Abstract
Necrotizing pneumonia is a severe complication of pneumonia, characterized by local destruction of lung tissue with development of multiple small cavities (abscesses) and may be associated with empyema. Empyema is an unusual complication in neonates with limited data reported. We present a healthy term neonate with late-onset sepsis caused by Methicillin-resistant Staphylococcus aureus (MRSA) bacteremia due to severe necrotizing pneumonia associated with advanced stage empyema. To the best of our knowledge this is the youngest reported patient with multifocal lung abscesses associated with stage 2 empyema treated successfully without surgical intervention.
Keywords: Necrotizing pneumonia, Methicillin resistant staphylococcus aureus, Empyema
1. Introduction
Staphylococcus aureus (SA) is a Gram-positive, coagulase-positive bacteria that belongs to the family Staphylococcaceae. It is often present in human body parts as commensal with about 20% of individuals are persistent nasal carriers [1]. SA infections can present in healthy neonates with no apparent risk factors for infection. Clinical presentation includes soft tissue infection, urinary tract infection, perinephric abscess, necrotizing pneumonia, bacteremia, septic shock, and even death [2]. The underlying mechanism of necrotizing pneumonia (NP) is poorly understood, but likely related to host susceptibility and bacterial virulence factors [3]. NP is a severe complication of pneumonia characterized by pulmonary inflammation with consolidation, peripheral necrosis, producing local destruction of lung tissue, development of multiple small cavities (abscesses), and mostly accompanied by empyema. Although rare, the incidence of NP has increased over time since first reported in children in 1994 [3]. Between 2006 and 2009 to 2009–2011 the incidence of NP among children increased from 4.5% to 9% [4]. The most common causes of NP include Streptococcus pneumonia and SA [3,5].
NP complicates between 0.8 and 7% of community-acquired pneumonia and 20% of those with empyema end up admitted to tertiary pediatric hospitals [5]. In a study of 47 children with empyema, half of those had concomitant NP. There was no difference in microbiology, oxygen requirement, and hospital length of stay. 13% with isolated empyema had complications as pneumothorax and pleural fistula compared to 16% in patients with concomitant NP [6].
We present a patient with late-onset sepsis caused by MRSA bacteremia due to severe NP and advanced stage empyema. To the best of our knowledge, this is the youngest patient with multifocal lung abscesses associated with stage 2 empyema successfully treated without surgical intervention.
2. Case presentation
A previously healthy 3-week-old Hispanic female presented to our emergency department (ED) with fever and difficulty breathing for 1 day. The patient was a full-term infant delivered via cesarean-section. The maternal group B streptococcus (GBS) status was unknown. The pregnancy was complicated by decreased fetal movement and second-hand smoking exposure. At delivery, the patient was hypoglycemic requiring admission to the Neonatal Intensive Care Unit (NICU) for intravenous fluids for one day. The patient was discharged home without complications.
On day of life 15, the patient was evaluated by her pediatrician for 2 weeks of nasal congestion and cough. Supportive treatment was recommended after which point the patient improved for 2 days. On day of life 21, the patient was re-evaluated with one day of subjective fever and fussiness where she was noted to have a temperature of 100.4 °F. The patient was referred to the nearest ED, where the patient was noted to be cyanotic with desaturations, in respiratory distress, and febrile to 100.8 °F.
Chest radiograph (CXR) showed left-sided pneumonia and left pleural effusion. Fig. 1. The initial laboratory revealed leukocytosis with white blood cell count (WBC) 32 x109/L, hyponatremia (132 mmol/L), and combined respiratory and metabolic alkalosis. Blood culture was drawn, and she was started on Ampicillin and Cefotaxime. Subsequently the patient was transferred for a higher level of care
Fig. 1.
Chest X-rays: Image (A) on day of admission showing hazy bilateral airspace opacities with diffuse consolidation of the left lung associated with a left pleural effusion, image (B) on Day 6 of antimicrobial therapy showing hazy confluent opacity in the left hemithorax represents necrotizing pneumonia with lung abscesses and normal right lung, image (C) on Day 19 of therapy showing continued resolution of the left upper lobe pneumonia and decreased size of the abscess cavity, image (D) on Day 30 of treatment and upon discharge showed clear lungs with mild left basilar residual pleural thickening.
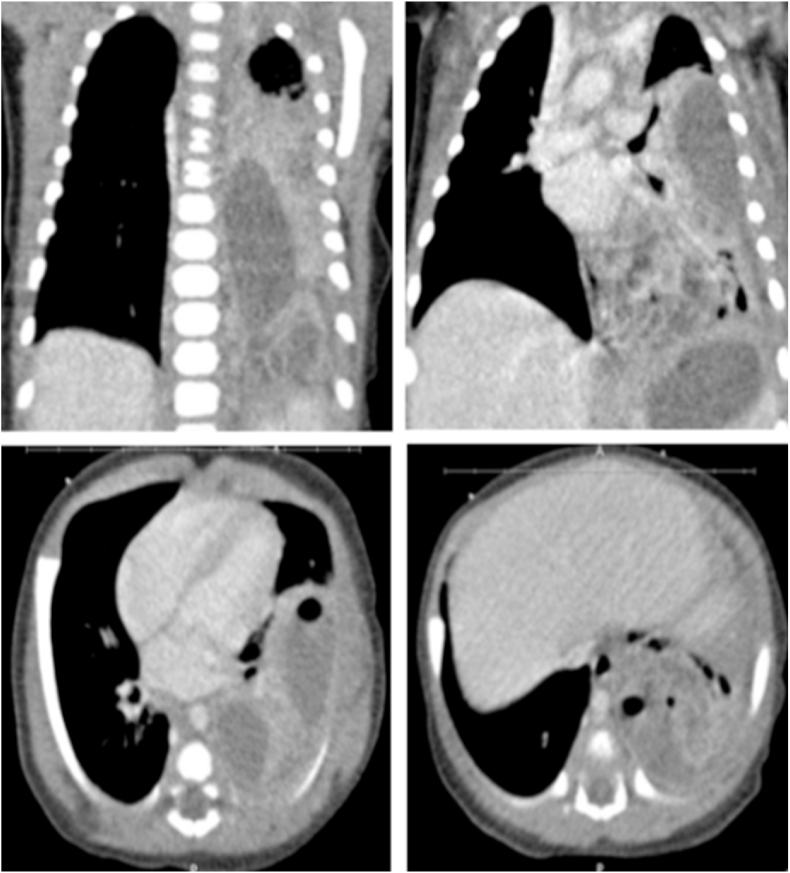
Upon admission, the patient was tachycardic, normotensive, and with decreased oxygen saturation (70%) on room air. On examination, she had diminished breath sounds in the left lung with moderate airway movement. She was placed on a 2L nasal cannula. WBC count was 34.1x109/L with 72% neutrophils. C-reactive protein and erythrocyte sedimentation rate were elevated (109mg/L and 28mm/h respectively). Repeat CXR confirmed hazy bilateral airspace opacities with diffuse consolidation of the left lung consistent with pneumonia associated with left pleural effusion. Chest Ultrasound (US) showed moderate complex left pleural effusion with internal debris and septations with consolidation of the left lung base. A chest computerized tomography (CT) scan showed air space consolidation of the left lower lobe, a 2 large peripherally enhancing fluid collections present in the left lower lobe with gas consistent with abscesses (superior abscess 1.6 x 4.7 × 5.6 cm and inferior abscess 2 x 3 × 4.2 cm) and small loculated left pleural effusion. Fig. 2. Cerebral spinal fluid (CSF) studies showed normal glucose (63 mg/dL), protein (34 mg/dL) with no pleocytosis (nucleated cells 4). Complete viral respiratory panel including Influenza, Respiratory Syncytial Virus, Adenovirus, Parainfluenza were all negative. Chlamydophila pneumonia and Bordetella pertussis polymerase chain reaction were also negative. Ampicillin, Cefepime, and Azithromycin were initiated then narrowed to Ampicillin/Sulbactam on hospital day 03. On hospital day 04, MRSA was isolated from blood culture. At this point Vancomycin was added. Nasal swab MRSA culture and sputum culture were also positive for MRSA. The patient remained bacteremic for 4 days. The echocardiogram was normal.
Fig. 2.
Chest CT scan on the third day of therapy demonstrates air space consolidation of the left lower lobe, small loculated left pleural effusion, and a 2 large peripherally enhancing fluid collections present in the left lower lobe with gas consistent with abscesses. The more superior and anterior abscess measures approximately 1.6 x 4.7 × 5.6 cm. The inferior and posterior abscess measures approximately 2 x 3 × 4.2 cm. Small loculated left pleural effusion was present. No pneumothorax was seen. Both components intraparenchymal and pleural empyema.
As the patient was clinically stable, pediatric surgery preferred to continue medical management given the complications of draining the abscess such as the risk of anesthesia, bronchopleural fistula formation, hemothorax, and pneumothorax. Our patient completed Vancomycin for 2 weeks followed by IV Clindamycin IV for 2 weeks. Serial Imaging showed dramatic improvement over the course of her hospitalization. A CXR before discharge showed well-expanded lungs and clear.
3. Discussion
In this report we describe a 3-week-old term infant with MRSA bacteremia due to severe NP associated with advanced stage empyema who was successfully treated without surgical intervention. Common pathogens associated with late-onset sepsis or pneumonia among infants include GBS, Escherichia coli, Listeria, and SA. Viral infections, especially influenza, frequently precede the development of SA pneumonia [8].
Our patient had a potential risk factor for MRSA infection due to 1-day previous hospitalization in the NICU. SA colonization is common in the first week of life (23%) especially in premature neonates who required intensive care admission with only 2.3% developed infection [9]. The rate of SA infection is 15–30 per 1000 neonatal admission [7]. In newborns with complicated pneumonia, non-infectious etiologies need to be considered including pulmonary malformation, bronchogenic cyst, tracheoesophageal fistula, and cystic fibrosis as well as immunodeficiency. Her imaging was not suggestive of a congenital defect and immunology evaluation was normal including quantitative immunoglobulins (IgG, IgM, IgE, and IgA), Mitogens phytohemagglutinin (PHA) and Concanavalin A (ConA), dihydrorhodamine flow cytometry test (DHR), lymphocyte phenotyping and lymphoproliferation assay.
Our patient presented with complicated pneumonia. In a recent review of 2358 children hospitalized with pneumonia very few [23 patients (1%)] were associated with SA and even less with MRSA [17 (<1%)]. The median age was 15 months with male predominance (71%). 26% had MRSA bacteremia. Although less common, children with MRSA pneumonia compared to non-SA pneumonia more commonly had higher associated parapneumonic effusions (88% vs 12%), prolonged hospital stay (10 vs 3 days), more frequent Intensive Care Unit (ICU) admissions (83% vs 21%), intubation (65% vs 7%) and higher mortality rate (6% vs less than 1%) [10]. Another study on 117 pediatric patients admitted with SA pneumonia between 2001 and 2009, the rate of admission increased by 4.94 per 10,000 admissions. MRSA patients were younger compared to MSSA. Median hospital stay for MRSA was 13 days, 50% required ICU admission with 27.9% required intubation [8]. The overall number of community-acquired MRSA infections and the incidence of empyema has increased in the early 2000s but it continues to decline since 2006 [2,11]. There was a 4-fold increase in thoracic empyema in the pediatric population from 0.6 cases per 100,000 children from 1996 to 1998 to 2.5 cases per 100,000 children from 2005 to 2007 in children less than 2 years of age and was most frequently caused by SA [8].
Our patient's course was complicated by empyema. Empyema progresses through 3 stages. Stage 1 is the early exudative phase, stage 2 is the fibro purulent phase with large quantities of white cells and fibrin deposition, resulting in loculations formation and stage 3 is the organizing phase. Management of empyema remains controversial. Medical decision therapy depends on the stage of the disease [12]. Early-stage empyema may respond to antibiotics but as loculations develop, advanced stages usually require referral for surgical consultation [12,13]. For these patients, surgical options include chest drain alone, chest drain with fibrinolysis (CDF), thoracotomy and VATS, with the latter preferred over thoracotomy [[12], [13], [14], [15]]. A systematic review and meta-analysis showed that VATS and CDF for empyema have similar rates of perioperative complications, however, VATS is associated with reduced need for re-intervention and shorter postoperative hospital stay [13,16,17]. VATS has its own complications such as the risk of anesthesia and bronchopleural fistula formation [15]. There are limited case reports for the use of non-operative interventions in neonates. Our patient was treated without surgical intervention.
Empiric IV vancomycin is recommended in patients who have necrotizing cavities or empyema or in severe clinical status requiring ICU admission. IV clindamycin can be used for stable patients without bacteremia and Linezolid can be used in children less than 12 years of age [18].
In summary, reports of empyema in newborns are rare. This case advances the literature by providing a rare instance of NP in a previously healthy neonate and highlights the challenges of management and the importance of medical treatment without surgical intervention. However, more studies are required to further investigate the efficacy of these alternative strategies in management.
Declaration of competing interest
The authors whose names are listed below certify that they have NO affiliations with or involvement in any organization or entity with any financial interest.
Contributor Information
Henry Zapata, Email: Henryal87@hotmail.com.
Andrew Wahba, Email: Andrew.A.Wahba@uth.tmc.edu.
References
- 1.Lakhundi S., Zhang K. Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018;31(4) doi: 10.1128/CMR.00020-18. e00020-18. Published 2018 Sep. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fortunov Regine, Hultén Kristina, Hammerman Wendy, Mason Edward, Kaplan Sheldon. Community-acquired Staphylococcus aureus infections in term and near-term previously healthy Neonates. Pediatrics. 2006;118:874–881. doi: 10.1542/peds.2006-0884. [DOI] [PubMed] [Google Scholar]
- 3.Masters Ian, Isles Alan, Grimwood Keith. Necrotizing pneumonia: an emerging problem in children? Pneumonia. 2017;9 doi: 10.1186/s41479-017-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemaître C., Angoulvant F., Gabor F. Necrotizing pneumonia in children: report of 41 cases between 2006 and 2011 in a French tertiary care center. Pediatr. Infect. Dis. J. 2013 Oct;32(10):1146–1149. doi: 10.1097/inf.0b013e31829be1bb. [DOI] [PubMed] [Google Scholar]
- 5.Spencer D.A., Thomas M.F. Necrotising pneumonia in children. Paediatr. Respir. Rev. 2014;15:240. doi: 10.1016/j.prrv.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Blanchon Sylvain, Anastaze Karine, Mornand Anne, Bajwa Nadia, Vidal Isabelle, Dumont Mehrak, Argiroffo Constance. Should empyema be managed differently in case of necrotizing pneumonia? Eur. Respir. J. 2013;42 [Google Scholar]
- 7.Carey A., Duchon J., Della-Latta P. The epidemiology of methicillin-susceptible and methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit, 2000–2007. J. Perinatol. 2010;30:135–139. doi: 10.1038/jp.2009.119. [DOI] [PubMed] [Google Scholar]
- 8.Carrillo-Marquez M.A., Hulten K.G., Hammerman W., Lamberth L., Mason E.O. SL Kaplan Staphylococcus aureus pneumonia in children in the era of community-acquired methicillin-resistance at Texas Children's Hospital Pediatr. Inf. Disp. J. 2011;30:545–550. doi: 10.1097/INF.0b013e31821618be. [DOI] [PubMed] [Google Scholar]
- 9.Gooch J.J., Britt E.M. Staphylococcus aureus colonization and infection in newborn nursery patients. Am. J. Dis. Child. 1978;132:893–896. doi: 10.1001/archpedi.1978.02120340069014. [DOI] [PubMed] [Google Scholar]
- 10.Frush Jennifer M., Zhu Y., Edwards Kathryn M., Grijalva C.G., Thomsen Isaac P., Self W.H., Jain S., Anderson Evan J., Ampofo Krow, Pavia Andrew T., Arnold Sandra R., McCullers Jonathan A., Williams D.J. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J. Hosp. Med. 2018;12:848–852. doi: 10.12788/jhm.3093. Published online first October 31, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otter Jon, French Gary. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect. Dis. 2010;10:227–239. doi: 10.1016/S1473-3099(10)70053-0. [DOI] [PubMed] [Google Scholar]
- 12.Avansino Jeffrey, Goldman Bryan, Sawin Robert, Flum David. Primary operative versus nonoperative therapy for pediatric empyema: a meta-analysis. Pediatrics. 2005;115:1652–1659. doi: 10.1542/peds.2004-1405. [DOI] [PubMed] [Google Scholar]
- 13.Pacilli M., Nataraja R.M. Management of paediatric empyema by video-assisted thoracoscopic surgery (VATS) versus chest drain with fibrinolysis: systematic review and meta-analysis. Paediatr. Respir. Rev. 2019;30:42–48. doi: 10.1016/j.prrv.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Li Su-Ting, Gates Robert. Primary operative management for pediatric empyema: decreases in hospital length of stay and charges in a national sample. Arch. Pediatr. Adolesc. Med. 2008;162:44–48. doi: 10.1001/archpediatrics.2007.10. [DOI] [PubMed] [Google Scholar]
- 15.Hilliard Tom, Henderson John, Hewer Simon. Management of parapneumonic effusion and empyema. Arch. Dis. Child. 2003;88:915–917. doi: 10.1136/adc.88.10.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexiou Christos, Goyal Anuj, Firmin Richard, Hickey Mark. Is open thoracotomy still a good treatment option for the management of empyema in children? Ann. Thorac. Surg. 2004;76:1854–1858. doi: 10.1016/S0003-4975(03)01076-2. [DOI] [PubMed] [Google Scholar]
- 17.Redden M.D., Chin T.Y., van Driel M.L., Daum Robert S., Fridkin Scott K., Gorwitz Rachel J., Kaplan Sheldon L., Karchmer Adolf W., Levine Donald P., Barbara . Surgical versus Non-surgical Management for Pleural Empyema. Cochrane Database Syst Rev. vol. 3. 2017. E. Murray, michael; p. CD010651. Catherine Liu, Arnold Bayer, Sara E. Cosgrove. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rybak J., Talan David A., Chambers Henry F. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 1 February 2011;52(Issue 3):e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]