Abstract
Malaria is one the leading health problem of the Ethiopia. Previously, areas above 2,000 m elevation were considered as malaria free areas. However, the major malaria epidemics were seen in areas at an altitude up to 3,000 m above sea level. These epidemics were due to climate and land-use changes (ecological changes) and still malaria is a growing health problem in highland parts of Ethiopia. This study aimed to investigate the species diversity, abundance and distribution of Anopheles mosquitoes in highland fringe of Bure district, Northwestern Ethiopia. It was done in the three different agroecological villages, Bukta (Irrigated), Workimdr (non-irrigated with few dry season breeding habitats) and Shnebekuma (non-irrigated with many dry season breeding habitats). Anopheles mosquitoes were collected by the Centers for Disease Control and Prevention Light Traps Catches, Pyrethrum Spray Catches, and Artificial Pit Shelters (APSs) from twenty-seven houses, thirty houses, and six APSs, respectively. Anopheles mosquitoes were identified morphologically to species using standard keys. Furthermore, molecular identification of Anopheles gambiae s.l was carried out using species-specific Polymerase Chain Reaction. Independent T-Test and One-way- ANOVA were employed to compare the mean mosquito's density between villages and species, indoor and outdoor host seeking mosquitoes. Descriptive statistic was used to calculate the proportion of each Anopheles species. Nine Anopheles mosquito species were identified in the study area which includes: Anopheles demeilloni, An. arabiensis, An. funestus group, An. coustani, An. squamosus, An. cinereus, An. pharoensis, An. rupicolus, and An. natalensis. Of the 4,703 Anopheles mosquitoes collected, An. demeilloni was the most prominent (50.7%, n = 2383) whereas An. rupicolus (0.03%, n = 3), and An. natalensis (0.02%, n = 1) were the least abundant. Higher mean density of Anopheles mosquitoes was collected from the non-irrigated village (2.395 ± 0.100) than irrigated (1.351 ± 0.109) (p = 0.001). In conclusion, three of the most important malaria vectors (An. arabiensis, An. funestus group and An. pharoensis) of Ethiopia were recorded in the study sites, especially the first two was found thought-out the year. Most of the Anopheles mosquitoes were collected from non-irrigated villages. Thus, breeding habitat management must be practiced throughout the year together with long-lasting insecticide-treated nets and insecticide residual sprays.
Keywords: Ecology, Molecular biology, Zoology, Malaria, Highland part, Bure district, Anopheles arabiensis, Anopheles funestus group, Non-irrigated village
Ecology; Molecular biology; Zoology; Malaria; Highland part; Bure district; Anopheles arabiensis; Anopheles funestus complex; Non-irrigated village.
1. Introduction
Malaria is a complex disease caused by protozoan parasites (genus Plasmodium) and transmitted by blood-feeding infectious mosquitoes (Dutta and Dutt, 1978). Over 3,500 mosquitoes have been recorded worldwide (Fang, 2010; Harbach and Kitching, 2016); however, about 537 species are Anopheles (Harbach, 2013) and only 70–80 is known to transmit human malaria worldwide (Robert et al., 2011). Of these, 41 are considered to be dominant vector species (Hay et al., 2010; Sinka et al., 2012) and capable of transmitting malaria by large. In Africa, there are 140 Anopheles species, but only twenty are known to transmit malaria to human (Hay et al., 2000; Sinka et al., 2012). In Ethiopia, over 42 species of Anopheles were recorded (Gaffigan et al., 2013), but the major malaria vector is Anopheles arabiensis while An. pharoensis, An. funestus and An. nili are secondary vectors (MoH, 2012; PMI, 2017).
In Ethiopia, malaria is the leading health problem of the country (Carter Center, 2013) because three-fourth (75%) of the total area of the country is malarious and more than two-third (approximately 68%) of the total populations live below 2,000 m above sea level/m.a.s.l/(Ayele et al., 2012). However, many studies have confirmed the occurrence of malaria disease up to 2,500 m.a.s.l. in many highland fringes of Ethiopia, such as in the outskirts of Addis Ababa (Woyessa et al., 2004), in Amhara region (Kassa et al., 2015; Lake et al., 2016) and in South Nation Nationality People Region (Tesfaye et al., 2011). Totally, many studies indicated that malaria morbidity and mortality had been significantly reduced in Ethiopia (PMI, 2017) and in Bure district in particular (Toyama et al., 2016).
The Amhara region is one of the highland parts of Ethiopia where malaria is a common disease (Alemu et al., 2013; Lake et al., 2016). However, entomological monitoring in the Amhara region is incomplete (Vajda and Webb, 2017) and little information is known about the dynamics of malaria vectors in the region (Ndenga et al., 2006). Bure district is one of the malarious areas in Amhara region, where there was no any study conducted on the diversity, abundance and spatiotemporal distribution of Anopheles mosquitoes. Therefore, study on the species compositions, dynamics, distributions of mosquitoes at the local level (Grillet, 2000; Coetzee, 2004) and how they differ with each other biologically (Ramirez et al., 2009; Eckhoff, 2011) can help to design and apply appropriate control measures including integrated vector control strategies to defeat malaria and to develop early warning systems for predicting malaria epidemics (Ramirez et al., 2009; Kihadye et al., 2010). Hence, this study aimed to assess the diversity, abundance, and the distribution of Anopheles mosquitoes in Bure district, Northwestern Ethiopia.
2. Materials and methods
2.1. Study area
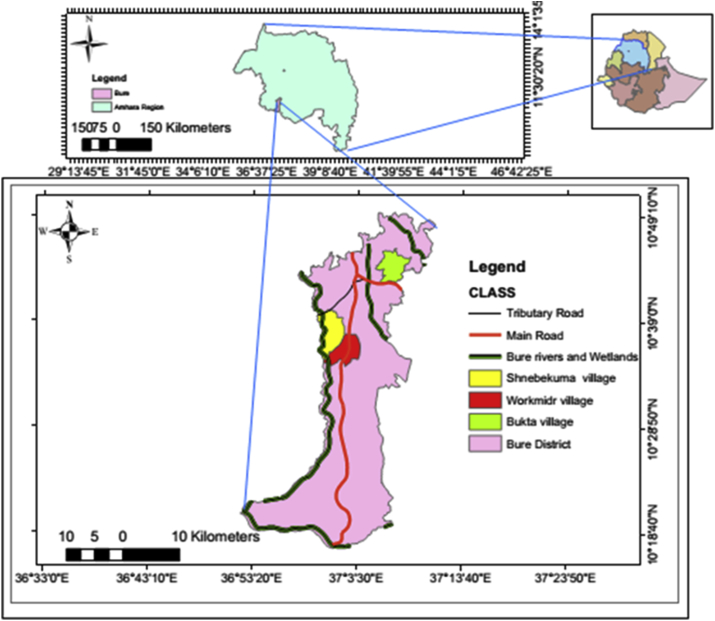
The study was conducted in Bure district, northwestern of Ethiopia, from July 2015 to June 2016. Geographically, Bure district is situated at an altitude ranging from 700 (Blue Nile gorge) to 2,350 m.a.s.l. (Figure 1). Socioeconomically, the majority (85%) of the populations are farmers who grow maize, teff (Eragrostis teff), pepper, potatoes, wheat, millets, followed by bean & pea, sunflower, niger, spices, vegetation's, and others; and the rests are merchants (6.8%) and others (non-governmental organizations, civil servants) (8.2%). Animals such as cattle, sheep, hens, mules, and donkeys are reared by the farmers. Additionally, both modern and traditional bee-keepers were present. The majority of the populations in the district live in houses made of mud and corrugated iron roofs.
Figure 1.
Map of the study area. a) Ethiopia, b) Amhara region, and c) Bure district.
The majority of Bure districts has subtropical zone (Woina-Dega) climate with annual mean minimum and maximum temperature of 9.9 °C and 29.2 °C, respectively and 2,000 mm mean annual rainfall range being 1,350–2,500 mm. The major rainy season of the district is from July to September, and a small amount is obtained from May to June and from October to December. The rest of the months (January–April) are dry seasons (Midekisa et al., 2015).
The study was conducted in three rural villages: Bukta, Workmidr and Shnebekuma, from July 2015–June 2016. Bukta Village: This village is found 8 km away from Bure town. Its geographic coordination is 10° 43.734′ N and 037° 06.555′ E. The village had 100 households with a total population of 403 individuals. The village has one annually flowing stream, and two marshlands. In this village, both traditional and modern irrigation was practiced. Workmidr Village: It is located about 12.5 km away from Bure Town at 10° 37.376′ N and 037° 02.392′ E. This village had 167 households and 542 inhabitants. Near to the village, one highly extended marshland is found. Moreover, annually flowing spring water and manmade pits were found, which were used for watering seedlings of pepper and eucalyptus trees. In this village, agriculture was based on rain. Shnebekuma Village: This village is found 8.5 km away from Bure town at 10° 38.932′ N and 037° 02.251′ E geographical coordinates. The village had about 136 households and 542 occupants. In this village, one highly extended and three small marshlands and one small stream were found. Agriculture was based on rain only. Generally, the three studied villages were surplus producers, but farmers in Bukta village are very rich. Totally, these villages are malarious, bed nets were distributed for the three villages once per 3-years before malaria infestation begins, on the first week of September. Moreover, anti-malaria chemical spraying (IRS) (Deltamethrin, K-Othrine Flow) was administered to the three villages according to the national spraying operation guidelines (MoH, 2012).
2.2. Adult mosquito collection, identification and processing
2.2.1. Mosquito collection
Anopheles mosquitoes were sampled longitudinally from July 2015–June 2016. Entomological surveys were conducted monthly in each village, for one year using Center for Disease Control and Prevention Light Trap Catches (LTCs), Pyrethrum Spray Catches (PSCs) and Artificial Pit- Shelters (APSs). In each village, 9 houses for LTCs and 10 houses for PSCs were randomly selected and scattered in near to the breeding sites, in the middle and periphery sides of the village. In parallel, 27-miniature light traps were prepared to collect the outdoor host seeking mosquitoes for the three villages, each had 9- LTCs. Additionally, six APSs were prepared in three villages to collect outdoor resting mosquitoes; each village had two.
Indoor host-seeking Anopheles mosquitoes were collected from 6:00 PM (sunset) to 6:00 AM (sunrise) by using miniature LTCs (Model 512; J. W. Hock Co., Atlanta, USA) once per month per house (Lines et al., 1991; Mboera et al., 1998). In the same trends, the outdoor host seeking mosquitoes were collected by LTCs from 06:00 AM to 06:00 PM hrs once per month.
Indoor-resting mosquitoes were collected in the mornings from 6:00 AM to 8:30 AM hrs using PSCs for the study period. Collection was made using white floor sheets, hand lenses, Baygon aerosol (Tetramethrin:0.4% and Permethrin:0.4%; SC. Johnson & Son. Inc, USA), small petri-dishes, paper cups with net covers, forceps, cotton wool, and a torch (WHO, 2003). Additionally, outdoor-resting mosquitoes were collected in the morning from 6:30 AM - 7:30 AM from APSs (1.5 m depths, 1.0 m width, and 1.2 m length) using a handheld mouth aspirator (WHO, 1975). The number of human occupants and other potential vertebrate hosts in each surveyed house during the previous night were recorded. Moreover, the house condition of each surveyed house was recorded; including the types of house, type of wall, and number of long-lasting insecticide treated nets (LLINs) used and spray status.
Outdoor-resting mosquitoes were collected from artificially made pit shelters by using a handheld mouth aspirator, paper cup with net covers, cotton wool, torch, and pencil. APSs were constructed under the shade of various dense shrub trees 10–15 m away from the resident villages. APSs had 1.5 m depths, 1.0 m width, and 1.2 m length (1.5 m × 1.0 m × 1.2 m). Approximately 0.5 m from the bottom of each pit-shelter, a 30-cm horizontal deep cavity was prepared in each of the four sides (WHO, 1975). A collection was made from 6:30 AM - 7:30 AM hrs. Before collection begins, the mouth of each pit shelter was covered with insecticide untreated white net to prevent mosquitoes from escaping and for visibility purpose. Resting mosquitoes was collected for about 10–20 min in each pit.
Totally, collection of mosquitoes were carried out after obtaining ethical approval from the ethical review committee of Addis Ababa University (Reference No.: CNSDO/382/07/15), Amhara Health Regional Bureau (Permission Reference No.: H/M/TS/1/350/07) and the Head of the Bure District Health Office (Permission Reference No.: BH/3/519L/2). Moreover, informed consent was obtained from the head of the selected households.
Anopheles Mosquito Species Identification: Mosquitoes collected by LTCs, PSCs and APSs were identified morphologically at genus level using taxonomic keys (Verrone, 1962; Gillies and Coetzee, 1987; Glick, 1992). Culex and male Anopheles were recorded and discarded.
Molecular Identification of Anopheles gambiae complex: Morphological identified and individually preserved An. gambiae specimens were identified by species-specific PCR (Wilkins et al., 2006) at the Molecular Biology Laboratory of Tropical and Infectious Diseases Research Centre, Jima University.
DNA was extracted from individual preserved An. gambiae complex species based on DNeasy Blood and Tissue Kits (2011). Then, DNA amplification was carried out. Following this, gel electrophoresis was carried out (Wilkins et al., 2006). At the end, agarose-gel was placed on UVP (Photo Doc-It-imaging system or UV-Trans- illuminator) to see the nature of the bands. Those mosquitoes that remained unamplified (without any band on the gel) were tested three times in an independent manner.
2.3. Data analysis
Anopheles mosquito data was entered into Microsoft Excel (Window-7) data sheets, for cleaning and analyzed using SPSS version-20 (SPSS, Inc., Chicago, IL, USA). Before any analysis, non-normalized data were transformed [log10 (x+1)]. Mean variations between Anopheles and Culex, between the indoor and outdoor host seeking mosquitoes were tested using independent samples T-test (p < 0.05). Variation in mean densities between species and species among villages were analyzed using one-way analysis of variance (ANOVA) (p < 0.05). Significant means (ANOVA) were separated using Tukey test (HSD). Simple descriptive statistics (count, percentage, tables, and figures) were used to assess different variables. Moreover, Anopheles mosquitoes biting densities (host seeking) across villages were calculated as the sum of each species female Anopheles caught in the village during the 12-month sampling period divided by the total number of LTCs for night-biting mosquitoes in each village (mosquito/LTCs/night). Similarly, indoor and outdoor mosquito biting densities were calculated as the sum of the female Anopheles catches caught in the villages during the 12-months sampling period divided by the total number of LTCs for night-biting mosquitoes in the villages (Okello et al., 2006). All statistical analyses were performed at the 5 % significance level.
3. Results
3.1. Diversity and abundance of Anopheles mosquitoes
A total of 11,625 female mosquitoes were collected in Bure district using all collection methods. Of these, 59.5% (n = 6922) belonged to the genus Culex, while the rest 40.5% (n = 4703) were from the genus Anopheles. The proportions of the two genera have not shown statistically significant difference (t = 1.165; df = 22; p = 0.257). Morphologically, nine species of the genus Anopheles were identified in the three villages, belonged to Anopheles demeilloni (50.7%), An. gambiae s.l (16.0%), An. funestus group (13.6%), An. coustani (12.9%), An. squamosus (5.0%), An. cinereus (1.5%), An. pharoensis (0.32%), An. rupicolus (0.006%) and An. natalensis (0.002%). The majority of Anopheles mosquitoes were collected by LTs (99.6%) as compared to PSCs (0.4%) and APSs (0.0%) (Table 1). Moreover, a total of 66 specimens of An. gambiae s.l was identified to species by PCR, of which 63 (95.5%) were successfully amplified and identified as An. arabiensis. Only three (4.5%) specimens which were checked for three times were not amplified. Hence, all An. gambiae s.l collected in this study were An. arabiensis.
Table 1.
Species composition, abundance and distribution of Anopheles mosquitoes by village and collection method in Bure District, Ethiopia.
| Study Sites | Anopheles Species, No (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Methods | An.ar | An. ph | An. fu | An. co | An. sq | An. Ci | An.dem | An.rup | An.nat | Total | |
| Bukta | LTs | 72 (100) | 3 (100) | 53 (100) | 103 (99) | 22 (100) | 45 (90) | 51 (100) | 1 (100) | 0 | 350 (98.3) |
| PSCs | 0 | 0 | 0 | 1 (1.0) | 0 | 5 (10) | 0 | 0 | 0 | 6 (1.7) | |
| APSs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 72 (100) | 3 (100) | 53 (100) | 104 (100) | 22 (100) | 50 (100) | 51 (100) | 1 (100) | 0 | 356 (100) | |
| Workmidr | LTs | 90 (98.9) | 3 (100) | 34 (100) | 113 (100) | 17 (100) | 1 (100) | 211 (100) | 2 (100) | 0 | 471 (99.8) |
| PSCs | 1 (1.1) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.2) | |
| APSs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 91 (100) | 3 (100) | 34 (100) | 113 (100) | 17 (100) | 1 (100) | 211 (100) | 2 (100) | 0 | 472 (100) | |
| Shnebekuma | LTs | 588 (100) | 9 (100) | 544 (98.4) | 389 (100) | 196 (100) | 15 (83.3) | 2121 (100) | 0 | 1 (100) | 3863 (99.7) |
| PSCs | 0 | 0 | 9 (1.6) | 0 | 0 | 3 (16.7) | 0 | 0 | 0 | 12 (0.3) | |
| APSs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 588 (100) | 9 (100) | 553 (100) | 389 (100) | 196 (100) | 18 (100) | 2121 (100) | 0 | 1 (100) | 3875 (100) | |
| Total Indoor-LTs | 343 | 10 | 261 | 225 | 80 | 30 | 828 | 0 | 1 | 1778 | |
| Total Outdoor-LTs | 407 | 5 | 370 | 380 | 155 | 31 | 1555 | 3 | 0 | 2906 | |
| Overall of each species, by LTs, No (%) | 750 (99.9) | 15 (100) | 631 (98.6) | 605 (99.8) | 235 (100) | 61 (88.4) | 2383 (100) | 3 (100) | 1 (100) | 4684 (99.6) | |
| Overall of each species, by PSCs, No (%) | 1 (0.1) | 0 (0.0) | 9 (1.4) | 1 (0.2) | 0 | 8 (11.6) | 0 | 0 | 0 | 19 (0.4) | |
| Overall of each species, by APSs (No %) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| All total, No (%) | 751 (100) | 15 (100) | 640 (100) | 606 (100) | 235 (100) | 69 (100) | 2383 (100) | 3 (100) | 1 (100) | 4703 (100) | |
| Overall % species | 16 | 0.32 | 13.6 | 12.9 | 5 | 1.5 | 50.7 | 0.006 | 0.002 | 100 | |
Note: An. ar = An.arabiensis, An. ph = An.pharoensis, An. fu = An. funestus group, An. co = An.coustani, An. squ = An. squamosus, An.ci = An.cinereus, An. dem = An.demeilloni, An.rup = An.rupicolus and An.nat = An.natalensis.
From all species, the most prominent was An. demeilloni, followed by An. arabiensis, An. funestus group and An. coustani; whereas An. pharoensis, An. cinereus, An. rupicolus and An. natalensis were the least representatives (F 8, 99 = 24.593, p = 0.0001) (Tables 1, 2 and 3).
Table 2.
Mean Anopheles mosquito density by village and species in Bure district, Ethiopia.
| Sites | Mean ± se | P - value |
|---|---|---|
| Bukta | 1.35 ± 0.11b | <0.0001 |
| Workmidr | 1.28 ± 0.20b | |
| Shnebekuma | 2.39 ± 0.10a | |
| Anopheles Species | Mean ± se | P - value |
| An. arabiensis | 0.31 ± 0.04b | <0.0001 |
| An. pharoensis | 0.01 ± 0 .01d | |
| An. funestus group | 0.25 ± 0.05bc | |
| An. coustani | 0.23 ± 0.06bc | |
| An. squamosus | 0.12 ± 0.04cd | |
| An. cinereus | 0.04 ± 0.01d | |
| An. demeilloni | 0.61 ± 0.07a | |
| An. rupicolus | 0.001 ± 0.002d | |
| An. natalensis | 0.001 ± 0.001d |
Note: Means followed by the same letter (s) in each column are not significantly different from each other at p < 0.05 (Tukey HSD). se = Standard Error.
Table 3.
Mean density of Anopheles species by village, Bure district, Ethiopia.
| Bukta |
Workmidr |
Shnebekuma |
P - value | |
|---|---|---|---|---|
| Species | Mean ± se | Mean ± se | Mean ± se | |
| An. arabiensis | 0.73 ± 0.10b | 0.79 ± 0.13b | 1.55 ± 0.12a | 0.001 |
| An. pharoensis | 0.06 ± 0.05 | 0.06 ± 0.05 | 0.15 ± 0.071 | 0.438 |
| An. funestus group | 0.51 ± 0.13b | 0.45 ± 0.11b | 1.48 ± 0.11a | 0.001 |
| An. coustani | 0.73 ± 0.16 | 0.49 ± 0.12 | 1.11 ± 0.20 | 0.082 |
| An. squamosus | 0.28 ± 0.11b | 0.27 ± 0.09b | 1.01 ± 0.12a | 0.001 |
| An. cinereus | 0.47 ± 0.12a | 0.03 ± 0.03b | 0.25 ± 0.09ab | 0.005 |
| An. demeilloni | 0.60 ± 0.10b | 0.90 ± 0.20b | 2.13 ± 0.10a | 0.001 |
Note: Mean (s) followed by the same letter (s) in the same row are not significantly different from each other at p < 0.05, Tukey HSD. se = Standard Error.
3.2. Abundance and spatiotemporal dynamics of Anopheles mosquitoes in the study area
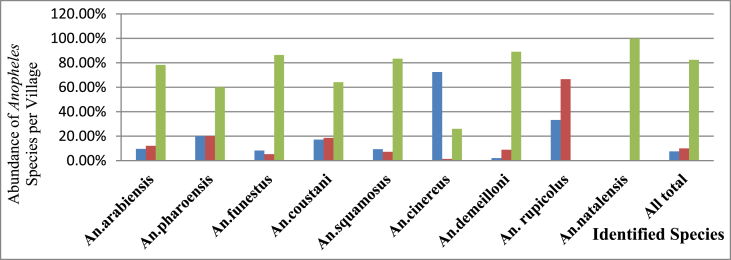
Greater number of adult Anopheles mosquitoes were collected in each of the two non-irrigated villages, Shnebekuma (n = 3875) and Workmidr (n = 472) than irrigated village, Bukta (n = 356) (ANOVA F = 19.202, p = 0.0001) (Figure 2) (Table 2).
Figure 2.
Percentage comparisons of Anopheles species by village, Bure district, Ethiopia.
Blue color represents Bukta, red color represents Workmidr, and green color represents Shnebekuma villages.
The abundance and mean density of each Anopheles mosquito by the village are presented in Figure 2 and Table 3, respectively. Significantly higher density of An. arabiensis (ANOVA F = 16.057, p = 0.0001), An. funestus group (ANOVA F = 23.935, p = 0.0001), An. squamosus (ANOVA F = 15.375, p = 0.0001) and An. demeilloni (ANOVA F = 33.832, p = 0.0001) were recorded from Shnebekuma than other villages. In Bukta, only An. cinereus was predominant. An. pharoensis, An. rupicolus and An. natalensis were very scarce and not distributed uniformly in the three villages; however, the remaining species were abundant in all villages. An. rupicolus was found in only Bukta and Workmidr; while An. natalensis was recorded from Shnebekuma Village (Table 1).
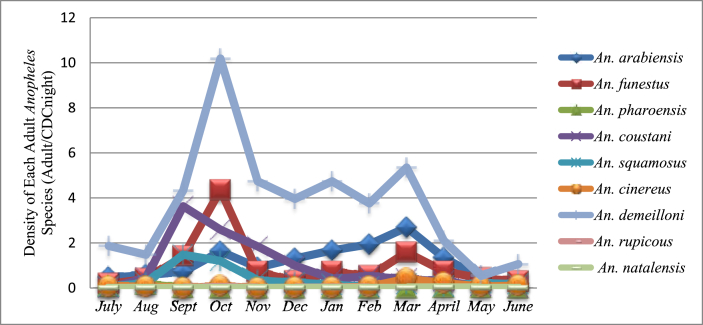
Figure 3 shows dynamics of Anopheles species over one-year study period. Anopheles demeilloni was the predominant species throughout the study period. Higher mean density of An. demeilloni (10.2 mosquito/night trap) and An. funestus group (4.4 mosquito/night trap) were recorded during October. However, mean density of An. arabiensis, the major malaria vector in Ethiopia was higher during March (2.7 mosquito/trap night).
Figure 3.
Densities of adult Anopheles mosquitoes across months (LTCs only).
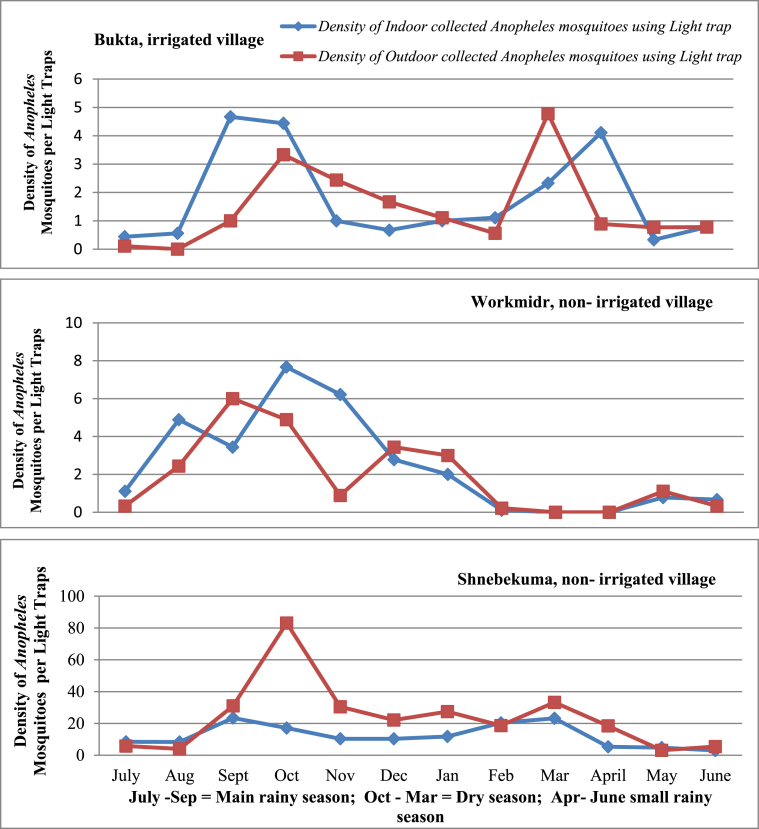
3.3. Density of indoor and outdoor host seeking Anopheles mosquitoes
Monthly indoor and outdoor hosts seeking Anopheles mosquito densities are shown in Figure 4. There was no significant difference in mean indoor and outdoor mosquito density among different species and villages (p > 0.05).
Figure 4.
Mean indoor and outdoor mosquito density by month of collection in three study villages, Bure district, Ethiopia.
4. Discussion
The overall aim of this study was to examine the diversity, abundance, and distribution of Anopheles mosquitoes in the three villages. A total of nine Anopheles species belonging to An. arabiensis, An. funestus group, An. pharoensis, An. coustani, An. squamosus, An. cinereus, An. demeilloni, An. rupicolus and An. natalensis were recorded in highland fringes of Bure district. An. arabiensis, the main malaria vector in Ethiopia (Fontaine et al., 1961; White et al., 1980), was also among others recorded in such high-altitude area (range 2,000–2,157 m.a.s.l.). This is in agreement with Woyessa (2001), Woyessa et al. (2004), and Dejenie et al. (2012) findings, where An. arabiensis from the outskirts of Addis Ababa and in Tigray region had been recorded at an altitude of 2,110 m.a.s.l. and 2,170m.a.s.l., respectively. Similarly, Animut et al. (2013a) and Tesfaye et al. (2011) recorded An. arabiensis at an altitude of 2,200 m.a.s.l. and 2,280 m.a.s.l. in south-central and southern Ethiopia, respectively. Moreover, this result is in agreement with other studies conducted in the highlands of Kenya (Mulambalah et al., 2011; Kweka et al., 2015).
In this study, what have been historically known the secondary malaria vectors (An. funestus and An. pharoensis) in Ethiopia, were recorded from the study area. An. funestus group was the second most abundant vector which was recorded in the higher altitude. Previously, An. funestus group had been reported from Gojam (O'connor, 1967). However, it is inconsistent with other reports from Ethiopia where this species had been recorded in areas below 2,000 m.a.s.l. (Ototo et al., 2015). The occurrence of this species up to 2,157 m.a.s.l. could be attributed to the presence of increased temperature as a result of climate change, land cover and land use changes (Afrane et al., 2012; Kweka et al., 2015). An. pharoensis was among the least prevalent species in the study area and it had been reported from high altitude areas of Ethiopia. Woyessa (2001) and Woyessa et al. (2004) reported An. pharoensis from the outskirt of Addis Ababa (2,110 m.a.s.l.). Recently, Animut et al. (2013b) reported An. pharoensis from Wurib at 2,200 m.a.s.l, in southern central Ethiopia.
Anopheles coustani was another mosquito recorded at 2,157 m.a.s.l. This observation is in line with Woyessa et al. (2004) and Chrispinus et al. (2011), who found An. coustani in highlands of Ethiopia and Kenya. An. coustani is a known malaria vector in Kenya (Mwangangi et al., 2013; Ogola et al., 2017) and was known to be a suspected vector in Ethiopia (Yewhalaw et al., 2014; Degafa et al., 2015). These scholars have detected sporozoite-infected An. coustani in southern central Ethiopia.
Overall, the proportion of An. demeilloni, An. arabiensis, An. funestus group, An. coustani, and An. squamosus were very high in this study as compared with previous reports (Woyessa et al., 2004; Dejenie et al., 2012; Animut et al., 2013b). The largest proportions of Anopheles mosquitoes were collected using LTCs (99.6%). The effectiveness of LTCs over the other methods is in agreement with Mala et al. (2011), and Animut et al. (2013a & 2013b). The inefficient of PSCs in this study could be due to change in resting behavior of mosquitoes, from endophilic to exophilic behavior. Similarly, it was impossible to collect single species of Anopheles using APSs; this is probably associated with the presence of other alternative hiding areas for exophilic mosquitoes.
Anopheles arabiensis showed both exophagic and endophagic behavior. This kind of feeding pattern of this species was observed in various parts of Ethiopia and Kenya (Olanga et al., 2015; Kenea et al., 2016; Taye et al., 2016). However, some reports from Ethiopia and Nigeria (Woyessa et al., 2004; Oyewole et al., 2007; Taye et al., 2017) showed that An. Arabiensis showed exophagic behavior while endophagic behavior of An. arabiensis had been reported from many areas of Ethiopia and Africa (Kibret et al., 2017; Ogola et al., 2017). The observed exophilic and endophilic behavior is connected with the host availability inside the house or outside of the house.
This study revealed that An. funestus group showed both endophagic and exophagic biting habit. Olanga et al. (2015) was observed both endophagic and exophagic behavior of An. funestus in Kenya. However, Degefa et al. (2017) and Ogola et al. (2017) were collected predominantly endophagic An. funestus in some parts of Africa. Similarly, Krafsur (1970) has documented endophagic An. funestus in Ethiopia. Contradictory to these biting habits, exophagic tendency of An. funestus was reported in other parts of Ethiopia (Kenea et al., 2016). Different from our results, Taye et al. (2016 & 2017) and Ogola et al. (2017) found more exophagic An. pharoensis and An. coustani in Ethiopia and Kenya.
Anopheles mosquitoes were collected in three villages with various agroecological zones. Higher proportions of adult Anopheles mosquitoes were collected in non-irrigated villages (Shnebekuma and Workmidr) than irrigated village (Bukta). This trend was true for all species, except An. coustani, An. cinereus and An. rupicolus. It was not comparable to the finding of Kibret et al. (2010), who were found higher of Anopheles mosquitoes in irrigated (85.2%, 94%, 92%) than non-irrigated (14.8%, 6%, 2%) villages in central Ethiopia. Other findings from Ethiopia and Africa also indicated the presence of a higher proportion of Anopheles mosquitoes in irrigated (villages very near to dam) than non-irrigated villages (villages very far from dam) (Dolo et al., 2004; Muturi et al., 2008; Mboera et al., 2010; Dejenie et al., 2012).
In the present study, the higher proportion of mosquito in non-irrigated village could be due to the presence of more productive breeding habitats throughout the study period (Mwangangi et al., 2012) than irrigated village. During surveying time, marches, stream pockets, and water rich pits were observed in non-irrigated villages than in the non-irrigated areas. Altitudinal difference (temperature variation) could also be the other possible source of variation, non-irrigated village has 2,025 m.a.s.l. than irrigated village (average elevation 2,157 m.a.s.l.) (Kulkarni et al., 2006; Animut et al., 2013a; Kibret et al., 2017). In Tanzanian, Maxwell et al. (2003) was collected 12 times greater Anopheles mosquitoes in the lowland than highland areas. Similarly, Kulkarni et al. (2006) and Animut et al. (2013a) were found higher proportion of Anopheles mosquitoes in lowland than highland altitude villages in Africa.
Moreover, the lower densities of Anopheles in irrigated villages (and areas near a dam) is due to a greater wealth created in the community via irrigation, which helped to construct good houses, resulted in the prohibition of the mosquitoes to enter in the house; thereby fewer numbers of mosquitoes were collected. Many studies have proved the purpose of well-constructed houses in reducing the abundances mosquito in the house (Atieli et al., 2009; Njie et al., 2009; Animut et al., 2013b). In irrigated village, the health center was established at the center of the settlement (inhabitants) than non-irrigated villages. Being very near, the villagers may have better treatment seeking behavior about the control and prevention of mosquitoes (Sissoko et al., 2004).
Distribution of Anopheles mosquitoes was found to be associated with surveyed seasons. Overall Anopheles mosquito density peaked after the end of the main rainy season in October. Generally, mosquito density started to increase from June and reached its peak in October. This finding agrees with Kibret et al. (2017), who found a higher density of Anopheles mosquito between October and November in Ethiopia. However, in different studies (Babatunde, 2009; Taye et al., 2016; Abraham et al., 2017), peaked mosquito density was shown during the main rainy season, from August to September. In our study, mosquito density peaked in October could be due to the presence permanent breeding sites together with elevated temperature; these provide very conducive breeding ground for Anopheles mosquitoes. In this study, IRS was applied between 20-30/12/2015 (Bure District Health Office, Unpub. Report, 2016); however, it had no any influence on the density of mosquitoes because the reduction was begun on November. This indicate the necessity of resistance measurement and the availability of other appropriate intervention measures.
5. Conclusions
The current study recorded the largest proportions of Anopheles mosquitoes in non-irrigated villages than the irrigated village throughout the study months. This finding documented the most important malaria vectors of Ethiopia, An. arabiensis and An. funestus group in all surveyed months and other potential vectors. The density of both indoor and outdoor host seeking mosquitoes was equally. Generally, irrigation has not any influence on the densities of mosquitoes. Therefore, the presence of An. arabiensis, An. funestus group and other potential vectors throughout the year warrants the top priority of breeding habitat management, parallel to using LLINs and IRSs. Environmental management must be applied throughout the year, especially in non-irrigated villages. The presence of insecticide resistant mosquitoes must be checked and other alternative measures should be in place because the density of mosquitoes was not decreased after the application of IRS and the use of new LLINs. Health education on malaria should be given strictly too.
Declarations
Author contribution statement
Tilahun Adugna: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Emana Getu, Delenasaw Yewhalaw: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Addis Ababa, Jima and Mizan-Tepi Universities.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abraham M., Massebo F., Lindtjorn B. High entomological inoculation rate of malaria vectors in area of high coverage of interventions in southwest Ethiopia: implication for residual malaria transmission. Parasit. Epidemiol. Contr. 2017;2:61–69. doi: 10.1016/j.parepi.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afrane Y.A., Githeko A.K., Yan G. The ecology of Anopheles mosquitoes under climate change: case studies from the effects of environmental changes in east Africa highlands. Ann. N. Y. Acad. Sci. 2012;1249:1–7. doi: 10.1111/j.1749-6632.2011.06432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemu K., Worku A., Berhane Y. Malaria infection has spatial, temporal and spatio- temporal heterogeneity in unstable malaria transmission areas in Northwest Ethiopia. PloS One. 2013;8(11) doi: 10.1371/journal.pone.0079966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animut A., Balkew M., Gebre-Michael T., Lindtjorn B. Blood meal sources and entomological inoculation rates of Anophelines along a highland altitudinal transect in south- central Ethiopia. Malar. J. 2013;12(76):112–121. doi: 10.1186/1475-2875-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animut A., Balkew M., Lindtjorn B. Impact of housing condition on indoor biting and indoor-resting Anopheles arabiensis density in a highland area, central Ethiopia. Malar. J. 2013;12(393):123–130. doi: 10.1186/1475-2875-12-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atieli H., Menya D., Githeko A., Scott A. House design modifications reduce indoor resting malaria vector densities in rice irrigation scheme area in western Kenya. Malar. J. 2009;8(108):1–9. doi: 10.1186/1475-2875-8-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayele D.G., Zewotir T.T., Mwambi H.G. Prevalence and risk factors of malaria in Ethiopia. Malar. J. 2012;11(195):1–8. doi: 10.1186/1475-2875-11-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babatunde T. Distribution and seasonal abundance of Anopheline mosquito species in Nguru, Yobe state, north-eastern Nigeria. A.R.I. 2009;6(1):949–952. [Google Scholar]
- Carter Center . Carter Center; Atlanta, Georgia: 2013. Summary Proceedings 4th Annual Malaria Control Program Review Ethiopia and Nigeria; pp. 1–19. [Google Scholar]
- Chrispinus S.M., Moses M.N., Donald N.S., John M.V. Diversity of Anopheles and prevalence of malaria in a highland area of Western Kenya. J. Parasitol. Vector Biol. 2011;3(3):33–39. [Google Scholar]
- Coetzee M. Distribution of the African malaria vectors of Anopheles gambiae complex. Am. J. Trop. Med. Hyg. 2004;70:103–104. [PubMed] [Google Scholar]
- Degefa T., Zeynudin A., Godesso A., Haile-Michael Y., Eba K., Zemene E., Emana D., Birlie B., Tushune K., Yewhalaw D. Malaria incidence and assessment of entomol- ogical indices among resettled communities in Ethiopia: a longitudinal study. Malar. J. 2015;14(24):1–10. doi: 10.1186/s12936-014-0532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degefa T., Yewhalaw D., Zhou G., Lee M., Atieli H., Githeko A.K., Yan G. Indoor and outdoor malaria vector surveillance in western Kenya: implications for better under- standing of residual transmission. Malar. J. 2017;16(443):1–12. doi: 10.1186/s12936-017-2098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejenie T., Yohannes M., Assmelash T. Adult mosquito populations and their health impact around and far from dams in Tigray Region, Ethiopia. Ethiop. J. Health Sci. 2012;4(2):40–51. [Google Scholar]
- DNeasy Blood and Tissue Kits . 2011. DNeasy Blood and Tissue Handbook.www.qiagen.com.handbooks Accessed on: 1/1/ 2011. From: [Google Scholar]
- Dolo G., Briet O.J., Dao A., Traore S.F., Bouare M., Sogoba N., Niare O., Bagayogo M., Sangare D., Teuscher T., Toure Y.T. Malaria transmission in relation to rice cultivation in the irrigated Sahel of Mali. Acta Trop. 2004;89:147–159. doi: 10.1016/j.actatropica.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Dutta H.M., Dutt A.K. Malarial ecology: a global perspective. Soc. Sci. Med. 1978;1:69–84. [PubMed] [Google Scholar]
- Eckhoff P.A. A malaria transmission-directed model of mosquito life cycle and ecology. Malar. J. 2011;10(303):1–17. doi: 10.1186/1475-2875-10-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J. World without mosquitoes. Nature. 2010;466(22):432–434. doi: 10.1038/466432a. [DOI] [PubMed] [Google Scholar]
- Fontaine R.E., Najjar A.E., Prince J.S. The 1958 malaria epidemic in Ethiopia. Am. J. Trop. Med. Hyg. 1961;10:795–803. doi: 10.4269/ajtmh.1961.10.795. [DOI] [PubMed] [Google Scholar]
- Gaffigan T.V., Wilkerson R.C., Pecor J.E., Stoffer J.A., Anderson T. 2013. Systematic Catalog of Culicidae. Walter Reed Biosystematics Unit, Division of Ecology, Walter Reed Army institute of Research, silver spring.http://www.mosquitocatalog.org Accessed on: 9/28/2016. From. [Google Scholar]
- Gillies M.T., Coetzee M. A supplement to the anophelinae of Africa south of the Sahara (afrotropical region). Johannesburg. South Afr. Insect Med. Res. 1987;55:1–125. [Google Scholar]
- Glick J.I. Illustrated key to the female Anopheles of Southwestern Asia and Egypt (Diptera: Culicide) Mosq. Systemat. 1992;24(2):125–151. [Google Scholar]
- Grillet M.E. Factors associated with the distribution of Anopheles aquasalis and An. oswaldoi (Diptera: Culicidae) in a malarious area, Northeastern Venezuela. J. Med. Entomol. 2000;37:231–238. doi: 10.1603/0022-2585-37.2.231. [DOI] [PubMed] [Google Scholar]
- Harbach R.E. 2013. The Phylogeny and Classification of Anopheles; pp. 1–13. [Google Scholar]
- Harbach R.E., Kitching I.J. The phylogeny of Anophelinae revisited: inferences about the origin and classification of Anopheles (Diptera: Culicidae) Zool. Scripta. 2016;55:34–47. [Google Scholar]
- Hay S.I., Rogers D.J., Toomer J.F., Snow R.W. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, internet access and review. Trans. R. Soc. Trop. Med. Hyg. 2000;94:113–127. doi: 10.1016/s0035-9203(00)90246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay S.I., Sinka M.E., Okara R.M. Developing global maps of the dominant Anopheles vectors of human malaria. PLoS Med. 2010;7(2):1–3. doi: 10.1371/journal.pmed.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassa A.W., Tamiru M.A., Yeshanew A.Z. Assessment of control measure and trends of malaria in Burie-Zuria district, west Gojjam zone, Amhara region, north west Ethiopia. Malar. Res. Treatment. 2015:1–4. doi: 10.1155/2015/302194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenea O., Balkew M., Tekie H., Gebre-Michael T., Deressa W., Loha E., Lindtjorn B., Overgaard H.J. Human-biting activities of Anopheles species in south central Ethiopia. Parasites Vectors. 2016;9(527):1–10. doi: 10.1186/s13071-016-1813-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibret S., Alemu Y., Boelee E., Tekie T., Alemu D., Petros B. The impact of a small scale irrigation scheme on malaria transmission in Ziway area, Central Ethiopia. Trop. Med. Int. Health. 2010;15:41–50. doi: 10.1111/j.1365-3156.2009.02423.x. [DOI] [PubMed] [Google Scholar]
- Kibret S., Wilson G.G., Ryder D., Tekie H., Petros B. Malaria impact of large dams at different eco-epidemiological settings in Ethiopia. Trop. Med. Health. 2017;45(41):1–14. doi: 10.1186/s41182-017-0044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihadye E.S.P., Nkwengulila G., Magesa M.S., Abdulla S. Diversity, spatial and temporal abundance of Anopheles gambiae complex in the Rufiji Riverbasin, south-eastern Tanzania. Tanzania. J. Health Res. 2010;12(1):1–4. doi: 10.4314/thrb.v12i1.56320. [DOI] [PubMed] [Google Scholar]
- Krafsur E.S. Anopheles nili as a vector of malaria in a lowland region of Ethiopia. Bull. World Health Organ. 1970;42(3):466–471. [PMC free article] [PubMed] [Google Scholar]
- Kulkarni M.A., Kweka E., Nyale E., Lyatuu E., Mosha F.W., Chandramohan D., Rau M.E., Drakeley C. Entomological evaluation of malaria vectors at different altitudes in Hai district, Northeastern Tanzania. J. Med. Entomol. 2006;43(3):580–588. doi: 10.1603/0022-2585(2006)43[580:eeomva]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kweka E.J., Munga S., Himeidan Y., Githeko A.K., Yan G. Assessment of mosquito larval productivity among different land use types for targeted malaria vector control in the western Kenya highlands. Parasites Vectors. 2015;8(356):1–6. doi: 10.1186/s13071-015-0968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake M.W., Mebratur M., Mehari D., Dessie K. Epidemiological analysis of malaria outbreak in Ankesha district, Awi zone, Amhara region, Ethiopia, 2012: Weaknesses in control measures and risk factors. Sci. J. Publ. Health. 2016;4:132–137. [Google Scholar]
- Lines J.D., Curtis C.F., Wilkes T.J., Njunwa K.J. Monitoring human-biting mosquitoes (Diptera: Culicidae) in Tanzania with light-traps hung beside mosquito. Bull. Entomol. Res. 1991;81:77–84. [Google Scholar]
- Mala A.O., Irungu L.W., Shililu J.I., Muturi E.J., Mbogo C.M., Njagi J.K., Mukabana W.R., Githure J.I. Plasmodium falciparum transmission and aridity: a Kenya experience from the dry lands of Baringo and its implications for Anopheles arabiensis control. Malar. J. 2011;10(121):1–9. doi: 10.1186/1475-2875-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell C.A., Chambo W., Mwaimu M., Magogo M., Carneiro I.A., Curtis C.F. Variation of malaria transmission and morbidity with altitude in Tanzania and with introduction of alphacypermethrin treated nets. Malar. J. 2003;2(28):1–9. doi: 10.1186/1475-2875-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mboera L.E.G., Kihonda J., Braks M.A., Knols B.G.J. Influence of centers for disease control light trap position, relative to a human-baited bednet, on catches of Anopheles gambiae and Culex quinquefasciatus in Tanzania. Am. J. Trop. Med. Hyg. 1998;59:595–596. doi: 10.4269/ajtmh.1998.59.595. [DOI] [PubMed] [Google Scholar]
- Mboera L.E.G., Senkoro K.P., Mayala B.K., Rumisha S.F., Rwegoshora R.T., Mlozi M.R.S., Shayo E.H. Spatio-temporal variation in malaria transmission intensity in five agro- ecosystem in Mvomero district Tanzania. Geopat. Health. 2010;4:167–178. doi: 10.4081/gh.2010.198. [DOI] [PubMed] [Google Scholar]
- Midekisa A., Beyene B., Mihretie A., Bayabil E., Wimberly M.C. Seasonal associations of climatic drivers and malaria in the highlands of Ethiopia. Parasites Vectors. 2015;8(339):1–11. doi: 10.1186/s13071-015-0954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health . 2012. National Malaria Guidelines. Addis Ababa, Ethiopia. Ethiopia Ministry of Health. Addis Ababa, Ethiopia.https://www.medbox.org/national-malaria-guidelines-ethiopia/download.pdf From. Accessed 30/3/2018. [Google Scholar]
- Mulambalah C.S., Siamba D.N., Ngeiywa M.M., Vulule J.M. Anopheles species diversity and breeding habitat distribution and the prospect for focused malaria control in the western highlands of Kenya. Int. J. Trop. Med. 2011;6:44–51. [Google Scholar]
- Muturi E.J., Muriu S., Shililu J., Mwangangi S., Jacob B.G., Mbogo C., Githure J., Novak R.J. Effect of rice cultivation on malaria transmission in central Kenya. Am. J. Trop. Med. Hyg. 2008;78(2):270–275. [PubMed] [Google Scholar]
- Mwangangi J.M., Midega J., Kahindi S., Njoroge L., Nzovu J., Githure J., Mbogo C.M., Beier J.C. Mosquito species abundance and diversity in Malindi, Kenya and their potential implication in pathogen transmission. Parasitol. Res. 2012;110:61–71. doi: 10.1007/s00436-011-2449-6. [DOI] [PubMed] [Google Scholar]
- Mwangangi J.M., Mbogo C.M., Orindi B.O., Muturi E.J., Midega J.T., Nzovu J., Gatakaa H., Githure J., Borgemeister C., Keating J., Beier J.C. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20-years. Malar. J. 2013;12(13):1–9. doi: 10.1186/1475-2875-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndenga B., Githeko A., Omukunda E., Munyekenye G., Atieli H., Wamai P., Mbogo C., Minakawa N., Zhou G., Yan G. Population dynamics of malaria vectors in western Kenya highlands. J. Med. Entomol. 2006;43(2):200–206. doi: 10.1603/0022-2585(2006)043[0200:pdomvi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Njie M., Dilger E., Lindsay S.W., Kirby M.J. Importance of eaves to house entry by Anopheline, but not Culicine mosquitoes. J. Med. Entomol. 2009;46:505–510. doi: 10.1603/033.046.0314. [DOI] [PubMed] [Google Scholar]
- Ogola E., Villinger J., Mabuka D., Omondi D., Orindi B., Mutunga J., Owino V., Masiga D.K. Composition of Anopheles mosquitoes, their blood-meal hosts and Plasmodium falciparum infection rates in three islands with disparate bed net coverage in Lake Victoria, Kenya. Malar. J. 2017;16(360):1–11. doi: 10.1186/s12936-017-2015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okello P.E., Van-Bortel W., Anatol M.B., Anne C., Patricia R., Talisuna A., D'Alessandro U. Variation in malaria transmission intensity in seven sites throughout Uganda. Am. J. Trop. Med. Hyg. 2006;75(2):219–225. [PubMed] [Google Scholar]
- Olanga E.A., Okombo L., Irungu L.W., Mukabana W.R. Parasites and vectors of malaria on Rusinga Island, western Kenya. Parasites Vectors. 2015;8(250):1–8. doi: 10.1186/s13071-015-0860-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ototo E.N., Mbugi J.P., Wanjala C.L., Zhou G., Githeko A.K., Yan G. Surveillance of malaria vector population density and biting behaviour in western Kenya. Malar. J. 2015;14(244):1–9. doi: 10.1186/s12936-015-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyewole I., Awololab T., Ibidapo C., Oduola A., Okwac O., Obansa J. Behavior and population dynamics of the major Anopheline vectors in a malaria endemic area in southern Nigeria. J. Vector Borne Dis. 2007;44:56–64. [PubMed] [Google Scholar]
- O’Connor C.T. The distribution of Anopheline mosquitoes in Ethiopia. Mosq. News. 1967;27(1):42–55. [Google Scholar]
- PMI . 2017. The President's Malaria Initiative: Eleventh Annual Report to congress; pp. 1–17. [Google Scholar]
- Ramirez J.L., Garver L.S., Dimopoulos J. Challenges and approaches for mosquito targeted malaria control. Curr. Mol. Med. 2009;9(2):116–130. doi: 10.2174/156652409787581600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V., Rocamora G., Julienne S., Goodman S.M. Why are Anopheline mosquitoes not present in the Seychelles? Malar. J. 2011;10(31):1–10. doi: 10.1186/1475-2875-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinka M.E., Bangs M.J., Manguin S., Rubio-Palis Y. A global map of dominant malaria vectors. Parasites Vectors. 2012;5(69):1–10. doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sissoko M.S., Dicko A., Briet O.J., Sissoko M., Sagara I., Keita H.D., Sogoba M., Rogier C., Toure Y.T., Doumbo O.K. Malaria incidence in relation to rice cultivation in the irrigated in Sahel of Mali. Acta Trop. 2004;89:161–170. doi: 10.1016/j.actatropica.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Taye B., Lelisa K., Emana D., Asale A., Yewhalaw D. Seasonal dynamics, longevity and biting activity of Anopheline mosquitoes in southwest Ethiopia. J. Insect Sci. 2016;16(1):1–7. doi: 10.1093/jisesa/iev150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taye B., Seid M., Gindab A. Entomological study on species composition, behavior, longevity and probability of surviving sporogony of Anopheles mosquitoes in Lare District, Ethiopia. J. Parasitol. Vector Biol. 2017;9(9):37–145. [Google Scholar]
- Tesfaye S., Belyhun Y., Teklu T., Mengesha T., Petros B. Malaria prevalence pattern observed in the highland fringe of Butajira, Southern Ethiopia: a longitudinal study from parasitological and entomological survey. Malar. J. 2011;10(153):1–9. doi: 10.1186/1475-2875-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama Y., Ota M., Molla G., Beyene B.B. Sharp decline of malaria cases in the Burie Zuria, Dembia, and Mecha districts, Amhara region, Ethiopia, 2012–2014: descriptive analysis of surveillance data. Malar. J. 2016;15(104):1–8. doi: 10.1186/s12936-016-1133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajda E.A., Webb C.E. Assessing the risk factors associated with malaria in the highlands of Ethiopia: what do we need to know? Trop. Med. Infect. Dis. 2017;2(4):1–13. doi: 10.3390/tropicalmed2010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrone G.A. Outline for the determination of malaria mosquitoes in Ethiopia: Part- I- Adult female Anopheles. Mosq. News. 1962;22(1):37–50. [Google Scholar]
- White G.B., Tesfaye F., Boreham P.F., Lemma G. Malaria vector capacity of Anopheles arabiensis and Anopheles quadriannulatus in Ethiopia: chromosomal inter- pretation after six years storage of field preparations. Trans. R. Soc. Trop. Med. Hyg. 1980;74:683–684. [Google Scholar]
- WHO . Malaria Entomology and Vector Control: Learner’s Guide. In: Trial, editor. 2003. https://apps.who.int/iris/bitstream/handle/10665/67450/WHO_CDS_CPE_SMT_2002.18_Rev.1_PartI.pdf?sequence=1 (World Health Organization HIV/AIDS, Tuberculosis and Malaria, Roll Back Malaria). HO/CDS/CPE/SMT/2002.18. Rev.1: Part I. From: Accessed on. [Google Scholar]
- Wilkins E.E., Howell P.I., Benedict M.Q. IMP PCR primers detect single nucleotide polymorphisms for Anopheles gambiae species identification, Mopti and Sahavanna rDNA types, and resistance to dieldrin in Anopheles arabiensis. Malar. J. 2006;5(125):1–7. doi: 10.1186/1475-2875-5-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 1975. WHO Manual on Practical Entomology in Malaria, Part-II.https://apps.who.int/iris/bitstream/handle/10665/42481/WHO_OFFSET_13_%28part1%29.pdf?sequence=1 From. Accessed on 23/4/2018. [Google Scholar]
- Woyessa A. Addis Ababa University; Addis: 2001. The Elucidation of Malaria Transmission and its Prevalence in highland Urban Area of Akaki Town; pp. 67–75. Ababa, Ethiopia. [Google Scholar]
- Woyessa A., Gebre-Micheal T., Ali A. An indigenous malaria transmission in the outskirts of Addis Ababa, Akaki town and its environs. Ethiop. J. Health. 2004;81:2–7. [Google Scholar]
- Yewhalaw D., Kelel M., Getu E., Temam S., Wessel G. Blood meal sources and sporozoite rates of Anophelines in Gilgel-Gibe dam area, Southwestern Ethiopia. 2014. https://www.researchgate.net/publication/292615632_Blood_meal_sources_and_sporozoite_rates_of_Anophelines_in_Gilgel-Gibe_dam_area_Southwestern_Ethiopia From. Accessed on 9/20/2020.