Abstract
Innate and adaptive immune mechanisms have emerged as critical regulators of CNS homeostasis and mental health. A plethora of immunologic factors have been reported to interact with emotion- and behavior-related neuronal circuits, modulating susceptibility and resilience to mental disorders. However, it remains unclear whether immune dysregulation is a cardinal causal factor or an outcome of the pathologies associated with mental disorders. Emerging variations in immune regulatory pathways based on sex differences provide an additional framework for discussion in these psychiatric disorders. In this review, we present the current literature pertaining to the effects that disrupted immune pathways have in mental disorder pathophysiology, including immune dysregulation in CNS and periphery, microglial activation, and disturbances of the blood-brain barrier. In addition, we present the suggested origins of such immune dysregulation and discuss the gender and sex influence of the neuroimmune substrates that contribute to mental disorders. The findings challenge the conventional view of these disorders and open the window to a diverse spectrum of innovative therapeutic targets that focus on the immune-specific pathophenotypes in neuronal circuits and behavior.
SIGNIFICANCE STATEMENT
The involvement of gender-dependent inflammatory mechanisms on the development of mental pathologies is gaining momentum. This review addresses these novel factors and presents the accumulating evidence introducing microglia and proinflammatory elements as critical components and potential targets for the treatment of mental disorders.
Introduction
Psychiatric or mental disorders include several syndromes manifested through physiologic, behavioral, emotional, and cognitive symptoms. Over a billion people worldwide suffer from mental disorders, with anxiety-related disorders, major depressive disorder (MDD), bipolar disorder (BD), post-traumatic stress disorder (PTSD), and schizophrenia (SCZ) accounting for more than 80% GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018). The World Health Organization estimates that the global burden of mental disorders translates to ∼32% of years lived with disability (Vigo et al., 2016), as the current pharmacotherapies prove ineffective for up to 50% of patients (Pfau et al., 2018). Insufficient understanding of underlying disease mechanisms as well as gender influence in disease manifestation is thought to cause the observed treatment resistance.
A growing body of evidence suggests that neuroimmunologic processes affect both neuronal integrity and neuropathology, revealing new targets for the development of effective therapeutics (Russo and Nestler, 2013; Miller et al., 2017; Pape et al., 2019). Multiple clinical studies have reported through genome-wide association studies (GWASs) (Howard et al., 2019; Marques et al., 2019) and postmortem histopathological findings (Mechawar and Savitz, 2016) that many patients with mental disorders exhibit chronic inflammation and immune system dysregulation accompanied by increased peripheral and central nervous system (CNS) inflammatory markers (Sandiego et al., 2015; Goldsmith et al., 2016; Wohleb et al., 2016).
Here, we provide an overview of the interplay between neuroinflammation and CNS homeostasis during neuropsychiatric dysfunctions. CNS neuroinflammation is predominantly initiated by the resident immune macrophage-like cells, the microglia, along with brain macrophages and astrocytes (Prinz and Priller, 2014). We discuss upstream causes of immune dysregulation in mental disorders, focusing on recent insights suggesting that inflammatory mechanisms are implicated in the mental disorder pathophysiology. We also summarize the sex and gender effects upon mental disorder prevalence and severity through the prism of neuroinflammatory mechanisms and suggest the necessity to take them into account in mental disorder therapeutics.
Neuroimmune Interactions in Brain Homeostasis
The CNS has been considered an “immune-privileged” system guarded by the blood-brain barrier (BBB) (Forrester et al., 2018). Despite its protection, the brain exhibits significant immunologic properties and is in constant interaction with the peripheral immune system (Pape et al., 2019). The immune system along with neurons and glial cells orchestrate the cognitive, emotional, and social properties in the healthy brain (Blank and Prinz, 2013). Significantly, although microglia account only for ∼10% of the cells in CNS, they have emerged as crucial neuroinflammatory effectors of these functions (Tay et al., 2018; Pape et al., 2019). They continuously survey their microenvironment with their short, fine, and highly motile processes, regulating CNS homeostasis (surveillance state) (Davalos et al., 2005; Nimmerjahn et al., 2005) from development through adulthood (Blank and Prinz, 2013; Prinz and Priller, 2014; Forrester et al., 2018). In the healthy adult brain, homeostatic microglia phagocytose cellular debris (Fourgeaud et al., 2016), modulate myelin levels (Miron et al., 2013; Safaiyan et al., 2016; Hagemeyer et al., 2017), monitor neurogenesis (Sierra et al., 2010; Gemma and Bachstetter, 2013), release cell signaling factors (Parkhurst et al., 2013), and act as vital components of synapse formation, plasticity, and function (Hanisch and Kettenmann, 2007; Wake et al., 2009; Tremblay et al., 2011; Bialas and Stevens, 2013). Acting as professional phagocytes of the CNS, microglia engulf axon parts, terminals, and dendritic spines, thereby contributing to synaptic activity modulation in CNS areas implicated in behavioral experiences (such as learning/memory, fear-anxiety, anhedonia, and social tasks) via a transforming growth factor-β–dependent complement cascade (Paolicelli et al., 2011; Tremblay et al., 2011; Schafer et al., 2012; Miyamoto et al., 2016; Torres et al., 2016; Tay et al., 2018). Their process motility can dramatically change in response to neuronal activity and neurotransmitter levels (Li et al., 2012; Abiega et al., 2016). Interestingly, a recent study demonstrated that microglia-mediated synaptic reorganization is responsible for the dissociation of hippocampal engrams during adulthood and affects previously encoded memories (Wang et al., 2020).
Microglial activation drives the neuroinflammation evident in neurologic (Ransohoff, 2016a) and neuropsychiatric diseases (Mondelli et al., 2017; Li and Barres, 2018). Microglia respond swiftly to a variety of environmental cues (e.g., immune challenges, injury, diseases) through various cell surface receptors, including toll-like receptors (TLRs), complement receptors (CR3, CR4), and scavenger receptors (CD36, CD91). During this response, they significantly alter their morphology and release cytokines, chemokines, reactive oxygen species (ROS)/reactive nitrogen species, and trophic factors. Previously, microglial activation was conceptually categorized into a bimodal scheme based on the study of peripheral macrophages, resulting in either “cytotoxic” effects on neurons and oligodendrocytes (M1 type) or “protective” effects through phagocytic capacity and support of neurite outgrowth (M2 type) (Ransohoff, 2016b). More recently, however, microglial activation is considered multidimensional, with several activation stages and overlap in gene expression (Ransohoff, 2016b; Salter and Stevens, 2017; Li and Barres, 2018).
There are three other CNS macrophage types: perivascular, meningeal, and choroid plexus macrophages, located at the interface between the circulation and the parenchyma (Prinz et al., 2017). Furthermore, circulating myeloid cells, such as monocytes, granulocytes, and dendritic cells, reside in the CNS vasculature network (Li and Barres, 2018). In several diseases or injuries, monocytes may promptly infiltrate the brain parenchyma and differentiate into microglia-like cells to alleviate or exacerbate disease progression (Ginhoux et al., 2010; Prinz and Priller, 2014; Li and Barres, 2018).
Another piece of the neuroimmune cross talk is regulated by astrocytes that express chemokine, cytokine, and complement receptors, allowing them to interact with microglia and macrophages and get activated to serve several functions, including neurotrophic support, synaptic homeostasis, mitigating oxidative stress, neuron-glia signaling, and others (Sofroniew and Vinters, 2010; Khakh and Sofroniew, 2015; Liddelow and Barres, 2015). Acute astrocytic activation may exert both reparative functions [e.g., through secretion of neurotrophic factors (BDNF, neurotrophic growth factor) and glutamate clearance] or neurotoxic functions, leading to neuronal loss and behavioral alterations (Sanacora and Banasr, 2013; Khakh and Sofroniew, 2015; Liddelow and Barres, 2015; Haroon et al., 2017). Astrocytes can also act as “gate keepers” through their BBB regulation, controlling trafficking of peripheral immune cells to the CNS (Abbott et al., 2006).
Involvement of Immune Dysregulation in Mental Disorders: An Evolutionary Perspective
Recent converging human and animal data show that stress-related neurocircuitry and immune system coevolved to act synergistically and shield organisms from environmental threats. During a stressful experience, a “fight or flight” reaction is usually observed, involving activation of inflammatory pathways [e.g., nuclear factor–κΒ (NF-κB)] and leading to significant increases in circulating levels of proinflammatory cytokines, such as interleukin-6 (IL-6) (Pace et al., 2006; Koo et al., 2010; Sasayama et al., 2013; Wohleb et al., 2016; Felger et al., 2020). These inflammatory responses do not target specific pathogens but rather environmental stressors, commonly observed in acute inflammatory diseases and mental disorders (such as MDD, SCZ, BD) (Pace and Miller, 2009; Derry et al., 2013; Goldsmith et al., 2016).
From an evolutionary perspective, it is hypothesized that modern humans have a hereditary genomic predisposition toward inflammation as a defensive response to environmental dangers and threatening stimuli (psychosocial stressors). The behavioral responses that are now adapted to mental disorders may have been used to enhance survival and reproduction in highly pathogenic and threatening environments many years ago (the pathogen-host hypothesis) (Miller and Raison, 2016). Consequently, mammals had to conserve energy for healing wounds and infections (social avoidance and anhedonia support this metabolic shunt) while maintaining hypervigilance against an attack from enemies (hyperalertness in stress and manic disorders) (Raison and Miller, 2013; Slavich and Irwin, 2014).
Equally important to the pathogen-host hypothesis of mental disorders is our detachment from an array of microbes that were previously ubiquitous in our microbiota (skin, gut, oral, and nasal) (Rook et al., 2015). Studies suggest that these microbes (commensals and symbiotes) have significantly contributed to the suppression of inflammatory responses through transforming growth factor-β signaling (Raison et al., 2010b; Rook et al., 2015). In modern times, sanitized urban environments resulted in decreased exposure to microbes and their immunoregulatory input. In the absence of these inflammatory regulators, the increased current psychosocial challenges and stressors have elicited increased immune responses, accounting for the high comorbidity of mental and inflammatory disorders (Prinz and Priller, 2014; Mechawar and Savitz, 2016; Miller and Raison, 2016; Wohleb et al., 2016).
Causal Factors of Immune Dysregulation in Mental Disorders
Two broad etiologic factors have been recognized as causes of mental disorder pathology: genetic susceptibility and environmental factors (Smoller, 2016). The latter include chronic stress, traumatic life events (physical, emotional, sexual abuse, bullying), malnutrition, drug abuse, social isolation, and prenatal environment (poor nutrition, exposure to drugs or toxins, and maternal infections or stress) (Wong and Licinio, 2001). Genetic sensitivity to environmental risks (Jaffee and Price, 2007; Belsky et al., 2009) can induce epigenetic changes at different levels, (e.g., neuroinflammatory, neurotransmitter, and brain connectivity), thus modifying the ability to adapt to subsequent stressors (Arango et al., 2018). Below, we discuss several causal factors of immune dysregulation affecting mental disorders.
Stress
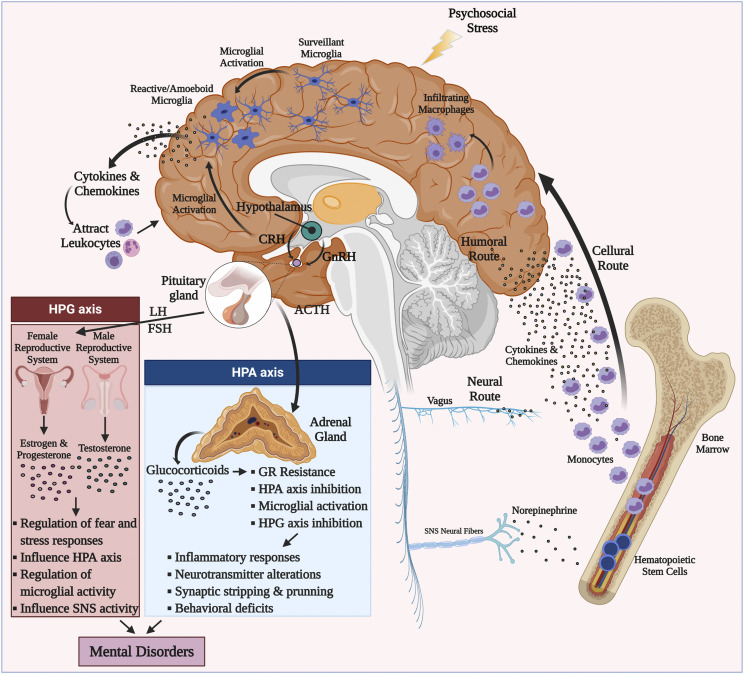
Stress is believed to trigger psychiatric symptoms through immune system overactivation and escalation of the risk of mental disorder occurrence (Bergink et al., 2014). The immune system swiftly responds to stress through hormones (cortisol, epinephrine, and norepinephrine) and sympathetic nervous system (SNS). This can happen via the hypothalamic-pituitary-adrenal (HPA) axis activation, which is the cardinal stress-mediated neuroendocrine system, resulting from corticotropin releasing hormone (CRH) and arginine vasopressin secretion, which stimulate the secretion of adrenocorticotropic hormone (ACTH) (Faravelli et al., 2012). ACTH can then stimulate glucocorticoid release, specifically cortisol, from the adrenal cortex, mobilizing immune cell trafficking in the body (Dhabhar et al., 2012; Faravelli et al., 2012). This acute reaction can potentially cause chronic low-grade inflammatory responses characterized by intensified proinflammatory responses of tumor necrosis factor-α (TNF-α), IL-6, and interferon-γ (IFN-γ) (Glaser and Kiecolt-Glaser, 2005) (Fig. 1).
Fig. 1.
Sex hormone influence and neuroimmune interplay in mental disorders. The HPA and hypothalamic-pituitary-gonadal (HPG) axes interact with each other and shape the downstream inflammatory responses, neurotransmitters, synaptic plasticity, and behavioral deficits observed in mental disorders. During psychosocial environmental stressors, microglia are stimulated through activation of immune receptors (TLRs, CRH receptors, and cytokine and chemokine receptors). Subsequent cytokine and chemokine secretion attracts activated myeloid cells to the brain via the cellular route. Once in the brain, infiltrating macrophages can drive central inflammatory responses. During psychosocial stress, catecholamines (e.g., noradrenaline released by activated SNS fibers) stimulate increases in myeloid cells (e.g., monocytes) in the periphery. Through induction of inflammatory signaling pathways (such as NF-κB and NLRP3 inflammasome), more proinflammatory cytokines and chemokines are produced that contribute to glucocorticoid resistance through glucocorticoid receptor cleavage. Proinflammatory cytokines and chemokines can access CNS through humoral and neural routes. Monocytes then infiltrate CNS through a compromised blood-brain barrier and differentiate to activated macrophages. Sex hormones affect risk for mental disorders by modulating these pathways at several levels: they 1) influence the perception, processing, and regulation of threat and fear; 2) modulate SNS/HPA reactivity to psychosocial stressors; and importantly 3) alter microglial and macrophage signal transduction through post-translational modifications and epigenetic changes. FSH, follicle stimulating hormone; GnRH, gonadotropin releasing hormone; LH, luteinizing hormone.
Recurrent stress (chronic stress) affects the production, reactivity, and circulation of immune cells, promoting detrimental inflammatory responses (Trottier et al., 2008). Several animal studies have demonstrated that chronic stress can induce immune dysregulation via glucocorticoid receptor (GR) resistance and inhibition of the HPA axis feedback loop, leading to proinflammatory cytokine production and a concomitant suppression of anti-inflammatory cytokines and immunosuppressive pathways (Stark et al., 2001; Frank et al., 2007; Engler et al., 2008; Heidt et al., 2014).
Early human studies in long-term social anxiety (bullying, social status changes, or hierarchy) reported higher rates of persistent anxiety, depression, low self-esteem, and incidence of illness (Marmot and Feeney, 1997; Griffin et al., 2002; Stansfeld et al., 2003). Correspondingly, clinical studies have concluded that chronic or early-life stress can induce transcriptional changes that promote susceptibility to hyperinflammatory responses, leading to a “biological imprinting” (Pace et al., 2006; Miller et al., 2008; Danese et al., 2011). For instance, teenagers with a history of childhood adversity have high IL-6 levels, correlating with subsequent development of depression (Miller and Cole, 2012). Peripheral C-reactive protein (CRP) and proinflammatory cytokine levels were shown to predict future PTSD development after traumatic and acute stressful events (Pervanidou et al., 2007; Eraly et al., 2014). Similarly, a meta-analysis of clinical studies revealed an enhanced proinflammatory profile (CRP, IL-6, and TNF-α) in adults with early-life childhood trauma and maltreatment (Baumeister et al., 2016). Changes in inflammatory gene expression mediated by epigenetic mechanisms [e.g., FK506 binding protein 5 (FKBP5), a factor associated with glucocorticoid sensitivity and mental disorders] have been linked with childhood traumatic experiences and elevated inflammatory responses (Jones, 2013; Klengel et al., 2013).
In the CNS, preclinical data have indicated that repeated psychosocial stress and early-life traumatic events can induce microglial activation (through stress hormones, cytokines, pattern recognition receptor agonists, and neurotransmitters), raising the risk of mental disorders later in life (Giovanoli et al., 2013; Howes and McCutcheon, 2017). In the hippocampus (HPC) and prefrontal cortex (PFC), psychosocial stress increases the levels of extracellular ATP, which induces the NLRP3-dependent inflammasome, leading to microglial interleukin IL-1β release (Pantazatos et al., 2017). Likewise, toll-like receptors TLR-2 and TLR-4 can mediate repeated stress-induced gene expression and TNF-α/IL-1α secretion from the PFC microglia (Wohleb et al., 2012; Nie et al., 2018; Furuyashiki and Kitaoka, 2019). Finally, restraint stress induces alterations to neurotransmitters such as glutamate and GABA, which influence microglial activation, proliferation, and motility (Nair and Bonneau, 2006; Fontainhas et al., 2011). Together, these findings suggest causal relationships between psychosocial stressors and early-life stress/trauma with immune dysregulation, inflammation, and development of mental disorders.
Genetic Factors
Since genetics, along with stress, are the most important psychopathological contributors, the “diathesis-stress” (diathesis being the genetic component) model constitutes the principal etiologic hypothesis for mental disorders (Smoller, 2016). The diathesis-stress model suggests that genetic vulnerability and environmental stressors can escalate the predisposition to disorder, which in turn occurs once the threshold of sufficient liability is crossed. Previous studies using family members and twins revealed that mental disorders had heritable components to varying degrees. More recently, molecular studies have started identifying specific genetic variations related with psychiatric disorder phenotypes.
Significantly, genome studies have associated MDD with immune-related genes involved in the IL-6, IFN, and natural killer cell signaling pathways. Specifically, genes upregulated in MDD include TNF-α receptor (TNFRSF10C), mitogen-activated protein kinase (MAPK) 14, IL-6 receptor, STAT3 (Wong et al., 2017), and several IFN-related genes (e.g., MX1, OAS1, IFIT3, PTPN6, and IRF7) (Mostafavi et al., 2014). Natural killer–related genes are downregulated (GZMB, KLRK1, PRF1, SH2D1B, KLRD1, and NFATC2) (Jansen et al., 2016). The TRPM2 gene (which influences reactive oxygen species production and exacerbates NLRP3 inflammasome) was also significantly associated with MDD (Wong et al., 2017). Finally, a recent study points to a stress-related neuroinflammatory association with MDD, potentially via p75NTR/neurotrophic growth factor (nerve growth factor) and innate immune TLR signaling (Chan et al., 2020). These results support and link the neurotrophic (Duman and Li, 2012) and neuroimmune hypotheses of depression (Hodes et al., 2015).
SCZ typically emerges in early adulthood and is characterized by episodic or continuous alterations of the perception of reality, behavior, and cognition (Foley et al., 2017). GWASs have identified promising target genes that are widely involved in dopaminergic (DRD2) and glutamatergic neurotransmission (GRM3, GRIN2A, SRR, CLCN3, and GRIA1), neuronal calcium signaling (e.g., CACNB2, CACNA1l, CACNA1C, RIMS1), and synaptic function (PAK6, KCTD13, CNTN4) (Foley et al., 2017). Significantly, the top genetic correlations in schizophrenia come from the major histocompatibility complex (MHC) and B-cell activation loci (CD19, CD20), rendering immune pathways at the center of schizophrenia research (Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium, 2015).
BD is a manic-depressive disorder that causes sudden changes in mood, concentration, energy, and activity levels, ranging from manic to depressive episodes. Several studies have associated BD within single-nucleotide polymorphisms (SNPs) in genes encoding cytokines or immune function (IFN-γ, IL-6, IL-1, TLR2, TLR4, PTGS2, CCL2, and CCL3) (Fries et al., 2019). A transcriptome-wide analysis included 1600 patients with SCZ and BD and reported the upregulation of members of IFN and NF-κB pathways (Guan et al., 2019).
Based on these studies, inflammatory, monoaminergic, and glutamatergic elements constitute the genetic “diathesis” and interact to coordinate the genetic predisposition of individuals to mental disorders.
Pathogen-Related Inflammation and Autoimmunity
Chronic disturbances in the innate and adaptive immunity systems are likely to dysregulate CNS function and alter cognitive performance. Frequently, pathogenic infection of the CNS drives the adaptive systemic inflammation, called “sickness behavior,” which is manifested as social withdrawal, depressed mood, anhedonia, irritability, fatigue, impaired concentration, muscle pain, and fever. The symptoms overlap significantly with those observed in several mental disorders and are considered the result of reallocating energy resources to combat infection and enhance host survival. Evidence from long-term studies has demonstrated that hospitalization due to infection increases the risk for major depression by 62% (Benros et al., 2011). In rodents, perinatal infection and systemic inflammatory responses induce cognitive deficits comparable to the psychosis observed in young adults after early infection (Khandaker et al., 2015). Similarly, Streptococcus pyogenes infection is frequently correlated with subsequent mental and autoimmune psychiatric disorders.
In a prospective study of severe infection [Helicobacter pylori, Chlamydia pneumoniae, Cytomegalovirus (CMV), Herpes simplex virus (HSV-1, and HSV-2)], high viral burden was associated with cognitive decline (Katan et al., 2013). In addition, high CMV antibody titers have been identified in MDD subjects relative to controls (Rector et al., 2014), whereas infection with the protozoan Toxoplasma gondii has been associated with increased risk for SCZ (Torrey et al., 2007), MDD, and manic and suicidal behavior (Dickerson et al., 2014; Sugden et al., 2016). Studies suggest that T. gondii encodes proteins homologous to neurotrophic factors and dopamine metabolism, possibly modulating dopaminergic neurotransmission (Carruthers and Suzuki, 2007), leading to activation of kynurenine (KYN) pathway metabolites in the brain (Notarangelo et al., 2014).
The mechanistic associations between inflammation and mental disorders remain incomplete. Microglial inflammasome activation constitutes a potential response to proinflammatory mediators (Heneka et al., 2018; Zhang et al., 2018), thereby increasing the risk for mental disorders (Misiak et al., 2019). The systemic proinflammatory cytokine (such as TNF-α, IL-1β, and IL-6) released in response to infection or injury can also affect the CNS through activation of cerebral endothelial cells and microglia (D’Mello and Swain, 2017). Cytokines can exert neurotoxic effects through ROS production and alterations of glutamatergic and monoamine transmission.
The equilibrium between CNS and the immune system resembles a double-edged sword: on one side are the positive effects of the evolutionarily advantageous sickness behavior; on the other side are the detrimental effects of chronic inflammation, which lead to neurotoxicity, cognitive decline, and mental dysfunction.
Such equilibrium imbalances are observed in autoimmune inflammatory responses and mental disorders. In fact, there is significantly higher comorbidity of autoimmune disorders with patients with mental disorders than there is in the general population (Vonk et al., 2007; Korczak et al., 2011; Kosmidis et al., 2012). At the same time, autoimmune diseases have a high risk factor for subsequent diagnosis of mental disorder (45% increase) (Benros et al., 2013). A subset of patients with mental disorders exhibit increased levels of circulating autoantibodies: patients with MDD and BD may present comorbidity with autoimmune thyroiditis, evident by the presence of thyroperoxidase antibodies (Pop et al., 1998; Kupka et al., 2002); patients with multiple sclerosis may experience neuropsychological alterations and chronic anxiety, as in MDD (Feinstein et al., 2014) or SCZ (Andreassen et al., 2015).
Whether autoimmune antibodies are a causal factor or outcome of the psychopathological process of mental disorders is still unknown. Nevertheless, there are cases of psychosis and depression that report the presence of autoantibodies targeting neurotransmission in patients with limbic encephalitis (Dalmau et al., 2011; Kayser et al., 2013). Therefore, it becomes gradually recognized that a subset of patients with mental disorders may in fact suffer from an autoimmune disease. Lennox et al. (2012) and Dahm et al. (2014) wrote the following in an editorial: “Antibody screening in young people presenting with psychosis, seizures and cognitive disturbance is now part of routine clinical practice in neurological and intensive care settings.”
Integrating Neuroimmune Systems in Mental Disorder Pathogenesis
Neuroinflammatory Mechanisms in Anxiety and Depressive Disorders
Anxiety and depressive disorders represent the leading class of mental disorders, with MDD affecting yearly 300 million people worldwide (Ferrari et al., 2013; GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). They persist throughout life and have high comorbidity with other disorders, including PTSD, BD, and SCZ (Conway et al., 2006; Hettema et al., 2011). Family and GWASs implicate genetic factors [∼30% contribution (Otte et al., 2016)] in the etiology of the disorders (monoaminergic, glutamatergic, neurotrophic, and stress hormone genes) (Smoller, 2016). However, exposure to chronic stress, traumatic experiences, and environmental factors are the most essential contributors for the onset of these disorders (Abelson et al., 2007; Dieleman et al., 2015).
In anxiety and depressive disorders, the fear-emotion processing network is stimulated in frontal [such as anterior cingulate cortex (ACC)] and limbic areas [amygdala (AMY)] (Gorman et al., 2000). Enhanced AMY-ACC connectivity has been correlated with augmentation of threatening stimuli in anxiety disorders, MDD, and PTSD (Killgore et al., 2014; Fonzo et al., 2015) and is accompanied by increased IL-6 levels (Muscatell et al., 2015). These increases are linked to social withdrawal, cognitive disturbances, depression (Harrison et al., 2009; Muscatell et al., 2015), and lower serotonin status (Hornboll et al., 2018). Exposure to stressful and traumatic events induces the HPA axis and cortisol release to counteract the norepinephrine-induced immune system activation and proinflammatory responses via NF-κB inhibition (Fig. 1). This balance is dysregulated during chronic activation of the HPA axis, leading to negative feedback and GR resistance.
In anxiety disorders, studies have demonstrated that patients with generalized anxiety disorder, panic disorder, and phobias have significantly increased cortisol levels (Mantella et al., 2008; Staufenbiel et al., 2013) and subsequent upsurges of sympathetic tone (Blechert et al., 2007; Alvares et al., 2013), leading to immune activation and inflammation. Patients with anxiety disorders (children and adults) exhibit elevated proinflammatory (CRP, TNF-α, IL-1β, and IL-6) and reduced anti-inflammatory (IL-2, IL-4) responses when compared with healthy controls (Brambilla et al., 1999; Hoge et al., 2009; Copeland et al., 2012; Vogelzangs et al., 2013; Wagner et al., 2015), as well as increased and highly sensitized lymphocytic T-cell populations (T helper 17) (Boscarino and Chang, 1999; Vieira et al., 2010). However, some other reports have described small or no changes in proinflammatory responses (Brambilla et al., 1999; Vogelzangs et al., 2013; Wagner et al., 2015). These discrepancies could be attributed to 1) the wide phenotypic and etiologic spectrum of anxiety disorders, 2) notions that only severe anxiety cases manifest increased inflammatory responses, 3) gender, and 4) comorbid mental or physical health problems.
In depressive disorders, early hypotheses had proposed that pathology stems from monoaminergic (Heninger et al., 1996) and glutamatergic alterations in the CNS (Kendell et al., 2005; Northoff and Sibille, 2014). However, approximately 40%–50% of patients with MDD are not responsive to antidepressants (Krishnan and Nestler, 2008), potentially reflecting that other disease mechanisms may be at play. The initial association between inflammation and depression was formed after the development of depression symptoms after long-term IFN-α treatment in patients with hepatitis C (Renault et al., 1987; Conversano et al., 2015). In a subsequent study that investigated the association of peripheral immune system and depression, Maes et al. (1992) identified elevated numbers of Ly6Chi monocytes and neutrophils in the blood of patients with MDD, proposing the inflammatory hypothesis of depression (Smith, 1991; Maes, 1995).
Since then, a plethora of studies have reported high levels of proinflammatory markers (CRP, TNF-α, IL-6, and IL-1β) in patients with depression (Maes et al., 1997; Howren et al., 2009; Miller et al., 2009; Dowlati et al., 2010; Khandaker et al., 2014; Mostafavi et al., 2014; Strawbridge et al., 2015; Goldsmith et al., 2016; Miller and Raison, 2016; Felger et al., 2020). Epidemiologic studies (Whitehall II) on large community samples (>3000 individuals) and a decade of follow-up demonstrated that elevated blood levels of IL-6 and CRP could be used as prognostic markers of depressive symptoms (Gimeno et al., 2009). In support of that, elevated cortisol levels, GR insensitivity and dysregulation of the HPA axis have been consistently correlated with the inflammatory manifestations during MDD (Turecki and Meaney, 2016). In a recent single-nucleus RNA sequencing study of PFC in patients with MDD, co-chaperones of GRs (Heat shock protein 90 and FKBP5) were downregulated (Nagy et al., 2020), whereas in a systematic meta-analysis, it was demonstrated that increased inflammation (TNF-α, IL-6) is correlated with glucocorticoid resistance and elevated levels of cortisol in patients with MDD (Perrin et al., 2019).
Respective increases of innate immune markers (TNF-α, IL-1β, IL-6, TLR3, and TLR4) (Miller et al., 2009; Miller and Raison, 2016), along with microglial and astrocytic activation in several brain areas (PFC, HPC, and ACC), have been reported in postmortem MDD brain samples (Steiner et al., 2008; Rao et al., 2010; Torres-Platas et al., 2014; Nagy et al., 2015). Microarray analyses of MDD individuals identified significant upregulation of immune transcripts (cytokines, complement) (Shelton et al., 2011; Kim et al., 2016). Neuroinflammation in patients with MDD has also been visualized by positron emission tomography (PET) using the translocator protein (TSPO; microgliosis and astrogliosis marker) (Setiawan et al., 2015).
In animal studies, exposure to psychosocial/environmental stressors (stress section) has similarly revealed high cortisol and ACTH levels in plasma, elevated hypothalamic CRH expression, and concomitant increases of proinflammatory plasma cytokines (Engler et al., 2005; Ramirez et al., 2016). Chronic stress can induce neuronal activation in the anxiety/threat appraisal areas (PFC, AMY, and HPC) through glutamatergic and norepinephrine signaling (Perrotti et al., 2004; Musazzi et al., 2015), leading to neuroendocrine stimulation and glucocorticoid release (Ulrich-Lai and Herman, 2009). In a mouse depression model [repeated social defeat stress (RSDS)], innate immune GR resistance was correlated with high cortisol levels (Avitsur et al., 2002) and increased IL-6 plasma levels (Janssen et al., 2010), and these effects were dependent on Il-1β or adrenergic signaling (Jankord et al., 2010). Accordingly, chronic stress–induced activation of β3 adrenergic receptor and downregulation of chemokine ligand-12 have been shown to induce increases in hematopoietic stem cell activity and peripheral elevation of monocytes and neutrophils (Engler et al., 2004; Wohleb et al., 2013; Heidt et al., 2014).
Microglial Interplay in Anxiety and Depressive Disorders
Microglia and macrophages are believed to have a pivotal role in depressive-like behavior (Wohleb et al., 2014b, 2015; Reader et al., 2015; Ramirez et al., 2016, 2017; Stein et al., 2017; Bollinger and Wohleb, 2019). Early work has demonstrated that in chronic stress models, β-adrenergic receptor signaling can induce microglial hypertrophy, proinflammatory cell activation (CD14, CD86, and TLR4), and cytokine expression (IL-6, IL-1β, TNF-α, and IL-1β), leading to depressive phenotype (Johnson et al., 2005; Blandino et al., 2006; Wohleb et al., 2011). These microglial-mediated effects are reduced by 1) GR antagonist RU486 (Wohleb et al., 2018; Horchar and Wohleb, 2019), 2) propranolol administration (β-adrenergic receptor antagonist), or 3) knocking out IL-1R in mice (Wohleb et al., 2011). These results suggest that chronic stress–induced inhibition of HPA axis may readily engage microglia and propagate inflammatory responses, driving associated behavioral consequences (Fig. 1).
Contributors to the chronic stress signal propagation are the pattern recognition receptor family of TLRs, associated with increased release of damage-associated molecular patterns, which in turn promote NLRP3 inflammasome activation, TSPO increase (Wang et al., 2018), and IL-1β release (Pan et al., 2014; Fleshner et al., 2017). Recently, a study by Nie et al. (2018) reported that RSDS can activate microglia through TLR2 and TLR4 and increased IL-1α and TNF-α expression, leading to atrophy of PFC neurons and social avoidance (Nie et al., 2018). The use of a double-knockout mouse model (TLR double knockout) or neutralizing antibodies for the cytokines rescued those effects, highlighting the pivotal role of TLRs on microglial activation during chronic stress.
Associated with microglial activation and concomitant inflammatory responses are activation markers (CD68), specific morphologic features (e.g., branch length and number, soma volume), phagocytic activity, and oxidative stress. Interestingly, RSDS studies have demonstrated that microglial activation (CD68), ROS production, and phagocytic activity (ex vivo) are upregulated in groups susceptible to stress, suggesting microglia-mediated neuronal dysfunction (Lehmann et al., 2016, 2018, 2019; Nie et al., 2018). Several studies have also attempted to visualize stress-induced morphologic changes in microglia, but the results are mixed (Wohleb et al., 2011, 2012, 2013, 2014a; Hinwood et al., 2012; Walker et al., 2013; Lehmann et al., 2016; McKim et al., 2016). Possible reasons for these discrepancies in the literature might be the differences in the stress paradigms [the nature and intensity of stressor (acute or chronic)] as well as lack of sensitivity and inconsistencies in the quantification of microglial morphology.
To examine the role of microglia in the onset of depression, some studies used an a priori microglial ablation (∼95%) by the colony stimulating factor-1 receptor antagonist PLX5622 (McKim et al., 2018; Lehmann et al., 2019; Weber et al., 2019). Ablation before RSDS resulted in resilience to chronic stress, reductions of ROS formation, monocyte recruitment, proinflammatory cytokines, and depression-related behavioral tests (McKim et al., 2018; Weber et al., 2019; Lehmann et al., 2019). Remarkably, repopulation of microglia after PLX5622 withdrawal [clonal expansion of microglia (Tay et al., 2017)] was sufficient to reinitiate this cascade of events and recapitulate the depression-like effects in mice. These results, however, unveil more questions regarding the role of microglia during depression: 1) Could chronic stress potentially induce microglial epigenetic changes at depression onset? 2) Is the neuronal sensitization (adverse activated areas) also contributing to a microglial “re-education” after repopulation? 3) What are the significance and microglial phenotype of the relatively uncharacterized resilient-to-stress animals? And 4) what is the contribution of peripheral leukocytes in these processes?
Regarding the latter, bone marrow–derived leukocytes can enter the brain through the BBB epithelial lining and contribute to depression pathophysiology (Banks et al., 1994, 1995), a finding both in postmortem studies in patients with MDD (Torres-Platas et al., 2014) and in RSDS animals (CNS infiltration and differentiation of proinflammatory Ly6Chi monocytes to macrophages) (Varvel et al., 2012; Wohleb et al., 2013) (Fig. 1). A recent study exhibited that this recruitment can be mediated by neurovascular adhesion of IL-1β–producing monocytes (vascular cell adhesion molecule-1 and ICAM-1) to the CNS parenchyma (McKim et al., 2018). This finding is of particular interest, considering that chronic stress may disrupt BBB integrity through alterations in the tight junction protein claudin-5 (Reader et al., 2015; Menard et al., 2017; Lehmann et al., 2018; Dudek et al., 2020). Interestingly, microglial activation and extracellular matrix degradation are believed to significantly contribute to BBB leakiness (Lehmann et al., 2016, 2018).
However, immune activation is not consistently reported in all depression cases (Lamers et al., 2013; Gold, 2015). For instance, a recent study by de Punder et al. (2018) demonstrated that only the patients with MDD with history of childhood adversity exhibited heightened inflammation, whereas microglial activation in postmortem studies is detectable in patients with MDD who committed suicide (Steiner et al., 2008, 2011, (Schnieder et al., 2014)). These observations suggest that immune activation may manifest only in moderate to severe depressive cases, accounting also for the treatment resistance reported in them (Fig. 2).
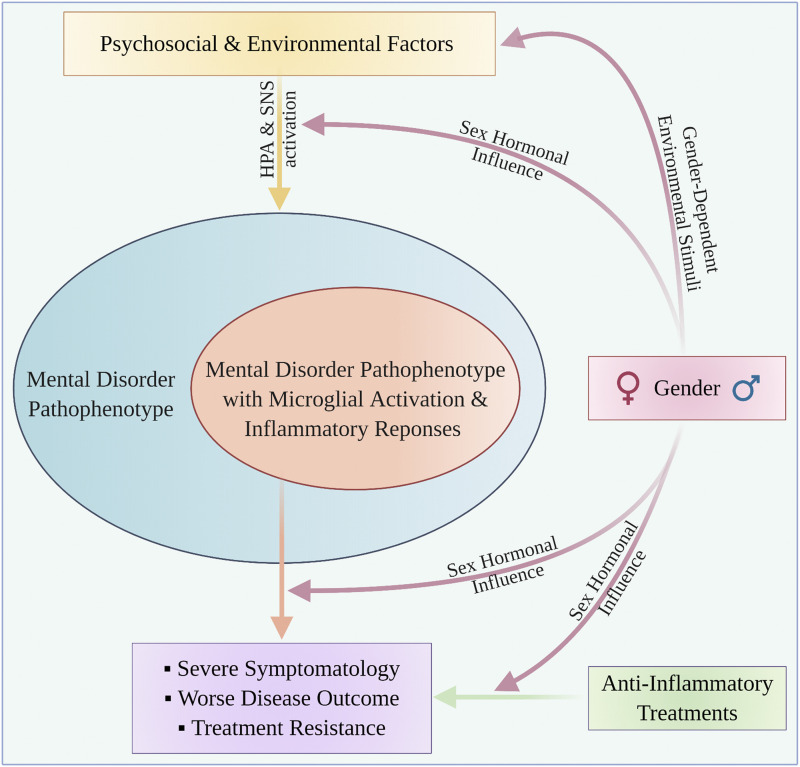
Fig. 2.
Gender influence and neuroimmune interplay in mental disorders. This conceptual model proposes that a wide range of psychosocial and environmental factors (e.g., stress, trauma, abuse, discrimination) induce CNS/peripheral inflammatory responses and microglial activation in a subset of patients with mental disorders. These patients exhibit moderate to severe pathophenotype, worse disease outcome, and resistance to conventional treatments. The gender can influence the underlying disease and treatment mechanisms on several levels, either via gender-dependent environmental factors or sex hormonal effects. Anti-inflammatory treatments can be used to supplement the current therapeutic regimens in this subset of patients.
Neuroinflammatory Highlights in Traumatic, Bipolar, and Schizophrenia Disorders
Post-Traumatic Stress Disorder
PTSD is a severe and heterogeneous psychiatric condition that develops in individuals who have experienced traumatic or dangerous events (i.e., threat of death, injury, sexual violence) and is characterized by significant comorbidities with MDD and panic disorder (Dedert et al., 2010; Norrholm et al., 2011). Innate immune response genes and anxiety/stress vulnerability genes are thought to contribute to PTSD heritability (Hauger et al., 2012; Skelton et al., 2012; Smoller, 2016), whereas exposure to trauma is a risk factor for dysregulated HPA axis function (elevated CRH levels) (Carpenter et al., 2004; Lee et al., 2005; Michopoulos et al., 2017) and inflammation (Michopoulos et al., 2015a). In fact, individuals exposed to childhood abuse, maltreatment, socioeconomic difficulties, or parental separation may exhibit increased proinflammatory activity during adulthood (Taylor et al., 2006; Hartwell et al., 2013; Lacey et al., 2013; McDade et al., 2013; Matthews et al., 2014; Tursich et al., 2014; Baumeister et al., 2016; Lin et al., 2016; Michopoulos et al., 2017). Studies have reported in patients with PTSD elevated circulating concentrations of TNF-α, INF-γ, IL-1β, IL-2, IL-6, and ICAM-1, which correlated positively with PTSD symptoms (von Kanel et al., 2007; Hoge et al., 2009; Oganesyan et al., 2009; Vidovic et al., 2011; Guo et al., 2012; Plantinga et al., 2013; Newton et al., 2014; Bersani et al., 2016). Respectively, cerebrospinal fluid (CSF) levels of CRP and IL-6 have been found elevated in PTSD (Baker et al., 2001; Heath et al., 2013; Plantinga et al., 2013; Lindqvist et al., 2014; Bersani et al., 2016), constituting risk factors for diagnosis (Michopoulos et al., 2015b). However, there are also studies that have described no change or even decreased levels of CRP, IL-6, and IL-2 in individuals with PTSD (Song et al., 2007; McCanlies et al., 2011; Muhtz et al., 2011). Similar discrepancies have been reported in studies examining anti-inflammatory plasma cytokine levels in patients with PTSD (IL-4, IL-8, and IL-10) (von Kanel et al., 2007; Hoge et al., 2009; Smith et al., 2011; Guo et al., 2012; Lindqvist et al., 2014; Jergovic et al., 2015; de Oliveira et al., 2018).
A meta-analysis of 20 PTSD independent studies sought to address these inconsistencies, revealing that proinflammatory markers (IL-1β, IL-6, TNF-α, and IFN-γ) are elevated and positively correlated with the illness duration in patients with PTSD (Passos et al., 2015). Another recent correlational study in traumatized women reported significant associations between higher concentrations of CRP, disease severity, and PTSD symptoms (Powers et al., 2019).
To summarize, these findings suggest that pharmaceutical interventions targeting inflammatory responses could potentially supplement the traditional psychotropic medications for severe cases of PTSD.
Bipolar Disorder
BD is a mental disorder characterized by frequent shifts in mood, activity levels, and concentration, ranging from manic episodes to depressive states. Studies have reported immune dysregulation during acute manic or depressive episodes, which is characterized by increased plasma levels of proinflammatory cytokines (TNF-α, IL-1β, IL-2R, IL-6, and IL-10) (Hope et al., 2011; Soderlund et al., 2011; Cetin et al., 2012; Munkholm et al., 2013; Stertz et al., 2013; Muneer, 2016). Similarly, postmortem analyses in the frontal cortices of patients with BD have demonstrated elevated mRNA and protein levels of IL-1b, CD11b, and inducible nitric oxide synthase (iNOS) (Rao et al., 2010).
Exacerbated inflammatory responses have been recorded by PET imaging, showing a significant increase of TSPO binding in the HPC of patients with BD (Haarman et al., 2014). Interestingly, studies have found microglial and monocytic activation with subsequent serum BDNF losses during manic or depressive phases, suggesting synaptic alterations between episodes (Drexhage et al., 2011; Parkhurst et al., 2013).
At present, research regarding the cross talk between inflammatory responses and cognitive performance in BD is extremely limited. The above studies, however, provide preliminary evidence of proinflammatory contributions on BD pathophysiology.
Schizophrenia
SCZ is a chronic, heterogeneous, and severe mental disorder affecting 1%–3% of the general population worldwide. It presents positive (hallucinations and movement disorders), negative (anhedonia, fatigue, asocial or atonic behavior), and cognitive symptoms (executive and memory functions) (van Os and Kapur, 2009). Epidemiologic studies have reported that prenatal maternal infection (influenza, T. gondii, herpes simplex virus type 2, and cytomegalovirus) constitutes a risk factor for the offspring to develop SCZ during adulthood (Brown and Derkits, 2010; Khandaker et al., 2013; Canetta et al., 2014). This correlation between maternal infection and SCZ is also supported by rodent models of maternal immune activation with polyinosinic:polycytidylic acid in midgestation (Hui et al., 2018), with the offspring displaying SCZ behavioral phenotypes (Meyer et al., 2009; Patterson, 2009; Giovanoli et al., 2013).
Equally important, SCZ pathophysiology has been associated with the genetic loci of MHCII (Shi et al., 2009; Stefansson et al., 2009), predominantly genes involving complement C4, suggesting microglial involvement (Sekar et al., 2016). High C4 expression has been detected in neuron and astrocyte subsets from postmortem samples from patients with SCZ (HPC and PFC), whereas C4 knockout mice display impaired synaptic refinement (Sekar et al., 2016). Significantly, PET studies reveal increased TSPO binding in the HPC and frontal cortex of patients with SCZ (van Berckel et al., 2008; Doorduin et al., 2009; Bloomfield et al., 2016; Marques et al., 2019), whereas the density of MHCII-positive amoeboid microglia is increased (Wierzba-Bobrowicz et al., 2005; Busse et al., 2012; Fillman et al., 2013).
A meta-analysis of blood cytokine levels in patients with SCZ revealed elevated expression of IFN-γ, IL-6, IL-8, IL-1RA, IL-1β, IL-10, and TNF-α during psychotic episodes (Goldsmith et al., 2016), whereas a recent study of frontal cortex areas in patients with SCZ demonstrated elevated macrophage numbers and vascular adhesion molecules expression (ICAM-1, vascular cell adhesion molecule-1), further highlighting the presence of inflammation in SCZ (Cai et al., 2020).
Inflammatory Effects on Neurotransmitter Metabolism
There are several proposed inflammatory mechanisms by which monoamine (serotonin, dopamine) and glutamate neurotransmission may be affected.
Monoamines
Compelling evidence supports the idea that monoamine synaptic deficits result from excessive inflammatory cytokine levels in mental disorders. Many studies have focused on the impact of inflammatory cytokines on serotonin reuptake transporter (SERT) function, a primary target for anxiety- and depression-related disorders. In a lipopolysaccharide-induced depressive-like model, interleukin-1β (IL-1β) and TNF-α induction resulted in induction of SERT expression (through p38 MAPK) and diminished serotonin synaptic levels (Zhu et al., 2010). This has been replicated in human studies, correlating blood TNF-α concentrations with increased SERT binding activity (Krishnadas et al., 2016), supporting the hypothesis that inflammation promotes resistance to selective serotonin reuptake inhibitors (SSRIs), as observed in patients with mood and anxiety disorders (Strawbridge et al., 2015).
Cytokine-induced activation of the immunosuppressive enzyme indoleamine 2,3 dioxygenase also significantly alters serotonin production. Indoleamine 2,3 dioxygenase activity can be induced by several inflammatory signaling mechanisms, such as NF-κB, and can divert tryptophan metabolism from serotonin into KYN (Muller and Schwarz, 2007), which is metabolized to neurotoxic quinolinic acid by activated microglia and brain-infiltrating macrophages (Raison et al., 2010a). Studies of patients with MDD demonstrated direct correlation of plasma inflammatory markers (e.g., TNF-α) with plasma KYN and CSF KYN/tryptophan levels, exhibiting greater depression severity in those patients (Haroon et al., 2020).
Dopamine is a monoamine neurotransmitter with essential roles in the regulation of reward, motivation, and psychomotor activity (Haber, 2014). Alterations in dopamine levels are responsible for some of the most characteristic symptoms of mental disorders: anhedonia, persistent fatigue, loss of interest, and psychomotor deficits. Within weeks after administration of the cytokine IFN-α, patients experience symptoms (Capuron et al., 2002; Capuron and Miller, 2004) due to dopamine loss (Capuron et al., 2012). Similar results were reported in animal studies (Felger et al., 2007, 2013) in which lipopolysaccharide or cytokine administration (IL-1β or IL-6) in mice resulted in loss of interest and reduction in reward sensitivity (Yohn et al., 2016; Bartlett et al., 2018). Another essential cofactor in monoamine synthesis, tetrahydrobiopterin, decreases in response to inflammation-induced oxidative stress (ROS and reactive nitrogen species), inducing anxiety and depressive symptoms (Neurauter et al., 2008; Haroon et al., 2012; Felger et al., 2013).
Despite the rich literature reporting dopamine level alterations in response to cytokine administration, little research has been conducted to associate inflammation with analogous dopamine responses in patients with mental disorders. For instance, patients with MDD and chronic fatigue syndrome with elevated inflammatory markers (such as CRP and cytokines) demonstrated aberrant connectivity within reward-related corticostriatal neurocircuitry (Miller et al., 2014; Felger et al., 2016).
Altogether, these data suggest that the current first-line treatment regimens, which activate monoamine receptors, support dopamine synthesis, and block dopamine reuptake, would ultimately have greater likelihood of success and longer efficacy if they were to be combined with anti-inflammatory medications. In agreement with that, patients with MDD with high inflammation exhibit greater responses to SSRIs used in combination with the dopamine transporter blocker bupropion than to SSRI monotherapy (Jha et al., 2017).
Glutamate
Glutamatergic neurotransmission is another system through which cytokines influence reward-, motor-, and threat-related circuitry (Tilleux and Hermans, 2007; Ida et al., 2008; Miller et al., 2009; Vezzani and Viviani, 2015; Birey et al., 2017; Murrough et al., 2017). Inflammatory cytokines at physiologic levels can induce synaptogenesis by 1) inducing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid activity postsynaptically, 2) promoting glutamate clearance [through excitatory amino acid transporters (EAAT)], and 3) maintaining and protecting synapses by NMDAR stimulation through cytokine receptor activation (Santello et al., 2011; Pribiag and Stellwagen, 2014; Haroon et al., 2017).
During chronic inflammatory diseases, cytokine levels can dramatically increase, resulting in persistent NF-κB activity and neurotoxicity (Santello and Volterra, 2012): TNF-α and IL-1β enhance risk for excitotoxicity by activating NMDAR signaling, (Vezzani and Viviani, 2015) or inhibiting EAATs (which clear glutamate) in HPC slices (Zou and Crews, 2005). Interestingly, use of NMDAR antagonists blocks the cytokine-potentiated glutamate neurotoxicity, signifying the role of N-methyl-d-aspartate signaling in inflammatory-mediated neurotoxicity and mental disorders (Zou and Crews, 2005).
Inflammatory cytokines and related components have been shown to induce glutamate neurotoxicity through microglia and astrocytes (Tilleux and Hermans, 2007; Ida et al., 2008; Haroon et al., 2017). Importantly, upon cytokine stimulation and subsequent microglial activation, large quantities of glutamate can be synthesized and released into synapses (Takeuchi et al., 2006). In addition, glutamate can be directly trafficked from microglia into astrocytes (through gap junctions), leading to EAAT dysfunction (Takeuchi et al., 2008). This glial glutamate release can stimulate extrasynaptic neuronal NMDARs and lead to BDNF loss and excitotoxicity (Hardingham et al., 2002; Hardingham and Bading, 2010).
Sex and Gender Differences in Immunoneuropsychiatry
The definition and usage of the terms sex and gender in the literature of mental disorders have sometimes been elusive and difficult to define. The term “sex differences” describes the biologic dissimilarities between male and female subjects, whereas the term “gender differences” describes the differential effects that psychosocial, cultural, and environmental factors have on men and women (Muehlenhard and Peterson, 2011). As such, the term sex is used for the animal studies assessing sex hormone influences (progesterone, estrogen, estradiol) or genetics of mental disorders, and both the sex (biologic) and gender (environment and experience) components are used in human cases (Oertelt-Prigione, 2012; Kuehner, 2017).
Justifying the Gender Gap in Mental Disorders
Differences in the epidemiology and symptomatology of mental disorders in men and women are well established, and with the exception of late-onset schizophrenia, women have significantly higher chronic prevalence of anxiety, depressive, and bipolar disorders (Boyd et al., 2015; Riecher-Rössler, 2017a,b; GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018; Yu, 2018; Rehm and Shield, 2019). A compelling body of evidence suggests that women are more vulnerable to psychosocial environmental stressors (due to sex hormone influence and blunted HPA axis stress responses), leading to higher prevalence of mental disorders.
Sex hormones can regulate various brain neurotransmitter pathways (serotonergic, dopaminergic, and GABAergic), modulating the sensitivity toward psychosocial influences. For instance, estradiol and progesterone fluctuations during menstrual cycle augment vulnerability of women toward psychosocial stressors but also significantly promote the overactivation, consolidation, and nonextinction of stressful experiences (Li and Graham, 2017). Stressful and emotion-negative stimuli (Kemp et al., 2004) induce greater activation of locus coeruleus (nucleus responding to stress and panic) in women compared with men (Filkowski et al., 2017; Bangasser et al., 2018).
Animal studies suggest that this difference in coping with anxiety and trauma stems from a sex-dependent HPA axis hypoactivation (Kajantie and Phillips, 2006). Specifically, low estradiol and progesterone levels (during menstruation, postpartum, and postmenopausal periods) attenuate the SNS-adrenal and HPA axis responsiveness, leading to repressed stress-coping capability (Fig. 1) (Kajantie and Phillips, 2006; Shansky et al., 2010). Studies demonstrate, however, that the decrease in cortisol release after stress exposure leads to lack of buffering mechanisms in emotional pathways (PFC-AMY) (Het et al., 2012; Kuehner, 2017). The most compelling evidence is the increased anxiety and depression prevalence postpuberty/-menarche (Kessler, 2003; Angold and Costello, 2006; Bale and Epperson, 2015), attributing these effects on ovarian hormonal level fluctuation (Costello et al., 2007). Similarly, atypical depression (a characteristic example of HPA hypoactivation predominantly observed in women) also presents this pathophenotype (Albert, 2015; Kuehner, 2017). Estradiol fluctuations can also exert pronounced effects on fear/memory and emotional extinction in anxiety-related and PTSD pathophysiology (Li and Graham, 2017).
Environmental and psychosocial influence is equally responsible for the gender differences observed in mental disorders (Kuehner, 2017; Riecher-Rössler, 2017b). Notably, reports demonstrate that domestic violence, sexual abuse (Kuehner, 2017; Oram et al., 2017), discrimination, and other risk factors can also contribute to the higher incidence of mental disorder in women (Hankin et al., 2007; Zahn-Waxler et al., 2008). As the prevalence of childhood sexual and emotional abuse is significantly higher in women than men, it constitutes an important contributor to gender differences in mental disorders (Kuehner, 2017).
Sex Influence and Inflammation in Mental Disorders
Several physiologic systems (HPA axis, immune dysregulation, neuroplasticity) have been implicated in the etiopathogenesis of mental disorders, and sex differences (hormone regulation and genetics) significantly modulate these processes (Rubinow and Schmidt, 2019) (Fig. 2). Importantly, sex hormones are key regulators of both innate and adaptive immune cell function (Rubinow and Schmidt, 2019; Slavich and Sacher, 2019), affecting immune cell progeny, proliferation, and cytokine production (Oertelt-Prigione, 2012; Trigunaite et al., 2015).
Anxiety and Depressive Disorders
Epidemiologic studies show robust gender-related differences in prevalence (2- to 3-fold higher in women), severity, and comorbidity reported in anxiety and traumatic and depressive disorders (Li and Graham, 2017; Rehm and Shield, 2019). Sex hormonal fluctuations are associated with heightened inflammatory responses in women compared with men (Giletta et al., 2018). In a meta-analysis of 26 studies, women were more prone to developing MDD after IFN-α treatment (Udina et al., 2012). Similar sensitivity to inflammatory components (endotoxin exposure) was observed in women with MDD, more than men, despite the similar magnitude in cytokine responses (IL-6, TNF-α) (Moieni et al., 2015). A recent longitudinal study highlighted the association between systemic inflammation and depression, demonstrating higher depression scores and inflammatory levels (CRP, IL-6, and fibrinogen) in women compared with men (Beydoun et al., 2020). Recently, a study focusing on severe suicidal peripartum depression implicated KYN pathway dysregulation followed by increased plasma inflammatory responses (IL-6, IL-8, IL-2, and quinolinic acid) and serotonergic system alterations (Achtyes et al., 2020).
Besides the demonstrated gender-based differences of inflammatory expression, a recent study reported gender-dependent molecular signatures in the transcriptome of patients with MDD (Seney et al., 2018). Both genders revealed elevated expression levels for MHC and antigen-processing genes in corticolimbic areas (AMY, PFC, cingulate gyrus), but microglial-related gene expression showed significant region-dependent decreases in women and respective increases in men. Gender differences were also reported in synaptic function and plasticity genes (higher in women than men), suggesting increased microglial phagocytosis of dendritic neuronal spines (Seney et al., 2018). However, considering the grave effects that gonadal hormones exert in mental disorders during adolescence, it is important to further explore the gender differences emerging in early adulthood.
Microglia have been considered in the past as a homogeneous cell population (Ginhoux and Guilliams, 2016). However, single-cell RNA sequencing has revealed signatures associated with distinct physiologic microglial functions and subtypes (Li and Barres, 2018; Hammond et al., 2019). Interestingly, Homeobox B8 (Hoxb8)-expressing microglia, which account for one-third of all microglia in the adult mouse brain, could be related to sex differences in mood disorder pathophysiology (De et al., 2018): loss of function of microglial Hoxb8 caused anxiety-related symptoms and obsessive compulsion with higher severity in female mice, potentially regulated by progesterone and β-estradiol (Tränkner et al., 2019).
Animal work has provided additional insights on the sex differences observed in microglia-mediated immune mechanisms during mental disorders. Restraint stress can augment microglial density and affect microglial fractalkine receptor (CX3CR1) expression only in the PFC of female rats (Bollinger et al., 2016). Microglia-related transcriptional alterations in iNOS, Arginase1, and CD200 suggested that male and female rat microglia respond differently (Bollinger et al., 2016). Sex differences were also obvious in microglial morphologic features in several brain regions (AMY, orbitofrontal cortex, and HPC) (Bollinger et al., 2017), possibly affecting differentially neuronal plasticity.
Traumatic and Schizophrenia Disorders
Females have higher PTSD prevalence than males (Olff et al., 2007) and higher heritability (Duncan et al., 2018). Sex hormones influence the noradrenergic response to aversive stimuli (Segal and Cahill, 2009; Lithari et al., 2010) and show greater AMY sensitivity after threatening stimuli (Williams et al., 2005), corroborating previous reports of inflammatory gender-dependent differences in PTSD individuals (Neylan et al., 2011). A recent PTSD transcriptome mega-analysis of seven types of trauma (intrapersonal, assault, combat, childhood, and others) demonstrated a shared molecular convergence in inflammatory cytokine, innate immune, and IFN-signaling cascades in both genders (Breen et al., 2018). However, the study also revealed gender-dependent alterations in several signaling modules (IL-12, MAPK, wound healing, and lipid metabolism), which were associated with specific modes of trauma (Breen et al., 2018). Correspondingly, in a recent PTSD rodent model, researchers detected sex-specific transcriptional responses to trauma involving NF-κB activation (TNF-α upregulation) and dysregulated synaptic plasticity in female HPC (Kim and Uddin, 2020).
In SCZ, higher disease severity and frequency is evident in men (Abel et al., 2010), and the mean disease onset is 5 years earlier than women (Andersen, 2003). Interestingly, there is an increase of SCZ psychotic episodes in women when postpartum (when estrogen levels drop suddenly) (Riecher-Rössler, 2017a), as well as around menopause (Castle and Murray, 1993). These data support the “estrogen hypothesis,” which postulates that estrogen can exert a protective effect in SCZ (Grigoriadis and Seeman, 2002).
The SCZ etiopathogenesis, compared with other mental disorders, involves predominantly neurodevelopmental abnormalities correlated with genetic factors and maternal infections as pathogenic contributors. It is believed that estrogen modulates microglial receptors and activity (Sierra et al., 2008), reducing inflammation (Sarvari et al., 2011). This sex-dependent hypothesis in SCZ human studies has focused on MHCII-microglial activation, evident in postmortem studies of patients with SCZ (Sekar et al., 2016; Mondelli et al., 2017). In a recent study, variations of complement component C4 in blood, brain, and lymphoblastoid cells drove stronger vulnerability and severity in men than in women (Kamitaki et al., 2020). Both C4 and its effector, complement component 3, are found at higher levels in plasma and CSF from men aged between 20 and 50 years, whereas in women, this increase occurs after menopause (40–50 years) (Ritchie et al., 2004; Gaya da Costa et al., 2018). As C4 increase occurs during the same time frame as disease vulnerability, it is suggested that microglial complement receptors in SCZ disorder may be affected differently by sex.
This hypothesis is also supported by several rodent maternal immune activation studies, in which male offspring are more vulnerable than female (Mattei et al., 2014; Deane et al., 2017; Hui et al., 2018; Notter et al., 2018). Interestingly, studies have revealed sex-based increases of inflammatory responses and “dark” microglial density (the cells appear dark in electron microscopy and interact with blood vessels and synapses), as well as extensive synapse interaction and oxidative stress in the HPC of males after exposure to prenatal polyinosinic:polycytidylic acid. Therefore, prenatal infection may differentially affect microglial responses in each gender (Hui et al., 2018).
Overall, microglia emerge as key players in traumatic and schizophrenia disorder onset, but it is still not clear whether these inflammatory effects occur only in a subset of severe disease cases or whether these mechanisms are exerted in a sex-dependent manner (Fig. 2).
Conclusions
Increasing evidence supports inflammatory mechanisms underlying the pathophysiology of mental disorders. Immune mechanisms have pronounced regulatory effects on the psychosocial stressor–genetic diathesis interaction (Fig. 1), demonstrating heightened inflammatory load in mental disorders. However, despite the evident induction of peripheral and CNS immune components and mediators in patients with mental disorders, these effects are not consistently reported. Could this possibly mean that inflammatory responses and microglial activation are evident only on a subset of patients with distinct pathophenotype or disease severity (Fig. 2)?
Postmortem studies from severe cases of depression, trauma, and psychoses have reported association with microglial activation and increases of peripheral and central proinflammatory responses (Haarman et al., 2014; Setiawan et al., 2015; Cattaneo et al., 2016; Mondelli et al., 2017; Raison, 2017; Wittenberg et al., 2020). These observations suggest that inflammatory cytokines, along with microglial activity, may serve as prognostic markers of disease development, severity, and expected resistance to treatment. With this in mind, anti-inflammatory regimens could be used to supplement the current antidepressant treatments: nonsteroidal anti-inflammatory drugs, minocycline, and other immunosuppressive drugs (Miller et al., 2017; Raison, 2017; Pfau et al., 2018; Wittenberg et al., 2020), targeting moderate to severe disease cases.
Sex hormones play an equally important role in mental disease modulation (Fig. 2). Gender effects have come to provide an additional layer of complexity to immunopsychiatry, integrating psychosocial, genetic, and developmental factors under the prism of sex hormonal influence. However, this complexity, if used correctly, would introduce gender as a valuable part of this equation and unveil novel pharmacological interventions for modern psychiatry.
Acknowledgments
We thank members of the laboratory for helpful discussions.
Abbreviations
- ACC
anterior cingulate cortex
- ACTH
adrenocorticotropic hormone
- AMY
amygdala
- BBB
blood-brain barrier
- BD
bipolar disorder
- BDNF
brain-derived neurotrophic factor
- CNS
central nervous system
- CRH
corticotropin releasing hormone
- CRP
C-reactive protein
- CSF
cerebrospinal fluid
- EAAT
excitatory amino acid transporter
- GR
glucocorticoid receptor
- GWAS
genome-wide association study
- HPA
hypothalamic-pituitary-adrenal
- HPC
hippocampus
- ICAM-1
intercellular adhesion molecule-1
- IFN
interferon
- IL
interleukin
- KYN
kynurenine
- MAPK
mitogen-activated protein kinase
- MDD
major depressive disorder
- MHC
major histocompatibility complex
- PFC
prefrontal cortex
- NF-κB
nuclear factor–κΒ
- NMDAR
N-methyl-d-aspartate receptor
- PD
panic disorder
- PET
positron emission tomography
- PTSD
post-traumatic stress disorder
- ROS
reactive oxygen species
- RSDS
repeated social defeat stress
- SCZ
schizophrenia
- SERT
serotonin reuptake transporters
- SNS
sympathetic nervous system
- SSRI
selective serotonin reuptake inhibitor
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
- TSPO
translocator protein
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Kokkosis, Tsirka.
Footnotes
This work was partially supported by American Heart Association predoctoral fellowship 19PRE34370044 (A.G.K.) and by National Institutes of Health National Institute of Mental Health [grant R01MH123093].
References
- Abbott NJ, Rönnbäck L, Hansson E. (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53. [DOI] [PubMed] [Google Scholar]
- Abel KM, Drake R, Goldstein JM. (2010) Sex differences in schizophrenia. Int Rev Psychiatry 22:417–428. [DOI] [PubMed] [Google Scholar]
- Abelson JL, Khan S, Liberzon I, Young EA. (2007) HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depress Anxiety 24:66–76. [DOI] [PubMed] [Google Scholar]
- Abiega O, Beccari S, Diaz-Aparicio I, Nadjar A, Layé S, Leyrolle Q, Gómez-Nicola D, Domercq M, Pérez-Samartín A, Sánchez-Zafra V, et al. (2016) Neuronal Hyperactivity Disturbs ATP Microgradients, Impairs Microglial Motility, and Reduces Phagocytic Receptor Expression Triggering Apoptosis/Microglial Phagocytosis Uncoupling. PLoS Biol 14:e1002466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtyes E, Keaton SA, Smart L, Burmeister AR, Heilman PL, Krzyzanowski S, Nagalla M, Guillemin GJ, Escobar Galvis ML, Lim CK, et al. (2020) Inflammation and kynurenine pathway dysregulation in post-partum women with severe and suicidal depression. Brain Behav Immun 83:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert PR. (2015) Why is depression more prevalent in women? J Psychiatry Neurosci 40:219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvares GA, Quintana DS, Kemp AH, Van Zwieten A, Balleine BW, Hickie IB, Guastella AJ. (2013) Reduced heart rate variability in social anxiety disorder: associations with gender and symptom severity. PLoS One 8:e70468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL. (2003) Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27:3–18. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Harbo HF, Wang Y, Thompson WK, Schork AJ, Mattingsdal M, Zuber V, Bettella F, Ripke S, Kelsoe JR, et al. Psychiatric Genomics Consortium (PGC) Bipolar Disorder and Schizophrenia Work Groups; International Multiple Sclerosis Genetics Consortium (IMSGC) (2015) Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: differential involvement of immune-related gene loci. Mol Psychiatry 20:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ. (2006) Puberty and depression. Child Adolesc Psychiatr Clin N Am 15:919–937, ix. [DOI] [PubMed] [Google Scholar]
- Arango C, Díaz-Caneja CM, McGorry PD, Rapoport J, Sommer IE, Vorstman JA, McDaid D, Marín O, Serrano-Drozdowskyj E, Freedman R, et al. (2018) Preventive strategies for mental health. Lancet Psychiatry 5:591–604. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Stark JL, Dhabhar FS, Sheridan JF. (2002) Social stress alters splenocyte phenotype and function. J Neuroimmunol 132:66–71. [DOI] [PubMed] [Google Scholar]
- Baker DG, Ekhator NN, Kasckow JW, Hill KK, Zoumakis E, Dashevsky BA, Chrousos GP, Geracioti TD., Jr. (2001) Plasma and cerebrospinal fluid interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation 9:209–217. [DOI] [PubMed] [Google Scholar]
- Bale TL, Epperson CN. (2015) Sex differences and stress across the lifespan. Nat Neurosci 18:1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Eck SR, Telenson AM, Salvatore M. (2018) Sex differences in stress regulation of arousal and cognition. Physiol Behav 187:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Broadwell RD. (1995) Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 2:241–248. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Gutierrez EG. (1994) Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett 179:53–56. [DOI] [PubMed] [Google Scholar]
- Bartlett EA, DeLorenzo C, Sharma P, Yang J, Zhang M, Petkova E, Weissman M, McGrath PJ, Fava M, Ogden RT, et al. (2018) Pretreatment and early-treatment cortical thickness is associated with SSRI treatment response in major depressive disorder. Neuropsychopharmacology 43:2221–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. (2016) Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry 21:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. (2009) Vulnerability genes or plasticity genes? Mol Psychiatry 14:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benros ME, Nielsen PR, Nordentoft M, Eaton WW, Dalton SO, Mortensen PB. (2011) Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry 168:1303–1310. [DOI] [PubMed] [Google Scholar]
- Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, Mortensen PB. (2013) Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry 70:812–820. [DOI] [PubMed] [Google Scholar]
- Bergink V, Gibney SM, Drexhage HA. (2014) Autoimmunity, inflammation, and psychosis: a search for peripheral markers. Biol Psychiatry 75:324–331. [DOI] [PubMed] [Google Scholar]
- Bersani FS, Wolkowitz OM, Lindqvist D, Yehuda R, Flory J, Bierer LM, Makotine I, Abu-Amara D, Coy M, Reus VI, et al. (2016) Global arginine bioavailability, a marker of nitric oxide synthetic capacity, is decreased in PTSD and correlated with symptom severity and markers of inflammation. Brain Behav Immun 52:153–160. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Obhi HK, Weiss J, Canas JA, Beydoun HA, Evans MK, Zonderman AB. (2020) Systemic inflammation is associated with depressive symptoms differentially by sex and race: a longitudinal study of urban adults. Mol Psychiatry 25:1286–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialas AR, Stevens B. (2013) TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci 16:1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Birey F, Kokkosis AG, Aguirre A. (2017) Oligodendroglia-lineage cells in brain plasticity, homeostasis and psychiatric disorders. Curr Opin Neurobiol 47:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandino P, Jr., Barnum CJ, Deak T. (2006) The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol 173:87–95. [DOI] [PubMed] [Google Scholar]
- Blank T, Prinz M. (2013) Microglia as modulators of cognition and neuropsychiatric disorders. Glia 61:62–70. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. (2007) Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosom Med 69:935–943. [DOI] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MA, Bonoldi I, Kalk N, Turkheimer F, et al. (2016) Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [(11)C]PBR28 PET brain imaging study. Am J Psychiatry 173:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JL, Bergeon Burns CM, Wellman CL. (2016) Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun 52:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JL, Collins KE, Patel R, Wellman CL. (2017) Behavioral stress alters corticolimbic microglia in a sex- and brain region-specific manner. PLoS One 12:e0187631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JL, Wohleb ES. (2019) The formative role of microglia in stress-induced synaptic deficits and associated behavioral consequences. Neurosci Lett 711:134369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino JA, Chang J. (1999) Higher abnormal leukocyte and lymphocyte counts 20 years after exposure to severe stress: research and clinical implications. Psychosom Med 61:378–386. [DOI] [PubMed] [Google Scholar]
- Boyd A, Van de Velde S, Vilagut G, de Graaf R, O’Neill S, Florescu S, Alonso J, Kovess-Masfety V, EU-WMH Investigators (2015) Gender differences in mental disorders and suicidality in Europe: results from a large cross-sectional population-based study. J Affect Disord 173:245–254. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Bellodi L, Perna G. (1999) Plasma levels of tumor necrosis factor-alpha in patients with panic disorder: effect of alprazolam therapy. Psychiatry Res 89:21–27. [DOI] [PubMed] [Google Scholar]
- Breen MS, Tylee DS, Maihofer AX, Neylan TC, Mehta D, Binder EB, Chandler SD, Hess JL, Kremen WS, Risbrough VB, et al. (2018) PTSD Blood Transcriptome Mega-Analysis: Shared Inflammatory Pathways Across Biological Sex and Modes of Trauma. Neuropsychopharmacology 43:469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. (2010) Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167:261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse S, Busse M, Schiltz K, Bielau H, Gos T, Brisch R, Mawrin C, Schmitt A, Jordan W, Müller UJ, et al. (2012) Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun 26:1273–1279. [DOI] [PubMed] [Google Scholar]
- Cai HQ, Catts VS, Webster MJ, Galletly C, Liu D, O’Donnell M, Weickert TW, Weickert CS. (2020) Increased macrophages and changed brain endothelial cell gene expression in the frontal cortex of people with schizophrenia displaying inflammation. Mol Psychiatry 25:761–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta S, Sourander A, Surcel HM, Hinkka-Yli-Salomäki S, Leiviskä J, Kellendonk C, McKeague IW, Brown AS. (2014) Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry 171:960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. (2002) Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 26:643–652. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. (2004) Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry 56:819–824. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH. (2012) Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry 69:1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, Price LH. (2004) Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology 29:777–784. [DOI] [PubMed] [Google Scholar]
- Carruthers VB, Suzuki Y. (2007) Effects of Toxoplasma gondii infection on the brain. Schizophr Bull 33:745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle DJ, Murray RM. (1993) The epidemiology of late-onset schizophrenia. Schizophr Bull 19:691–700. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Ferrari C, Uher R, Bocchio-Chiavetto L, Riva MA, Pariante CM, MRC ImmunoPsychiatry Consortium (2016) Absolute Measurements of Macrophage Migration Inhibitory Factor and Interleukin-1-β mRNA Levels Accurately Predict Treatment Response in Depressed Patients. Int J Neuropsychopharmacol 19:pyw045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetin T, Guloksuz S, Cetin EA, Gazioglu SB, Deniz G, Oral ET, van Os J. (2012) Plasma concentrations of soluble cytokine receptors in euthymic bipolar patients with and without subsyndromal symptoms. BMC Psychiatry 12:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RF, Turecki G, Shabalin AA, Guintivano J, Zhao M, Xie LY, van Grootheest G, Kaminsky ZA, Dean B, Penninx BWJH, et al. (2020) Cell type-specific methylome-wide association studies implicate neurotrophin and innate immune signaling in major depressive disorder. Biol Psychiatry 87:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conversano C, Carmassi C, Carlini M, Casu G, Gremigni P, Dell’Osso L. (2015) Interferon α therapy in patients with chronic hepatitis C infection: quality of life and depression. Hematol Rep 7:5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. (2006) Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 67:247–257. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. (2012) Generalized anxiety and C-reactive protein levels: a prospective, longitudinal analysis. Psychol Med 42:2641–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Worthman C, Erkanli A, Angold A. (2007) Prediction from low birth weight to female adolescent depression: a test of competing hypotheses. Arch Gen Psychiatry 64:338–344. [DOI] [PubMed] [Google Scholar]
- D’Mello C, Swain MG. (2017) Immune-to-brain communication pathways in inflammation-associated sickness and depression. Curr Top Behav Neurosci 31:73–94. [DOI] [PubMed] [Google Scholar]
- Dahm L, Ott C, Steiner J, Stepniak B, Teegen B, Saschenbrecker S, Hammer C, Borowski K, Begemann M, Lemke S, et al. (2014) Seroprevalence of autoantibodies against brain antigens in health and disease. Ann Neurol 76:82–94. [DOI] [PubMed] [Google Scholar]
- Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. (2011) Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 10:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, Werts H, Freeman J, Pariante CM, Moffitt TE, et al. (2011) Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry 16:244–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan W-B. (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752–758. [DOI] [PubMed] [Google Scholar]
- De S, Van Deren D, Peden E, Hockin M, Boulet A, Titen S, Capecchi MR. (2018) Two distinct ontogenies confer heterogeneity to mouse brain microglia. Development 145:dev152306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane AR, Millar J, Bilkey DK, Ward RD. (2017) Maternal immune activation in rats produces temporal perception impairments in adult offspring analogous to those observed in schizophrenia. PLoS One 12:e0187719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedert EA, Calhoun PS, Watkins LL, Sherwood A, Beckham JC. (2010) Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med 39:61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira JF, Wiener CD, Jansen K, Portela LV, Lara DR, Souza LDM, da Silva RA, Moreira FP, Oses JP. (2018) Serum levels of interleukins IL-6 and IL-10 in individuals with posttraumatic stress disorder in a population-based sample. Psychiatry Res 260:111–115. [DOI] [PubMed] [Google Scholar]
- de Punder K, Entringer S, Heim C, Deuter CE, Otte C, Wingenfeld K, Kuehl LK. (2018) Inflammatory measures in depressed patients with and without a history of adverse childhood experiences. Front Psychiatry 9:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry HM, Fagundes CP, Andridge R, Glaser R, Malarkey WB, Kiecolt-Glaser JK. (2013) Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendocrinology 38:2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Malarkey WB, Neri E, McEwen BS. (2012) Stress-induced redistribution of immune cells--from barracks to boulevards to battlefields: a tale of three hormones--Curt Richter Award winner. Psychoneuroendocrinology 37:1345–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Katsafanas E, Khushalani S, Yolken R. (2014) Antibodies to Toxoplasma gondii in individuals with mania. Bipolar Disord 16:129–136. [DOI] [PubMed] [Google Scholar]
- Dieleman GC, Huizink AC, Tulen JH, Utens EM, Creemers HE, van der Ende J, Verhulst FC. (2015) Alterations in HPA-axis and autonomic nervous system functioning in childhood anxiety disorders point to a chronic stress hypothesis. Psychoneuroendocrinology 51:135–150. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. (2009) Neuroinflammation in schizophrenia-related psychosis: a PET study, J Nucl Med 50, pp 1801–1807. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457. [DOI] [PubMed] [Google Scholar]
- Drexhage RC, Hoogenboezem TH, Versnel MA, Berghout A, Nolen WA, Drexhage HA. (2011) The activation of monocyte and T cell networks in patients with bipolar disorder. Brain Behav Immun 25:1206–1213. [DOI] [PubMed] [Google Scholar]
- Dudek KA, Dion-Albert L, Lebel M, LeClair K, Labrecque S, Tuck E, Ferrer Perez C, Golden SA, Tamminga C, Turecki G, et al. (2020) Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc Natl Acad Sci USA 117:3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Li N. (2012) A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci 367:2475–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Ratanatharathorn A, Aiello AE, Almli LM, Amstadter AB, Ashley-Koch AE, Baker DG, Beckham JC, Bierut LJ, Bisson J, et al. (2018) Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol Psychiatry 23:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Sheridan JF. (2004) Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol 148:106–115. [DOI] [PubMed] [Google Scholar]
- Engler H, Bailey MT, Engler A, Stiner-Jones LM, Quan N, Sheridan JF. (2008) Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology 33:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler H, Engler A, Bailey MT, Sheridan JF. (2005) Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. J Neuroimmunol 163:110–119. [DOI] [PubMed] [Google Scholar]
- Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O’Connor DT, Baker DG, Marine Resiliency Study Team (2014) Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry 71:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faravelli C, Lo Sauro C, Godini L, Lelli L, Benni L, Pietrini F, Lazzeretti L, Talamba GA, Fioravanti G, Ricca V. (2012) Childhood stressful events, HPA axis and anxiety disorders. World J Psychiatry 2:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein A, Magalhaes S, Richard JF, Audet B, Moore C. (2014) The link between multiple sclerosis and depression. Nat Rev Neurol 10:507–517. [DOI] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH. (2007) Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry 62:1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, Le N-A, Feinberg R, Tansey MG, Miller AH. (2020) What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry 25:1301–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li L, Marvar PJ, Woolwine BJ, Harrison DG, Raison CL, Miller AH. (2013) Tyrosine metabolism during interferon-alpha administration: association with fatigue and CSF dopamine concentrations. Brain Behav Immun 31:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, Miller AH. (2016) Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry 21:1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA. (2013) Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med 10:e1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkowski MM, Olsen RM, Duda B, Wanger TJ, Sabatinelli D. (2017) Sex differences in emotional perception: meta analysis of divergent activation. Neuroimage 147:925–933. [DOI] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, Cairns M, Weickert CS. (2013) Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol Psychiatry 18:206–214. [DOI] [PubMed] [Google Scholar]
- Fleshner M, Frank M, Maier SF. (2017) Danger Signals and Inflammasomes: Stress-Evoked Sterile Inflammation in Mood Disorders. Neuropsychopharmacology 42:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley C, Corvin A, Nakagome S. (2017) Genetics of schizophrenia: ready to translate? Curr Psychiatry Rep 19:61. [DOI] [PubMed] [Google Scholar]
- Fontainhas AM, Wang M, Liang KJ, Chen S, Mettu P, Damani M, Fariss RN, Li W, Wong WT. (2011) Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One 6:e15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Ramsawh HJ, Flagan TM, Sullivan SG, Letamendi A, Simmons AN, Paulus MP, Stein MB. (2015) Common and disorder-specific neural responses to emotional faces in generalised anxiety, social anxiety and panic disorders. Br J Psychiatry 206:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester JV, McMenamin PG, Dando SJ. (2018) CNS infection and immune privilege. Nat Rev Neurosci 19:655–671. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Través PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, Callaway P, Zagórska A, Rothlin CV, Nimmerjahn A, et al. (2016) TAM receptors regulate multiple features of microglial physiology. Nature 532:240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. (2007) Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS proinflammatory cytokine responses. Brain Behav Immun 21:47–59. [DOI] [PubMed] [Google Scholar]
- Fries GR, Walss-Bass C, Bauer ME, Teixeira AL. (2019) Revisiting inflammation in bipolar disorder. Pharmacol Biochem Behav 177:12–19. [DOI] [PubMed] [Google Scholar]
- Furuyashiki T, Kitaoka S. (2019) Neural mechanisms underlying adaptive and maladaptive consequences of stress: roles of dopaminergic and inflammatory responses. Psychiatry Clin Neurosci 73:669–675. [DOI] [PubMed] [Google Scholar]
- Gaya da Costa M, Poppelaars F, van Kooten C, Mollnes TE, Tedesco F, Würzner R, Trouw LA, Truedsson L, Daha MR, Roos A, et al. (2018) Age and sex-associated changes of complement activity and complement levels in a healthy caucasian population. Front Immunol 9:2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017 [published correction appears in Lancet (2019) 393:e44]. Lancet 392:1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemma C, Bachstetter AD. (2013) The role of microglia in adult hippocampal neurogenesis. Front Cell Neurosci 7:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giletta M, Slavich GM, Rudolph KD, Hastings PD, Nock MK, Prinstein MJ. (2018) Peer victimization predicts heightened inflammatory reactivity to social stress in cognitively vulnerable adolescents. J Child Psychol Psychiatry 59:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno D, Kivimäki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Kumari M, Lowe GD, Rumley A, Marmot MG, et al. (2009) Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med 39:413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Guilliams M. (2016) Tissue-resident macrophage ontogeny and homeostasis. Immunity 44:439–449. [DOI] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Feldon J, et al. (2013) Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice [published correction appears in Science (2014) 343:1077]; [published correction appears in Science (2014) 344:151]. Science 339:1095–1099. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. (2005) Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 5:243–251. [DOI] [PubMed] [Google Scholar]
- Gold PW. (2015) The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry 20:32–47. [DOI] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ. (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 21:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, Kent JM, Sullivan GM, Coplan JD. (2000) Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry 157:493–505. [DOI] [PubMed] [Google Scholar]
- Griffin JM, Fuhrer R, Stansfeld SA, Marmot M. (2002) The importance of low control at work and home on depression and anxiety: do these effects vary by gender and social class? Soc Sci Med 54:783–798. [DOI] [PubMed] [Google Scholar]
- Grigoriadis S, Seeman MV. (2002) The role of estrogen in schizophrenia: implications for schizophrenia practice guidelines for women. Can J Psychiatry 47:437–442. [DOI] [PubMed] [Google Scholar]
- Guan J, Cai JJ, Ji G, Sham PC. (2019) Commonality in dysregulated expression of gene sets in cortical brains of individuals with autism, schizophrenia, and bipolar disorder. Transl Psychiatry 9:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Liu T, Guo JC, Jiang XL, Chen F, Gao YS. (2012) Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac J Trop Med 5:323–325. [DOI] [PubMed] [Google Scholar]
- Haarman BC, Riemersma-Van der Lek RF, de Groot JC, Ruhé HG, Klein HC, Zandstra TE, Burger H, Schoevers RA, de Vries EF, Drexhage HA, et al. (2014) Neuroinflammation in bipolar disorder - a [(11)C]-(R)-PK11195 positron emission tomography study. Brain Behav Immun 40:219–225. [DOI] [PubMed] [Google Scholar]
- Haber SN. (2014) The place of dopamine in the cortico-basal ganglia circuit. Neuroscience 282:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeyer N, Hanft K-M, Akriditou M-A, Unger N, Park ES, Stanley ER, Staszewski O, Dimou L, Prinz M. (2017) Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol 134:441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J, et al. (2019) Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50:253–271.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch U-K, Kettenmann H. (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, Roesch L. (2007) Sex differences in adolescent depression: stress exposure and reactivity models. Child Dev 78:279–295. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. (2010) Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci 11:682–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. (2002) Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci 5:405–414. [DOI] [PubMed] [Google Scholar]
- Haroon E, Miller AH, Sanacora G. (2017) Inflammation, glutamate, and glia: a trio of trouble in mood disorders. Neuropsychopharmacology 42:193–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Raison CL, Miller AH. (2012) Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 37:137–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroon E, Welle JR, Woolwine BJ, Goldsmith DR, Baer W, Patel T, Felger JC, Miller AH. (2020) Associations among peripheral and central kynurenine pathway metabolites and inflammation in depression. Neuropsychopharmacology 45:998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, Critchley HD. (2009) Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry 66:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell KJ, Moran-Santa Maria MM, Twal WO, Shaftman S, DeSantis SM, McRae-Clark AL, Brady KT. (2013) Association of elevated cytokines with childhood adversity in a sample of healthy adults. J Psychiatr Res 47:604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Olivares-Reyes JA, Dautzenberg FM, Lohr JB, Braun S, Oakley RH. (2012) Molecular and cell signaling targets for PTSD pathophysiology and pharmacotherapy. Neuropharmacology 62:705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath NM, Chesney SA, Gerhart JI, Goldsmith RE, Luborsky JL, Stevens NR, Hobfoll SE. (2013) Interpersonal violence, PTSD, and inflammation: potential psychogenic pathways to higher C-reactive protein levels. Cytokine 63:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, et al. (2014) Chronic variable stress activates hematopoietic stem cells. Nat Med 20:754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, McManus RM, Latz E. (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19:610–621. [DOI] [PubMed] [Google Scholar]
- Heninger GR, Delgado PL, Charney DS. (1996) The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry 29:2–11. [DOI] [PubMed] [Google Scholar]
- Het S, Schoofs D, Rohleder N, Wolf OT. (2012) Stress-induced cortisol level elevations are associated with reduced negative affect after stress: indications for a mood-buffering cortisol effect. Psychosom Med 74:23–32. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Webb BT, Guo AY, Zhao Z, Maher BS, Chen X, An SS, Sun C, Aggen SH, Kendler KS, et al. (2011) Prioritization and association analysis of murine-derived candidate genes in anxiety-spectrum disorders. Biol Psychiatry 70:888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood M, Morandini J, Day TA, Walker FR. (2012) Evidence that microglia mediate the neurobiological effects of chronic psychological stress on the medial prefrontal cortex, Cereb Cortex 22, pp 1442–1454. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Kana V, Menard C, Merad M, Russo SJ. (2015) Neuroimmune mechanisms of depression. Nat Neurosci 18:1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. (2009) Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety 26:447–455. [DOI] [PubMed] [Google Scholar]
- Hope S, Dieset I, Agartz I, Steen NE, Ueland T, Melle I, Aukrust P, Andreassen OA. (2011) Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorders but not in schizophrenia. J Psychiatr Res 45:1608–1616. [DOI] [PubMed] [Google Scholar]
- Horchar MJ, Wohleb ES. (2019) Glucocorticoid receptor antagonism prevents microglia-mediated neuronal remodeling and behavioral despair following chronic unpredictable stress. Brain Behav Immun 81:329–340. [DOI] [PubMed] [Google Scholar]
- Hornboll B, Macoveanu J, Nejad A, Rowe J, Elliott R, Knudsen GM, Siebner HR, Paulson OB. (2018) Neuroticism predicts the impact of serotonin challenges on fear processing in subgenual anterior cingulate cortex. Sci Rep 8:17889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, Coleman JRI, Hagenaars SP, Ward J, Wigmore EM, et al. 23andMe Research Team; Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium (2019) Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 22:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, McCutcheon R. (2017) Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl Psychiatry 7:e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. (2009) Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71:171–186. [DOI] [PubMed] [Google Scholar]
- Hui CW, St-Pierre A, El Hajj H, Remy Y, Hébert SS, Luheshi GN, Srivastava LK, Tremblay ME. (2018) Prenatal immune challenge in mice leads to partly sex-dependent behavioral, microglial, and molecular abnormalities associated with schizophrenia. Front Mol Neurosci 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida T, Hara M, Nakamura Y, Kozaki S, Tsunoda S, Ihara H. (2008) Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci Lett 432:232–236. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. (2007) Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Mol Psychiatry 12:432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankord R, Zhang R, Flak JN, Solomon MB, Albertz J, Herman JP. (2010) Stress activation of IL-6 neurons in the hypothalamus. Am J Physiol Regul Integr Comp Physiol 299:R343–R351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R, Penninx BW, Madar V, Xia K, Milaneschi Y, Hottenga JJ, Hammerschlag AR, Beekman A, van der Wee N, Smit JH, et al. (2016) Gene expression in major depressive disorder. Mol Psychiatry 21:339–347. [DOI] [PubMed] [Google Scholar]
- Janssen DG, Caniato RN, Verster JC, Baune BT. (2010) A psychoneuroimmunological review on cytokines involved in antidepressant treatment response. Hum Psychopharmacol 25:201–215. [DOI] [PubMed] [Google Scholar]
- Jergović M, Bendelja K, Savić Mlakar A, Vojvoda V, Aberle N, Jovanovic T, Rabatić S, Sabioncello A, Vidović A. (2015) Circulating levels of hormones, lipids, and immune mediators in post-traumatic stress disorder - a 3-month follow-up study. Front Psychiatry 6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Minhajuddin A, Gadad BS, Greer T, Grannemann B, Soyombo A, Mayes TL, Rush AJ, Trivedi MH. (2017) Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology 78:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. (2005) Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 135:1295–1307. [DOI] [PubMed] [Google Scholar]
- Jones R. (2013) Neurogenetics: trauma and stress, from child to adult. Nat Rev Genet 14:77. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. (2006) The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology 31:151–178. [DOI] [PubMed] [Google Scholar]
- Kamitaki N, Sekar A, Handsaker RE, de Rivera H, Tooley K, Morris DL, Taylor KE, Whelan CW, Tombleson P, Loohuis LMO, et al. Schizophrenia Working Group of the Psychiatric Genomics Consortium (2020) Complement genes contribute sex-biased vulnerability in diverse disorders. Nature 582:577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M, Moon YP, Paik MC, Sacco RL, Wright CB, Elkind MS. (2013) Infectious burden and cognitive function: the Northern Manhattan Study. Neurology 80:1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser MS, Titulaer MJ, Gresa-Arribas N, Dalmau J. (2013) Frequency and characteristics of isolated psychiatric episodes in anti–N-methyl-d-aspartate receptor encephalitis. JAMA Neurol 70:1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Silberstein RB, Armstrong SM, Nathan PJ. (2004) Gender differences in the cortical electrophysiological processing of visual emotional stimuli. Neuroimage 21:632–646. [DOI] [PubMed] [Google Scholar]
- Kendell SF, Krystal JH, Sanacora G. (2005) GABA and glutamate systems as therapeutic targets in depression and mood disorders. Expert Opin Ther Targets 9:153–168. [DOI] [PubMed] [Google Scholar]
- Kessler RC. (2003) Epidemiology of women and depression. J Affect Disord 74:5–13. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV. (2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18:942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB. (2015) Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2:258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. (2014) Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry 71:1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Zimbron J, Lewis G, Jones PB. (2013) Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychol Med 43:239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Britton JC, Schwab ZJ, Price LM, Weiner MR, Gold AL, Rosso IM, Simon NM, Pollack MH, Rauch SL. (2014) Cortico-limbic responses to masked affective faces across ptsd, panic disorder, and specific phobia. Depress Anxiety 31:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GS, Uddin M. (2020) Sex-specific and shared expression profiles of vulnerability and resilience to trauma in brain and blood. Biol Sex Differ 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Hwang Y, Webster MJ, Lee D. (2016) Differential activation of immune/inflammatory response-related co-expression modules in the hippocampus across the major psychiatric disorders. Mol Psychiatry 21:376–385. [DOI] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, et al. (2013) Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci 16:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. (2010) Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA 107:2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczak DJ, Pereira S, Koulajian K, Matejcek A, Giacca A. (2011) Type 1 diabetes mellitus and major depressive disorder: evidence for a biological link. Diabetologia 54:2483–2493. [DOI] [PubMed] [Google Scholar]
- Kosmidis MH, Bozikas VP, Giannouli V, Karavatos A, Fokas K. (2012) Familial comorbidity of bipolar disorder and multiple sclerosis: genetic susceptibility, coexistence or causal relationship? Behav Neurol 25:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadas R, Nicol A, Sassarini J, Puri N, Burden AD, Leman J, Combet E, Pimlott S, Hadley D, McInnes IB, et al. (2016) Circulating tumour necrosis factor is highly correlated with brainstem serotonin transporter availability in humans. Brain Behav Immun 51:29–38. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. (2008) The molecular neurobiology of depression. Nature 455:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner C. (2017) Why is depression more common among women than among men? Lancet Psychiatry 4:146–158. [DOI] [PubMed] [Google Scholar]
- Kupka RW, Nolen WA, Post RM, McElroy SL, Altshuler LL, Denicoff KD, Frye MA, Keck PE, Jr., Leverich GS, Rush AJ, et al. (2002) High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biol Psychiatry 51:305–311. [DOI] [PubMed] [Google Scholar]
- Lacey RE, Kumari M, McMunn A. (2013) Parental separation in childhood and adult inflammation: the importance of material and psychosocial pathways. Psychoneuroendocrinology 38:2476–2484. [DOI] [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. (2013) Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry 18:692–699. [DOI] [PubMed] [Google Scholar]
- Lee R, Geracioti TD, Jr., Kasckow JW, Coccaro EF. (2005) Childhood trauma and personality disorder: positive correlation with adult CSF corticotropin-releasing factor concentrations. Am J Psychiatry 162:995–997. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Cooper HA, Maric D, Herkenham M. (2016) Social defeat induces depressive-like states and microglial activation without involvement of peripheral macrophages. J Neuroinflammation 13:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Weigel TK, Cooper HA, Elkahloun AG, Kigar SL, Herkenham M. (2018) Decoding microglia responses to psychosocial stress reveals blood-brain barrier breakdown that may drive stress susceptibility. Sci Rep 8:11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Weigel TK, Poffenberger CN, Herkenham M. (2019) The behavioral sequelae of social defeat require microglia and are driven by oxidative stress in mice. J Neurosci 39:5594–5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox BR, Coles AJ, Vincent A. (2012) Antibody-mediated encephalitis: a treatable cause of schizophrenia. Br J Psychiatry 200:92–94. [DOI] [PubMed] [Google Scholar]
- Li Q, Barres BA. (2018) Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 18:225–242. [DOI] [PubMed] [Google Scholar]
- Li SH, Graham BM. (2017) Why are women so vulnerable to anxiety, trauma-related and stress-related disorders? The potential role of sex hormones. Lancet Psychiatry 4:73–82. [DOI] [PubMed] [Google Scholar]
- Li Y, Du X-F, Liu C-S, Wen Z-L, Du J-L. (2012) Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell 23:1189–1202. [DOI] [PubMed] [Google Scholar]
- Liddelow S, Barres B. (2015) SnapShot: astrocytes in health and disease. Cell 162:1170–1170.e1. [DOI] [PubMed] [Google Scholar]
- Lin JE, Neylan TC, Epel E, O’Donovan A. (2016) Associations of childhood adversity and adulthood trauma with C-reactive protein: a cross-sectional population-based study. Brain Behav Immun 53:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Mellon S, Yehuda R, Flory JD, Henn-Haase C, Bierer LM, Abu-Amara D, Coy M, Neylan TC, et al. (2014) Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun 42:81–88. [DOI] [PubMed] [Google Scholar]
- Lithari C, Frantzidis CA, Papadelis C, Vivas AB, Klados MA, Kourtidou-Papadeli C, Pappas C, Ioannides AA, Bamidis PD. (2010) Are females more responsive to emotional stimuli? A neurophysiological study across arousal and valence dimensions. Brain Topogr 23:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M. (1995) Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 19:11–38. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. (1997) Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine 9:853–858. [DOI] [PubMed] [Google Scholar]
- Maes M, Van der Planken M, Stevens WJ, Peeters D, DeClerck LS, Bridts CH, Schotte C, Cosyns P. (1992) Leukocytosis, monocytosis and neutrophilia: hallmarks of severe depression. J Psychiatr Res 26:125–134. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, Reynolds CF, Lenze EJ. (2008) Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology 33:773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M, Feeney A. (1997) General explanations for social inequalities in health. IARC Sci Publ 207–228. [PubMed] [Google Scholar]
- Marques TR, Ashok AH, Pillinger T, Veronese M, Turkheimer FE, Dazzan P, Sommer IEC, Howes OD. (2019) Neuroinflammation in schizophrenia: meta-analysis of in vivo microglial imaging studies. Psychol Med 49:2186–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei D, Djodari-Irani A, Hadar R, Pelz A, de Cossío LF, Goetz T, Matyash M, Kettenmann H, Winter C, Wolf SA. (2014) Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun 38:175–184. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Chang YF, Thurston RC, Bromberger JT. (2014) Child abuse is related to inflammation in mid-life women: role of obesity. Brain Behav Immun 36:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCanlies EC, Araia SK, Joseph PN, Mnatsakanova A, Andrew ME, Burchfiel CM, Violanti JM. (2011) C-reactive protein, interleukin-6, and posttraumatic stress disorder symptomology in urban police officers. Cytokine 55:74–78. [DOI] [PubMed] [Google Scholar]
- McDade TW, Hoke M, Borja JB, Adair LS, Kuzawa C. (2013) Do environments in infancy moderate the association between stress and inflammation in adulthood? Initial evidence from a birth cohort in the Philippines. Brain Behav Immun 31:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, Godbout JP. (2016) Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. J Neurosci 36:2590–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim DB, Weber MD, Niraula A, Sawicki CM, Liu X, Jarrett BL, Ramirez-Chan K, Wang Y, Roeth RM, Sucaldito AD, et al. (2018) Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry 23:1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechawar N, Savitz J. (2016) Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry 6:e946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, Takahashi A, Flanigan ME, Aleyasin H, LeClair KB, et al. (2017) Social stress induces neurovascular pathology promoting depression. Nat Neurosci 20:1752–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. (2009) In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev 33:1061–1079. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Norrholm SD, Jovanovic T. (2015a) Diagnostic biomarkers for posttraumatic stress disorder: promising horizons from translational neuroscience research. Biol Psychiatry 78:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T. (2017) Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 42:254–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, Gillespie CF, Ressler KJ. (2015b) Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry 172:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Felger JC. (2017) Therapeutic Implications of Brain–Immune Interactions: Treatment in Translation. Neuropsychopharmacology 42:334–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Jones JF, Drake DF, Tian H, Unger ER, Pagnoni G. (2014) Decreased basal ganglia activation in subjects with chronic fatigue syndrome: association with symptoms of fatigue. PLoS One 9:e98156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL. (2016) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. (2008) A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry 64:266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cole SW. (2012) Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biol Psychiatry 72:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao J-W, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJM, et al. (2013) M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16:1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiak B, Ricceri L, Sąsiadek MM. (2019) Transposable elements and their epigenetic regulation in mental disorders: current evidence in the field. Front Genet 10:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A, Wake H, Ishikawa AW, Eto K, Shibata K, Murakoshi H, Koizumi S, Moorhouse AJ, Yoshimura Y, Nabekura J. (2016) Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun 7:12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. (2015) Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology 40:1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli V, Vernon AC, Turkheimer F, Dazzan P, Pariante CM. (2017) Brain microglia in psychiatric disorders. Lancet Psychiatry 4:563–572. [DOI] [PubMed] [Google Scholar]
- Mostafavi S, Battle A, Zhu X, Potash JB, Weissman MM, Shi J, Beckman K, Haudenschild C, McCormick C, Mei R, et al. (2014) Type I interferon signaling genes in recurrent major depression: increased expression detected by whole-blood RNA sequencing. Mol Psychiatry 19:1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlenhard CL, Peterson ZD. (2011) Distinguishing between sex and gender: history, current conceptualizations, and implications. Sex Roles 64:791–803. [Google Scholar]
- Muhtz C, Godemann K, von Alm C, Wittekind C, Goemann C, Wiedemann K, Yassouridis A, Kellner M. (2011) Effects of chronic posttraumatic stress disorder on metabolic risk, quality of life, and stress hormones in aging former refugee children. J Nerv Ment Dis 199:646–652. [DOI] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ. (2007) The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry 12:988–1000. [DOI] [PubMed] [Google Scholar]
- Muneer A. (2016) The neurobiology of bipolar disorder: an integrated approach. Chonnam Med J 52:18–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkholm K, Braüner JV, Kessing LV, Vinberg M. (2013) Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res 47:1119–1133. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Abdallah CG, Mathew SJ. (2017) Targeting glutamate signalling in depression: progress and prospects. Nat Rev Drug Discov 16:472–486. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Treccani G, Popoli M. (2015) Functional and structural remodeling of glutamate synapses in prefrontal and frontal cortex induced by behavioral stress. Front Psychiatry 6:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, Irwin MR, Eisenberger NI. (2015) Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav Immun 43:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy C, Maitra M, Tanti A, Suderman M, Théroux JF, Davoli MA, Perlman K, Yerko V, Wang YC, Tripathy SJ, et al. (2020) Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat Neurosci 23:771–781. [DOI] [PubMed] [Google Scholar]
- Nagy C, Suderman M, Yang J, Szyf M, Mechawar N, Ernst C, Turecki G. (2015) Astrocytic abnormalities and global DNA methylation patterns in depression and suicide. Mol Psychiatry 20:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Bonneau RH. (2006) Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol 171:72–85. [DOI] [PubMed] [Google Scholar]
- Neurauter G, Schröcksnadel K, Scholl-Bürgi S, Sperner-Unterweger B, Schubert C, Ledochowski M, Fuchs D. (2008) Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab 9:622–627. [DOI] [PubMed] [Google Scholar]
- Newton TL, Fernandez-Botran R, Miller JJ, Burns VE. (2014) Interleukin-6 and soluble interleukin-6 receptor levels in posttraumatic stress disorder: associations with lifetime diagnostic status and psychological context. Biol Psychol 99:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylan TC, Sun B, Rempel H, Ross J, Lenoci M, O’Donovan A, Pulliam L. (2011) Suppressed monocyte gene expression profile in men versus women with PTSD. Brain Behav Immun 25:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X, Kitaoka S, Tanaka K, Segi-Nishida E, Imoto Y, Ogawa A, Nakano F, Tomohiro A, Nakayama K, Taniguchi M, et al. (2018) The innate immune receptors TLR2/4 mediate repeated social defeat stress-induced social avoidance through prefrontal microglial activation. Neuron 99:464–479.e7. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. (2011) Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry 69:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Sibille E. (2014) Why are cortical GABA neurons relevant to internal focus in depression? A cross-level model linking cellular, biochemical and neural network findings. Mol Psychiatry 19:966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarangelo FM, Wilson EH, Horning KJ, Thomas MA, Harris TH, Fang Q, Hunter CA, Schwarcz R. (2014) Evaluation of kynurenine pathway metabolism in Toxoplasma gondii-infected mice: implications for schizophrenia. Schizophr Res 152:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notter T, Coughlin JM, Gschwind T, Weber-Stadlbauer U, Wang Y, Kassiou M, Vernon AC, Benke D, Pomper MG, Sawa A, et al. (2018) Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol Psychiatry 23:323–334. [DOI] [PubMed] [Google Scholar]
- Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium (2015) Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways [published correction appears in Nat Neurosci (2015) 18:926]. Nat Neurosci 18:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertelt-Prigione S. (2012) The influence of sex and gender on the immune response. Autoimmun Rev 11:A479–A485. [DOI] [PubMed] [Google Scholar]
- Oganesyan LP, Mkrtchyan GM, Sukiasyan SH, Boyajyan AS. (2009) Classic and alternative complement cascades in post-traumatic stress disorder. Bull Exp Biol Med 148:859–861. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BP. (2007) Gender differences in posttraumatic stress disorder. Psychol Bull 133:183–204. [DOI] [PubMed] [Google Scholar]
- Oram S, Khalifeh H, Howard LM. (2017) Violence against women and mental health. Lancet Psychiatry 4:159–170. [DOI] [PubMed] [Google Scholar]
- Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF. (2016) Major depressive disorder. Nat Rev Dis Primers 2:16065. [DOI] [PubMed] [Google Scholar]
- Pace TW, Miller AH. (2009) Cytokines and glucocorticoid receptor signaling. Relevance to major depression. Ann N Y Acad Sci 1179:86–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. (2006) Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry 163:1630–1633. [DOI] [PubMed] [Google Scholar]
- Pan Y, Chen XY, Zhang QY, Kong LD. (2014) Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun 41:90–100. [DOI] [PubMed] [Google Scholar]
- Pantazatos SP, Huang YY, Rosoklija GB, Dwork AJ, Arango V, Mann JJ. (2017) Whole-transcriptome brain expression and exon-usage profiling in major depression and suicide: evidence for altered glial, endothelial and ATPase activity. Mol Psychiatry 22:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333:1456–1458. [DOI] [PubMed] [Google Scholar]
- Pape K, Tamouza R, Leboyer M, Zipp F. (2019) Immunoneuropsychiatry - novel perspectives on brain disorders. Nat Rev Neurol 15:317–328. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, III, Lafaille JJ, Hempstead BL, Littman DR, Gan W-B. (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155:1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, Salum G, Magalhães PV, Kapczinski F, Kauer-Sant’Anna M. (2015) Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2:1002–1012. [DOI] [PubMed] [Google Scholar]
- Patterson PH. (2009) Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res 204:313–321. [DOI] [PubMed] [Google Scholar]
- Perrin AJ, Horowitz MA, Roelofs J, Zunszain PA, Pariante CM. (2019) Glucocorticoid resistance: is it a requisite for increased cytokine production in depression? A systematic review and meta-analysis. Front Psychiatry 10:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. (2004) Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci 24:10594–10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C, Lazaropoulou C, Papassotiriou I, Tsiantis J, Chrousos GP. (2007) Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology 32:991–999. [DOI] [PubMed] [Google Scholar]
- Pfau ML, Ménard C, Russo SJ. (2018) Inflammatory mediators in mood disorders: therapeutic opportunities. Annu Rev Pharmacol Toxicol 58:411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, Goldberg J, Vaccarino V. (2013) Association between posttraumatic stress disorder and inflammation: a twin study. Brain Behav Immun 30:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop VJ, Maartens LH, Leusink G, van Son MJ, Knottnerus AA, Ward AM, Metcalfe R, Weetman AP. (1998) Are autoimmune thyroid dysfunction and depression related? J Clin Endocrinol Metab 83:3194–3197. [DOI] [PubMed] [Google Scholar]
- Powers A, Dixon HD, Conneely K, Gluck R, Munoz A, Rochat C, Mendoza H, Hartzell G, Ressler KJ, Bradley B, et al. (2019) The differential effects of PTSD, MDD, and dissociation on CRP in trauma-exposed women. Compr Psychiatry 93:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribiag H, Stellwagen D. (2014) Neuroimmune regulation of homeostatic synaptic plasticity. Neuropharmacology 78:13–22. [DOI] [PubMed] [Google Scholar]
- Prinz M, Erny D, Hagemeyer N. (2017) Ontogeny and homeostasis of CNS myeloid cells. Nat Immunol 18:385–392. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J. (2014) Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15:300–312. [DOI] [PubMed] [Google Scholar]
- Raison CL. (2017) The promise and limitations of anti-inflammatory agents for the treatment of major depressive disorder. Curr Top Behav Neurosci 31:287–302. [DOI] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. (2010a) CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry 15:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Lowry CA, Rook GA. (2010b) Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch Gen Psychiatry 67:1211–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. (2013) The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D). Mol Psychiatry 18:15–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez K, Fornaguera-Trías J, Sheridan JF. (2017) Stress-induced microglia activation and monocyte trafficking to the brain underlie the development of anxiety and depression. Curr Top Behav Neurosci 31:155–172. [DOI] [PubMed] [Google Scholar]
- Ramirez K, Niraula A, Sheridan JF. (2016) GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain Behav Immun 51:154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. (2016a) How neuroinflammation contributes to neurodegeneration. Science 353:777–783. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. (2016b) A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19:987–991. [DOI] [PubMed] [Google Scholar]
- Rao JS, Harry GJ, Rapoport SI, Kim HW. (2010) Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry 15:384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF. (2015) Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation, and anxiety. Neuroscience 289:429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector JL, Dowd JB, Loerbroks A, Burns VE, Moss PA, Jarczok MN, Stalder T, Hoffman K, Fischer JE, Bosch JA. (2014) Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain Behav Immun 38:133–141. [DOI] [PubMed] [Google Scholar]
- Rehm J, Shield KD. (2019) Global Burden of Disease and the Impact of Mental and Addictive Disorders. Curr Psychiatry Rep 21:10. [DOI] [PubMed] [Google Scholar]
- Renault PF, Hoofnagle JH, Park Y, Mullen KD, Peters M, Jones DB, Rustgi V, Jones EA. (1987) Psychiatric complications of long-term interferon alfa therapy. Arch Intern Med 147:1577–1580. [PubMed] [Google Scholar]
- Riecher-Rössler A. (2017a) Oestrogens, prolactin, hypothalamic-pituitary-gonadal axis, and schizophrenic psychoses. Lancet Psychiatry 4:63–72. [DOI] [PubMed] [Google Scholar]
- Riecher-Rössler A. (2017b) Sex and gender differences in mental disorders. Lancet Psychiatry 4:8–9. [DOI] [PubMed] [Google Scholar]
- Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. (2004) Reference distributions for complement proteins C3 and C4: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal 18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA, Lowry CA, Raison CL. (2015) Hygiene and other early childhood influences on the subsequent function of the immune system. Brain Res 1617:47–62. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ. (2019) Sex differences and the neurobiology of affective disorders. Neuropsychopharmacology 44:111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14:609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaiyan S, Kannaiyan N, Snaidero N, Brioschi S, Biber K, Yona S, Edinger AL, Jung S, Rossner MJ, Simons M. (2016) Age-related myelin degradation burdens the clearance function of microglia during aging. Nat Neurosci 19:995–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, Stevens B. (2017) Microglia emerge as central players in brain disease. Nat Med 23:1018–1027. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Banasr M. (2013) From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry 73:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandiego CM, Gallezot J-D, Pittman B, Nabulsi N, Lim K, Lin S-F, Matuskey D, Lee J-Y, O’Connor KC, Huang Y, et al. (2015) Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci USA 112:12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Bezzi P, Volterra A. (2011) TNFα controls glutamatergic gliotransmission in the hippocampal dentate gyrus. Neuron 69:988–1001. [DOI] [PubMed] [Google Scholar]
- Santello M, Volterra A. (2012) TNFα in synaptic function: switching gears. Trends Neurosci 35:638–647. [DOI] [PubMed] [Google Scholar]
- Sárvári M, Hrabovszky E, Kalló I, Solymosi N, Tóth K, Likó I, Széles J, Mahó S, Molnár B, Liposits Z. (2011) Estrogens regulate neuroinflammatory genes via estrogen receptors α and β in the frontal cortex of middle-aged female rats. J Neuroinflammation 8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasayama D, Hattori K, Wakabayashi C, Teraishi T, Hori H, Ota M, Yoshida S, Arima K, Higuchi T, Amano N, et al. (2013) Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res 47:401–406. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnieder TP, Trencevska I, Rosoklija G, Stankov A, Mann JJ, Smiley J, Dwork AJ. (2014) Microglia of prefrontal white matter in suicide. J Neuropathol Exp Neurol 73:880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SK, Cahill L. (2009) Endogenous noradrenergic activation and memory for emotional material in men and women. Psychoneuroendocrinology 34:1263–1271. [DOI] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, et al. Schizophrenia Working Group of the Psychiatric Genomics Consortium (2016) Schizophrenia risk from complex variation of complement component 4. Nature 530:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Huo Z, Cahill K, French L, Puralewski R, Zhang J, Logan RW, Tseng G, Lewis DA, Sibille E. (2018) Opposite molecular signatures of depression in men and women. Biol Psychiatry 84:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL, Rekkas PV, Houle S, et al. (2015) Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. (2010) Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway, Cereb Cortex 20, pp 2560–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, Mirnics K. (2011) Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry 16:751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I, Dudbridge F, Holmans PA, Whittemore AS, Mowry BJ, et al. (2009) Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 460:753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJP, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. (2010) Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7:483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. (2008) Steroid hormone receptor expression and function in microglia. Glia 56:659–674. [DOI] [PubMed] [Google Scholar]
- Skelton K, Ressler KJ, Norrholm SD, Jovanovic T, Bradley-Davino B. (2012) PTSD and gene variants: new pathways and new thinking. Neuropharmacology 62:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. (2014) From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull 140:774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Sacher J. (2019) Stress, sex hormones, inflammation, and major depressive disorder: Extending Social Signal Transduction Theory of Depression to account for sex differences in mood disorders. Psychopharmacology (Berl) 236:3063–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ. (2011) Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet 156B:700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS. (1991) The macrophage theory of depression. Med Hypotheses 35:298–306. [DOI] [PubMed] [Google Scholar]
- Smoller JW. (2016) The Genetics of Stress-Related Disorders: PTSD, Depression, and Anxiety Disorders. Neuropsychopharmacology 41:297–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund J, Olsson SK, Samuelsson M, Walther-Jallow L, Johansson C, Erhardt S, Landén M, Engberg G. (2011) Elevation of cerebrospinal fluid interleukin-1ß in bipolar disorder. J Psychiatry Neurosci 36:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Li X, Kang Z, Kadotomi Y. (2007) Omega-3 fatty acid ethyl-eicosapentaenoate attenuates IL-1beta-induced changes in dopamine and metabolites in the shell of the nucleus accumbens: involved with PLA2 activity and corticosterone secretion. Neuropsychopharmacology 32:736–744. [DOI] [PubMed] [Google Scholar]
- Stansfeld SA, Head J, Fuhrer R, Wardle J, Cattell V. (2003) Social inequalities in depressive symptoms and physical functioning in the Whitehall II study: exploring a common cause explanation. J Epidemiol Community Health 57:361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. (2001) Social stress induces glucocorticoid resistance in macrophages. Am J Physiol Regul Integr Comp Physiol 280:R1799–R1805. [DOI] [PubMed] [Google Scholar]
- Staufenbiel SM, Penninx BW, Spijker AT, Elzinga BM, van Rossum EF. (2013) Hair cortisol, stress exposure, and mental health in humans: a systematic review. Psychoneuroendocrinology 38:1220–1235. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, Werge T, Pietiläinen OP, Mors O, Mortensen PB, et al. Genetic Risk and Outcome in Psychosis (GROUP) (2009) Common variants conferring risk of schizophrenia. Nature 460:744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Vasconcelos MF, Albrechet-Souza L, Ceresér KMM, de Almeida RMM. (2017) Microglial over-activation by social defeat stress contributes to anxiety- and depressive-like behaviors. Front Behav Neurosci 11:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein H-G, Bogerts B. (2008) Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res 42:151–157. [DOI] [PubMed] [Google Scholar]
- Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z, Mawrin C, Brisch R, Bielau H, Meyer zu Schwabedissen L, et al. (2011) Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation 8:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stertz L, Magalhães PV, Kapczinski F. (2013) Is bipolar disorder an inflammatory condition? The relevance of microglial activation. Curr Opin Psychiatry 26:19–26. [DOI] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ. (2015) Inflammation and clinical response to treatment in depression: a meta-analysis. Eur Neuropsychopharmacol 25:1532–1543. [DOI] [PubMed] [Google Scholar]
- Sugden K, Moffitt TE, Pinto L, Poulton R, Williams BS, Caspi A. (2016) Is toxoplasma gondii infection related to brain and behavior impairments in humans? Evidence from a population-representative birth cohort. PLoS One 11:e0148435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Jin S, Suzuki H, Doi Y, Liang J, Kawanokuchi J, Mizuno T, Sawada M, Suzumura A. (2008) Blockade of microglial glutamate release protects against ischemic brain injury. Exp Neurol 214:144–146. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A. (2006) Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem 281:21362–21368. [DOI] [PubMed] [Google Scholar]
- Tay TL, Béchade C, D’Andrea I, St-Pierre MK, Henry MS, Roumier A, Tremblay ME. (2018) Microglia Gone Rogue: Impacts on Psychiatric Disorders Across the Lifespan. Front Mol Neurosci 10:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay TL, Mai D, Dautzenberg J, Fernández-Klett F, Lin G, Sagar, Datta M, Drougard A, Stempfl T, Ardura-Fabregat A, et al. (2017) A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci 20:793–803. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. (2006) Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry 60:296–301. [DOI] [PubMed] [Google Scholar]
- Tilleux S, Hermans E. (2007) Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res 85:2059–2070. [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. (2014) Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav Immun 42:50–59. [DOI] [PubMed] [Google Scholar]
- Torres L, Danver J, Ji K, Miyauchi JT, Chen D, Anderson ME, West BL, Robinson JK, Tsirka SE. (2016) Dynamic microglial modulation of spatial learning and social behavior. Brain Behav Immun 55:6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Lun ZR, Yolken RH. (2007) Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull 33:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tränkner D, Boulet A, Peden E, Focht R, Van Deren D, Capecchi M. (2019) A Microglia Sublineage Protects from Sex-Linked Anxiety Symptoms and Obsessive Compulsion. Cell Rep 29:791–799.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M-È, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. (2011) The role of microglia in the healthy brain. J Neurosci 31:16064–16069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigunaite A, Dimo J, Jørgensen TN. (2015) Suppressive effects of androgens on the immune system. Cell Immunol 294:87–94. [DOI] [PubMed] [Google Scholar]
- Trottier MD, Newsted MM, King LE, Fraker PJ. (2008) Natural glucocorticoids induce expansion of all developmental stages of murine bone marrow granulocytes without inhibiting function. Proc Natl Acad Sci USA 105:2028–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turecki G, Meaney MJ. (2016) Effects of the social environment and stress on glucocorticoid receptor gene methylation: a systematic review. Biol Psychiatry 79:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tursich M, Neufeld RW, Frewen PA, Harricharan S, Kibler JL, Rhind SG, Lanius RA. (2014) Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Transl Psychiatry 4:e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udina M, Castellví P, Moreno-España J, Navinés R, Valdés M, Forns X, Langohr K, Solà R, Vieta E, Martín-Santos R. (2012) Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J Clin Psychiatry 73:1128–1138. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, et al. (2008) Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 64:820–822. [DOI] [PubMed] [Google Scholar]
- van Os J, Kapur S. (2009) Schizophrenia. Lancet 374:635–645. [DOI] [PubMed] [Google Scholar]
- Varvel NH, Grathwohl SA, Baumann F, Liebig C, Bosch A, Brawek B, Thal DR, Charo IF, Heppner FL, Aguzzi A, et al. (2012) Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc Natl Acad Sci USA 109:18150–18155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Viviani B. (2015) Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96 (Pt A):70–82. [DOI] [PubMed] [Google Scholar]
- Vidović A, Gotovac K, Vilibić M, Sabioncello A, Jovanović T, Rabatić S, Folnegović-Šmalć V, Dekaris D. (2011) Repeated assessments of endocrine- and immune-related changes in posttraumatic stress disorder. Neuroimmunomodulation 18:199–211. [DOI] [PubMed] [Google Scholar]
- Vieira MM, Ferreira TB, Pacheco PA, Barros PO, Almeida CR, Araújo-Lima CF, Silva-Filho RG, Hygino J, Andrade RM, Linhares UC, et al. (2010) Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol 229:212–218. [DOI] [PubMed] [Google Scholar]
- Vigo D, Thornicroft G, Atun R. (2016) Estimating the true global burden of mental illness. Lancet Psychiatry 3:171–178. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. (2013) Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry 3:e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk R, van der Schot AC, Kahn RS, Nolen WA, Drexhage HA. (2007) Is autoimmune thyroiditis part of the genetic vulnerability (or an endophenotype) for bipolar disorder? Biol Psychiatry 62:135–140. [DOI] [PubMed] [Google Scholar]
- von Känel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. (2007) Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res 41:744–752. [DOI] [PubMed] [Google Scholar]
- Wagner EY, Wagner JT, Glaus J, Vandeleur CL, Castelao E, Strippoli MP, Vollenweider P, Preisig M, von Känel R. (2015) Evidence for chronic low-grade systemic inflammation in individuals with agoraphobia from a population-based prospective study. PLoS One 10:e0123757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. (2009) Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 29:3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker FR, Nilsson M, Jones K. (2013) Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets 14:1262–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yue H, Hu Z, Shen Y, Ma J, Li J, Wang XD, Wang L, Sun B, Shi P, et al. (2020) Microglia mediate forgetting via complement-dependent synaptic elimination. Science 367:688–694. [DOI] [PubMed] [Google Scholar]
- Wang YL, Han QQ, Gong WQ, Pan DH, Wang LZ, Hu W, Yang M, Li B, Yu J, Liu Q. (2018) Microglial activation mediates chronic mild stress-induced depressive- and anxiety-like behavior in adult rats. J Neuroinflammation 15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, McKim DB, Niraula A, Witcher KG, Yin W, Sobol CG, Wang Y, Sawicki CM, Sheridan JF, Godbout JP. (2019) The Influence of Microglial Elimination and Repopulation on Stress Sensitization Induced by Repeated Social Defeat. Biol Psychiatry 85:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Lechowicz W, Stepień T, Pasennik E. (2005) Quantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol 43:81–89. [PubMed] [Google Scholar]
- Williams LM, Barton MJ, Kemp AH, Liddell BJ, Peduto A, Gordon E, Bryant RA. (2005) Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. Neuroimage 28:618–626. [DOI] [PubMed] [Google Scholar]
- Wittenberg GM, Stylianou A, Zhang Y, Sun Y, Gupta A, Jagannatha PS, Wang D, Hsu B, Curran ME, Khan S, et al. MRC ImmunoPsychiatry Consortium (2020) Effects of immunomodulatory drugs on depressive symptoms: a mega-analysis of randomized, placebo-controlled clinical trials in inflammatory disorders. Mol Psychiatry 25:1275–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E, McKim D, Sheridan J, Godbout J. (2014a) Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci 8:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. (2012) Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology 37:1491–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Franklin T, Iwata M, Duman RS. (2016) Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci 17:497–511. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. (2011) β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci 31:6277–6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Sheridan JF, Godbout JP. (2015) Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci 8:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. (2014b) Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci 34:2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF. (2013) Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 33:13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Terwilliger R, Duman CH, Duman RS. (2018) Stress-induced neuronal colony stimulating factor 1 provokes microglia-mediated neuronal remodeling and depressive-like behavior. Biol Psychiatry 83:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Arcos-Burgos M, Liu S, Vélez JI, Yu C, Baune BT, Jawahar MC, Arolt V, Dannlowski U, Chuah A, et al. (2017) The PHF21B gene is associated with major depression and modulates the stress response. Mol Psychiatry 22:1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M-L, Licinio J. (2001) Research and treatment approaches to depression. Nat Rev Neurosci 2:343–351. [DOI] [PubMed] [Google Scholar]
- Yohn SE, Arif Y, Haley A, Tripodi G, Baqi Y, Müller CE, Miguel NS, Correa M, Salamone JD. (2016) Effort-related motivational effects of the proinflammatory cytokine interleukin-6: pharmacological and neurochemical characterization. Psychopharmacology (Berl) 233:3575–3586. [DOI] [PubMed] [Google Scholar]
- Yu S. (2018) Uncovering the hidden impacts of inequality on mental health: a global study. Transl Psychiatry 8:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn-Waxler C, Shirtcliff EA, Marceau K. (2008) Disorders of childhood and adolescence: gender and psychopathology. Annu Rev Clin Psychol 4:275–303. [DOI] [PubMed] [Google Scholar]
- Zhang CJ, Jiang M, Zhou H, Liu W, Wang C, Kang Z, Han B, Zhang Q, Chen X, Xiao J, et al. (2018) TLR-stimulated IRAKM activates caspase-8 inflammasome in microglia and promotes neuroinflammation. J Clin Invest 128:5399–5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. (2010) Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology 35:2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JY, Crews FT. (2005) TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res 1034:11–24. [DOI] [PubMed] [Google Scholar]