Abstract
Alternative splicing (AS), an essential process for the maturation of mRNAs, is involved in tumorigenesis and tumor progression, including angiogenesis, apoptosis, and metastasis. AS changes can be frequently observed in different tumors, especially in geriatric lung adenocarcinoma (GLAD). Previous studies have reported an association between AS events and tumorigenesis but have lacked a systematic analysis of its underlying mechanisms. In the present study, we obtained splicing event information from SpliceSeq and clinical information regarding GLAD from The Cancer Genome Atlas. Survival-associated AS events were selected to construct eight prognostic index (PI) models. We also constructed a correlation network between splicing factors (SFs) and survival-related AS events to identify a potential molecular mechanism involved in regulating AS-related events in GLAD. Our study findings confirm that AS has a strong prognostic value for GLAD and sheds light on the clinical significance of targeting SFs in the treatment of GLAD.
Keywords: alternative splicing, geriatric, lung adenocarcinoma, prognostic index models, splicing factors
Introduction
Cancer morbidity and mortality rates are rapidly increasing worldwide. Lung cancer is the most common cause of cancer-related mortality [1]. In 2018, the numbers of new cases and deaths globally for lung carcinomas were 2.1 million (11.6% of the total cancer cases) and 1.8 million (18.4% of total cancer deaths), respectively [2]. In the new World Health Organization (WHO) classification, non–small cell lung carcinomas (NSCLCs) are classified into squamous cell carcinomas, adenocarcinomas, large cell carcinomas, and mixed cell carcinomas. Among them, adenocarcinoma is the most common type, accounting for approximately 60% of NSCLCs [3]. According to the latest statistics from the American Cancer Society, lung cancer mainly occurs in the elderly. Most people diagnosed with lung cancer are 65 or older; very few people are diagnosed under 45 years of age. The average age at diagnosis is approximately 70 [1]. Considering that elderly patients always have more complications and poor prognosis, many clinical trial inclusion criteria exclude elderly patients. Thus, more attention should be paid to geriatric patients. A study analyzing the genetic characteristics of 184 patients with lung adenocarcinoma showed that distinctive genetic profile including the Kristen rat sarcoma viral oncogene, serine/threonine kinase 11 (STK11), and epidermal growth factor receptor (EGFR) exon 20 mutation were common in the older patient group. However, EGFR/tumor protein 53 (TP53) mutations, anaplastic lymphoma kinase (ALK), and human epidermal growth factor receptor 2 (HER2) genetic alterations were more prevalent in younger patients [4]. Currently, tumor biomarkers, tumor stages, and molecular markers are common indicators that predict the prognosis of patients with lung adenocarcinoma (LUAD) [5–7]. However, the number of biomarkers that can be used clinically is limited and no prognostic model was built exclusively for elderly patients. Therefore, novel and effective prediction methods are needed to predict the prognosis of GLAD.
RNA splicing is a complex and sophisticated process used to generate mature mRNAs by removing introns and joining exons. This process is highly controlled by the spliceosome [8]. The spliceosome consists of more than 300 proteins and five small nuclear RNAs (snRNAs), U1, U2, U4, U5, and U6. Spliceosome and associated RNA splicing factors (SFs) are involved in the regulation of alternative splicing (AS) events [9]. AS is an essential process for regulating gene expression and ensuring proteome diversity [10]. AS can increase the diversity of mRNAs through seven types of splicing events: alternate acceptor site (AA), alternate donor site (AD), alternate promoter (AP), alternate terminator (AT), retained intron (RI), mutually exclusive exons (ME), and exon skip (ES) [10]. Abnormal AS events can produce aberrant protein isoforms, which may contribute to the development of tumors [11–13]. Genome-wide studies have publicized more than 15,000 tumor-related splice variants in 27 types of cancers [14]. David et al. showed that splicing events were related to tumorigenesis and to progression, including angiogenesis, metastasis, and apoptosis [15]. Other studies have indicated that AS changes are involved in the prognosis of different tumors, such as prostate cancer, papillary thyroid cancer, and uterine corpus endometrial cancer [16–18]. These studies reported an association between AS events and tumorigenesis. However, systematic survival analyses of AS in GLAD are lacking. Studies have revealed specific roles for SFs in lung cancer development [19], and some of these studies have suggested that SFs can regulate the aberrant process of AS with effects on the occurrence and development of lung cancer [20]. Multiple studies have indicated that AS events have diagnostic value and could be considered potential drug targets [21–24]. Given the high incidence of splicing defects in lung adenocarcinoma, the potential connection between SFs and AS events in GLAD deserves further exploration and supporting evidence.
The Cancer Genome Atlas (TCGA) is a project that classifies the major cancer-related genomic alterations [25]. Currently, a single gene or molecular marker cannot accurately predict the progression of the disease. With the development of high-throughput sequencing technology, many studies have established predictive models that pay closer attention to the interaction of multiple genes [26–28]. However, no study currently has provided evidence supporting the prognostic value of AS events in GLAD. Thus, for the present study we downloaded splicing data from TCGA of geriatric patients with LUAD to build the GLAD prognosis model. A splicing network between SFs and survival-related AS events was established to demonstrate potential molecular mechanisms involved in regulating AS-related events in GLAD.
Materials and methods
Data acquisition and organization
The clinical information of 251 elderly patients with LUAD (age 65–89) and mRNA expression data were downloaded from the TCGA Genomic Data Commons (GDC) (https://portal.gdc.cancer.gov/) database. Cancer-related AS events were selected in 59 normal controls and 513 tumor tissues from the TCGA SpliceSeq database (https://bioinformatics.mdanderson.org/TCGASpliceSeq/). In addition, we obtained the percent-sliced-in (PSI) values for seven types of AS events from RNA-seq to quantify them in GLAD patients.
Statistical analysis
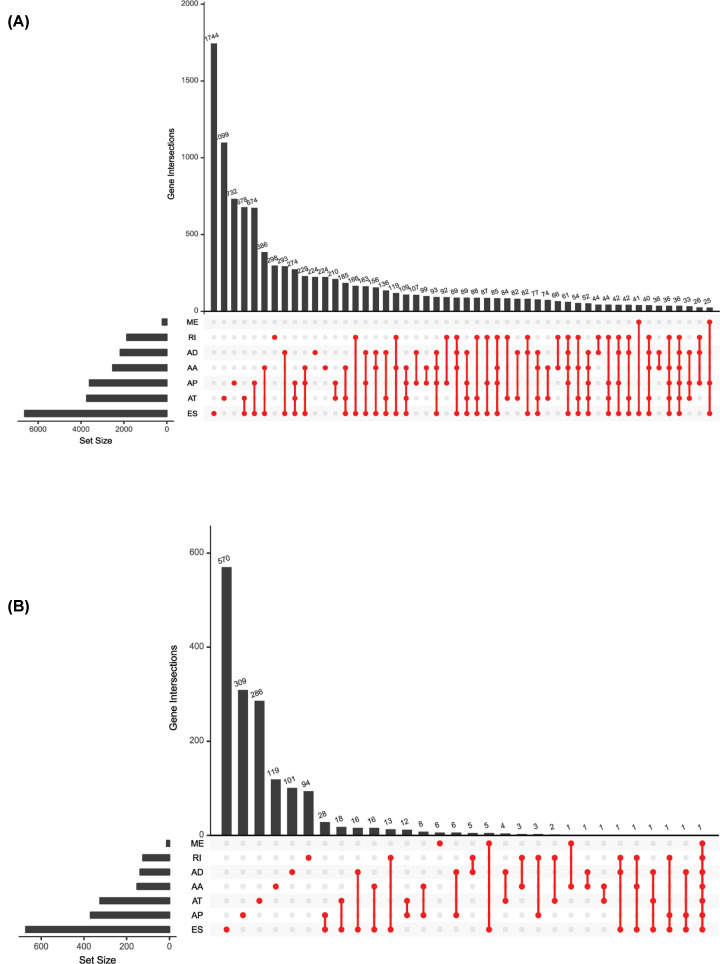
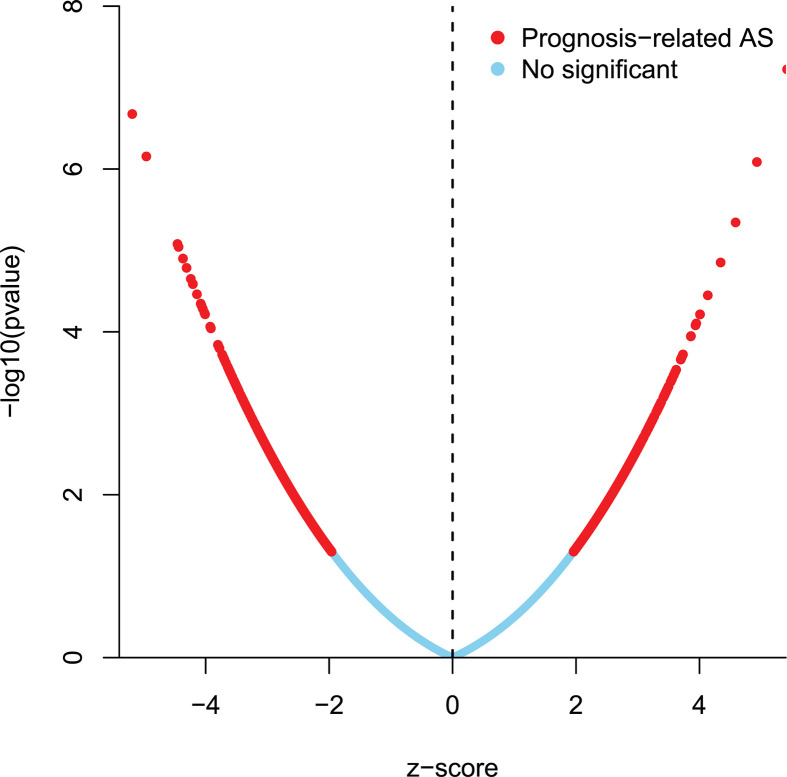
Univariate Cox regression analyses were conducted to select survival associated AS events. UpSet, a novel technique was used to visualize intersecting sets [29]. Specific splicing events were illustrated in the UpSet plots generated by the R package (Figure 1A,B). The R package was also used to create Volcano plots and Bubble charts to reveal the prognostic related AS events. Further, univariate analyses and multivariate analyses were performed to explore independent survival-related prognostic factors.
Figure 1. UpSet plots of alternative splicing (AS) events in geriatric lung adenocarcinoma (GLAD).
(A) UpSet plots of AS events in GLAD. The horizontal axis represents the number of genes in each AS type. The vertical axis represents the number of genes for one or several splicing types. (B) UpSet plots of survival-related AS events in GLAD.
Construction of the prognostic index models
Univariate Cox regression analyses were conducted to select survival associated AS events (P<0.05). Least absolute shrinkage and selection operator (LASSO) regression analysis was used to screen and eliminate genes with high correlation to construct a credible prognostic index model. Genes with high survival correlations selected by LASSO regression were used to construct PI models. In addition, risk curves were generated using the R package based on the risk score. We calculated the risk value for each case based on the following formula:
where PSI is the percent that was spliced in to indicate AS event changes [30], and C is the regression coefficient.
Evaluation of the prognostic value of the prognostic index models
Based on the median value of the risk score calculated by the PI model, cases were divided into high-risk and low-risk groups. Kaplan–Meier curve (K-M) analysis was used to describe survival probabilities. To certify the reliability of the model in predicting prognosis, the survival receiver-operator characteristic (ROC) package in R was used to calculate the area under the curve (AUC) of the ROC curve for each model. Models with AUC > 0.7 were more effective models. We then substituted data from non-elderly lung adenocarcinoma patients (age 33–64) into the PI models to demonstrate that AS events differed between elderly and non-elderly lung adenocarcinoma patients. Differences that may arise from the differential expression of genes in elderly and non-elderly patients.
Correlation network between alternative splicing and splicing factors
SFs played a significant role in regulating splicing events. We obtained the information regarding SFs from the database SpliceAid2 (http://www.introni.it/splicing.html). The mRNA expression of SFs in geriatric lung adenocarcinoma was downloaded from the TCGA database. Survival-related SFs were screened by univariate Cox regression analysis. Pearson correlation analysis was used to analyze the correlation between survival-related SFs and AS events with independent predictive significance (|correlation coefficient| > 0.6, P<0.001). We explored the correlation network of survival-associated SFs and prognosis-associated AS events to further understand the underlying molecular mechanism of AS events in GLAD. Cytoscape v3.7.1 software was used to visualize network data such as genetic, protein–DNA, and protein–protein interactions [31] and generated a potential regulatory network between AS and SFs. Then, based on the R package, the Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were used to assess the functions associated with the most significant prognosis-related AS events.
Results
Clinical characteristics
In the present study, cancer-related AS events were selected in 59 normal controls and 513 tumor tissues from the TCGA SpliceSeq database. A total of 251 patients were included in our analysis. We processed the clinical information and TCGA SpliceSeq files of geriatric patients with LUAD. Univariate analyses and multivariate analyses were performed to explore independent survival-related prognostic factors (Table 1). In the univariate Cox hazard analyses, the TNM stage (hazard ratio (HR) = 1.653, 95% CI: 1.300–2.103; P<0.001), tumor stage (HR = 1.64, 95% CI: 1.206–2.233; P<0.05), lymph node metastasis (HR = 2.199, 95% CI: 1.625–2.977; P<0.001), and risk score of eight PI models were significantly correlated with the survival time of elderly patients with LUAD. However, no significant correlations were observed between survival time and age, sex, or distant metastasis. According to the multivariate cox hazard analysis, only the risk score of eight PI models significantly correlated with the survival time of geriatric patients with LUAD. This demonstrated that the PI model could be used as an independent prognostic factor to predict the prognosis of LUADs in geriatric patients.
Table 1. Univariate and multivariate Cox regression analysis of clinical variables and eight prognostic index models of lung adenocarcinoma in geriatric patients.
| Clinical variable | Group | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | ||
| Age | <75 | 1.022 (0.970–1.076) | 0.413 | 1.001 (0.955–1.068) | 0.726 |
| ≧75 | |||||
| Sex | Male | 0.980 (0.586–1.639) | 0.938 | 0.832 (0.485–1.426) | 0.503 |
| Female | |||||
| Stage-TMN | I-II | 1.653 (1.300–2.103) | 4.25E-05 | 1.112 (0.437–2.833) | 0.824 |
| III-IV | |||||
| T | T1-T2 | 1.641 (1.206–2.233) | 0.002 | 1.167 (0.747–1.825) | 0.498 |
| T3-T4 | |||||
| M | M0 | 1.023 (0.308–3.403) | 0.97 | 0.998 (0.069–14.501) | 0.999 |
| M1 | |||||
| N | N0 | 2.199 (1.625–2.977) | 3.33E-07 | 1.738 (0.776–3.892) | 0.178 |
| N1 | |||||
| Risk score of AA | High-risk | 1.096 (1.066–1.126) | 4.62E-11 | 1.082 (1.051–1.115) | 1.81E07 |
| Low-risk | |||||
| Risk score of AD | High-risk | 1.122 (1.085–1.161) | 2.50E-11 | 1.100 (1.060–1.141) | <0.001 |
| Low-risk | |||||
| Risk score of AP | High-risk | 1.195 (1.139–1.253) | 2.21E-13 | 1.169 (1.111–1.230) | 1.54E09 |
| Low-risk | |||||
| Risk score of AT | High-risk | 1.155 (1.108–1.205) | 2.13E-11 | 1.125 (1.072–1.180) | 1.75E-06 |
| Low-risk | |||||
| Risk score of ES | High-risk | 1.102 (1.071–1.134) | 2.37E-11 | 1.096 (1.058–1.135) | 2.83E-07 |
| Low-risk | |||||
| Risk score of ME | High-risk | 1.233 (1.146–1.328) | 2.50E-08 | 1.254 (1.153–1.363) | 1.07E-07 |
| Low-risk | |||||
| Risk score of RI | High-risk | 1.065 (1.041–1.089) | 2.84E-08 | 1.056 (1.031–1.081) | 5.05E-06 |
| Low-risk | |||||
| Risk score of ALL | High-risk | 1.071 (1.050–1.095) | 1.10E-10 | 1.062 (1.038–1.087) | 1.79E-07 |
| Low-risk | |||||
Abbreviations: AA, alternate acceptor site; AD, alternate donor site; AP, alternate promoter; AT, alternate terminator; ES, exon skip; HR, hazard ratio; ME, mutually exclusive exons; RI, retained intron.
Survival-associated alternative splicing events
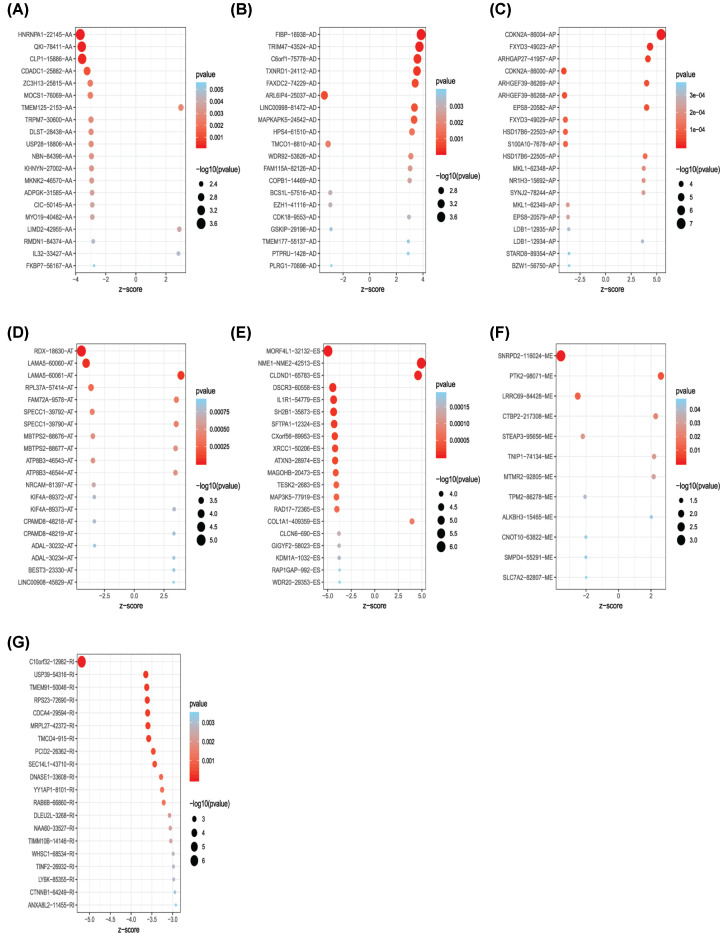
The intersections of genes and AS events in GLAD were described using the following UpSet plot (Figure 1A,B), which indicated that a single gene may be involved in different AS events. Of seven types of AS events, ES was the most common type. A total of 16,793 ES events were observed in 6475 genes, of which only 1744 genes were involved in the ES event. We also identified 3559 AA events in 2306 genes, 3057 AD events in 1710 genes, 8992 AP events in 3398 genes, 8546 AT events in 3522 genes, 220 ME events in 65 genes, and 2781 RI events in 1681 genes (Figure 1A). After combining AS data and survival data, a total of 2381 survival-related AS events in 1633 genes were reported through the univariate Cox regression analysis (P<0.05), including 158 AA events in 151 genes, 145 AD events in 137 genes, 580 AP events in 369 genes, 527 AT events in 325 genes, 825 ES events in 672 genes, 12 ME events in 13 genes, and 124 RI events in 124 genes (Figure 1B). The distribution of AS events correlated with patient survival are displayed in the Volcano plot (P<0.05) shown in Figure 2. Furthermore, the bubble charts in Figure 3 revealed the 20 most significant prognostic-related AS events; however, only 12 prognostic related ME events were included. As shown, each AS event is defined by a unique code. For example, for the code ATAD3A-176-AA, ATAD3A is the name of gene, 176 is the sequence number of the splicing event in the TCGA database, and AA is the splicing type.
Figure 2. Volcano plot of AS events (P<0.05).
The red dots indicate prognosis-related AS events; the blue dots indicate the events with no significance.
Figure 3. Bubble plots of the most significant genes with seven types of survival-associated alternative splicing events.
The ID of the specific AS event is displayed on the vertical axis. Events with greater significance are represented by larger circles and are colored in red. (A) 20 prognosis-related AA events; (B) 20 prognosis-related AD events; (C) 20 prognosis-related AP events; (D) 20 prognosis-related AT events; (E) 20 prognosis-related ES events; (F) 12 prognosis-related ME events; (G) 20 prognosis-related RI events.
Prognostic value of prognostic indexes in survival analysis
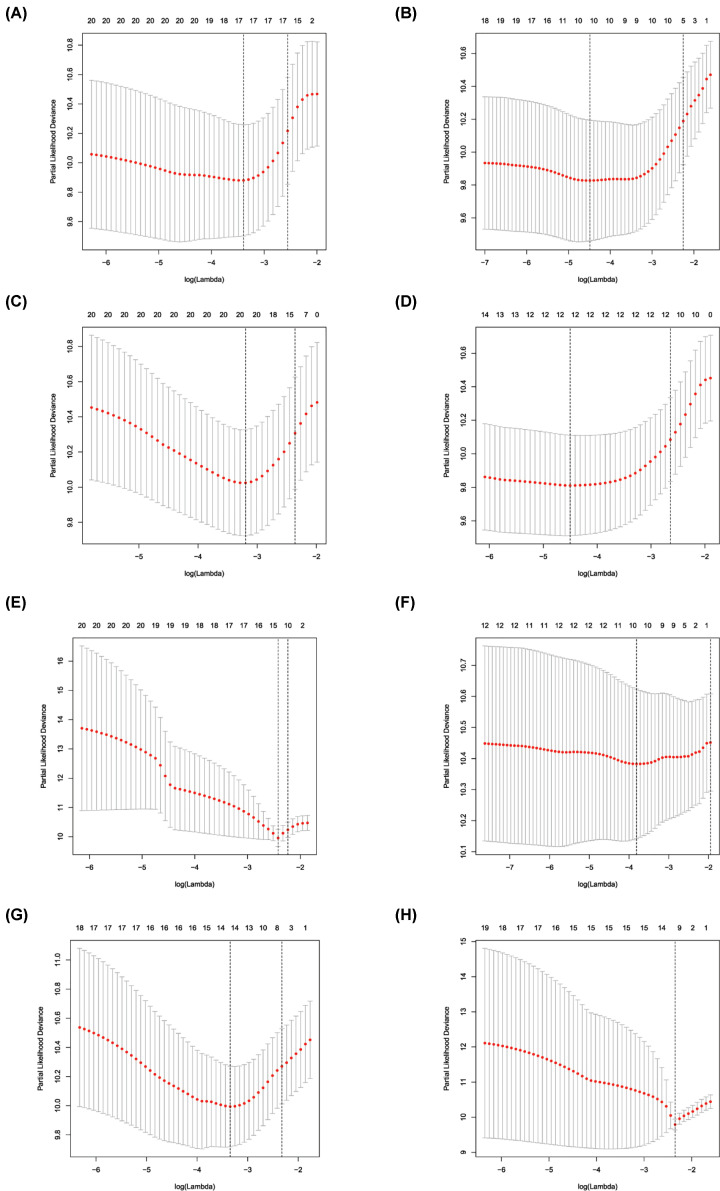
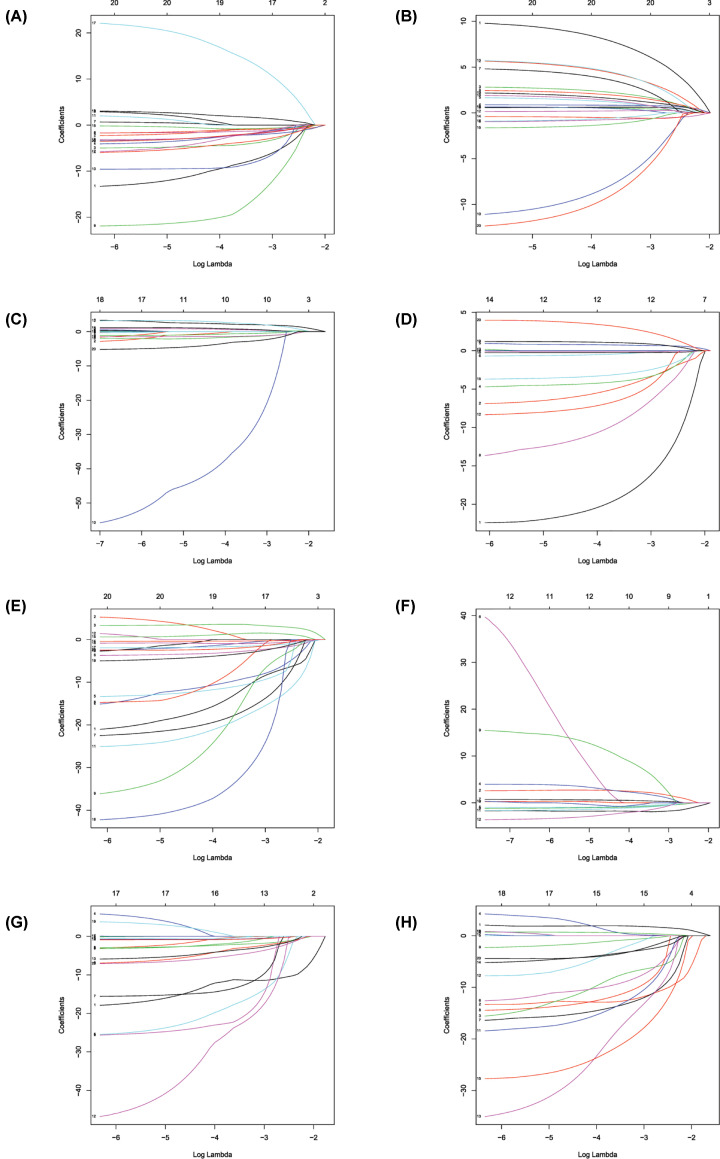
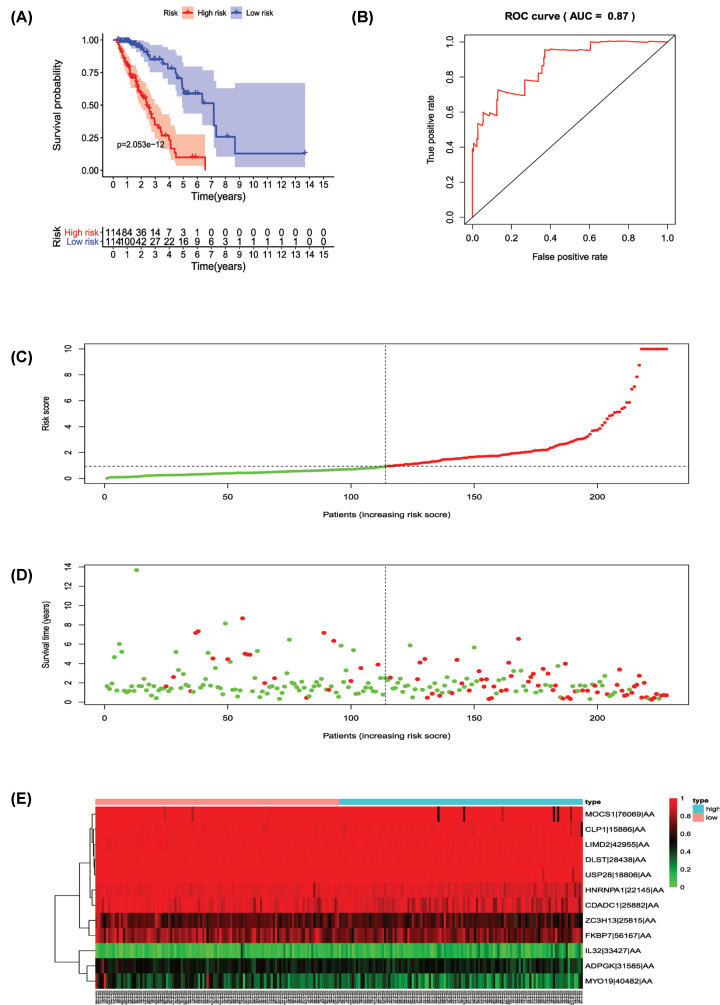
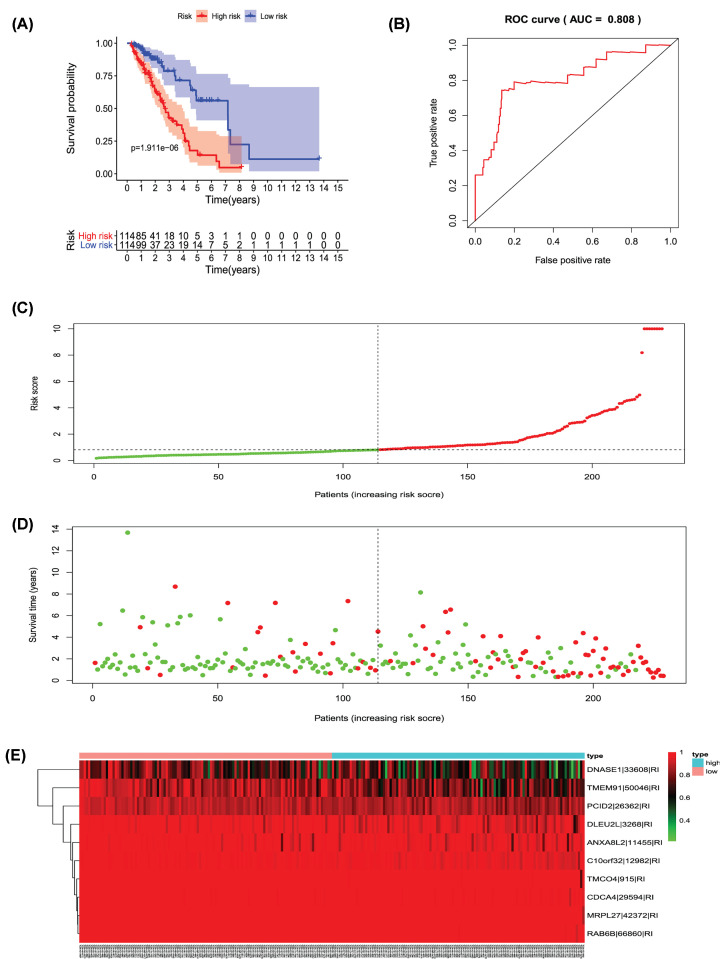
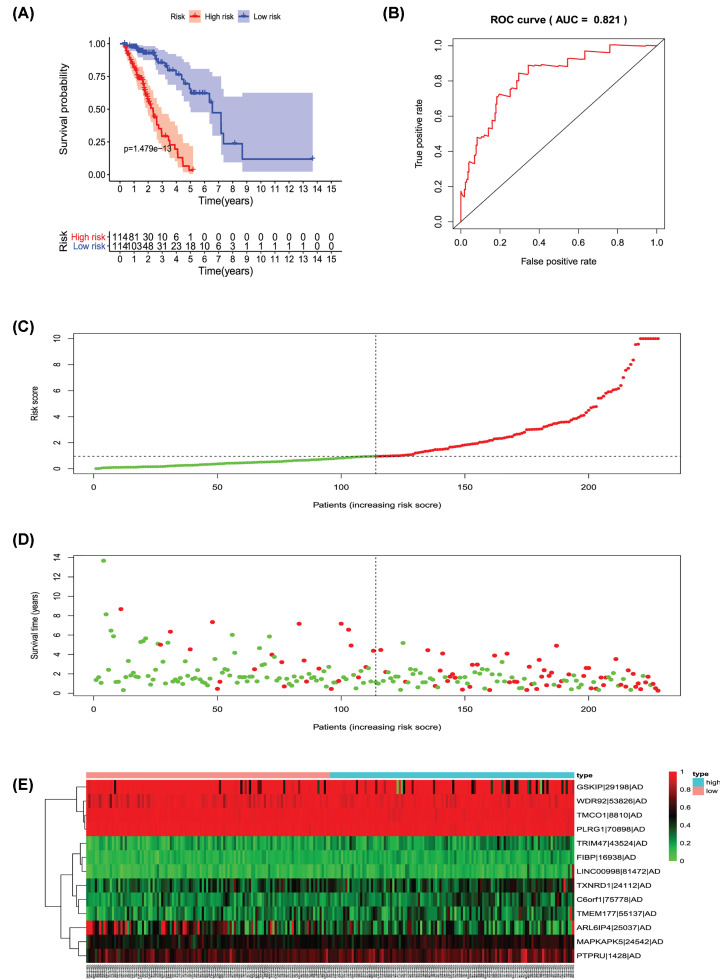
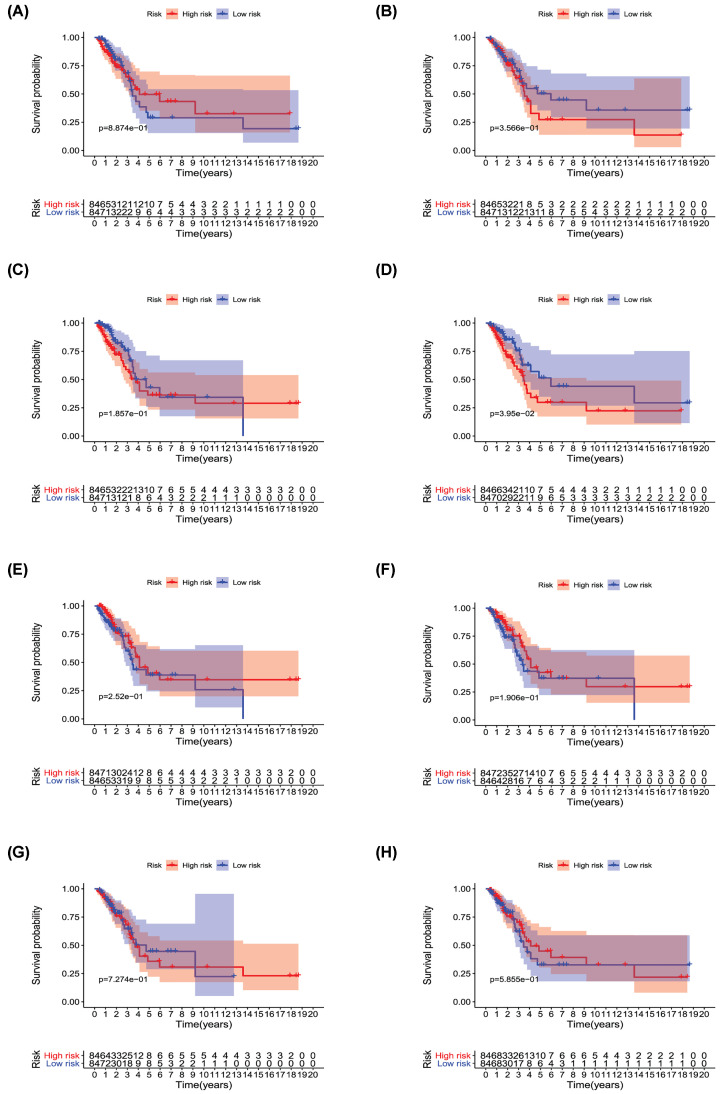
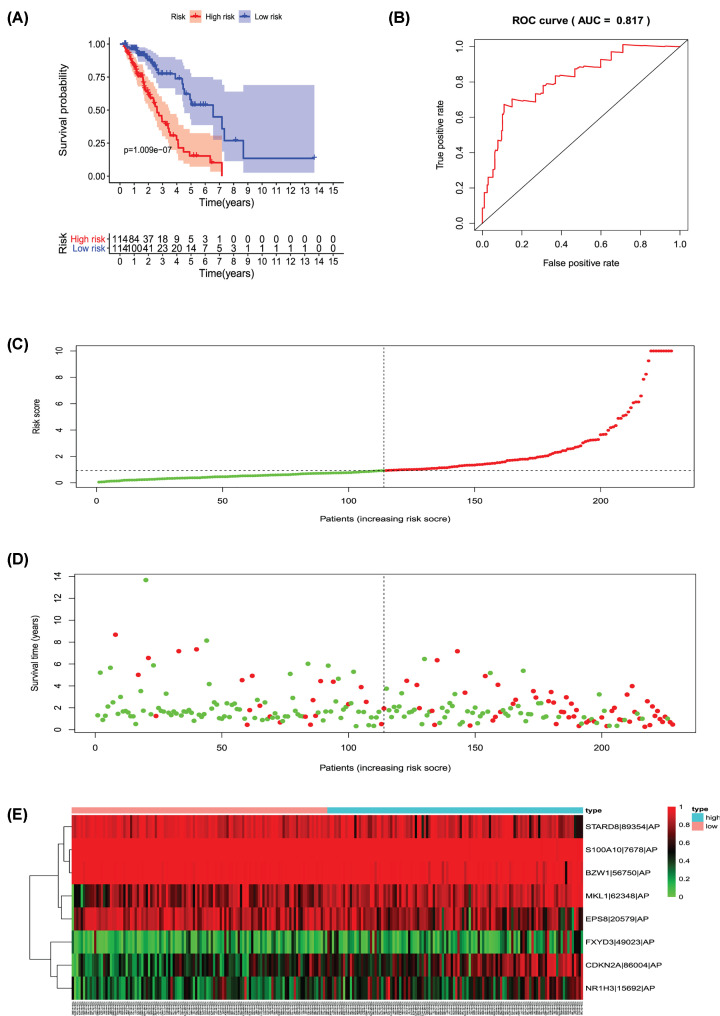
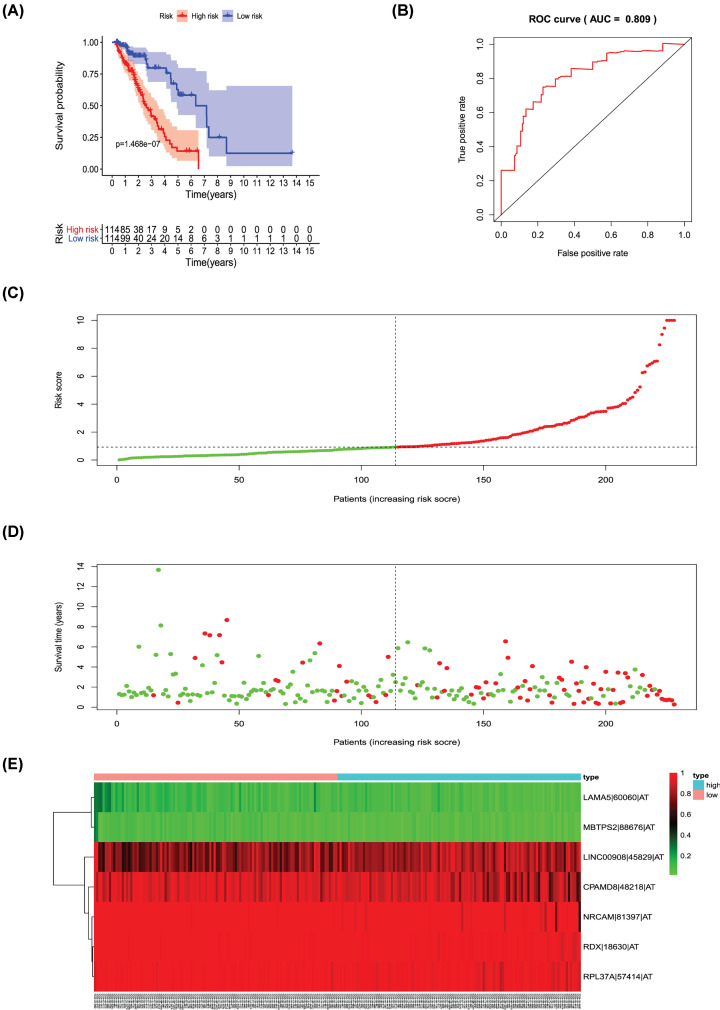
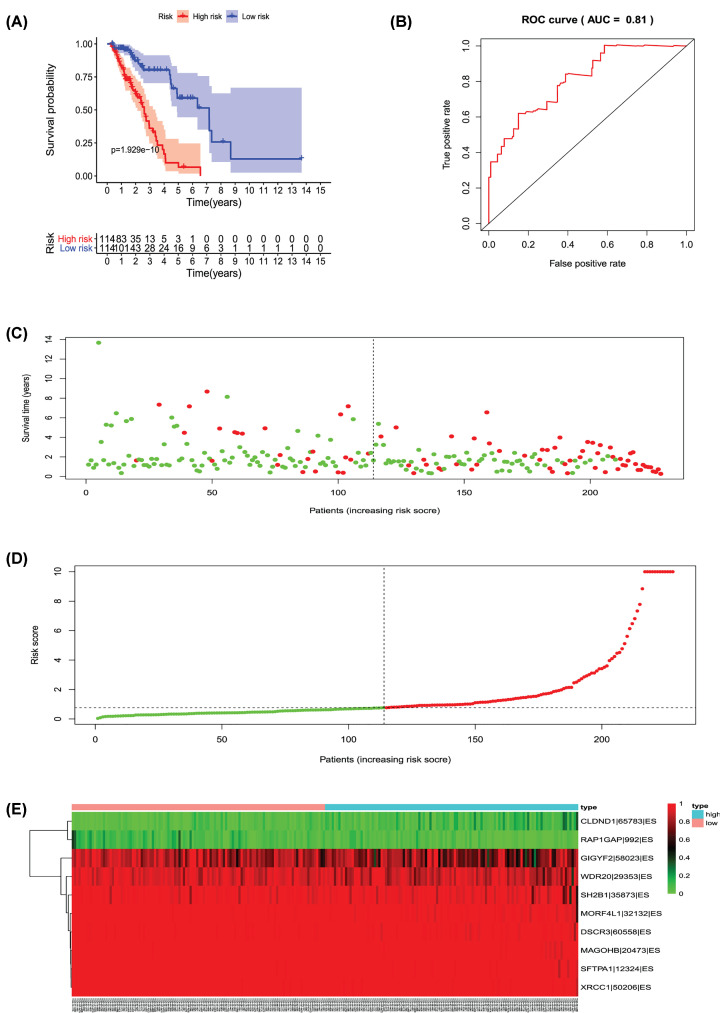
Univariate Cox regression analysis (P<0.001) was used to select significant survival associated with AS events for the construction of PI models. Then LASSO Cox analysis was conducted to eliminate interacting genes after cross-validation of minimum error (Figure 4A–H), and to screen significant survival-associated genes (Figure 5A–H). Genes with high survival correlations selected by LASSO regression were used to construct the PI models. The ROC curve analyses showed that eight models had predictive significance for prognosis. The PI model of AA events was the most effective at estimating the prognosis of geriatric patients with LUADs, with an AUC value of 0.87 (Figure 6B), followed by PI of all types and the PI of AD events, with an AUC value of 0.857 and 0.821, respectively (Figures 13B and 7B). Cases were then divided into high-risk and low-risk groups according to the median PI value. The results showed that all the PI models could achieve good stratification of the prognosis of the low- and high-risk groups (Figures 6–13). Kaplan–Meier curve analysis showed that the survival time of the low-risk group was significantly longer than the high-risk group (Figures 6A–13A). Patients with lower risk experienced a longer survival (P<0.001). According to the univariate and multivariate Cox regression analysis, PI models of AD splicing events showed the most significant survival time, with a value of 1.479E-13 (Figure 7A), followed by the PI of AA events and PI of all types of splicing events, with values of 2.053E-12 and 1.377E-11, respectively (Figures 6A and 13A). In all PI models, as the risk score increased, geriatric patients with LUAD were more inclined to experience a poorer prognosis (Figures 6C–13C). Heat maps were used to illustrate the relationship between AS events and the risk score. If the risk value positively correlated with the PSI value of the AS events, the AS event was considered as high-risk event (Figures 6E–13E). After substituting the data of non-elderly lung adenocarcinoma patients into the prognosis model, we found that there was no significant difference in survival between the high-risk group and low-risk group (Figure 14A–H).
Figure 4. Construction of prognostic signatures based on least absolute shrinkage and selection operator (LASSO) Cox analysis.
(A–H) The lowest point of the ordinate is the minimum point of the cross-validation error.
Figure 5. LASSO COX analysis of seven types of events.
(A–H) The horizontal axis represents the Log Lambda. The vertical axis represents the coefficients. As the value of the Log Lambda increased, the coefficient approached 0.
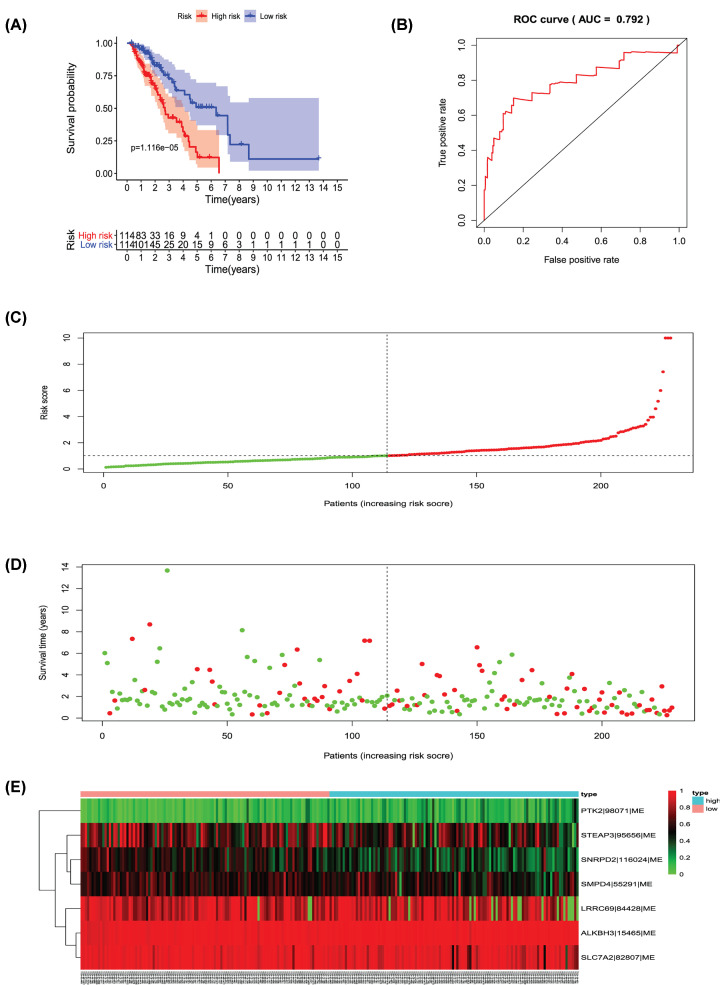
Figure 6. Analysis of the prognostic index (PI)-AA.
The 12 prognosis-associated events of AA were selected by multivariate Cox regression analysis to construct the PI-AA model. (A) The risk curve of the PI evaluating the survival time of geriatric patients with LUAD stratified by the median PI value into a low-risk and high-risk groups. (B) Time-dependent receiver-operator characteristic curve of the PI for predicting the tumor status. (C) PI value curve for geriatric patients with LUAD. (D) Survival conditions and survival time of GLAD patients distributed according to risk score (green dots represent survivors; red dots represent deaths). (E) Heat map indicating the relationship between the PSI value of the AS events and the risk score.
Figure 13. Analysis of the PI-ALL.
The ten prognosis-associated events of all types were selected by multivariate Cox regression analysis to construct the PI-ALL model. (A) The risk curve of PI to evaluate the survival time of geriatric patients with LUAD stratified by the median PI value into low-risk and high-risk groups. (B) Time-dependent receiver-operator characteristic curve of PI to predict the tumor status. (C) PI value curve for geriatric patients with LUAD. (D) Survival conditions and survival time of GLAD patients distributed according to risk score (green dots represent survivors; red dots represent deaths). (E) Heat map indicating the relationship between the PSI value of the AS events and the risk score.
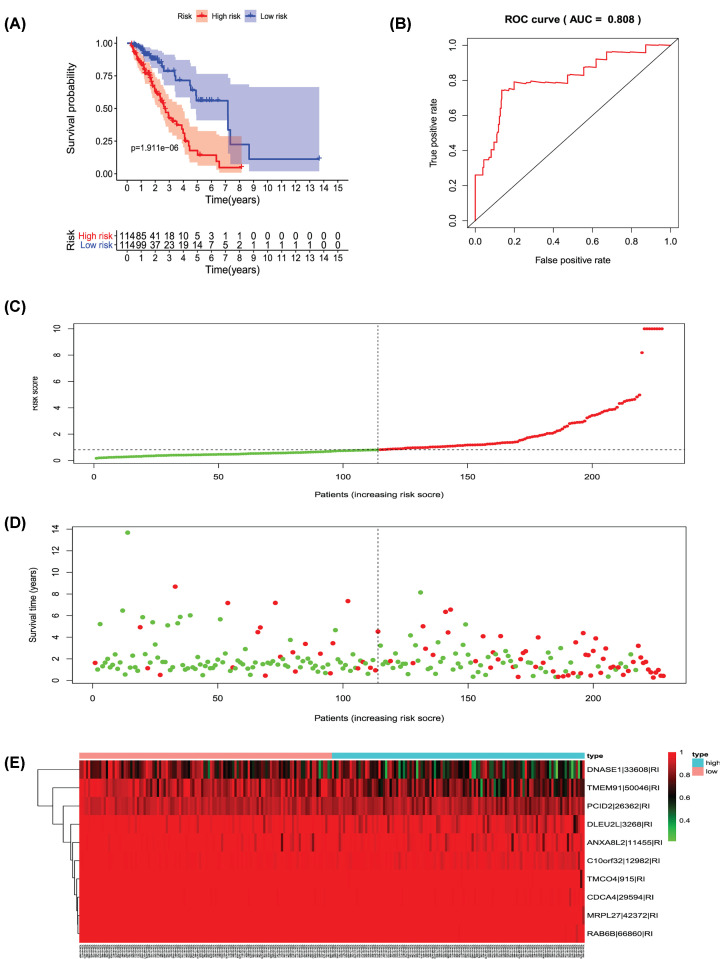
Figure 7. Analysis of the PI-AD.
Thirteen prognosis-associated events of AD were selected by multivariate Cox regression analysis to construct the PI-AD model. (A) The risk curve of the PI for evaluating the survival time of geriatric patients with LUAD stratified by the median PI value into a low-risk and high-risk group. (B) Time-dependent receiver-operator characteristic curve of PI for predicting the tumor status. (C) PI value curve for geriatric patients with LUAD. (D) Survival conditions and survival time of GLAD patients distributed according to risk score (green dots represent survivors; red dots represent deaths). (E) Heat map indicating the relationship between the PSI value of the AS events and the risk score.
Figure 14. Non-elderly patients with lung adenocarcinoma data substituted into the PI model.
(A) PI-AA model (B) PI-AD model (C) PI-AP model (D) PI-AT model (E) PI-ES model (F) PI-ME model (G) PI-RI model and (H) PI-All model.
Figure 8. Analysis of the PI-AP.
The eight prognosis-associated events of AP were selected by multivariate Cox regression analysis to construct the PI-AP model. (A) The risk curve of the PI for evaluating the survival time of geriatric patients with LUAD stratified by the median PI value into low-risk and high-risk groups. (B) Time-dependent receiver-operator characteristic curve of PI for predicting the tumor status. (C) PI value curve for geriatric patients with LUAD. (D) Survival conditions and survival time of GLAD patients distributed according to risk score (green dots represent survivors; red dots represent deaths). (E) Heat map indicating the relationship between the PSI value of the AS events and the risk score.
Figure 9. Analysis of the PI-AT.
The seven prognosis-associated events of AT were selected by multivariate Cox regression analysis to construct the PI-AT model. (A) The risk curve of PI for evaluating the survival time of geriatric patients with LUAD stratified by the median PI value into a low-risk and high-risk groups. (B) Time-dependent receiver-operator characteristic curve of PI for predicting the tumor status. (C) PI value curve for geriatric patients with LUAD. (D) Survival conditions and survival time of GLAD patients distributed according to risk score (green dots represent survivors; red dots represent deaths). (E) Heat map indicating the relationship between the PSI value of the AS events and the risk score.
Figure 10. Analysis of the PI-ES.
The 10 prognosis-associated events of ES were selected by multivariate Cox regression analysis to construct the PI-ES model. (A) The risk curve of the PI for evaluating survival geriatric patients with LUAD stratified by the median PI value into a low-risk and high-risk groups. (B) Time-dependent receiver-operator characteristic curve of PI for predicting the tumor status. (C) PI value curve for geriatric patients with LUAD. (D) Survival conditions and survival time of GLAD patients distributed according to risk score (green dots represent survivors; red dots represent deaths). (E) Heat map indicating the relationship between the PSI value of the AS events and the risk score.
Figure 11. Analysis of the PI-ME.
The seven prognosis-associated events of ME were selected by multivariate Cox regression analysis to construct the PI-ME model. (A) The risk curve of the PI for evaluating the survival time of geriatric patients with LUAD stratified by the median PI value into low-risk and high-risk groups. (B) Time-dependent receiver-operator characteristic curve of PI for predicting the tumor status. (C) PI value curve for geriatric patients with LUAD. (D) Survival conditions and survival time of GLAD patients distributed according to risk score (green dots represent survivors; red dots represent deaths). (E) Heat map indicating the relationship between the PSI value of the AS events and the risk score.
Figure 12. Analysis of the PI-RI.
The 10 prognosis-associated events of RI were selected by multivariate Cox regression analysis to construct the PI-RI model. (A) The risk curve of the PI for evaluating the survival time of geriatric patients with LUAD stratified by the median PI value into a low-risk and high-risk groups. (B) Time-dependent receiver-operator characteristic curve of the PI for predicting the tumor status. (C) PI value curve for geriatric patients with LUAD. (D) Survival conditions and survival time of GLAD patients distributed according to risk score (green dots represent survivors; red dots represent deaths). (E) Heat map indicating the relationship between the PSI value of the AS events and the risk score.
Survival-associated splicing factors-alternative splicing networks
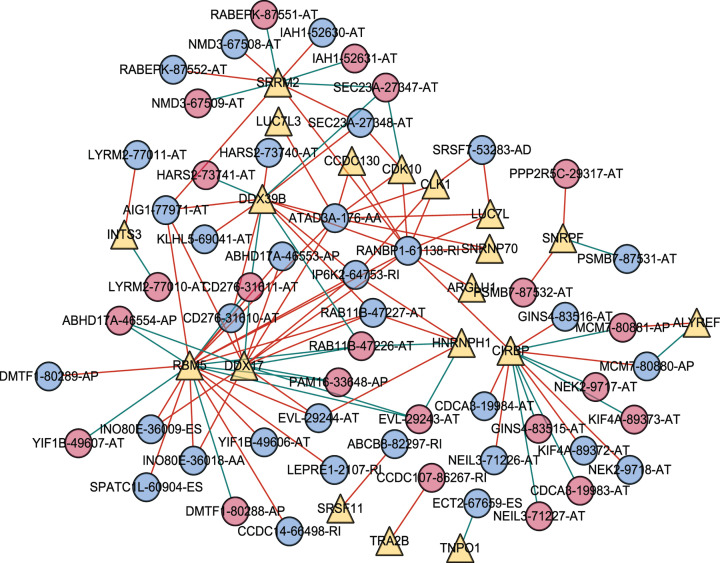
Previous studies have suggested that the dysregulation of SF plays a crucial role in tumorigenesis or progression [8]. Data relative to the mRNA expression of SFs in geriatric lung adenocarcinoma were downloaded from the TCGA database. Pearson’s correlation analysis was used to analyze the correlation between survival-related SFs and AS events with independent predictive significance (screening criteria: |correlation coefficient| > 0.6, P<0.001). A total of 19 SF and 54 AS events were selected by Pearson’s analysis. A correlation network was established using Cytoscape to examine the potential regulatory association between the SF and the survival-associated AS events (Figure 15). A shown in Figure 15, a single SF can be involved in regulating multiple AS events, and an AS event can be regulated by multiple SFs. Generally, AS events with high-risk were mainly negatively correlated with SFs, whereas AS events with low-risk were mainly positively correlated. However, some SFs are negatively correlated with low-risk AS events. For example, SNRPF showed a negative correlation with PSMB7-87531-AT but a positive correlation with PSMB7-87532-AT.
Figure 15. Correlation network between survival-associated splicing factors and splicing in geriatric patients with LUAD.
The PSI values of survival-related AS events are represented by red/blue dots. Survival-associated SFs are represented by yellow dots. The positive/negative correlations between expressions of the SFs and PSI values for AS are represented by red/green lines.
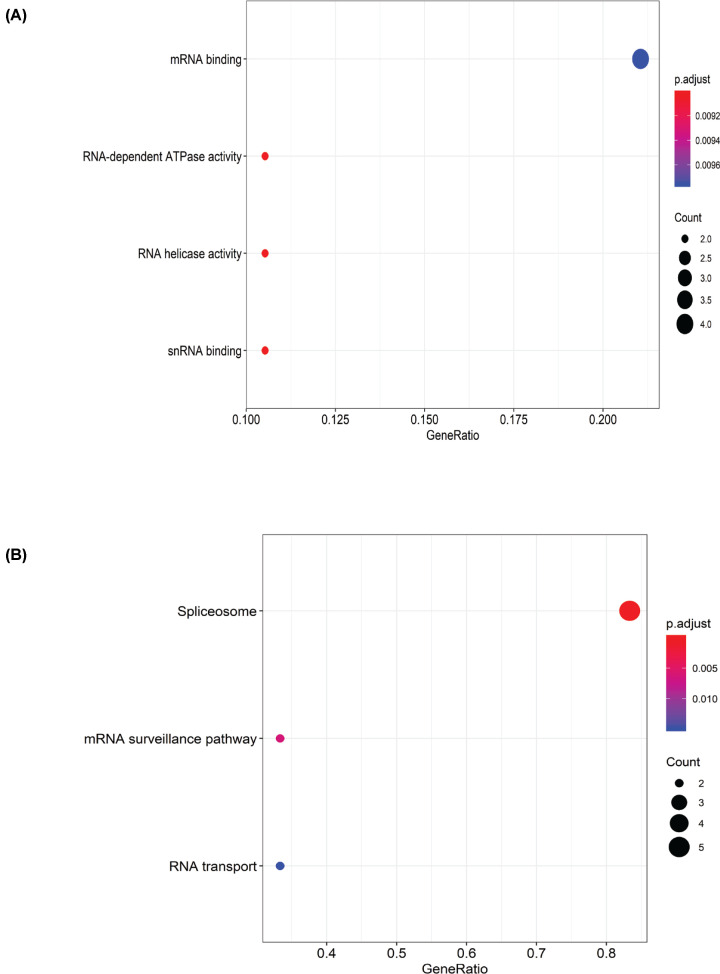
To investigate the potential biological function of the 19 SFs, we analyzed the biological pathways involved and identified enriched pathways using the R package. In GO terms, genes were mostly enriched in terms involving RNA-dependent ATPase activity, RNA helicase activity, snRNA binding, and mRNA binding (Figure 16A). Further, three KEGG pathways were enriched in the AS-SFs network, including the spliceosome, the mRNA surveillance pathway, and RNA transport (Figure 16B). We determined that the gene DDX39B was involved in seven biological functions (Table 2).
Figure 16. Biological function analysis.
(A) Terms identified by Gene Ontology (GO) analysis. The dot size represents the number of the enriched genes, and FDR values are shown by the color scale; FDR, false discovery rate. (B) Enrichment pathways identified by Kyoto Encyclopedia of Genes and Genomes (KEGG).
Table 2. The enrichment results in the alternative splicing- splicing factor regulatory network.
| Category | Description | Count | P-value | Gene ID | |
|---|---|---|---|---|---|
| GO_Biology process | GO: 0008186 | RNA-dependent ATPase activity | 2 | 0.0004 | DDX17, DDX39B |
| GO: 0003724 | RNA helicase activity | 2 | 0.0007 | DDX17, DDX39B | |
| GO: 0017069 | snRNA binding | 2 | 0.0007 | SNRNP70, DDX39B | |
| GO: 0003729 | mRNA binding | 4 | 0.0011 | CIRBP, TRA2B, RBM5, LUC7L3 | |
| KEGG pathway | hsa03040 | Spliceosome | 5 | 1.2747E-08 | SNRPF, SNRNP70, TRA2B, ALYREF, DDX39B |
| hsa03015 | mRNA surveillance pathway | 2 | 0.0020 | ALYREF, DDX39B | |
| hsa03013 | RNA transport | 2 | 0.0075 | ALYREF, DDX39B | |
Abbreviations: GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Discussion
Splicing of pre-mRNA is essential for the maturation of mRNAs, and it is an important step in regulating the expression of protein and genes. Abnormal AS in pre-mRNA splicing may lead to the development of tumors. Previous studies have revealed that SFs can regulate aberrant AS events that affect the occurrence and development of lung cancer. For example, Liu et al. showed that abnormal splicing of BIN1 was controlled by serine and arginine-rich factor 1 (SRSF1) in NSCLC [32]. Lin et al. showed that SRSF1 and RBM4 had differential impact on HIF-1α in a CU element-dependent manner [33]. However, these studies were limited to a single splicing factor or splicing event and did not group the types of lung cancer evaluated. Our study focused on analyzing prognosis related AS events and SFs in elderly lung adenocarcinoma patients. We screened the SFs and AS events related to the prognosis of geriatric patients with LUAD and established eight PI models to analyze the risk score of different AS events. Then, we screened the SFs and AS events that were highly correlated with each other using Pearson correlation analysis. A total of 54 AS events and 19 SFs were selected to construct a correlation network that could help elucidate the potential mechanisms involved in the splicing pathway in GLAD.
The ROC curve analyses verified that all the models had a certain guiding significance for prognosis, and the PI model of AA events was the most significant. In the AA model, we analyzed 12 AS events closely related to the prognosis of GLAD, related genes mainly include LIMD2, USP28, IL32, and ZC3H13. LIM domain containing 2 (LIMD2) is a small LIM-only protein that has been revealed to promote tumor progression. A recent study showed that LIMD2 serves as an oncogenic in NSCLC and was regulated by miR-34a and miR-124 [34,35]. Ubiquitin-specific proteases (USP) regulate physiological homeostasis of the ubiquitination process, a high level of USP28 is related to poor overall survival and prognosis in NSCLC patients [35]. IL32 and ZC3H13 also play an important role in tumorigenesis and metastasis of lung adenocarcinoma [36,37]. These are consistent with our findings. AS is a complex and sophisticated process and SFs can regulate the aberrant AS events that affect the occurrence and development of lung cancer. Therefore, the correlation between SFs and AS events with independent prognostic value is worth studying.
In our study, a total of 19 SFs were included in the correlation network. We analyzed the regulation relationship between SFs and AS events, our findings may provide new insight for precise treatment and explain the potential molecular mechanism of GLAD. Different SFs often have a synergistic effect when regulating the same AS event. For example, ATAD3A is positively regulated by several SF such as RBM5, DDX17, CDK-10, CLK1, CCDC130, LUC7L, SNRNP70, and LUC7L3. Besides, one SF may positively or negatively affect different AS events. For example, SRRM2 positively regulates RANBP1-61138-RI, whereas it negatively regulates SEC23A-27347-AT. In our network about GLAD, positive correlations between SFs and AS events were more common than negative ones. Generally, AS events with high-risk are mainly negatively correlated with SFs, whereas AS events with low-risk are mainly positively correlated. In other words, most of the SF in our study may delay the progression of GLAD except SNRPF, ALYREF, and TNPO1.
Several SFs in key nodes were frequently related to splicing events in GLAD, mainly including DDX39B, DDX17, SRRM2, CIRBP, and RBM5. Prior studies have shown that these SF are closely related to tumor formation. Overexpression of DDX39B enhances cell proliferation and global translation to promote tumor formulation [38]. DDX17 promotes the formation of hepatocellular carcinoma by inhibiting Klf4 transcriptional activity [39]. SRRM2 is a main component of the spliceosome, and mutation in SRRM2 is associated with the predisposition of papillary thyroid carcinoma [40]. Abnormal expression of CIRBP is involved in the progression and migration of nasopharyngeal carcinoma and bladder cancer [41,42]. Besides, RBM5 can be acted as a tumor suppressor [43], and it inhibits the formation of lung adenocarcinoma through several apoptotic signaling pathways [44]. It was consistent with our study findings that RBM5 could up-regulate several low-risk AS events and down-regulate high-risk AS events. However, previous studies did not discuss the role of DDX39B, DDX17, SRRM2, and CIRBP in GLAD. In our analyses, these SF were found to affect tumor prognosis by regulating several AS events. For example, DDX39B positively regulates the ATAD3A-176-AA, whereas negatively regulates the RAB11B-47226-AT, and GO function terms and KEGG pathway analysis showed that DDX39B was involved in seven biological functions. DDX17 positively regulates the AIG1-77971-AT, whereas negatively regulates the RAB11B-47227-AT. Furthermore, we found that DDX39B, DDX17, and RBM5 were simultaneously positively regulated the ATAD3A-176-AA. ATAD3A is a kind of mitochondrial enzyme, and it is a low-risk gene associated with AA event [45]. The deregulation of ATAD3A is crucial in the tumor microenvironment because it promotes tumor metastasis [46]. As such, ATAD3A is a promising drug target in the treatment of GLAD. However, there are few studies on ATAD3A in lung adenocarcinoma, the clinical significance of targeting ATAD3A in the treatment of GLAD deserves further exploration and demonstration. Therefore, our study may highlights a possible mechanism in GLAD tumorigenesis.
The results of the GO analysis indicated that the genes were mainly involved in RNA-dependent ATPase activity, RNA helicase activity, snRNA binding, and mRNA binding. Furthermore, three KEGG pathways were enriched in the AS-SFs network, including the spliceosome, the mRNA surveillance pathway, and RNA transport. It is worth noting that DDX39B was involved in seven biological functions. The AS events generated from these genes may affect the development of GLAD by interfering with the above biological processes and pathways.
In conclusion, we assessed the prognostic value of survival-related AS events in GLAD and established eight PI models with high prognostic values. The regulatory network and enrichment analyses revealed the distinct relationship between AS and SFs. The process of these specific regulatory mechanisms in spliceosomes may serve as crucial starting points for further exploration of splicing events in GLAD.
Acknowledgements
Thanks to Charlesworth for providing language help.
Abbreviations
- AA
alternate acceptor site
- AD
alternate donor site
- AP
alternate promoter
- AS
alternative splicing
- AT
alternate terminator
- AUC
area under the curve
- ES
exon skip
- GDC
Genomic Data Commons
- GLAD
geriatric lung adenocarcinoma
- GO
Gene ontology
- HR
hazard ratio
- K-M
Kaplan–Meier curve
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LASSO
least absolute shrinkage and selection operator
- LUAD
lung adenocarcinoma
- ME
mutually exclusive exons
- NSCLC
non–small cell lung carcinoma
- PI
prognostic index
- PSI
percent-sliced-in
- RI
retained intron
- ROC
receiver-operator characteristic
- SF
splicing factor
- TCGA
The Oncology Genome Atlas
Data Availability
The datasets used and analyzed during the current study are available from the online tools.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This study was sponsored by the Program for Science and Technology Innovation Talents in Universities of Henan Province [grant number 19HASTIT001].
Author Contribution
Conceptualization: Yidi Wang, Yaxuan Wang, and Wang Ma; Formal analysis: Yidi Wang, Yaxuan Wang, Hong Li, and Wang Ma; Methodology: Kenan Li, Yabing Du, Kang Cui, and Pu Yu; Validation: Tengfei Zhang, and Wang Ma; Writing – original draft, Yidi Wang; Writing – review & editing, Yidi Wang and Yaxuan Wang.
Ethics Approval
There were no cell, tissue, or animal studies. No ethical requirements are involved.
Consent for Publication
All authors agree to publish the paper.
References
- 1.(2020) Key Statistics for Lung Cancer https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html American Cancer Society
- 2.Adeloye D., Sowunmi O.Y., Jacobs W., David R.A., Adeosun A.A., Amuta A.O. et al. (2018) Estimating the incidence of breast cancer in Africa: a systematic review and meta-analysis. J. Glob. Health 8, 010419–010419 10.7189/jogh.08.010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travis W.D., Brambilla E., Nicholson A.G., Yatabe Y., Austin J.H.M., Beasley M.B. et al. (2015) The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 10, 1243–1260 10.1097/JTO.0000000000000630 [DOI] [PubMed] [Google Scholar]
- 4.Hou H., Zhu H., Zhao H., Yan W., Wang Y., Jiang M. et al. (2018) Comprehensive Molecular Characterization of Young Chinese Patients with Lung Adenocarcinoma Identified a Distinctive Genetic Profile. Oncologist 23, 1008–1015 10.1634/theoncologist.2017-0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yerukala Sathipati S. and Ho S.Y. (2017) Identifying the miRNA signature associated with survival time in patients with lung adenocarcinoma using miRNA expression profiles. Sci. Rep. 7, 7507 10.1038/s41598-017-07739-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodard G.A., Jones K.D. and Jablons D.M. (2016) Lung Cancer Staging and Prognosis. Cancer Treat. Res. 170, 47–75 10.1007/978-3-319-40389-2_3 [DOI] [PubMed] [Google Scholar]
- 7.Yoon B.W., Chang B. and Lee S.H. (2020) High PD-L1 Expression is Associated with Unfavorable Clinical Outcome in EGFR-Mutated Lung Adenocarcinomas Treated with Targeted Therapy. OncoTargets Ther. 13, 8273–8285 10.2147/OTT.S271011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvinge H., Kim E., Abdel-Wahab O. and Bradley R.K. (2016) RNA splicing factors as oncoproteins and tumour suppressors. Nat. Rev. Cancer 16, 413–430 10.1038/nrc.2016.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urbanski L.M., Leclair N. and Anczuków O. (2018) Alternative-splicing defects in cancer: Splicing regulators and their downstream targets, guiding the way to novel cancer therapeutics. Wiley Interdisciplinary Rev. RNA 9, e1476 10.1002/wrna.1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omenn G.S., Menon R. and Zhang Y. (2013) Innovations in proteomic profiling of cancers: alternative splice variants as a new class of cancer biomarker candidates and bridging of proteomics with structural biology. J. Proteomics 90, 28–37 10.1016/j.jprot.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J. and Manley J.L. (2013) Misregulation of pre-mRNA alternative splicing in cancer. Cancer Discov. 3, 1228–1237 10.1158/2159-8290.CD-13-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coomer A.O., Black F., Greystoke A., Munkley J. and Elliott D.J. (2019) Alternative splicing in lung cancer. Biochim. Biophys. Acta Gene Regul. Mech. 1862, 194388 10.1016/j.bbagrm.2019.05.006 [DOI] [PubMed] [Google Scholar]
- 13.Climente-González H., Porta-Pardo E., Godzik A. and Eyras E. (2017) The Functional Impact of Alternative Splicing in Cancer. Cell Rep. 20, 2215–2226 10.1016/j.celrep.2017.08.012 [DOI] [PubMed] [Google Scholar]
- 14.He C., Zhou F., Zuo Z., Cheng H. and Zhou R. (2009) A global view of cancer-specific transcript variants by subtractive transcriptome-wide analysis. PLoS ONE 4, e4732 10.1371/journal.pone.0004732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.David C.J. and Manley J.L. (2010) Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes Dev. 24, 2343–2364 10.1101/gad.1973010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Z.G., He R.Q. and Mo Z.N. (2018) Prognostic value and potential function of splicing events in prostate adenocarcinoma. Int. J. Oncol. 53, 2473–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin P., He R.Q., Huang Z.G., Zhang R., Wu H.Y., Shi L. et al. (2019) Role of global aberrant alternative splicing events in papillary thyroid cancer prognosis. Aging 11, 2082–2097 10.18632/aging.101902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao L., Xie Z.C., Pang J.S., Li T.T. and Chen G. (2019) A novel alternative splicing-based prediction model for uteri corpus endometrial carcinoma. Aging 11, 263–283 10.18632/aging.101753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seiler M., Peng S., Agrawal A.A., Palacino J., Teng T., Zhu P. et al. (2018) Somatic Mutational Landscape of Splicing Factor Genes and Their Functional Consequences across 33 Cancer Types. Cell Rep. 23, 282.e284–296.e284 10.1016/j.celrep.2018.01.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheng J., Zhao Q., Zhao J., Zhang W., Sun Y., Qin P. et al. (2018) SRSF1 modulates PTPMT1 alternative splicing to regulate lung cancer cell radioresistance. EBioMedicine 38, 113–126 10.1016/j.ebiom.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkman B.M. (2004) Splice variants as cancer biomarkers. Clin. Biochem. 37, 584–594 10.1016/j.clinbiochem.2004.05.015 [DOI] [PubMed] [Google Scholar]
- 22.de Fraipont F., Gazzeri S., Cho W.C. and Eymin B. (2019) Circular RNAs and RNA Splice Variants as Biomarkers for Prognosis and Therapeutic Response in the Liquid Biopsies of Lung Cancer Patients. Front. Genet. 10, 390 10.3389/fgene.2019.00390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salton M. and Misteli T. (2016) Small Molecule Modulators of Pre-mRNA Splicing in Cancer Therapy. Trends Mol. Med. 22, 28–37 10.1016/j.molmed.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Montiel N., Rosas-Murrieta N.H., Anaya Ruiz M., Monjaraz-Guzman E. and Martinez-Contreras R. (2018) Alternative Splicing as a Target for Cancer Treatment. Int. J. Mol. Sci. 19, 545 10.3390/ijms19020545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomczak K., Czerwińska P. and Wiznerowicz M. (2015) The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemporary Oncol. (Poznan, Poland) 19, A68–A77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun G., Li Y., Peng Y., Lu D., Zhang F., Cui X. et al. (2019) Identification of a five-gene signature with prognostic value in colorectal cancer. J. Cell. Physiol. 234, 3829–3836 10.1002/jcp.27154 [DOI] [PubMed] [Google Scholar]
- 27.Selvaraj G., Kaliamurthi S., Kaushik A.C., Khan A., Wei Y.K., Cho W.C. et al. (2018) Identification of target gene and prognostic evaluation for lung adenocarcinoma using gene expression meta-analysis, network analysis and neural network algorithms. J. Biomed. Inform. 86, 120–134 10.1016/j.jbi.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 28.Ma B., Geng Y., Meng F., Yan G. and Song F. (2020) Identification of a Sixteen-gene Prognostic Biomarker for Lung Adenocarcinoma Using a Machine Learning Method. J. Cancer 11, 1288–1298 10.7150/jca.34585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lex A., Gehlenborg N., Strobelt H., Vuillemot R. and Pfister H. (2014) UpSet: Visualization of Intersecting Sets. IEEE Trans. Vis. Comput. Graph. 20, 1983–1992 10.1109/TVCG.2014.2346248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin K.T. and Krainer A.R. (2019) PSI-Sigma: a comprehensive splicing-detection method for short-read and long-read RNA-seq analysis. Bioinformatics 35, 5048–5054 10.1093/bioinformatics/btz438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D. et al. (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J., Liu T., Wang M., Lv W., Wang Y., Jia Y. et al. (2020) SRSF1-dependent alternative splicing attenuates BIN1 expression in non-small cell lung cancer. J. Cell. Biochem. 121, 946–953 10.1002/jcb.29366 [DOI] [PubMed] [Google Scholar]
- 33.Chang H.L. and Lin J.C. (2019) SRSF1 and RBM4 differentially modulate the oncogenic effect of HIF-1α in lung cancer cells through alternative splicing mechanism. Biochim. Biophys.Acta Mol. Cell Res. 1866, 118550 10.1016/j.bbamcr.2019.118550 [DOI] [PubMed] [Google Scholar]
- 34.Wang F., Li Z., Xu L., Li Y., Li Y., Zhang X. et al. (2018) LIMD2 targeted by miR-34a promotes the proliferation and invasion of non-small cell lung cancer cells. Mol. Med. Rep. 18, 4760–4766 [DOI] [PubMed] [Google Scholar]
- 35.Zhang F., Qin S., Xiao X., Tan Y., Hao P. and Xu Y. (2019) Overexpression of LIMD2 promotes the progression of non-small cell lung cancer. Oncol. Lett. 18, 2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng Q., Li S., Zhou Y., Ou W., Cai X., Zhang L. et al. (2014) Interleukin-32 contributes to invasion and metastasis of primary lung adenocarcinoma via NF-kappaB induced matrix metalloproteinases 2 and 9 expression. Cytokine 65, 24–32 10.1016/j.cyto.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 37.Li N. and Zhan X. (2020) Identification of pathology-specific regulators of m(6)A RNA modification to optimize lung cancer management in the context of predictive, preventive, and personalized medicine. EPMA J. 11, 485–504 10.1007/s13167-020-00220-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Awasthi S., Verma M., Mahesh A., Khan M.I.K., Govindaraju G., Rajavelu A. et al. (2018) DDX49 is an RNA helicase that affects translation by regulating mRNA export and the levels of pre-ribosomal RNA. Nucleic Acids Res. 46, 6304–6317 10.1093/nar/gky231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue Y., Jia X., Li C., Zhang K., Li L., Wu J. et al. (2019) DDX17 promotes hepatocellular carcinoma progression via inhibiting Klf4 transcriptional activity. Cell Death Dis. 10, 814 10.1038/s41419-019-2044-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomsic J., He H., Akagi K., Liyanarachchi S., Pan Q., Bertani B. et al. (2015) A germline mutation in SRRM2, a splicing factor gene, is implicated in papillary thyroid carcinoma predisposition. Sci. Rep. 5, 10566–10566 10.1038/srep10566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu M., Ge Q., Wang G., Luo Y., Wang X., Jiang W. et al. (2018) CIRBP is a novel oncogene in human bladder cancer inducing expression of HIF-1α. Cell Death Dis. 9, 1046–1046 10.1038/s41419-018-1109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin T.-Y., Chen Y., Jia J.-S., Zhou C., Lian M., Wen Y.-T. et al. (2019) Loss of Cirbp expression is correlated with the malignant progression and poor prognosis in nasopharyngeal carcinoma. Cancer Manag. Res. 11, 6959–6969 10.2147/CMAR.S211389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jamsai D., Watkins D.N., O'Connor A.E., Merriner D.J., Gursoy S., Bird A.D. et al. (2017) In vivo evidence that RBM5 is a tumour suppressor in the lung. Sci. Rep. 7, 16323 10.1038/s41598-017-15874-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao C., Yang B., Zhao L., Wang S., Zhang J. and Wang K. (2013) Tumor suppressor gene RBM5 delivered by attenuated Salmonella inhibits lung adenocarcinoma through diverse apoptotic signaling pathways. World J. Surg. Oncol. 11, 123 10.1186/1477-7819-11-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X., Li G., Ai L., Ye Q., Yu T. and Yang B. (2019) Prognostic value of ATAD3 gene cluster expression in hepatocellular carcinoma. Oncol. Lett. 18, 1304–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Teng Y., Ren X., Li H., Shull A., Kim J. and Cowell J.K. (2016) Mitochondrial ATAD3A combines with GRP78 to regulate the WASF3 metastasis-promoting protein. Oncogene 35, 333–343 10.1038/onc.2015.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the online tools.