Abstract
Alzheimer’s disease is one of the most progressive forms of dementia, ultimately leading to death in aged populations. The major hallmarks of Alzheimer’s disease include deposition of extracellular amyloid senile plaques and intracellular neurofibrillary tangles in brain neuronal cells. Although there are classical therapeutic options available for the treatment of the diseases, however, they provide only a symptomatic relief and do not modify the molecular pathophysiological course of the disease. Recent research advances in Alzheimer’s disease have highlighted the potential role of anti-amyloid, anti-tau, and anti-inflammatory therapies. However, these therapies are still in different phases of pre-clinical/clinical development. In addition, drug repositioning/repurposing is another interesting and promising approach to explore rationalized options for the treatment of Alzheimer’s disease.
This review discusses the different aspects of the pathophysiological mechanism involved in the progression of Alzheimer’s disease along with the limitations of current therapies. Furthermore, this review also highlights emerging investigational drugs along with recent drug repurposing approaches for Alzheimer’s disease.
Keywords: Alzheimer’s disease, drug repurposing, inflammatory cytokines, chronic neuroinflammation, drug repurposing
1. Introduction
Alzheimer’s disease (AD) is one of the most prevalent age-related, serious and irreversible neurodegenerative diseases progressively leading to death. It is pathologically characterized by marked memory impairment and cognitive deficit in aged adult population. The pathological hallmarks of AD include deposition of amyloid beta (Aβ) plaque in extra-neuronal parenchyma and neurofibrillary tangles (NFTs) in brain tissues [1-4]. Additionally, tau-positive neuronal threads, dystrophic neurites as well as pro-inflammatory microglial cells and reactive astrocytes are also present. These structural and inflammatory lesions lead to a loss of neurons in vulnerable regions of the brain, leading to AD. Evidences suggest that amyloid deposition and tau pathology in AD can precede structural changes in the brain, including progressive hippocampal volume loss and decreased glucose metabolism. It is eventually followed by the manifestation of clinical features such as memory loss, social dependence, and motor abnormalities.
Numerous literature reports indicate that the chronic neuroinflammatory process in the brain contributes to the development of AD pathophysiology and has been linked directly to memory impairment, cognitive deficit, and dementia. This is evident by an elevated level of inflammatory proteases, cytokines, and chemokines, along with increased oxidative stress due to the accumulation of microglial cells around senile Aβ-plaques [5-7]. Such reports have also been confirmed by in-vitro and in-vivo studies along with data from the transgenic animals [4, 8-10].
2. Current therapeutic options in Alzheimer’s disease
The number of individuals affected by AD is expected to be ~40 million worldwide, with a continuously increasing number of 7.7 million new cases every year [11-13]. Thus, AD has been ranked at 6th position among the top ten major causes of death. In spite of an alarming situation, there are only limited, approved therapeutic options available for the treatment of AD (Table 1). Currently, AChEIs (acetylcholinesterase inhibitors) are used for the treatment of AD which are based on the classical cholinergic hypothesis, taking into consideration the key role of acetylcholine (ACh) in cognitive functions of the human brain (Table 1). According to this theory, there is a decrease in activity of the key enzymes involved in acetylcholine synthesis: choline acetyltransferase (ChAT) and pyruvate dehydrogenase (PDH) complex. Furthermore, the declined functions of muscarinic M1 receptor subtypes and nicotinic receptors in the brain due to Aβ deposition also lead to impaired cholinergic neurotransmission in AD [6, 7, 14, 15]. Therefore, AChEIs present a logical approach for the treatment of AD pathology by inhibition of acetylcholine decomposition. Researchers are still focused on the discovery of safe and efficacious drugs that stimulate the cholinergic transmission by selective activation of either central M1 muscarinic or nicotinic receptors. However, any selective M1 agonist has not been discovered yet. This was mainly due to a lack of M1 subtype selectivity of compounds designed so far and the incidence of serious adverse effects.
Table 1.
Approved clinical therapies for treatment of Alzheimer’s disease.
| Drug Name | Current Stage | Mechanism of Action in AD | Refs. |
|---|---|---|---|
| Tacrine | Marketed | Non-competitive and non-selective reversible inhibitor AChE | [2, 4] |
| Donepezil | Marketed | Highly selective reversible non-competitive inhibitor of AChE | [2, 4] |
| Rivastigmine | Marketed | Pseudo-selective irreversible inhibitor of AChE and BuChE | [2, 4] |
| Galantamine | Marketed | Selective, competitive & reversible AChE inhibitor | [2, 4] |
| Huperzine A | Approved in China | Distinctive affinity for AChE with strong anti-oxidant and anti-inflammatory properties | [2, 4, 6] |
| Memantine | Marketed | Non-competitive NMDA receptor antagonist | [2, 4, 6, 15] |
The currently approved drugs to be used in AD treatment are AChEIs (Table 1) e.g. Rivastigmine, Gallantamine, Donepezil), and N-methyl-D-aspartic acid (NMDA) receptor antagonist (Memantine). Thus, AChEIs were the first drugs approved for the treatment of AD [4, 10, 14]. Tacrine was one of the first approved AChEI drugs for the treatment of AD, however, it is now obsolete. It is a non-competitive and non-selective reversible inhibitor to AChE, having dose-related efficacy, short half-life, and a high incidence of adverse effects and hepatotoxicity. Donepezil is another drug approved in 1996 for AD, which is a highly selective, reversible inhibitor of AChE without any serious adverse effects. Rivastigmine is a pseudo-selective irreversible inhibitor of both AChE and butyryl cholinesterase (BuChE) and it has shown good improvement in cognition and neuroprotective effects. Galantamine is a tertiary alkaloid obtained from various species of Amaryllidaceae approved in 2001 for the treatment of AD. It is a selective, competitive, and reversible inhibitor of AChE and also exerts a modulating action on nicotinic receptors with low hepatotoxicity. Huperzine A is another widely used drug in eastern medicine, which shows a distinctive affinity towards AChE along with strong antioxidant and anti-inflammatory properties [16]. However, it is approved by the State Food and Drug Administration of China for AD therapy. Memantine is a non-competitive NMDA receptor antagonist approved for the treatment of AD in mild to severe stages. It reduces excitotoxicity and neurodegeneration caused by excessive glutamatergic neurotransmission. It may also decrease tau-hyperphosphorylation and protects against Aβ-induced neuronal toxicity [2, 15].
Nonetheless, the available therapies have a very limited effect in the management of the pathophysiological aspects of AD. Since the etiology of AD is still not fully known, the treatment strategies focus more on its risk factors and prevention, rather than finding the exact cause of this disease. Therefore, there is an urgent need for rationalized exploration of novel therapeutic targets to control the molecular pathophysiology and chronic neuroinflammation associated with AD.
3. Molecular pathways involved in the pathophysiology of Alzheimer’s disease
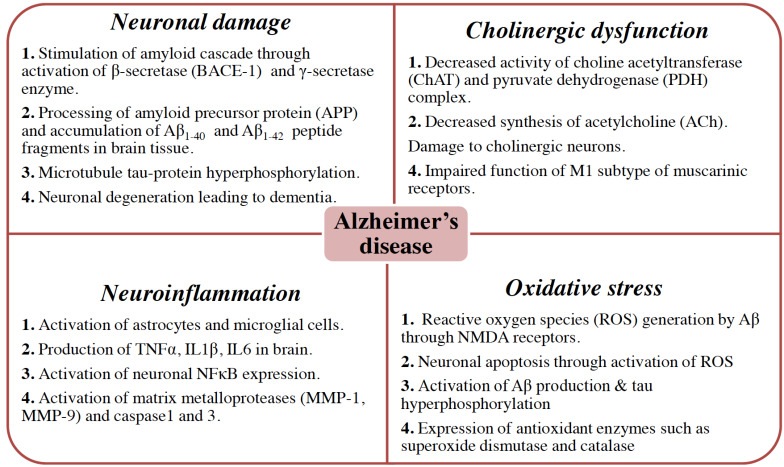
The pathophysiology of AD is multi-dimensional and complex and has been explained by the involvement of various molecular pathways. The potential molecular pathways in AD include neuronal damage due to the accumulation of Aβ and NFTs, cholinergic dysfunction, neuroinflammation, and oxidative stress (Fig. 1).
Fig. (1).
The multidimensional pathological characteristics in AD. The major hallmarks of AD include Aβ and hyperphosphorylated tau proteins called NFTs. However, the pathophysiology of AD is complex and multi-dimensional including cholinergic neuronal damage, cholinergic dysfunction, neuronal inflammation and loads of oxidative stress. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.1. Amyloid-beta Hypothesis: Production and Clearance of Aβ, the Role of Monomeric and Oligomeric Forms of Aβ
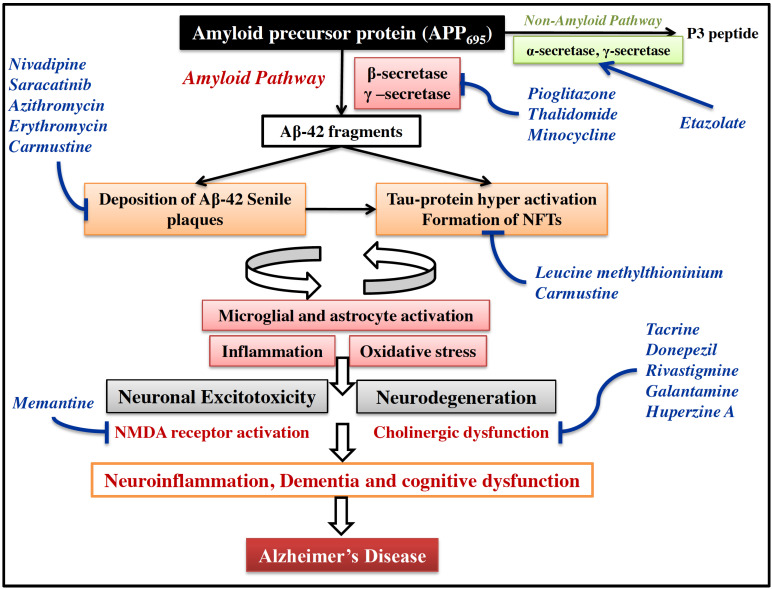
Among various pathways, the most popular and consistent pathway explained by several research groups is the Aβ hypothesis [17, 18]. It has consistently been reported that there is a progressive increase in the concentration of amyloid plaques and NFT in the brain of AD patients. The amyloid plaques are extracellular deposits of Aβ42 fragments in the brain parenchyma and cerebral blood vessels [19-21]. These fragments are formed by cleavage of APP (amyloid precursor proteins) by different secretase enzymes (α, β, and γ-isoforms) in the brain neurons (Fig. 2). APP (APP695) is a transmembrane glycoprotein expressed by several types of cells, including microglia, astrocyte, brain neuronal cells in CNS. APP (APP751 and APP770) is also expressed in the peripheral cells and tissues such as adrenal gland, kidney, heart, liver, spleen, pancreas, muscles, platelets, leukocytes, and endothelial cells. The α-secretase cleaves APP mainly outside CNS in the peripheral cells and tissues [7, 22, 23]. The β and ɣ-secretase cleaves APP particularly in the CNS neuronal cells leading to the generation of shorter insoluble peptide fragments (~39-43 amino acids) known as Aβ-fragments [24]. In particular, Aβ42 fragments possess a distinct neurotoxic effect leading to neurodegeneration, inflammation, amyloid angiopathy, and mitochondrial dysfunction in CNS (Fig. 2). These fragments form peculiar insoluble structures around the brain neuronal cells and constitute a histopathological hallmark of AD pathophysiology called senile plaque [17, 24, 25].
Fig. (2).
Molecular pathogenesis in AD. The APP695 expressed predominantly in brain is cleaved by amyloid pathway through β and γ-secretases to form insoluble Aβ42 peptide fragments. These fragments accumulate to form senile plaques around brain cholinergic neurons. Deposition of such insoluble plaques around CNS neuron causes hyperphosphorylation of tau protein leading to formation of NFTs. Usually these pathological features are cleared from brain by microglia and astrocytes under normal conditions. However, due to imbalance in deposition and clearance of these pathological features, the microglia and astrocytes are hyper-activated leading to inflammation and oxidative stress. Due to this, a vicious cycle of inflammation is formed between Aβ accumulation, activated microglia, and microglial inflammatory mediators, which further enhance Aβ deposition and neuroinflammation. All these structural and biochemical changes in brain cause neuronal excitotoxicity and neurodegeneration. This ultimately leads to dementia and cognitive deficit in AD. Drugs are shown in blue colour at respective points in this signaling based on their suggested mechanism of action. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
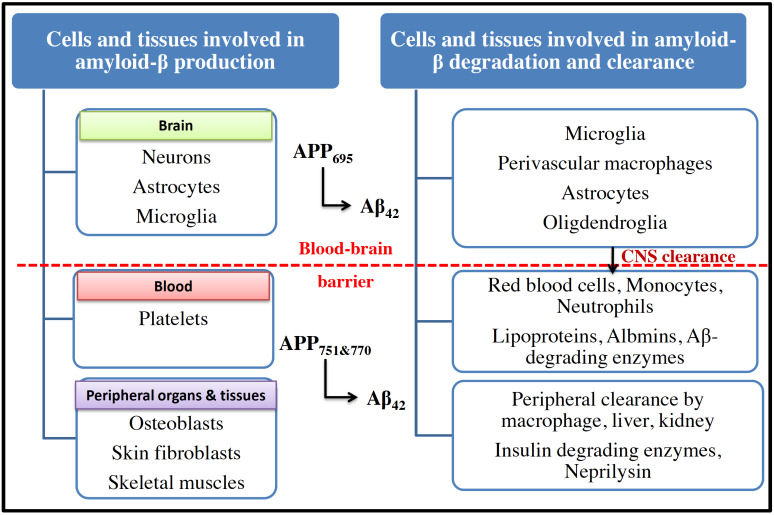
Generally, these fragments undergo proteolytic degradation by certain enzymes, including neprilysin, insulin-degrading enzyme, and matrix metalloproteinase in CNS. In addition, these fragments can also be taken away from CNS by various processes. These include phagocytosis, endocytosis, macropinocytosis by various cells, including microglia, perivascular macrophages, astrocytes, oligodendroglia [17, 24]. In addition, these Aβ fragments are also cleared from CNS by direct efflux to the peripheral circulation (Fig. 3). In the peripheral system, several cells and tissues participate in Aβ catabolism and constitute potential Aβ clearance pathways. The peripheral cells, such as monocytes, macrophages, neutrophils, lymphocytes, and erythrocytes, mediate the uptake and phagocytosis or endocytosis of Aβ-fragments. These fragments may also undergo proteolytic degradation by Aβ-degrading enzymes followed by their blood clearance by Aβ-binding proteins (albumin, antithrombin III and apolipoproteins, ApoE and ApoJ). During the progression of AD, there is a marked imbalance between the production and clearance of amyloid plaques in the brain and peripheral organs and tissues [19, 25, 26]. This imbalance between production and clearance of Aβ-fragments has become an important pathophysiological marker for the characterization of amyloid hypothesis in AD [18].
Fig. (3).
Production, degradation and clearance of amyloid-β in different organs and tissues in brain and periphery. APP695 is expressed particularly in CNS and is cleaved by β and γ-secretases to form insoluble Aβ42 fragments. Normally, these Aβ42 fragments are cleared off the brain by resident brain immune cells to the periphery. However, due to excessive production of Aβ42 during AD, there is an imbalance in production and clearance. This ultimately activates brain immune cells leading to a significant increase in inflammation and oxidative stress. Another isoform of APP, APP751 and 770 is expressed by platelets, skin and bone cells in peripheral tissues. The peripheral amyloid is cleared off from the body by macrophages through liver and kidney through the action of various enzymes. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The Aβ42 is mainly monomeric at physiological concentrations and is present in the brain and CSF of normal individuals [22, 27, 28]. It has been reported that the Aβ42 monomers activate type-1 insulin-like growth factor receptors and enhance the glucose uptake in neurons by promoting translocation of the Glut3 glucose transporter from the cytosol to the plasma membrane. In neurons, activity-dependent glucose uptake was blunted after blocking endogenous monomeric Aβ42 production and re-established by the administration of exogenous Aβ42 monomers. Furthermore, the APP-null neurons failed to enhance depolarization-stimulated glucose uptake unless exogenous monomeric Aβ42 was added. These data suggest that Aβ42 monomers were critical for maintaining neuronal glucose homeostasis [29, 30], and have a protective role in normal brain functions.
Under physiological conditions, insulin binding to its cell surface insulin receptors triggers its autophosphorylation and subsequent tyrosine phosphorylation of insulin receptor substrate-1. The neuroprotective action of Aβ42 monomers was mediated by the activation of phosphatidylinositol-3-kinase pathway and downstream cellular responses that facilitate synaptic plasticity and memory. During AD, the Aβ42 monomers self-aggregate into oligomers causing synaptic dysfunction and neuronal loss. The accumulation of Aβ42 oligomers leads to increased TNFα levels and activation of various stress kinases that causes inhibition of insulin receptor substrate-1. Aβ42 oligomers also instigate the additional removal of insulin receptors from the cell surface and redistribution to the cell body. These combined events block normal neuronal insulin signaling. Such defective insulin signaling further accelerates Aβ42 production and aggregation in the brain by enhancing amyloidogenic processing of the APP. Thus, Aβ42 oligomer-induced insulin resistance may create a vicious cycle to upregulate their own production and aggregation by disrupting physiological actions of insulin in brain [29-31]. Such mechanisms could account in part for Aβ42 oligomers build-up and its damaging effects in the brain during AD progression.
3.2. Tau-hyperphosphorylation and Formation of Neurofibrillary Tangles
Under normal conditions, the tau protein promotes strength to an assembly of tubulin protein, providing microtubular stability. Due to a significantly high concentration of Aβ42 fragments in the brain in AD, the microtubule-associated tau proteins are hyperphosphorylated. When these tau proteins are hyperphosphorylated, they are not able to form stable structures with tubulin, but rather they form insoluble misfolded aggregates as NFTs. The abnormal tau hyperphosphorylation and aggregation play an important role in neurofibrillary degeneration [32-34]. NFTs may reduce normal tau function, compromise normal cellular physiology, and disrupt tau-mediated regulation of microtubule dynamics resulting in neurodegeneration. Deposition of these NFT proteins in the brain tissue leads to neuronal and synaptic damage [35, 36]. Literature evidences have shown that there exists a strong level of interdependence between Aβ accumulation and formation of NFTs during initiation and progression of neuroinflammation and neurodegeneration in AD [32].
3.3. Role of a Cholinergic and Glutaminergic Neuronal System
The cholinergic hypothesis of AD is another most extensively studied approach to describe the cellular and molecular pathophysiology. The cholinergic neurons in the hippocampus, frontal cortex, amygdala, nucleus basalis, and medial septum regions serve an important functional role in awareness, attention, learning, memory, and other cognitive processes. During the onset of AD, these basal forebrain cholinergic neurons are primarily damaged by a primary degenerative process. Such cholinergic neuronal loss was seen up to 90% in advanced stages of progressive AD [5, 37, 38]. Due to this, there is a significant deficit in cholinergic neurotransmission in CNS, leading to the onset of cognitive impairment. It has been reported that the non-selective muscarinic antagonist, scopolamine-induced cognitive impairment, favors the production of Aβ peptide and decreases the activity of α-secretase in the CNS [4, 5, 38]. The exact molecular mechanisms of the effect of cholinergic drugs in learning and memory, and their clinical relevance are still being studied for the improvement of AD pathophysiology. Recent studies have reported that Aβ interacts with cholinergic receptors in the brain, affecting their function. In general, the cholinergic and glutaminergic systems significantly interact during alteration in CNS neurotransmission. There is an adjustment in glutaminergic signaling noted during the cholinergic disruptions in AD [37, 38]. The physiological glutaminergic neurotransmission in the hippocampus produces a cytosolic calcium signal, which mediates synaptic plasticity phenomena such as long-term potentiation (LTP), encouraging learning and memory consolidation. However, a sustained increase in calcium, sodium, and chloride ions, as a result of the hyper-activation of NMDA receptors, has been associated with excessive depolarization of the postsynaptic membrane, onset neurodegenerative processes, and cell death. Such an increase in intra-neuronal calcium due to dysfunctional glutaminergic neurotransmission may generate a long-lasting depression in the cerebellum. This may ultimately lead to increased calcium overload in mitochondria, activation of nitric oxide synthesis, generation of free radicals, oxidative stress, and neuronal death [37-39]. The cholinergic hypothesis has served as a basis for the majority of treatment strategies and diverse drug development approaches (acetylcholinesterase inhibitors, cholinergic precursors, cholinergic receptor agonists, NMDA receptor blockers) for AD in the recent past. However, this hypothesis does not provide an explanation to establish a definitive causative factor for multifaceted pathophysiology of AD.
3.4. Role of Inflammatory Mediators
The production versus clearance imbalance in Aβ homeostasis leads to its accumulation within the extracellular space. The extracellular Aβ deposits trigger activation of microglia (resident immune cells of the brain), and astrocytes in the surrounding parenchyma [40, 41]. They are also activated by hyperphosphorylated tau protein aggregates (NFTs) inside neuronal cell bodies and neuritis [22, 28]. These pathological features ultimately lead to progressive inflammation and oxidative stress through microglia and astrocyte activation. The microglia and astrocytes detect the pathogen or danger-associated molecular patterns (PAMPs or DAMPs) through cell-surface pattern recognition receptors (PRRs). Glial cells direct the neuroimmune responses by phagocytic mechanism by the release of pro- and anti-inflammatory cytokines, chemokines, and growth factors [42]. Under normal conditions, there is a resolution signal to maintain glial homeostasis after a time-gap. However, a chronic activation of the innate immune system remains in the brain due to long term deposition of Aβ and NFTs during AD; and hence there is no resolution stage. Such persistent alteration of the glia cell phenotype is believed to contribute to AD severity and progression [43]. These inflammatory changes appear in subcortical nuclei such as the locus coeruleus in the early stages of AD and eventually, spread to affect cholinergic neurons in the hippocampus and cortical areas with the progression of the disease [7, 34, 41].
The activation of microglial cells results in clearance of Aβ and has been demonstrated to exert a positive effect on animal models of AD [42, 44, 45]. However, prolonged microglial activation may result in exacerbation of AD pathology [45-47]. The capacity of microglia for Aβ phagocytosis is compromised during their prolonged activation, while their immunological activation continues simultaneously. This subsequently results in sustained pro-inflammatory cytokine signaling along with progressive accumulation of Aβ in the surrounding environment. The sustained release of cytokines and associated neurotoxins from microglia serves to exacerbate neuroinflammation and neurodegeneration [40, 41, 44, 47]. This creates a vicious cycle of activated immune cells recruitment, activation of pro-inflammatory signaling, increased oxidative stress, and neuronal damage. Thus, microglial cells are thought to be primarily responsible for orchestrating neuroinflammation and neurodegeneration in AD. Recent literature reports indicate that as microglia become less able to clear Aβ, peripheral macrophages may be recruited for Aβ plaque deposition in an effort to clear Aβ. The recruitment and activation of the peripheral macrophages contribute to intensify cytokine milieu in the brain [9, 43, 48, 49].
Aβ aggregates can also mimic damage-associated molecular patterns (DAMPs) and stimulate toll-like receptors (TLR2 and TLR4) on microglia, thereby mounting an inflammatory response by the NLRP3 (NACHT, LRR and PYD domains-containing protein 3) inflammasome complex [50, 51]. Consequently, caspase-1 is recruited to the inflammasome, and a number of pro-inflammatory mediators, such as TNFα and IL1β, are released by microglia [14, 28, 43, 52]. Repeated peripheral administration of lipopolysaccharides in wild-type mice has shown to increase Aβ levels, activate microglia, and induce cognitive impairment [53]. Studies within PS1/APP TLR4 knockout mice confirm an increase in plaque pathology, resulting in reduced glial activation and improved cognitive function [54]. Therapeutic blockade of TLR4 signaling by intracerebroventricular injections of cyanobacterial product was able to reduce memory impairment and glial cell activation induced by intra-cerebroventricular administration of Aβ oligomers [27, 53, 54]. TLR4 pathway is associated with the activation of p38MAPK pathway, which primarily regulates the release of pro-inflammatory cytokines, such as TNFα, IL1β, and IL6. Thus, it would be quite interesting to check the role of p38MAPK (mitogen-activtaed protein kinase) and MK2 (mitogen-activtaed protein kinase-activated protein kinase-2) in neuroinflammation in AD.
In addition, the ATP-gated ion channel P2X7 is expressed abundantly in microglia and responds to extracellular ATP during stress or tissue damage. This can lead to activation of the NLRP3 inflammasome complex, producing pro-inflammatory cytokines, IL1β and IL18. Thus, the P2X7–NLRP3 axis has recently been documented for its potential role in neuroinflammation and neurodegeneration in AD [51, 55, 56]. Based on this concept, the brain-penetrant, P2X7 antagonists, have progressed to phase I clinical studies (Table 2) for neuroinflammatory and neurodegenerative diseases [57-59]. P2X7 up-regulation has been reported in post-mortem AD brains and in animal models of tauopathy and amyloid deposition [55]. It has also been reported that P2X7 activation prevents APP processing by α-secretase and facilitates the formation of toxic Aβ through P2X7-mediated activation of glycogen synthase kinase 3 (GSK-3) in J20 mice model of AD (over-expressing human APP gene with AD-linked mutations). Inhibition of P2X7 with Brilliant Blue G in the same mouse model increased α-secretase activity and reduced the formation of Aβ plaques, supporting a beneficial role of P2X7 antagonism in AD. Recently, it has also been reported that mice lacking P2X7 have reduced Aβ load and cognitive impairment in the AD model. Moreover, MCC950 (NLRP3 inhibitor) has also been reported to prevent cognitive decline in APP/PS1 expressing mice model [42, 50, 56].
Table 2.
Potential list of repositioned drugs under development for Alzheimer’s disease.
| Drug Name | Current Stage | Mechanism of Action in AD | Refs. |
|---|---|---|---|
| leucine methylthioninium | Phase III | Inhibition of tau-hyperphosphorylation | [16, 64, 86, 87] |
| Pioglitazone | Phase III | Regulation of β-secretase enzyme, BACE-1 modulator | 16, 64, 86, 87] |
| Thalidomide | Phase III | Regulation of β-secretase enzyme, BACE-1 modulator | [16, 103] |
| Etazolate | Phase III | α-secretase stimulation, GABAA-receptor modulator | [10, 16, 103] |
| Montelukast | Phase II | Dual antagonist of CysLT1 receptor and GPR17 receptors in brain | [89, 90, 94] |
| Rilapladib | Phase II | cPLA2 inhibitor, Inhibits LOX and COX pathway | [13, 67] |
| ABT-126 | Phase II | α7-nicotinic receptor agonist for improvement of cognitive dementia | [13, 67] |
| Taldalafil | Phase II | PDE-5 inhibition leading to increased cGMP & inhibition of Aβ deposits | [16, 102] |
| Acetritin | Phase II | Acts on retinoid receptors (RXR and RAR) | [16] |
| Bexarotene | Phase II | Activates RXR receptors | [16] |
| Riluzole | Phase II | Inhibition of glutamate release Inactivation of voltage dependent sodium channels |
[16] |
| Nivadipine | Phase II | Reduction of Aβ oligomerization and deposits, neuronal toxicity | [88, 105] |
| Minocycline | Phase II | Regulation of β-secretase | [16, 88, 105] |
| Saracatinib | Phase I | Inhibition of Src/Abl family of kinases and Fyn kinase, Inhibition of Aβ formation. |
[16, 86-88, 105] |
|
JNJ-55308942 JNJ-54175446 |
Phase I | Blocked ex-vivo human IL-1β, Brain penetrant | [16, 86-88, 105] |
| Zileuton | Preclinical | 5-LOX inhibitor | [16, 88, 105] |
| Azithromycin, Erythromycin | Preclinical | Inhibition of APP and reduced cerebral Aβ deposits | [16, 88, 105] |
| Carmustine | Preclinical | Reduction of Aβ deposits and inhibition of tau pathology | [16, 88, 105] |
| Dapsone | Preclinical | Improvement of cognitive functions and dementia | [16, 88, 105] |
3.5. Role of Oxidative Stress Mediators
Brain neurons are particularly at a high risk of excessive reactive oxygen species (ROS) generation and oxidative damage. This is because brain functions utilize high oxygen (20%) and high glucose (25%) for energy production. Although oxidative damage is certainly found to play an important role in AD pathology, it remains uncertain whether this is a cause or a consequence of pathogenic processes in AD [38, 60]. One of the hypotheses suggesting oxidative damage as an early event in the disease is supported by the association of reduced ApoE4 genotype with higher oxidative insults. It has been shown that ApoE protein expression is reduced in ApoE4 carriers, and there is an association between decreased amount of ApoE protein and higher oxidative stress to lipids. The levels of thiobarbituric acid reactive substance (TBARS), isoprostanes, markers of lipid peroxidation have been reported to be increased in cerebrospinal fluid in ApoE4-positive healthy individuals and in AD patients [28, 60, 61].
Certain metals are crucial to many physiological processes in the brain and their homeostasis is controlled by various metal importers, exporters, and metal sequestering proteins. However, the dyshomeostasis of metal ions and their neurotoxic effects in brain during AD pathology have been documented in literature. The imbalance of metal ions is generally associated with reduced enzymatic activities, elevated protein aggregation, and oxidative stress in the brain, leading to cell death and neurodegeneration. The metal ions such as iron, copper, and zinc are associated with an increase in Aβ40 and Aβ42 aggregation in vitro and generation of free radicals in vivo, thus contributing to neuronal toxicity [62, 63]. The Fenton reaction between reduced forms of metals (iron (II) or copper (II)) and hydrogen peroxide is particularly harmful, as it yields the hydroxyl radical. RNA-bound iron poses a significant threat to neuronal viability in vulnerable neurons in AD due to a great reduction in normal protein synthesis. Copper exerts similar oxidative disturbances in neuronal tissue because it interacts with a regulatory protein, ceruloplasmin, which converts redox-active iron (II) to a less reactive iron (III). Copper also interacts with APP in an electron transfer reaction that reduces copper (II) to copper (I), enhancing the production of a hydroxyl radical intermediate [64]. It has been found that APP contains an iron-responsive element on the 5′ untranslated region of its mRNA with sequence homology to the iron-responsive element of the iron-storage ferritin protein. Studies on primary neuronal cultures indicate a ferroxidase activity of APP, which facilitates iron export from cells to minimize ROS production. APP has also been reported to prevent the release of iron (II) from heme, thereby further reducing the toxic accumulation of redox-active iron. Zinc (II) seems to simultaneously inhibit the ferroxidase activity of APP, and further contribute to an aberrant iron accumulation and ROS-generation [65, 66]. Such destructive effects of metal dyshomeostasis in brain suggest an important role in oxidative damage in AD. Based on these concepts, desferrioxamine (an FDA-approved drug for iron overload disease) has been tested in AD clinical trials, as it is involved in chelation and clearance of iron (III) from the brain. However, its brain bioavailability is very low due to hydrophilic nature and high molecular weight [66].
Several studies have demonstrated that there is an overlap between pathophysiological mechanisms implicated in type 2 diabetes and AD. Hyper-insulinemia, in type 2 diabetes may inhibit the degradation of extracellular Aβ through the insulin-degrading enzyme (IDE) [4, 15, 28]. Moreover, hyperglycemia may result in enhanced metabolism of glucose leading to increased levels of NADH and FADH2, which are used by mitochondria to generate ATP. The overproduction of NADH leads to a greater proton gradient and consequently, to an excessive generation of ROS. In addition, the increased levels of intracellular glucose lead to the non-enzymatic glycosylation of cellular proteins, lipoproteins, or nucleic acids. The resulting products are known as advanced glycation end products (AGEs) [43, 44, 61]. Receptors for AGE (RAGE) are pattern recognition receptors that bind multiple ligands derived from damaged cell environment as well as Aβ. The interaction of RAGE with its ligands results in enhanced ROS generation, due to the activation of NADPH oxidase (NOX). Increased expression of RAGE has been demonstrated in the brains of AD mouse models. Administration of high-AGE diet has been found to lead to spatial memory impairments, oxidative damage to the vasculature, and increased levels of insoluble Aβ in mouse hippocampus [4, 28, 40, 61].
Exposure of neurons to oligomeric Aβ leads to enhanced ROS production, probably through a mechanism involving stimulation of NMDA receptors and consequent activation of NOX. ROS derived from NOX may lead to the activation of kinases, including ERK1/2, downstream activation of calcium-dependent phospholipase A2 (cPLA2), release of arachidonic acid, and perturbations in the membrane phospholipids [67, 68].
3.6. Role of Structural and Functional Integrity of Blood-brain Barrier
Pathological injury or damage of BBB is a relatively new frontier area in AD research. Normal BBB is a highly specialized monolayer of endothelial cells lining the blood vessels in the brain. This monolayer of endothelial cells separates the circulating blood from the brain parenchyma. Aβ binds to low-density lipoprotein receptor-related protein-1 (LPR1) on the endothelial cells of the brain capillaries and is then released into the bloodstream. Dysfunction in BBB endothelial monolayer may lead to decreased Aβ clearance from the brain parenchyma [14, 43, 69, 70]. The integrity of the BBB depends critically on the functional state of the associated astrocytes and microglia and is compromised during neuroinflammation. Moreover, the interaction between astrocytes and endothelial cells is important to limit the influx of peripheral immune cells after brain injury. The astrocytes react to inflammatory stimuli leading to release of pro-inflammatory mediators such as astrocytic NF-κB, TNFα, IL6, and IL1β. These pro-inflammatory cytokines may be responsible for the increased permeability of the blood-brain barrier (BBB), initially in order to recruit immune cells [47, 58]. It has been shown in vitro that TNFα may increase the permeability of endothelial monolayer cultures via actin remodeling. Moreover, transgenic over-expression of IL1β in mice was found to increase BBB permeability [35, 41, 59]. These inflammatory changes lead to an increased expression of chemokines and chemokine receptors (CXCL2, CXCR2, CCL2, CXCR4), which further recruit neutrophils. Thus, it causes a significant influx of peripheral immune cells into the brain, leading to edema and neuronal damage in AD [28, 60, 61]. Thus, changes in the structural and functional integrity of BBB may lead to increased Aβ deposits in the brain [19, 69, 71].
4. Recent approaches to control Alzheimer’s disease
4.1. Drugs Targeting Amyloid-beta: Secretase Inhibitors
Currently, the amyloid hypothesis has been the most explored mechanism, based on the hallmark feature of extracellular Aβ plaques accumulation and aggregation. The two main forms of toxic Aβ fragments are produced by proteolytic cleavage of APP695 by β-secretase and γ-secretase: (i) Aβ1-40 (ii) Aβ1-42. These fragments have a greater tendency to form extracellular senile plaques, which further leads to neuronal toxicity and death [18, 58, 72]. In addition, it has also been evident that there is an imbalance between the production and clearance of these toxic peptide fragments [17, 25, 40, 73]. In spite of several extensive efforts to decipher the molecular processes of the amyloid pathway, it is not clearly understood. Despite continuous efforts, none of the drugs aimed to target this pathway has been approved.
One of the most extensively explored approaches to control the amyloid processing of APP protein is to modulate the activity of β-secretase (BACE-1) and γ-secretase enzymes [25]. These secretase inhibitors can block the enzymes that cleave APP to form toxic form of Aβ peptide and formation of senile plaques [45, 74, 75]. Typical inhibitors bind to the catalytic domains of these proteases and block their proteolytic activities [25, 76, 77]. However, BACE-1 and γ-secretase are versatile proteases regulating many substrates in the brain and CNS. Therefore, the modulation of activity of these proteases may have a risk of interference with different critical signaling processes. Ghosh et al., found that some bioisosteres of isophthalamide had good cell-free and cell-based BACE-1 inhibitory activity. These compounds (GRL-7234 and GRL-8234) have shown a considerable reduction in the production of toxic Aβ peptides [78]. Currently, small molecule BACE inhibitors, such as LY2811376, LY2886721, and E2609, are in different phases of clinical trials [25, 77, 78]. LY2811376 was reported to be one of the first oral, non-peptide BACE-1 inhibitor in clinical trials. However, it displayed a significant toxicity of the retinal epithelial cells in animals, thus suggesting a need for safety assessment of this type of drugs. Recently a γ-secretase modulator, semagacestat, was tested to specifically target the γ-site cleavage of APP and reduce Aβ42 levels, and it reached up to phase III clinical trials. However, it did not show an adequate benefit in controlling the progression of AD as compared to the placebo group. Furthermore, its administration increased the risk of skin cancer [76] in the treated population.
Another enzyme, α-secretase, participates in so-called non-amyloid decomposition of APP, resulting in a so-called soluble form of APP with neuroprotective and memory enhancing effects. Stimulation of this enzyme by etazolate (originally developed as a selective GABAA receptor modulator) is currently being evaluated in phase III clinical trials [10].
Among the natural compounds used for inhibition of BACE-1, there are some compounds used in traditional chinese medicine, such as 2,2',4'-trihydroxichalcone (TDC) and ginsenoside Rg1, and other synthetic compounds such as OM99-2, hydroxyethylamine isosteres (HEA), isophthalamide, and des(dimethylamino) compound [79]. One of the natural drugs, Rg1 ginsenoside (obtained from Panax notoinseng) has been extensively used over the years in traditional medicine for improving the memory function. It has shown about 80% inhibitory activity against BACE-1, revealing its potential protection against the degenerative effects of Aβ. TDC is a family of flavonoid chalcones extracted from Glycyrrhiza glabra, and is used traditionally as an emollient in stomach disorders and respiratory problems. Recently, this drug is under evaluation as a potential candidate for BACE-1 inhibition in AD.
These reports suggest promising alternative therapeutic strategies for specifically modulating APP processing either by inhibition of amyloid pathway (BACE-1 or γ-secretase) or, by stimulation of non-amyloid processing (α-secretase).
4.2. Inhibition of Tau-protein Hyperphosphorylation
A possible intervention for the therapy aimed at tau protein can be in the form of inhibition of hyperphosphorylation or desegregation of the filaments of this hyperphosphorylated protein [32]. Clinically, lithium (in form of carbonate salt) and valproic acid were tested to inhibit the degree of tau-hyperphosphorylation, and both of them act by inhibition of glycogen synthase kinase-3β (GSK-3β) enzyme. The development of lithium was stopped in phase II clinical trials due to contradictory results. Valproic acid reached phase III clinical trials, however, it did not show any improvement of cognitive parameters as compared to control. In addition, methylene blue has also been tested and has shown to dissolve the filaments of hyperphosphorylated tau protein in-vitro. This drug reached phase II trials due to its clinical relevance, but has not shown the desirable criteria for the improvement of cognitive functions. Another derivative of methylene blue, leucine methylthioninium is currently in phase III clinical trials in AD [5, 10, 12].
4.3. Drugs Targeting Neuroinflammation and Neurotoxicity in AD
PLA2 inhibitors are one of the emerging novel therapeutic targets for the treatment of inflammatory neurodegenerative diseases such as AD. Under normal conditions, PLA2 is involved in the generation of lipid mediators that are closely associated with phospholipids turnover, neurotransmitter release, long-term potentiation, memory processes, membrane repair, ion channel function, and gene transcription processes. However, there is an increased degradation of phospholipids due to the activation of PLA2. This leads to changes in membrane permeability and stimulation of enzymes involved in lipolysis, resulting in disruption of membrane structure during certain pathological conditions [67, 68]. The effects are closely associated with the activation of microglia and astrocytes, which release inflammatory cytokines (TNFα, IL1β, and IL6). These inflammatory mediators propagate and intensify neuroinflammation by a number of mechanisms [80-83]. These mechanisms include further up- regulation of PLA2, activation of inflammatory mediators, and increased oxidative stress resulting in various pathological changes in brain [41, 47, 84]. PLA2 inhibition has also been shown to decrease the levels of tau protein phosphorylation and Aβ deposition in the rat’s brain [67, 68]. An important mechanism through which both, Aβ and tau, could be affected via p25/Cdk5 pathway. The latter produces hyperphosphorylated tau and neurofibrillary tangles as well as aberrant APP processing, and recent evidence indicates that increased cPLA2 is essential for triggering p25-mediated inflammatory and neurodegenerative processes [40, 59, 68, 85]. Based on such mechanistic data, rilapladib is one of the potent PLA2 inhibitors currently being tested in phase II clinical trials of AD [13, 67].
4.4. Drug Repurposing Approaches in Alzheimer’s Disease
Drug repurposing or drug repositioning is an innovative drug development approach predicated on the reuse of existing approved drugs for new medical indications. Due to high failure rates and higher costs involved in traditional drug discovery and development process, many pharmaceutical companies are primarily focused on drug repurposing/repositioning strategy [16, 86, 87]. Several recent studies have been carried out for repurposing of drugs for possible treatment in AD. The main purpose of such efforts is to find newer treatments that can affect different molecular markers and control the pathological disease mechanism in AD. Drug repurposing approach can be particularly helpful to develop a new drug much faster and at a cheaper cost as compared to traditional drug discovery process [11, 88]. Several drugs are under pre-clinical and clinical development by using such repurposing approach (listed in Table 2).
Cysteinyl leukotrienes (CysLTs) have been documented in literature reports as a major inflammatory lipid mediator in AD. The CNS is capable of producing CysLTs, particularly by transcellular biosynthesis in neurons and glial cells. Such transcellular biosynthesis of CysLT affects the functioning of CNS neuronal cells, via corresponding CysLT receptors. In human brain, CysLT1 receptors have a lower expression level than CysLT2 receptor. The expression of both CysLT1 and CysLT2 receptors has been found to be increased in astrocyte, microglial cells, and neuronal and glial-appearing cells during chronic brain inflammation and aging in AD. One of the marketed CysLT1 receptor antagonists for asthma, montelukast, is currently in phase II clinical trials for AD [89, 90]. In preclinical studies, montelukast has shown elevation of hippocampal neurogenesis, reduction of neuroinflammation, and improvement of learning and memory in a model of brain aging and mild cognitive impairment [91-93]. However, this drug has a limitation of low bioavailability. Hence, a Phase I clinical study (ClinicalTrials.gov: NCT03402503) has been carried out to evaluate pharmacology of montelukast by using an oral film formulation for improving its bioavailability. It was found to be safe and tolerable in healthy subjects, reduced the first-pass-effect, and had a 52% higher bioavailability in cerebrospinal fluid compared to existing formulation [89, 90, 94]. Such formulations of the CysLT1 receptor antagonist, montelukast might be a novel effective repurposed drug for the treatment of AD. In addition, recent studies have suggested the beneficial effects of zileuton (5-LOX inhibitor used for asthma) in AD [33, 95]. It has been reported that the expression of 5-LOX is increased in the brain of AD patients and this hypothesis makes it a promising target for AD. 5-LOX pathway has a major role in CysLT production and CysLT receptor activation leading to the activation of amyloid pathway [77, 96-101]. Preclinical studies using zileuton have clearly shown an attenuation of amyloid deposition in the brain tissues of mice model [33].
Apart from the repurposing of drugs affecting CysLT pathways, including zileuton and CysLT1 receptor antagonists, some other important classes of drugs are also currently being explored for AD treatment. The role of phosphodiesterase-5 (PDE-5) enzyme in the regulation of cyclic guanosine monophosphate (cGMP) has also been studied and linked with AD. An increase in cGMP levels activates the intracellular protein kinase in the brain and phosphorylates several proteins leading to a detrimental role in neuroplasticity, tau hyperphosphorylation, and Aβ accumulation. Recently, sildenafil and tadalafil (PDE-5 inhibitor used for erectile dysfunction) have been studied in pre-clinical and clinical models of AD. Sildenafil has shown to be successful in inhibition of neuroinflammation and Aβ deposition in aged mice models [102]. Tadalafil displayed a better efficacy and exhibited distinct neuroprotection and memory improvement than sildenafil, as it could inhibit PDE-5 enzyme more effectively due to high blood-brain barrier penetration [16, 102].
Recently, the repurposing of anticancer drugs for AD treatment was also found to be interesting due to the fact that both cancer and AD share some of the common signaling pathways, including mitochondrial dysfunction, increased oxidative stress, and development of misfolded proteins and compromised cell metabolism. Interestingly, it has been observed that there is a lower risk of AD development in cancer patients of advanced ages, who were treated with chemotherapy, as compared to control group [16, 103, 104]. Imatinib (an approved tyrosine kinase inhibitor for chronic leukemia) has been suggested to be useful for the therapy of AD, because it has shown neuroprotection due to the reduction of Aβ deposition. However, imatinib does not cross the blood-brain barrier effectively and is also readily effluxed by P-glycoprotein. The hyperphosphorylation of tau proteins reduces its ability to bind to microtubules, ultimately leading to the formation of NFTs. Paclitaxel (an approved drug for ovarian, breast, and non-small cell lung cancer) has been shown to reduce such hyper phosphorylation and has shown efficacy in the reduction of brain tauopathies in preclinical models. Thalidomide is another anticancer drug being tested in phase III clinical trials, with established potential in AD pathology and has demonstrated BACE-1 modulation, inhibition of endothelial cell proliferation, angiogenesis, and breakdown of the blood-brain barrier. It has also shown a reduction in hippocampal neuronal loss through inhibition of TNFα. In addition, carmustine (a small, lipophilic, and non-ionized nitrosourea molecule used as an alkylating agent in brain cancer) showed a strong reduction in Aβ production and improved tau pathologies in preclinical models, at a non-toxic dose [16, 103, 104].
Other important classes of drugs that are currently being investigated in AD are peroxisome-proliferated activated receptor γ (PPARγ) agonists (pioglitazone), dihydropyridine calcium channel blockers (nivadipine) and angiotensin II receptor blocker (valsartan). The first two classes of drugs are currently in clinical trials of AD, whereas the latter is under preclinical testing [70, 88, 105]. It is now clearly evident that the inflammatory processes play an important role in the progressive loss of memory and dementia in AD. Studies have shown that PPARγ receptors agonists could reduce inflammatory responses in Aβ-induced microglial cells in the brain during AD [9, 40, 48, 49, 83]. Based on this mechanistic fact, pioglitazone has shown favorable clinical efficacy and safety profiles in diabetic patients and can also have a great potential in reducing the progression of AD. The dihydropyridine calcium channel blocker, such as nilvadipine has shown to reduce the production, oligomerization, and accumulation of Aβ in vitro. It also improves cell survival and reduces neurotoxicity. This drug has a good blood-brain barrier penetration and can also increase brain blood flow through its vasodilatory property. This drug is currently being investigated in phase II clinical trial for AD. It has been suggested that there is an elevation of brain angiotensin II levels during chronic adverse environmental stress in AD, which acts further at AT1 and AT2 receptor subtypes. Such increasein brain angiotensin II levels has been linked to amyloidogenesis and memory impairment. Valsartan has shown a reduction in amyloid deposition, improved memory and cognition. It has also displayed a significant inhibition of inflammation, vasoconstriction, and mitochondrial dysfunction, in addition to activation of acetylcholine release by in-vitro and in-vivo studies. However, further extensive studies are needed to establish its clinical potential in AD [11, 15, 88].
Antimicrobials have also recently been explored in various preclinical and clinical studies as one of the potential classes of drugs for possible treatment of AD [34, 106]. Macrolides, such as azithromycin and erythromycin, have shown inhibition of APP resulting in reduced cerebral levels of Aβ in preclinical models. Doxycycline has shown potential in reducing Aβ deposition along with an increase in the disassembly of preformed fibrils. In addition, the combination of doxycycline with rifampicin has shown beneficial effects in the Aβ reduction, probably due to decreased production and increased clearance of Aβ. Dapsone is an antibiotic used to treat leprosy. This drug received attention in the 1990s, when a decreased incidence of dementia was noticed in leprosy patients treated with dapsone. Currently, dapsone is under preclinical evaluation in AD models to establish its potential against Aβ deposition. Such protection by antimicrobials in AD has further been confirmed by data from the clinical studies on leprosy and tuberculosis patients, showing similar instances of AD prevalence [86, 87].
In addition, several potential copper and iron-chelating drugs may be repurposed for the treatment of neurodegenerative diseases, provided there are sufficient evidences about their mechanism of action, pre-clinical and clinical efficacy [64]. The available literature evidences clearly point towards the drug repurposing/repositioning approach as an interesting strategy for the development of drugs for AD, in addition to their approved therapeutic use. Moreover, it also seems to be an attractive alternative, due to already established safety and tolerability profile of such drugs rendering to require smaller sample sizes for clinical studies, and hence reducing the overall cost and time of the clinical development of the repurposed drugs.
Conclusion and future perspectives
The molecular pathophysiology of AD is quite complex and multifaceted. The currently available therapies only provide a symptomatic relief, merely delay disease progression, but do not modify the disease mechanism. Due to this, several researchers across the world are continuously looking for the development of new mechanistic treatment of AD. In a recent interesting study by Cummings et al., it has been reported that there are a total of 132 agents (includes small molecules and biological therapies) being tested in different phases of drug developments for AD [107]. Many of these molecules target amyloid as one of the key and common mechanisms of action. However, most of them are still either in pre-clinical phase or provide only a marginal clinical benefit as compared to approved therapies. Another rigorous and continuous approach in developing anti-AD drugs is by either inhibiting the enzymes involved in APP processing (modulation of γ-secretase or BACE-1) or, by inhibition of tau-hyper-phosphorylation. Unfortunately, most of these molecules have failed in clinical trials because of the severe side effects associated with them. Furthermore, there is also a major concern in the treatment of the diverse disease population from moderate to advanced stages of AD. Even some of the available therapies do not provide, even a symptomatic relief, in moderate to advanced stages of the disease. The lack of an effective therapy for these populations also represents a significant area of drug discovery and development opportunity for researchers. Indeed, there is an urgent need to develop more effective treatments for moderate to advanced stages of this disease. Another major crucial, unmet need for AD drug development is to identify, validate, and include rationalized efficient clinical biomarkers as end-points. Such specific biomarkers would provide a basis for the measurement of disease severity in order to set a clinical end-point and help predict the results of clinical trial, clearly.
On the other hand, there is a continuous effort being made towards drug repurposing/repositioning approach for AD in order to modify the molecular pathophysiology of the disease. The pharmaceutical industries have diverted their focus from the new drug discovery and development programs to drug repurposing approaches, mainly due to high attrition rates and lack of funds. This approach is much easier and faster as compared to the traditional drug discovery process due to the current advancement of technological and computational screening methods. Such emerging concepts on drug repurposing owe huge expectations to controlling molecular hallmarks of AD.
A constant progress is being made in terms of defining new targets for the treatment of AD, developing new agents, introducing innovative clinical trial designs, incorporating a broader range of populations in clinical trials, and developing new biomarkers that provide insight into the impact of emerging therapies. Such strategies will surely be helpful to accelerate effective drug development pipeline for AD therapy.
Acknowledgements
Declared none.
list of Abbreviations
- 5-LOX
5-lipoxygenase
- AchE/AchEI
Acetylcholinesterase/Acetylcholinesterase inhibitors
- AD
Alzheimer’s disease
- AGE
Advanced glycation end products
- ApoE and ApoJ
Apolipoproteins E and J
- APP
Amyloid precursor protein
- AT1
Angiotensin 1 receptor
- AT2
Angitensin 2 receptor
- ATP
Adenosine triphosphate
- Aβ
Amyloid beta
- BACE-1
β-secretase
- BBB
Blood brain barrier
- BuChE
Butyryl cholinesterase
- cGMP
Cyclic guanosine monophosphate
- ChAT
Choline acetyl transferase
- CNS
Central nervous system
- cPLA2
Cyclic phospholipase A2
- CysLTs
Cysteinyl leukotrienes
- DAMP
Damage-associated molecular patterns
- ERK1/2
Extracellular signal-regulated kinase
- FADH2
Reduced flavin adenine dinucleotide
- GABAA receptor
Gamma-amino butyric acid A receptor
- GSK-3β
Glycogen synthase kinase-3 beta
- IL1β
Interleukin 1β
- IL6
Interleukin 6
- MAPK
Mitogen-activated protein kinase
- MK2
Mitogen-activated protein kinase-activated protein kinase 2
- NADH
Nicotinamide adenine dinucleotide
- NFTs
Neurofibrillary tangles
- NFκB
Nuclear factor κB
- NLRP3
NACHT, LRR and PYD domains-containing protein 3
- NMDA
N-methyl-D-aspartic acid
- NOX
NADPH-oxidase
- PAMP
Pathogen-associated molecular patterns
- PDE-5
Phosphodiesterase-5
- PDH
Pyruvate dehydrogenase
- PPARγ
Peroxisome-proliferated-activated receptors-γ
- PRR
Pattern recognition receptors
- RAGE
Receptors for advanced glycation end products
- ROS
Reactive oxygen species
- TBARS
Thio-barbituric acid reactive substance
- TDC
2,2’,4’-trihydroxy chalcone
- TLR
Toll-like receptors
- TNFα
Tumor necrosis factor α
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Sala Frigerio C., De Strooper B. Alzheimer’s disease mechanisms and emerging roads to novel therapeutics. Annu. Rev. Neurosci. 2016;39:57–79. doi: 10.1146/annurev-neuro-070815-014015. [DOI] [PubMed] [Google Scholar]
- 2.Bondi M.W., Edmonds E.C., Salmon D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017;23(9-10):818–831. doi: 10.1017/S135561771700100X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kozlov S., Afonin A., Evsyukov I., Bondarenko A. Alzheimer’s disease: as it was in the beginning. Rev. Neurosci. 2017;28(8):825–843. doi: 10.1515/revneuro-2017-0006. [DOI] [PubMed] [Google Scholar]
- 4.Dos Santos Picanco L.C., Ozela P.F., de Fatima de Brito Brito M., Pinheiro A.A., Padilha E.C., Braga F.S., de Paula da Silva C.H.T., Dos Santos C.B.R., Rosa J.M.C., da Silva Hage-Melim L.I. Alzheimer’s Disease: A review from the pathophysiology to diagnosis, new perspectives for pharmacological treatment. Curr. Med. Chem. 2018;25(26):3141–3159. doi: 10.2174/0929867323666161213101126. [DOI] [PubMed] [Google Scholar]
- 5.Mufson E.J., Ikonomovic M.D., Counts S.E., Perez S.E., Malek-Ahmadi M., Scheff S.W., Ginsberg S.D. Molecular and cellular pathophysiology of preclinical Alzheimer’s disease. Behav. Brain Res. 2016;311:54–69. doi: 10.1016/j.bbr.2016.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanabria-Castro A., Alvarado-Echeverría I., Monge-Bonilla C. Molecular pathogenesis of Alzheimer’s Disease: An update. Ann. Neurosci. 2017;24(1):46–54. doi: 10.1159/000464422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J., Gu B.J., Masters C.L., Wang Y-J. A systemic view of Alzheimer disease - insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol. 2017;13(10):612–623. doi: 10.1038/nrneurol.2017.111. [DOI] [PubMed] [Google Scholar]
- 8.Cummings J., Aisen P.S., DuBois B., Frölich L., Jack C.R., Jr, Jones R.W., Morris J.C., Raskin J., Dowsett S.A., Scheltens P. Drug development in Alzheimer’s disease: the path to 2025. Alzheimers Res. Ther. 2016;8:39. doi: 10.1186/s13195-016-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Eldik L.J., Carrillo M.C., Cole P.E., Feuerbach D., Greenberg B.D., Hendrix J.A., Kennedy M., Kozauer N., Margolin R.A., Molinuevo J.L., Mueller R., Ransohoff R.M., Wilcock D.M., Bain L., Bales K. The roles of inflammation and immune mechanisms in Alzheimer’s disease. Alzheimers Dement. (N. Y.) 2016;2(2):99–109. doi: 10.1016/j.trci.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuca K., Soukup O., Maresova P., Korabecny J., Nepovimova E., Klimova B., Honegr J., Ramalhob T.C., França T.C.C. Current Approaches Against Alzheimer’s Disease in Clinical Trials. J. Braz. Chem. Soc. 2016;27(4):641–649. [Google Scholar]
- 11.Corbett A., Pickett J., Burns A., Corcoran J., Dunnett S.B., Edison P., Hagan J.J., Holmes C., Jones E., Katona C., Kearns I., Kehoe P., Mudher A., Passmore A., Shepherd N., Walsh F., Ballard C. Drug repositioning for Alzheimer’s disease. Nat. Rev. Drug Discov. 2012;11(11):833–846. doi: 10.1038/nrd3869. [DOI] [PubMed] [Google Scholar]
- 12.Cummings J., Lee G., Ritter A., Zhong K. Alzheimer’s disease drug development pipeline. Alzheimers Dement. (N. Y.) 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar A., Singh A. Ekavali, A review on Alzheimer’s disease pathophysiology and its management: an update. Pharmacol. Rep. 2015;67(2):195–203. doi: 10.1016/j.pharep.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Vickers J.C., Mitew S., Woodhouse A., Fernandez-Martos C.M., Kirkcaldie M.T., Canty A.J., McCormack G.H., King A.E. Defining the earliest pathological changes of Alzheimer’s disease. Curr. Alzheimer Res. 2016;13(3):281–287. doi: 10.2174/1567205013666151218150322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vargas D.M., De Bastiani M.A., Zimmer E.R., Klamt F. Alzheimer’s disease master regulators analysis: search for potential molecular targets and drug repositioning candidates. Alzheimers Res. Ther. 2018;10(1):59–70. doi: 10.1186/s13195-018-0394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durães F., Pinto M., Sousa E. Old drugs as new treatments for neurodegenerative diseases. Pharmaceuticals (Basel) 2018;11(2):44. doi: 10.3390/ph11020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunan J., Small D.H. Regulation of APP cleavage by alpha-, beta- and gamma-secretases. FEBS Lett. 2000;483(1):6–10. doi: 10.1016/S0014-5793(00)02076-7. [DOI] [PubMed] [Google Scholar]
- 18.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 19.Chai Q., He W.Q., Zhou M., Lu H., Fu Z.F. Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J. Virol. 2014;88(9):4698–4710. doi: 10.1128/JVI.03149-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wierenga C. E., Hays C. C., Zlatar Z. Z. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer's disease. 2014. [DOI] [PMC free article] [PubMed]
- 21.Hays C.C., Zlatar Z.Z., Wierenga C.E. The utility of cerebral blood flow as a biomarker of preclinical Alzheimer’s disease. Cell. Mol. Neurobiol. 2016;36(2):167–179. doi: 10.1007/s10571-015-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiernan C.T., Mufson E.J., Kanaan N.M., Counts S.E. Tau Oligomer pathology in nucleus basalis neurons during the progression of Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2018;77(3):246–259. doi: 10.1093/jnen/nlx120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahman S.O., Singh R.K., Hussain S., Akhtar M., Najmi A.K. A novel therapeutic potential of cysteinyl leukotrienes and their receptors modulation in the neurological complications associated with Alzheimer’s disease. Eur. J. Pharmacol. 2019;842:208–220. doi: 10.1016/j.ejphar.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 24.Miners J.S., Baig S., Palmer J., Palmer L.E., Kehoe P.G., Love S. Abeta-degrading enzymes in Alzheimer’s disease. Brain Pathol. 2008;18(2):240–252. doi: 10.1111/j.1750-3639.2008.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui J., Wang X., Li X., Wang X., Zhang C., Li W., Zhang Y., Gu H., Xie X., Nan F., Zhao J., Pei G. Targeting the γ-/β-secretase interaction reduces β-amyloid generation and ameliorates Alzheimer’s disease-related pathogenesis. Cell Discov. 2015;1(1):15021. doi: 10.1038/celldisc.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalra J., Khan A. Reducing Aβ load and tau phosphorylation: Emerging perspective for treating Alzheimer’s disease. Eur. J. Pharmacol. 2015;764:571–581. doi: 10.1016/j.ejphar.2015.07.043. [DOI] [PubMed] [Google Scholar]
- 27.Balducci C., Frasca A., Zotti M., La Vitola P., Mhillaj E., Grigoli E., Iacobellis M., Grandi F., Messa M., Colombo L., Molteni M., Trabace L., Rossetti C., Salmona M., Forloni G. Toll-like receptor 4-dependent glial cell activation mediates the impairment in memory establishment induced by β-amyloid oligomers in an acute mouse model of Alzheimer’s disease. Brain Behav. Immun. 2017;60:188–197. doi: 10.1016/j.bbi.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Simunkova M., Alwasel S.H., Alhazza I.M., Jomova K., Kollar V., Rusko M., Valko M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019;93(9):2491–2513. doi: 10.1007/s00204-019-02538-y. [DOI] [PubMed] [Google Scholar]
- 29.Giuffrida M.L., Caraci F., Pignataro B., Cataldo S., De Bona P., Bruno V., Molinaro G., Pappalardo G., Messina A., Palmigiano A., Garozzo D., Nicoletti F., Rizzarelli E., Copani A. β-amyloid monomers are neuroprotective. J. Neurosci. 2009;29(34):10582–10587. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giuffrida M.L., Caraci F., De Bona P., Pappalardo G., Nicoletti F., Rizzarelli E., Copani A. The monomer state of beta-amyloid: where the Alzheimer’s disease protein meets physiology. Rev. Neurosci. 2010;21(2):83–93. doi: 10.1515/REVNEURO.2010.21.2.83. [DOI] [PubMed] [Google Scholar]
- 31.Giuffrida M.L., Tomasello M.F., Pandini G., Caraci F., Battaglia G., Busceti C., Di Pietro P., Pappalardo G., Attanasio F., Chiechio S., Bagnoli S., Nacmias B., Sorbi S., Vigneri R., Rizzarelli E., Nicoletti F., Copani A. Monomeric ß-amyloid interacts with type-1 insulin-like growth factor receptors to provide energy supply to neurons. Front. Cell. Neurosci. 2015;9:297. doi: 10.3389/fncel.2015.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunden K.R., Yao Y., Potuzak J.S., Ferrer N.I., Ballatore C., James M.J., Hogan A.M., Trojanowski J.Q., Smith A.B., III, Lee V.M. The characterization of microtubule-stabilizing drugs as possible therapeutic agents for Alzheimer’s disease and related tauopathies. Pharmacol. Res. 2011;63(4):341–351. doi: 10.1016/j.phrs.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Meco A., Lauretti E., Vagnozzi A.N., Praticò D. Zileuton restores memory impairments and reverses amyloid and tau pathology in aged mice. Neurobiol. Aging. 2014;35:2458–2464. doi: 10.1016/j.neurobiolaging.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H., Liu C-C., Zheng H., Huang T.Y. Amyloid, tau, pathogen infection and antimicrobial protection in Alzheimer’s disease -conformist, nonconformist, and realistic prospects for AD pathogenesis. Transl. Neurodegener. 2018;7(1):34. doi: 10.1186/s40035-018-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang X.Q., Zhang X.Y., Wang X.R., Yu S.Y., Fang S.H., Lu Y.B., Zhang W.P., Wei E.Q. Transforming growth factor β1-induced astrocyte migration is mediated in part by activating 5-lipoxygenase and cysteinyl leukotriene receptor 1. J. Neuroinflammation. 2012;9:145. doi: 10.1186/1742-2094-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ciaramella A., Salani F., Bizzoni F., Orfei M.D., Caltagirone C., Spalletta G., Bossù P. Myeloid dendritic cells are decreased in peripheral blood of Alzheimer’s disease patients in association with disease progression and severity of depressive symptoms. J. Neuroinflammation. 2016;13:18. doi: 10.1186/s12974-016-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teaktong T., Graham A.J., Court J.A., Perry R.H., Jaros E., Johnson M., Hall R., Perry E.K. Nicotinic acetylcholine receptor immunohistochemistry in Alzheimer’s disease and dementia with Lewy bodies: differential neuronal and astroglial pathology. J. Neurol. Sci. 2004;225(1-2):39–49. doi: 10.1016/j.jns.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Singh P., Sharma B. Reversal in cognition impairments, cholinergic dysfunction, and cerebral oxidative stress through the modulation of ryanodine receptors (RyRs) and Cysteinyl leukotriene-1 (CysLT1) receptors. Curr. Neurovasc. Res. 2016;13(1):10–21. doi: 10.2174/1567202612666151026105610. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka M., Shih P.Y., Gomi H., Yoshida T., Nakai J., Ando R., Furuichi T., Mikoshiba K., Semyanov A., Itohara S. Astrocytic Ca2+ signals are required for the functional integrity of tripartite synapses. Mol. Brain. 2013;6:6. doi: 10.1186/1756-6606-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai Z., Hussain M.D., Yan L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014;124(5):307–321. doi: 10.3109/00207454.2013.833510. [DOI] [PubMed] [Google Scholar]
- 41.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Münch A.E., Chung W.S., Peterson T.C., Wilton D.K., Frouin A., Napier B.A., Panicker N., Kumar M., Buckwalter M.S., Rowitch D.H., Dawson V.L., Dawson T.M., Stevens B., Barres B.A. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biber K., Bhattacharya A., Campbell B.M., Piro J.R., Rohe M., Staal R.G.W., Talanian R.V., Möller T. Microglial drug targets in ad: opportunities and challenges in drug discovery and development. Front. Pharmacol. 2019;10:840. doi: 10.3389/fphar.2019.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ardura-Fabregat A., Boddeke E.W.G.M., Boza-Serrano A., Brioschi S., Castro-Gomez S., Ceyzériat K., Dansokho C., Dierkes T., Gelders G., Heneka M.T., Hoeijmakers L., Hoffmann A., Iaccarino L., Jahnert S., Kuhbandner K., Landreth G., Lonnemann N., Löschmann P.A., McManus R.M., Paulus A., Reemst K., Sanchez-Caro J.M., Tiberi A., Van der Perren A., Vautheny A., Venegas C., Webers A., Weydt P., Wijasa T.S., Xiang X., Yang Y. Targeting neuroinflammation to treat Alzheimer’s Disease. CNS Drugs. 2017;31(12):1057–1082. doi: 10.1007/s40263-017-0483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wes P.D., Sayed F.A., Bard F., Gan L. Targeting microglia for the treatment of Alzheimer’s Disease. Glia. 2016;64(10):1710–1732. doi: 10.1002/glia.22988. [DOI] [PubMed] [Google Scholar]
- 45.McGeer P.L., McGeer E.G. Targeting microglia for the treatment of Alzheimer’s disease. Expert Opin. Ther. Targets. 2015;19(4):497–506. doi: 10.1517/14728222.2014.988707. [DOI] [PubMed] [Google Scholar]
- 46.Hamelin L., Lagarde J., Dorothée G., Leroy C., Labit M., Comley R.A., de Souza L.C., Corne H., Dauphinot L., Bertoux M., Dubois B., Gervais P., Colliot O., Potier M.C., Bottlaender M., Sarazin M. Clinical IMABio3 team. Early and protective microglial activation in Alzheimer’s disease: a prospective study using 18F-DPA-714 PET imaging. Brain. 2016;139(Pt 4):1252–1264. doi: 10.1093/brain/aww017. [DOI] [PubMed] [Google Scholar]
- 47.Bouvier D.S., Murai K.K. Synergistic actions of microglia and astrocytes in the progression of Alzheimer’s disease. J. Alzheimers Dis. 2015;45(4):1001–1014. doi: 10.3233/JAD-143156. [DOI] [PubMed] [Google Scholar]
- 48.Chen W.W., Zhang X., Huang W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016;13(4):3391–3396. doi: 10.3892/mmr.2016.4948. [Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang F., Jiang L. Neuroinflammation in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2015;11:243–256. doi: 10.2147/NDT.S75546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thawkar B.S., Kaur G. Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J. Neuroimmunol. 2019;326:62–74. doi: 10.1016/j.jneuroim.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 51.Bhattacharya A., Jones D.N.C. Emerging role of the P2X7-NLRP3-IL1β pathway in mood disorders. Psychoneuroendocrinology. 2018;98:95–100. doi: 10.1016/j.psyneuen.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 52.McGeer P.L., Guo J.P., Lee M., Kennedy K., McGeer E.G. Alzheimer’s Disease Can Be Spared by nonsteroidal anti-inflammatory drugs. J. Alzheimers Dis. 2018;62(3):1219–1222. doi: 10.3233/JAD-170706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rangasamy S.B., Jana M., Roy A., Corbett G.T., Kundu M., Chandra S., Mondal S., Dasarathi S., Mufson E.J., Mishra R.K., Luan C.H., Bennett D.A., Pahan K. Selective disruption of TLR2-MyD88 interaction inhibits inflammation and attenuates Alzheimer’s pathology. J. Clin. Invest. 2018;128(10):4297–4312. doi: 10.1172/JCI96209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pourbadie H.G., Sayyah M., Khoshkholgh-Sima B., Choopani S., Nategh M., Motamedi F., Shokrgozar M.A. Early minor stimulation of microglial TLR2 and TLR4 receptors attenuates Alzheimer’s disease-related cognitive deficit in rats: behavioral, molecular, and electrophysiological evidence. Neurobiol. Aging. 2018;70:203–216. doi: 10.1016/j.neurobiolaging.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 55.Illes P., Rubini P., Huang L., Tang Y. The P2X7 receptor: a new therapeutic target in Alzheimer’s disease. Expert Opin. Ther. Targets. 2019;23(3):165–176. doi: 10.1080/14728222.2019.1575811. [DOI] [PubMed] [Google Scholar]
- 56.Bhattacharya A., Biber K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia. 2016;64(10):1772–1787. doi: 10.1002/glia.23001. [DOI] [PubMed] [Google Scholar]
- 57.Heneka M.T., McManus R.M., Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat. Rev. Neurosci. 2018;19(10):610–621. doi: 10.1038/s41583-018-0055-7. [DOI] [PubMed] [Google Scholar]
- 58.Thal D.R. The role of astrocytes in amyloid β-protein toxicity and clearance. Exp. Neurol. 2012;236(1):1–5. doi: 10.1016/j.expneurol.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 59.Pekny M., Pekna M. Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol. Rev. 2014;94(4):1077–1098. doi: 10.1152/physrev.00041.2013. [DOI] [PubMed] [Google Scholar]
- 60.Wojsiat J., Zoltowska K.M., Laskowska-Kaszub K., Wojda U. Oxidant/antioxidant imbalance in Alzheimer’s Disease: therapeutic and diagnostic prospects. Oxid. Med. Cell. Longev. 2018;2018:6435861. doi: 10.1155/2018/6435861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Z., Zhong C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014;30(2):271–281. doi: 10.1007/s12264-013-1423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonda D.J., Lee H.G., Blair J.A., Zhu X., Perry G., Smith M.A. Role of metal dyshomeostasis in Alzheimer’s disease. Metallomics. 2011;3(3):267–270. doi: 10.1039/c0mt00074d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown D.R. Metals in neurodegenerative disease. Metallomics. 2011;3(3):226–228. doi: 10.1039/c1mt90005f. [DOI] [PubMed] [Google Scholar]
- 64.Lanza V., Milardi D., Di Natale G., Pappalardo G. Repurposing of Copper(II)-chelating drugs for the treatment of neurodegenerative diseases. Curr. Med. Chem. 2018;25(4):525–539. doi: 10.2174/0929867324666170518094404. [DOI] [PubMed] [Google Scholar]
- 65.Li Y., Jiao Q., Xu H., Du X., Shi L., Jia F., Jiang H. Biometal dyshomeostasis and toxic metal accumulations in the development of Alzheimer’s Disease. Front. Mol. Neurosci. 2017;10:339. doi: 10.3389/fnmol.2017.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garza-Lombó C., Posadas Y., Quintanar L., Gonsebatt M.E., Franco R. Neurotoxicity linked to dysfunctional metal ion homeostasis and xenobiotic metal exposure: redox signaling and oxidative stress. Antioxid. Redox Signal. 2018;28(18):1669–1703. doi: 10.1089/ars.2017.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ong W-Y., Farooqui T., Kokotos G., Farooqui A.A. Synthetic and natural inhibitors of phospholipases A2: their importance for understanding and treatment of neurological disorders. ACS Chem. Neurosci. 2015;6(6):814–831. doi: 10.1021/acschemneuro.5b00073. [DOI] [PubMed] [Google Scholar]
- 68.Ha J.S., Dho S.H., Youm T.H., Kwon K.S., Park S.S. Astrocytic phospholipase A2 contributes to neuronal glutamate toxicity. Brain Res. 2014;1590:97–106. doi: 10.1016/j.brainres.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 69.Sweeney M.D., Sagare A.P., Zlokovic B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018;14(3):133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engelhardt B., Carare R.O., Bechmann I., Flügel A., Laman J.D., Weller R.O. Vascular, glial, and lymphatic immune gateways of the central nervous system. Acta Neuropathol. 2016;132(3):317–338. doi: 10.1007/s00401-016-1606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong K.H., Riaz M.K., Xie Y., Zhang X., Liu Q., Chen H., Bian Z., Chen X., Lu A., Yang Z. Review of current strategies for delivering alzheimer’s disease drugs across the blood-brain barrier. Int. J. Mol. Sci. 2019;20(2):381–406. doi: 10.3390/ijms20020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fisher Y., Nemirovsky A., Baron R., Monsonego A. Dendritic cells regulate amyloid-β-specific T-cell entry into the brain: the role of perivascular amyloid-β. J. Alzheimers Dis. 2011;27(1):99–111. doi: 10.3233/JAD-2011-102034. [DOI] [PubMed] [Google Scholar]
- 73.Salminen A., Kauppinen A., Kaarniranta K. Hypoxia/ischemia activate processing of Amyloid Precursor Protein: impact of vascular dysfunction in the pathogenesis of Alzheimer’s disease. J. Neurochem. 2017;140(4):536–549. doi: 10.1111/jnc.13932. [DOI] [PubMed] [Google Scholar]
- 74.Chen C.H., Zhou W., Liu S., Deng Y., Cai F., Tone M., Tone Y., Tong Y., Song W. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012;15(1):77–90. doi: 10.1017/S1461145711000149. [DOI] [PubMed] [Google Scholar]
- 75.Montagne A., Nation D.A., Pa J., Sweeney M.D., Toga A.W., Zlokovic B.V. Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol. 2016;131(5):687–707. doi: 10.1007/s00401-016-1570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Henley D.B., Sundell K.L., Sethuraman G., Dowsett S.A., May P.C. Safety profile of semagacestat, a gamma-secretase inhibitor: IDENTITY trial findings. Curr. Med. Res. Opin. 2014;30(10):2021–2032. doi: 10.1185/03007995.2014.939167. [DOI] [PubMed] [Google Scholar]
- 77.Chu J., Praticò D. The 5-Lipoxygenase as modulator of Alzheimer’s γ-secretase and therapeutic target. Brain Res. Bull. 2016;126(Pt 2):207–212. doi: 10.1016/j.brainresbull.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghosh A.K., Brindisi M., Tang J. Developing β-secretase inhibitors for treatment of Alzheimer’s disease. J. Neurochem. 2012;120(1) Suppl. 1:71–83. doi: 10.1111/j.1471-4159.2011.07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang C. Natural compounds that modulate BACE1-processing of amyloid-beta precursor protein in Alzheimer’s disease. Discov. Med. 2012;14(76):189–197. [PubMed] [Google Scholar]
- 80.Llano D.A., Li J., Waring J.F., Ellis T., Devanarayan V., Witte D.G., Lenz R.A. Cerebrospinal fluid cytokine dynamics differ between Alzheimer disease patients and elderly controls. Alzheimer Dis. Assoc. Disord. 2012;26(4):322–328. doi: 10.1097/WAD.0b013e31823b2728. [DOI] [PubMed] [Google Scholar]
- 81.Gezen-Ak D., Dursun E., Hanağası H., Bilgiç B., Lohman E., Araz Ö.S., Atasoy İ.L., Alaylıoğlu M., Önal B., Gürvit H., Yılmazer S. BDNF, TNFα, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer’s disease or mild cognitive impairment. J. Alzheimers Dis. 2013;37(1):185–195. doi: 10.3233/JAD-130497. [DOI] [PubMed] [Google Scholar]
- 82.Honma T., Hatta K., Hitomi Y., Kambayashi Y., Hibino Y., Konoshita T., Nakamura H. Increased systemic inflammatory interleukin-1ß and interleukin-6 during agitation as predictors of Alzheimer’s disease. Int. J. Geriatr. Psychiatry. 2013;28(3):233–241. doi: 10.1002/gps.3816. [DOI] [PubMed] [Google Scholar]
- 83.Hsieh H.L., Yang C.M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. BioMed Res. Int. 2013;2013:484613. doi: 10.1155/2013/484613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Falkowska A., Gutowska I., Goschorska M., Nowacki P., Chlubek D., Baranowska-Bosiacka I. Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism. Int. J. Mol. Sci. 2015;16(11):25959–25981. doi: 10.3390/ijms161125939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pekny M., Wilhelmsson U., Pekna M. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 2014;565:30–38. doi: 10.1016/j.neulet.2013.12.071. [DOI] [PubMed] [Google Scholar]
- 86.Siavelis J.C., Bourdakou M.M., Athanasiadis E.I., Spyrou G.M., Nikita K.S. Bioinformatics methods in drug repurposing for Alzheimer’s disease. Brief. Bioinform. 2016;17(2):322–335. doi: 10.1093/bib/bbv048. [DOI] [PubMed] [Google Scholar]
- 87.Williams G., Gatt A., Clarke E., Corcoran J., Doherty P., Chambers D., Ballard C. Drug repurposing for Alzheimer’s disease based on transcriptional profiling of human iPSC-derived cortical neurons. Transl. Psychiatry. 2019;9(1):220. doi: 10.1038/s41398-019-0555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim T-W. Drug repositioning approaches for the discovery of new therapeutics for Alzheimer’s disease. Neurotherapeutics. 2015;12(1):132–142. doi: 10.1007/s13311-014-0325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lai J., Mei Z.L., Wang H., Hu M., Long Y., Miao M.X., Li N., Hong H. Montelukast rescues primary neurons against Aβ1-42-induced toxicity through inhibiting CysLT1R-mediated NF-κB signaling. Neurochem. Int. 2014;75:26–31. doi: 10.1016/j.neuint.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 90.Rozin S.I. Case series using montelukast in patients with memory loss and dementia. Open Neurol. J. 2017;11:7–10. doi: 10.2174/1874205X01711010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ilarraza R., Wu Y., Adamko D.J. Montelukast inhibits leukotriene stimulation of human dendritic cells in vitro. Int. Arch. Allergy Immunol. 2012;159(4):422–427. doi: 10.1159/000338818. [DOI] [PubMed] [Google Scholar]
- 92.Kumar A., Prakash A., Pahwa D., Mishra J. Montelukast potentiates the protective effect of rofecoxib against kainic acid-induced cognitive dysfunction in rats. Pharmacol. Biochem. Behav. 2012;103(1):43–52. doi: 10.1016/j.pbb.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 93.Al-Amran F.G., Hadi N.R., Hashim A.M. Cysteinyl leukotriene receptor antagonist montelukast ameliorates acute lung injury following haemorrhagic shock in rats. Eur. J. Cardiothorac. Surg. 2013;43(2):421–427. doi: 10.1093/ejcts/ezs312. [DOI] [PubMed] [Google Scholar]
- 94.Lai J., Hu M., Wang H., Hu M., Long Y., Miao M.X., Li J.C., Wang X.B., Kong L.Y., Hong H. Montelukast targeting the cysteinyl leukotriene receptor 1 ameliorates Aβ1-42-induced memory impairment and neuroinflammatory and apoptotic responses in mice. Neuropharmacology. 2014;79:707–714. doi: 10.1016/j.neuropharm.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 95.Shi S.S., Yang W.Z., Tu X.K., Wang C.H., Chen C.M., Chen Y. 5-Lipoxygenase inhibitor zileuton inhibits neuronal apoptosis following focal cerebral ischemia. Inflammation. 2013;36(6):1209–1217. doi: 10.1007/s10753-013-9657-4. [DOI] [PubMed] [Google Scholar]
- 96.Joshi Y.B., Praticò D. The 5-lipoxygenase pathway: oxidative and inflammatory contributions to the Alzheimer’s disease phenotype. Front. Cell. Neurosci. 2015;8(436):436. doi: 10.3389/fncel.2014.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giannopoulos P.F., Chu J., Sperow M., Li J.G., Yu W.H., Kirby L.G., Abood M., Praticò D. Pharmacologic inhibition of 5-lipoxygenase improves memory, rescues synaptic dysfunction, and ameliorates tau pathology in a transgenic model of tauopathy. Biol. Psychiatry. 2015;78(10):693–701. doi: 10.1016/j.biopsych.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chu J., Giannopoulos P.F., Ceballos-Diaz C., Golde T.E., Praticò D. 5-Lipoxygenase gene transfer worsens memory, amyloid, and tau brain pathologies in a mouse model of Alzheimer disease. Ann. Neurol. 2012;72(3):442–454. doi: 10.1002/ana.23642. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Manev H., Chen H., Dzitoyeva S., Manev R. Cyclooxygenases and 5-lipoxygenase in Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35(2):315–319. doi: 10.1016/j.pnpbp.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ikonomovic M.D., Abrahamson E.E., Uz T., Manev H., Dekosky S.T. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer’s disease. J. Histochem. Cytochem. 2008;56(12):1065–1073. doi: 10.1369/jhc.2008.951855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Firuzi O., Zhuo J., Chinnici C.M., Wisniewski T., Praticò D. 5-Lipoxygenase gene disruption reduces amyloid-beta pathology in a mouse model of Alzheimer’s disease. FASEB J. 2008;22(4):1169–1178. doi: 10.1096/fj.07-9131.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sheng M., Lu H., Liu P., Li Y., Ravi H., Peng S-L., Diaz-Arrastia R., Devous M.D., Womack K.B. Sildenafil improves vascular and metabolic function in patients with Alzheimer’s Disease. J. Alzheimers Dis. 2017;60(4):1351–1364. doi: 10.3233/JAD-161006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tousi B. The emerging role of bexarotene in the treatment of Alzheimer’s disease: current evidence. Neuropsychiatr. Dis. Treat. 2015;11:311–315. doi: 10.2147/NDT.S61309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Okereke O.I., Meadows M-E. More evidence of an inverse association between cancer and Alzheimer Disease. JAMA Netw. Open. 2019;2(6):e196167. doi: 10.1001/jamanetworkopen.2019.6167. [DOI] [PubMed] [Google Scholar]
- 105.Shoaib M., Kamal M.A., Rizvi S.M.D. Repurposed drugs as potential therapeutic candidates for the management of Alzheimer’s Disease. Curr. Drug Metab. 2017;18(9):842–852. doi: 10.2174/1389200218666170607101622. [DOI] [PubMed] [Google Scholar]
- 106.Moir R.D., Lathe R., Tanzi R.E. The antimicrobial protection hypothesis of Alzheimer’s disease. Alzheimers Dement. 2018;14(12):1602–1614. doi: 10.1016/j.jalz.2018.06.3040. [DOI] [PubMed] [Google Scholar]
- 107.Cummings J., Lee G., Ritter A., Sabbagh M., Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement. (N. Y.) 2019;5:272–293. doi: 10.1016/j.trci.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]