Abstract
Goblet cells (GCs) are single-cell glands that produce and secrete mucin. Mucin forms a mucus layer, which can separate the materials in cavities from the intestinal epithelium and prevent the invasion of pathogenic microorganisms in various ways. GCs can also participate in the immune response through nonspecific endocytosis and goblet cell-associated antigen passages (GAPs). GCs endocytose soluble substances from the lumen and transmit antigens to the underlying antigen-presenting cells (APCs). A variety of immuno-regulatory factors can promote the differentiation, maturation of GCs, and the secretion of mucin. The mucin secreted by GCs forms a mucus layer, which plays an important role in resisting the invasion of foreign bacteria and intestinal inherent microorganisms, regulating the immune performance of the body. Therefore, the present study mainly reviews the barrier function of the mucus layer, the mucus secreted by goblet cells, the protective effect against pathogenic bacteria, the delivery of luminal substances through GAPs and the relationship between GCs and the immune response.
Keywords: Goblet cells, Immune response, Mucins
Introduction
The intestine is one of the important digestive organs of the human and animal body, but there are large numbers of bacteria, viruses and parasites in the intestine, which is a potential source of infection [1]. In general, intestinal mucosal barriers include mechanical, immune, and biological barriers [2]. These barriers play a significant role in maintaining the intestinal micro-ecological balance and the stability of the internal environment, preventing the invasion of pathogens and the displacement of endotoxins, and regulating the microbial–host immune response [3]. The mucus on the surface of the intestinal mucosa participates in forming a mucus barrier and plays an important role in protecting the epithelium [4]. Goblet cells (GCs) are formed by the differentiation of intestinal epithelial cells. GCs are single-cell glands whose main function is to synthesize and secrete mucins. Mucin 2 (MUC2), water, and inorganic salts constitute the mucus layer, in which water accounts for more than 90%; thus, the layer is colloidal. The mucus layers in the small and large intestines are different. In the small intestine, the mucus and antibacterial peptides/proteins form an antibacterial gradient, reducing the number of bacteria that can reach the epithelial cells. In the colon, normally, bacteria cannot penetrate its two mucus layers. The penetration of the inner layer by bacteria can lead to inflammation [5,6]. Mucin can prevent the loss of sIgA antibody molecules on the intestinal cavity side of epithelial cells because sIgA can interact with mucin and bacterial cell surface proteins to stabilize the bacterial biofilm [7]. sIgA in the mucus layer will be phagocytosed and cleared by macrophages after binding to bacteria or antigens.
Therefore, the main content of this review includes the defense function of the mucus layer and sIgA, the classification and function of GCs, the secretory function of mucin, the regulatory pathway of immune factors on mucin secretion, and the mechanism of immune factors on GCs and mucin. The introduction of the above content will lay a theoretical foundation for further study of the relationship between GCs and the immune response.
The histomorphology and distribution of goblet cells
GCs are single-cell glands that exist in humans and mammals and are present in the epithelium, including the respiratory, digestive tracts, and genital atrium. GCs are present in the intestinal epithelial cells in the early postnatal period [8]. As a highly polarized columnar epithelial cell, the tops of GCs are enlarged, and the bottom of the cytoplasm is narrow and located on the basement membrane. The nucleus is small, located at the base, triangular or oblate, and stained deeply. GC has a large perinuclear region with the endoplasmic reticulum, Golgi apparatus, and concentrated vesicles. The pluripotent stem cells at the base of the intestinal crypt differentiate to form GCs [9]. The cytoplasm is filled with thick secretory granules containing mucin, a glycoprotein. GCs are known for their secretion of mucin, providing the mucosal surfaces with a thick mucus lining that acts as a barrier to limit interactions with microbes. The migration and differentiation to the shedding of GCs occur over a total of 2–4 days [10]. The distribution of GCs in the intestinal epithelium increases gradually from the duodenum to the distal colon [11], from 4% to 16%, consistent with the increase in the distribution of the intestinal flora.
The defense function of the mucus layer
Protective effect of the mucosal layer on the intestinal epithelium
There are a large number of microorganisms in the intestine. The normal human gut microbiota comprises two major phyla, namely Bacteroidetes and Firmicutes [12]. The intestinal microflora is divided into physiological bacteria, conditioned pathogenic bacteria and pathogenic bacteria [13]. These bacteria may cause damage to the body through the mucosal barrier, making the intestine a potential source of infection [14]. The relationship between intestinal bacteria and the mucosal barrier is well-balanced in the stable state; intestinal bacteria cannot contact the intestinal epithelium. However, when the mucosal barrier is dysfunctional, intestinal bacteria can approach the intestinal immune cells and cause inflammatory bowel disease (IBD) [15]. Mucus serves as a semi-permeable gel layer, which allows the exchange of gases, water and nutrients with the underlying epithelium [16]. The mucosal epithelium is characterized by a mucus layer on the surface, which plays an important role in separating the intestinal epithelium from the cavity material, resisting the invasion of exogenous bacteria and intestinal microorganisms and maintaining the intestinal micro-ecological balance.
The thickness of the gel-forming mucins formed by the secreted glycoproteins on the surfaces of intestinal epithelial cells ranges from 300 to 700 μm, preventing microorganisms from penetration. The transmembrane mucins, such as MUC1 and MUC3, MUC12, MUC13 and MUC17, cover the apical surfaces of the enterocytes at a thickness of 30–500 nm in a structure called glycocalyx, which can also play a physically protective role. The mucous layer is constantly updated and removes pathogenic substances from the intestinal lumen.
The mucus layer not only plays roles as a source of lubrication [17] and a physical barrier but also captures microorganisms and acts as a trap for microbes. The mucus provides a matrix for a rich array of antimicrobial molecules [18]; mucin oligosaccharides can bind to microorganisms, and the structures and negatively charged properties of glycoproteins are beneficial for encapsulating bacteria. The exposed chemical groups are similar to the surface structure of the intestinal epithelium, making it easy for bacteria to recognize and attach. Mucin oligosaccharides have an antibacterial activity or can carry other antibacterial molecules in some cases. Mucins can bind antibacterial molecules such as histatins and statherin to make them better protect the host in the correct mucosal microenvironment [19]. Experiments have shown that MUC2 binds to luminal antigens (especially bacteria) and reacts with dendritic cells (DCs) to suppress inflammatory [20].
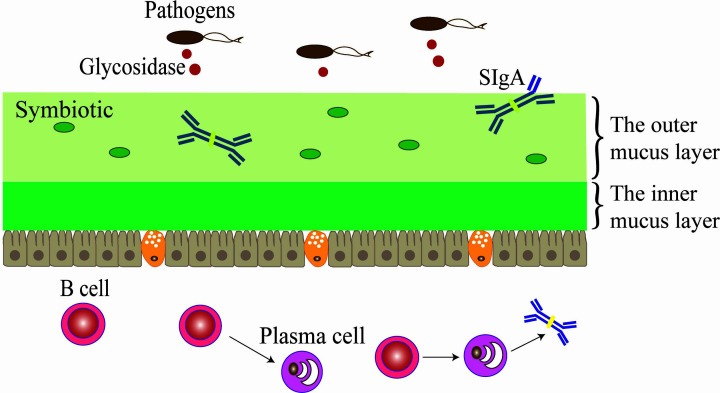
The colon has two layers of mucus, while the small intestine has one [21]. The loose outer mucus layer of the colon is the habitat of commensal bacteria. The relatively dense inner mucus layer is firmly attached to the epithelial cells and the concentration of MUC2 is higher. The inner mucus layer is impervious to bacteria [22] and is renewed every hour by surface GCs [23] (Figure 1). The mucus of the small intestine and antibacterial peptides function together to form an antibacterial mucus gradient, preventing bacteria from reaching epithelial cells.
Figure 1. Regulatory mechanism of sIgA on the barrier function of the colon mucus layer.
The colon has two layers of mucus layers. The inner mucous layer of the colon is tightly attached to the epithelial cells and cannot be infiltrated by bacteria, and the outer mucus layer is relatively loose; symbiotic bacteria and sIgA are colonized in the outer mucus layer.
Microbes, microbial products and cytokines regulate the production and secretion of mucus [24]. Microbiota affects the composition and thickness of mucus. Germ-free mice have fewer GCs and a thinner mucus layer than conventionally raised mice [25,26]. PH affects the rheological property of mucus [27,28]. Helicobacter pylori can invade the mucus barrier by increasing the pH in the environment and reducing the viscoelasticity of mucin [29]. Parasympathetic drugs promote mucus secretion of crypt goblet cells [30]. Immunomodulatory prostaglandins (cAMP-mediated agonists) and carbachol (Ca2+-mediated agonists) produce significant mucus release [31]. The prostaglandin stimulation rate can reach about 350 pl/min, which is a long-lasting stimulus [32].
Under normal circumstances, pathogenic bacteria rarely colonize the intestinal lumen; however, when the intestinal flora is displaced or the flora is out of balance, a large number of pathogenic bacteria can colonize and expand, causing disease. The bacteria’s exoglycosidase breaks down the polysaccharides in the mucin, and the mucin layer dissolves when it touches the core of the protein [33]. The depletion of GCs leads to defects in the mucus layer, increased adhesion of bacteria on the surface of the epithelium, and reduced digestion and absorption of nutrients [34,35]. Mucin changes and the dysfunction of the mucosal barrier is related to the occurrence and development of IBD such as ulcerative colitis (UC) and Crohn’s disease (CD) [36,37].
Mucin and sIgA contribute to the barrier function
Secretory immunoglobulin A (sIgA) is the main immunoglobulin on the intestinal mucosal surface and plays an important role in preventing the attachment and colonization of pathogenic bacteria and protecting the gastrointestinal mucosa [38,39]. sIgA is secreted into the intestinal cavity and is mixed in the mucous layer, which covers the surface of epithelial cells and binds microorganisms or food antigens to form antigen–antibody complexes, facilitates phagocytosis and removal of macrophages. sIgA cannot bind to mucus in the absence of carbohydrates and cannot stop infections caused by bacteria in a murine respiratory infection model [40]. It has been proven that sIgA–mucin protein interaction is an important factor for sIgA to capture pathogens.
Immunofluorescence microscopy determined that sIgA co-located with intestinal bacteria in the outer mucous layers of mouse and human colon. It was demonstrated that sIgA anchored the outer mucous layer by acting in conjunction with mucinous proteins and intestinal bacteria [41], thus playing a role in the immune defense to germs and maintaining a mutually beneficial symbiosis with symbiotic bacteria, protecting epithelial cells. Using pIgR and the mucin-2 deficient mice demonstrated that Muc2 is needed instead of sIgA for bacteria excreting from the inner mucus layer of the colon [42]. The above points indicated that sIgA promotes mucosal colonization of microbiota with beneficial properties, whereas disease states may induce sIgA responses to pathogens that disrupt the equilibrium of the healthy microbiome.
The mucus of secretory goblet cells
As secretory intestinal epithelial cells, GCs function to synthesize and secrete mucin. Mucin is a high-molecular-weight glycoprotein, the synthesis process of which includes dimerization in the endoplasmic reticulum, Golgi glycosylation, and finally oligomerization [43]. It is stored in particles in the GCs, and after being released, it forms a network structure on the surface of the intestinal epithelium. The major mucin in the intestine is MUC2, which is highly glycosylated mucin. Its protein skeleton binds to various O-link oligosaccharides [44].
Mucin genes are named MUC1 to MUC21 according to their sequence of discovery [45]. In terms of their structure and location, mucins can be divided into the secretory type and membrane-bound type. Secretory mucins include MUC2, MUC5AC, MUC5B, MUC6, MUC19 [46,47] (secreted gel-forming mucins) and MUC7 [48], MUC8 [49], MUC9 [50] (secreted non-gel-forming mucins). Membrane-bound types include MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC16, MUC17, and MUC20. According to their functions, they are divided into transmembrane mucin and secretory mucin. Structurally, transmembrane mucin includes a transmembrane domain in its C-terminus, which is involved in intracellular signal transduction. Different mucins are expressed at different sites in vivo: the stomach and duodenum can express MUC6; MUC5B is expressed in the human colon at low levels; and MUC5AC is expressed in the stomach and the intestine after infection [51].
All mucins contain a large number of serine (P), threonine (T) and proline (S) residues, called PTS domains. These residues are also highly glycosylated, and their N- and C-termini are rich in asparagine and cysteine. Secretory granules, which are formed by completely glycosylated mucins, are filled and stored in the GCs. The secretion including the constitutive and regulatory pathways, regulated by a variety of biological activity factors. The constitutive pathway continuously secretes mucin to maintain the renewal of the intestinal mucus layer, while the regulatory pathway functions in cases of pathological or environmental stimulation, such as bacterial infection and inflammation [52].
Sentinel goblet cells and nonspecific endocytosis
Previous studies have shown that GCs secretion of mucin is associated with autophagy, NLRP6 (NOD-like receptor family pyrin domain-containing 6) and caspase 1/11 [53–55]. GCs secrete mucin granules in the colon exposed to ischemia–reperfusion, remove bacteria from the colonic crypts, and restore the mucus layer during reperfusion [56].
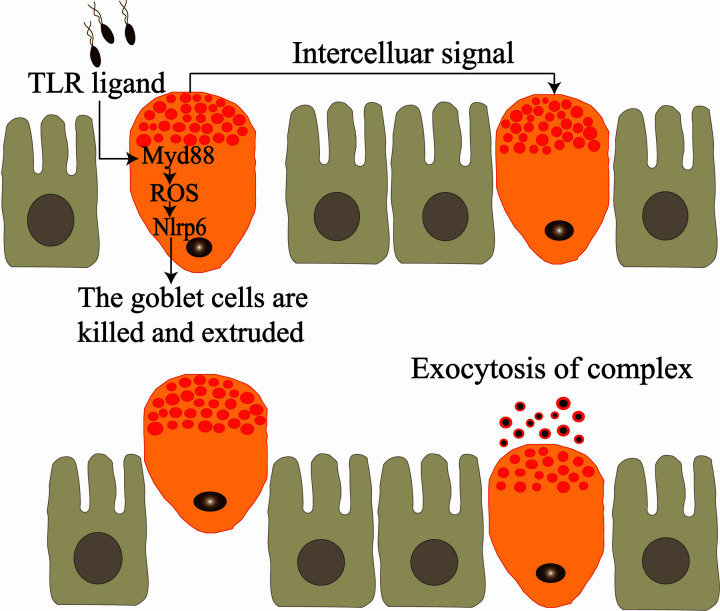
Stem cells at the bottom and middle of the intestinal crypts continue to differentiate to maintain the self-renewal of the intestinal epithelium [57]. Recently, it has been discovered that some GCs at the entrance of colonic crypts can undergo nonspecific endocytosis, called sentinel goblet cells (senGCs). The senGCs, in turn, activate the TLR (Toll-like receptor) signal and MyD88 (myeloid differentiation factor 88) dependent reactive oxygen species synthesis of Nox/Duox (NADPH oxidase/dual oxidase) and the NLRP6 inflammasome-mediated activation of caspases 1 and 11, thereby reacting with and endocytosing the ligands of TLR2/1, TLR4 and TLR5, causing the complex to trigger the exocytosis of Muc2 and intercellular signaling connections. Adjacent responsive GCs are induced to secrete MUC2, and the bacteria are washed out from the opening of the crypt, protecting it (Table 1). The endocytosed senGCs are killed after the complex exocytosis occurs and are discharged from the epithelium into the lumen, a process driven by the activation of the NLRP6 inflammasome (Figure 2) [58].
Table 1. Mucus production and immunomodulatory molecules and pathways.
| Trigger | Cell | Pathway | Result |
|---|---|---|---|
| TLR ligand | Sentinel goblet cells (senGCs) | TLR, MyD88, NLRP6 | Exocytosis of Muc2 and adjacent responsive GCs are induced to secrete MUC2 |
| adverse conditions such as bacterial infection or antibioticS | GCs in the small intestine and distal colon | Myd88, EGRF and p42/p44 MAPK | Inhibit the formation of GAPs |
Mucin production is regulated by many factors, including temperature, pH value, infection, and inflammatory factors. When the body is threatened by bacterial infection, it will activate the GCs pathway and secrete mucus to prevent pathogens from approaching the intestinal epithelium.
Figure 2. Regulatory mechanism of the goblet cell endocytosis signaling pathway.
After stimulating goblet cells, the bacterial TLR ligand activates the intracellular signaling pathway, which in turn activates Myd88, ROS synthesis, and Nlrp6, triggering the exocytosis of the complex and the secretion of MUC2 by adjacent goblet cells. The goblet cells that undergo non-specific endocytosis are killed and expelled to the lumen.
GCs can rapidly deal with pathogens that may cause intestinal epithelial infection by infiltrating the mucosal barrier through non-specific endocytosis, secreting mucus to maintain the mucous layer without activating the adaptive immune system. Although GCs die in this process, they play an important role in protecting intestinal mucosa and intestinal epithelium [59].
The immune signal mechanisms of Goblet cell-associated antigen passages
GCs in the small intestine and distal colon can form goblet cell-associated antigen passages (GAPs). GAPs are the main mechanism for delivering luminal small soluble antigens to intestine lamina propria (LP) DCs in a stable state [60–62]. GAPs are formed by GCs when acetylcholine (ACh) acts on muscarinic acetylcholine receptor 4 (mAChR4), antigen-presenting cells (APCs) migrate to the intestinal epithelium and acquire antigen in a mAChR4-dependent manner.
GAPs in the small intestine form 18 days after weaning. Colon GAPs are formed before weaning and are suppressed after weaning [63]. The formation of the proximal colon GAPs is inhibited by GC intrinsic microbial sensing. Colon microbes inhibit the formation of the GAPs in a myd88-dependent manner, Myd88 activates epidermal growth factor receptor (EGRF), and p42/p44 mitogen-activated protein kinase (MAPK), which makes them phosphorylated to inhibit the formation of colon GAPs (Table 1) [64]. The proximal colon has a higher density of bacteria than the small intestine [65], and a thinner mucus layer than the distal colon [66]. Through the inhibition of microbial sensing, the immune system of the proximal colon is prevented from being exposed to the bacteria in the cavity and the inflammatory reaction is avoided. Opening colonic GAPs for 4 days leads to increased levels of neutrophil chemokine CXCL1 and inflammatory factors IL-6, IL-17, as well as the influx of colonic neutrophils.
The antigen delivered by the GAPs in the small intestine is mainly delivered to CD103+ DCs, which can stimulate the proliferation of T cells and induce an adaptive immune response. Particles smaller than 20 nm can enter the small intestine GAPs. LP-DCs slowly collect or actively detect antigens. Some GCs in the small intestine form GAPs when they secrete, but not all GCs secretion is related to the formation of the GAPs. GAPs are also found in human jejunum [67]. The intestine LP-APCs include CD11b+CD103−CX3CR1+ APCs and CD11b+CD103+CX3CR1− APCs [68], collectively known as mononuclear phagocytes (MNPs), both of which can react with GAPs in the small intestine and colon. The more frequent reaction between CD103+ LP-APCs and GAPs may be due to the stronger migration ability of CD103+ LP-APCs, the response ability to granulocytes and inflammatory factors, and the stimulation ability to T cells [69].
The use of antibiotics causes inflammation and damages the intestinal mucosa and epithelium. Antibiotics reduce the inhibition of the formation of colon GAPs, allowing commensal bacteria and protein antigens in the intestinal lumen to translocate across the intestinal epithelium through the GAPs [70]. LP-MNPs ingest commensal bacteria and protein antigens and migrate to the draining lymph nodes (LNs) to promote inflammatory response, leading to the response of inflammatory T cells to other innocuous antigens [71].
Salmonella can pass through the intestinal epithelium through GAPs in the early stage of infection [72]. IL-1β participates in the inhibition of GAP formation in the small intestine during Salmonella infection. Besides, Listeria monocytogenes has also been proved to pass through the intestinal epithelium through targeting GCs [73], indicating that the pathogenic bacteria may use GAPs as an invasion portal. It is speculated that under disadvantaged conditions such as bacterial infection, GCs may secrete mucus to maintain the mucus layer, inhibit the formation of GAPs, and thus prevent the immune system from being inappropriately exposed to intraluminal substances. GAP formation is highly adaptable to different luminal conditions [74].
GAPs are also related to oral tolerance [75]. Oral tolerance is the state in which the immune system has no immune response to innocuous antigens such as food antigens. Oral tolerance generates regulatory T cells (Tregs), which play an important role in maintaining immune homeostasis [76]. GAPs support the induction and maintenance of oral tolerance by delivering luminal antigens, maintaining pre-existing LP Tregs, and imprinting tolerogenic properties on LP-APCs [77].
Effects of inflammatory factors on goblet cells and mucus secretion
Effects of Th2 secreted cytokines on goblet cells and mucus secretion
Intestinal infection with parasites causes the immune response to skew toward type 2 helper (Th2) cells, leading to increased secretion of cytokines such as IL-4 [78], IL-5, IL-9, and IL-13. Among them, IL-13 plays a dominant role and a vital cytokine regulating GC proliferation. IL-13 can act on epithelial cells by acting on signal transducer and activator of transcription-6 (STAT6) and promotes GC proliferation. Experiments have shown that GC proliferation was detected in IL-13 overexpressing mice, and the overexpression of exogenous IL-25 and IL-9 also promoted GC proliferation and mucin expression through an IL-13-dependent pathway [79]. In the LP of the small intestine, IL-13 plays a role in expelling worm through GCs hyperplasia [80]. IL-4 and IL-13 up-regulate intestinal trefoil factor (ITF) and MUC2 transcription in human colon cancer cell lines [81,82]. GCs proliferation caused by helminth infection is mainly controlled by IL-4 and IL-13 in the Th2 immune response, but the GCs proliferation caused by Syphacia obvelata and Schistosoma mansoni infection is independent of IL-4 and IL-13 [83].
Th2 cytokines, including IL-4, 1L-6, IL-9, 1L-10, and IL-13 [84]. IL-9 and IL-10 have also been shown to regulate mucin expression. Interleukin-9 up-regulates mucus expression in the airways [85]. IL-10 promotes the production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in GCs [86].
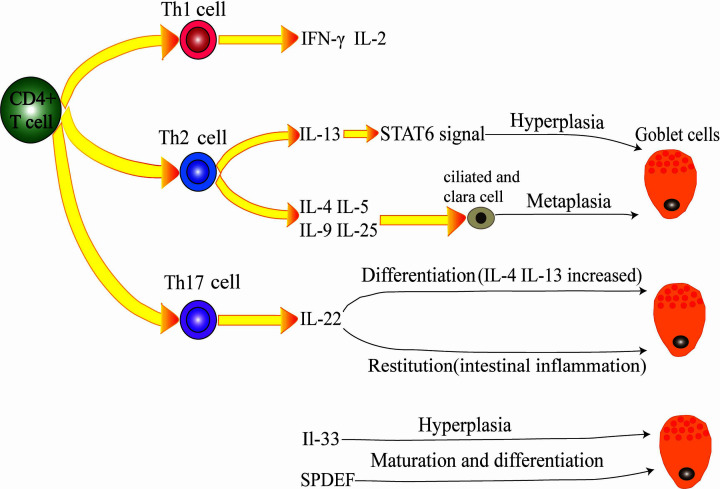
Th2 cells promote not only the proliferation of GCs but also the metaplasia of ciliated and Clara cells into GCs by releasing IL-4, IL-5, IL-9 and IL-25, followed by increased mucus secretion and airway epithelial thickening [87,88] (Figure 3).
Figure 3. Inflammatory factors regulate goblet cell secretion.
CD4 Th cells include Th1, Th2, and Th17 cells. Th2 cells secrete IL-13, which can promote goblet cell proliferation through STAT6 signaling. Th2 cells can also secrete IL-4, IL-5, IL-9, and IL-25 to promote the metaplasia of ciliated cells and Clara cells into goblet cells. Th17-secreted IL-22 can regulate goblet cell differentiation and promote goblet cells in the case of intestinal inflammation. Other cytokines, such as IL-33 and SPDEF, can also affect goblet cells. IL-33 promotes the proliferation of goblet cells, and SPDEF regulates the maturation and differentiation of goblet cells.
Effects of Th17 secreted cytokines on goblet cells and mucus secretion
Th17-related IL-22 can regulate GC differentiation and mucin expression. IL-22−/− mice had lower levels of mucin expression and GC numbers in response to Nippostrongylus brasiliensis and Trichuris muris infections compared with wild-type mice and increased contents of IL-4 and IL-13 [89]. During intestinal inflammation, IL-22 can promote MUC1 levels and the restitution of GCs, increasing the mucus barrier and epithelial regeneration. This process is mediated by Th1 or Th2 [90]. There may be overlap in the process by which inflammatory factors promote GC differentiation and mucin expression. IL-22 gene delivery leads to increased STAT3 (signal transducer and activator of transcription-3)-dependent mucin secretion and GCs repair, rapidly alleviate local intestinal inflammation [91].
A typical cytokine, IL-17A, is also released by Th17 cells. IL-17A can induce the release of many cytokines, and TNF-ɑ, IL-1β, G-CSF, IL-6, CXCL2 and IL-8, IL-17A and IL-6 together can promote the expression of MUC5AC and MUC5B [92]. Both Th2 and Th17 cells exhibit significantly increased expression of the MUC5AC gene in airway epithelial cells in vitro and in vivo [93].
Effects of other cytokines on goblet cells and mucus secretion
IL-33 promotes GC proliferation and mucin expression. IL-33 induce MUC5AC expression, and also GC hyperplasia at air–liquid interface culture in human nasal epithelial cells [94]. IL-33 does not directly act on epithelial cells but indirectly induces GCs differentiation through IL-13 produced by group 2 innate lymphoid cells [95]. The transcription factor SPDEF (SAM pointed domain ETS factor) can regulate GCs in multiple organs. SPDEF regulates the proliferation of GCs in the airway, which is related to its maturation; SPDEF is also related to the maturation and differentiation of GCs in the small intestine and plays a role in promoting maturation and differentiation of GCs in the lung [96,97].
Conclusion
The mucus layer prevents microorganisms from approaching the gastrointestinal epithelial cells and moves continuously to remove residual material. Mucus can capture pathogenic bacteria and can also serve as a matrix for antibacterial substances. Thus, the pathogen needs a unique pathway to break into the mucin and invade the mucous layer. The dynamic properties of the intestinal mucus layer and its regulation of the intestinal microflora and local immune system are very complex. The different distributions of the mucous layer in the intestinal lumen and the pleiotropic effect of mucin during inflammation suggest that the intestinal mucous barrier is not only an independent component but also closely related to the intestinal mucosal physics and immune barrier. The temporal and spatial distributions of the bacteria in mucus and their interaction with the local immune system remain challenges for future research. Our research group is interested in this research work and hopes to provide strong evidence for the function of the mucus layer barrier.
Abbreviations
- ACh
acetylcholine
- APC
antigen-presenting cell
- CD
Crohn’s disease
- DC
dendritic cell
- EGRF
epidermal growth factor receptor
- GAP
goblet cell-associated antigen passages
- GC
Goblet cell
- IBD
inflammatory bowel disease
- ITF
intestinal trefoil factor
- LN
lymph nodes
- LP
lamina propria
- mAChR4
muscarinic acetylcholine receptor 4
- MAPK
mitogen-activated protein kinase
- MUC2
mucin 2
- MNP
mononuclear phagocytes
- NLRP6
NOD-like receptor family pyrin domain-containing 6
- Nox/Duox
NADPH oxidase/dual oxidase
- senGC
sentinel goblet cell
- sIgA
secretory immunoglobulin A
- SPDEF
SAM pointed domain ETS factor
- STAT3
signal transducer and activator of transcription-3
- STAT6
signal transducer and activator of transcription-6
- Th2
type 2 helper
- TLR
Toll-like receptor
- Treg
regulatory T cell
- UC
ulcerative colitis
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This study was financed by the grants from the Shaanxi Province Science Foundation (2017JQ3012) and Fundamental Research Funds for the Central Universities [grant number Z109021548] and the Special Scientific Research Fund of Agriculture Public Welfare Industry [grant number 201203062].
References
- 1.Huang C. and Zeng Y. (2017) Study on the function of goblet cells and intestinal mucus barrier. Int. J. Digestive Dis. 37, 357–360 [Google Scholar]
- 2.Li W. and Chen Q. (2008) Research progress of intestinal mucosal immune barrier and its relationship with body health. Food Sci. 29, 649–654 [Google Scholar]
- 3.Lei C. and Dong G. (2012) Regulation of intestinal flora on intestinal mucosal immunity in animals. J. Animal Nutrition 24, 416–422 [Google Scholar]
- 4.Liu Y., Ding Y. et al. (2017) Research progress of intestinal goblet cells and their functions. World Chin. J. Digestion 25, 1279–1286 10.11569/wcjd.v25.i14.1279 [DOI] [Google Scholar]
- 5.Yang W., Lai L. et al. (2018) Research progress on the role of intestinal goblet cells in intestinal immune regulation. J. Cell Mol. Immunol. 34, 1046–1050 [PubMed] [Google Scholar]
- 6.Johansson M.E. et al. (2013) The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 352–361 10.1038/nrgastro.2013.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macpherson A.J., McCoy K.D. et al. (2008) The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 10.1038/mi.2007.6 [DOI] [PubMed] [Google Scholar]
- 8.Birchenough G.M.H. et al. (2013) Altered Innate Defenses in the Neonatal Gastrointestinal Tract in Response to Colonization by Neuropathogenic Escherichia coli. Infection Immunity 81, 3264–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Flier L.G. and Clevers H. (2009) Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71, 241–260 10.1146/annurev.physiol.010908.163145 [DOI] [PubMed] [Google Scholar]
- 10.van der Flier L.G., van Gijn M.E. et al. (2009) Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 136, 903–912 10.1016/j.cell.2009.01.031 [DOI] [PubMed] [Google Scholar]
- 11.Barker N. et al. (2008) The intestinal stem cell. Genes Dev. 22, 1856–1864 10.1101/gad.1674008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M. and Nageshwar Reddy D. (2015) Role of the normal gut microbiota. World J. Gastroenterol. 21, 8787–8803 10.3748/wjg.v21.i29.8787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L. and Zhou X. (2017) Research progress of intestinal flora and intestinal mucosal immunity and related intestinal diseases. Chin. J. Microecol. 29, 494–497 [Google Scholar]
- 14.Miyata N., Morris L.L. et al. (2018) Microbial Sensing by Intestinal Myeloid Cells Controls Carcinogenesis and Epithelial Differentiation. Cell Rep. 24, 2342–2355 10.1016/j.celrep.2018.07.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okumura R. and Takeda K. (2018) Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 38, 10.1186/s41232-018-0063-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornicky S., Tawiahy A. and Chadee K. (2015) Roles and regulation of the mucus barrier in the gut. Tissue Barriers 3, 1–2, eCollection 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCauley H.A. et al. (2015) Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 21, 492–503 10.1016/j.molmed.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 18.Linden S.K., Sutton P. et al. (2008) Mucins in the mucosal barrier to infection. Mucosal Immunol. 1, 183–197 10.1038/mi.2008.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruno L.S. et al. (2005) Two-hybrid analysis of human salivary mucin MUC7 interactions. Biochim. Biophys. Acta 1746, 65–72 10.1016/j.bbamcr.2005.08.007 [DOI] [PubMed] [Google Scholar]
- 20.Shan M., Gentile M. et al. (2013) Mucus Enhances Gut Homeostasis and Oral Tolerance by Delivering Immunoregulatory Signals. Science 342, 447–453 10.1126/science.1237910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ermund A., Schutte A. et al. (2013) Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer's patches. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G341–G347 10.1152/ajpgi.00046.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson M.E.V. et al. (2008) The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. 105, 15064–15069 10.1073/pnas.0803124105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson M.E. et al. (2013) The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 352–361 10.1038/nrgastro.2013.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrianifahanana M., Moniaux N. and Batra S.K. (2006) Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim. Biophys. Acta 1765, 189–222 [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa K., Satoh Y., Oomori Y., Yamano M., Matsuda M. and Ono K. (1989) Influence of conventionalization on cecal wall structure of germfree Wistar rats: quantitative light and qualitative electron microscopic observations. Anat. Embryol. (Berl.) 180, 191–198 10.1007/BF00309771 [DOI] [PubMed] [Google Scholar]
- 26.Szentkuti L., Riedesel H., Enss M.L., Gaertner K. and von Engelhardt W. (1990) Pre-epithelial mucus layer in the colon of conventional and germfree rats. Histochem. J. 22, 491–497 10.1007/BF01007234 [DOI] [PubMed] [Google Scholar]
- 27.Cao X., Bansil R., Bhaskar K.R., Turner B.S., LaMont J.T., Niu N. et al. (1999) pH-dependent conformational change of gastric mucin leads to sol-gel transition. Biophys. J. 76, 1250–1258 10.1016/S0006-3495(99)77288-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celli J.P., Turner B.S., Afdhal N.H., Ewoldt R.H., McKinley G.H., Bansil R. et al. (2007) Rheology of gastric mucin exhibits a pH-dependent sol-gel transition. Biomacromolecules 8, 1580–1586 10.1021/bm0609691 [DOI] [PubMed] [Google Scholar]
- 29.Celli J.P., Turner B.S., Afdhal N.H., Keates S., Ghiran I., Kelly C.P. et al. (2009) Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc. Natl. Acad. Sci. 106, 14321–14326 10.1073/pnas.0903438106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Specian R.D. and Neutra M.R. (1982) Regulation of intestinal goblet cell secretion. I. Role of parasympathetic stimulation. Am. J. Physiol. 242, G307–G309 [DOI] [PubMed] [Google Scholar]
- 31.Garcia M.A.S., Yang N. and Quinton P.M. (2009) Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J. Clin. Invest. 119, 2613–2622 10.1172/JCI38662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halm D.R. and Halm S.T. (2000) Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am. J. Physiol. Cell Physiol. 278, C212–C233 10.1152/ajpcell.2000.278.1.C212 [DOI] [PubMed] [Google Scholar]
- 33.Backhed F. et al. (2015) Host-Bacterial Mutualism in the Human Intestine. Science 307, 1915–1920 10.1126/science.1104816 [DOI] [PubMed] [Google Scholar]
- 34.Shao Y. et al. (2013) β-1,3/1,6-Glucan alleviated intestinal mucosal barrier impairment of broiler chickens challenged with Salmonella enterica serovar Typhimurium. Poult. Sci. 92, 10.3382/ps.2013-03029 [DOI] [PubMed] [Google Scholar]
- 35.Li S. et al. (2019) Protective effects of γ;-irradiated Astragalus polysaccharides on intestinal development and mucosal immune function of immunosuppressed broilers. Poult. Sci. 0, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gersemann M., Becker S., Kübler I., Koslowski M., Wang G., Herrlinger K.R. et al. (2009) Differences in goblet cell differentiation between Crohn's disease and ulcerative colitis. Differentiation 77, 84–94 10.1016/j.diff.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 37.Okumura R. and Takeda K. (2017) Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 49, 1–8 10.1038/emm.2017.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantis N.J. et al. (2011) Secretory IgA's Complex Roles in Immunity and Mucosal Homeostasis in the Gut. Mucosal Immunol. 4, 603–611 10.1038/mi.2011.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pabst O. (2012) New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 12, 821–832 10.1038/nri3322 [DOI] [PubMed] [Google Scholar]
- 40.Phalipon A., Cardona A. et al. (2002) Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17, 107–115 10.1016/S1074-7613(02)00341-2 [DOI] [PubMed] [Google Scholar]
- 41.Kaetzel C.S. (2014) Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunol. Lett. 162, 10.1016/j.imlet.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogier E.W., Frantz A.L. et al. (2014) Secretory IgA is Concentrated in the Outer Layer of Colonic Mucus along with Gut Bacteria. Pathogens (Basel, Switzerland) 3, 390–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasnain S.Z., Tauro S., Das I. et al. (2013) IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 144, 357.e359–368.e359 10.1053/j.gastro.2012.10.043 [DOI] [PubMed] [Google Scholar]
- 44.Arike L. et al. (2017) Intestinal Muc2 mucin O-glycosylation is affected by microbiota and regulated by differential expression of glycosyltransferases. Glycobiology 27, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dekker J., Rossen J.W. et al. (2002) The MUC family: an obituary. Trends Biochem. Sci. 27, 126–131 10.1016/S0968-0004(01)02052-7 [DOI] [PubMed] [Google Scholar]
- 46.Perez-Vilar J. (2007) Mucin Granule Intraluminal Organization. Am. J. Respir. Cell Mol. Biol. 36, 183–190 10.1165/rcmb.2006-0291TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y., Zhao Y.H., Kalaslavadi T.B., Hamati E., Nehrke K., Le A.D. et al. (2003) Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am. J. Respir. Cell Mol. Biol. 30, 155–165 10.1165/rcmb.2003-0103OC [DOI] [PubMed] [Google Scholar]
- 48.Bobek L.A., Tsai H., Biesbrock A.R. and Levine M.J. (1993) Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7). J. Biol. Chem 268, 20563–20569 [PubMed] [Google Scholar]
- 49.Shankar V., Pichan P., Eddy R.L. Jr, Tonk V., Nowak N., Sait S.N. et al. (1997) Chromosomal localization of a human mucin gene (MUC8) and cloning of the cDNA corresponding to the carboxy terminus. Am. J. Respir. Cell Mol. Biol 16, 232–241 10.1165/ajrcmb.16.3.9070607 [DOI] [PubMed] [Google Scholar]
- 50.Lapensee L., Paquette Y. and Bleau G. (1997) Allelic polymorphism and chromosomal localization of the human oviduct in gene (MUC9). Fertil. Steril. 68, 702–708 10.1016/S0015-0282(97)00317-8 [DOI] [PubMed] [Google Scholar]
- 51.Johansson M.E. and Hansson G.C. (2016) Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 16, 639–649 10.1038/nri.2016.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu Y. and Liu X. (2013) Research progress on intestinal goblet cell structure and function. Int. J. Pathol. Clin. Med. 33, 424–430 [Google Scholar]
- 53.Wlodarska M. et al. (2014) NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059 10.1016/j.cell.2014.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johansson M.E.V. and Hansson G.C. (2014) Is the Intestinal Goblet Cell a Major Immune Cell? Cell Host Microbe. 15, 251–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen G.Y. and Stappenbeck T.S. (2014) Mucus, It Is Not Just a Static Barrier. Sci. Signal 7, 10.1126/scisignal.2005357 [DOI] [PubMed] [Google Scholar]
- 56.Grootjans J. et al. (2013) Goblet cell compound exocytosis in the defense against bacterial invasion in the colon exposed to ischemia-reperfusion. Gut Microbes. 4, 232–235 10.4161/gmic.23866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shahriyari L., Komarova N.L. and Jilkine A. (2016) The role of cell location and spatial gradients in the evolutionary dynamics of colon and intestinal crypts. Biol. Direct 11, 42 10.1186/s13062-016-0141-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Birchenough G.M., Nystrom E.E. et al. (2016) A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science (New York, N.Y.) 352, 1535–1542 10.1126/science.aaf7419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McGuckin M.A. and Hasnain S.Z. (2017) Goblet cells as mucosal sentinels for immunity. Mucosal Immunol. 10, 1118–1121 10.1038/mi.2016.132 [DOI] [PubMed] [Google Scholar]
- 60.Howe S.E., Lickteig D.J. et al. (2014) The uptake of soluble and particulate antigens by epithelial cells in the mouse small intestine. PLoS ONE 9, 10.1371/journal.pone.0086656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tordesillas L. et al. (2018) Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immunol. 62, 107–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guerville M. et al. (2016) Gastrointestinal and hepatic mechanisms limiting entry and dissemination of lipopolysaccharide into the systemic circulation. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G1–G15 10.1152/ajpgi.00098.2016 [DOI] [PubMed] [Google Scholar]
- 63.Knoop K.A. et al. (2017) Microbial antigen encounter during a preweaning interval is critical for tolerance to gut bacteria. Sci. Immunol. 2, 10.1126/sciimmunol.aao1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Knoop K.A., McDonald K.G. et al. (2015) Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 8, 198–210 10.1038/mi.2014.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu R., Li Y. et al. (2016) Research progress on microecological balance of intestinal flora and human health. Food Industry Technol. 37, 383–391 [Google Scholar]
- 66.van Bergenhenegouwen J. and Kraneveld A.D. (2016) Lipoproteins attenuate TLR2 and TLR4 activation by bacteria and bacterial ligands with differences in affinity and kinetics. BMC Immunol. 17, 10.1186/s12865-016-0180-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McDole J.R., Wheeler L.W. et al. (2012) Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349 10.1038/nature10863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varol C. et al. (2009) Intestinal Lamina Propria Dendritic Cell Subsets Have Different Origin and Functions. Immunity 31, 502–512 10.1016/j.immuni.2009.06.025 [DOI] [PubMed] [Google Scholar]
- 69.Schulz O., Jaensson E. et al. (2009) Intestinal CD103+, but Not CX3CR1+, Antigen Sampling Cells Migrate in Lymph and Serve Classical Dendritic Cell Functions. J. Exp. Med. 206, 3101–3114 10.1084/jem.20091925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knoop K.A. and McDonald K.G. (2016) Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 65, 1100–1109 10.1136/gutjnl-2014-309059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knoop K.A. and Gustafsson J.K. (2017) Antibiotics promote the sampling of luminal antigens and bacteria via colonic goblet cell associated antigen passages. Gut Microbes 8, 400–411 10.1080/19490976.2017.1299846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kulkarni D.H. and McDonald K.G. (2018) Goblet cell associated antigen passages are inhibited during Salmonella typhimurium infection to prevent pathogen dissemination and limit responses to dietary antigens. Mucosal Immunol. 11, 1103–1113 10.1038/s41385-018-0007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nikitas G. et al. (2011) Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. J. Exp. Med. 208, 2263–2277 10.1084/jem.20110560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knoop K.A. et al. (2020) In vivo labeling of epithelial cell-associated antigen passages in the murine intestine. Lab Anim. (NY) 49, 79–88 10.1038/s41684-019-0438-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Knoop K.A. and Newberry R.D. (2018) Goblet cells: multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 11, 1551–1557 10.1038/s41385-018-0039-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pabst O. and Mowat A.M. (2012) Oral tolerance to food protein. Mucosal Immunol. 5, 232–239 10.1038/mi.2012.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kulkarni D.H. et al. (2019) Goblet cell associated antigen passages support the induction and maintenance of oral tolerance. Mucosal Immunol. 13, 271–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oeser K., Schwartz C. et al. (2015) Conditional IL-4/IL-13-deficient mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal Immunol. 8, 672–682 10.1038/mi.2014.101 [DOI] [PubMed] [Google Scholar]
- 79.Lee J.J., Kim D. et al. (2013) STAT6 expression and IL-13 production in association with goblet cell hyperplasia and worm expulsion of Gymnophalloides seoi from C57BL/6 mice. Korean J. Parasitol. 51, 589–594 10.3347/kjp.2013.51.5.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller M.M. et al. (2020) BATF acts as an essential regulator of IL-25-responsive migratory ILC2 cell fate and function. Sci. Immunol. 5, 10.1126/sciimmunol.aay3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iwashita J., Sato Y., Sugaya H., Takahashi N., Sasaki H. and Abe T. (2003) mRNA of MUC2 is stimulated by IL-4, IL-13 or TNF-α through a mitogen-activated protein kinase pathway in human colon cancer cells. Immunol. Cell Biol. 81, 275–282 10.1046/j.1440-1711.2003.t01-1-01163.x [DOI] [PubMed] [Google Scholar]
- 82.Blanchard C., Durual S., Estienne M., Bouzakri K., Heim M.H., Blin N. et al. (2004) IL-4 and IL-13 up-regulate intestinal trefoil factor expression: requirement for STAT6 and de novo protein synthesis. J. Immunol. 172, 3775–3783 10.4049/jimmunol.172.6.3775 [DOI] [PubMed] [Google Scholar]
- 83.Marillier R.G., Michels C. et al. (2008) IL-4/IL-13 independent goblet cell hyperplasia in experimental helminth infections. BMC Immunol. 9, 11 10.1186/1471-2172-9-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lucey D.R., Clerici M. and Shearer G.M. (1996) Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin. Microbiol. Rev. 9, 532–562 10.1128/CMR.9.4.532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Louahed J., Toda M., Jen J., Hamid Q., Renauld J.C., Levitt R.C. et al. (2000) Interleukin-9 upregulates mucus expression in the airways. Am. J Respir Cell Mol Biol 22, 649–656 10.1165/ajrcmb.22.6.3927 [DOI] [PubMed] [Google Scholar]
- 86.Hasnain S.Z., Tauro S., Das I., Tong H., Chen A.C., Jeffery P.L. et al. (2013) IL-10 promotes production of intestinal mucus by suppressing protein misfolding and endoplasmic reticulum stress in goblet cells. Gastroenterology 144, 357–368 10.1053/j.gastro.2012.10.043 [DOI] [PubMed] [Google Scholar]
- 87.Reddehase M.J. (2019) Adverse immunological imprinting by cytomegalovirus sensitizing for allergic airway disease. Med. Microbiol. Immunol. (Berl) 208, 469–473 10.1007/s00430-019-00610-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lambrecht B.N. and Hammad H. (2012) The airway epithelium in asthma. Nat. Med. 18, 684–692 10.1038/nm.2737 [DOI] [PubMed] [Google Scholar]
- 89.Birchenough G.M. and Johansson M.E. (2015) New developments in goblet cell mucus secretion and function. Mucosal Immunol. 8, 712–719 10.1038/mi.2015.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mizoguchi A. (2012) Healing of intestinal inflammation by IL-22. Inflamm. Bowel Dis. 18, 1777–1784 10.1002/ibd.22929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sugimoto K., Ogawa A., Mizoguchi E., Shimomura Y., Andoh A., Bhan A.K. et al. (2008) IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Y. et al. (2003) Stimulation of Airway Mucin Gene Expression by Interleukin (IL)-17 through IL-6 Paracrine/Autocrine Loop. J. Biol. Chem. 278, 17036–17043 [DOI] [PubMed] [Google Scholar]
- 93.Xia W., Bai J. et al. (2014) Interleukin-17A promotes MUC5AC expression and goblet cell hyperplasia in nasal polyps via the Act1-mediated pathway. PLoS One 9, 10.1371/journal.pone.0098915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ishinaga H., Kitano M. et al. (2017) Interleukin-33 induces mucin gene expression and goblet cell hyperplasia in human nasal epithelial cells. Cytokine 90, 60–65 10.1016/j.cyto.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 95.Waddell A., Vallance J.E. et al. (2019) IL-33 Induces Murine Intestinal Goblet Cell Differentiation Indirectly via Innate Lymphoid Cell IL-13 Secretion. J. Immunol. 202, 598–607 10.4049/jimmunol.1800292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park K.S., Korfhagen T.R. et al. (2007) SPDEF regulates goblet cell hyperplasia in the airway epithelium. J. Clin. Invest. 117, 978–988 10.1172/JCI29176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen G. et al. (2009) SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J. Clin. Invest. 119, 2914–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]