Abstract
Introduction
Achyranthes aspera, Chenopodium murale, Satureja punctata, Rumex abyssinicus and Aloe pulcherrima are traditionally used to treat urolithiasis in Ethiopia. However, there are limited reports on toxicity studies.
Objective
This study was intended to evaluate the acute and sub-acute toxicity effects of plants.
Materials and Methods
The crude extracts of A. aspera and C. murale leaves, S. punctata aerial parts, R. abyssinicus rhizomes, and A. Pulcherrima gel were prepared using 70 % ethanol. In acute toxicity, 125, 500 and 2000 mg/kg were tested in a stepwise manner; whereas 2000 mg/kg administrated to female rats using gavage during sub-acute toxicity. On day 14 and 28, blood samples were collected from retro-orbital sinus; liver and kidneys of each animal were collected under anaesthesia. Data were analyzed using one-way ANOVA, Dunnett's comparison test of the Graph Pad Prism.
Results
No mortality and significant weight loss for all extracts in both toxicity tests. In acute toxicity, C. murale extract significantly reduced hemoglobin and platelets (P < 0.01) compared with the control. Likewise, S. punctata (P < 0.05) and R. abyssinicus (P < 0.01) extracts revealed significant reduction in platelet count. An exposure to C. murale and R. abyssinicus extracts reduced the concentrations of platelet distribution width and platelet larger cell ratio (p < 0.05) during sub-acute toxicity test. The level of creatinine reduced due to A. aspera extract administrations(P < 0.05). Liver histopathological examinations revealed focal periportal hepatitis following sub-acute toxicity test of C. murale. Histopathological studies of liver demonstrated that R. abyssinicus, A. aspera and S. punctata extracts showed mild acute liver injury. A. pulcherrima was not associated with any toxicity.
Conclusion
C. murale extract showed hematological, and histopathological toxicity profiles in rats. Furthermore, chronic toxicity studies of A. aspera, S. punctata and R. abyssinicus extracts would be beneficial to ensure safety.
Keywords: Acute toxicity, Albino wistar female rats, Antiurolithiatic plant extracts, Sub-acute toxicity
1. Introduction
Herbal medicine is an alternative natural remedy in primary health care in developing countries [1]. In Africa, it was estimated that 70–80 % patients are treated by traditional medicine (TM) [2]. In Ethiopia, it has been estimated that 70 % of humans and 90 % of livestock depends on TM [3]. In the world, about 25 % of modern drugs developed from plants [4]. Plants synthesize a variety of metabolites, some of which may be beneficial or potentially toxic to mankind [5]. Also, it has been true that pharmaceutical drugs may be therapeutic at one dose and toxic at another [6]. In order to ensure safety, there must be a study to show safety profiles of herbs claimed to be beneficial to humans and the animals before deciding to use them [7]. The selective uptake or accumulation of a particular xenobiotic in a specific tissue or cell, the inhibition of the normal export of a potentially toxic metabolite from a cell to the outside, and the activation of cellular receptors could lead to toxicity [8,9]. In modern drug development, about one-third of the drug candidates required high cost for its toxicity studies [10].
Acute and sub-acute toxicity tests are routinely performed for the investigation of natural products or drugs. Acute toxicity test is the first step to determine the adverse effects of substances within 14 days of administration of single dose [11,12]. It is usually administered via oral routes to determine the median lethal dose (LD50) for a particular toxic substance in testing animals (usually rats or mice) [13]. In sub-acute toxicity test, a drug is daily administered [12], usually from three weeks to three months in rodents (rats), dogs and monkeys [14,15]. The starting dose could be the expected therapeutic level selected from one of the four fixed levels (5, 50, 300 and 2000 mg/kg body weight) of test agents for acute toxicity test. Ideally, the starting dose should be that which is most likely to produce mortality and other toxic effects in the dosed animals [16]. Furthermore, previous studies had administered extracts at 2000 and 5000 mg/kg dose for 28 days to determine sub-acute toxicity [17].
Liver and kidneys are the primary organs affected by metabolic reactions of toxicants [9,18], and are useful in predicting toxicity effects of phytotherapeutic products or drugs [19]. The liver is the main target for toxic compounds because of its prior exposure to foreign substances absorbed in the intestine before reaching into the blood circulation [20,21]. Although toxins may harm the liver, it detoxifies toxins [22]. Thus, in experimental animals, liver function test would allow to understand the toxic effects, which can be extrapolated for safety if used in humans.
Blood parameters are relevant indicators for potential health hazards and have a higher predictive value for toxicity [23,24]. The calculated red blood cell indices, that is, the mean corpuscular volume (MCV) and the mean corpuscular hemoglobin concentration (MCHC) are used for diagnosis of anemia [25]. Hematological indices such as packed cell volume (PCV) and hemoglobin (HB) are associated with the total population of red blood cells (RBCs). MCV reflects the size of red blood cells; whereas the mean corpuscular hemoglobin (MCH) and MCHC are used mathematically to define the concentration of hemoglobin [26]. The packed cell volume (PCV) or hematocrit represents the percentage of RBCs of whole blood volume, which is clinically used to determine anemia [27]. These blood biomarkers help to determine toxicity in relation to dose and time-response [28]. Furthermore, the considerations of platelet indices such as mean platelet volume (MPV), platelet distribution width (PDW) and a platelet large cell ratio (P-LCR) are also used to screen toxicity [[29], [30], [31]]. In vitro platelet toxicity assays can be used to evaluate the predictive values of hypersensitivity reactions against drugs [32]. Increasing or decreasing in platelet counts result in clotting or bleeding abnormalities, respectively [31].
Despite the wider use of folk medicine in developing nations, there are limited scientific evidences regarding safety profiles [23]. In Ethiopia, various medicinal plants such as Achyranthes aspera [[33], [34], [35]], Satureja punctata [33,34], Rumex abyssinicus, Chenopodium murale and Aloe pulcherrima [34] have been claimed to possess antiurolithiatic effects. To date, there is insufficiency of scientific evidence reported on the toxicity of these plants. Therefore, the objective of the present study was to evaluate acute and/or sub acute toxicity profiles of the aforementioned medicinal plants in albino Wistar female rats.
2. Materials and methods
2.1. Plant material identification and collection
The plant materials were collected from natural habitats between October 2017 to November 2018. The plant specimens were submitted to the National Herbarium, Department of Plant Biology and Biodiversity Management, Addis Ababa University (AAU) for taxonomic authentication and the corresponding collection number (a voucher number) was given as follows. A. aspera L. leaves (TA231) were collected from Addis Ababa University "Arat kilo" Campus; R. abyssinicus Jacq. rhizomes (TA232) in Arada Sub-city, at Sheger park near to Sheraton Hotel from Addis Ababa; and S. punctata (Benth.) Briq. aerial parts (TA233) at "Entoto" forest region, Northwest to Addis Ababa; C. murale L. leaves (TA234) in "Keyit" locality of Debre Birhan, 130 km to North of Addis Ababa; and A. pulcherrima Gilbert & Sebsebe gel (TA235) from Ankober District of North Shewa Zone, 40 km Northeast of Debre Berhan Town. These specimens were deposited in the National Herbarium of AAU for future reference. Some medicinal plants used traditionally to treat various diseases including urolithiasis are indicated in Table 1.
Table 1.
Selected medicinal plants investigated in the present study.
| Scientific name (Family) | Local name (Amharic) | Parts used | Traditional uses |
|---|---|---|---|
| Achyranthes aspera L. (Amaranthaceae) | Telenji | Leaves & roots | Treat cough, colic, debility, dropsy, dog bite, asthma, stop bleeding and gynaecological disorders, antiarthritic, antifertility (spermicidal), laxative for gastric disorders, ecbolic, abortifacients, anti-helminthic, aphrodisiac, antiviral, anti-plasmodic, antihypertensive, anticoagulant, diuretic and anti-tumor [38]. It is also used to treat a scorpion bite, gonorrhea, obstetric disorders, and diabetes mellitus, fever, dysentery, and prevents hypersensitivity reactions (anti-allergic) of the skin [39]. It also shows the effects of nephroprotective, antiparasitic, hypoglycaemic, analgesic, antipyretic, and purgative [40]. |
| Rumex abyssinicus Jacq. (Polygonaceae) | Mekimeko | Rhizomes | Useful for treatment of gonorrhea, lung TB, leprosy, fever, liver disease, hypertension, hemorrhoids, scabies, antiemetic, aphrodisiac, cough, rabies, vermifuge, rheumatism, and migraine. It is used as a diuretic, anti-microbial, anti-inflammatory and analgesic activities. Root powder paste with lime juice applied for Tinea nigra, T. versicolor [41,42]. |
| Satureja punctata (Benth.) Briq. (Lamiaceae) | Yelomi-eshet (Lomishet) | Aerial parts | To treat headache, stop menstruation, relieve stomach pains [33], and improve the quality of milk [43]; muscle pain relievers, tonic, carminative agents to treat stomach and intestinal disorders such as cramps, nausea, indigestion, and diarrhea [44,45]. Essential oils of Satureja species are used in various industrial applications as flavoring, medicine, and perfumes [46]. |
| Chenopodium murale L. (Chenopodiaceae) | Amedmado | Leaves | Used as a diuretic, mild purgative, emollient, anthelmintic, tranquilizer, laxative agent, and tonic for liver [47]. It also has antibacterial, antifungal, insecticidal, cytotoxic, anthelmintic, anti-diaphoretic, stomachic, antispasmodic, emmenagogue, anti-asthmatic, abortifacients, migraine, digestive problems, sterility, hair loss, anxiolytic, antidepressant and anti-hypertensive effects [48], and to treat jaundice [49]. |
| Aloe pulcherrima Gilbert & Sebsebe (Aloaceae) | Sete-Eret | Gel | The gel/latex is used to treat various infectious diseases such as malaria and used for wound healing [50,51]. |
2.2. Preparation of plant crude extracts
The leaves of A. aspera, the rhizomes of R. abyssinicus, the aerial parts S. punctata and the leaves of C. murale were cleaned thoroughly with tap water to remove contaminants, and dried in shed at room temperature from 2 to 3 weeks in the Biomedical Sciences laboratory, AAU. These dried plant parts were finely powdered using a kitchen grinder (Mortar and Pestle, sized about 9 in. in diameter). These powders were put through a sieve of 3 mesh sizes so as to filter a gross solid matter. The gel of A. pulcherrima was collected by spill-out its fresh leaves with the Knife. In Ethiopia, traditional healer's use various ways of extract preparations, including water, and local alcoholic beverages like "Araki or katikala" (alcohol content from 45 % to 80 %), local beer "Tella" (alcohol content of filtered "Tella" from 5 % to 6 %), and "Tej" (alcohol content from 7 % to 11 %) [36].
The extracts were prepared using a procedure similar to that often used by traditional healer's or patients, with some minor modifications. The plant powders were soaked in hydro-ethanol (70 %), which was placed on a shaker for 72 h. The mixture was filtered through cotton/gauze, then through Whatman filter paper number 1 (pore size: 11 μm) to remove fine solid plant particles or insoluble constituents. The entire extracts were concentrated to dryness using a Rotary Evaporator by removing ethanol (EtOH) at 45 °C under reduced pressure. The distilled water extracts were concentrated using Lyophilizer machine. Then, the semi-solid concentrates poured into a glass Petri-plates and allowed to completely dry in water bath adjusted to 45 °C. The final dried extracts were collected and stored in labeled sterile bottles covered with tightly stopper and kept in Freezer on −20 °C until used in the experiments.
2.3. Experimental animals
Adult albino Wistar non-pregnant female rats weighing 200−220 g and between 8–10 weeks of age were used. Previous reports suggested that female rats are more susceptible than male rats for toxicants [37]. As a result, the experimental female rats were purchased from the Ethiopian Public Health Institute (EPHI) animal breeding center. The experiment was carried out in Biomedical laboratory, at Addis Ababa University (AAU), and in Traditional Medicine and Modern Drug Research Laboratory of EPHI.
Experimental animals were housed in groups of three and six maintained under a controlled room temperature (22 ± 2 °C) with a constant humidity (55 ± 5 %), ventilation, no noise, and light (12 h light/12 h dark) cycles. Every day, they were fed with pellets (standard diet) produced by Alema Farms PLC (Bishoftu, Ethiopia), and had access to tap water ad libitum. They were acclimatized to standard laboratory conditions (in plastic cages with stainless steel top) for seven days prior to the experiment. Toxicity assays were conducted in accordance with the standard guidelines of the Organization for Economic Cooperation and Development (OECD, 2001) for use of animals in scientific research. That is, animal experiments were conducted in compliance with the ARRIVE guidelines for the care and use of laboratory animals in scientific research. It was also carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments.
2.4. Chemicals and reagents
All chemicals/reagents, and assay kits used were of analytical grade. These were purchased from local distributors in Ethiopia. Chemicals such as absolute Ethanol from Wisteam PLC, Potassium dihydrogen phosphate (anhydrous), and Sodium phosphate (dibasic anhydrous, extra pure) were purchased from Micron International Trading House PLC. Formaldehyde and Isoflurane from Neway Chemicals PLC, Addis Ababa. The Kits for liver function test from a Roshi PLC through EPHI, EDTA tubes, Serum Separator tubes, Capillary tubes, and surgical blade were purchased from Micro Pharma PLC in Addis Ababa, Ethiopia.
2.5. Toxicity determination
Prior to extract administrations, rats were fasted overnight (12 h), but had free access to water. The weight of each rat was recorded just before test extract administrations and the dose of extracts was calculated in relation to the body weight of each rat. Extracts were reconstituted as homogeneous suspensions in distilled water (vehicle) and administered using an oral gavage feeding needle (PY252-4215, 16gauge, Harvard). The dosing volume of test substances was 2 mL/100 g of body weight, and the time of dosing was at 12:30am throughout the study. After the test substance has been administered, food was withheld for 4 h [16].
2.5.1. Experimental design for acute toxicity (14 days)
In acute toxicity tests, a total of 18 Wistar female rats was randomly allocated into 6 groups consisting of 3 rats per group. That is, Group I (Control), Group II (R. abyssinicus), Group III (S. punctata), Group IV (C. murale), Group V (A. pulcherrima) and Group VI(A. aspera). Each group of rats was administered with a single fixed dose of the respective TM extracts tested in a stepwise procedure (125, 500, and 2000 mg/kg body weight). Group I rats received distilled water (vehicle) until the end of the experiment.
Any indications of clinical toxicity were closely monitored within 4 h of treatment period up to 24 h. Thereafter, observations for toxic manifestations (rising fur, draping, tremors, excitability, twitching, salivation and mortality) were made until the end of the 14 day period [16]. After 14 days, they were humanly sacrificed.
2.5.2. Experimental design for sub-acute toxicity (28 days)
As recommended by OECD (2001), the selection of the starting dose of the sub-acute toxicity test was based on a dose, which is not expected to produce mortality or severe acute toxicity. The 2000 mg/kg body weight of the extracted material was the highest dose determined not to induce acute toxicity in rats during the first phase of the present study.
The experimental study of sub-acute toxicity was parallel design, in which each group given unique extracts. A total of 36 Wistar female rats were randomly divided into 6 groups consisting of 6 rats per group with matching body weights. That is, Group I (Control), Group II (C. murale), Group III (R. abyssinicus), Group IV (S. punctata), Group V (A. pulcherrima), and Group VI (A. aspera). Group II to VI were given a single dose 2000 mg/kg body weight once daily for 28 days, consecutively. The dosing volume was 2 mL/100 g of body weight. The control group received distilled water once daily throughout the experiment.
2.5.3. Body weight measurements
The weight of the control and experimental rats were recorded using Mettler PE 1600 analytical balance(+0.01 g, Switzerland) on the first day of the study (prior to the administration of test extracts), and at the end of the experiment at day 14th (acute), and day 28th (sub-acute).
2.5.4. Hematological studies
At the end of the experimental period, euthanasia was administered using Isoflurane (C3H2ClF5O, Forane) inhalant followed by gentle cervical dislocation. Euthanized rats were placed in a sealed glass chamber where high levels of anesthetic gas (3 %) were introduced, which depresses the central nervous system resulting in peaceful death. Blood samples were collected once unconsciousness has been achieved. About 1 mL blood samples were collected from each rat in ethylenediamine tetraacetic acid (EDTA) anticoagulant tubes from the retro-orbital vein by a glass capillary tube puncture. Hematological examinations were carried out using Automated Hematology Analyzer (XT-1800i, Japan).
2.5.5. Estimation of biochemical markers
Liver enzymes such as Alkaline phosphatase (ALP), Aspartate aminotransferase (AST), and Alanine aminotransferase (ALT) were assayed as indicators of liver toxicity. This was done by enzyme marker kits using Clinical Chemistry Auto-analyzer (Cobas 6000 analyzer, Germany). The manufacturer's instruction was followed in the course of each biochemical analysis.
2.5.6. Histopathological examinations
Histopathological examinations were done for liver and kidney tissues of the experimental rats. All rats were sacrificed in a humane manner using Isoflurane anesthesia at the end of the 14th day for acute toxicity and at the end of the 28th day for sub-acute toxicity studies. Liver and kidneys were examined macroscopically for its gross pathological changes (lesions developed) due to exposures of test extracts as compared to control groups in toxicity studies. The tissues were microscopically examined for sub-acute toxicity test. In sub-acute toxicity, the tissues were fixed by 10 % buffered neutral formalin solution, and subsequently embedded in paraffin wax. The sections (5 μm thick) were cut using Rotary Microtome 4060E (Germany), and mounted on glass slides and stained with Hematoxylin and Eosin to study the histopathological changes [52]. All fields of the tissue morphology were examined under a light microscope (100x magnification) (Wagtech Thatcham, Berkshire, RG19 4QD, United Kingdom), and the photomicrographs were captured using a digital Camera manually mounted on it (Sony Cyber-shot DSC-W180 10.1 M P with 3x optical Zoom, New Jersey, USA) for further reference.
2.6. Statistical analysis
The data were analyzed using Graph Pad Prism version 6 Software (Graph Pad Software, San Diego, CA, USA). Analysis of one way ANOVA followed by Dunnett's comparison test was applied to compare each group with the control. The data values were expressed as mean ± standard deviation (SD). A p-value < 0.05 was considered as statistically significant.
3. Results
3.1. Acute toxicity test
The administrations of A. aspera (Aa) leaves, R. abyssinicus (Ra) rhizomes, S. punctata (Sp) aerial parts, C. murale (Cm) leaves and A. pulcherrima (Ap) gel extracts in rats did not show mortality at fixed dose 2000 mg/kg body weight. Moreover, there were no visible signs of acute toxicity, i.e., food and water consumptions were unaffected; and salivation, aggression, rising furs, and writhing were not observed for 14 days.
3.1.1. Acute-Effect on body weight
The administration of extracts at 125 and 500 mg/kg body weight to rats resulted in an increase in body weight over time similar to the control group, which indicates that the extracts were not acutely toxic. The extracts of S. punctata, A. pulcherrima, and A. aspera did not acutely affect body weight at a given dose 2000 mg/kg body weight in rats. These supports the safety of these extracts at a given dose in rats, which resulted an increase in body weight over time similar to the control group. However, the administrations of C. murale and R. abyssinicus extracts at dose 2000 mg/kg were associated with weight loss compared to their initial weight, although it was not significant (Fig. 1C).
Fig. 1.
Acute toxicity effects of plant extracts on body weight changes in non-pregnant female Wistar albino rats at dose (A) 125 mg/kg, (B) 500 mg/kg, and (C) 2000 mg/kg b.w. Note: D0 = day zero, and D14 = day 14. Comparisons were made with a control group before and after the experiment. The data illustrated the mean + SD of 3 rats per treatment groups (n = 3).
3.1.2. Hematological parameters of acute toxicity
The administrations of C. murale and R. abyssinicus extracts significantly (P < 0.05) reduced HGB concentrations(13.11 + 1.31 g/dl) and 14.10 + 2.52 g/dl, respectively, compared with control (18.23 + 1.00/g/dl). Clinically meaningful increment of RBC concentration was observed, although it did not reach statistical significance compared with the control (4.75 × 106/mm3 Cells). Most of the hematological biomarkers remain within the normal limit in comparison with the control group (P > 0.05) (Table 2).
Table 2.
. Hematological parameters of non-pregnant female Wistar rats exposed to extracts (dose 2000 mg/kg) on14th day follow up.
| Hematological Parameters | Normal control | Cm | Ra | Ap | Sp | Aa |
|---|---|---|---|---|---|---|
| WBC (Cell x 103/mm3) | 6.69 + 1.21 | 5.44 + 1.23 | 6.10 + 2.67 | 5.99 + 0.31 | 6.12 + 1.47 | 6.67 + 0.91 |
| RBC (Cell x 106/mm3) | 4.75 + 0.40 | 4.46 + 0.67 | 4.52 + 1.39 | 4.55 + 0.28 | 5.00 + 0.16 | 4.94 + 0.53 |
| HGB (g/dl) | 18.23 + 1.00 | 13.11 + 1.31** | 14.10 + 2.52* | 16.25 + 1.20 | 17.36 + 0.20 | 17.36 + 1.06 |
| HCT (%) | 48.23 + 3.37 | 44.80 + 4.03 | 43.46 + 7.33 | 46.65 + 2.75 | 50.50 + 0.43 | 50.46 + 2.68 |
| MCV (fl/cell) | 56.36 + 1.25 | 57.33 + 1.07 | 56.50 + 1.21 | 54.50 + 1.41 | 56.13 + 1.43 | 56.46 + 0.61 |
| MCH (pg/cell) | 19.43 + 0.25 | 19.20 + 0.34 | 19.03 + 0.20 | 18.95 + 0.77 | 19.26 + 0.49 | 19.43 + 0.40 |
| MCHC (g/dl) | 34.50 + 0.30 | 33.40 + 0.10 | 33.63 + 0.32 | 34.85 + 0.49 | 34.36 + 0.25 | 34.40 + 0.43 |
| RDW-SD (fl) | 28.96 + 2.80 | 27.76 + 0.60 | 27.60 + 1.67 | 26.95 + 0.63 | 27.93 + 1.25 | 28.20 + 0.85 |
| RDW-CV(%) | 17.53 + 1.49 | 15.06 + 1.19 | 15.76 + 2.56 | 17.20 + 1.13 | 18.43 + 0.20 | 17.33 + 1.58 |
| PDW (fl) | 8.70 + 0.20 | 8.53 + 0.45 | 8.73 + 0.25 | 8.30 + 0.28 | 9.06 + 0.32 | 8.80 + 0.30 |
| MPV (fl) | 8.00 + 0.20 | 7.96 + 0.35 | 7.90 + 0.34 | 7.55 + 21.21 | 8.06 + 0.23 | 7.90 + 0.30 |
| P-LCR (%) | 10.83 + 1.20 | 10.13 + 2.41 | 10.06 + 2.40 | 8.40 + 1.41 | 11.14 + 1.70 | 10.33 + 1.70 |
| PCT (ng/mL) | 0.67 + 0.13 | 0.35 + 0.14 | 0.43 + 0.31 | 0.75 + 0.03 | 0.35 + 0.11 | 0.63 + 0.17 |
| NEUT (%) | 0.83 + 0.08 | 0.73 + 0.02 | 0.70 + 0.27 | 0.78 + 0.26 | 0.84 + 0.18 | 1.06 + 0.26 |
| LYM (%) | 5.39 + 0.98 | 4.09 + 1.00 | 5.08 + 2.29 | 5.01 + 0.69 | 4.83 + 1.05 | 5.15 + 0.84 |
| MONO (103/mL) | 0.17 + 0.17 | 0.12 + 0.05 | 0.20 + 0.16 | 0.14 + 0.14 | 0.24 + 0.13 | 0.22 + 0.05 |
| EO(x103/mL) | 0.08 + 0.00 | 0.06 + 0.00 | 0.06 + 0.01 | 0.05 + 0.02 | 0.19 + 0.05 | 0.08 + 0.02 |
Note: WBC = White Blood Cells; RBC = Red Blood Cells; HGB =Hemoglobin; HCT = Hematocrit (called Packed Cell Volume, PCV); MCV = Mean Corpuscular Volume; MCH = Mean Corpuscular Hemoglobin; MCHC = Mean Corpuscular Hemoglobin Concentration; RDW-SD = Standard Deviation in Red Cell Distribution Width; RDW-CV = Coefficient of Variation in Red Cell Distribution Width; PDW = Platelet Distribution Width; MPV = Mean Platelet Volume; PLT = Platelet; P-LCR = Platelet Larger Cell Ratio; PCT = Procalcitonin; NEUT = Neutrophils; LYM = Lymphocyte Count; MONO = Monocytes; EO= Eosinophils; pg(pictograms); Cm=C. murale leaves; Ra=R. abyssinicus rhizome; Ap=A. pulcherrima gel; Sp= S. punctata aerial parts, and Aa=A. aspera leaves. Values are represented as mean + SD of triplicates (n = 3). *p < 0.05, **p < 0.01,***p < 0.001 indicate significant changes in comparison with the normal control.
The extracts of C. murale, R. abyssinicus and S. punctata revealed a significant reduction in platelet count as compared with the control (P < 0.05) (Fig. 2).
Fig. 2.
Effects of plant extracts on platelet concentrations in Wistar female rats exposed for a dose 2000 mg/kg in acute toxicity study. Note: Aa=A. aspera, Sp= S. punctata, Ra=R. abyssinicus, Cm=C. murale and Ap=A. pulcherrima. *P < 0.05, **p < 0.01,***p < 0.001 indicate significant changes in comparison with the normal control.
3.2. Sub-acute toxicity study
No mortality for extracts of A. aspera leaves, R. abyssinicus rhizomes, S. punctata aerial parts, C. murale leaves and A. pulcherrima gel at dose 2000 mg/kg body weight.
3.2.1. Sub-acute effect on body weight
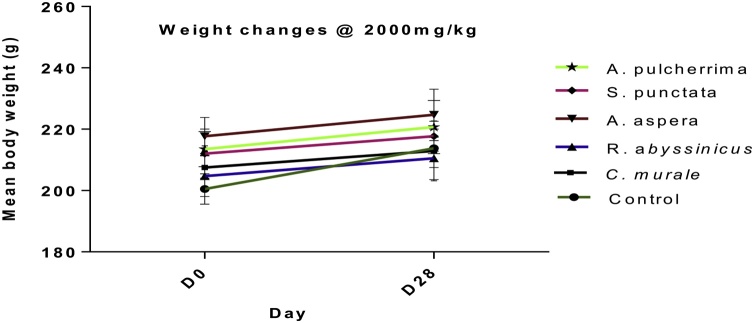
Results revealed no significant weight changes for all extracts at dose 2000 mg/kg (Fig. 3).
Fig. 3.
Effects of plant extracts on body weight changes in non-pregnant female Wistar rats during sub-acute toxicity study. Note: D0= day zero, and D28 = day 28. Comparisons were made with a control group before and after the experiment. Data illustrated the mean + SD of 6 rats per treatment groups (n = 6).
3.2.2. Hematological markers of sub-acute toxicity
Comparisons made between the control (5.68 + 0.77 × 106/mm3 cells) and the treated groups showed no significant difference for Cm, Ra, Sp, Aa and Ap extracts (Table 3).
Table 3.
Hematological parameters of sub-acute toxicity in non-pregnant female Wistar albino rats exposed to plant extracts at single dose 2000 mg/kg for 28 days.
| Hematological parameters | Normal control | Cm | Ra | Sp | Aa | Ap |
|---|---|---|---|---|---|---|
| WBC(cell × 103/mL) | 7.46 + 0.96 | 6.22 + 2.34 | 6.99 + 1.04 | 6.57 + 1.13 | 7.28 + 0.54 | 6.92 + 1.38 |
| RBC (cell× 106/mL) | 5.68 + 0.77 | 4.26 + 0.25 | 4.37 + 0.23 | 4.49 + 0.44 | 4.63 + 0.05 | 4.50 + 1.12 |
| MCV (fl/cell) | 60.50 + 2.40 | 60.26 + 0.40 | 61.63 + 1.23 | 61.23 + 1.19 | 60.56 + 0.80 | 60.53 + 2.99 |
| MCH (pg/dl) | 18.00 + 0.56 | 17.76+0.35 | 18.10 + 0.17 | 17.76 + 0.15 | 17.60 + 0.10 | 17.93 + 0.70 |
| MCHC (g/dl) | 29.80 + 0.28 | 29.50 + 0.40 | 29.36 + 0.40 | 29.00 + 0.36 | 29.10 + 0.43 | 29.60 + 0.65 |
| PLT (103/μl) | 762.00 + 94.75 | 729.33 + 20.13 | 688.33 + 7.09 | 642.33 + 11.80 | 818.66 + 81.05 | 580.00 + 278.26 |
| RDW-SD (fl) | 38.90 + 0.84 | 39.56 + 0.81 | 39.86 + 1.80 | 41.13 + 0.90 | 39.83 + 1.40 | 39.00 + 1.92 |
| RDW-CV (%) | 22.35 + 1.34 | 21.73 + 0.15 | 21.33 + 0.60 | 22.06 + 0.50 | 21.83 + 0.20 | 21.16 + 0.87 |
| PCT (ng/mL) | 0.86 + 0.01 | 0.67 + 0.03 | 0.61 + 0.02 | 0.58 + 0.11 | 0.75 + 0.06 | 0.53 + 0.24 |
| MONO (x103/mL) | 0.37 + 0.31 | 0.23 + 0.03 | 0.19 + 0.05 | 0.30 + 0.06 | 0.49 + 0.22 | 0.45 + 0.10 |
| EO x (103/mL) | 0.05 + 0.01 | 0.08 + 0.04 | 0.34 + 0.02 | 0.05 + 0.01 | 0.04 + 0.01 | 0.07 + 0.04 |
Note: WBC = White Blood Cells; RBC = Red Blood Cells; MCV = Mean Corpuscular Volume; MCH = Mean Corpuscular Hemoglobin; MCHC = Mean Corpuscular Hemoglobin Concentration; PLT = Platelet; RDW-SD = Standard Deviation in Red Cell Distribution Width; RDW-CV = Coefficient of Variation in Red Cell Distribution Width; PCT = Procalcitonin; MONO = Monocytes; EO= Eosinophils; pg = pictograms; Ra=R. abyssinicus rhizome, Cm=C.murale leaves, Sp=S. punctata aerial parts, Aa=A. aspera leaves and Ap=A. pulcherrima gel. Values are represented as mean ± SD (n = 6).
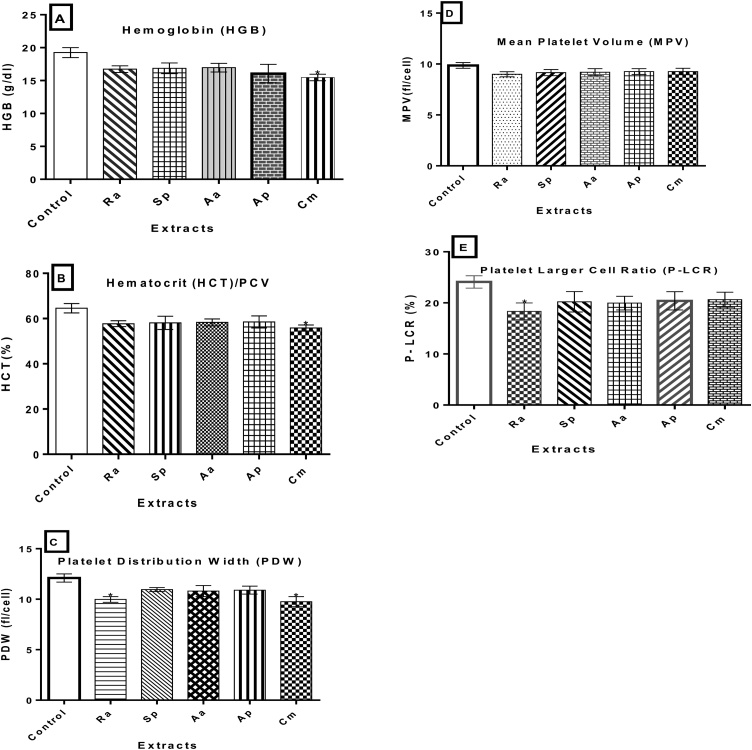
Hemoglobin (HGB) concentration (19.25 g/dl) and platelet distribution width (PDW) (9.76 fl/cell) were reduced significantly for C. murale extract administrations (P < 0.05). Similarly, PDW (9.98 fl/cell) and a platelet larger cell ratio (P-LCR) concentrations reduced(18.33 %) significantly following exposures to R. abyssinicus extract (P < 0.05). Although it was not statistically significant, there was a decrease in the mean platelet volume (MPV) for all extracts (Ra, Sp, Aa and AP) administered compared with the control (9.86 fl/cell). C. murale extract were associated with a reduced PCV (55.83 %) compared with the control (64.55 %) (Fig. 4A-E).
Fig. 4.
Hematological markers of toxicity in non-pregnant female Wistar albino rats exposed for 28 days. Values are expressed as mean ± SD (n = 6). (A) HGB, (B) PCV, (C) PDW, (D) MPV, and (E) P-LCR. Note: Aa=A. aspera leaves, Sp=S. punctata aerial parts, Ra=R. abyssinicus rhizome, Cm=C. murale leaves, and Ap=A. pulcherrima gel. *P < 0.05 statistically significant compared to the control group.
3.2.3. Effects of plant extracts on biochemical markers of liver toxicity
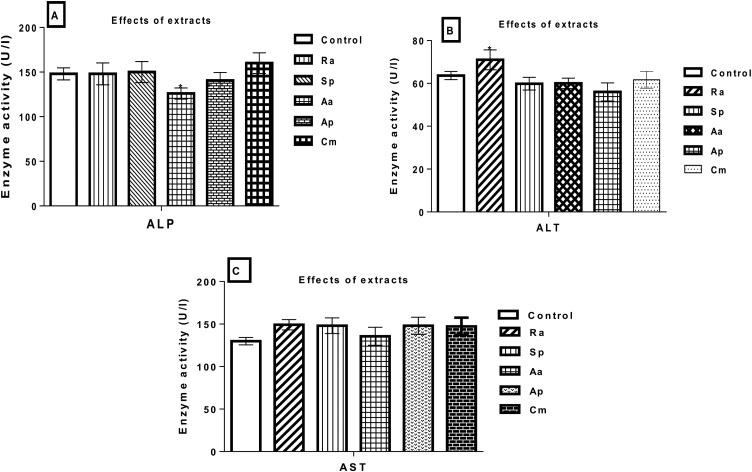
Following an exposure to A. aspera extract, the level of ALP was decreased (126U/L) compared to the control (148U/L). Similarly, ALT levels increased significantly(p < 0.05) in the administration of R. abyssinicus extract (71U/L) compared to the control (63.65U/L). However, all tested extracts did not show effect on AST levels (Fig. 5A-C).
Fig. 5.
Estimation of (A) ALP, (B) ALT, and (C) AST in serum indicating the effects of plant extracts on liver functional indices of non-pregnant female Wistar rats exposed for 28 days. Note: ALP = Alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, Aa=A. aspera leaves, Sp=S. punctata aerial parts, Ra=R. abyssinicus rhizomes, Cm=Chenopodium murale leaves, and Ap=A. pulcherrima gel. The data illustrated the mean + SD of six rats per treatment groups (n = 6). *P < 0.05 statistically significant compared to the control group.
3.2.4. Histopathological examination for extract toxicity of the liver and kidneys
3.2.4.1. Liver histopathology
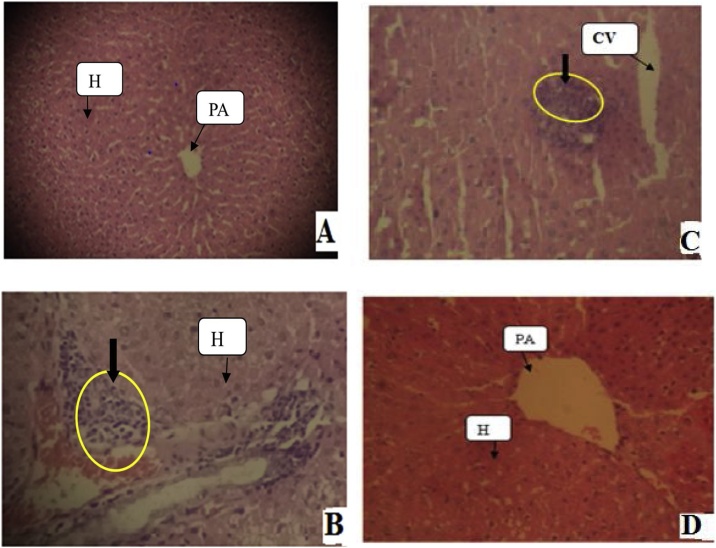
Histopathological studies of liver demonstrated that the extract of C. murale was toxic at given dose revealing focal periportal hepatitis (Fig. 6B), whereas R. abyssinicus, A. aspera and S. punctata extracts showed mild acute injury on liver tissues in the given dose 2000 mg/kg body weight in comparison with the control group(Fig. 6C). Indications of sub-acute toxicity in the liver, following exposure of rats to plant extracts were characterized microscopically by fine septa of extracellular matrix (chiefly collagen) that divided hepatocyte plates into small clusters of individual hepatocyte (Fig. 6).
Fig. 6.
Effects of plant extracts on liver tissues of non-pregnant female rats exposed for 28 days. (A) Liver tissues from control rats had normal architecture with hepatocytes arranged around the central vein; (B) Liver showing periportal hepatitis (C. murale extract); (C)Liver tissue revealed local inflammatory infiltrations (R. abyssinicus, A. aspera and S. punctata extracts), and (D) A. pulcherrima extracts with normal view. Photomicrographs were at 100x magnification using light Microscope, and 5 μm thick paraffin sections, Hematoxylene and Eosin stain. Arrows indicate on tissues with yellow circle shows lobular hepatitis. Note: PA = Portal Area; H= Hepatocytes; and CV = Central Vein.
3.2.4.2. Kidney histopathology
The extract of C. murale showed a patch of mild acute kidney tubular injuries (Fig. 7B). Some minor tubular inflammations were also observed following exposure to R. abyssinicus, A. aspera and S. punctata extracts (Fig. 7C).
Fig. 7.
Sub-acute toxic effects of plant extracts on kidney tissues of on-pregnant female rats after exposure for 28 days. (A) Kidney tissues of control group showing normal morphology of the glomeruli and tubules; (B) Mild tubular injury with congestions (C. murale extract); (C) Mild tubular inflammations (R. abyssinicus, A. aspera and S. punctata extracts), (D) A. pulcherrima extract with normal view. Photomicrographs were at 100x magnification using a light microscope, and 5 μm thick paraffin sections, Hematoxylene and Eosin stain. Arrows with yellow circles indicate tissues affected as a result of herbal extracts. Note: G = Glomerulus, BS = Bowman’s Space, DT = Distal Convoluted Tube.
4. Discussion
The body weight loss observed in the experimental rats, following R. abyssinicus and C. murale extracts administered at 2000 mg/kg during acute toxicity study was similar to the reports on other plant extracts [19,55,53]. The weight loss in the rats may be due to anorexia and disturbances in carbohydrates, proteins or fat metabolism, which may have been affected by administering extracts as suggested by Ghelani et al. [56].
A significant reduction in platelet count by administrations of C. murale, R. abyssinicus and S. punctata extracts can be an indication of acute toxicity to the test rats. The reduction in Mean Corpuscular Hemoglobin Concentration (MCHC) following C. murale extract administration is also an indication that induces anemia. This may be supported by the fact that short-term exposure of erythrocytes to cytotoxic agents could result in a hemoglobin reduction [58]. Although earlier work on S. punctata essential oils revealed cytotoxicity and hemolytic properties of human monocytic leukemia cells (THP-1 cell lines) [57] and erythrocytes [45], no toxic effects were detected in the present study, which used crude extracts of the plant's aerial parts.
Acutely non-toxic compounds may be toxic on prolonged exposures due to its accumulation, effects on enzyme levels, and the disruption of physiological and biochemical homeostasis [14]. In sub-acute toxicity study, the concentrations of hemoglobin (HGB) and platelet distribution width (PDW) were reduced significantly during C. murale extract administrations. Similarly, PDW and the platelet larger cell ratio (P-LCR) concentrations significantly declined for R. abyssinicus extract compared with the control. A previous study reported that R. abyssinicus extract did not cause significant change in hematological indices, but increased HCT concentrations at dose 1500 mg/kg b.w [66]. Thus, reductions in the HGB, PDW and P-LCR concentrations might imply that the test extract interfered with the normal production of hemoglobin within RBCs. This context is supported by the fact that short-term exposure of erythrocytes to cytotoxic agents result in hemoglobin reductions [58].
A significant reduction in the activities of alkaline phosphatase (ALP) following A. aspera extract administration is suggestive of its anti-urolithic potential of the plant extract similar to a study on Phyllanthus niruri extract, which had demonstrated that the decrease in ALP level contributes to a reduction in the number of renal calculi [59]. Furthermore, it was reported that A. aspera extract have hepatoprotective activity against paracetamol-induced toxicity at dose 250 mg/kg b.w [60] and it is non-toxic administered intraperitoneally in rats [40], which is a good indication that would justify its use as an antiurolithic treatment.
The extracts of R. abyssinicus, A. aspera and S. punctata showed normal glomeruli with slight necrosis of tubular cells. A previous study demonstrated the nephroprotective effects of R. abyssinicus extract [54] indicating the need for further evaluations of this plant extract. Because of the disruptions of blood flow through the liver and the failure of hepatocytes to come in contact with blood, there was profound hepatic dysfunction. The patches of tubular epithelial injuries observed in liver sections were an indication that C. murale causes sub-acute toxicity. It was also reported that R. abyssinicus extract revealed congestions and cellular infiltrations in the liver and kidneys of rats at dose 1500 mg/kg b.w [66]. Although R. abyssinicus extract showed slight necrosis of renal tubular cells, previous studies demonstrated its nephroprotective effects [60] indicating the need for further evaluations of the extract.
The mode of action of most phytotoxins associated with toxicants is unclear [61]. However, it is reported that toxicants may exert on microtubules [62], causes cellular hypersensitivity, or cellular modifications [10], interfere with nutrient absorptions [63], and being pro-oxidants (inducing oxidative stress or inhibiting antioxidant systems) when there are high doses of phytochemicals such as polyphenols and flavonoids [64]. Moreover, it has been also suggested that soil contaminants such as heavy metals, aflatoxins, and pathogenic microbes during plant extract preparation causes toxicity [65].
5. Conclusion
The findings of the present study demonstrated that hydro-ethanolic extracts of A. aspera, S. punctata, R. abyssinicus, C. murale, and A. pulcherrima did not cause mortality in experimental rats at dose 2000 mg/kg in sub-acute toxicity tests. However, C. murale extract induced toxicity as supported by hematological and histological findings. The information obtained from this study can serve as a baseline for further pharmacological studies of these medicinal plants. Furthermore, chronic toxicity studies and phytochemical characterizations of A. aspera, S. punctata, and R. abyssinicus extracts would be beneficial.
Ethical approval
The use of animal experimental protocols was approved by the Animal Ethics Committee, College of Natural Sciences Institutional Review Board (CNS-IRB) (Approval Minute No. IRB/020/2016), Addis Ababa University.
Availability of data and materials
Data supporting the findings are presented within the manuscript and data are secured (not publicly available), and the research is ongoing for some other purposes.
Authors' contributions
TA: Conceived the project idea, designed the study protocol, performed the experiment, collected and analyzed data, and drafted the manuscript; BP: Involved in evaluation of the study protocol, and manuscript editing; BP and TS: Involved in fundraising; AD: contribute in evaluation of the study protocol, and manuscript overview; NF: Participate in plant material collections and partly involved in animal surgery; DS: Deals with histopathology and proofreading; and DC: Involved in hematological studies. All authors approved the final manuscript.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
The authors would like to thank Addis Ababa University for financial support and laboratory facilities. We also thank the Ethiopian Public Health Institute (EPHI) providing laboratory space and associated facilities. Moreover, we thank Mrs. Zinayitu Tafere for her inputs during data collection. We also would like to thank Dr. Guadie Sharew (MD) for his vital comments on the manuscript.
References
- 1.Ankur C., Amarchand P., Aadarsh C., Deepa I., Pawar R.S., Patil U.K. Potential of medicinal plants in kidney, gall and urinary stones. Int. J. Drug Dev. Res. 2010;2(2):431–447. [Google Scholar]
- 2.Diallo D., Paulsen B.S., Hvemm B. Production of traditional medicine: preparations accepted as medicines in Mali. In: Hostettmann K., Chinyanganya F., Maillard M., Wolfender J.L., editors. Chemistry, Biological and Pharmacological Properties of African Medicinal Plants: Proceedings of the First International IOCD-Symposium. UZ Publications; Victoria Falls, Zimbabwe: 1996. pp. 235–243. February 25-28 Harare. [Google Scholar]
- 3.Endashaw B. Study on actual situation of medicinal plants in Ethiopia. Japan Assoc. Int.Coll. Agric. Fores. 2007 http://www.jaicaf.or.jp/publications/ethiopiaac.pdf [Google Scholar]
- 4.Rates S.M. Plants as source of drugs. Toxicon. 2001;39(5):603–613. doi: 10.1016/s0041-0101(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 5.Kale O.E., Awodele O., Akindele A.J. Subacute and subchronic oral toxicity assessments of Acridocarpus smeathmannii (DC.) Guill. & Perr. Root in Wistar rats. Toxicol. Rep. 2019;6:161–175. doi: 10.1016/j.toxrep.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharif H., Mukhtar M., Mustapha Y., Baba G., Lawal A. Acute and subchronic toxicity profile of Euphorbia pulcherrima methanol extract on Wistar albino rats. Adv. Pharm. J. 2015:9. Article ID 539646. [Google Scholar]
- 7.Moreira S.S., Tamashiro L.K., Jorge B.C., Balin P.S., Heredia-Vieira S.C., Almeida G.L., Cardoso C.A.L., Kassuya C.A.L., Arena A.C. Toxicological safety evaluation in acute and 28-day studies of aqueous extract from Serjania marginata Casar. (Sapindaceae) levels in rats. J. Ethnopharmacol. 2018 doi: 10.1016/j.jep.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Boelsterli U.A. 1st ed. Taylor and Francis Inc.; London and New York: 2003. Mechanistic Toxicology.THe Molecular Basis of How Chemicals Disrupt Biological Targets; pp. 1–314. [Google Scholar]
- 9.Saad B., Azaizeh H., Abu-Hijleh G., Said O. Safety of traditional Arab herbal medicine, Evid.-Based compl. Altern. Med. 2006;3:433–439. doi: 10.1093/ecam/nel058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guengerich F.P. Drug Metabolism and Pharmacokinetics (DMPK) Advance Publication by J-STAGE; 2010. Mechanisms of drug toxicity and relevance to pharmaceutical development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhiouani H., El-Hilaly J., Israili Z.H., Lyoussi B. Acute and sub-chronic toxicity of an aqueous extract of the leaves of Herniaria glabra in rodents. J. Ethnopharmacol. 2008;118:378–386. doi: 10.1016/j.jep.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Bhardwaj S., Gupta D. Study of acute, subacute and chronic toxicity test. Int. J. Adv. Res. Pharmaceu. BioSci. 2012;2:103–129. [Google Scholar]
- 13.Gadanya A., Sule M., Atiku M. Acute toxicity study of “GADAGI” Tea on rats. Bayero J. Pur. Appl. Sci. 2011;4:147–149. [Google Scholar]
- 14.Gandhare B., Kavimani S., Rajkapoor B. Acute and subacute toxicity study of methanolic extract of Ceiba pentandra (Linn.) Gaertn. on rats. Int. J. Sci. Res. 2013;5:315–324. [Google Scholar]
- 15.Singh A.K., Attrey D.P., Deep P., Dubey S., Naved T., Roy B. Acute and subacute toxicity studies of pharmacologically active Seabuckthorn leaf extract. Int. J. Pharm. Sci. 2014;6:415–419. [Google Scholar]
- 16.OECD Guidelines for the testing of chemicals. Acute Oral Toxicity-Acute Toxic Class Method. 2001;(423):1–14. [Google Scholar]
- 17.Unuofin J.O., Otunola G.A., Afolayan A.J. Evaluation of acute and subacute toxicity of whole-plant aqueous extract of Vernonia mespilifolia less. in Wistar rats. J. Integr. Med. 2018 doi: 10.1016/j.joim.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Dybing E., Doe J., Groten J., Kleiner J., O’brien J., Renwick A., Schlatter J., Steinberg P., Tritscher A., Walker R. Hazard characterization of chemicals in food and diet: dose response, mechanisms and extrapolation issues. Food Chem. Toxicol. 2002;40:37–282. doi: 10.1016/s0278-6915(01)00115-6. [DOI] [PubMed] [Google Scholar]
- 19.Bello I., Bakkouri A.S., M.Tabana Y., Al-Hindi B., Al-Mansoub M.A., Mahmud R., Asmawi M.Z. Acute and sub-acute toxicity evaluation of the methanolic extract of Alstonia scholaris stem bark. Med. Sci. 2016;4(4) doi: 10.3390/medsci4010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuel A.J.S.J., Mohan S., Chellappan D.K., Kalusalingam A., Ariamuthu S. Hibiscus vitifolius (Linn.) root extracts shows potent protective action against anti-tubercular drug induced hepatotoxicity. J. Ethnopharmacol. 2012;141:396–402. doi: 10.1016/j.jep.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 21.Rhiouani H.R., Nazari P., Kamli-Nejad M., Lyoussi B. Acute and subchronic oral toxicity of an aqueous extract of leaves of Herniaria glabra in rodents. J. Ethnopharm. 2008;118:378–386. doi: 10.1016/j.jep.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Ravikumar S., Gnanadesigan M. Hepatoprotective and antioxidant properties of Rhizophora mucronata Mangrove Plant in CCl4 intoxicated rats. Int. J. Clin. Exp. Med. 2012;4:66–72. [Google Scholar]
- 23.Arsad S.S., Esa N.M., Hamzah H., Othman F. Evaluation of acute, subacute and subchronic oral toxicity of Rhaphidophora decursiva (Roxb.) Schott extract in male Sprague Dawley rats. J. Med. Plant Res. 2013;7:3030–3040. [Google Scholar]
- 24.Ghadirkhomi A., Safaeian L., Zolfaghari B., Aghaye-Ghazvini M.R., Rezaei P. Evaluation of acute and sub-acute toxicity of Pinus eldarica bark extract in Wistar rats. Avicenna J. Phytomed. 2016;6(5):558–566. [PMC free article] [PubMed] [Google Scholar]
- 25.Amna O.F., Nooraain H., Noriham A., Azizah A., Husna R.N. Acute and oral subacute toxicity study of ethanolic extract of Cosmos caudatus leaf in Sprague Dawley rats. Int. J. Biosci. Biochem. Bioinforma. 2013;3(4):301–305. [Google Scholar]
- 26.Mahmoud A.M. Hematological alterations in diabetic rats- Role of adipocytokines and effect of citrus flavonoids. Exp. Clin. Sci. Int. J. 2013;12:647–657. [PMC free article] [PubMed] [Google Scholar]
- 27.Wintrobe M.M., Greer J.P. Lippincott Williams & Wilkins; Philadelphia: 2009. Wintrobe’s Clinical Hematology. [Google Scholar]
- 28.WHO . World Health Organization; Geneva: 1993. I.P.C.S Environmental Health Criteria 155: Biomarkers and Risk Assessment: Concepts and Principles; pp. 11–17. [Google Scholar]
- 29.Elsewefy D.A., Farweez B.A., Ibrahim R.R. Platelet indices: consideration in thrombocytopenia. Egypt J. Haematol. 2014;3(39):134–138. [Google Scholar]
- 30.Kaito K., Otsubo H., Usui N., Yoshida M., Tanno J., Kurihara E., Matsumoto K., Hirata R., Domitsu K., Kobayashi M. Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity, and specificity in the diagnosis of immune thrombocytopenia. Br. J. Haematol. 2005;128:698–702. doi: 10.1111/j.1365-2141.2004.05357.x. [DOI] [PubMed] [Google Scholar]
- 31.Hawkins R.I. The importance of platelet function tests in toxicological screening using laboratory animals. Lab. Anim. 1972;6:155–167. doi: 10.1258/002367772781006383. [DOI] [PubMed] [Google Scholar]
- 32.Elzagallaai A.A., Koren G., Rieder M.J. The predictive value of the in vitro platelet toxicity assay (iPTA) for the diagnosis of hypersensitivity reactions to sulfonamides. J. Clin. Pharmacol. 2013;53(6):626–632. doi: 10.1002/jcph.85. [DOI] [PubMed] [Google Scholar]
- 33.Abate G. In: Etse Debdabe: Ethiopian Traditional Medicine. 1st`ed. Demissew S., editor. Department of Biology, Science Faculty, Addis Ababa University Press; Addis Ababa: 1989. pp. 33–183. Amharic version) [Google Scholar]
- 34.Belay M.A. Book of Ethiopian Traditional Medicine. 2014. Metsafe adhnote, tikmu mulu Tena lehulu. Addis Ababa, Language (“Amharic”). Artistic P.E.B/11256. [Google Scholar]
- 35.Aggarwal A., Tandon S., Singla S.K., Tandon C. Reduction of oxalate-induced renal tubular epithelial (NRK-52E) cell injury and inhibition of calcium oxalate crystallization in vitro by aqueous extract of Achyranthes aspera. Int. J. Green Pharm. 2010;4(3):159–164. doi: 10.1590/s1677-55382010000400011. [DOI] [PubMed] [Google Scholar]
- 36.WHO . World Health Organization; Ethiopia: 2004. Global Status Report on Alcohol.www.who.int/substance abuse/ publications /en/ethiopia.pdf. [Google Scholar]
- 37.Ferreira P., Cardoso T., Ferreira F., Fernandes-Ferreira M., Piper P., Sousa M.J. Mentha piperita essential oil induces apoptosis in yeast associated with both cytosolic and mitochondrial ROS-mediated damage. FEMS Yeast Res. 2014;14(7):1006–1014. doi: 10.1111/1567-1364.12189. [DOI] [PubMed] [Google Scholar]
- 38.Uniyal S.K., Singh K.N., Jamwal P., Lal B. Traditional use of medicinal plants among the tribal communities of Chhota Bhangal, Western Himalaya. J. Ethnobiol. 2006;2:1–14. doi: 10.1186/1746-4269-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okon B.E., Essien E.E., Poh C.F., Orji M.H. An evaluation of the sub acute toxicity and haemostatic effects of leaves extract of Achyranthes aspera in mice and albino rats. Eur. J. Med. Plant. 2015;7(1):16–25. [Google Scholar]
- 40.Reddy C.V., Kamble A.K. Toxicity study of Achyranthus aspera. Int. Lett. Nat. Sci. 2014;9:85–96. [Google Scholar]
- 41.Getie M., Gebre-Mariam T., Rietz R., Höhne C., Huschka C., Schmidtke M., Abate A., Neubert R.H.H. Evaluation of the Anti-microbial and Antiinflammatory activities of the Medicinal plants Dodonaea viscosa, Rumex nervosus and Rumex abyssinicus. Fitoterapia. 2003;74(1-2):139–143. doi: 10.1016/s0367-326x(02)00315-5. [DOI] [PubMed] [Google Scholar]
- 42.Mekonnen T., Urga K., Engidawork E. Evaluation of the diuretic and analgesic activities of the rhizomes of Rumex abyssinicus Jacq in mice. J. Ethnopharmacol. 2010;127(2):433–439. doi: 10.1016/j.jep.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Hedberg I., Kelbessa E., Edwards S., Demissew S., Persson E. Flora of Ethiopia and Eritrea, addis ababa university. Addis Ababa. 2006;5:516–517. [Google Scholar]
- 44.Momtaz S., Abdollahi M. An update on pharmacology of Satureja species; from antioxidant, antimicrobial, anti-diabetes and anti-hyperlipidemic to reproductive stimulation. Int. J. Pharmacol. 2010;6:346–353. [Google Scholar]
- 45.Tepe B., Cilkiz M. A pharmacological and phytochemical overview on Satureja. Pharm. Biol. 2015;54(3):375–412. doi: 10.3109/13880209.2015.1043560. [DOI] [PubMed] [Google Scholar]
- 46.Teklu S., Alemayehu G., Abegaz B.M. Chemical composition of the essential oil of Satureja punctata. J. Essent. Oil Res. 1998;10(3):339–341. [Google Scholar]
- 47.Andrews F.W. 1st ed. Volume 1-III. Arbroath, T. Buncle and Co. Ltd., Arbroath, Scotland, for the Sudan Government; 2009. (The Flowering Plants of Anglo-Egyptian Sudan (1950-1956)). [Google Scholar]
- 48.Ahmad B., Jan Q., Choudhary M.I., Nisar M. Phytochemical evaluation of Chenopodium murale L. Asian J. Plant Sci. 2003;2:1072–1078. [Google Scholar]
- 49.Jan G., Khan M.A., Jan F. Medicinal value of the Asteraceae of Dir Kohistan Valley, NWFP, Pakistan. J. Ethnob. Leaflets. 2009;13:1205–1215. [Google Scholar]
- 50.Demissew S., Friis I., Awas T., Wilkin P., Weber O., Bachman S., Nordal I. Four new species of Aloe (Aloaceae) from Ethiopia, with notes on the ethics of describing new taxa from foreign countries. Kew Bull. 2011;66:111–121. [Google Scholar]
- 51.Abdissa D., Geleta G., Bacha K., Abdissa N. Phytochemical Investigation of Aloe pulcherrima roots and evaluation for its Antibacterial and Antiplasmodial activities. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0173882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doddola S., Pasupulati H., Koganti B., Prasad K.V.S.R.G. Evaluation of Sesbania grandiflora for Antiurolithiatic and antioxidant properties. J. Nat. Med. 2008;62:300–307. doi: 10.1007/s11418-008-0235-2. [DOI] [PubMed] [Google Scholar]
- 53.Ogbuehi I.H., Ebong O.O., Obianime A.W. Oral acute toxicity (LD50) study of different solvent extracts of Abrus precatorius Linn leaves in Wistar rats. Eur. J. Exp. Biol. 2015;5:18–25. [Google Scholar]
- 54.Jaganathan R., Ravinayagam V., Panchanadham S., Palanivelu S. Toxicological, biochemical and histopathological evaluation of Tridham, a siddha medicine in Wistar albino rats. J. Biochem. Technol. 2012;4(1):541–548. [Google Scholar]
- 55.Prasanth K.M., Suba V., Ramireddy B., Srinivasa B.P. Acute and subchronic oral toxicity assessment of the ethanolic extract of the root of Oncoba spinosa (Flacourtiaceae) in rodents. J. Biochem. Technol. 2015;14:1849–1855. [Google Scholar]
- 56.Ghelani H., Chapala M., Jadav P. Diuretic and antiurolithiatic activities of an ethanolic extract of Acorus calamus L. rhizome in experimental animal models. J. Tradit. Complement. Med. 2016;6:431–436. doi: 10.1016/j.jtcme.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tarikua Y., Hymeteb A., Hailuc A., Rohlof J. Essential oil composition, Antileishmanial, and toxicity study of Artemisia abyssinica and Satureja punctata sp. from Ethiopia. Chem. Biodivers. 2010;7:1009–1018. doi: 10.1002/cbdv.200900375. [DOI] [PubMed] [Google Scholar]
- 58.da Silva M.G.C., Amorim R.N.L., Câmara C.C., Fontenele-Neto J.D., Soto-Blanco B. Acute and sub-chronic toxicity of aqueous extracts of Chenopodium ambrosioides leaves in rats. J. Med. Food. 2014;17:979–984. doi: 10.1089/jmf.2013.0134. [DOI] [PubMed] [Google Scholar]
- 59.Pucci N.D., Marchini G.S., Mazzucchi E., Reis S.T., Srougi M., Evazian D., Nahas W.C. Effect of Phyllanthus niruri on metabolic parameters of patients with kidney stone: a perspective for disease prevention. Int. Braz J Urol. 2018;44(4):758–764. doi: 10.1590/S1677-5538.IBJU.2017.0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar S.V.S., Chandrika G., Mahesh K., Meghanath P.V.S. Hepatoprotective Activity of Achyranthes aspera linn against Paracetamol induced toxicity. Int. J. Pharm. Sci. 2012;4(5):299–302. [Google Scholar]
- 61.Agarwal A.K., Baerson S.R., Rogers P.D., Jacob M.R., Barker K.S., Cleary J.D., Walker L.A., Nagle D.G., Clark A.M. Genome-wide expression profiling of the response to polyene, pyrimidine, azole, and echinocandin antifunal agents in Saccharomyces cerevisiae. J. Biol. Chem. 2003;278:34998–35015. doi: 10.1074/jbc.M306291200. [DOI] [PubMed] [Google Scholar]
- 62.Iwasaki S. Natural organic compounds that affect to microtubule functions. Yakugaku Zasshi. 1998;118(4):112–126. [PubMed] [Google Scholar]
- 63.Leonard B., Takayuki S. 2nd ed. Elsevier; Burlington: 2009. Introduction to Food Toxicology; p. 124. ISBN 9780080921532. [Google Scholar]
- 64.Halliwell B. Dietary polyphenols: good, bad, or indifferent for your health? Cardiovasc. Res. 2007;73(2):341–347. doi: 10.1016/j.cardiores.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Parshuram N.S., Sateesh B. Acute and 28-day oral toxicity studies of methanolic extract of Lagenaria siceraria (Cucurbitaceae) fruit in rats. Drug Chem. Toxicol. 2019:1–9. doi: 10.1080/01480545.2019.1617302. [DOI] [PubMed] [Google Scholar]
- 66.Mugisha M.K., Ndukui J.G., Namutembi A., Waako P., Karlson A.-K.B., Vudriko P. Acute and sub-acute toxicity of ethanolic leaf extracts of Rumex abyssinica Jacq. (Polygonaceae) and Mentha spicata L.(Lamiaceae) J. Pharm. Pharmacol. 2014;5(3):10. Article ID:44242. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the findings are presented within the manuscript and data are secured (not publicly available), and the research is ongoing for some other purposes.