Highlights
-
•
Higher nuclear ENO-1/ MBP-1 staining was detected in low-grade serous cancer cases compared to high-grade ones.
-
•
Nuclear ENO-1/ MBP-1 expression correlated with different Wnt signaling markers in EOC.
-
•
High nuclear ENO-1/ MBP-1 expression was associated with improved overall survival in epithelial ovarian cancer (EOC).
-
•
Nuclear ENO-1/ MBP-1 may be a promising new therapeutic target in EOC.
Keywords: Enolase-1, MBP-1, Immunohistochemistry, Wnt signaling, Ovarian cancer
Abstract
Background:
Enolase-1, primarily known for its role in glucose metabolism, is overexpressed in various cancer entities. In contrast its alternative spliced nuclear isoform MBP-1 acts as a tumor suppressor. The aim of this study is to analyze the prognostic impact of Enolase-1/ MBP-1 and its functional significance in epithelial ovarian cancer (EOC).
Methods:
By immunohistochemistry, Enolase-1 staining was examined in 156 EOC samples. Evaluation of Enolase-1 staining was conducted in the nucleus and the cytoplasm using the semi-quantitative immunoreactive score. Expression levels were correlated with clinical and pathological parameters as well as with overall survival to assess for prognostic impact.
Results:
Cytoplasmic and nuclear Enolase-1 expression did not show a significant difference between the histological subtypes (p = 0.1). High nuclear Enolase-1/ MBP-1 staining negativly correlated with the tumor grading (p<0.001; Cc= −0.318). Cytoplasmic Enolase-1 did not correlate with clinicopathological data. Higher nuclear Enolase-1/ MBP-1 staining was detected in low-grade serous cancer cases compared to high-grade ones (median IRS 3 (range 0–8) vs. median IRS 2 (range 0–4), p<0.001). Nuclear Enolase-1/ MBP-1 expression correlated with the Wnt signaling markers membranous beta-catenin (p = 0.007; Cc=0.235), serine residue 9-phosphorylated glycogen synthase kinase 3 beta (p<0.001; Cc=0.341) and snail/slug (p = 0.004; Cc= −0.257). High nuclear Enolase-1/ MBP-1 expression was associated with improved overall survival (88.6 vs. 33.1 months, median; p = 0.013).
Conclusion:
Additional knowledge of Enolase-1/ MBP-1 as a biomarker and its interactions within the Wnt signaling pathway and epithelial–mesenchymal transition potentially improve the prognosis of therapeutic approaches in EOC.
Introduction
Epithelial ovarian cancer (EOC) is one of the most lethal tumor entities [47]. Lack of adequate screening methods and rising resistances towards chemotherapy over the clinical course further contribute to the relatively low 5-year survival at around 45% [4,47]. Histologically EOC is classified into five main subtypes: high-grade serous, low-grade serous, mucinous, endometrioid, and clear-cell histology [43]. Standard treatment for advanced EOC consists of primary cytoreductive surgery, followed by platinum-based combination chemotherapy followed by targeted therapies like the anti-angiogenic antibody bevacizumab or poly-ADP-ribose-polymerase inhibitors [11]. To date, most reliable prognostic factors include the presence of residual disease after initial debulking surgery, the International Federation of Gynecology and Obstetrics (FIGO) –stage, ascites volume, patient age, and histological subtype [14,55,1,17]. However, widely accepted prognostic biomarkers are missing. Taking the heterogeneity of ovarian cancer into account appears crucial for developing new prognostic and therapeutic strategies.
Enolase, a glycolytic metalloenzyme, catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate and therefore plays a pivotal role in glycolysis and gluconeogenesis. Enolase-1/ alpha-Enolase (ENO-1) is one of three different isoforms and ubiquitously expressed in the cytoplasm of most human tissue including the ovaries [42,16]. Beyond its cellular housekeeping function in glucose metabolism, increasing evidence suggests that ENO-1 is an important mediator in pathological conditions like infection, inflammation, autoimmunity and malignancy [2,29,39,35,5,34,31,32,21]. Hence, ENO-1 is overexpressed in a subset of cancers – a fact that is partially described by the Warburg effect, an adaptive response of anaerobic glycolysis in tumor cells to hypoxia [54,8].
In contrast, myc promoter-binding protein 1 (MBP-1) as alternatively spliced nuclear isoform of the ENO1 gene binds and suppresses the activity of c-myc transcription factor, a master regulator of cellular functions, and therefore acts as a tumor suppressor [50,13]. Thus, the dual function of cytoplasmic ENO-1 and nuclear MBP-1 is of great interest for a better understanding of ovarian cancer biology.
The expression analysis of ENO-1/ MBP-1 in different histological subtypes of EOC and its correlation with survival was the primary aim of the current study which is the first of its kind.
Material and methods
Patients and specimens
156 EOC samples of patients that underwent surgery between 1990 and 2002 at the Department of Obstetrics and Gynecology, Ludwig Maximilian University in Munich, Germany, were analyzed (Table 1). The clinical data were received from the patient's charts, whereas the follow-up data derived from the Munich Cancer Registry (MCR). Only patients with pathologically confirmed EOC were included, while benign tumors, just as borderline tumors were excluded. Also, none of the patients had neoadjuvant chemotherapy. Previously, other pathological parameters were investigated in the same patients’ collective, which enables correlation analysis. After the samples had been formalin-fixed and paraffin-embedded (FFPE), they were evaluated by a specialized pathologist at the department of Pathology, Ludwig Maximilian University, who classified them into histological subtypes (serous (n = 110), endometrioid (n = 21), mucinous (n = 13), clear-cell (n = 12)) and rated the tumor grading. Serous ovarian cancer samples were divided into low and high grading. Endometrioid ovarian cancer was graded according to G1 to G3. For mucinous carcinoma, there is no WHO classification; however, the subtype is often classified into G1 to G3. Clear cell cancer was always categorized as G3. Staging was performed using the FIGO (2014) and TNM classification. I (n = 35), II (n = 10,) III (n = 103), and IV (n = 3). Data on primary tumor extension was available in 155 cases: T1 (n = 40), T2 (n = 18), T3 (n = 93), T4 (n = 4) and on lymph node involvement in 95 cases: N0 (n = 43), N1 (n = 52). In nine cases the data on distant metastasis was available: M0 (n = 3), M1 (n = 6). Information to FIGO stage and grading are missing in 5 and 9 cases respectively.
Table 1.
Clinicopathologic characteristics of the ovarian cancer patients.
| Clinicopathologic parameters | n | Percentage (%) |
|---|---|---|
| Histology | ||
| Serous | 110 | 70.5 |
| Clear cell | 12 | 7.7 |
| Endometrioid | 21 | 13.5 |
| Mucinous | 13 | 8.3 |
| Primary tumor expansion | ||
| TX | 1 | 0.6 |
| T1 | 40 | 25.6 |
| T2 | 18 | 11.5 |
| T3 | 97 | 62.3 |
| Nodal status | ||
| pNX | 61 | 39.1 |
| pN0 | 43 | 27.6 |
| pN1 | 52 | 33.3 |
| Distant Metastasis | ||
| pMX | 147 | 94.2 |
| pM0 | 3 | 1.9 |
| pM1 | 6 | 3.8 |
| Grading Serous | ||
| Low | 24 | 21.8 |
| High | 80 | 72.7 |
| Grading Endometrioid | ||
| G1 | 6 | 28.6 |
| G2 | 5 | 23.8 |
| G3 | 8 | 38.1 |
| Grading Mucinous | ||
| G1 | 6 | 46.2 |
| G2 | 6 | 46.2 |
| G3 | 0 | 0 |
| Grading Clear cell | ||
| G3 | 12 | 100.0 |
| FIGO | ||
| I | 35 | 22.4 |
| II | 10 | 6.4 |
| III | 103 | 66.0 |
| IV | 3 | 1.9 |
| Age | ||
| ≤ 60 years | 83 | 53.2 |
| > 60 years | 73 | 46.8 |
Ethical approval
This study was approved by the Ethics Committee of the Ludwig-Maximilians-University, Munich, Germany (approval number 227-09, 18-392 and 19-972). All tissue samples used for this study were obtained from leftover material from the archives of the Department of Gynecology and Obstetrics, Ludwig-Maximilians-University, Munich, Germany, initially used for pathological diagnostics. The diagnostic procedures were completed before the current study was performed. During all experimental and statistical analysis, the observers were fully blinded to patient's data. All experiments were performed according to the standards of the Declaration of Helsinki (1975). As per declaration of our ethics committee no written informed consent of the participants or permission to publish is needed given the circumstances described above.
Immunohistochemistry
The formalin-fixed and paraffin-embedded ovarian cancer tissue samples were dewaxed in xylene for 20 min, before adding 100% ethanol to completely remove the Xylol. Unspecific color responses were avoided by blocking the endogenous peroxidase with 3% H2O2 in methanol, followed by rehydrating it in 70%, then 50% ethanol. Afterwards the slides were placed in a pressure cooker for 10 min, using sodium citrate buffer (pH=6; 0.1 M citric acid and 0.1 M sodium citrate). After cooling down, this was followed by washing the samples first in distilled water, then in phosphate buffered saline (PBS) twice. After preprocessing, the slides were incubated in a blocking solution (ZytoChem Plus HRP Polymer System, Berlin, Germany, POLHRP-100) for 5 min to prevent an unspecific staining reaction. This was followed by a 16-hour incubation overnight at 4 °C with the primary antibodies: anti-ENO-1, mouse IgG, monoclonal, Abcam, ab190365 at a 1:3000 dilution; anti-beta-catenin, rabbit IgG, polyclonal, Novus Biologicals, NLS2231 at a 1:100 dilution, anti- GSK-3β[pS9], rabbit IgG, polyclonal, Acris, SP4594P at a 1:800 dilution and anti-snail/slug, rabbit IgG, polyclonal, Lifespan Biosciences, LS92467 at a 1:600 dilution – then washed in PBS twice. Next step was the application of reagent 2 (ZytoChem Plus HRP Polymer System, Berlin, Germany, POLHRP-100), consisting of a corresponding biotinylated secondary anti-mouse/rabbit IgG antibody and the associated avidin-biotin-peroxidase complex, for 20 min. For the visualization reaction 3,3 Diaminobenzidine (DAB) and a substrate buffer (Liquid DAB and Substrate Chromogen System, Dako, Munich, Germany, K3468) was used for 30 min, followed by distilled water, to stop the reaction. After counterstaining the slides with Mayer's acidic hematoxylin (Waldeck-Chroma, Münster, Germany, catalog-number 2E-038) for two minutes, they were dehydrated in an ascending series of alcohol (70%, 96%, 100%), brightened by adding xylol and covered. Negative and positive controls were used to assess the specificity of the immunoreactions. Negative controls were performed in kidney and placental tissue by replacement of the primary antibodies by species-specific (mouse/rabbit) isotype control antibodies (Dako, Glostrup, Denmark). For positive controls, placental, fallopian tube, vulva and colon tissues were used. (Supplementary Fig. 1)
Staining evaluation
All EOC specimens were examined with a Leitz photomicroscope (Wetzlar, Germany) and specific ENO-1 immunohistochemically staining reaction was observed in the cytoplasm and nucleus of the cells. The intensity and distribution pattern of ENO-1 staining was rated using the semi-quantitative immunoreactive score (IRS) [45]. To obtain the IRS result, the staining intensity (0=no, 1=weak, 2=moderate, and 3=strong staining) and the percentage of positive stained cells (0=no staining, 1=<10% of the cells, 2 = 11–50% of the cells, 3 = 51–80% of the cells and 4=>81%) were multiplied. Cut-off points for the IRS were selected for the ENO-1 staining considering the distribution pattern of IRS in the collective. Nuclear ENO-1 staining was regarded as low with IRS 0–2 and as high with IRS≥3. Staining evaluation of the Wnt signaling markers was equally performed.
Statistical analysis
IBM SPSS Statistics, version 25.0 (IBM Corporation, Armonk, NY, USA) was used for collecting and analysing all data. To compare the distribution of more than two independent samples Kruskal–Wallis H-test was used [30]. Bivariate correlations between clinical and pathological data have been calculated with Pearson's chi-squared test and Spearman's analysis [49]. Overall-survival was compared with Kaplan–Meier curves and log-rank testing was used to detect differences in patients’ overall survival times. To identify an appropriate cut-off, the ROC curve was drawn. It is considered as one of the most reliable methods for cut-off point selection. In this context, the ROC curve is a plot representing sensitivity on the y-axis and (1-specificity) on the x-axis [37]. Consecutively Youden index, defined as the maximum (sensitivity+specificity-1) [57], was used to find the optimal cut-off maximizing the sum of sensitivity and specificity [19,41]. For multivariate analyses, the cox regression was performed. For all analysis a P-value of less than 0.05 was considered to be statistically significant.
Results
Nuclear ENO-1/ MBP-1 expression correlates with clinicopathologic characteristics
The clinicopathologic characteristics of the analyzed ovarian cancer patients are listed in Table 1. Out of 156 successfully stained ovarian cancer specimens, 142 (91%) showed positive cytoplasmic and nuclear ENO-1/ MBP-1 expression. Median (range) immunoreactivity scores (IRS) for ENO-1/ MBP-1 in cytoplasm and nucleus were 6 (0,8) and 2 (0,8), respectively.
Cytoplasmic and nuclear ENO-1/ MBP-1 expression did not show a significant difference between the histological subtypes (p = 0.1) (Fig. 1).
Fig. 1.
Detection of Enolase-1 with immunohistochemistry. Notes: (A) Enolase-1 staining in ovarian cancer with serous, (B) clear cell, (C) endometrioid, (D) and mucinous histology. (E) Nuclear and (G) cytoplasmic Enolase-1 expression in histological subtypes. Cytoplasmic and nuclear Enolase-1 expression did not show a significant difference between the histological subtypes (p = 0.1). Images representative of 3 independent experiments. scale bar = 100 μm.
ENO-1/ MBP-1 expression displayed correlations to clinicopathologic characteristics (Table 2). A negative correlation was observed between high nuclear ENO-1/ MBP-1 staining and grading (p<0.001; Cc= −0.318). In the cytoplasm, ENO-1 does not correlate with clinicopathological data. Nuclear and cytoplamic expression correllated to each other (p = 0.001; Cc= 0.278).
Table 2.
Correlation between cytoplasmic/ nuclear ENO-1/ MBP-1 expression and clinicopathological data.
| Cytoplasmic ENO-1 expression |
Nuclear ENO-1/MBP-1 expression |
|||
|---|---|---|---|---|
| Variables | p | Correlation coefficient | p | Correlation coefficient |
| Age | 0.429 | 0.067 | 0.393 | −0.072 |
| Histology | 0.379 | 0.074 | 0.819 | 0.019 |
| FIGO | 0.925 | −0.008 | 0.482 | −0.061 |
| Grading | 0.444 | 0.067 | <0.001 | −0.318** |
Clinicopathologic data and ENO-1 expresssion were correlated to each other using Pearson's chi-squared test. Significant correlations are indicated by asterisks (*: p<0.05; **: p<0.01).
p: two-tailed significance.
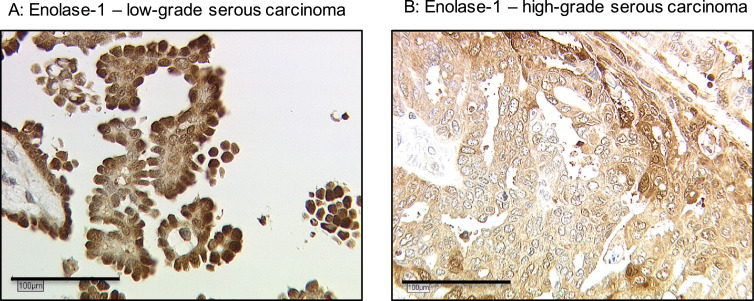
Higher nuclear ENO-1/ MBP-1 staining was detected in low-grade serous cancer cases compared to high-grade ones (median IRS 3 (range 0–8) vs. median IRS 2 (range 0–4), p<0.001) (Fig. 2). The distribution pattern of nuclear ENO-1/ MBP-1is shown in Supplementary Table 1.
Fig. 2.
Detection of nuclear Enolase-1/ MBP-1 in low/high-grade serous carcinoma. Notes: Higher nuclear ENO-1/ MBP-1 staining was detected in (A) low-grade serous carcinoma compared to (B) high-grade serous carcinoma (median IRS 3 (range 0–8) vs. median IRS 2 (range 0–4), p<0.001).
Nuclear ENO-1/ MBP-1 expression correlates with Wnt signaling markers
We then analyzed the correlation between nuclear ENO-1/ MBP-1 expression and Wnt signaling markers in the same ovarian cancer cohort (Table 3). A positive correlation was detected to the expression of membranous beta-catenin (p = 0.007; Cc=0.235) and serine residue 9-phosphorylated glycogen synthase kinase 3 beta (GSK-3β[pS9]) (p<0.001; Cc=0.341), a negative correlation to the transcription factor snail/slug (p = 0.004; Cc=−0.257).
Table 3.
Correlation analysis between nuclear ENO-1/ MBP-1 expression and different Wnt signaling markers.
| Staining | Nuclear ENO-1/ MBP-1 | Membranous beta-catenin | GSK-3β[pS9] | Snail/slug |
|---|---|---|---|---|
| Nuclear ENO-1/ MBP-1 | ||||
| Cc | 1.000 | 0.235 | 0.341 | −0.257 |
| p | – | 0.007* | <0.001* | 0.004* |
| n | 142 | 129 | 112 | 126 |
| Membranous beta-catenin | ||||
| Cc | 0.235 | 1.000 | 0.393 | −0.390 |
| p | 0.007* | – | <0.001* | <0.001* |
| n | 129 | 147 | 126 | 134 |
| GSK-3β[pS9] | ||||
| Cc | 0.341 | 0.393 | 1.000 | −0.076 |
| p | <0.001* | <0.001* | – | 0.419 |
| n | 112 | 126 | 140 | 116 |
| snail/slug | ||||
| Cc | −0.257 | −0.390 | −0.076 | 1.000 |
| p | 0.004* | <0.001* | 0.419 | – |
| n | 126 | 134 | 116 | 145 |
IRS of nuclear ENO-1/ MBP-1 was correlated to different Wnt signaling markers using Spearman's correlation analysis. Significant correlations are indicated by asterisks (*: p<0.05).
Cc: correlation coefficient, p: two-tailed significance, n: number of patients.
High nuclear ENO-1/ MBP-1 expression is associated with improved overall survival
The median age of the patients was 58.7 (standard deviation [SD] 31.4) years, with a range of 31–88 years. The median follow-up OS of the EOC patients was 34.4 (SD 57.8) months. Nuclear ENO-1/ MBP-1 expression was significantly associated with a longer OS in the whole cohort (Fig. 3, median 88.6 vs. 33.1 months; p = 0.013). Association of high MBP-1 expression with OS in the different EOC subtypes was not statistically significant. (Supplementary Fig. 2)
Fig. 3.
Kaplan–Meier estimate of nuclear ENO-1/ MBP-1. Notes: High ENO-1/ MBP-1 expression (IRS≥3) was associated with improved overall survival (88.6 vs. 33.1 months, median; p = 0.013). Censoring events have been marked in the graphs.
Clinicopathological parameters as independent prognostic factors
A multivariate cox-regression analysis was performed to detect which parameters were independent prognostic factors for overall survival in the present cohort. In this analysis, patients’ age (p<0.001), FIGO stage (p = 0.002) and serous grading (p<0.001) were independent factors for overall survival. High nuclear ENO-1/ MBP-1 expression (p = 0.676), however, was not confirmed as an independent prognostic factor (Table 4).
Table 4.
Multivariate analysis of the analyzed ovarian cancer patients (n = 156).
| Covariate | Hazard ratio (95% CI) | p-value |
|---|---|---|
| Patient's Age | 1.009* (1.004–1.014) | <0.001 |
| FIGO (I, II vs. III, IV) | 2.723* (1.434–5.170) | 0.002 |
| Grading serous (low vs. high) | 2.845* (1.590–5.089) | <0.001 |
| nuclear ENO-1/ MBP-1 (high vs. low) | 0.885 (0.500–1.569) | 0.676 |
A multivariate Cox regression model was established to investigate independency of prognostic factors.
Significant independent factors are indicated by asterisks (*: p<0.05). CI: confidence interval.
Discussion
This present study focused on ENO-1 expression in the cytoplasm and nucleus of different histologic subtypes of EOC and its correlation with clinicopathological parameters and different Wnt signaling markers. Whereas cytoplasmic ENO-1 expression has no impact in our EOC cohort, nuclear ENO-1/ MBP-1 was significantly higher expressed in patients with low-grade serous histology and lower expressed in high-grade serous histology, respectively.
Nuclear ENO-1/ MBP-1 expression showed correlations to the Wnt signaling markers membranous beta-catenin, GSK-3β[pS9] and the transcriptionfactor snail/slug. Moreover patients with an increased nuclear ENO-1/MBP-1 expression confirmed to have a significantly improved OS. Our results suggest a functional role of MBP-1 in ovarian cancerogenesis, which merits further investigations.
In recent years, increasing evidence suggests that ENO-1`s molecular function differs depending on its cellular localization [7,15,16]. The primary catalytic function of the cytoplasmic glycolytic enzyme ENO-1 is essential for glucose metabolism [40]. Dysregulation of this physiological process in context with the Warburg effect is a relevant condition in different kinds of cancer including glioma, lymphoma, thyroid carcinoma, lung cancer, colorectal cancer, gastric cancer, pancreatic cancer, hepatocellular carcinoma, endometrial cancer and breast cancer [51,53,22,9,48,20,60,59,44,58]. Overexpression of ENO-1 in these malignant tumors promotes cell proliferation, migration and invasion through FAK-mediated PI3K/AKT pathway and influencing plasminogen signaling [2,36,20,44].
Alternative splicing of the ENO1 gene results in a 36 kDa nuclear isoform, called myc promoter-binding protein 1 (MBP-1) [50]. This shorter gene product binds to the c-myc promoter and prevents the formation of an active transcription initiaton complex [50,18]. Thus the proto-oncogene c-myc, a key transcriptional factor influencing cell proliferation, migration, differentiation and apoptosis, is negatively regulated in the context of a tumor suppressor [13].
Indeed studies in gastric, prostate and breast cancer revealed that elevated levels of MBP-1 reduce proliferation, migration and invasion in respective cancer cells [24,26,28]. In addition MBP-1 seems to be a regulator in epithelial–mesenchymal transition (EMT) [26]. Immunohistochemical analyses in breast cancer showed that MBP-1 was highly downregulated compared to benign breast tissue and correlated with better progression free survival [33,6].
The regulating mechanism of ENO-1/ MBP-1 ratio is not well understood so far. Present studies revealed that ENO-1 translation is mostly triggered by hypoxia whereas MBP-1 translation by endoplasmic reticulum stress. In addition cellular glucose concentration may influence ENO-1/ MBP-1 ratio: whereas breast cancer cells under normal or higher concentrations show lower MBP-1 levels, the contrary effect is observed under lower glucose concentrations [46].
Our present study distinguished the expression profile of ENO-1/ MBP-1 in ovarian cancer for the first time. Up to date only few in-vitro studies in ovarian cancer exist. Former analyses showed an association of higher ENO-1 expression and chemotherapy resistance in ovarian cancer cells and suggested ENO-1 as putative target to overcome these drug resistance [23,52,12]. In addition ENO-1 expression was elevated in brain metastases of ovarian cancer compared with the primary tumor tissue [56]. No previous studies examined nuclear ENO-1/ MBP-1 in ovarian cancer before. Our expression analysis of nuclear ENO-1/ MBP-1 with its beneficial survival impact is in line with the above described results in other cancer entities. The putative positive impact of nuclear ENO-1/ MBP-1 on ovarian cancer biology is supported by the fact that nuclear ENO-1/ MBP-1 expression is reduced in high-grade serous ovarian cancer compared to the low-grade cases.
As mentioned before MBP-1 reduces EMT, an essential process in the initation of metastasis in cancer progression [25]. The Wnt signaling pathway, which is activated in EOC and therefore plays a pivotal role in cancerogenesis, is one of the major signaling pathways thought to be involved in EMT [3,38]. The correlation analysis between nuclear ENO-1/ MBP-1 expression and the Wnt signaling markers membranous beta-catenin, GSK-3β[pS9] and snail/slug revealed a putative association of nuclear ENO-1/ MBP-1 with an inactivated Wnt signaling and EMT. Nuclear ENO-1/ MBP-1 positively correlated to membranous beta-catenin and inactivated GSK-3β[pS9], which represent an inactivated Wnt signaling. In contrast nuclear ENO-1/ MBP-1 negatively correlated to snail/slug, an important transcription factor in the activated Wnt signaling. Thus our findings support nuclear ENO-1/ MBP-1 as possible new target involved in the Wnt signaling and EMT. In vitro studies showed that ENOblock, a unique small molecule inhibitor of the non-glycolytic functions of enolase, caused higher expression of MBP-1 linked to c-myc repression and negativly influenced cancer cell migration and invasion [27,10]. Further studies are needed to verify our findings and prove ENOblock as putative new therapeutical approach in EOC.
Taken together, we indicate that nuclear ENO-1/ MBP-1 may be a promising new target in EOC so that clinical implications should be further addressed in future research.
CRediT authorship contribution statement
Bastian Czogalla: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Project administration, Software, Visualization, Writing - original draft. Alexandra Partenheimer: Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing - review & editing. Susann Badmann: Formal analysis, Writing - review & editing. Elisa Schmoeckel: Formal analysis, Validation, Writing - review & editing. Doris Mayr: Formal analysis, Supervision, Validation, Writing - review & editing. Thomas Kolben: Writing - review & editing. Susanne Beyer: Writing - review & editing. Anna Hester: Writing - review & editing. Alexander Burges: Writing - review & editing. Sven Mahner: Supervision, Writing - review & editing. Udo Jeschke: Conceptualization, Methodology, Project administration, Supervision, Writing - review & editing. Fabian Trillsch: Conceptualization, Project administration, Supervision, Writing - original draft.
Declaration of Competing Interest
Thomas Kolben holds stock of Roche AG and his relative is employed at Roche AG. Anna Hester has received a research grant from the “Walter Schulz” foundation and advisory board, speech honoraria and travel expenses from Roche and Pfizer. Alexander Burges has received advisory board and honoraria from AstraZeneca, Clovis, Roche and Tesaro. Research support, advisory board, honoraria, and travel expenses from AstraZeneca, Clovis, Medac, MSD, Novartis, PharmaMar, Roche, Sensor Kinesis, Tesaro, Teva have been received by Sven Mahner and from AstraZeneca, Medac, PharmaMar, Roche, Tesaro by Fabian Trillsch. All other authors declare no conflict of interest.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors." in main text as the funding statement
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.100910.
Appendix. Supplementary materials
References
- 1.Aletti G.D., Gostout B.S., Podratz K.C., Cliby W.A. Ovarian cancer surgical resectability: relative impact of disease, patient status, and surgeon. Gynecol. Oncol. 2006;100:33–37. doi: 10.1016/j.ygyno.2005.07.123. [DOI] [PubMed] [Google Scholar]
- 2.Altenberg B., Greulich K.O. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–1020. doi: 10.1016/j.ygeno.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Arend R.C., Londoño-Joshi A.I., Straughn J.M., Jr., Buchsbaum D.J. The Wnt/β-catenin pathway in ovarian cancer: a review. Gynecol. Oncol. 2013;131:772–779. doi: 10.1016/j.ygyno.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin L.A., Huang B., Miller R.W. Ten-year relative survival for epithelial ovarian cancer. Obstet. Gynecol. 2012;120:612–618. doi: 10.1097/AOG.0b013e318264f794. [DOI] [PubMed] [Google Scholar]
- 5.Bergmann S., Schoenen H., Hammerschmidt S. The interaction between bacterial Enolase and plasminogen promotes adherence of streptococcus pneumoniae to epithelial and endothelial cells. Int. J. Med. Microbiol. 2013;303:452–462. doi: 10.1016/j.ijmm.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Cancemi P., Buttacavoli M., Roz E., Feo S. Expression of Alpha-Enolase (ENO1), Myc Promoter-Binding Protein-1 (MBP-1) and Matrix Metalloproteinases (MMP-2 and MMP-9) reflect the nature and aggressiveness of breast tumors. Int. J. Mol. Sci. 2019;20:3952. doi: 10.3390/ijms20163952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capello M., Ferri-Borgogno S., Cappello P., Novelli F. α-Enolase: a promising therapeutic and diagnostic tumor target. FEBS J. 2011;278:1064–1074. doi: 10.1111/j.1742-4658.2011.08025.x. [DOI] [PubMed] [Google Scholar]
- 8.Capello M., Ferri-Borgogno S., Riganti C. Targeting the Warburg effect in cancer cells through ENO1 knockdown rescues oxidative phosphorylation and induces growth arrest. Oncotarget. 2016;7:5598–5612. doi: 10.18632/oncotarget.6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S., Duan G., Zhang R., Fan Q. Helicobacter pylori cytotoxin-associated gene a protein upregulates α-enolase expression via Src/MEK/ERK pathway: implication for progression of gastric cancer. Int. J. Oncol. 2014;45:764–770. doi: 10.3892/ijo.2014.2444. [DOI] [PubMed] [Google Scholar]
- 10.Cho H., Um J., Lee J.-.H. ENOblock, a unique small molecule inhibitor of the non-glycolytic functions of enolase, alleviates the symptoms of type 2 diabetes. Sci. Rep. 2017;7:44186. doi: 10.1038/srep44186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombo N., Sessa C., du Bois A. ESMO–ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019;30:672–705. doi: 10.1093/annonc/mdz062. [DOI] [PubMed] [Google Scholar]
- 12.Cruz I.N., Coley H.M., Kramer H.B. Proteomics analysis of ovarian cancer cell lines and tissues reveals drug resistance-associated proteins. Cancer Genom. Proteom. 2017;14:35–51. doi: 10.21873/cgp.20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dang C.V. MYC on the path to cancer. Cell. 2012;149:22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dembo A.J., Davy M., Stenwig A.E. Prognostic factors in patients with stage I epithelial ovarian cancer. Obstet. Gynecol. 1990;75:263—273. [PubMed] [Google Scholar]
- 15.Díaz-Ramos A., Roig-Borrellas A., García-Melero A., López-Alemany R. α-Enolase, a multifunctional protein: its role on pathophysiological situations. J. Biomed. Biotechnol. 2012;2012 doi: 10.1155/2012/156795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Didiasova M., Schaefer L., Wygrecka M. When place matters: shuttling of Enolase-1 across cellular compartments. Front. Cell Dev. Biol. 2019;7:61. doi: 10.3389/fcell.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.du Bois A., Reuss A., Pujade-Lauraine E. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials. Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 18.Feo S., Arcuri D., Piddini E. ENO1 gene product binds to the c-myc promoter and acts as a transcriptional repressor: relationship with Myc promoter-binding protein 1 (MBP-1) FEBS Lett. 2000;473:47–52. doi: 10.1016/S0014-5793(00)01494-0. [DOI] [PubMed] [Google Scholar]
- 19.Fluss R., Faraggi D., Reiser B. Estimation of the Youden index and its associated cutoff point. Biometrical J. 2005;47:458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 20.Fu Q.-.F., Liu Y., Fan Y. Alpha-enolase promotes cell glycolysis, growth, migration, and invasion in non-small cell lung cancer through FAK-mediated PI3K/AKT pathway. J. Hematol. Oncol. 2015;8:22. doi: 10.1186/s13045-015-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funk J., Schaarschmidt B., Slesiona S. The glycolytic enzyme enolase represents a plasminogen-binding protein on the surface of a wide variety of medically important fungal species. Int. J. Med. Microbiol. 2016;306:59–68. doi: 10.1016/j.ijmm.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Gao J., Zhao R., Xue Y. Role of enolase-1 in response to hypoxia in breast cancer: exploring the mechanisms of action. Oncol. Rep. 2013;29:1322–1332. doi: 10.3892/or.2013.2269. [DOI] [PubMed] [Google Scholar]
- 23.Georges E., Bonneau A.-.M., Prinos P. RNAi-mediated knockdown of α-enolase increases the sensitivity of tumor cells to antitubulin chemotherapeutics. Int. J. Biochem. Mol. Biol. 2011;2:303–308. [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh A.K., Steele R., Ray R.B. c-myc Promoter-binding protein 1 (MBP-1) regulates prostate cancer cell growth by inhibiting MAPK pathway. J. Biol. Chem. 2005;280:14325–14330. doi: 10.1074/jbc.M413313200. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Hsu K.-.W., Hsieh R.-.H., Wu C.-.W. MBP-1 suppresses growth and metastasis of gastric cancer cells through COX-2. Mol. Biol. Cell. 2009;20:5127–5137. doi: 10.1091/mbc.e09-05-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung D.-.W., Kim W.-.H., Park S.-.H. A Unique small molecule inhibitor of Enolase clarifies its role in fundamental biological processes. ACS Chem. Biol. 2013;8:1271–1282. doi: 10.1021/cb300687k. [DOI] [PubMed] [Google Scholar]
- 28.Kanda T., Raychoudhuri A., Steele R. MBP-1 inhibits breast cancer growth and metastasis in immunocompetent mice. Cancer Res. 2009;69:9354. doi: 10.1158/0008-5472.CAN-09-2974. LP-9359. [DOI] [PubMed] [Google Scholar]
- 29.Kinloch A., Tatzer V., Wait R. Identification of citrullinated alpha-enolase as a candidate autoantigen in rheumatoid arthritis. Arthritis Res. Ther. 2005;7:R1421–R1429. doi: 10.1186/ar1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruskal W.H., Wallis W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952;47:583–621. doi: 10.2307/2280779. [DOI] [Google Scholar]
- 31.Li J., Zhang H., Xie M. NSE, a potential biomarker, is closely connected to diabetic peripheral neuropathy. Diabetes Care. 2013;36:3405–3410. doi: 10.2337/dc13-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M., Li J., Wang J. Serum level of anti-α-enolase antibody in untreated systemic lupus erythematosus patients correlates with 24-hour urine protein and d-dimer. Lupus. 2017;27:139–142. doi: 10.1177/0961203317721752. [DOI] [PubMed] [Google Scholar]
- 33.Lo Presti M., Ferro A., Contino F. Myc promoter-binding protein-1 (MBP-1) is a novel potential prognostic marker in invasive ductal breast carcinoma. PLoS One. 2010;5:e12961. doi: 10.1371/journal.pone.0012961. e12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehra S., Walker J., Patterson K., Fritzler M.J. Autoantibodies in systemic sclerosis. Autoimmun. Rev. 2013;12:340–354. doi: 10.1016/j.autrev.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Montes A., Dieguez-Gonzalez R., Perez-Pampin E. Particular association of clinical and genetic features with autoimmunity to citrullinated α-enolase in rheumatoid arthritis. Arthritis Rheum. 2011;63:654–661. doi: 10.1002/art.30186. [DOI] [PubMed] [Google Scholar]
- 36.Moreno-Sánchez R., Rodríguez-Enríquez S., Marín-Hernández A., Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 37.Nakas C.T., Alonzo T.A., Yiannoutsos C.T. Accuracy and cut-off point selection in three-class classification problems using a generalization of the Youden index. Stat. Med. 2010;29:2946–2955. doi: 10.1002/sim.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nusse R., Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 39.Owen J.B., Di Domenico F., Sultana R. Proteomics-determined differences in the concanavalin-A-fractionated proteome of hippocampus and inferior parietal lobule in subjects with Alzheimer's disease and mild cognitive impairment: implications for progression of AD. J. Proteome Res. 2009;8:471–482. doi: 10.1021/pr800667a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pancholi V. Multifunctional α-enolase: its role in diseases. Cell. Mol. Life Sci. C. 2001;58:902–920. doi: 10.1007/PL00000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perkins N.J., Schisterman E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am. J. Epidemiol. 2006;163:670–675. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petrak J., Ivanek R., Toman O. Déjà vu in proteomics. A hit parade of repeatedly identified differentially expressed proteins. Proteomics. 2008;8:1744–1749. doi: 10.1002/pmic.200700919. [DOI] [PubMed] [Google Scholar]
- 43.Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460:237–249. doi: 10.1007/s00428-012-1203-5. [DOI] [PubMed] [Google Scholar]
- 44.Principe M., Borgoni S., Cascione M. Alpha-enolase (ENO1) controls alpha v/beta 3 integrin expression and regulates pancreatic cancer adhesion, invasion, and metastasis. J. Hematol. Oncol. 2017;10:16. doi: 10.1186/s13045-016-0385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Remmele W., Stegner H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;1987(8):138–140. [PubMed] [Google Scholar]
- 46.Sedoris K.C., Thomas S.D., Miller D.M. c-myc Promoter binding protein regulates the cellular response to an altered glucose concentration. Biochemistry. 2007;46:8659–8668. doi: 10.1021/bi7003558. [DOI] [PubMed] [Google Scholar]
- 47.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 48.Song Y., Luo Q., Long H. Alpha-enolase as a potential cancer prognostic marker promotes cell growth, migration, and invasion in glioma. Mol. Cancer. 2014;13:65. doi: 10.1186/1476-4598-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spearman C. The proof and measurement of association between two things. Am. J. Psychol. 1987;100:441–471. doi: 10.2307/1422689. [DOI] [PubMed] [Google Scholar]
- 50.Subramanian A., Miller D.M. Structural analysis of α-Enolase: mapping the functional domains involved in down-regulation of the c-myc protooncogene. J. Biol. Chem. 2000;275:5958–5965. doi: 10.1074/jbc.275.8.5958. [DOI] [PubMed] [Google Scholar]
- 51.Takashima M., Kuramitsu Y., Yokoyama Y. Overexpression of alpha enolase in hepatitis C virus-related hepatocellular carcinoma: association with tumor progression as determined by proteomic analysis. Proteomics. 2005;5:1686–1692. doi: 10.1002/pmic.200401022. [DOI] [PubMed] [Google Scholar]
- 52.Teng P.-.N., Bateman N.W., Wang G. Establishment and characterization of a platinum- and paclitaxel-resistant high grade serous ovarian carcinoma cell line. Hum. Cell. 2017;30:226–236. doi: 10.1007/s13577-017-0162-1. [DOI] [PubMed] [Google Scholar]
- 53.Trojanowicz B., Winkler A., Hammje K. Retinoic acid-mediated down-regulation of ENO1/MBP-1 gene products caused decreased invasiveness of the follicular thyroid carcinoma cell lines. J. Mol. Endocrinol. 2009;42:249–260. doi: 10.1677/JME-08-0118. [DOI] [PubMed] [Google Scholar]
- 54.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vergote I., De Brabanter J., Fyles A. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet. 2001;357:176–182. doi: 10.1016/S0140-6736(00)03590-X. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida A., Okamoto N., Tozawa-Ono A. Proteomic analysis of differential protein expression by brain metastases of gynecological malignancies. Hum. Cell. 2013;26:56–66. doi: 10.1007/s13577-012-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 58.Zhan P., Zhao S., Yan H. α-enolase promotes tumorigenesis and metastasis via regulating AMPK/mTOR pathway in colorectal cancer. Mol. Carcinog. 2017;56:1427–1437. doi: 10.1002/mc.22603. [DOI] [PubMed] [Google Scholar]
- 59.Zhao M., Fang W., Wang Y. Enolase-1 is a therapeutic target in endometrial carcinoma. Oncotarget. 2015;6:15610–15627. doi: 10.18632/oncotarget.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu X., Miao X., Wu Y. ENO1 promotes tumor proliferation and cell adhesion mediated drug resistance (CAM-DR) in Non-Hodgkin's Lymphomas. Exp. Cell Res. 2015;335:216–223. doi: 10.1016/j.yexcr.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 61.D.G. Mutch. FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol. Oncology. 2014;133(3):401–404. doi: 10.1016/j.ygyno.2014.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.