Abstract
Mononuclear macrophages derived from the bone marrow (myeloid cells) are key cellular components of the innate immune system in different organs. In this minireview, we are focused on both brain and blood macrophages, known as microglia and monocytes, respectively. We provide a succinct summary of the cells’ functions under both normal and pathologic conditions, with particular reference to common neurodegenerative disorders, such as Alzheimer and Parkinson disease.
SIGNIFICANCE STATEMENT
In this minireview, we aim to summarize available literature on microglial and myeloid involvement in CNS disease, directing the reader toward relevant and translatable interpretations of myeloid cell function in CNS health and neurodegeneration.
Introduction
Mononuclear macrophages are essential cellular components of the innate immune system, and as such, they are present in all organs. They are called “mononuclear” to distinguish them from polymorphonuclear macrophages, also known as neutrophils, which are residents of blood tissue, along with multiple other lineages of immune cells, both innate and adaptive. The term “myeloid” refers to the cells’ origin from the bone marrow and, ultimately, from the yolk sac from which the earliest macrophage progenitors arise. These primordial, so-called fetal macrophages (or early myeloid progenitors) are present throughout the embryo and fetus beginning during early developmental stages. Myeloid cells arise as the result of distinct hematopoietic waves in the embryonic yolk sac, followed by the embryonic liver and, finally, bone marrow resident stem cells (Goldmann et al., 2016). Microglia, the resident myeloid cells in the central nervous system (CNS), arise from primitive yolk sac macrophages that engraft the developing neuroectoderm destined to become the CNS parenchyma. As the embryo develops, subsequent hematopoietic events generate additional populations of both tissue-resident and circulating macrophages (Kierdorf et al., 2019).
In adults, depending on which organ they occupy, mononuclear phagocytes may assume characteristic morphologies that reflect a given organ’s or tissue’s unique cytoarchitecture. A good example of tissue-specific morphology is microglial cells, which reside in the CNS parenchyma, and like all neural cells there, they are process-bearing (ramified) cells. In the blood, monocytes represent the second-most-prevalent group of macrophages in addition to neutrophils. Unlike microglia, monocytes are rounded similar to all other blood leukocytes. We use the term “myeloid cells” to refer collectively to microglia, other CNS-associated macrophages, and monocytes because they are closely related cell types. They are distinguished most easily by their locations in the healthy state: microglia reside in the CNS parenchyma, other CNS-associated macrophages reside outside the CNS parenchyma but inside the skull, and monocytes reside in the bloodstream.
CNS-Associated Macrophages and CNS-Resident Microglia
We only briefly describe CNS-associated macrophages, directing the interested reader to comprehensive recent reviews on these cells (Mrdjen et al., 2018; Kierdorf et al., 2019). In essence, CNS-associated macrophages refer to cells located within structures that border the CNS parenchyma, i.e., the perivascular space, the leptomeningeal (subarachnoid) space, and the choroid plexus. The perivascular and leptomeningeal spaces are compartments containing cerebrospinal fluid (CSF), produced by the choroid plexus, and they serve as drainage and barrier systems of the CSF. They are somewhat analogous to lymphatic channels in other tissues. Their existence serves important protective roles for the CNS in that they afford a means for eliminating waste products while also providing an immunologic border preventing entry of infectious microorganisms or blood-borne leukocytes into the CNS parenchyma. They are an essential component of CNS border security, so to speak. The security guards are the macrophages that reside there, which are the perivascular macrophages, leptomeningeal macrophages, and choroid plexus macrophages (epiplexus or Kolmer cells). Further details and subclassifications of these guardian cells are described elsewhere (Kierdorf et al., 2019).
In the context of CNS disease or injury, other CNS macrophages can present CNS antigens or may serve as entry points for peripheral immune cell infiltration. Microglia, the CNS-resident macrophages, participate in maintaining parenchymal homeostasis and respond to insult or injury, also acting as gatekeepers for entry of other peripheral immune subtypes (Kierdorf et al., 2019; Van Hove et al., 2019). CNS myeloid cells are also present in the form of choroid plexus macrophages. Because choroid plexus ependymal cells are responsible for maintaining and filtering CSF, choroid plexus–associated macrophages and dendritic cells are ideally positioned to encounter CNS antigens and cytokines released into CSF (Ransohoff and Engelhardt, 2012). Additionally, CNS antigen presentation in cervical lymph nodes, even in the absence of dedicated lymphatic vessels in the CNS, may occur via CSF drainage to the nasal mucosa. Finally, although choroid plexus–associated macrophages have not been shown to traffic to cervical lymph nodes for antigen presentation, both choroid plexus macrophages and dendritic cells have been shown to present CNS antigens during CNS disease, such as multiple sclerosis (Ransohoff and Engelhardt, 2012).
The meninges, composed of three distinct tissue layers, play host to resident meningeal macrophages and also to a variety of other peripheral leukocytes (Wilson et al., 2010). Bathed in CSF, meningeal macrophages in the subarachnoid space are key antigen-presenting cells in the context of pathologic conditions and participate in local control of CNS pathogens (Wilson et al., 2010; Van Hove et al., 2019). Perivascular macrophages are particularly specialized to the CNS microenvironment, arising from an embryonic hematopoietic wave distinct from that giving rise to microglia. Similar to choroid plexus and meningeal macrophages, perivascular macrophages are bathed in CSF; however, unlike other CNS-associated macrophage populations, perivascular cells are very close to most CNS regions, being separated from the parenchyma only by a basement membrane (Graeber and Streit, 1990b). They are ideally positioned to sample the changing local CNS milieu.
Mononuclear macrophages, both CNS and peripheral, balance between removal and nonremoval of damaged or injured cells in efferocytosis—the balance between “eat-me” and “don’t-eat-me” signals (Lagasse and Weissman, 1994; Li, 2012; Weiskopf et al., 2016). Dysfunction in these and associated pathways may induce immune dysregulation, which could contribute to CNS diseases and their peripheral correlates.
Peripheral Myeloid Cell Subtypes
In contrast to both CNS-resident microglia and CNS-associated macrophages, peripheral myeloid cells (monocytes) are continuously repopulated by hematopoietic stem cells in the bone marrow niche. Thus, monocytes have a much shorter lifespan than microglia in the CNS parenchyma, which are thought to be long-lived (Lawson et al., 1992; Askew et al., 2017). Normally, monocytes reside in the blood stream for relatively short periods before migrating into various other organs and tissues, where they differentiate into tissue-specific macrophages. A notable exception to this is the CNS, which does not allow for entry of monocytes under normal conditions. This can change dramatically both in acute pathologic situations, such as CNS trauma and stroke (Streit et al., 1998; Gelderblom et al., 2009; Hu et al., 2012), and in chronic diseases, notably in multiple sclerosis, in which a massive influx of mononuclear cells (both monocytes and lymphocytes) from the bloodstream occurs. This type of immense and rapid mononuclear cell infiltration causes pathology; it represents an autoimmune attack directed against certain myelin antigens that, for reasons unknown, have become encephalitogenic, i.e., causative of encephalitic neuroinflammation. Mononuclear cell infiltration in multiple sclerosis represents the best example of a CNS autoimmune disease, and this kind of autoantigen-induced encephalitic neuroinflammation causes debilitating demyelination. There is no evidence to suggest that this type of autoimmune neuroinflammatory response occurs in neurodegenerative diseases (see below).
Neuroimmune Cells in the Healthy Brain
Monocytes and other blood leukocytes are notably absent from the normal CNS parenchyma. Instead, the CNS has evolved to harbor its own specialized immune cells called microglia (Graeber and Streit, 1990a). Microglia are distributed ubiquitously throughout the entire CNS, and they continuously monitor the parenchymal microenvironment, on the lookout for perturbations that may disrupt homeostasis (Fig. 1A). As mononuclear phagocytes, they are the primary source of endogenous brain macrophages, and as such, their key role is clearance of debris, which facilitates reorganization of neuronal circuits and triggers repair (Fig. 1, B and C) (Neumann et al., 2009). Thus, phagocytosis of debris not only serves the purpose of maintaining a clean microenvironment but also is important for contributing to normal neuronal functioning, allowing changes in neuronal connectivity and synaptic plasticity (Graeber, 2010; Paolicelli et al., 2011). Perhaps the most important and essential function of microglia is neuroprotection (Streit, 2002), which is no different from that of other neuron-supporting glial cells. Phagocytosis of microorganisms in the parenchyma is one way for microglia to be neuroprotective. However, viruses and bacteria do not easily get past the aforementioned “border security” of meninges, CSF, and other CNS-associated macrophages. Thus, the CNS is well protected by different layers of structural and immunologic barriers, and brain infections are not very common in adults with healthy immune systems. However, these barriers break down when individuals become immunocompromised, human immunodeficiency virus/AIDS probably being the best example.
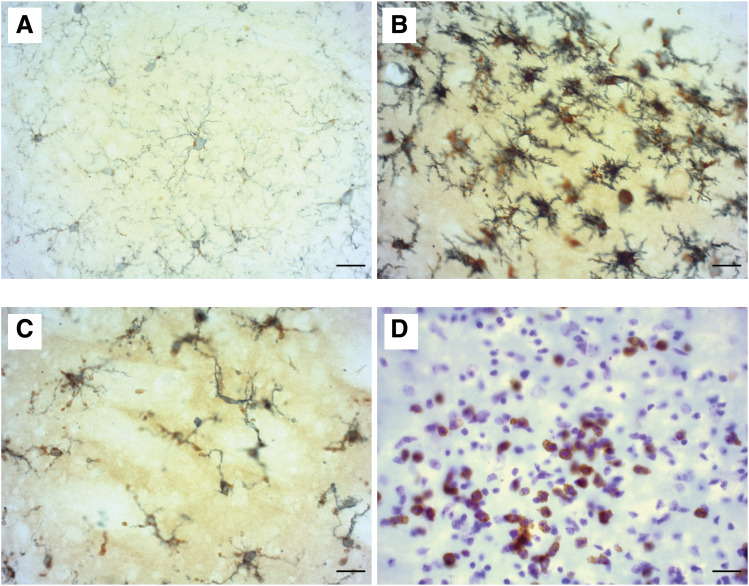
Fig. 1.
Microglia at resting, activated, and dystrophic states are seen in MCAO (middle cerebral artery occlusion) unilateral stroke model brain tissue, accompanied by extensive peripheral myeloid cell infiltration. Within a coronal brain section from a mouse at 24 hours after the stroke in the contralateral hemisphere, (A) resting microglia are labeled with myeloid cell marker IBA1 (gray) and a marker of phagocytic and lysosomal function, CD68 (brown). (B) In the ipsilateral hemisphere, activated microglia at the edge of the lesion display a characteristic phenotype with increased IBA1 and CD68 expression, indicating active phagocytosis. (C) Dystrophic, fragmenting microglia can be observed within the stroke core. In addition to microglial activation, lesioned CNS tissue displays (D) extensive peripheral myeloid cell infiltration shown by Ly6B.2 immunostaining, specific to infiltrating peripheral myeloid cells. Identification of myeloid cell subtypes can be made using nuclear Nissl counterstain, as monocytes (mononuclear) or neutrophils (polymorphonuclear). Original magnification, 40× images. Scale bar, 20 μm.
Neuroimmune Cell Function in the Diseased Brain
Microglial activation occurs when perturbations of CNS homeostasis occur, usually in the form of acute traumatic or ischemic insults or onset of disease (Kreutzberg, 1996). Microglial activation represents the first and quintessential cellular response to CNS injury and, as such, the first step in the wound healing process that ensues over subsequent days and weeks (Fig. 1B). Inflammation is defined as the cellular response to injury (Streit et al., 2014); thus, microglial activation represents a neuroinflammatory response of CNS endogenous cells. Exogenous (blood-borne) immune cells may also become involved and enter into the damaged CNS if indeed the injury involves blood-brain barrier (BBB) breakdown (Fig. 1D), which allows blood leukocytes to migrate into the parenchyma. Endogenous neuroinflammation in the CNS is also known as “reactive gliosis,” a term that refers to both microglial and astrocytic mobilization after injury or disease (Fig. 1B). In terms of chronic CNS neurodegenerative disease, it is worth noting that both Alzheimer disease (AD) and Parkinson disease (PD) have an endogenous neuroinflammatory component as part of their neuropathology, but neither of these conditions is an inflammatory disease, meaning inflammation is not the central cause of degenerative phenomena. In both AD and PD, microglia exhibit macrophage responses—i.e., in AD, microglia become activated in response to amyloid aggregation (foreign body response, Fig. 2B) (Streit et al., 2018); in PD, microglia become activated in response to dying dopaminergic neurons (necroptosis) (Fig. 2C) and release of neuromelanin pigment in the substantia nigra (Langston et al., 1999; Imamura et al., 2003; Gordon et al., 2018).
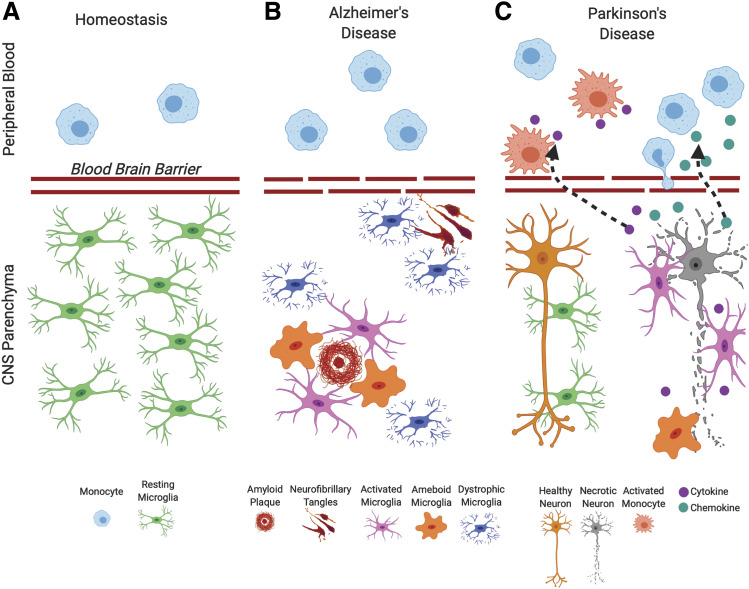
Fig. 2.
Microglia and myeloid cell states in health and disease. While at homeostasis, (A) microglial activation in the CNS and alterations in peripheral blood myeloid cells are seldom seen, and dramatic changes in immune activation are seen in degenerative diseases such as AD and PD. (B) In AD, microglia become activated in response to amyloid formation and form phagocytic clusters attempting to remove this insoluble material. Neurofibrillary degeneration does not elicit microglial activation but coincides with microglial dystrophy; (C) in PD microglial activation, phagocytic activity and secretion of soluble signaling molecules are associated with response to dopaminergic neurons undergoing necroptosis in the midbrain. Secreted chemical factors also induce changes in peripheral myeloid populations, altering peripheral immune function and potentially inducing peripheral myeloid participation in dopaminergic degeneration.
Alzheimer Disease
Our discussion here will be restricted to postmortem findings from human beings with late-onset Alzheimer disease (LOAD), which is the most common form of AD and accounts for more than 95% of all cases. It is beyond the scope of the current review to discuss the many transgenic AD mouse models that have been developed because these models attempt to model certain molecular aspects of AD but do not recapitulate human disease progression. With regard to LOAD, it is important to realize that the condition is marked by a prolonged preclinical phase during which individuals are nonsymptomatic but develop lesions identical to those seen in clinical, symptomatic disease, although to a lesser extent (Price et al., 2009; Braak and Del Tredici, 2015). Recent work focused on delineating the sequence of pathologic lesion development during the preclinical phase of LOAD in nonselected individuals (Streit et al., 2018) has clearly shown that appearance of neurofibrillary degeneration precedes amyloid β protein (Aβ) deposition, confirming the results of others generated in much larger human cohorts (Braak et al., 2011). Our work has also shown that microglial activation (neuroinflammation) occurs only after both neurofibrillary degeneration (NFD) and amyloid aggregates are already present (Fig. 2B). Thus, as mentioned above, it is clear that onset of NFD is not the result of neuroinflammation, as claimed in the past (McGeer and McGeer, 2001; Hardy and Selkoe, 2002; Heneka et al., 2015), but that the inflammatory component of LOAD occurs only after aggregation of soluble Aβ into insoluble amyloid has occurred (Streit et al., 2018). This transformation of Aβ into amyloid represents the formation of a foreign body (Gray et al., 1990) that attracts the attention of CNS microglia because their job as mononuclear phagocytes is to remove undesirable material, including foreign bodies, from the extracellular space, as shown in Fig. 2B. The formation of insoluble amyloid aggregates causes microglia to become activated and form phagocytic cell clusters around amyloid deposits producing the characteristic inflammatory neuropathology described in the literature for over 30 years (McGeer et al., 1987; Itagaki et al., 1989; Perlmutter et al., 1990; Sasaki et al., 1997; Rogers et al., 2002). Importantly, the neuroinflammatory component of LOAD is limited to involvement of microglia and does not involve parenchymal infiltration of other CNS-associated macrophages or peripheral monocytes. Microglial activation is directed toward amyloid elimination and not toward neurodegeneration. That said, one cannot exclude the possibility that myeloid cells other than microglia may be affected by LOAD pathophysiology. The literature in human tissues, as well as studies in cultured human cells, indicates that BBB function in patients with AD is compromised (Alafuzoff et al., 1983; Wada, 1998; Farrall and Wardlaw, 2009). Peripheral monocytes and macrophages in patients with AD show altered phagocytic and chemotactic activity when exposed to Aβ aggregates in vitro (Fiala et al., 2005). In this context, defective BBB function might conceivably allow peripheral myeloid cells to infiltrate the CNS, but an invasion of peripheral immune cells has not been demonstrated in humans with AD.
What is the relationship between microglia and neurofibrillary degeneration in LOAD? It has been shown that areas in the human AD brain containing high levels of NFD do not show presence of activated microglia (Streit et al., 2009). Instead, these regions are characterized by abundant presence of so-called dystrophic microglia (Fig. 2B), which are cells that display an abnormal morphology thought to reflect cell senescence (Streit et al., 2004). Microglial dystrophy increases with normal aging but becomes highly prevalent in LOAD, as well as in Down syndrome (Xue and Streit, 2011), in which it is prominently colocalized with NFD. In fact, it appears that during preclinical and clinical LOAD, the extent of microglial dystrophy largely parallels the extent of NFD, suggesting that both degenerative phenomena may have a common underlying cause related to advanced age, the greatest risk factor of LOAD (Streit et al., 2020).
Parkinson Disease
Our discussion of myeloid cell function and dysregulation in PD will focus on potential myeloid cell interactions with the midbrain dopaminergic regions, with relevant forays into secreted chemical mediators that may provide clues to altered peripheral immune states in patients with PD and animal models of PD-like degeneration. In some human studies, as well as in rodent models of PD, immunologic changes are observable in the microglial secretome, microglial morphology, and both CNS and peripheral cytokine and chemokine concentrations (Vawter et al., 1996; Hurley et al., 2003; Gordon et al., 2018). Postmortem analysis of human brain tissues shows altered cytokine and chemokine secretion profiles compared with healthy, age-matched controls (Blum-Degen et al., 1995; Vawter et al., 1996; Nagatsu et al., 2000; Mount et al., 2007). Given, however, that secreted signaling factors can have varying effects on immune cells, depending on tissue-specific cues and cytokine combinations and concentrations, the presence of elevated cytokines in postmortem PD brain tissues does not necessarily imply an inflammatory scenario (Mantovani et al., 2004; Lucin and Wyss-Coray, 2009). Indeed, the myeloid cell response to insult, injury, or cell death varies depending on the milieu chemical cues in the microenvironment. The terms “activation” and “inflammation,” often used to describe pathologic changes in myeloid cells, fail to convey the complexity of the extremes of monocyte and microglial function, ranging from adaptive inflammation to injury resolution and healing (Streit et al., 2014; Lively and Schlichter, 2018).
Degenerative conditions affecting the CNS, such as AD (above) and PD, induce changes in a wide range of cytokines with pleiotropic effects—cytokine milieus that induce either proinflammatory or regenerative microglial phenotypes have both been repeatedly shown over the last three decades to be altered in Parkinson disease, with studies often contradicting one another. Reflecting complexity of the condition, the combinations of cytokines present naturally depend on the nature of the insult. In the context of PD, the insult is chronic and would result in a distinct cytokine milieu (Blum-Degen et al., 1995; Mount et al., 2007). Adding interest to the story is the fact that the BBB, traditionally thought to separate CNS from periphery into discrete compartments, in fact acts as an educational gate (Park et al., 2016) that allows CNS-originating signaling molecules (and even antigens) to enter systemic circulation with the potential to influence the peripheral immune system (Fig. 2C). That CNS molecules can exit the parenchyma, enter peripheral circulation, and retain the ability to modulate peripheral immune homeostasis in much the same way that cytokines modulate microglial response raises intriguing questions for neurodegeneration. Indeed, studies within the last 5 years have begun to investigate such changes in peripheral myeloid cell populations in PD, along with the beginnings of mechanistic studies in animal models. Although the infiltration of peripheral myeloid cells into the CNS in PD remains largely unexplored, patients with PD have been shown to exhibit elevated circulating classic monocytes along with a concurrent increase in chemokine ligand 2 (CCL2) (Grozdanov et al., 2014), a chemokine that promotes myeloid cell egress from bone marrow (Fujimura et al., 2015). Further investigation revealed that inhibition of the CD95/CD95L axis, commonly considered an apoptosis pathway (Park et al., 2003), resulted in an elevation in nonclassic monocytes (Grozdanov et al., 2014), possibly restoring a more healthy immune profile. Gao et al. (2015) went on to demonstrate that both genetic and pharmacological inhibition of this pathway is protective against MPTP-induced degeneration. Parallel studies to assess stem cell proliferation reveal that circulating hematopoietic stem cells from patients with PD, both idiopathic and familial, have the potential to generate monocytes more robustly and with greater expression of chemokine receptor type 2 (CCR2) than healthy controls, along with dramatically elevated CCL2 expression (Funk et al., 2013). In other words, expression of both chemokine (CCL2, chemokine ligand 2) and receptor (CCR2) is elevated, suggesting peripheral monocyte dysregulation at a hematopoietic level. These alterations in the basic characteristics of myeloid cell genesis in Parkinson disease, the implications of which remain unexplored, warrant detailed investigation.
Resident microglia in Parkinson disease have been pointed out as culprits in dopaminergic neuron degeneration (Lecours et al., 2018), but evidence is often contradictory. In an attempt to address this question, Kim et al. (2018) studied LRRK2 (leucine-rich repeat kinase 2) mutant model systems and human peripheral myeloid cells derived from patients with Parkinson disease to determine whether phagocytic activity differed from healthy controls. Guided by genetic studies in flies, it was demonstrated that murine microglia and human macrophages exhibited increased phagocytic capacity, which was abrogated by knockdown of LRRK2 (Kim et al., 2018). These data suggest that, rather than inducing inflammation and contributing to neuronal degeneration, microglia may elevate their phagocytic activity in PD, possibly as an attempt to keep up with ongoing necroptosis (Fig. 2C). A separate study of gene expression sought to use peripheral myeloid cells as a proxy for CNS myeloid cells, considering likely differences between mononuclear phagocytes as a result of residence in different tissue compartments. Inspired by genome-wide association studies that indicated the potential for an inflammatory state in PD (Nalls et al., 2011), differential gene expression in patients with PD and healthy control subjects did not reveal such an association (Schlachetzki et al., 2018). This study was not, however, adequately powered to detect a difference between patients with PD and controls; a larger cohort study could reveal different results and show dysregulated myeloid cell function.
In the discussion of CNS and peripheral myeloid cells, it is important to note that the innate immune system may cascade into adaptive immune activation and should be at least considered in the context of CNS and peripheral myeloid cells in Parkinson disease. Groups exploring this area have studied patients with PD (Sulzer et al., 2017), postmortem tissue (González et al., 2013), and in vitro systems to explore possible mechanisms of interaction between myeloid cells and T cells in PD (Sommer et al., 2016). Sulzer et al. (2017) have elaborated on the identification of antigen-specific T cells in patients with PD, whereas another group has shown evidence of CD4+ T cell infiltration in postmortem PD midbrain tissue (González et al., 2013). However, much work remains to be done to identify the role, if any, played by these cells in PD, as T cells are known to patrol the CNS compartment in homeostasis (Ransohoff and Engelhardt, 2012) and during acute injury/infection (Russo and McGavern, 2015). Experimental conditions using in vivo and in vitro overexpression systems (Sommer et al., 2016) have shown that T cell–mediated suppression of microglial phagocytosis occurs in the presence of α synuclein burden and that genetic ablation of T cell development appears to resolve this. One should note that α synuclein overexpression does not represent the human condition and is of limited translational value—results should be interpreted as such. Given that T cell infiltration into the midbrain in Parkinson disease is a rare occurrence, T cell–mediated suppression of microglial function in the physiologic condition would be limited and likely would not impede microglial function.
Conclusions
A diverse and well documented set of microglial responses occurs in the CNS of patients with degenerative disease. In more recent years, the peripheral myeloid cell response in Alzheimer and Parkinson disease has been more extensively characterized, in which complex and yet-to-be-understood changes occur. Such a complex mix of phenotypic, chemotactic, secretory, and functional myeloid cell responses suggests a level of complexity in neurodegenerative conditions that cannot be described simply as “inflammation.” Immune dysregulation would be a better term to capture the complexity of immunologic changes in various diseases.
Acknowledgments
We would like to thank Eduardo Candelario-Jalil (University of Florida, Department of Neuroscience) for the generous gift of middle cerebral artery occlusion stroke tissues and Fatemeh Shaerzadeh (University of Florida, Department of Neuroscience) for assistance with imaging.
Abbreviations
- Aβ
amyloid β protein
- AD
Alzheimer disease
- BBB
blood-brain barrier
- CNS
central nervous system
- CSF
cerebrospinal fluid
- LOAD
late-onset Alzheimer disease
- NFD
neurofibrillary degeneration
- PD
Parkinson disease
Authorship Contributions
Participated in research design: Gopinath, Khoshbouei, Streit.
Conducted experiments: Gopinath, Collins, Khoshbouei, Streit.
Performed data analysis: Gopinath.
Wrote or contributed to the writing of the manuscript: Gopinath, Khoshbouei, Streit.
Footnotes
We acknowledge funding from University of Florida Research Developmental Fund (Moonshot), from the Fixel Institute for Neurologic Diseases, and from National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke Grants 2R01-NS071122-07A1 to H.K.; shared instrumentation Grant S10OD020026 and Grant 8AZ19 awarded to W.J.S. from the Florida Department of Health; and multi-PI 5R21NS103108-02 awarded to H.K. and W.J.S. We also acknowledge funding from the National Center for Advancing Translational Sciences of the NIH under University of Florida Clinical and Translational Science Awards TL1TR001428 and UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Alafuzoff I, Adolfsson R, Bucht G, Winblad B. (1983) Albumin and immunoglobulin in plasma and cerebrospinal fluid, and blood-cerebrospinal fluid barrier function in patients with dementia of Alzheimer type and multi-infarct dementia. J Neurol Sci 60:465–472. [DOI] [PubMed] [Google Scholar]
- Askew K, Li K, Olmos-Alonso A, Garcia-Moreno F, Liang Y, Richardson P, Tipton T, Chapman MA, Riecken K, Beccari S, et al. (2017) Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep 18:391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum-Degen D, Müller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. (1995) Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 202:17–20. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. (2015) The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 138:2814–2833. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70:960–969. [DOI] [PubMed] [Google Scholar]
- Farrall AJ, Wardlaw JM. (2009) Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging 30:337–352. [DOI] [PubMed] [Google Scholar]
- Fiala M, Lin J, Ringman J, Kermani-Arab V, Tsao G, Patel A, Lossinsky AS, Graves MC, Gustavson A, Sayre J, et al. (2005) Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J Alzheimers Dis 7:221–232, discussion 255–262. [DOI] [PubMed] [Google Scholar]
- Fujimura N, Xu B, Dalman J, Deng H, Aoyama K, Dalman RL. (2015) CCR2 inhibition sequesters multiple subsets of leukocytes in the bone marrow. Sci Rep 5:11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk N, Wieghofer P, Grimm S, Schaefer R, Bühring HJ, Gasser T, Biskup S. (2013) Characterization of peripheral hematopoietic stem cells and monocytes in Parkinson’s disease. Mov Disord 28:392–395. [DOI] [PubMed] [Google Scholar]
- Gao L, Brenner D, Llorens-Bobadilla E, Saiz-Castro G, Frank T, Wieghofer P, Hill O, Thiemann M, Karray S, Prinz M, et al. (2015) Infiltration of circulating myeloid cells through CD95L contributes to neurodegeneration in mice. J Exp Med 212:469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, et al. (2009) Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40:1849–1857. [DOI] [PubMed] [Google Scholar]
- Goldmann T, Wieghofer P, Jordão MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, et al. (2016) Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol 17:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González H, Contreras F, Prado C, Elgueta D, Franz D, Bernales S, Pacheco R. (2013) Dopamine receptor D3 expressed on CD4+ T cells favors neurodegeneration of dopaminergic neurons during Parkinson’s disease. J Immunol 190:5048–5056. [DOI] [PubMed] [Google Scholar]
- Gordon R, Albornoz EA, Christie DC, Langley MR, Kumar V, Mantovani S, Robertson AAB, Butler MS, Rowe DB, O’Neill LA, et al. (2018) Inflammasome inhibition prevents α-synuclein pathology and dopaminergic neurodegeneration in mice. Sci Transl Med 10:eaah4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber MB. (2010) Changing face of microglia. Science 330:783–788. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. (1990a) Microglia: immune network in the CNS. Brain Pathol 1:2–5. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. (1990b) Perivascular microglia defined. Trends Neurosci 13:366. [DOI] [PubMed] [Google Scholar]
- Gray F, Vinters HV, Le Noan H, Salama J, Delaporte P, Poirier J. (1990) Cerebral amyloid angiopathy and granulomatous angiitis: immunohistochemical study using antibodies to the Alzheimer A4 peptide. Hum Pathol 21:1290–1293. [DOI] [PubMed] [Google Scholar]
- Grozdanov V, Bliederhaeuser C, Ruf WP, Roth V, Fundel-Clemens K, Zondler L, Brenner D, Martin-Villalba A, Hengerer B, Kassubek J, et al. (2014) Inflammatory dysregulation of blood monocytes in Parkinson’s disease patients. Acta Neuropathol 128:651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, et al. (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. (2012) Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43:3063–3070. [DOI] [PubMed] [Google Scholar]
- Hurley SD, O’Banion MK, Song DD, Arana FS, Olschowka JA, Haber SN. (2003) Microglial response is poorly correlated with neurodegeneration following chronic, low-dose MPTP administration in monkeys. Exp Neurol 184:659–668. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. (2003) Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol 106:518–526. [DOI] [PubMed] [Google Scholar]
- Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. (1989) Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol 24:173–182. [DOI] [PubMed] [Google Scholar]
- Kierdorf K, Masuda T, Jordão MJC, Prinz M. (2019) Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci 20:547–562. [DOI] [PubMed] [Google Scholar]
- Kim KS, Marcogliese PC, Yang J, Callaghan SM, Resende V, Abdel-Messih E, Marras C, Visanji NP, Huang J, Schlossmacher MG, et al. Canadian Lrrk2 in Inflammation Team (CLINT) (2018) Regulation of myeloid cell phagocytosis by LRRK2 via WAVE2 complex stabilization is altered in Parkinson’s disease. Proc Natl Acad Sci USA 115:E5164–E5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. (1996) Microglia: a sensor for pathological events in the CNS. Trends Neurosci 19:312–318. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Weissman IL. (1994) bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med 179:1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. (1999) Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol 46:598–605. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Gordon S. (1992) Turnover of resident microglia in the normal adult mouse brain. Neuroscience 48:405–415. [DOI] [PubMed] [Google Scholar]
- Lecours C, Bordeleau M, Cantin L, Parent M, Paolo TD, Tremblay ME. (2018) Microglial implication in Parkinson’s disease: loss of beneficial physiological roles or gain of inflammatory functions? Front Cell Neurosci 12:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. (2012) Eat-me signals: keys to molecular phagocyte biology and “appetite” control. J Cell Physiol 227:1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lively S, Schlichter LC. (2018) Microglia responses to pro-inflammatory stimuli (LPS, IFNγ+TNFα) and reprogramming by resolving cytokines (IL-4, IL-10). Front Cell Neurosci 12:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, Wyss-Coray T. (2009) Immune activation in brain aging and neurodegeneration: too much or too little? Neuron 64:110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Tago H, McGeer EG. (1987) Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett 79:195–200. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. (2001) Inflammation, autotoxicity and Alzheimer disease. Neurobiol Aging 22:799–809. [DOI] [PubMed] [Google Scholar]
- Mount MP, Lira A, Grimes D, Smith PD, Faucher S, Slack R, Anisman H, Hayley S, Park DS. (2007) Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci 27:3328–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D, et al. (2018) High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease [published correction appears in Immunity (2018) 48:599]. Immunity 48:380–395.e6. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. (2000) Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm Suppl:277–290. [DOI] [PubMed] [Google Scholar]
- Nalls MA, Plagnol V, Hernandez DG, Sharma M, Sheerin UM, Saad M, Simón-Sánchez J, Schulte C, Lesage S, Sveinbjörnsdóttir S, et al. International Parkinson Disease Genomics Consortium (2011) Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet 377:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Kotter MR, Franklin RJ. (2009) Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132:288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333:1456–1458. [DOI] [PubMed] [Google Scholar]
- Park DR, Thomsen AR, Frevert CW, Pham U, Skerrett SJ, Kiener PA, Liles WC. (2003) Fas (CD95) induces proinflammatory cytokine responses by human monocytes and monocyte-derived macrophages. J Immunol 170:6209–6216. [DOI] [PubMed] [Google Scholar]
- Park HS, Park MJ, Kwon MS. (2016) Central nervous system-peripheral immune system dialogue in neurological disorders: possible application of neuroimmunology in urology. Int Neurourol J 20 (Suppl 1):S8–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter LS, Barron E, Chui HC. (1990) Morphologic association between microglia and senile plaque amyloid in Alzheimer’s disease. Neurosci Lett 119:32–36. [DOI] [PubMed] [Google Scholar]
- Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, et al. (2009) Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 30:1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Engelhardt B. (2012) The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol 12:623–635. [DOI] [PubMed] [Google Scholar]
- Rogers J, Strohmeyer R, Kovelowski CJ, Li R. (2002) Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia 40:260–269. [DOI] [PubMed] [Google Scholar]
- Russo MV, McGavern DB. (2015) Immune surveillance of the CNS following infection and injury. Trends Immunol 36:637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Yamaguchi H, Ogawa A, Sugihara S, Nakazato Y. (1997) Microglial activation in early stages of amyloid beta protein deposition. Acta Neuropathol 94:316–322. [DOI] [PubMed] [Google Scholar]
- Schlachetzki JCM, Prots I, Tao J, Chun HB, Saijo K, Gosselin D, Winner B, Glass CK, Winkler J. (2018) A monocyte gene expression signature in the early clinical course of Parkinson’s disease. Sci Rep 8:10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A, Fadler T, Dorfmeister E, Hoffmann AC, Xiang W, Winner B, Prots I. (2016) Infiltrating T lymphocytes reduce myeloid phagocytosis activity in synucleinopathy model. J Neuroinflammation 13:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ. (2002) Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 40:133–139. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Del Tredici K, Leyh J, Lier J, Khoshbouei H, Eisenlöffel C, Müller W, Bechmann I. (2018) Microglial activation occurs late during preclinical Alzheimer’s disease. Glia 66:2550–2562. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xue QS, Bechmann I. (2009) Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol 118:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Khoshbouei H, Bechmann I. (2020) Dystrophic microglia in late-onset Alzheimer’s disease. Glia 68:845–854. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. (2004) Dystrophic microglia in the aging human brain. Glia 45:208–212. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT. (1998) Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol 152:74–87. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Xue QS, Tischer J, Bechmann I. (2014) Microglial pathology. Acta Neuropathol Commun 2:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, et al. (2017) T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 546:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, De Prijck S, Vandamme N, De Schepper S, Van Isterdael G, Scott CL, et al. (2019) A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci 22:1021–1035. [DOI] [PubMed] [Google Scholar]
- Vawter MP, Dillon-Carter O, Tourtellotte WW, Carvey P, Freed WJ. (1996) TGFbeta1 and TGFbeta2 concentrations are elevated in Parkinson’s disease in ventricular cerebrospinal fluid. Exp Neurol 142:313–322. [DOI] [PubMed] [Google Scholar]
- Wada H. (1998) Blood-brain barrier permeability of the demented elderly as studied by cerebrospinal fluid-serum albumin ratio. Intern Med 37:509–513. [DOI] [PubMed] [Google Scholar]
- Weiskopf K, Jahchan NS, Schnorr PJ, Cristea S, Ring AM, Maute RL, Volkmer AK, Volkmer JP, Liu J, Lim JS, et al. (2016) CD47-blocking immunotherapies stimulate macrophage-mediated destruction of small-cell lung cancer. J Clin Invest 126:2610–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EH, Weninger W, Hunter CA. (2010) Trafficking of immune cells in the central nervous system. J Clin Invest 120:1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue QS, Streit WJ. (2011) Microglial pathology in Down syndrome. Acta Neuropathol 122:455–466. [DOI] [PubMed] [Google Scholar]