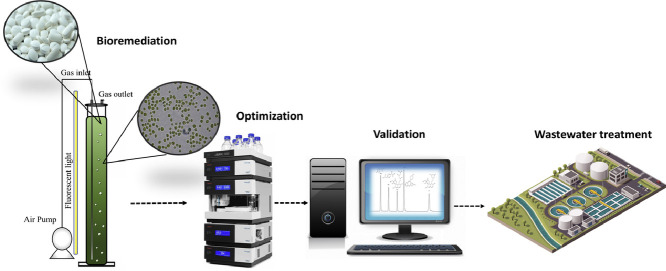
Abstract
A rapid reverse phase high-performance liquid chromatography (RP-HPLC) method was developed and validated for the simultaneous quantification of paracetamol, ibuprofen, olanzapine, simvastatin and simvastatin acid in the context of microalgae bioremediation. The method was validated according to the guidelines of the US Food and Drug Administration (FDA), the International Conference on Harmonization (ICH), and Eurachem with respect to system suitability, linearity, accuracy, precision, recovery, limits of detection and quantification, ruggedness, selectivity and specificity. The estimated limits of detection and quantification were, respectively, 0.03 and 0.10 µg mL−1 for paracetamol, 0.03 and 0.09 µg mL−1 for ibuprofen, 0.04 and 0.13 µg mL−1 for olanzapine, 0.27 and 0.83 µg mL−1 for simvastantin, and 0.05 and 0.14 µg mL−1 for simvastantin acid. The inter-day and intra-day precision results were within the acceptance limit of relative standard deviation (%RSD) of less than 2, and the percentage recovery was found to be within the required limits of 80–110%. The developed method is rapid, linear, precise, robust and accurate, and has been successfully applied to the determination of the above common pharmaceutical products during microalgae bioremediation.
Keywords: Bioremediation, HPLC, Method validation, Microalgae, Pharmaceuticals, Wastewater
Graphical abstract
Specifications table
| Subject Area | Environmental Science |
| More specific subject area | Analytical Chemistry |
| Method name | A rapid reverse phase high-performance liquid chromatography (RP-HPLC) method |
| Name and reference of original method | If applicable, include full bibliographic details of the main reference(s) describing the original method from which the new method was derived. |
| Resource availability | If applicable, include links to resources necessary to reproduce the method (e.g. data, software, hardware, reagent) |
Introduction
Water pollution is a severe global threat to human health and wildlife. As a result of domestic, agricultural and industrial water usage, effluents from wastewater frequently contains pollutants that can persist in the environment, bioaccumulate through the food web and reach drinking water. Several environmentally ubiquitous organic chemicals, e.g. pharmaceuticals, plasticizers, persistent organic pollutants (POPs) are not eliminated by conventional treatment methods, and may be found in drinking water. The U.S. EPA estimates that 20% of the total dietary exposure to emerging pollutants comes from drinking water [1,2]. Conventional wastewater treatment technology includes preliminary, primary and secondary treatments [3], which remove the majority of the biochemical oxygen demand (BOD) and suspended solids, found in wastewaters. However, the conventional treatment cannot produce effluents of high quality, since the apparently clean water is loaded with emerging pollutants, together with nitrogen and phosphorous which cause eutrophication. Advanced wastewater treatments include tertiary treatment, using physicochemical techniques, such as chemical precipitation, ozonation, UV light, reverse osmosis, together with quaternary treatment. The quaternary treatment is designed for the removal of heavy metals, organic pollutants and soluble mineral ions (also denoted as quinternary). The use of microalgae for wastewater treatment is not new and has been exploited for decades. The removal of certain pollutants from wastewater has been reported in the treatment of industrial textile [4], dairy effluents [5] and urban wastewater [6]. Currently, the use of microalgae has been implemented in several stations for the treatment of diverse wastewater sources, including municipal and industrial ones [7,8]. Wastewater treatment is an expensive process but microalgae are a source of multiple products such as pigments, bioplastics and secondary metabolites [9], which may help lessen the respective burden.
In order to reduce the effects of discharges of pollutants and comply with present and future regulations for disposal of wastewaters, more advanced systems are needed. Also, more and better methods for monitoring and validation of emerging pollutants are required.
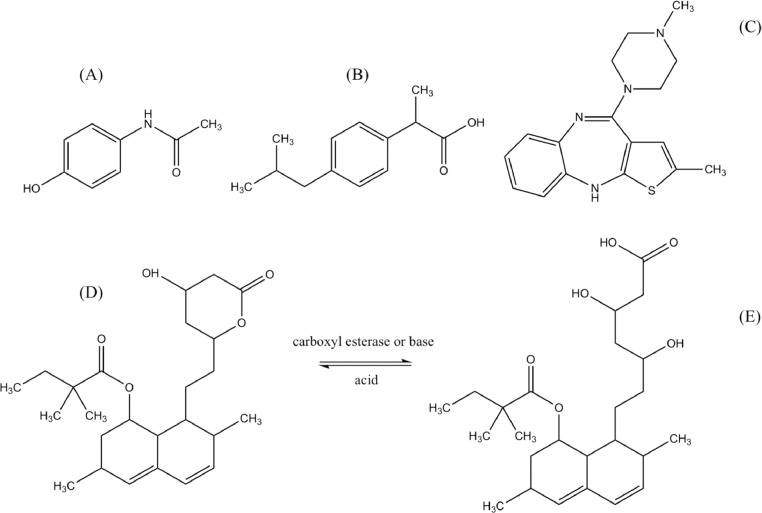
The pharmaceuticals used in this study include paracetamol (or acetaminophen) (PAR), ibuprofen (IBU), olanzapine (OLA) and simvastatin (SIM). They were chosen for their occurrence or persistence in the environment; it should be noted that paracetamol and ibuprofen are generally classified as harmful to aquatic organisms [10]. Paracetamol is the most commonly used analgesic drug and is heavily present in effluents. Ibuprofen is one of the most frequently used nonsteroidal anti-inflammatory drug. Olanzapine, an antipsychotic drug, used for the treatment of schizophrenia, is resistant to photodegradation by sunlight, and is found in surface waters [11,12]. Simvastatin is a lipid regulator and also often found in effluents. This pharmaceutical is a lactone that is hydrolysed in water to the corresponding β-hydroxyacid, the simvastatin acid (SIMA) (Fig. 1). In influents, concentrations of 492340–6924 ng L−1 of PAR, 1681–33764 ng L−1 of IBU, 7–115 ng L−1 of OLA and 1230 ng L−1 of SIM have been reported [10,13].
Fig. 1.
Chemical structures of (a) paracetamol, (b) ibuprofen, (c) olanzapine, (d) simvastatin and (e) simvastatin acid.
HPLC is widely used by the pharmaceutical industry to quantify pharmaceuticals, with particular focus on its quantification in tablets [14,15]. The quantification of emerging pollutants, such as pharmaceuticals in wastewaters is a field needing further exploration. In this context, the objective of this study was to develop and validate a RP-HPLC method for four common pharmaceuticals, with relevance for bioremediation purposes. The validated method was successfully applied to the assessment of the performance and efficiency of free and immobilised cells of microalgae Nannochloropsis sp. in removing the four pharmaceuticals [16].
Experimental
Reagents and chemicals
Paracetamol was purchased from Fagron Iberica (Spain) and Ibuprofen supplied by Laboratórios Medinfar (Lisboa, Portugal). Olanzapine was acquired from Zhejiang MYOY Import & Export Co., Ltd (Hangzhou, China). Simvastatin was kindly provided by Labesfal, Laboratórios Almiro, S.A. (Santiago de Besteiros, Portugal). Microalgae medium f2 was obtained from Varicon Aqua Solution (Malvern, UK). All other reagents and solvents were of analytical or HPLC grade (Fig. 2).
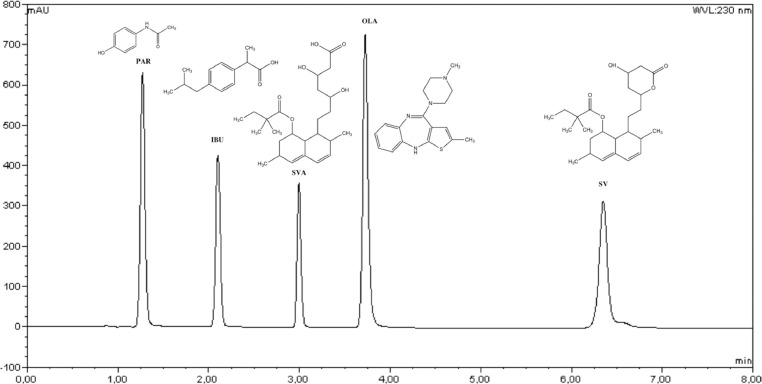
Fig. 2.
Chromatogram of the standard 75 µg mL−1 solution of PAR, IBU, SIMA and SIM considered for the evaluation of the system suitability.
Instrumentation
The HPLC analysis of PAR, IBU, OLA, SIM, and SIMA was carried out using a Dionex Ultimate 3000 system equipped with an auto injector and four variable UV/VIS dual wavelength detectors. The column used for the analysis was a Luna Phenyl-Hexyl, PhenomenexⓇ (Torrance, USA), with 5 µm particle size, 3 mm internal diameter and 150 mm length, supported with a SecurityGuard™ cartridge PhenomenexⓇ (Torrance, USA), with 3.0 mm internal diameter, which was in an oven at a temperature of 35 ˚C. The data were recorded using Chromeleon software. Chromatographic analysis was conducted in multistep gradient mode, as indicated in Table 1. Preferentially, the UV detector was set at 230 nm for the simultaneous detection of PAR, IBU, OLA, SIM, and SIMA. The injection volume was 10 µL for standard and samples. Before analysis, every standard and sample was filtered through 0.22 µm filters. A run time of 8 min was found adequate for the separation of the five analytes, followed by a washing step of 3 min with buffer between runs.
Table 1.
Chromatographic conditions of the gradient HPLC method.
| Time (min) | Eluent A (%) | Eluent B (%) | Flow rate (ml min−1) |
|---|---|---|---|
| 0 | 30 | 70 | 0.8 |
| 1 | 40 | 60 | 0.8 |
| 2 | 60 | 40 | 0.8 |
| 5 | 65 | 35 | 0.8 |
| 7 | 70 | 30 | 0.8 |
| 8 | 30 | 70 | 0.8 |
Eluent A: acetonitrile; Eluent B: phosphate buffer at pH 7.3.
Preparation of mobile phase, stock and standard solutions and quality controls
The mobile phase consisted of a mixture of a buffer and acetonitrile according to the gradient mode, Table 1. The buffer was prepared by dissolving 1.15 g of dipotassium hydrogen phosphate salt in 650 mL of ultrapure water. The value of the pH was adjusted to 7.3 ± 0.1 with phosphoric acid. All solutions were filtered through a 0.22 membrane filter and sonicated to degas. Five acetonitrile stock solutions at 1 mg mL−1 of PAR, IBU, OLA, SIM and SIMA were prepared. SIMA was obtained by alkaline hydrolysis of SIM; a SIM methanol solution of 2 mg mL−1 was prepared and mixed to an equal volume of 0.04 M NaOH solution. This mixture was heated to 60 °C for 45 min and then neutralized with 1 M HCl. A SIMA solution of ca. 1 mg mL−1 is obtained and confirmed by the absence of the SIM peak in HPLC analysis [17]. Two working standard solutions containing the five analytes were prepared at the concentrations of 100 and 10 µg mL−1 by dilution of each stock solution with the mobile phase (acetonitrile:buffer, 50:50). For the determination of the limits of detection and quantification, six standard solutions (0.1, 0.25, 0.5, 0.75, 1 and 1.25 µg mL−1) were prepared from the 10 µg mL−1 working solution. Eight standard solutions (0.5, 1, 5, 10, 25, 50, 75, 100 µg mL−1) were prepared by dilution of the working standard solution with the mobile phase (acetonitrile:buffer, 50:50). For the quality control solutions, six replicates of 0.5, 1.5, 50 and 100 µg mL−1 standards containing the five analytes were considered. All stock solutions were stored at -20 °C and working solutions were freshly prepared as needed.
Method validation
The method for PAR, IBU, OLA, SIM, and SIMA quantification was validated according to the US Food and Drug Administration (FDA) [18], and the International Conference on Harmonization (ICH) guidelines [19] in addition to Eurachem [20], with respect to system suitability, linearity, accuracy, precision, recovery, limits of detection and quantification, selectivity and specificity.
System suitability
The system suitability test ensures that the complete testing system, including instrument, reagents, column and analyst, is suitable for the intended application. For that purpose, six consecutives injections were made with the standard solution of PAR, IBU, OLA, SIM and SIMA at a concentration of 75 µ mL−1. The parameters theoretical plate number (N), capacity factor (k´), resolution (R), tailing factor (T) were analysed. N is indicative of column efficiency, k´ is a measure of where the peak of interest is located with respect to the void volume, R is a measure of how well two peaks are separated, and T is a measure of peak symmetry.
Limits of detection (LOD) and quantification (LOQ)
LOD is the lowest amount of analyte in a sample which can be detected but not necessarily quantified as an exact value, and LOQ is the lowest amount of analyte in a sample which can be quantitatively determined with acceptable accuracy and precision. The LOD and LOQ values were determined by using regression parameters from a calibration curve from six standard solutions (0.1, 0.25, 0.5, 0.75, 1 and 1.25 µg mL−1) containing the five analytes (3.3σ/S and 10σ/S, respectively, where σ is the standard deviation of the residues and S is the slope) [19].
Linearity
Linearity is a critical criterion for quantitative analysis and constitutes a measure of accuracy over the range of the method. The linearity of the proposed method was evaluated through calibration curves, constructed with eight standard solutions, containing the five analytes, ranging from 0.5 to 100 µg mL−1, to calculate coefficient of correlation, slope, and intercept values. Data were analysed using the Analysis ToolPak of Microsoft ExcelⓇ (Microsoft Corp., Redmond, WA) with linear regression by the least squares method.
Accuracy and precision
The accuracy of an analytical method expresses the closeness between the reference value and the value that was actually found. The mean value should be within 15% of the theoretical value, except at the LLOQ, where it should not deviate by more than 20% [18]. The accuracy of the method was established by analysing six replicates of the four quality controls and by calculating the trueness for each analyte. Trueness is expressed in terms of bias, and represents the systematic deviation from a true central value and was calculated as
% accuracy = (observed concentration/nominal concentration) x 100.
Precision represents the degree of concordance between the measured value and the reference value [20]. Precision is generally dependent on analyte concentration, and must therefore be determined within the range of concentrations of interest. Evaluation of precision was determined by repeatability (intra-day) and intermediate precision (inter-day) for three consecutive days. Six replicates of four quality control solutions (0.5, 1.5, 50 and 100 µg mL−1) were prepared and analysed according to intra-day and inter-day precision. The relative standard deviation (RSD) of the results should be lower than 15%, according with the requirements, except for the LLOQ, where it should be lower than 20% [18].
Selectivity and specificity
Analytical selectivity could be interpreted as “the extent to which the method can be used to determine particular analytes in mixtures or matrices without interferences from other components of similar behaviour” [21]. IUPAC recommends the term selectivity, while other areas, e.g. pharmaceutical field, use the term specificity, yet agreement exists on the interpretation. In the developed method, the response of the solution containing only PAR, IBU, OLA, SIM and SIMA was compared with microalgae culture medium f2.
Recovery
Recovery studies may be used to address the level of bias. The recovery of PAR, IBU, OLA, SIM and SIMA from the microalgae culture medium was determined by comparison of the respective concentrations with those of standard solutions in the mobile phase at three different concentrations (1, 50 and 100 µg mL−1).
Method applicability
Nannochloropsis sp. culture conditions
Nannochloropsis sp. was obtained from Varicon Aqua Solution, Malvern, UK, and was cultivated for 6 days in 2 L f/2 medium. 100 cm3 of a Nannochloropsis sp. culture were filtered and washed, and cells were subsequently transferred to a sterilized closed photobioreactor, to which 100 cm3 of culture medium Cell-hi TEViT (Varicon Aqua Solution, Malvern, UK) were added. This is based upon the f/2 medium deprived of nitrates. Nitrate concentration was 0.30 g L−1 and salinity 25 g L−1. The culture media was previously sterilized by microwave irradiation.
Removal of pharmaceuticals from water by Nannochloropsis sp.
To the 100 cm3 Nannochloropsis sp. culture, a concentration of 50 µg mL−1 of each pharmaceutical, PAR, IBU, OLA, SIM and SIMA, was added. Similarly, a blank with 50 µg mL−1 of each pharmaceutical, PAR, IBU, OLA, SIM and SIMA, was added to 100 cm3 of culture medium f2, without cells. The cultures and the f2 media were m-2s−1 with 16:8 photoperiod and kept for 60 h. Millipore water were added when needed to ensure the same volume due to water loss by evaporation. Samples of 30 mL were replaced with Millipore water. The cultures were aerated by bubbling atmospheric air, at a rate of 300 cm3 min−1, and grown at 25±2 °C under light with an irradiance level of ± 100 µmol. Each experiment was carried out in triplicate.
Results and discussion
Method development and optimization
A RP-HPLC method for the simultaneous analysis of PAR, IBU, OLA, SIM and SIMA was developed. According to the published literature, an earlier HPLC method for the simultaneous determination of OLA, SIM and SIMA, used a Phenyl-Hexyl column for the analysis of the three analytes with good separation and short retention times, which motivated the choice for this column. Regarding the analysis of PAR and IBU, there are a number of reported methods in literature [22].
The pKa of the analytes was also considered; pKa´s from PAR, IBU, OLA, SIM are 9.46, 4.85, 7.24 and 14.91, respectively. Following the usual rule of thumb, the pH of the mobile phase should be selected two units above or below the pKa of the analyte. Attaining pH values near the higher and lower values of pH, 12.91 and 2.85, is, however, detrimental to the column. For strongly acidic values of pH, IBU is not dissociated and shows a strong hydrophobic attraction with the silica bed, resulting in retention times near 4 min. However, at pH 3, the retention times of PAR and OLA are 1.103 and 1.047 resulting in a poor separation of the peaks and low resolution. Therefore, a pH value around 7 was chosen for the separation of the five analytes. Regarding mobile phase, methanol was discarded since, in the presence of small amounts of acid, the transesterification of ibuprofen to the corresponding methyl ester may occur. Thus, acetonitrile was the choice for eluent A and phosphate buffer for eluent B. Different ratios of acetonitrile and buffer was tested until the best separation was found in gradient mode, as described in Table 1. Under the described conditions, PAR, IBU, OLA, SIM and SIMA eluted at 1.26, 2.10, 3.72, 6.35 and 2.99 min, respectively. The method was validated over the range of 05–100 µg mL−1.
Method validation
System suitability
The system suitability parameters, summarised in Table 2, including %RSD, theoretical plates, tailing factor and resolution, met the required criteria. Peaks show symmetry and high resolution. The %RSD of the peak area and retention time for PAR, IBU, SIM and SIMA were lower than 2% (Table 2), indicating that the system is appropriate to simultaneously analyse the five compounds. Capacity factor values for PAR and IBU are below 2, however, in a gradient chromatogram, the values from the capacity factor have no theoretical meaning. The results obtained with the system suitability indicate that the selected chromatographic parameters are suitable to identify PAR, IBU, OLA, SIM and SIMA.
Table 2.
System suitability test parameters.
| Chromatographic parameters | PAR (75 µg mL−1) |
IBU (75 µg mL -1) |
SIMA (75 µg mL−1) |
OLA (75 µg mL−1) |
SIM (75 µg mL -1) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Retention time (min.) | Peak area | Retention time (min.) | Peak area | Retention time (min.) | Peak area | Retention time (min.) | Peak area | Retention time (min.) | Peak area | Acceptance criteria | |
| Mean (n=6) | 1.26 | 37.15 | 2.10 | 25.0 | 2.99 | 17.6 | 3.72 | 51.0 | 6.35 | 31.3 | - |
| S.D. | 0.002 | 0.51 | 0.002 | 0.50 | 0.004 | 0.25 | 0.004 | 0.67 | 0.006 | 0.50 | - |
| %RSD | 0.13 | 1.38 | 0.11 | 2.00 | 0.13 | 1.42 | 0.11 | 1.30 | 0.09 | 1.58 | ≤ 2.0% a |
| Theoretical plates (N) | 2816 | 8670 | 23708 | 20120 | 64570 | > 1000a | |||||
| Capacity factor (k´) | 0.52 | 1.52 | 2.59 | 3.47 | 6.62 | > 2.0b | |||||
| Tailing factor (T) | 1.08 | 1.06 | 1.14 | 1.23 | 0.98 | ≤ 2.0b | |||||
| Resolution (rs) | 9.00 | 10.6 | 8.03 | 20.0 | 14.4 | > 2.0b (> 1.5a,c) | |||||
Limits of detection (LOD) and quantification (LOQ)
The estimated values for LOD and LOQ are summarized in Table 3. The LOD and LOQ values show the sensitivity of the method for the detection and quantification of PAR, IBU, OLA, SIM and SIMA.
Table 3.
Estimated limits of detection LOD and quantification LOQ for PAR, IBU, SIMA, OLA and SIM.
| Analyte | LOD (µg mL−1) | LOQ (µg mL−1) |
|---|---|---|
| PAR | 0.03 | 0.10 |
| IBU | 0.03 | 0.09 |
| SIMA | 0.05 | 0.14 |
| OLA | 0.04 | 0.13 |
| SIM | 0.27 | 0.83 |
Linearity
For the assessment of the linearity of the method, solutions with concentrations in the range of 0.5–100 µg mL−1 were evaluated. The correlation coefficient was found to be more than 0.9999 for the five analytes, indicating good linearity of the calibration curve (Table 4). The residual standard deviations close to zero, 0.17, 0.15, 0.11, 0.25, 0.5 for PAR, IBU, OLA, SIM and SIMA respectively, confirm linearity. However, by visual inspection of the plot response against concentration, a systematic trend in the distribution, reflects a change in variance with level. The residuals plot for simvastatin indicates that the model is good fit for high concentrations, and a good predictor of lower concentration values (Table 4).
Table 4.
Results obtained from the regression analysis by the weighted least squares method for PAR, IBU, SIMA, OLA and SIM (n=6).
| Analyte | Mean R2 | Mean slope ± S.E. | Mean intercept ± S.E. |
|---|---|---|---|
| PAR | 0.99994 | 0.50 ± 0.070 | -0.004 ± 0.07 |
| IBU | 0.99991 | 0.34 ± 0.001 | -0.060 ± 0.07 |
| SIMA | 0.99997 | 0.44 ± 0.001 | 0.010 ± 0.05 |
| OLA | 0.99994 | 0.80 ± 0.003 | 0.230 ± 0.12 |
| SIM | 0.99940 | 0.50 ± 0.005 | 0.367 ± 0.24 |
Accuracy and precision
The data obtained with the evaluation of the accuracy and precision are shown in Table 5. All the results met the acceptance criteria. The intra-day and inter-day %RSD and bias values fall within the acceptance criteria of 15% of the theoretical value demonstrating that the developed method is accurate, reliable and reproducible [18].
Table 5.
Intraday and interday precision and accuracy for PAR, IBU, SIMA, OLA and SIM (n=6).
| Nominal concentration (µg mL−1) | Intraday (n=6) |
Interday (n=18) |
||||
|---|---|---|---|---|---|---|
| Measured concentration (µg mL−1) mean ± SD | Precision %RSD | Accuracy %bias | Measured concentration (µg mL−1) mean ± SD | Precision %RSD | Accuracy %bias | |
| PAR (0.5) | 0.49 ± 0.01 | 3.80 | -2.82 | 0.50 ± 0.01 | 4.75 | 0.28 |
| PAR (1.5) | 1.53 ± 0.02 | 2.29 | 2.12 | 1.52 ± 0.01 | 1.61 | 1.23 |
| PAR (50) | 51.22 ± 0.55 | 2.14 | 2.44 | 50.74 ± 0.41 | 1.61 | 1.47 |
| PAR (100) | 100.56 ± 0.92 | 1.82 | 0.56 | 102.52 ± 1.04 | 2.02 | 2.52 |
| IBU (0.5) | 0.52 ± 0.01 | 5.20 | 4.69 | 0.52 ± 0.01 | 4.27 | 3.59 |
| IBU (1.5) | 1.60 ± 0.01 | 2.66 | 6.61 | 1.57 ± 0.02 | 3.34 | 4.52 |
| IBU (50) | 50.50 ±0.54 | 3.10 | 1.01 | 49.78 ± 0.38 | 2.21 | -0.43 |
| IBU (100) | 98.90 ± 0.73 | 0.73 | -1.10 | 102.73 ± 1.18 | 3.36 | 2.73 |
| SIMA (0.5) | 0.50 ± 0.01 | 5.54 | 0.95 | 0.50 ± 0.01 | 5.44 | -0.03 |
| SIMA (1.5) | 1.49 ± 0.03 | 4.29 | -0.60 | 1.50 ± 0.02 | 2.60 | -0.29 |
| SIMA (50) | 53.24 ± 0.93 | 3.96 | 6.48 | 51.68 ±0.80 | 3.51 | 3.36 |
| SIMA (100) | 100.44 ±0.89 | 2.01 | 0.44 | 102.42 ± 2.40 | 5.31 | 2.41 |
| OLA (0.5) | 0.51 ± 0.02 | 3.98 | 2.89 | 0.51 ± 0.02 | 4.84 | 2.65 |
| OLA (1.5) | 1.49 ± 0.04 | 2.98 | -0.59 | 1.49 ± 0.03 | 2.70 | -0.29 |
| OLA (50) | 49.51 ± 1.26 | 3.10 | -0.97 | 48.99 ±0.85 | 2.10 | -2.03 |
| OLA (100) | 96.87 ± 1.13 | 1.41 | -3.13 | 97.08 ±1.65 | 2.06 | -2.92 |
| SIM (0.5) | 0.45 ± 0.01 | 5.25 | -9.98 | 0.44 ± 0.01 | 5.00 | -11.55 |
| SIM (1.5) | 1.52 ±0.02 | 2.81 | 1.57 | 1.52 ± 0.02 | 2.60 | 1.08 |
| SIM (50) | 45.05 ± 0.96 | 0.96 | 3.97 | 46.07 ±0.76 | 3.07 | -7.86 |
| SIM (100) | 91.48 ± 0.77 | 1.57 | -8.52 | 92.76 ± 0.88 | 1.76 | 7.24 |
Selectivity and specificity
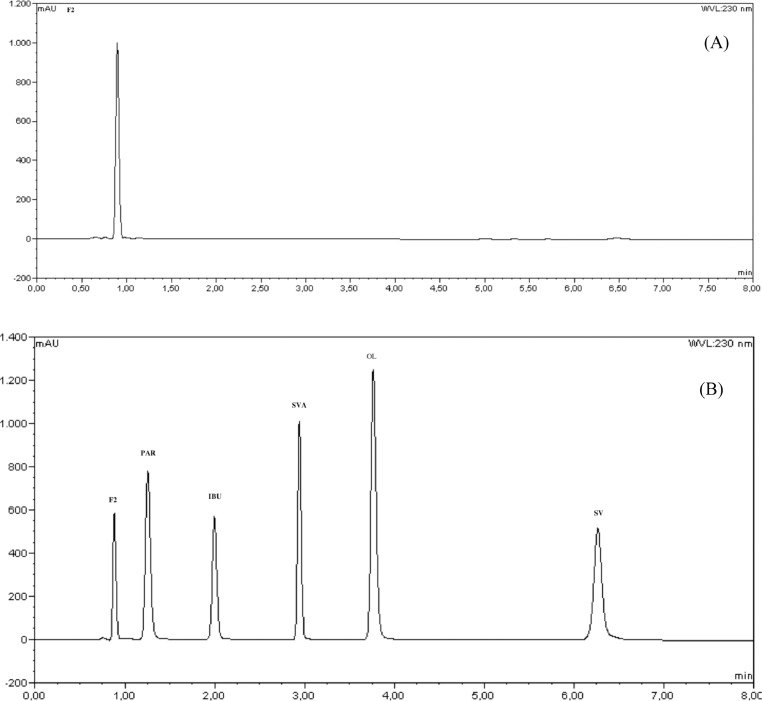
The selectivity of the method was evaluated by analysing standard solutions in the presence of the components of the culture medium (Fig. 3). The absence of any signal at the same elution time as the five analytes suggests that there were no matrix interferences.
Fig. 3.
Chromatograms (A) of f2 medium and (B) of the standards PAR, IBU, SIMA and SIM considered for the evaluation of the matrix effect.
Recovery
As can be seen from Table 6, all calculated mean recovery (trueness) are in the range of 81-109% being within in the acceptable recovery percentage of 80-110% for the analytes concentration of 1 ppm indicating the adequacy of simultaneous quantify the five analytes.
Table 6.
Percentage of recovery of PAR, IBU, SIMA, OLA and SIM from the microalgae culture medium (n=6).
| µg mL−1 | PAR |
IBU |
SIMA |
OLA |
SIM |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 50 | 100 | 1 | 50 | 100 | 1 | 50 | 100 | 1 | 50 | 100 | 1 | 50 | 100 | ||
| %Recovery | 102.5 | 98.0 | 98.7 | 99.8 | 96.9 | 95.7 | 99.6 | 108.7 | 108.9 | 80.8 | 93.0 | 101.3 | 104.7 | 93.0 | 101.3 | |
Method applicability
The method applicability was assessed by evaluating the efficiency of bioremediation, using the microalgae Nannochloropsis sp. For removal of the five pharmaceuticals from contaminated water. To quantify PAR, IBU, OLA, SIM and SIMA in contaminated water, microalgae cells were filtered, and the supernatant was analysed by RP-HPLC by the method developed in this work. Nannochloropsis sp. removed 11.33, 7.98 and 35.64 µg mL -1 of PAR, IBU and OLA, respectively. The results for SIM and SIMA were inconclusive; due to some level of precipitation of SIM and SIMA, it could not be established clearly whether the disappearance of both compounds was due to bioremediation or precipitation. However, some additional peaks appeared at different retention times but with the same wavelength of SIM. This could suggest metabolization of the pharmaceutical with the correspondent excreted metabolite. The present study demonstrates that the specie used for the removal of the pharmaceuticals is suitable for the bioremediation of PAR, IBU, OLA, SIM and SIMA in highly concentrated wastewaters.
Conclusions
An HPLC method was developed and optimized for the determination and quantification of PAR, IBU, OLA, SIM and SIMA during bioremediation using microalgae. The developed method was shown to be rapid, linear, precise, robust and accurate, and is suitable for the evaluation of microalgae bioremediation efficiency.
Author contributions
The conception and design of the study, all the calculations, analysis and interpretation of data has been done by TE. TE, AA and CP performed optimization of HPLC; AS provided resources; The manuscript was written by TE; HB and ACCP reviewed the calculations and the manuscript; The work was supervised by HB, ACCP and MC.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the Fundação para a Ciência e Tecnologia (FCT), Portuguese Agency for Scientific Research, for the PhD research grant to TE, SFRH/BD/81385/2011. We are grateful for funding from the Coimbra Chemistry Centre (CQC) which is supported by the FCT through the programmes UID/QUI/UI0313/2019 and COMPETE. The authors also thank Labesfal-Laboratórios Almiro, S.A. for kind donation of simvastatin.
Footnotes
Direct Submission or Co-Submission: Co-submissions are papers that have been submitted alongside an original research paper accepted for publication by another Elsevier journal Direct Submission
References
- 1.A. Reade, T. Quinn, J.S. Schreiber, Scientific and Policy assessment for addressing per-and polyfluoroalkyl substances (PFAS) in Drinking Water - MI 2019, (2019) 1–102.
- 2.U.S. Environmental Protection Agency, No Title, About Risk Assessment, 2018. (n.d.). https://www.epa.gov/risk/about-risk-assessment (accessed June 2019).
- 3.Sonune A., Ghate R. Developments in wastewater treatment methods. Desalination. 2004 doi: 10.1016/j.desal.2004.06.113. [DOI] [Google Scholar]
- 4.San Keskin N.O., Celebioglu A., Uyar T., Tekinay T. Microalgae immobilized by nanofibrous web for removal of reactive dyes from wastewater. Ind. Eng. Chem. Res. 2015 doi: 10.1021/acs.iecr.5b01033. [DOI] [Google Scholar]
- 5.Yadavalli R., Heggers G.R.V. Two stage treatment of dairy effluent using immobilized Chlorella pyrenoidosa. J. Environ. Heal. Sci. Eng. 2013 doi: 10.1186/2052-336x-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz-Marin A., Mendoza-Espinosa L.G., Stephenson T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour. Technol. 2010 doi: 10.1016/j.biortech.2009.02.076. [DOI] [PubMed] [Google Scholar]
- 7.http://www.clearaswater.com (accessedJune 2019).
- 8.https://algaerefinery.eu/#about (accessedJune 2019).
- 9.Encarnação T., Pais A.A.C.C., Campos M.G., Burrows H.D. Cyanobacteria and microalgae: A renewable source of bioactive compounds and other chemicals. Sci. Prog. 2015:98. doi: 10.3184/003685015X14298590596266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrie B., Barden R., Kasprzyk-Hordern B. A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015;72:3–27. doi: 10.1016/j.watres.2014.08.053. [DOI] [PubMed] [Google Scholar]
- 11.Karpinska J., Sokol A., Bernatowicz A., Szulecka A., Kotowska U. Studies on photodegradation of levomepromazine and olanzapine under simulated environmental conditions. Photochem. Photobiol. Sci. 2012;11:1575–1584. doi: 10.1039/c2pp25068c. [DOI] [PubMed] [Google Scholar]
- 12.Bercu J.P., Parke N.J., Fiori J.M., Meyerhoff R.D. Human health risk assessments for three neuropharmaceutical compounds in surface waters. Regul. Toxicol. Pharmacol. 2008;50:420–427. doi: 10.1016/j.yrtph.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Ottmar K.J., Colosi L.M., Smith J.A. Fate and transport of atorvastatin and simvastatin drugs during conventional wastewater treatment. Chemosphere. 2012 doi: 10.1016/j.chemosphere.2012.03.066. [DOI] [PubMed] [Google Scholar]
- 14.Franeta J.T., Agbaba D., Eric S., Pavkov S., Aleksic M., Vladimirov S. HPLC assay of acetylsalicylic acid, paracetamol, caffeine and phenobarbital in tablets. Farmaco. 2002 doi: 10.1016/S0014-827X(02)01265-X. [DOI] [PubMed] [Google Scholar]
- 15.El-Yazbi F.A., Amin O.A., El-Kimary E.I., Khamis E.F., Younis S.E. Simultaneous determination of methocarbamol and aspirin in presence of their pharmacopeial-related substances in combined tablets using novel HPLC-DAD method. Drug Dev. Ind. Pharm. 2019 doi: 10.1080/03639045.2018.1535603. [DOI] [PubMed] [Google Scholar]
- 16.Encarnação T., T C.Palito, Pais A.A.C.C., Valente A.J.M., Burrows H.D. Removal of Pharmaceuticals from Water by Free and Imobilised Microalgae. Molecules. 2020 doi: 10.3390/molecules25163639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vitorino C., Sousa J.J., Pais A.A.C.C. A rapid reversed-phase HPLC method for the simultaneous analysis of olanzapine and simvastatin in dual nanostructured lipid carriers. Anal. Methods. 2013;5:5058–5064. doi: 10.1039/c3ay40757h. [DOI] [Google Scholar]
- 18.FDA Guidance for Industry: bioanalytical method validation. US Dep. Heal. Hum. Serv. Food andDrug Adm. Cent. Drug Eval. andResearch Cent. Vet. Med. 2001 [Google Scholar]
- 19.ICH Guidance for industry: Q2B validation of analyticalprocedures, methodology. U.S. Dept. Heal. Human, Food Drug Adm. Cent. Drug Res. Cent. Biol. Eval. Res. 1996 Rockville, MD. [Google Scholar]
- 20.Eurachem, The Fitness for Purpose ofAnalytical Methods-A Laboratory Guide to Method Validation and Related Topics, (2014).
- 21.AOAC, Guidelines for collaborative study procedures to validate characteristics of a method ofanalysis, (2002). http://www.aoac.org.
- 22.Jahan S., Islam J., Begum R., Kayesh R., Rahman A. A Study of method development, validation, and forced degradation for simultaneous quantification of paracetamol and ibuprofen in pharmaceutical dosage form by RP-HPLC method. Anal. Chem. Insights. 2014;9:75–81. doi: 10.4137/Acici.S18651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Épshtein N.A. Validation of HPLC techniques for pharmaceutical analysis. Pharm. Chem. J. 2004 doi: 10.1023/b:phac.0000038422.27193.6c. [DOI] [Google Scholar]
- 24.A.M.T. Committee, Reviewer guidance - validation of chromatographic methods, 1994.
- 25.Wiggins D.E. System suitability in an optimized HPLC system. J. Liq. Chromatogr. 1991 doi: 10.1080/01483919108049375. [DOI] [Google Scholar]