Abstract
Surgical treatment of abdominal aortic aneurysm in patients with a transplanted kidney is true a challenge. Conventional open repair of the aneurysm requires aortic cross-clamping. Therefore, it can pose a risk of ischemic injury to the transplanted kidney. Endovascular repair, which limits the duration of interruption of blood flow to the transplanted kidney, is a suitable alternative for repair of abdominal aortic aneurysm, if feasible anatomically. Here, we present a case of a 62-year-old woman who was transferred to our hospital because of abdominal pain and had a history of renal transplant 14 years ago. Computed tomography confirmed a large infrarenal fusiform abdominal aortic aneurysm 6 cm in maximal diameter and another 4 cm fusiform aneurysm in the left common iliac artery. We successfully performed endovascular aneurysm repair combined with femoro-femoral bypass. The postoperative course was uneventful, and the patient was discharged on the 5th postoperative day.
Keywords: Abdominal aortic aneurysms, Endovascular aneurysm repair, Kidney transplant recipient
Introduction
The management of abdominal aortic aneurysm (AAA) in patients with a kidney transplant is always a significant challenge for clinicians because it can pose the risk of ischemic injury to the transplanted kidney. Conventional open repair of the AAA requires aortic cross-clamping. Therefore, to minimize the risk, a variety of proposed procedures for protection of transplanted kidney during aortic cross-clamping have been reported, such as cold renal perfusion, temporary shunts, and extracorporeal bypass [1, 2]. Currently, endovascular aortic aneurysm repair (EVAR) is an alternative option as it can be performed without the need for aortic cross-clamping. Moreover, the interruption of blood flow to the transplanted kidney is insignificant in EVAR and is a suitable choice for AAA repair, if feasible [3]. Here we present a case of successful EVAR for AAA in a kidney transplant recipient. The patient provided written informed consent and this report was approved by institutional review board.
Presentation of case
A 62-year-old woman was transferred to our hospital because of abdominal pain. Her medical history included kidney transplantation 14 years ago in Russia. Upon physical examination, she was normotensive with a blood pressure of 120/60 mm Hg, a heart rate of 70 beats per minute, and peripheral capillary oxygen saturation of 96%. A large pulsatile mass around the umbilical area was unexpectedly palpated on abdominal examination and the serum creatinine value was 0.78 mg/dL.
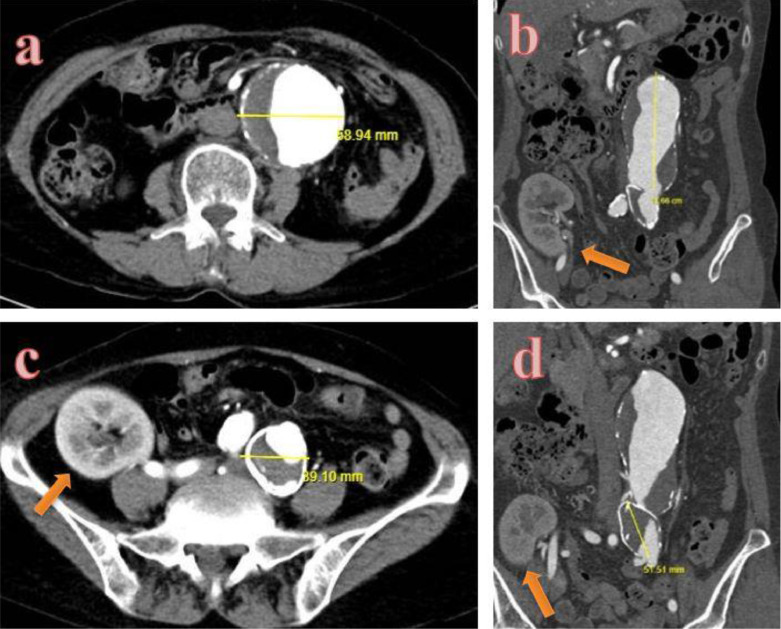
Abdominal computerized tomography angiography (CTA) was performed using a 128-slice machine with the administration of intravenous contrast material at a rate of 3.5 mL/min. A large infrarenal fusiform aneurysm without evidence of rupture was noticed. The diameter and the length of the aneurysm were measured to be approximately 6 cm and 11 cm, respectively (Fig. 1a, b). CTA also showed a large left iliac aneurysm with a calcified intramural thrombus. The maximal diameter of this aneurysm was approximately 4 cm in size (Fig. 1c, d). The transplanted kidney was also noted in the right lower quadrant receiving blood supply directly from the right internal iliac artery (Fig. 2).
Fig. 1.
Abdominal CT images. (a) Axial and (b) Coronal images obtained in the arterial phase show a large abdominal aortic aneurysm with intramural thrombus. (c) Axial and (d) coronal views obtained in the arterial phase shows a large left iliac aneurysm with calcified intramural thrombus. The transplanted kidney was also noted in the right lower quadrant.
Fig. 2.
Coronal volume render images: Anteroposterior (a) and posteroanterior (b) views show a large aortic and left iliac aneurysm with calcified intramural thrombus. The left external iliac artery angulated severely (green arrow).
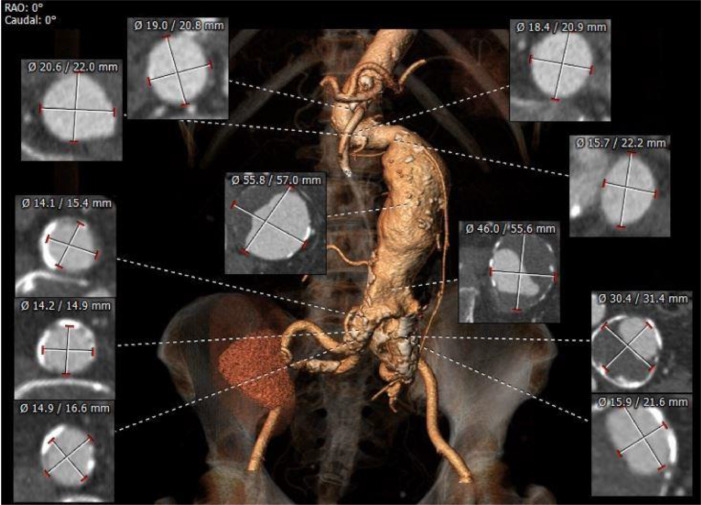
The three-dimensional analysis was performed using Mensio Vascular 10.1 software to obtain the sizing and planning for EVAR (Fig. 3). The results of sizing are shown in Figure 3. The distance from the lower renal artery to the aortic bifurcation was 160 mm. The lengths of the left and right common iliac arteries were 36 mm and 25 mm, respectively. The infrarenal and suprarenal angulations were 90° and 117°, respectively. The left external iliac artery was severely angulated (Fig. 2). We performed a hybrid approach with aorto-uni-iliac (AUI) stent grafting combined with coil embolization of the left common iliac artery and femoro-femoral bypass.
Fig. 3.
The three-dimension analysis was done with Mensio Vascular 10.1 software to obtain the sizing and planning for EVAR.
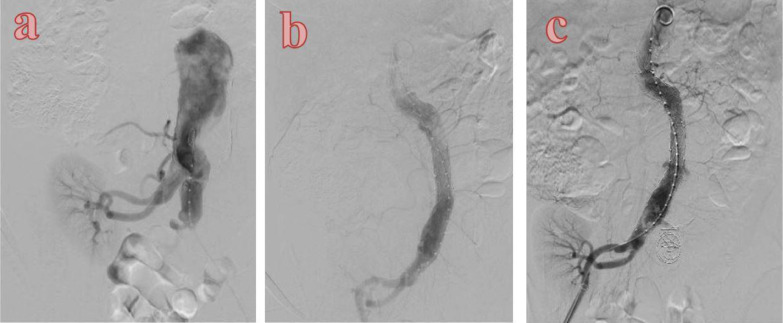
The procedure was carried out under general anesthesia, and arterial access was accomplished by exposing bilateral femoral arteries. The Endurant AUI Stent Graft System (including main body 25-14-102 mm, 16-20-124 mm stent for the right common iliac artery) was successfully expanded without any leakage (Fig. 4b). Coil embolization was successfully deployed to occlude the left common iliac artery (Fig. 4c). After performing EVAR, we performed a femoro-femoral crossover bypass procedure using Dacron graft 7. The postoperative course was uneventful, and the patient was discharged on the 5th postoperative day with a serum creatinine level of 1.05 mg/dL.
Fig. 4.
(a) Preintervention angiography; (b) Aorto-uni-iliac stent graft was expanded from infra abdominal aorta to right common iliac artery combined with occlusion of contralateral common iliac by coils (c).
Discussion
Renal transplant recipients have many risks of developing atherosclerotic vascular disease, such as hypertension, hyperlipidemia, diabetes mellitus, and prior hemodialysis. Increasing the number of aneurysmal or occlusive vascular disease is the result of such risks, particularly AAA.
Surgical treatment for AAA in kidney transplant recipients is a challenge. Aortic clamping results in ischemic injury for transplanted kidney in operation, causes functional failure of the transplanted kidney. Some additional therapies preventing transplanted kidney from ischemic injury have been reported successfully, such as cold perfusion, local hyperthermia, and pump-oxygenation bypass [4,5]. Consequently, the operation is more complex. There are also many factors related to the ischemic injury of the transplanted kidney, such as the technical problem. Therefore, aortic reconstruction is a challenge in kidney transplant recipients, especially in patients with poor performance status.
However, this problem can be resolved with the development of endovascular interventions. EVAR is being refined and is the most common minimally invasive technique for AAA repair [6]. In kidney transplant patients, endovascular repair is a suitable choice for AAA repair, if feasible anatomically, because of the limited duration of interruption of blood flow to the transplanted kidney [7]. In this case, the patient had an infrarenal fusiform AAA, a left fusiform common iliac arterial aneurysm, and a transplanted kidney receiving blood supply directly from the right internal iliac artery. The left external iliac artery was severely angulated. We decided to deploy an AUI stent graft from the infrarenal abdominal aorta to the right common iliac artery, occluded the left common iliac artery by coil, and performed femoro-femoral bypass on the same day. In this way, there was no significant disruption to the blood supply of the transplanted kidney, and blood flow to the pelvic organs and lower extremities was maintained. The postoperative course was uneventful, and the patient was discharged on the 5th postoperative day.
Although the advantage of EVAR in patients with transplanted kidneys is the advection of aortic cross-clamping, it still poses a potential risk of kidney failure due to contrast substance exposure. In this case, the postoperative serum creatinine was normal. However, we also need to be more concerned with the risk of progression of renal failure after intervention in such cases in the future. A study of Bostock IC et al. on a database of 17,213 patients who underwent EVAR to compare the risk of developing renal dysfunction in group of transplanted kidney patients and nontransplant patients. They concluded that EVAR in transplanted kidney patients was technically feasible with comparable 1-year mortality results and durable graft insertion in comparison to nontransplant patient outcomes. However, renal dysfunction was 3 times more frequent in transplanted kidney patients treated with EVAR than in the other groups [8]. Therefore, the clinical decision-making of EVAR in kidney transplant patients must carefully weigh the risks and benefits.
To conclude, endovascular repair for AAA in kidney transplant recipients may be feasible and safe in a suitable patient. Further studies are needed to evaluate the efficacy and safety of endovascular repair for AAA in kidney transplant recipient.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Patient consent
Written informed consent was obtained from the patient for the publication of this case report.
Footnotes
Declaration of competing interest: The authors declare that they have no conflict of interest regarding the publication of this case report.
References
- 1.Wolf W, Ayisi K, Ismail M, Kalmar P, Pokar H, Trautwein S. Abdominal aortic aneurysm repair after renal transplantation with extracorporeal bypass. Thorac Cardiovasc Surg. 1991;39(6):384–385. doi: 10.1055/s-2007-1020006. [DOI] [PubMed] [Google Scholar]
- 2.O'Mara CS, Flinn WR, Bergan JJ, Yao JS. Use of a temporary shunt for renal transplant protection during aortic aneurysm repair. Surgery. 1983;94(3):512–515. [PubMed] [Google Scholar]
- 3.Premaratne S, Hopkins J, Duddy M, Sang KT, Kay M, Rogoveanu R. Abdominal aortic aneurysm repair in renal and liver transplant recipients. Eur J Vasc Endovasc Surg. 2019;58(6):e848–e850. doi: 10.1177/1538574419880673. [DOI] [PubMed] [Google Scholar]
- 4.Callaghan CJ, Munday IT, Casey ND, Large SR, Gaunt ME. Abdominal aortic aneurysm repair in a renal transplant recipient: a modified pump-oxygenation bypass technique to reduce hypotension and myocardial ischaemia. EJVES Extra. 2005;10(5):120–121. [Google Scholar]
- 5.Sadat U, Huguet EL, Varty K. Abdominal aortic aneurysm surgery in renal, cardiac and hepatic transplant recipients. Eur J Vasc Endovasc Surg. 2010;40(4):443–449. doi: 10.1016/j.ejvs.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Ivancev K, Vogelzang R. A 35 year history of stent grafting, and how EVAR conquered the world. Eur J Vasc Endovasc Surg. 2020;59(5):685–694. doi: 10.1016/j.ejvs.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Karkos C, McMahon G, Fishwick N, Lambert K, Bagga A, McCarthy M. Endovascular abdominal aortic aneurysm repair in the presence of a kidney transplant: therapeutic considerations. Cardiovasc Interv Radiol. 2006;29:284–288. doi: 10.1007/s00270-005-0043-y. [DOI] [PubMed] [Google Scholar]
- 8.Bostock IC, Zarkowsky DS, Hicks CW, Stone DH, Eslami MH, Malas MB. Outcomes of endovascular aortic aneurysm repair in kidney transplant recipients: results from a national quality initiative. Am J Transplant. 2016;16(8):2395–2400. doi: 10.1111/ajt.13733. [DOI] [PMC free article] [PubMed] [Google Scholar]