisabeth Bondesson for their translations of Danish and Swedish language articles.
Funding Statement
J.A.D. is funded by a British Heart Foundation Non-Clinical PhD Studentship (FS/18/71/33938) and C.W.G is funded by a Wellcome Intermediate Clinical Fellowship (201440/Z/16/Z). The funders had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Charlotte Warren-Gash reports grants from Wellcome, grants from British Heart Foundation, during the conduct of the study; personal fees from Sanofi Pasteur, outside the submitted work. The authors declare no other potential conflicts of interest for this work.
References
- 1.World Health Organization. The top 10 causes of death. https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed February11, 2019.
- 2.European Heart Network. CVD statistics 2017; 2017. Available from: http://www.ehnheart.org/cvd-statistics/cvd-statistics-2017.html. Accessed October9, 2019..
- 3.Denaxas SC, Morley KI. Big biomedical data and cardiovascular disease research: opportunities and challenges. Eur Hear J - Qual Care Clin Outcomes. 2015;1(1):9–16. doi: 10.1093/ehjqcco/qcv005 [DOI] [PubMed] [Google Scholar]
- 4.Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the general practice research database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14. doi: 10.1111/j.1365-2125.2009.03537.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sund R. Quality of the Finnish hospital discharge register: a systematic review. Scand J Public Health. 2012;40(6):505–515. doi: 10.1177/1403494812456637 [DOI] [PubMed] [Google Scholar]
- 6.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubbo B, Fitzpatrick NK, Denaxas S, et al. Use of electronic health records to ascertain, validate and phenotype acute myocardial infarction: a systematic review and recommendations. Int J Cardiol. 2015;187:705–711. doi: 10.1016/j.ijcard.2015.03.075 [DOI] [PubMed] [Google Scholar]
- 9.Woodfield R, Grant I, Sudlow CLM; UK Biobank Follow-Up and Outcomes Working Group. Accuracy of electronic health record data for identifying stroke cases in large-scale epidemiological studies: a systematic review from the UK biobank stroke outcomes group. PLoS One. 2015;10(10):e0140533. doi: 10.1371/journal.pone.0140533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA, Guo Y. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS One. 2014;9(3):e92286. doi: 10.1371/journal.pone.0092286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PLoS One. 2014;9(8):e104519. doi: 10.1371/journal.pone.0104519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS One. 2015;10(8):e0135834. doi: 10.1371/journal.pone.0135834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poudel I, Tejpal C, Rashid H, Jahan N. Major adverse cardiovascular events: an inevitable outcome of ST-elevation myocardial infarction? A literature review. Cureus. 2019;11(7). doi: 10.7759/cureus.5280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson JA, Banerjee A, Muzambi R, Smeeth L, Warren-Gash C. Validity of acute cardiovascular outcome diagnoses in European electronic health records: a systematic review protocol. BMJ Open. 2019;9(10). doi: 10.1136/bmjopen-2019-031373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution. Eur Heart J. 2016;37(27):2129–2200. [DOI] [PubMed] [Google Scholar]
- 16.Luepker RV, Apple FS, Christenson RH, et al. Case definitions for acute coronary heart disease in epidemiology and clinical research studies. Circulation. 2003;108(20):2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA [DOI] [PubMed] [Google Scholar]
- 17.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. doi: 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529. doi: 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 19.The Cochrane Collaboration. Cochrane handbook for systematic reviews of interventions. Version 5. (Higgins JP, Green S, eds.); 2011. Available from: https://handbook-5-1.cochrane.org/. Accessed September9, 2020.
- 20.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):1–10. doi: 10.1186/2049-3258-72-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schünemann HJ, Oxman AD, Brozek J, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008;336:1106–1110. doi: 10.1136/bmj.39500.677199.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bork CS, Al-Zuhairi KS, Hansen SM, Delekta J, Joensen AM. Accuracy of angina pectoris and acute coronary syndrome in the Danish National Patient Register. Dan Med J. 2017;64(5). [PubMed] [Google Scholar]
- 23.Coloma PM, Valkhoff VE, Mazzaglia G, et al. Identification of acute myocardial infarction from electronic healthcare records using different disease coding systems: a validation study in three European countries. BMJ Open. 2013;3(6):e002862. doi: 10.1136/bmjopen-2013-002862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kümler T, Gislason GH, Kirk V, et al. Accuracy of a heart failure diagnosis in administrative registers. Eur J Heart Fail. 2008;10(7):658–660. doi: 10.1016/j.ejheart.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 25.Lühdorf P, Overvad K, Schmidt EB, Johnsen SP, Bach FW. Predictive value of stroke discharge diagnoses in the Danish National Patient Register. Scand J Public Health. 2017;45(6):630–636. doi: 10.1177/1403494817716582 [DOI] [PubMed] [Google Scholar]
- 26.Madsen M, Balling H, Eriksen LS. [The validity of the diagnosis of acute myocardial infarction in 2 registries: the Heart Registry compared to the National Patient Registry]. Ugeskr Laeger. 1990;152(5):308–314. Danish. [PubMed] [Google Scholar]
- 27.Madsen M, Davidsen M, Rasmussen S, Abildstrom SZ, Osler M. The validity of the diagnosis of acute myocardial infarction in routine statistics: a comparison of mortality and hospital discharge data with the Danish MONICA registry. J Clin Epidemiol. 2003;56(2):124–130. doi: 10.1016/S0895-4356(02)00591-7 [DOI] [PubMed] [Google Scholar]
- 28.Mard S, Nielsen FE. Positive predictive value and impact of misdiagnosis of a heart failure diagnosis in administrative registers among patients admitted to a university hospital cardiac care unit. Clin Epidemiol. 2010;2:235–239. doi: 10.2147/CLEP.S12457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6(11):e012832. doi: 10.1136/bmjopen-2016-012832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wildenschild C, Mehnert FW, Thomsen R, et al. Registration of acute stroke: validity in the Danish Stroke Registry and the Danish National Registry of Patients. Clin Epidemiol. 2013;6:27. doi: 10.2147/CLEP.S50449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalsgaard E-M, Witte DR, Charles M, Jørgensen ME, Lauritzen T, Sandbæk A. Validity of Danish register diagnoses of myocardial infarction and stroke against experts in people with screen-detected diabetes. BMC Public Health. 2019;19(1):228. doi: 10.1186/s12889-019-6549-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delekta J, Hansen S, AlZuhairi K, Bork C, Joensen A. The validity of the diagnosis of heart failure (I50.0-I50.9) in the Danish National Patient Register. Dan Med J. 2018;65(4):A5470. [PubMed] [Google Scholar]
- 34.Egholm G, Madsen M, Thim T, et al. Evaluation of algorithms for registry-based detection of acute myocardial infarction following percutaneous coronary intervention. Clin Epidemiol. 2016;8:415–423. doi: 10.2147/CLEP.S108906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frost L, Andersen LV, Vestergaard P, Husted S, Mortensen LS. Trend in mortality after stroke with atrial fibrillation. Am J Med. 2007;120(1):47–53. doi: 10.1016/j.amjmed.2005.12.027 [DOI] [PubMed] [Google Scholar]
- 36.Gaist D. Risk of subarachnoid haemorrhage in first degree relatives of patients with subarachnoid haemorrhage: follow up study based on national registries in Denmark. BMJ. 2000;320(7228):141–145. doi: 10.1136/bmj.320.7228.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joensen AM, Jensen MK, Overvad K, et al. Predictive values of acute coronary syndrome discharge diagnoses differed in the Danish National Patient Registry. J Clin Epidemiol. 2009;62(2):188–194. doi: 10.1016/J.JCLINEPI.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 38.Johnsen SP, Overvad K, Sørensen HT, Tjønneland A, Husted SE. Predictive value of stroke and transient ischemic attack discharge diagnoses in The Danish National Registry of Patients. J Clin Epidemiol. 2002;55(6):602–607. doi: 10.1016/S0895-4356(02)00391-8 [DOI] [PubMed] [Google Scholar]
- 39.Krarup L-H, Boysen G, Janjua H, Prescott E, Truelsen T. Validity of stroke diagnoses in a National Register of Patients. Neuroepidemiology. 2007;28(3):150–154. doi: 10.1159/000102143 [DOI] [PubMed] [Google Scholar]
- 40.Barer D, Ellul J, Watkins C. Correcting outcome data for case mix in stroke medicine. BMJ. 1996;313(7063):1005–1006. doi: 10.1136/bmj.313.7063.1005c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook M, Baker N, Lanes S, Bullock R, Wentworth C, Michael Arrighi H. Incidence of stroke and seizure in Alzheimer’s disease dementia. Age Ageing. 2015;44(4):695–699. doi: 10.1093/ageing/afv061 [DOI] [PubMed] [Google Scholar]
- 42.McAlpine R, Pringle S, Pringle T, Lorimer R, MacDonald TM. A study to determine the sensitivity and specificity of hospital discharge diagnosis data used in the MICA study. Pharmacoepidemiol Drug Saf. 1998;7(5):311–318. doi: [DOI] [PubMed] [Google Scholar]
- 43.Pfister R, Michels G, Wilfred J, Luben R, Wareham NJ, Khaw KT. Does ICD-10 hospital discharge code I50 identify people with heart failure? A validation study within the EPIC-Norfolk study. Int J Cardiol. 2013;168(4):4413–4414. doi: 10.1016/j.ijcard.2013.05.031 [DOI] [PubMed] [Google Scholar]
- 44.Ruigómez A, Martín‐Merino E, Rodríguez LAG. Validation of ischemic cerebrovascular diagnoses in the health improvement network (THIN). Pharmacoepidemiol Drug Saf. 2010;19(6):579–585. doi: 10.1002/PDS.1919 [DOI] [PubMed] [Google Scholar]
- 45.Sansom LT, Ramadan H. Stroke incidence: sensitivity of hospital data coding of acute stroke. Int J Stroke. 2015;10(6):E70. doi: 10.1111/ijs.12577 [DOI] [PubMed] [Google Scholar]
- 46.Van Staa TP, Abenhaim L. The quality of information recorded on a UK database of primary care records: a study of hospitalizations due to hypoglycemia and other conditions. Pharmacoepidemiol Drug Saf. 1994;3(1):15–21. doi: 10.1002/pds.2630030106 [DOI] [Google Scholar]
- 47.Wright FL, Green J, Canoy D, Cairns BJ, Balkwill A, Beral V. Vascular disease in women: comparison of diagnoses in hospital episode statistics and general practice records in England. BMC Med Res Methodol. 2012;12(1):161. doi: 10.1186/1471-2288-12-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou EH, Gelperin K, Levenson MS, Rose M, Hsueh YH, Graham DJ. Risk of acute myocardial infarction, stroke, or death in patients initiating olmesartan or other angiotensin receptor blockers – a cohort study using the clinical practice research datalink. Pharmacoepidemiol Drug Saf. 2014;23(4):340–347. doi: 10.1002/pds.3549 [DOI] [PubMed] [Google Scholar]
- 49.Davenport RJ, Dennis MS, Warlow CP. The accuracy of Scottish Morbidity Record (SMR1) data for identifying hospitalised stroke patients. Health Bull (Raleigh). 1996;54(5):402–405. [PubMed] [Google Scholar]
- 50.Donnan PT, Dougall HT, Sullivan FM. Optimal strategies for identifying patients with myocardial infarction in general practice. Fam Pract. 2003;20(6):706–710. doi: 10.1093/fampra/cmg614 [DOI] [PubMed] [Google Scholar]
- 51.Gaist D, Wallander M-A, González-Pérez A, García-Rodríguez LA. Incidence of hemorrhagic stroke in the general population: validation of data from The Health Improvement Network. Pharmacoepidemiol Drug Saf. 2013;22(2):176–182. doi: 10.1002/pds.3391 [DOI] [PubMed] [Google Scholar]
- 52.Hammad TA, McAdams MA, Feight A, Iyasu S, Dal Pan GJ. Determining the predictive value of Read/OXMIS codes to identify incident acute myocardial infarction in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2008;17(12):1197–1201. doi: 10.1002/pds.1672 [DOI] [PubMed] [Google Scholar]
- 53.Herrett E, Shah AD, Boggon R, et al. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ. 2013;346(may203):f2350–f2350. doi: 10.1136/bmj.f2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khand AU, Shaw M, Gemmel I, Cleland JGF. Do discharge codes underestimate hospitalisation due to heart failure? Validation study of hospital discharge coding for heart failure. Eur J Heart Fail. 2005;7(5):792–797. doi: 10.1016/j.ejheart.2005.04.001 [DOI] [PubMed] [Google Scholar]
- 55.Kirkman MA, Mahattanakul W, Gregson BA, Mendelow AD. The accuracy of hospital discharge coding for hemorrhagic stroke. Acta Neurol Belg. 2009;109(2):114–119. [PubMed] [Google Scholar]
- 56.Kivimäki M, Batty GD, Singh-Manoux A, Britton A, Brunner EJ, Shipley MJ. Validity of cardiovascular disease event ascertainment using linkage to UK hospital records. Epidemiology. 2017;28(5):735–739. doi: 10.1097/EDE.0000000000000688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ekker MS, Verhoeven JI, Vaartjes I, van Nieuwenhuizen KM, Klijn CJM, de Leeuw FE. Stroke incidence in young adults according to age, subtype, sex, and time trends. Neurology. 2019;92(21):e2444–e2454. doi: 10.1212/WNL.0000000000007533 [DOI] [PubMed] [Google Scholar]
- 58.Hammar N, Alfredsson L, Rosén M, Spetz CL, Kahan T, Ysberg AS. A national record linkage to study acute myocardial infarction incidence and case fatality in Sweden. Int J Epidemiol. 2001;30(Suppl 1):S30–4. doi: 10.1093/ije/30.suppl_1.S30 [DOI] [PubMed] [Google Scholar]
- 59.Heerdink ER, Leufkens HG, Herings RM, Ottervanger JP, Stricker BH, Bakker A. NSAIDs associated with increased risk of congestive heart failure in elderly patients taking diuretics. Arch Intern Med. 1998;158(10):1108–1112. doi: 10.1001/archinte.158.10.1108 [DOI] [PubMed] [Google Scholar]
- 60.Holmqvist M, Gränsmark E, Mantel Ä, et al. Occurrence and relative risk of stroke in incident and prevalent contemporary rheumatoid arthritis. Ann Rheum Dis. 2013;72(4):541–546. doi: 10.1136/annrheumdis-2012-201387 [DOI] [PubMed] [Google Scholar]
- 61.Nieuwkamp DJ, Vaartjes I, Algra A, Rinkel GJE, Bots ML. Risk of cardiovascular events and death in the life after aneurysmal subarachnoid haemorrhage: a nationwide study. Int J Stroke. 2014;9(8):1090–1096. doi: 10.1111/j.1747-4949.2012.00875.x [DOI] [PubMed] [Google Scholar]
- 62.Vila-Corcoles A, Satue-Gracia E, Ochoa-Gondar O, et al. Incidence and lethality of ischaemic stroke among people 60 years or older in the region of Tarragona (Spain), 2008–2011. Rev Neurol. 2014;59(11):490–496. [PubMed] [Google Scholar]
- 63.Varmdal T, Bakken IJ, Janszky I, et al. Comparison of the validity of stroke diagnoses in a medical quality register and an administrative health register. Scand J Public Health. 2016;44(2):143–149. doi: 10.1177/1403494815621641 [DOI] [PubMed] [Google Scholar]
- 64.Köster M, Asplund K, Johansson Å, Stegmayr B. Refinement of Swedish Administrative Registers to monitor stroke events on the national level. Neuroepidemiology. 2013;40(4):240–246. doi: 10.1159/000345953 [DOI] [PubMed] [Google Scholar]
- 65.Merry AHH, Boer JMA, Schouten LJ, et al. Validity of coronary heart diseases and heart failure based on hospital discharge and mortality data in the Netherlands using the cardiovascular registry Maastricht cohort study. Eur J Epidemiol. 2009;24(5):237–247. doi: 10.1007/s10654-009-9335-x [DOI] [PubMed] [Google Scholar]
- 66.Stegmayr B, Asplund K. Measuring stroke in the population: quality of routine statistics in comparison with a population-based stroke registry. Neuroepidemiology. 1992;11(4–6):204–213. doi: 10.1159/000110933 [DOI] [PubMed] [Google Scholar]
- 67.Mähönen M, Jula A, Harald K, et al. The validity of heart failure diagnoses obtained from administrative registers. Eur J Prev Cardiol. 2013;20(2):254–259. doi: 10.1177/2047487312438979 [DOI] [PubMed] [Google Scholar]
- 68.Pajunen P, Koukkunen H, Ketonen M, et al. The validity of the Finnish Hospital Discharge Register and causes of death register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12(2):132–137. [DOI] [PubMed] [Google Scholar]
- 69.Pietilä K, Tenkanen L, Mänttäri M, Manninen V. How to define coronary heart disease in register-based follow-up studies: experience from the Helsinki Heart Study. Ann Med. 1997;29(3):253–259. doi: 10.3109/07853899708999343 [DOI] [PubMed] [Google Scholar]
- 70.Rapola JM, Virtamo J, Korhonen P, et al. Validity of diagnoses of major coronary events in national registers of hospital diagnoses and deaths in Finland. Eur J Epidemiol. 1997;13(2):133–138. doi: 10.1023/A:1007380408729 [DOI] [PubMed] [Google Scholar]
- 71.Appelros P, Terént A. Validation of the Swedish inpatient and cause-of-death registers in the context of stroke. Acta Neurol Scand. 2011;123(4):289–293. doi: 10.1111/j.1600-0404.2010.01402.x [DOI] [PubMed] [Google Scholar]
- 72.Leppälä JM, Virtamo J, Heinonen OP. Validation of stroke diagnosis in the National Hospital Discharge Register and the Register of Causes of Death in Finland. Eur J Epidemiol. 1999;15(2):155–160. doi: 10.1023/A:1007504310431 [DOI] [PubMed] [Google Scholar]
- 73.Tolonen H, Salomaa V, Torppa J, et al. The validation of the Finnish Hospital Discharge Register and causes of death register data on stroke diagnoses. Eur J Cardiovasc Prev Rehabil. 2007;14(3):380–385. doi: 10.1097/01.hjr.0000239466.26132.f2 [DOI] [PubMed] [Google Scholar]
- 74.Baldereschi M, Balzi D, Di Fabrizio V, et al. Administrative data underestimate acute ischemic stroke events and thrombolysis treatments: data from a multicenter validation survey in Italy. PLoS One. 2018;13(3):e0193776. doi: 10.1371/journal.pone.0193776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barchielli A, Balzi D, Naldoni P, et al. Hospital discharge data for assessing myocardial infarction events and trends, and effects of diagnosis validation according to MONICA and AHA criteria. J Epidemiol Community Health. 2012;66(5):462–467. doi: 10.1136/jech.2010.110908 [DOI] [PubMed] [Google Scholar]
- 76.Bezin J, Girodet P-O, Rambelomanana S, et al. Choice of ICD-10 codes for the identification of acute coronary syndrome in the French hospitalization database. Fundam Clin Pharmacol. 2015;29(6):586–591. doi: 10.1111/fcp.12143 [DOI] [PubMed] [Google Scholar]
- 77.Bosco-Lévy P, Duret S, Picard F, et al. Diagnostic accuracy of the International Classification of Diseases, Tenth Revision, codes of heart failure in an administrative database. Pharmacoepidemiol Drug Saf. 2019;28(2):194–200. doi: 10.1002/pds.4690 [DOI] [PubMed] [Google Scholar]
- 78.Giroud M, Hommel M, Benzenine E, Fauconnier J, Béjot Y, Quantin C. Positive predictive value of French hospitalization discharge codes for stroke and transient ischemic attack. Eur Neurol. 2015;74:92–99. doi: 10.1159/000438859 [DOI] [PubMed] [Google Scholar]
- 79.Haesebaert J, Termoz A, Polazzi S, et al. Can hospital discharge databases be used to follow ischemic stroke incidence? Stroke. 2013;44(7):1770–1774. doi: 10.1161/STROKEAHA.113.001300 [DOI] [PubMed] [Google Scholar]
- 80.Hammar N, Larsen FF, de Faire U. Are geographical differences and time trends in myocardial infarction incidence in Sweden real? Validity of hospital discharge diagnoses. J Clin Epidemiol. 1994;47(6):685–693. doi: 10.1016/0895-4356(94)90216-x [DOI] [PubMed] [Google Scholar]
- 81.Heliövaara M, Reunanen A, Aromaa A, Knekt P, Aho K, Suhonen O. Validity of hospital discharge data in a prospective epidemiological study on stroke and myocardial infarction. Acta Med Scand. 1984;216(3):309–315. doi: 10.1111/j.0954-6820.1984.tb03809.x [DOI] [PubMed] [Google Scholar]
- 82.Hjerpe P, Merlo J, Ohlsson H, Bengtsson Boström K, Lindblad U. Validity of registration of ICD codes and prescriptions in a research database in Swedish primary care: a cross-sectional study in Skaraborg primary care database. BMC Med Inform Decis Mak. 2010;10(1). doi: 10.1186/1472-6947-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ingelsson E, Ärnlöv J, Sundström J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7(5):787–791. doi: 10.1016/j.ejheart.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 84.Joensuu T, Näyhä S. Reliability of hospital discharge diagnoses of acute myocardial infarction. Scand J Soc Med. 1992;20(2):85–86. doi: 10.1177/140349489202000204 [DOI] [PubMed] [Google Scholar]
- 85.Kaspar M, Fette G, Güder G, et al. Underestimated prevalence of heart failure in hospital inpatients: a comparison of ICD codes and discharge letter information. Clin Res Cardiol. 2018;107(9):778–787. doi: 10.1007/s00392-018-1245-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leone MA, Capponi A, Varrasi C, Tarletti R, Monaco F. Accuracy of the ICD-9 codes for identifying TIA and stroke in an Italian automated database. Neurol Sci. 2004;25(5):281–288. doi: 10.1007/s10072-004-0355-8 [DOI] [PubMed] [Google Scholar]
- 87.Lindblad U, Råstam L, Ranstam J, Peterson M. Validity of register data on acute myocardial infarction and acute stroke: the Skaraborg Hypertension Project. Scand J Soc Med. 1993;21(1):3–9. doi: 10.1177/140349489302100102 [DOI] [PubMed] [Google Scholar]
- 88.Nilsson AC, Spetz CL, Carsjö K, Nightingale R, Smedby B. [Reliability of the hospital registry. The diagnostic data are better than their reputation]. Lakartidningen. 1994;91(7):598,603–605. Swedish. [PubMed] [Google Scholar]
- 89.Øie LR, Madsbu MA, Giannadakis C, et al. Validation of intracranial hemorrhage in the Norwegian Patient Registry. Brain Behav. 2018;8(2):e00900. doi: 10.1002/brb3.900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rinaldi R, Vignatelli L, Galeotti M, Azzimondi G, de Carolis P. Accuracy of ICD-9 codes in identifying ischemic stroke in the General Hospital of Lugo di Romagna (Italy). Neurol Sci. 2003;24(2):65–69. doi: 10.1007/s100720300074 [DOI] [PubMed] [Google Scholar]
- 91.Rodrigo-Rincon I, Martin-Vizcaino MP, Tirapu-Leon B, Zabalza-Lopez P, Abad-Vicente FJ, Merino-Peralta A. Validity of the clinical and administrative databases in detecting post-operative adverse events. Int J Qual Health Care. 2015;27(4):267–275. doi: 10.1093/intqhc/mzv039 [DOI] [PubMed] [Google Scholar]
- 92.Sedova P, Brown RD, Zvolsky M, et al. Validation of stroke diagnosis in the National Registry of Hospitalized Patients in the Czech Republic. J Stroke Cerebrovasc Dis. 2015;24(9):2032–2038. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.019 [DOI] [PubMed] [Google Scholar]
- 93.Spolaore P, Brocco S, Fedeli U, et al. Measuring accuracy of discharge diagnoses for a region-wide surveillance of hospitalized strokes. Stroke. 2005;36(5):1031–1034. doi: 10.1161/01.STR.0000160755.94884.4a [DOI] [PubMed] [Google Scholar]
- 94.Valk MJ, Mosterd A, Broekhuizen BDL, et al. Overdiagnosis of heart failure in primary care: a cross-sectional study. Br J Gen Pract. 2016;66(649):e587–e592. doi: 10.3399/bjgp16X685705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Doorn S, Brakenhoff TB, Moons KGM, et al. The effects of misclassification in routine healthcare databases on the accuracy of prognostic prediction models: a case study of the CHA2DS2-VASc score in atrial fibrillation. Diagnostic Progn Res. 2017;1(1). doi: 10.1186/s41512-017-0018-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Verdú-Rotellar JM, Frigola-Capell E, Alvarez-Pérez R, et al. Validation of heart failure diagnosis registered in primary care records in two primary care centres in Barcelona (Spain) and factors related. A cross-sectional study. Eur J Gen Pract. 2017;23(1):107–113. doi: 10.1080/13814788.2017.1305104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gini R, Schuemie MJ, Mazzaglia G, et al. Automatic identification of type 2 diabetes, hypertension, ischaemic heart disease, heart failure and their levels of severity from Italian General Practitioners’ electronic medical records: a validation study. BMJ Open. 2016;6(12):e012413. doi: 10.1136/bmjopen-2016-012413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bernal JL, Barrabés JA, Íñiguez A, et al. Clinical and administrative data on the research of acute coronary syndrome in Spain. Minimum basic data set validity. Rev Española Cardiol (English Ed). 2019;72(1):56–62. doi: 10.1016/j.rec.2018.01.026 [DOI] [PubMed] [Google Scholar]
- 99.Mähönen M, Salomaa V, Brommels M, et al. The validity of hospital discharge register data on coronary heart disease in Finland. Eur J Epidemiol. 1997;13(4):403–415. doi: 10.1023/A:1007306110822 [DOI] [PubMed] [Google Scholar]
- 100.Palomäki P, Miettinen H, Mustaniemi H, et al. Diagnosis of acute myocardial infarction by MONICA and FINMONICA diagnostic criteria in comparison with hospital discharge diagnosis. J Clin Epidemiol. 1994;47(6):659–666. doi: 10.1016/0895-4356(94)90213-5 [DOI] [PubMed] [Google Scholar]
- 101.Aboa-Eboulé C, Mengue D, Benzenine E, et al. How accurate is the reporting of stroke in hospital discharge data? A pilot validation study using a population-based stroke registry as control. J Neurol. 2013;260(2):605–613. doi: 10.1007/s00415-012-6686-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ellekjaer H, Holmen J, Krüger O, Terent A. Identification of incident stroke in Norway: hospital discharge data compared with a population-based stroke register. Stroke. 1999;30(1):56–60. doi: 10.1161/01.STR.30.1.56 [DOI] [PubMed] [Google Scholar]
- 103.Ho KKL, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(SUPPL. 4):6–43. doi: 10.1016/0735-1097(93)90455-A [DOI] [PubMed] [Google Scholar]
- 104.Carlson KJ, Lee DCS, Goroll AH, Leahy M, Johnson RA. An analysis of physicians’ reasons for prescribing long-term digitalis therapy in outpatients. J Chronic Dis. 1985;38(9):733–739. doi: 10.1016/0021-9681(85)90115-8 [DOI] [PubMed] [Google Scholar]
- 105.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College Of Cardiology Foundation/American Heart Association Task Force On Practice Guidelines. Circulation. 2013;128(16). doi: 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 106.Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA project: registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90(1):583–612. doi: 10.1161/01.cir.90.1.583 [DOI] [PubMed] [Google Scholar]
- 107.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. Circulation. 2007;116(22):2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397 [DOI] [PubMed] [Google Scholar]
- 108.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035. doi: 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 109.Aho K, Harmsen P, Hatano S, Marquardsen J, Smirnov VE, Strasser T. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ. 1980;58(1):113–130. [PMC free article] [PubMed] [Google Scholar]
- 110.Thorvaldsen P, Kuulasmaa K, Rajakangas A-M, Rastenyte D, Sarti C, Wilhelmsen L. Stroke trends in the WHO MONICA project. Stroke. 1997;28(3):500–506. doi: 10.1161/01.STR.28.3.500 [DOI] [PubMed] [Google Scholar]
- 111.Brenner H, Gefeller O. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat Med. 1997;16(9):981–991. doi: [DOI] [PubMed] [Google Scholar]
- 112.Yao RJR, Andrade JG, Deyell MW, Jackson H, McAlister FA, Hawkins NM. Sensitivity, specificity, positive and negative predictive values of identifying atrial fibrillation using administrative data: a systematic review and meta-analysis. Clin Epidemiol. 2019;11:753–767. doi: 10.2147/CLEP.S206267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McGuinness LA, Warren-Gash C, Moorhouse LR, Thomas SL. The validity of dementia diagnoses in routinely collected electronic health records in the United Kingdom: a systematic review. Pharmacoepidemiol Drug Saf. 2019;28(2):244–255. doi: 10.1002/pds.4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nissen F, Quint JK, Morales DR, Douglas IJ. How to validate a diagnosis recorded in electronic health records. Breathe. 2019;15(1):64–68. doi: 10.1183/20734735.0344-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hemingway H, Asselbergs FW, Danesh J, et al. Big data from electronic health records for early and late translational cardiovascular research: challenges and potential. Eur Heart J. 2018;39(16):1481–1495. doi: 10.1093/eurheartj/ehx487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Banerjee A, Pasea L, Harris S, et al. Estimating excess 1-year mortality from COVID-19 according to underlying conditions and age in England: a rapid analysis using NHS health records in 3.8 million adults. medRxiv. 2020. doi: 10.1101/2020.03.22.20040287 [DOI] [PMC free article] [PubMed] [Google Scholar]