Abstract
Background
NPS belonging to the benzodiazepine (BZD) class, e.g., ‘legal/designer BZDs’/‘research chemicals’, have recently emerged in the drug (mainly online/virtual) market.
Objective
While certain NPS belonging to the BZD class possess pharmacological profiles similar to controlled pharmaceutical BZDs, clinical and pharmacological profiles of current emerging BZDs are still not well-described. Therefore, there is a need to increase clinicians’/public health knowledge/awareness, to incentive harm reduction strategies.
Method
A comprehensive overview was carried out by using the EMCDDA/EDND database regularly monitored by our research team, by specifically looking at the ‘new BZDs’ so far notified. Furthermore, given the limitation of peer-reviewed data published so far, a nonparticipant multilingual qualitative netnographic study was conducted to obtain further clinical/pharmacological/toxicological data, including psychonauts’ online trip reports.
Results
First designer BZDs appeared as NPS around 2007. So far, 29 designer BZDs have been notified to the EMCDDA, being some of them extremely powerful, also at lower dosages. They are sold as tablets/powder/pellets/capsules/blotters/liquids, at very affordable prices, and variably administered. Some are also sold on the illicit drugmarket as counterfeit forms of traditional BZDs or as either adulterants or diluents in heroin or other synthetic opioids/cannabinoids. Nowadays, there is no guarantee of the quality of designer BZDs composition/purification and, hence, most NPS consumers may be inadvertently exposed to unsafe and harmful compounds.
Conclusion
Given the limited information on their pharmacology/toxicity, variations in dosage, onset of effects, combination of substances, potency, and general patient or individual variability, the concomitant use of these substances with other drugs entails several and unpredictable risks.
Keywords: New benzodiazepines, NPS, novel psychoactive substances, benzodiazepines, designer benzodiazepines, synthetic benzodiazepines
1. Introduction
Benzodiazepines (BZDs) act as positive allosteric modulators on the gamma-aminobutyric acid (GABA)A receptor [1]. GABA represents the main inhibitor neurotransmitter in the brain and plays an important role in modulating the activity of many neurons, including those in the amygdala and prefrontal cortex [1]. The GABAA receptor is a ligand-gated chloride-selective ion channel build-up of five subunits: two α, two ß (the binding site for endogenous neurotransmitter) and one γ. BZDs bind to the pocket created by α and γ subunits and induce a conformational change in the GABAA receptor. This alteration, in turns, induces a conformational change in the GABAA receptor such as to increase the apparent affinity for channel gating by GABA at both agonist sites. As a result, maximal currents elicited by GABA remain unaffected, and the GABA concentration channel opening curve is shifted to lower GABA concentrations’ chloride channel that hyperpolarizes the cell and accounts for GABA’s inhibitory effect throughout the central nervous system [1]. These complex pharmacological activities explain the different clinical effects (i.e., anxiolytic, hypnotic, anticonvulsant, amnestic, and muscle relaxant) of BZDs. The pharmacological activity of BZDs is determined by the type of GABAA receptor α subunit to which they bind. Thus, the sedative, anterograde amnesic and anticonvulsant actions, as well as the addictive potential of these drugs, require the presence of α1-containing GABAA receptors, while the anxiolytic effects are mediated by GABAA receptors containing α2 subunits, and the myorelaxant actions by GABAA receptors containing α2, α3, and α5 subunits [2].
Overall, BZDs are generally classified according to their pharmacokinetic characteristics, i.e. a) plasma half-life (t½); and, b) hepatic metabolism [1, 3] (Table 1). Plasma half-life (t½) represents the hours required for the concentration of the drug in the body to be reduced to half of the maximum concentration. In detail, t½α represents the ‘phase distribution’ from the vascular system to the tissues; whilst t½β indicates the ‘elimination phase’ and represents, hence, an index of the metabolism and excretion of BZDs. This phase varies significantly among different BZDs (from 2-3 hours up to more than 100 hours) and is relevant for the accumulation of some BZDs in tissues after long-term use (see Table 1 for more details). Furthermore, BZDs may be classified according to their chemical structure and designer BZDs are mainly classified as either 1,4-benzodiazepines (a structure shared by most ‘traditional’ BZDs), triazolo-BZDs, or thienotriazolodiazepines (Table 1) [4]. BZD metabolism mainly occurs in the liver, primarily by oxidative metabolism mediated by the cytochrome P450 (CYP450) family, i.e. CYP3A4, CYP3A5, CYP2C19, CYP2C18, CYP2C9 and CYP2B6 [5]. Tolerance and dependence may occur shortly after consumption has started, which requires dose increase and may trigger drug-seeking behaviours [1, 3]. Acute intoxication may cause respiratory and central nervous system depression, even though is rarely lethal if the BDZ is taken alone [6]. However, BZDs are usually consumed in combination with other depressant drugs/substances (i.e., opioids, antidepressants, etc.), as commonly documented amongst opioid consumers in order to enhance their euphoric effects, alleviate withdrawal or abstinence symptoms, or temper highs induced by psychostimulants or synergistically enhance alcohol effect [1, 3].
As a relatively new phenomenon, novel psychoactive substances (NPS) belonging to the BZD class have emerged in the drug (mainly online/virtual) market, and are being sold under street names such as ‘legal benzodiazepines’, ‘designer benzodiazepines’ or ‘research chemicals’. This group of drugs includes substances that were tested but not approved as medicines in the pharmaceutical industry or that have been manufactured by modifying the core structure of existing pharmaceutical BZDs [7-9]. Whilst certain NPS belonging to the BZD class possess pharmacological profiles similar to the ‘controlled’ pharmaceutical BZDs, profiles of most of the current designer/NPS BZDs are not completely well-described, hence, their safety, toxicological and clinical profile are still unknown, posing serious health risks to consumers [9, 10]. Furthermore, the risk of polydrug use involving BZDs and opioids (both traditional and synthetic ones) are furtherly intensified by NPS belonging to these new/designer BZDs [10]. Given the limited information on the pharmacology and toxicity of these substances, variations in the dosage, onset of effects, combinations with other substances, potency, and general patient or individual variability, the concomitant use of these substances with other drugs entails several and unpredictable risks, particularly amongst high-risk opioid users [11]. Interestingly, over the long-term and in the last few years, a diverse range of NPS, including synthetic opioids and BZDs have been found [10]. In fact, by the end of 2018, EMCDDA monitored more than 730 NPS, of which 55 were reported for the first time in Europe in 2018, of which around 5% belonging to the BZD class [10]. So far, 29 NPS belonging to the BZD class have been reported by the Member States to the UNODC Early Warning Advisory (EWA), of which 23 were firstly detected in Europe during the last 5 years [10]. In 2008, phenazepam was the first new BZD to be reported to the EWA. In 2011, Germany, Norway and the United Kingdom were the first countries to report the emergence of another designer, BZD, etizolam. In the following years (2012-2013), a relatively stable number of BZDs were reported to the EMCDDA; with an increased number in 2014-2016, a reduction in 2017 and a further increase in 2018 [10].
Some new BZDs were sold as tablets, capsules or powders under their own names, marketed as ‘legal’ versions of authorised medicines. In other cases, counterfeiters used these substances to produce ‘fake’ versions of commonly prescribed anti-anxiety drugs, such as diazepam and alprazolam, which were sold directly on the illicit online drug market [10]. Furthermore, some new BZDs have been identified mixed with other NPS (i.e., synthetic cannabinoids) or have been labeled as diazepam tablets but containing a new potent synthetic opioid [11]. Overall, some of the new BZDs have been historically approved and marketed for use in some countries (i.e., phenazepam); whilst others have been previously investigated and may be found in some patent literature but subsequently not marketed; finally, the remaining ones represent completely new compounds [11]. Consequently, as an increasing number of BZD derivatives have appeared on the NPS market, many of them associated with hospitalizations and fatalities, several countries worldwide have placed some of these substances under national control [10, 11]. For example, in Europe, NPS belonging to the BZD class have been placed under national control in countries, such as Denmark, Finland, Sweden, Switzerland, Turkey and the United Kingdom. In South-East Asia, the Republic of Korea is also reported to have placed diclazepam under national control and in the Middle East, the United Arab Emirates has placed diclazepam, and etizolam, flubromazepam and pyrazolam under national control [11]. Indeed, the WHO’s Expert Committee on Drug Dependence will be reviewing both Etizolam and Flualprazolam at its 42nd meeting 21-25 October 2019, to bring these under international control [12].
Therefore, the present comprehensive overview aims at providing up-to-date insight into the world of new/designer/synthetic BZDs recently marketed in NPS marketplaces,
2. Materials and Methods
A comprehensive overview was carried out by using the EMCDDA/EDND database regularly monitored and analysed by a team member of our research team, by specifically looking at the so-called ‘new benzodiazepines’ so far marketed (last update: 25 September 2019). For each BZD here identified and selected, a PubMed/Medline search was also conducted to evaluate (if any) literature published (particularly, case-reports). Two team members of our research team combined the search strategy of free-text terms and exploded MESH headings for the topics of Benzodiazepines and Novel Psychoactive Substances as following: ((Benzodiazepines) [Title/Abstract]) AND (Designer [Title/Abstract])) and for each of the 29 BZDs here identified (Table 2). Secondary searches were performed using the reference list of included articles and relevant systematic reviews. All articles published without the time and/or language restriction were selected. Working independently and in parallel, two reviewers of our research team read the papers and determined whether they provided data on ‘new benzodiazepines’. To be included in the present overview, studies were required to meet the following criteria: a) empirical and peer-reviewed study; b) at least an abstract with estimates (for those papers not found in full text and/or with full text but not in English) and/or full results published in English; c) investigated ‘new benzodiazepines’. Studies evaluating ‘classical BZDs’, even though containing data on abuse and/or misuse were correctly excluded as not relevant to the aims of the present paper. As limited information is available, non-systematic review, reviews, letters to editors and meta-analyses were also considered for retrieving data (if available). Two team members of our research team independently extracted the data. Disagreements were resolved by discussion and consensus with a third member of the team. Data were collected using an ad-hoc developed data extraction spreadsheet. Table 2 provides a summary of the data collected by the present comprehensive review.
However, given the limitation of peer-reviewed data published so far, a preliminary nonparticipant multilingual qualitative study of a list of websites and other online resources (i.e. e-newsgroups, chat-rooms, bulletin boards, and e-newsletters), specifically addressed to psychonauts and NPS consumers, was additionally conducted in order to obtain more data (in terms of clinical, pharmacological and toxicological effects) about the 29 BZDs selected and analysed here. A systematic Internet search was conducted on Google® which included the following keywords: ‘benzodiazepine name’ and/or possible acronyms, street names etc. plus ‘to buy’, ‘experience’, ‘trip’, ‘legal high’, ‘abuse’, ‘misuse’. The first 5 pages recorded per search term and search engine were consequently selected and analyzed only if relevant in terms of information and data provided regarding ‘new/designer BZDs’. Within the time frame of January–September 2019, data were collected from 12 websites. Confidentiality measures applied to the dataset included storage in an online, password‐protected computer and removal of screen pseudonyms, URLs, country and city identifiers. Some 2,900 fora threads were screened. After the removal of Web pages which were either duplicates or nonrelevant to the aims of the study, 268 fora threads were used to retrieve and analyze the data presented here.
Ethical approval for the study was granted by the Department of Pharmacy Ethics Committee at the University of Hertfordshire (December 15, 2010, reference code PHAEC/1042), with further extensions of the approval granted in November 2013 and February 2019 (Protocol number: aLMS/SF/UH/02951(2).
All designer BZDs are here described and discussed according to a chronological order of appearance on the online drug market and according to the notification sent out to the EMCDDA.
3. Results
3.1. Phenazepam
Phenazepam is a long-acting BZD, belonging to the 1,4-BZDs, the same family as diazepam, oxazepam and temazepam, which was developed in the 1970s for the treatment of epilepsy, alcohol withdrawal syndrome, insomnia, anxiety, and as premedication in anesthesia procedures, which is currently the most prescribed BZD in Russia since 1978 [13-19]. Phenazepam has not been licensed in other European countries. Phenazepam is currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs. Phenazepam is controlled in Estonia, Latvia, Lithuania, Moldova, Norway, Sweden, and the Republic of Ireland [16, 20-22]. Whilst it is covered by prescription legislation in Estonia, Latvia, Lithuania, the Russian Federation and Belarus [16, 22]. Following the UK Advisory Council on the Misuse of Drugs (ACMD) advice, the Home Office imposed a ban (dated 22 July 2011) under the Open General Import License on the importation of phenazepam [23-25]. Following the ACMD recommendation, phenazepam became controlled in the UK, like other BZDs, as a Class C drug from June 2012. The recreational/unauthorized use of phenazepam has been reported during the recent last years, particularly in the USA, New Zealand, and some European countries, particularly in Scandinavian countries (i.e., Finland, Norway and Sweden) [16, 26-28].
From a pharmacological point of view, phenazepam is generally more potent than diazepam (5-10-fold) and possesses more severe and persistent side-effects (up to 5 days-3 weeks) by having a long elimination half-life of around 60 hours after ingestion [16, 26, 29-32]. Phenazepam has an active metabolite 3-hydroxyphenazepam, which is as well 5- to 10-fold more potent than diazepam. Various fatal cases have been reported following the intake of phenazepam as well as reports of abuse, especially in combination with opioids and/or other sedatives [19, 21, 27, 32-43]. The most frequently reported causes of poisoning occurring in children in Moscow to appear to be BZD-related, mainly involving cases of phenazepam intake [44]. Reported side-effects include amnesia, drowsiness, dizziness, somnolence, difficulty in waking up, muscle weakness, headache, weakening of attention, incoordination, blurred vision, slurred speech, ataxia, and muscle weakness [45]. At high doses, delirium and psychosis-like behaviour have been reported [46]. Phenazepam and its active metabolite are both GABAA receptor positive allosteric modulators [47, 48]. In Russia and in other countries in which is legally marketed, phenazepam is available as 0.5-1 mg tablets, injectable solutions (0.1%, 0,3%) and transdermal patches, with a usual therapeutic oral dosage of 0.5 mg 2-3 times per day, and a maximum tolerated dose of 10 mg daily [33, 49, 50]. In the NPS market, phenazepam has been sold as a powder, tablets, spiked in blotters similar to LSD, or, in the USA, sold as an air freshener known as “Zannie” which can be administered by spraying into the mouth [45]; it is easily available via the Internet, often produced in China [32, 51, 52]. The main routes of administration (ROA) reported include orally (most common), snorted, inhaled, administered transdermal or rectally, or injected (after crushing the tablet) [53]. Phenazepam has been reported to be used to enhance the euphoric effects of opioids (particularly, to “boost” methadone doses), to alleviate withdrawal or abstinence syndrome (i.e., between heroin ‘fixes’), to potentiate the effects of alcohol and to temper/balance cocaine ‘highs’ [28, 50, 54].
The Scottish police seized phenazepam for the first time in October 2008, sold as fake ‘diazepam’; then it appeared again in January 2011 in North Wales and in March 2011 in Germany [21]. Furthermore, phenazepam appeared to be presently mixed in some packages containing synthetic cannabinoids, JWH-018, JWH-073, JWH-122 and JWH-250 [55-57]. There are several anecdotal reports from psychonaut fora which describe it as “a very long lasting, potent and subtle benzodiazepine (…)” [52, 54]. In particular, a low dose is reported to range from 0.5 to 1 mg, a typical dose is 1-2 mg, and a high dose is 2-4 mg or more [58]. The onset of symptomatology is reported after 15-60 minutes, and effects may last more than 18 hours and after-effects more than 36 hours [58].
3.2. Etizolam
Etizolam is a short-acting BZD, belonging to the thienotriazolodiazepine family (in which a diazepine ring is fused to a thiophene ring, instead of benzene), marketed in some countries (i.e., Japan), which is used for the treatment of insomnia, anxiety disorders, and withdrawal symptomatology [59]. The recommended dosage of etizolam for medical use is approximately 1 mg to 1.5 mg daily up to a maximum of 4 mg daily [60]. It has elimination kinetics between those of short-intermediate derivatives and ultra-rapidly eliminated BZDs. Etizolam is pharmacologically similar to diazepam [60]. It has been implicated in fatalities [61]. Perhaps no more so than in Scotland. The number of deaths registered there involving etizolam has reached a crisis point; rising gradually from 1 in 2012, 8 in 2013, 37 in 2014, and to 43 in 2015. However, the number in 2016 was 225, with 300 in 2017 but an unprecedented 551 in 2018, out of a total of 1313 drug-related poisoning deaths [62]. There are no further data about clinical, pharmacological or toxicological properties. Etizolam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Etizolam was first reported to the EMCDDA by the UK’s Hampshire police in September 2011 in a seizure of tablets bought on line. Subsequently, powders, as well as ‘blotters’ (similar to LSD paper doses) were reported [21]. There are several anecdotal reports from psychonaut fora which describe it with a “high potency which allows an effective dose of a few milligrams to be present on a paper dose (…)” [52; 54]. In particular, a low dose is 0.5-1 mg, a typical dose is 1-2 mg, and a high dose is 2-4 mg or more [58]. The onset of symptomatology occurs in 10-40 minutes, effects last 5-8 hours and after-effects for 6-24 hours [58].
3.3. Pyrazolam
Pyrazolam is a triazolo-BZD with apparently very little information, structurally similar to alprazolam but is brominated rather than chlorinated and contains a pyridinyl group instead of a phenyl group. It was first developed and patented by Hoffman-La Roche in 1979 in a patent [63]. However, pyrazolam is the first BZD on the NPS market that is not marketed anywhere in the world by a pharmaceutical company for medical purposes [63]. It has been sold as tablets that contain 0.5 mg of active compound per tablet. From a pharmacological point of view, pyrazolam is generally more potent than diazepam (12-fold) and has an elimination half-life of around 6 hours after ingestion [63]. There are no further data about clinical, pharmacological or toxicological properties. Pyrazolam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Pyrazolam was first reported in a seizure of tablets in a mail package on 3 August 2011 by Finnish Customs officials [63, 64]. There are limited anecdotal reports from psychonaut fora which describe it as “quite sedating, amnesic and loss of inhibition at higher doses (…) anxiolytic effect at lower doses (…)” [52, 54]. In particular, a light dose is 1-2 mg, a typical dose is 2-3 mg, and a high dose is 3-4 mg [58]. The onset of symptomatology occurs in 10-15 minutes, and effects may last 5-8 hours and after-effects for 1-12 hours [58].
3.4. Flubromazepam
Flubromazepam is a long-lasting BZD, structurally similar to phenazepam, from which it differs due to substitution of a fluorine atom instead of a chlorine atom, and to triazolam and pyrazolam. It does not appear to be licensed for medical use. Flubromazepam was first described in 1962 when it was noted to be several times more potent than chlordiazepoxide, the reference substance used in several assays [65]. Derivatives of this substance may be used as antivirals [66]. Pharmacokinetic data suggest an elimination half-life of about 100 hours and anticonvulsant properties [63]. Published literature described a fatal case of poisoning by the synthetic opioid U-47700 in combination with flubromazepam in a 24-year-old man [67]. Furthermore, three hospitalizations from acute exposure have been reported in the US and 33 in Sweden between 2012-2017 [63, 68-71]. There are no further data about clinical, pharmacological or toxicological properties. Flubromazepam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Flubromazepam was first identified in a sample of capsules analysed by a German university forensic institute in March 2013 [69, 70, 72, 73]. There are several anecdotal reports from psychonauts fora which describe it as “of extreme duration, with effects for larger doses reaching up to three days” [52, 54]. In particular, a low dose is 2-4 mg, a typical dose is 4-8 mg, and a high dose is 8-12 mg or more [58]. Symptomatology occurs in 15-90 minutes, effects may last 12-18 hours and after-effects for more than 36 hours [58].
3.5. Diclazepam
Diclazepam is the 2'-chloro derivative of diazepam and a positional isomer of 4-chlorodiazepam. It was first synthetised by the Hoffman-La Roche in 1960 [74] and recently appeared in the ‘grey drug market’ as an alternative to etizolam [10]. Babbini et al. [75] reported a potency of approximately 4-8 times higher than diazepam in terms of reducing motor activity and conflict behaviour in rats whilst Sternbach et al. [76] described a potency similar to diazepam regarding to muscle relaxant and sedative effects in mice and twice as potent than diazepam investigating the same effects in cats [76]. It does not show differences in the behavioural activity if given to monkeys, compared to diazepam [77]. Diclazepam has a long half-life of approximately 42 hours and its pharmacokinetic profile follows a biphasic elimination [7]. There are no further data about clinical, pharmacological or toxicological properties. Diclazepam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Diclazepam was first identified in a sample of tablets analysed by a German university forensic institute in August 2013 [69, 70, 72]. There are several anecdotal reports on psychonauts fora which describe it as “sedative and hypnotic” with similar effects to diazepam, even though “10-fold times more potent and with an intermediate half-life” [54]. In particular, a low dose is 0.25-1 mg, a typical dose is 1-2 mg, and a strong dose is 2 mg or more [58]. Symptomatology occurs after 15-90 minutes, effects may last 8-12 hours and after-effects for 1-24 hours [58].
3.6. Alprazolam triazolobenzophenone derivative
The Alprazolam triazolobenzophenone derivative represents a product of hydrolysis, under acidic conditions, of alprazolam and, hence, a metabolite of alprazolam as well. At neutral pH, it rapidly converts to alprazolam. It was firstly developed by the Upjohn Company in the 1980s as a water-soluble pro-drug of alprazolam for the parenteral (intravenous or intramuscular) ROA [78]. There are no further data about clinical, pharmacological or toxicological properties. Alprazolam triazolobenzophenone is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Alprazolam triazolobenzophenone has been firstly identified in a seizure of 1000 grams of white powder, seized at Madrid Airport in March 2014 by Customs authorities in a package that had arrived from India; it also contained paracetamol. The compound was identified and characterised using the gas chromatography-mass spectrometry (GC-MS) and nuclear magnetic resonance (NMR) by the Spanish National Focal Point [79]. There are limited anecdotal reports from psychonaut fora but it is supposed to exert an effect similar to that of alprazolam [54].
3.7. Meclonazepam
Meclonazepam represents the 3-methyl-derivative of clonazepam, hence, it has been supposed that it exhibits similar sedative, anxiolytic and anti-parasitic effects [80]. Its synthesis was first developed and patented by Hoffman-La Roche in 1977 [81]. Its pharmacology has been investigated in clinical trials as an anxiolytic and as a schistosomicidal compound able to treat parasitic infections by Schistosoma haemayobium and Schistosoma Mansori [81-84]. Drowsiness, dizziness, slurred speech, ataxia, muscle weakness, reduced mental alertness and lateral nystagmus have been described as the main side-effects [82]. The effects appear to be dose-dependent with a narrow therapeutic range (0.3-0.4 mg/kg) [82]. Doses above 0.4 mg/kg have been described as causing severe adverse drug effects, with the most pronounced effects within 3 hours after oral intake of more than 1 mg of meclonazepam and amnesia after a 4-mg dose [82, 85]. An anxiolytic potency 3-fold that of diazepam has been reported [83]. From a pharmacokinetic perspective, a plasma t ½ of approximately 40-80 hours is reported [83, 86]. There are no further data about clinical, pharmacological or toxicological properties. Meclonazepam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Meclonazepam was first identified in a seizure of 145 capsules in May 2014 by Swedish police in Eskilstuna. The substance was identified by the Swedish National Laboratory of Forensic Science (SKL) using GC-MS and NMR analyses [69, 70, 79]. There are several anecdotal reports from psychonaut fora which describe it as “relatively fast sublingual onset” which gives it a strong ‘anti-panic effect’ [54]. In particular, a low dose is 0.25-0.5 mg, a typical dose is 0.5-1 mg, and a high dose is 1-2 mg or more [58]. The onset of symptomatology is in 20-45 minutes, effects may last 8-12 hours and after-effects for 8-48 hours [58].
3.8. Deschloroetizolam
Deschloroetizolam is a thieno diazepine, structurally similar to etizolam, from which it differs due to the absence of chlorine on the benzene ring; triazolam and alprazolam [87]. Deschloroetizolam is supposed to have a rapid onset of action, even though it appears to be half as potent as its parent compound etizolam with a duration twice as long, as supposed by the loss of the chlorine atom [76, 87, 88]. Sedation, respiratory distress, muscle relaxation, amnesia, dizziness, thought deceleration, disinhibition, the delusion of sobriety, and dream potentiation have been described following its intake [87]. The synthesis of deschloroetizolam was first described in a 1988 patent [88, 89]. There are no further data about clinical, pharmacological or toxicological properties. Deschloroetizolam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Deschloroetizolam was first identified in a UK seizure of blue tablets in August 2014. The substance was identified by the WEDINOS Project in Wales using TOF (time-of-flight mass spectrometry) analysis [69, 79]. There are limited anecdotal reports from psychonaut fora which describe it as “longer acting and slightly less potent than etizolam” [54]. In particular, a low dose is 2-4 mg, a typical dose is 4-6 mg, and a strong dose is 6-12 mg [58]. The onset of symptomatology happens in 1-5 minutes, and effects may last 8-10 hours and after-effects for 1-8 hours [58].
3.9. Flubromazolam
Flubromazolam is a substituted BZD, structurally related to pyrazolam from which it differs due to the substitution of a 2-fluorophenyl instead of a 2-pyridinyl group at position 6. Moreover, it is the triazolo-analogue of flubromazepam and it is structurally related to alprazolam and triazolam [90-93]. The substance has been researched in the patent literature for its anxiolytic properties and decreased sedative, hypnotic, and ataxic side-effects, but it does not appear to be licensed for medical use. Łukasik-Głebocka et al. [94] reported a case of severe intoxication following the intake of flubromazolam in an individual who presented with deep coma, bilateral pinpoint unreactive pupils, acute respiratory failure and hypotension complicated by hypoxic cerebral ischaemia. Huppertz et al. [90] reported muscle relaxation, sedation, difficulty following and participating in conversation and partial amnesia in a healthy volunteer following intake of 0.5 mg of flubromazolam. A case-report described a 36-year-old male affected with a schizoaffective disorder, opioid use disorder, seizures, anxiety and a posttraumatic stress disorder who presented to an inpatient facility and reported flubromazolam abuse [93]. There are no further data about clinical, pharmacological or toxicological properties. Flubromazolam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Flubromazolam was first identified in a sample of 10 white rectangle shaped tablets labelled “XANAX”, seized in Malmö by Swedish police in September. The substance was identified by the National Laboratory of Forensic Science (SKL) by using GC-MS and NMR [79]. There are several anecdotal reports from psychonaut fora which describe “mild anxiolytic and skeletal muscle relaxant effects” at low doses as 0.1 mg whilst “significant sedation” at doses of 0.5 mg [51]. Moreover, it has been described as “hard to dose” due to its unpredictable dose-response effects [52, 93]. In particular, a ‘threshold’ dose of 80 μg is described, with a low dose being 100-200 μg, a typical dose is 200-400 μg, and a high dose is 400-600 μg or more [58]. The onset of symptomatology occurs in 20-45 minutes, and effects may last 6-12 hours and after-effects for 6-24 hours [58].
3.10. Nifoxipam
Nifoxipam represents the 3-hydroxy-desmethyl-derivative (active metabolite) of the hypnotic BZD flunitrazepam, from which it differs due to the presence of an additional hydroxyl group and the deletion of a methyl group. Moreover, nifoxipam is also the 3-hydroxy metabolite of fonazepam [95]. It is normally consumed in tablet form, even though is also available in the powder form. Its typical ROA is orally or sublingually [95]. Little is known about the pharmacology and toxicology of nifoxipam. Nifoxipam likely possesses a pharmacological activity similar to flunitrazepam, by binding to the GABAA receptor, and with similar side-effects and toxicity [95]. Nifoxipam is extremely physically and psychologically addictive and presents cross-tolerance with all BZDs, thereby reducing their pharmacological effects [95]. There are no further data about clinical, pharmacological or toxicological properties. Nifoxipam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Nifoxipam was first identified in a seizure of 20 brown tablets made by the Swedish Police in April 2014. The substance was analytically confirmed by GC-MS, LC-MS (liquid chromatography-mass spectrometry) and NMR analysis by the Swedish National Laboratory of Forensic Sciences [69, 79]. In December 2014, four-round, light-brown tablets marked as ‘nifoxipam 1 mg’ were sent from the UK to Finland where they were seized and nifoxipam was analytically confirmed by GC-MS and LC-MS/MS analysis [9, 10]. Finally, in January 2015, the Norwegian Federal Police seized 101 brown tablets found in a mail package sent from the UK to Norway and nifoxipam was analytically confirmed by GC-MS analysis [10, 95]. There are several anecdotal reports from psychonaut fora which describe “a greater hypnotic effect” [51], anxiety relief, euphoria within 10-15 minutes after intake of 1 mg of nifoxipam along with a drink [52]. A low dose is 250-500 μg, a typical dose is 500-1000 μg, and high dose is 1000-2000 μg or more [58]. The onset of symptomatology occurs after 10-75 minutes, and effects may last 10-18 hours and after-effects for 1-24 hours [58].
3.11. Clonazolam/Clonitrazolam
Clonazolam is a triazolo BZD, structurally similar to flubromazolam, from which it differs due to possessing a nitro group in the 8th position and a 2-chlorophenyl group instead of a 2-fluorophenyl group. Clonazolam is the second most common single agent exposure responsible for 50 cases (21% of the sample) of BZD intoxications reported to the National Poison Data System (USA) in 2014-2017, according to a study by Carpenter et al. [64]. The most frequently reported motivations for intake are reported as: misuse (12%), abuse (60%) and suspected suicide (20%), being acute intoxication the most commonly described (78%). Subjects who took clonazolam described the onset of lethargy/drowsiness (68%), slurred speech (16%), tachycardia (14%), confusion (10%), agitation/irritability (6%), ataxia (6%), hypotension (6%), coma (6%), and bradycardia (6%). Most patients were treated with fluid infusion (34%), oxygen (12%), intubation or ventilation (6%), flumazenil (6%) and antiemetics (4%). Eleven patients were admitted to a noncritical care unit, ten to a critical care unit while 17 were treated and released; only five cases were admitted to a psychiatric facility, by reporting minor or moderate sequalae in 45% and 35% of cases, respectively [70]. There are no further data about clinical, pharmacological or toxicological properties.
Clonazolam was first synthesized in the 1970s by the Upjohn Company [96]. Clonazolam was first identified in a seizure of 25 yellow tablets containing a white powder by the Swedish Police on 16 October 2014. The substance was analytically confirmed by GC-MS and NMR analysis [69, 79]. Clonazolam was classified as a Class C drug by the May 2017 amendment to the Misuse of Drugs Act 1971 in the UK [97]. There are several anecdotal reports from psychonaut fora that describe “a totally relaxing and anxiolytic effect, a moderate sedation” [54]. A threshold dose is described as being 50-75 μg, a low dose is 75-200 μg, a typical dose is 200-400 μg, and a high dose is 500-1000 μg or more [58]. Symptomatology occurs in 10-30 minutes, with effects lasting 6-10 hours and after-effects for 1-12 hours [58]. The most commonly reported ROA is oral, with a described potency 10-fold higher than lorazepam 1 mg [98]. Moosmann et al. [8] reported that clonazolam, due to its higher potency, can cause sedation and amnesia at oral doses as low as 0.5 mg which are extremely difficult to measure for users handling bulk materials, and, being tablets often vary greatly in terms of clonazolam content, this can frequently lead to unintended overdosing and lead to drug facilitated crimes [99].
3.12. Adinazolam
Adinazolam is a short-acting BZD belonging to the triazolo-BZD class. The half-life of adinazolam is indicated as less than 3 hours [100]. Three hours were also considered the time of peak onset for adinazolam, i.e., the time after administration of the substance where subjective effects were most pronounced [101]. In-vivo metabolism of adinazolam occurs mainly through the liver and results in the formation of active metabolites, mostly N-demethyl-adinazolam (NDMAD) [102]. Alpha-hydroxy-alprazolam and estazolam are also metabolites of adinazolam [103]. Adinazolam has a high affinity for the GABAA receptor [102]. In vitro, both adinazolam and NDMAD bind to central BZD-receptors but NDMAD has an approximately 25-fold higher activity than adinazolam [102, 104-106]. The molecule has been studied for the treatment of depression, panic disorder, general anxiety and status epilepticus [106-108]. It can be used to induce sedation and anterograde amnesia (whereby it reduces the memory of an event following its administration) [100, 109]. Ataxia, dysarthria, weakness, diminished reflexes, confusion, coma and a paradoxical excitement in children have been reported, in addition to signs of overdose [100]. A human study comparing the subjective effects and abuse potential of adinazolam (30 mg and 50 mg) with diazepam, lorazepam and a placebo showed that adinazolam causes the most “mental and physical sedation” and the greatest “mental unpleasantness” [101]. The same study notes that despite reports of “unpleasantness” the participants of the study rated the substance of high “street value”, capable of producing “physical and mental highness” according to the Addiction Research Criteria Inventory (ARCI) [101]. There are no further data about clinical, pharmacological or toxicological properties. Adinazolam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Adinazolam was first identified in a sample of 1 gram of white powder collected by the Medical Centre in the Institute of Forensic Medicine at the University of Freiburg (Germany) on 5 September 2015. The substance was analytically confirmed by GC-MS analysis [70, 109]. A seizure of 105 tablets was reported shortly afterwards (8 September 2015) by the Swedish Focal point, following a seizure by the Swedish police in Gottenburg. The tablets were white in colour with the markings “D/CD” and weighed 0.35 grams on average. Moreover, the Swedish Poison Control Centre/STRIDA project reported a positive detection for adinazolam related to a hospital inquiry where an individual had taken “Xanor 2.0” (i.e., brand name for Xanax® in Sweden) [109]. Additionally, a sample of 5 g of adinazolam (as a white powder) was test-purchased on 18 September 2015 by the National Forensic Laboratory in Slovenia [110]. There are several anecdotal reports from psychonaut fora. A low dose is 5-15 mg, a typical dose is 15-30 mg, and a high dose is 30-50 mg or more [58]. The onset of symptomatology occurs after 10-25 minutes, effects may last 2-5 hours and after-effects for 1-16 hours [58].
3.13. Nitrazolam
Nitrazolam is a triazolo-BZD, structurally similar to clonazolam (previously notified on 30 December 2014), from which it differs by a chlorine atom on the ortho position of the benzene ring [111]. Its synthesis and activity were described in a 1976 patent [96, 112]. Animal studies show that nitrazolam can be several times more potent than diazepam in preventing of electroshock-induced tonic-extensor convulsions [111]. Moreover, nitrazolam appears to be less potent than clonazolam and triazolam [111]. Clonazolam may cause powerful sedation and amnesia at a total oral dose of 0.5 mg [88, 96, 113-115]. There are no further data about clinical, pharmacological or toxicological properties. Nitrazolam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Nitrazolam was first identified in a sample of light brown powder on the 20 October 2015 by the Medical Centre, Institute of Forensic Medicine at the University of Freiburg, Germany. The substance was analytically confirmed by GC-MS and NMR [110, 111]. There are several anecdotal reports from psychonaut fora, which described mostly an “anxiolytic and muscle relaxant effect […] with a low tolerance shows hypnotic effects. Amnesiac, but not a huge degree […] closer to etizolam than alprazolam” [116]. A low dose is 0.5-1 mg, a typical dose is 1-2 mg, and a high dose is 2-3 mg or more [58]. The onset of symptomatology happens in 15-30 minutes, and effects may last 5-10 hours and after-effects for 10-24 hours [58].
3.14. Metizolam
Metizolam is a thienodiazepine, structurally similar to etizolam, from which it differs by only a methyl ring in the thiophene moiety [117]. There are no data about clinical, pharmacological or toxicological properties. Metizolam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Metizolam was first identified in a sample of 20 light-blue round tablets by the Medical Centre, Institute of Forensic Medicine at the University of Freiburg, Germany, on 25 September 2015. The substance was analytically confirmed by GC-MS and NMR. The Danish National Focal Point reported a seizure of 55 blue round tablets containing metizolam on the 30 October 2015 by Danish Customs and related to a package sent from the UK to a private address in Denmark [110]. There are limited anecdotal reports from psychonaut fora. It has been reported that metizolam causes effects similar to etizolam, even though it is half as potent and with around a 60% longer t ½ [51]. It has also been described as exerting hypnotic and sedative effects, and may cause amnesia and lowered inhibition if taken at higher dosages [116]. A low dose is 1-2 mg, a typical dose is 2-4 mg, and a high dose at 4-6 mg, while a heavy dose is 6 mg or more [58]. Symptomatology occurs in 30-90 minutes, with effects lasting 5-8 hours and after-effects for 10-30 hours [58].
3.15. Cloniprazepam
Cloniprazepam shares structural similarities with the previously notified BZD clonazolam (clonitrazolam) and meclonazepam. It has also been described as a prodrug for clonazepam [58]. There are no data about clinical, pharmacological or toxicological properties. Cloniprazepam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Cloniprazepam was first identified in a seizure of 25 white capsules by Swedish Police in Sundsvall on 2 December 2015. The substance was analytically confirmed by GC-MS, GC-IR (gas chromatography infrared spectrometry), LC-HRMS (Liquid Chromatography-High Resolution Mass Spectrometry) and NMR [70, 110, 118, 119]. There are limited anecdotal reports from psychonaut fora. Cloniprazepam is usually sold online in 2.5 mg capsules and available is in packs of 20, 60, 120 and 240 and it appears to exert similar effects to clonazepam [51]. A low dose is 0.5-1 mg, a typical dose is 1-2 mg, and a high dose is 2-4 mg or more [58]. The onset of symptomatology occurs in 15-45 minutes, with effects lasting 6-9 hours and after-effects for 1-8 hours [58].
3.16. 3-hydroxyphenazepam
3-Hydroxyphenazepam is an active metabolite of both phenazepam (7-bromo-5-(2-chlorophenyl)-1,3-dihydro-1,4-benzodiazepin-2-one) and cinazepam [120, 121]. It can be quantified by LC-MS/MS in a variety of post-mortem fluids (subclavian blood, femoral blood, cardiac blood, urine, vitreous humour) and tissues (thalamus, liver and psoas muscle) [122]; and by GC-MS (limit of detection: 1 mg/L) [29, 123]. In a study in which a 5-mg oral dose of phenazepam was given to healthy volunteers, 3-hydroxyphenazepam was detected in urine samples but not in blood samples [29]. In research investigating its distribution in the plasma and brain of mice tranquillising and anticonvulsive properties were reported [124-126]. It is a full γ- GABAA receptor positive allosteric modulator [73, 126, 127]. 3-hydroxyphenazepam appears to be pharmacologically active with some 5- to 10-fold higher potency than diazepam, probably due to the bromine atom in the molecule [128]. In addition, 3-hydroxyphenazepam represents the main metabolite of levana (3-hydroxyphenazepam hemisuccinate) [125]. Researchers report that levana has a greater anticonvulsive effect than its metabolite 3-hydroxyphenazepam [125, 126, 129]. 3-Hydroxyphenazepam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
The 3-hydroxyphenazepam molecule was first identified in a collected sample of white powder by the Medical Centre in the University of Freiburg, Institute of Forensic Medicine, Forensic Toxicology Department on 19 October 2015 [69, 110]. It was also detected in a seizure of 21 tablets (12 white tablets and 9 pale blue tablets) by Swedish police on 1 December 2015 in Varberg [69, 110]. The STRIDA project described a case-series of consecutive patients with admitted or suspected NPS intake afferent to the emergency department of all Swedish hospitals in 2012-2016, of which eight cases had 3-hydroxyphenazepam implicated [69]. There are several significant anecdotal reports from psychonaut fora. A low dose is 0.5-1 mg, a typical dose is 1-2 mg, and a high dose is 2-4 mg [58]. Symptomatology occurs 30-90 minutes after oral and/or sublingual intake, with effects lasting 10-24 hours and after-effects for 2-24 hours [58]. The most commonly reported ROAs are oral and sublingually [51, 52, 54].
3.17. Fonazepam
Fonazepam is structurally related to the internationally controlled substance flunitrazepam (aka ‘Rohypnol®’), from which it differs only due to the absence of an N-methyl group [130]. Fonazepam is the desmethyl-derivative and one of the active metabolites of flunitrazepam [95, 130]. Therefore, it has been supposed to possess similar pharmacological and toxicological properties to flunitrazepam (i.e., hypnotic and pre-anesthetic effects) [95]. Furthermore, it also shares structural similarities with the previously notified BZD nifoxipam (3-hydroxydesmethylflunitrazepam), from which it differs by the absence of a hydroxy group. Fonazepam was included in research describing the synthesis of 1,3-dihydro-7-nitro-5-phenyl-2H-1,4-benzodiazepin-2-ones by direct nitration of the corresponding unsubstituted BZDs [131]. The synthesis of fonazepam was first described in a 1963 patent by Hoffman-La Roche and research determining 1,4-BZDs and -diazepin-2-ones in blood by Electron-Capture Gas-Liquid Chromatography (EC-GLC) [131, 132]. Research into the binding affinity to GABAA receptors has been predicted for fonazepam (Ro 05-4435) using artificial neural networks [133]. Fonazepam was reported to have a binding affinity (log IC50) of 0.176 and a predicted value of 0.565 [95, 133, 134]. Fonazepam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Fonazepam was first identified in a sample of 51 tablets (27 white, 15 blue and 9 grey tablets) found in Lidköping, by Swedish police on 10 March 2016. The substance was analytically confirmed by GC-MS using a reference standard [95, 135]. In addition, it was also detected in a collected sample of 1 gram of white/yellow powder received from an online research chemical company based in China on 13 January 2016. The sample was collected by the Medical Center at the University of Freiburg, Institute of Forensic Medicine, Forensic Toxicology Department [10]. There are several significant anecdotal reports on psychonaut fora. Fonazepam is normally consumed in a tablet (or in powder) form and administered in doses ranging from 0.5 to 3 mg [51, 52, 54]. The most commonly reported ROAs are oral and sublingual [51, 52, 54]. It is usually taken in association with other BZDs [52]. Fonazepam is a controlled substance on Schedule IV of the Controlled Substances Act in the U.S.A. as a derivative of flunitrazepam [95].
3.18. 4-chlorodiazepam
4-chlorodiazepam represents the 4-chloro-derivative of the internationally controlled substance diazepam, from which it differs due to the addition of a chloro substituent in the para or 4-position of the phenyl ring. It is a positional isomer of another designer BZD, diclazepam (Ro5-3448 or 2’-chlorodiazepam) [136]. First studies investigating 4-Chlorodiazepam (aka Ro 5-4864) began in the mid-1960s and it was mentioned in a 1964 patent on ‘Amino substituted benzophenone oximes and derivatives thereof’ [137]. The compound was researched for its anticonvulsant profile against experimental seizures in mice [138]. Initial clinical studies using healthy volunteers indicated that 4-Chlorodiazepam
had a pharmacological effect comparable to that of diazepam [139]. It was further investigated due to its binding and higher affinity to the translocator protein (TSPO), a peripheral-type BZD receptor, even though it does not bind to central-type BZD receptors [139-141]. Research in rodents indicates that 4-chlorodiazepam may exert analgesic, antidepressant, cardio-protective and neuro-protective effects [141-149]. A study by Viega et al. [145] indicated that there were several possible explanations for the neuro-protective effect of 4-Chlorodiazepam including modulation of the mitochondrial transmembrane potential protecting the neural cells from damage by reactive species, prevention of apoptosis or regulation of steroid synthesis. 4-Chlorodiazepam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
4-chlorodiazepam has been first identified in a sample of 5 grams of off-white powder by the Slovenian National Forensic Laboratory in Ljubljana. The sample was purchased from the Internet as part of the RESPONSE project and was received on 10 May 2016, shipped from China. The substance was analytically confirmed by GC-MS, HPLC-TOF (Time-of-Flight High-Performance Liquid Chromatography), FTIR-ATR (Spectrophotometry Infrared-Attenuated total reflectance), GC-MS-IR-(condensed phase) and ion chromatography [9, 135]. There are limited clinically significant anecdotal reports from psychonaut fora. It has been described as having pro-anxiety and pro-convulsive effects [54, 116].
3.19. Flunitrazolam
Flunitrazolam is a triazolo BZD, structurally related to the previously notified clonazolam (clonitrazolam), differing by the replacement of 2-chloro with 2-fluoro on the phenyl group. It is also the triazolo version of the internationally controlled substance flunitrazepam [87, 150]. Flunitrazolam was discovered in the 1960s but it has never been marketed [87]. No information about doses, effects, safety and tolerability has been published so far. However, based on its structural similarity to other triazolo-BZDs, it has been supposed that the potency of flunitrazolam is higher than of the already highly potent flunitrazepam [151]. A t ½ of around 8 hours in oral fluid is reported [87]. There are no further data on its pharmacological and/or toxicological profile so far. Flunitrazolam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Flunitrazolam was first identified by an analytical laboratory in Germany on 6 October 2016, although there was seizure of 80 grey tablets in Sweden on 7 June 2016. The molecule has not been previously described in the scientific or patent literature [9, 109, 135, 152]. There are limited clinically significant anecdotal reports from psychonaut fora. A threshold dose is stated to be 0.3-0.4 mg, a low dose as 0.4-0.8 mg, a typical dose is 0.8-1.5 mg, and a high dose as 1.5-3 mg [58]. The onset of symptomatology occurs within 10-30 minutes after oral intake, with effects lasting 4-5 hours and after-effects for 1-16 hours; whilst after sublingual administration, symptoms start within 5-15 minutes, may last around 4-5 hours with after-effects present for 1-12 hours [58].
3.20. Bromazolam
Bromazolam is a triazolo-BZD structurally related to flubromazepam, from which it differs due to the absence of a fluorine in the 2-position on the phenyl ring. Bromazolam is also structurally similar to pyrazolam, where the pyridinyl group has been replaced with a phenyl group. Moreover, bromazolam is the brominated version of the internationally controlled substance alprazolam [115, 153]. There are further data about clinical, pharmacological or toxicological properties. Bromazolam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Bromazolam was first identified in a sample of 0.74 grams of yellow powder seized by Swedish Customs in Stockholm on 3 August 2016. The substance was analytically confirmed by the Swedish National Forensic Centre using GC-MS, GC-IRD (Gas Chromatography with infrared detection), LC-HRMS and NMR [9, 135]. There are limited clinically significant anecdotal reports from psychonaut fora. A low dose is 0.5-1 mg, a typical dose is 1-3 mg, and a high dose is 3-5 mg or more [58]. Symptomatology onset occurs 15-45 minutes after oral intake, effects may last 5-8 hours and after-effects for 1-12 hours after administration [58].
3.21. Norfludiazepam
Norfludiazepam is a BZD, structurally related to the internationally controlled substance diazepam, from which it differs due to the addition of a fluoro substituent in the ortho or 2-position of the phenyl ring and by the absence of the methyl group attached to the amide in diazepam. It is also structurally related to the previously notified 4-chlorodiazepam (Ro 5-4864), from which it differs due to the replacement of the chlorine in the 4-position on the phenyl ring with a fluorine in the 2-position and the methyl group attached to the amide in 4-chlorodizepam is also absent [74]. It is the active metabolite of flurazepam and fludiazepam and is used in the synthesis of midazolam, either as an intermediate with 2-amino-5-chloro-2'-fluorobenzophenone as the starting substance or as the starting substance itself [74, 136, 154]. Norfludiazepam has a significantly longer half-life compared to flurazepam (up to 24-74 hours) [154]. Research into norfludiazepam began in the mid-1960s [74]. No further pharmacological and toxicological data are available. There are no further data about clinical, pharmacological or toxicological properties. Norfludiazepam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Norfludiazepam was first identified in 5 grams of white powder seized by Swedish Customs in Stockholm, on 22 November 2016, and in 50 orange tablets and in 1 gram of white powder by the Medical Center – University of Freiburg, Institute of Forensic Medicine, Forensic Toxicology Department, Freiburg Germany, on 4 March 2016. The substance was analytically confirmed by the Swedish National Forensic Centre using GC-MS [9, 135], There are no clinically significant anecdotal reports from psychonaut fora describing clinical effects of norfludiazepam so far.
3.22. Ro 07-4065
Ro 07-4065 is structurally related to the internationally controlled substances diazepam and fludiazepam. Ro 07-4065 differs from diazepam due to the addition of fluoro substituents in both the 2- and 6-positions on the phenyl ring and differs from fludiazepam due to the additional fluoro-substituent in the 6-position on the phenyl ring. It is also structurally related to norfludiazepam (Ro 5-3367), formally notified to the EMCDDA in January 2017, from which it differs due to the additional fluoro-substituent in the 6-position on the phenyl ring and due to the addition of a methyl group attached to the amide [10, 155]. Despite it being previously described in a 1972 patent, it is an extremely newly marketed BZD, mainly used as a research tool to help determining the shape and function of the GABA receptor complex [133, 134, 156, 157]. There is limited published information on its pharmacology and toxicology. The binding affinity of Ro 07-4065 to GABAA receptors has been predicted using in silico methods, such as for example artificial neural networks, and being reported as 0.613 [133, 134]. There are no further data about clinical, pharmacological or toxicological properties. Ro 07-4065 is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Ro 07-4065 was firstly identified in 1 gram of pale beige powder seized by Swedish Customs at FedEx Arlanda, Stockholm, on 14 March 2017 and notified as an NPS by Sweden to the EMCDDA in May 2017. The substance originated by China and the sample was declared as a “sample for research” [155]. The substance was analytically confirmed by the Swedish National Forensic Centre using GC-MS, GC-IRD, LC-HRMS and NMR [11, 135]. There are no clinically significant anecdotal reports from psychonaut fora describing the clinical effects of Ro 07-4065 so far.
3.23. Thionordazepam
Thionordazepam is structurally related to internationally controlled nordazepam and alprazolam. Thionordazepam differs from nordazepam due to the replacement of the oxygen with a sulfur and it differs from alprazolam due to the absence of the 1,2,4-triazole moiety [158]. It was previously described in a 1963 patent by Hoffman-La Roche [158]. Thionordazepam is used in the synthesis of alprazolam [159, 160]. The analysis and identification of the starting material and synthesis-related intermediates for alprazolam, including thionordazepam, using high performance thin layer chromatography is reported in the literature [159]. There is no information available on the pharmacology and toxicology of thionordazepam. There are no further data about clinical, pharmacological or toxicological properties. Thionordazepam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Thionordazepam was first identified in 2 grams of pale-yellow powder seized by Swedish Customs in Stockholm on 20 June 2017. The substance was analytically confirmed by the Swedish National Forensic Centre using GC-MS, GC-IRD, LC-HRMS and NMR [11]. There are no clinically significant anecdotal reports from psychonaut fora describing the clinical effects of thionordazepam so far.
3.24. Methyl-clonazepam
Methylclonazepam is a 1,4-BZD, structurally related to clonazepam, being its N-methyl derivative, and to internationally controlled flunitrazepam, from which it differs due to the substitution of the fluorine atom present in flunitrazepam with a chlorine atom [131]. Methylclonazepam also shares structural similarities with the previously notified meclonazepam, diclazepam and cloniprazepam [161-163]. Synthesis of Methylclonazepam was originally described by Sternbach et al. [131]. Behavioural effects of methyl clonazepam were compared with diazepam in rats and mice [164]. Methylclonazepam showed relatively potent muscle relaxant and extremely potent anti-pentetrazol convulsing action as compared to diazepam, even though it has been reported to be approximately 5-times more potent than diazepam in impairing rotarod performance in mice [164]. Methylclonazepam exerted muscle relaxant action in the rotarod method in rats and mice like clonazepam and nitrazepam and an anticonvulsant action like clonazepam [165]. Overall, it possesses a wider pharmacological spectrum than clonazepam, which is almost equal in potency to nitrazepam and is more potent than diazepam [165]. Methylclonazepam at a dose of 2.5 mg/kg showed a depressant effect on gamma motor activity in rats. The depressant effects of a dose of 5.0 mg/kg lasted for more than 60 minutes [166]. Methylclonazepam produced sedation with a general drowsy pattern in the electroencephalogram in rabbits [167]. Furthermore, it has also been reported to exert both facilitative and depressive effects on paradoxical sleep in cats, depending on the dose [168]. From a pharmacokinetic point of view, methylclonazepam has a long plasma t ½ (40 hours) [83]. A double-blind, randomised cross-over study recruiting 18 inpatients affected with Generalized Anxiety Disorder compared daily flexible doses (3-6 mg) of methylclonazepam vs 2.5 mg lorazepam vs placebo, by showing highly significant superiority of both BZDs over placebo and a significant superiority of methylclonazepam over lorazepam on the Hamilton Anxiety scale (p<.001), Clinical Global Impairment (CGI)(p<.01), without any significant differences in side-effects [83]. There are no further data about clinical, pharmacological or toxicological properties. Methylclonazepam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Methylclonazepam was been first identified in 100 grams of pale-yellow powder seized by Swedish Customs in Stockholm on 8 November 2017. The substance was analytically confirmed by the Swedish National Forensic Centre using GC-MS, GC-IRD, LC-HRMS, and NMR [11]. There are no clinically significant anecdotal reports from psychonaut fora describing the clinical effects of methylclonazepam so far.
3.25. Fluclotizolam
Fluclotizolam is a thienodiazepine, where the diazepine ring is fused to thiophene, instead of to a benzene ring, structurally related to internationally controlled brotizolam from which it differs in the halogen substituents at the tiophene and phenyl ring. Moreover, fluclotizolam also shares structural similarities with etizolam, formally notified to the EMCDDA in December 2011, from which it differs due to the replacement of a phenyl ring at the thiophene. Fluclotizolam was mentioned in a 1974 patent on thienotriazolodiazepines [169]. There are no data about clinical, pharmacological or toxicological properties. Fluclotizolam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Fluclotizolam was firstly seized in 94 pale green tablets by Swedish police in Gällivare, on 26 October 2017. The substance was analytically confirmed by the Swedish National Forensic Centre using GC-MS, GC-IRD, LC-HRMS and NMR [11]. It was also identified in 10 blotters seized by Danish customs at the Copenhagen International Post Office on 25 October 2017 [11]. There is no information available on the pharmacology and toxicology of this substance, as there are no published reports so far. Based on its chemical structure and similarity to brotizolam and etizolam, the substance is expected to have sedative-hypnotic effects [170]. There are limited clinically significant anecdotal reports from psychonauts fora. A low dose is 0.25 mg, a typical dose is 0.25-0.5 mg, a high dose is 0.5-0.75 mg, whilst a heavy effect is experienced after more than 0.75 mg [58]. Caution is strongly recommended at higher doses, with 2 mg being considered a ‘blackout dose’ [171]. The onset of symptomatology occurs 10-30 minutes after oral intake, effects may last 6-14 hours and after-effects for 1-36 hours after administration [58]. There are conflicting anecdotal reports on its dosage, though claims have been made that it has an approximately 3-fold higher potency and a shorter t ½, compared to etizolam [51, 58, 116, 171].
3.26. Tofisopam
Tofisopam is an atypical 2,3-benzodiazepine, which contains a stereogenic center. Hence, it possesses an S- and an R-enantiomer form [172, 173]. Tofisopam is a BZD first developed in Hungary and authorised in some European countries, marketed in the racemate form under the name Grandaxin®, orally administered at 300 mg daily for the treatment of neurotic and somatic disorders associated with tension, anxiety, vegetative disorders, lack of energy and motivation, apathy, fatigue, depressed mood and alcohol withdrawal syndrome [174, 175]. Moreover, tofisopam is marketed in other international countries, such as Japan, India, Russia etc. [176, 177]. Pellow et al. [178] described its behavioural and biochemical profile, in both animals and humans. Tofisopam does not act on the BZD site of the GABA receptor but has a good anxiolytic activity without having appreciable sedative, anticonvulsant, amnestic, or muscle-relaxant effects in humans [179, 180]; whilst it completely lacked anxiolytic and anticonvulsant properties in animals [178]. In addition, it appears to exert mixed dopamine agonist and antagonist-like properties in several in vivo and in vitro animal tests [181]. Moreover, under some circumstances, tofisopam may demonstrate stimulant properties as well [178, 182]. Tofisopam has multiple selective phosphodiesterase (PDE)-inhibiting actions (i.e. at PDE4A1, PDE10A1, PDE3 and PDE2A3) which are being actively evaluated for managing negative and cognitive symptoms of schizophrenia [176, 180, 183, 184]. Furthermore, it does not impair psychomotor and intellectual performance, like other BZDs and it has a potent capability in alleviating vegetative symptoms accompanying anxiety disorders [182, 185-188]. Due to these pharmacodynamic properties, tofisopam has been considered as an atypical BZD [178, 183]. Tofisopam is rapidly absorbed from the intestinal tract, peak plasma concentrations are reached within 1-1.5 hours in humans [189]. After oral absorption, it undergoes extensive first-pass hepatic metabolism, mainly by demethylation, and has a t ½ of around 6-8 hours [190]. Hatayama et al. [191] described a hypouricemic effect after 2-3 hours following oral administration of tofisopam (300 mg daily) comparable or greater than that of losartan and/or fenofibrate; hence, it may be suggested in patients with hyperuricemia and/or gout with concomitant autonomic dysfunction symptoms. There are no further data about clinical, pharmacological or toxicological properties. Tofisopam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Tofisopam was firstly identified in 80 white tablets, labelled as ‘GRANDAX’, seized in blister packs by Swedish Customs in Malmö on 22 November 2017. The substance was analytically confirmed using GC-MS, NMR and LC-HRMS by the Swedish National Forensic Centre [11]. There is considerable information available on the pharmacology, toxicology and clinical profile of this substance, as there are some published reports on tofisopam, particularly focusing on its anxiolytic properties and alleviating effects on gastrointestinal functional or psychosomatic disorders [177, 182, 185-188, 192-194]. A Japanese retrospective observational study carried out on a sample of patients affected with functional dyspepsia described a significant improvement (p<.05) at the Gastrointestinal Symptom Rating Scale (GSRS) total score, the State-Trait Anxiety Inventory (STAY) total score, and the Zung Self-rating Depression Scale (SDS) total score, and at the following GSRS domains: abdominal pain, indigestion and constipation [177]. A case-report described a clinically significant efficacy of tofisopam in the treatment of paroxysmal supraventricular tachycardia [194]. There are significant anecdotal reports on psychonaut fora which describe a “relaxing effect” with “excellent concentration, motivation, and sociability, without any muscle relaxant or any sedative or amnestic properties and without any apparent withdrawal effects” [51, 116].
3.27. Flualprazolam
Flualprazolam is a 1,2,4-triazolobenzodiazepine, where the diazepine ring is fused to a triazole ring. Flualprazolam is the 2-fluoro derivative of alprazolam and it differs from triazolam by replacement of the chlorine with fluorine at the 2-position (ortho position) on the phenyl ring attached to the benzodiazepine moiety. Both substances, alprazolam, and triazolam, are under international control. Flualprazolam is also structurally related to flubromazolam, formally notified to the EMCDDA in 2014 [152], from which it differs due to the replacement of bromine with chlorine at the 8-position on the benzodiazepine moiety [96, 195]. The synthesis of flualprazolam has been previously described [96, 195, 196]. Based on its chemical structure and similarity to alprazolam and triazolam the substance is expected to have sedative and hypnotic effects. The 1,4-triazolo ring present in triazolobenzodiazepines prevents the oxidative metabolism of classical BZDs (i.e., diazepam), which results in the formation of active metabolites with long elimination t ½ [197]. In some of the pharmacological tests conducted on mice, fluaprazolam was reported to be active at doses of less than 10 μg/kg [96]. There are no further data about clinical, pharmacological or toxicological properties. Flualprazolam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Flualprazolam was first identified in 89.8 grams of pale beige powder seized by Swedish police in Linköping on 29 November 2017. The substance was analytically confirmed using GC-MS, LC-HRMS and NMR by the Swedish National Forensic Centre [11]. It was also identified in 1 gram of yellow powder, collected by the Slovenian National Forensic Laboratory in Ljubljana, purchased from the Internet, as part of the RESPONSE 2 project and was received on 3 January 2018. The price was 129 US dollars per 1 gram and the sample was advertised as flualprazolam. The substance was analytically confirmed using GC-MS, HPLC-TOF, IC, FTIR-ATR, GC-(MS)-IR condensed phase at the Slovenian National Forensic Laboratory and by NMR at the Faculty of Chemistry and Chemical technology, University of Ljubljana [10]. There is limited information available on the pharmacology and toxicology of this substance, as there are not published reports so far. However, in a recent report by WHO ECDD on flualprazolam, more than 25 deaths with confirmed exposure to flualprazolam and around 30 non-fatal poisonings with suspected exposure have been reported so far [12]. The presence of this molecule in the material seized in relation to an ‘anesthesia robbery’ case has recently been reported [198]. There are limited clinically significant anecdotal reports from psychonauts fora. A longer half-life and higher potency compared to alprazolam is reported, with strong and heavy effects after oral intake of 0.5-1 mg and 1-2 mg respectively [58]. Symptomatology occurs 10-30 minutes after oral intake, effects may last 6-14 hours and after-effects for 36 hours [58]. Flualprazolam appears to be marketed as ‘fake’ alprazolam (labelled as ‘Xanax®’ or Xanor’ tablets) and some psychonauts defined it as the “king of the RC benzos” (i.e. the king of the research chemicals benzodiazepines) [54, 199, 200].
3.28. Clobromazolam/Phenazolam
Clobromazolam is a 1,2,4-triazolobenzodiazepine, where the diazepine ring is fused to a triazole ring. Clobromazolam is the 2-chloro derivative of bromazolam and shares structural similarities with clonazolam and flubromazolam, formally notified to the EMCDDA in 2016, 2015 and 2014 respectively [152]. Clobromazolam is structurally related to the internationally controlled substances phenazepam, alprazolam and triazolam. The synthesis of clobromazolam (compound V) has been previously described in the literature; it differs from triazolam due to the replacement of chlorine with bromine at the 8-position on the benzodiazepine moiety [201]. It has been pharmacologically evaluated following oral administration in mice and reported as “very potent in pharmacological tests for anticonvulsant, central depressant and discoordination activity in mice”, with a strong central depression, ataxia and convulsive reactions at doses of 0.2-1 g/kg, with symptoms lasting more than 24 hours [201]. The acute toxicity of clobromazolam was found to be very low in mice and, compared to triazolam, it has a similarly low toxicity with a similar anticonvulsant effect towards pentetrazol, with 25% of the triazolam activity in the test of electroshock, 12% of the locomotor inhibiting effect and 40% of the discoordination activity [201]. There are no further data about clinical, pharmacological or toxicological properties. Clobromazolam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Clobromazolam was first identified in 20 white capsules containing white powder seized by Swedish Police in Luleå on 11 March 2016 [11]. The substance was analytically confirmed using GC-MS, LC-HRMS and NMR by the Swedish National Forensic Centre [11]. There is limited information available on the pharmacology and toxicology of this substance, as there are no published reports so far. There are no clinically significant anecdotal reports from psychonaut fora describing the clinical effects of clobromazolam so far.
3.29. Bentazepam
Bentazepam is a thienodiazepine that differs from the previously notified thienodiazepines (i.e., etizolam, metizolam and fluclotizolam) due to the presence of cyclohexane fused to the thiophene instead of a triazole [201]. Bentazepam was used in the manufacture of a medicinal product for human use authorized in Spain and marketed as Tiadipona® (marketing authorization suspended on 6 March 2019) [202]. It exerts anxiolytic, anticonvulsant, sedative and muscle relaxant effects [203, 204]. Its peak plasma concentration is reached within 1-3 hours after oral administration and it has a t ½ of approximately 3.3 hours [202]. Hepatitis and severe liver damage have also been associated with bentazepam [205-209]. There are no further data about clinical, pharmacological or toxicological properties. Bentazepam is not currently controlled under the 1971 United Nations Convention on Psychotropic Substances or the 1961 Single Convention on Narcotic Drugs.
Bentazepam was firstly seized by the Swedish Police, on 3 March 2014, in Borlänge in a sample of 6 white round tablets. The substance was identified by the Swedish National Forensic Centre sing GC-MS and reference material [110]. There are limited clinically significant anecdotal reports from psychonauts fora. A low dose is 15-30 mg, a typical dose is 30-50 mg whilst a strong effect is experienced with 50-75 mg or more [58]. Symptomatology happens 15-45 minutes after oral intake, effects may last 3-6 hours and after-effects for 1-8 hours after administration [58].
4. Discussion
During recent years, the advent of NPS and the dissemination of internet purchasing has meant that some BZDs, that are not licensed in most countries, but which remain available in some others (i.e., Russia and ex-Soviet Union states), began to appear more widely and frequently in the NPS market as ‘legal and without medical prescription alternative’ to the common regulated pharmaceutical BZDs (i.e., phenazepam, etizolam, etc.). However, beside these BZDs, there are also completely new and recently synthetised NPS BZDs (i.e., pyrazolam, flubromazepam, etc.), some of them were previously research trial compounds whose development did not proceed to clinical use [79, 210]. These compounds are sold as tablets, powder, pellets, capsules or blotters, and recently liquids under their own names, at very affordable prices [37, 211, 212]. They are usually administered orally, intramuscularly, intravenously or insufflated nasally, or inhaled by smoking or vaporisation; occasionally rectally [70, 152, 211]. Most designer BZDs possess a liver metabolism, primarily by oxidative metabolism mediated by the CYP450 family, mainly CYP3A4 [152]. One of the major issues and concerns related to the emergence of NPS BZDs is that there is no guarantee of the quality of their composition and purification and, hence, most NPS consumers may be inadvertently taking not that BZD labelled but another compound (belonging or not to the BZD family) and potentially many times more harmful than expected, due to the lack of information regarding drug-drug interactions. In fact, some of them are also sold on the illicit drug market as counterfeit forms of other traditional/pharmaceutical BZDs (e.g., diazepam, alprazolam, etc.), which may increase the risk of unintentional overdose and intoxication [9, 10, 170]. Furthermore, there have been sporadic reports of the use of these designer BZDs as either adulterants or diluents in heroin or other synthetic opioids or cannabinoids, with consequent respiratory depression documented [8, 213, 214].
Designer BZDs began to appear as recreational drugs/NPS around 2007 [68, 152, 215]. Between 2012 and 2019, new compounds classified as ‘designer BZDs’ started to be distributed by online retailers, mostly labelled as ‘research chemicals’ [10]. Some of these compounds are extremely powerful, also at lower dosages (i.e., flubromazolam) [152, 215]. Indeed, psychonauts boosted the NPS market and shaped the drug market overall, by increasing NPS, including designer BZDs, popularity and interest by posting technical and accurately descriptive trip reports on the web. In fact, the increasing interest towards the ‘psychonauts’ world’, and contextually towards online platforms, specifically designed and disseminated as “pro-drug” virtual informative centers for e-psychonauts, facilitated the exchange of pharmaceutical and clinical ‘dirty’ (aka ‘unsafe’, ‘unverified’) information about NPS in general, and, specifically, on designer BZDs [216-218]. However, there is currently a “dramatic gap” in the information flow amongst clinicians working in the Addiction and/or in Mental Health services, as this gap is represented by the information available on the surface (i.e., mainly derived by the information provided by EMCDDA/other drug monitoring systems and literature) and those ‘hidden’ (i.e., accessible mainly amongst NPS consumers, as shared/posted by themselves; hence, mainly available online but not always ‘verified’). Moreover, it is assumed that clinicians working in Mental Health and Addiction Services may “underestimate” the real number of designer BZDs consumers and, hence, they may not collect clinically relevant data on their clinical, pharmacological and toxicological effects. This consideration mainly derived by the fact that there are not so far published epidemiological data on this relatively new emerging phenomenon, as it may be difficult to collect data for several reasons here listed: a) most designer BZDs are extremely new and unknown by clinicians who do not ask for that specific compound to their patients; b) most consumers (mainly those who buy drugs/NPS, including designer BZDs online) may not completely aware if they are taking traditional or designer BZDs which are frequently sold as counterfeit of traditional BZDs; c) most NPS consumers may not completely aware if they are taking designer BZDs as they are mainly contained as adulterants, etc. in NPS packages without specifying they are mixed with synthetic opioids/cannabinoids, etc.
Overall, the effects of designer BZDs largely vary depending on the dose(s) consumed, the route of administration and combination with other drugs/medicaments/substances. However, information on the effects of these designer BZDs is largely limited to self-reported experiences coming from online trip reports and limited case-reports and/or case-series [37, 152, 170, 216]. By analogy to traditional BZDs, the desired effects may include sociability, muscle relaxation, sleep-inducing and anxiolytic effects, as a reducer of chronic pain, and nervousness. At lower doses, BZDs may cause drowsiness, fatigue and lethargy; whilst, at higher doses motor coordination disorders, mood swings, dizziness and sometimes euphoria have been reported [152]. The use of BZDs, including ‘designer benzos’ varies from country to country, even within the UK. So, whilst ‘traditional’ BZDs account for most of BZD-related fatalities in Northern Ireland, in Scotland it is NPS varieties that dominate not only the BZD-related poisoning deaths, but all drug-poisoning related ones. For example, nearly 95% of all NPS-related deaths registered in Scotland during the period 2013-8 involved BZD analogues (unpublished data from National Records of Scotland). Furthermore, dependence and tolerance have been documented as well as withdrawal syndrome after abrupt discontinuation [150, 152, 219]. In fact, designer BZDs, like traditional ones, play an integral role in the reinforcing and addictive properties through the GABAA receptors in the mesolimbic dopaminergic pathway, by determining the onset of tolerance and a physical and psychological dependence [219].
5. Limitations of the present study
Therefore, the great limitation of the present overview arises from the fact that the information search strategy has been mainly performed on those designer BZDs so far notified to EMCDDA and not strictly limited to all designer BZDs currently available on the NPS market. The ongoing ‘NPS finder’ project performed by our research team is working to develop a useful online tool able to identify a much larger number of NPS, including BZDs, by specifically focussing on psychonauts’ entries only. Therefore, one of the major limitations of our work is directly related to these ‘information’ and ‘search strategy’ biases which indeed limit the reliability of the findings collected here. In addition, most data here discussed mainly derive from cases of intoxications (i.e., subjects coming to the emergency departments due to health risky reactions following designer BDZs intake) or trip experiences collected through a netnographic approach on the web.
Conclusion
BZDs are a class of drugs widely prescribed for the short-term treatment of anxiety, epilepsy, muscle spasm, alcohol withdrawal and insomnia. However, BZDs can also be misused and/or abused by various substance user groups, mainly in combination with other drugs of abuse (e.g., opioids, psychostimulants, etc.) [152, 215]. Their wide application and use facilitates the diffusion and growth of an extensive interest in research around their chemical structures, pharmacological properties and clinical effects, by incentivising the development of a wide variety of active compounds that did not obtain marketing authorisation and which are now being rediscovered and sold online as ‘legal alternatives to the prescribed-only BZDs’, becoming indeed NPS (aka ‘designer/synthetic/new BZDs’) [10]. Despite limited information so far available for most of these ‘designer BZDs’, a recent trend in the NPS market appears to be a greater diffusion of this class [10]. One could argue that a possible explanation may depend on country, i.e. in some countries such as the UK it is indeed extremely difficult to be prescribed with BZDs; other explanations could be that this dramatic increased trend is related to higher rates of anxiety disorders, particularly amongst substances users, and an increased number of subjects who self-medicate [220]. Furthermore, designer BZDs may represent a target of interest as well for those subjects who commonly abuse other substances (i.e., opioids, psychostimulants, etc.). The recent increase in the dissemination and seizures of this new NPS class, observed during the last several years, poses a great concern regarding health risks associated with their intake, particularly amongst those vulnerable subjects who consume other substances [211, 221]. In fact, combining designer BZDs with other drugs (e.g., ethanol, marijuana, stimulants, opioids, and other psychoactive substances) can prove to be risky, especially as there are no reliable data about any possible synergic and antagonistic effects.
Therefore, further clinical research should be conducted within this field in order to better identify and prevent potential risky behaviours and attenuate (if not actually eliminate) health risks associated with the intake of these designer/synthetic/new BZDs. A preventive and informative campaign should be aimed at clinicians and mental health professionals as well as amongst drug users about this new class of BZDs.
Table 1.
Pharmacokinetic and chemical structure classification of traditional and some new/designer BZDs.
| SHORT / ULTRA-SHORT PLASMA HALF-LIFE (t½) BZD - I GROUP |
|---|
| • Drug name (traditional): a) Triazolobenzodiazepines: e.g., Alprazolam; Estazolam; Triazolam b) Thienobenzodiazepines: e.g., Bentazepam; Brotizolam; Clotiazepam • NPS: a) Triazolobenzodiazepines: Adinazolam; Alprazolam triazolobenzophenone derivative; Bromazolam; Clobromazolam; Clonazolam (clonitrazolam); Flualprazolam; Flubromazolam; Flunitrazolam; Nitrazolam; Pyrazolam; Thionordazepam; Zapizolam b) Thienobenzodiazepines: thienotriazolodiazepines (Etizolam; Deschloroetizolam; Metizolam; Fluclotizolam) |
| • Pharmacokinetic features a) Plasma half-life: increased in elderly patients and in those with liver disease. a) Triazolobenzodiazepines: 6-24 hours. b) Thienobenzodiazepines: 2-6 hours. b) Metabolism: hydroxylation and conjugation with glucuronic acid. |
| • Accumulation: after prolonged use. |
| • Interactions: Cimetidine, propranolol, fluoxetine, paroxetine, fluvoxamine and oral contraceptives inhibit the processes of hepatic hydroxylation (P-450), and decrease the metabolism of these BZD, increasing their plasma levels. Excessive sedative effects and hypotension when combined with alcohol. |
| SHORT / ULTRASHORT PLASMA HALF-LIFE (t½) BZD - II GROUP |
| • Drug name (traditional): Oxazepam-like BZD: Camazepam; Lorazepam; Lormetazepam; Oxazepam; Temazepam. • NPS: Flutazolam; Nimetazepam (belonging to 1,4-BZD family) |
| c) Pharmacokinetic features: no modification in elderly patients or in those with liver disease. a) Plasma half-life: 10-24 hours. b) Metabolism: conjugation with glucuronic acid. No active metabolites. |
| • Accumulation: no accumulation. |
| • Interactions: No relevant pharmacokinetic interactions. Excessive sedative effect and risk of hypotension when combined with alcohol or CNS sedative drugs. |
| MEDIUM / LONG PLASMA HALF-LIFE (t½) BZD |
| • Drug name (traditional) a) Pronordiazepam-like BZD: Bromazepam; Chlorazepate; Chlordesmetildiazepam; Chlordiazepoxide; Clobazam; Desmethyldiazepam; Diazepam; Flurazepam; Ketazolam; Medazepam; Pinazepam; Prazepam; Quazepam. b) Nitro-BZD: Clonazepam; Flunitrazepam; Nitrazepam. • NPS: a) Pronordiazepam-like BZD: 4’-chlorodiazepam or Ro 5-4864 (belonging to 1,4-BZD family); Diclazepam or Ro 5-3448 (belonging to 1,4-BZD family); Flubromazepam (belonging to 1,4-BZD family); Meclonazepam (belonging to 1,4-BZD family); Norfludiazepam or Ro 5-3367 (belonging to 1,4-BZD family); Ro 07-4065 (belonging to 1,4-BZD family). b) Nitro-BDZ: 3-hydroxyphenazepam (belonging to 1,4-BZD family); Cloniprazepam (belonging to 1,4-BZD family); Fonazepam or Ro-4435 (belonging to 1,4-BZD family); Methylclonazepam (belonging to 1,4-BZD family); Nifoxipam or DP 370 (belonging to 1,4-BZD family); Phenazepam (belonging to 1,4-BZD family). |
| • Pharmacokinetic features a) Plasma half-life: 24-48 hours (nitro-BZD); >48 hours (pro-nordiazepam-like BZD). Plasma t½ increases in elderly and patients with liver diseases. b) Metabolism: demethylation to nordiazepam, hydroxylation and conjugation by glucuronic acid; nitro-reduction and conjugation by glucuronic acid. Active metabolites with long plasma half-life. |
| • Accumulation: after long-term treatment. |
| • Interactions: cimetidine, propranolol, fluoxetine, paroxetine, fluvoxamine, and oral contraceptives, decrease hepatic hydroxylation (CYP-450) and increase plasma levels of these BZD. Combination with alcohol and CNS depressant drugs induces severe sedative and hypotensive effects. |
Table 2.
List of current new/designer benzodiazepines.
| Name of BZD (CAS Registry Number) |
Chemical Formula
(Molecular Weight, g/mol) |
Molecular Structure |
Systematical Chemical Name (IUPAC Name)
Other or Street Names |
Year Patented
(Year Notified to the EMCDDA) |
Aspect and Characteristics |
User Reports of
Effects (Typical Recreational dose, mg) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenazepam (51753-57-2) | C15H10BrClN2O (349.6) |

|
(7-bromo-5-(2-chlorophenyl)-1,3-dihydro-1,4-benzodiazepin-2-one) Other/Street Names: Fenazepam; BD 98; Elzepam; Phezipam; Phenorelaxan; Phenzitat; Bonzai; Bonsai; Supersleep; Fenaz; Soviet Benzo; Zannie |
1974 (2007, UK) |
White crystalline powder with a greyish-yellow tinge, odorless and tasteless, insoluble in water and soluble in ethanol, dimethylformamide and chloroform | Anxiolytic, extremely sedating, sort-term memory loss, blackouts at higher doses, insomnia, delirium, psychotic episodes (0.5-1) |
|||||||||||||||
| Etizolam (40054-69-1) |
C17H15ClN4S (342.1) |
 |
4-(2-chlorophenyl)-2-ethyl-9-methyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine Other/Street Names: AHR 3219; Depas; Y-7131; Etizola; Sedekopan; Pasaden; Etizest; Etilaam; Etiz; Etizzy |
1978 (2011, UK) |
White crystalline powder Tablets Blotters similar to LSD paper doses |
Anxiolytic, muscle relaxation, sleep-aid, euphoria (0.25-3) | |||||||||||||||
| Pyrazolam (39243-02-2) |
C16H12BrN5 (354.2) |
 |
8-bromo-1-methyl-6-(pyridin-2-yl)-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine Other/Street Names: 1-methyl[1,2,4]triazo-6-(2-pyridinyl)-8-bromo-1,4-benzodiazepine |
1979 (2011, Finland) |
Tablets | Effects lasting 6-7 hours, anxiolytic, low sedation, low hypnotic effect, low recreational value (1) |
|||||||||||||||
| Flubromazepam (2647-50-9) |
C15H10BrFN2O (331.1) |
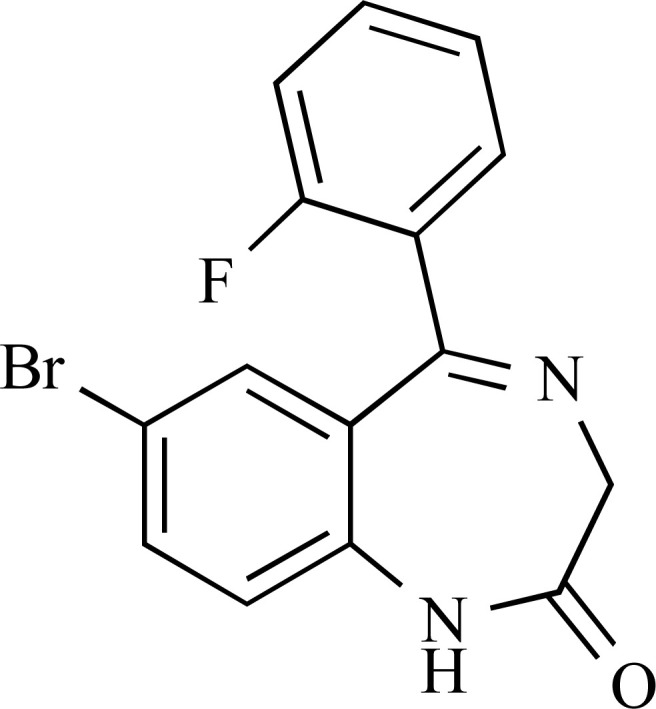 |
7-bromo-5-(2-fluorophenyl)-1,3-dihydro-2H-1,4-benzodiazepin-2-one Other/Street Names: NA |
1962 (2013, Germany) |
Capsules | Effects lasting 18-24 hours, anxiolytic, mild euphoria, blackouts, sedating and muscle relaxation, short-term memory loss (4) |
|||||||||||||||
| Diclazepam (2894-68-0) |
C16H12Cl2N2O (319.2) |
 |
7-chloro-5-(2-chlorophenyl)-1-methyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one Other/Street Names: Ro 5-3448; 2-chlorodiazepam; chlorodiazepam |
1964 (2013, Sweden) |
Blue tablets | Effects lasting 5-12 hours, anxiolytic, useful for ‘tapering’ dependence of other BZDs, low cognitive impairment, low recreational value (1-2) |
|||||||||||||||
| Name of BZD (CAS Registry Number) |
Chemical Formula (Molecular Weight, g/mol) |
Molecular Structure |
Systematical Chemical Name (IUPAC Name) Other or Street Names |
Year Patented (Year Notified to the EMCDDA) |
Aspect and Characteristics |
User Reports of Effects (Typical Recreational dose, mg) |
|||||||||||||||
| Alprazolam triazolobenzophenone derivative (125316-83-8) |
C17H15ClN4O (326.8) |
 |
[2-[3-(Aminomethyl)-5-methyl-4H-1,2,4-triazol-4-yl]-5-chlorophenyl] phenylmethanone Other/Street Names: NA |
1986 (2014, Spain) |
White powder | Anxiolytic (NA) |
|||||||||||||||
| Meclonazepam (S-enantiomer: 58662-84-3; Racemate: 67027-56-9) |
C16H12ClN3O3 (329.7) |
 |
(S)-5-(2-chlorophenyl)-3-methyl-7-nitro-1,3-dihydro-2H-1,4-benzodiazepin-2-one Other/Street Names: (S)-3-methylclonazepam; Ro 11-3128; Ro 11-3624; Meclonazepamum; Methylclonazepam; 3-methylclonazepam |
1975 (2014, Sweden) |
White powder | Low sedation, anxiolytic, muscle relaxation (2-3) |
|||||||||||||||
| Deschloroetizolam (40054-73-7) |
C17H16N4S (308.4) |
 |
2-ethyl-9-methyl-4-phenyl-6h-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin Other/Street Names: ETZ-2; etizolam-2 |
1998 (2014, UK) |
Blue tablets | Effects lasting 12-24 hours, anxiolytic, sedation, slight euphoria (4-6) |
|||||||||||||||
| Flubromazolam (612526-40-6) |
C17H12BrFN4 (371.2) |
 |
8-Bromo-6-(2-fluorophenyl)-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine Other/Street Names: JYI-73 |
1978 (2014, Sweden) |
White rectangle shaped tablets | Effects lasting 12 – 18 hours, anxiolytic, high tolerance to lower doses quickly observed, blackouts and memory loss, strongly sedating, higher doses of 2.5 – 4 mg have effects reported to last up to 3 days and strong memory loss and cognitive impairment. Ingestion of 3 mg of flubromazolam 19 hours prior to hospitalization has been reported in a patient. Severe respiratory failure, hypotension, central nervous system depression and brain damage were observed. (0.15-0.25) |
|||||||||||||||
| Name of BZD (CAS Registry Number) |
Chemical Formula (Molecular Weight, g/mol) |
Molecular Structure |
Systematical Chemical Name (IUPAC Name) Other or Street Names |
Year Patented (Year Notified to the EMCDDA) |
Aspect and Characteristics |
User Reports of Effects (Typical Recreational dose, mg) |
|||||||||||||||
| Nifoxipam (74723-10-7) |
C15H10FN3O4 (315.3) |
 |
5-(2-fluorophenyl)-3-hydroxy-7-nitro-1H-benzo[e][1,4]diazepin-2(3H)-one Other/Street Names: 5-(2-fluorophenyl)-3-methyl-7-nitro-1,3-dihydro-2H-1,4-benzodiazepin-2-one; 3-hydroxydesmethylflunitrazepam; DP-370 |
1985 (2015, Sweden) |
Brown tablets | Effects lasting 12-18 hours, anxiolytic, moderately sedating, mild euphoric, High doses can cause users to feel sleep-deprived, muscle relaxant (0.5-2) |
|||||||||||||||
| Clonazolam (33887-02-4) |
C17H12ClN5O2 (353.8) |
 |
6-(2-chlorophenyl)-1-methyl-8-nitro-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine Other/Street Names: clonitrazolam |
1971 (2014, Sweden) |
White powder in yellow tablets | Slight euphoria, strongly sedating (0.5-1) |
|||||||||||||||
| Adinazolam (37115-32-5) |
C19H18ClN5 (351.8) |
 |
1-(8-chloro-6-phenyl-4H-[1,2,4]triazolo[4,5-a][1,4]benzodiazepin-1-yl)-N,N-dimethylmethanamine Other/Street Names: Deracyn®; Adinazolamum; 8-chloro-1-[(dimethylamino) methyl]-6-phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepine |
1976 (2015, Germany, Sweden and Slovenia) |
White powder White tablet labelled “D/CD” |
Strongly sedating, anterograde amnesia (20) | |||||||||||||||
| Nitrazolam (28910-99-8) |
C17H13N5O2 (319.3) |
 |
1-methyl-8-nitro-6-phenyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine Other/Street Names: NA |
1971 (2015, Germany) |
Light brown powder | Anxiolytic, hypnotic, strongly sedating (0.5-2) | |||||||||||||||
| Metizolam (40054-68-0) |
C16H13ClN4S (328.8) |
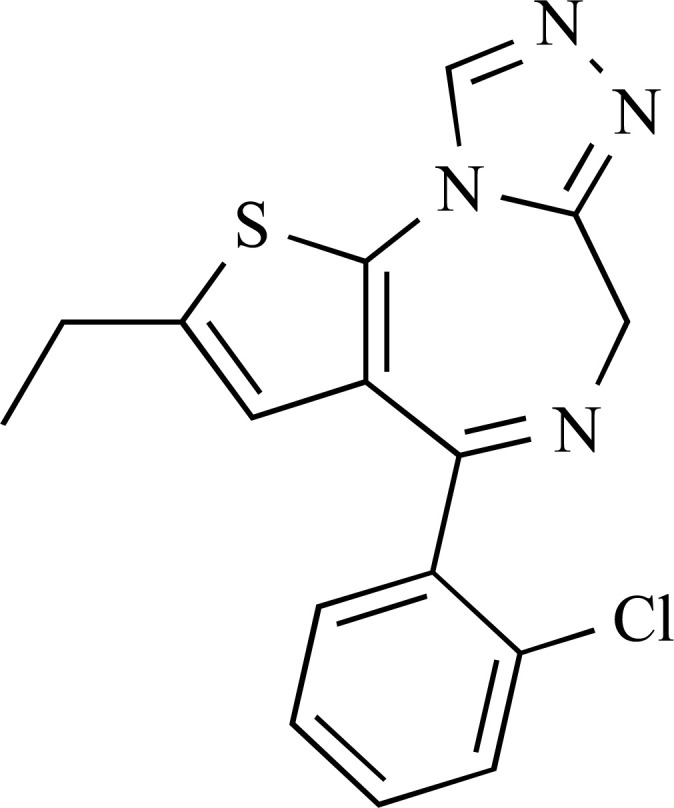 |
4-(2-chloro-phenyl)-2-ethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine Other/Street Names: desmethyletizolam |
1988 (2015, Germany and Denmark) |
Light-blue or blue round tablets | Anxiolytic and muscle relaxation, effects not as strong as etizolam (2) |
|||||||||||||||
| Name of BZD (CAS Registry Number) |
Chemical Formula (Molecular Weight, g/mol) |
Molecular Structure |
Systematical Chemical Name (IUPAC Name) Other or Street Names |
Year Patented (Year Notified to the EMCDDA) |
Aspect and Characteristics |
User Reports of Effects (Typical Recreational dose, mg) |
|||||||||||||||
| Cloniprazepam (1998158-84-1) |
C19H16ClN3O3 (369.8) |
 |
5-(2-Chlorophenyl)-1-(cyclopropylmethyl)-7-nitro-1,3-dihydro-2H-benzo[e][1,4]diazepin-2-one Other/Street Names: 7-nitro-1-(cyclo-propyl(methyl))-1,3-dihydro-5-(2-chlorophenyl)-2H-1,4-benzodiazepin-2-one; kloniprazepam; 2-chloro-7’-nitroprazepam; 1-cyclopropylmethylclonazepam |
Not reported (2015, Sweden) |
White capsule | Slight anxiolytic, higher doses (>5-10 mg) required for muscle relaxation, sedation in most users (2.5) | |||||||||||||||
| 3-hydroxyphenazepam (70030-11-4) |
C15H10BrClN2O2 (365.6) |
 |
7-bromo-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-2H-1,4-benzodiazepin-2-one Other/Street Names: 7-bromo-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy-2H-1,4-benzodiazepin-2-one; 3-HOP; HPNZ; 3-oxyfenazepam; 3-hydroxyfenazepam |
Not reported (2016, Sweden) |
White tablet and pale blue tablet | Anxiolytic, slight muscle relaxation, strongly sedating (0.5-2) | |||||||||||||||
| Fonazepam (2558-30-7) |
C15H10FN3O3 (299.3) |
 |
5-(2-fluorophenyl)-1,3-dihydro-7-nitro-2H-1,4-benzodiazepin-2-one Other/Street Names: Desmethyl-flunitrazepam; Norflunitrazepam; Ro 05-4435; N-desmethylflunitrazepam |
1963 (2016, Sweden) |
white, blue and grey tablets white/yellow powder |
Anxiolytic, muscle relation, sedation (0.6) | |||||||||||||||
| 4-chlorodiazepam (14439-61-3) |
C16H12ClN2O (319.2) |
 |
7-chloro-5-(4-chlorophenyl)-1-methyl-3H-1,4-benzodiazepin-2-one Other/Street Names: 7-chloro-5-(4-chlorophenyl)-1-methyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one; 7-chloro-1,3-dihydro-1-methyl-5-(p-chlorophenyl)-2H-1,4-benzodiazepine-2-one; 4-chlorodiazepam; 4’-chlorodiazepam; Ro 5-4864; Ro5-4864; Chlorodiazepam |
1964 (2016, Slovenia) |
Off-white powder | Pro-convulsing effects, also at lower doses neuroprotective, sedative (NA) |
|||||||||||||||
| Name of BZD (CAS Registry Number) |
Chemical Formula (Molecular Weight, g/mol) |
Molecular Structure |
Systematical Chemical Name (IUPAC Name) Other or Street Names |
Year Patented (Year Notified to the EMCDDA) |
Aspect and Characteristics |
User Reports of Effects (Typical Recreational dose, mg) |
|||||||||||||||
| Flunitrazolam (2243815-18-9) |
C17H12FN5O2 (337.1) |
 |
6-(2-fluorophenyl) -1-methyl-8-nitro-4H-[1,2,4] triazolo[4,3-a][1,4]benzodiazepine Other/Street Names: Fluclazolam |
1960 (2016, Germany) |
White Powder Grey and blue Tablets Pellets and soaked into paper strips |
Strong sedative, slight amnesia reported, anxiolytic (0.8-1.5) | |||||||||||||||
| Bromazolam (71368-80-4) |
C17H13BrN4 (353.2) |
 |
8-bromo-1-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine Other/Street Names: 4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine, 8-bromo-1-methyl-6-phenyl-;8- bromo-1-methyl-6-phenyl-4H-benzo[f][1,2,4]triazolo[4,3-a][1,4]diazepine; XLI-268 |
1976 (2016, Sweden) |
Yellow powder | No published reports (1-3) |
|||||||||||||||
| Norfludiazepam (2886-65-9) |
C15H10ClFN2O (288.7) |
 |
7-chloro-5-(2-fluorophenyl)-1,3-dihydro-1,4-benzodiazepin-2-one Other/Street Names: Chloro-5-(2-fluorophenyl)-1,3-dihydro-2H-[1,4]-benzodiazepin-2-one, 7-chloro-5-(2-fluorophenyl)-1,3-dihydro-2H-1,4-benzodiazepin-2-one, 7-chloro-5-(o-fluorophenyl)-1,3-dihydro-2H-1,4-benzodiazepine-2-one; N-desalkylflurazepam; Desalkylflurazepam; Norflurazepam; Desalkylflurazepam; Norflutoprazepam; Ro 5-3367 |
1962 (2016, Sweden and Germany) |
Orange tablet White powder |
Strongly sedating and long lasting effects (5) |
|||||||||||||||
| Ro 07-4065 (39080-67-6) |
C16H11ClF2N2O (320.7) |
 |
7-chloro-5-(2,6-difluorophenyl)-1-methyl-3H-1,4-benzodiazepin-2-one Other/Street Names: 7-chloro-5-(2,6-difluorophenyl)-1-methyl-1,3-dihydro-2H-benzo[e][1,4]diazepin-2-one, 7-chloro-5-(2,6-difluorophenyl)-1,3-dihydro-1-methyl-2H-1,4-benzodiazepin-2-one, 7-chloro-5-(2,6-difluorophenyl)-1-methyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one; Ro-07-4065; Difludiazepam |
1972 (2017, Sweden) |
Pale beige powder | No published reports (NA) |
|||||||||||||||
| Name of BZD (CAS Registry Number) |
Chemical Formula (Molecular Weight, g/mol) |
Molecular Structure |
Systematical Chemical Name (IUPAC Name) Other or Street Names |
Year Patented (Year Notified to the EMCDDA) |
Aspect and Characteristics |
User Reports of Effects (Typical Recreational dose, mg) |
|||||||||||||||
| Thionordiazepam (4547-02-8) |
C15H11ClN2S (286.8) |
 |
7-chloro-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-thione Other/Street Names: 7-chloro-5-phenyl-1,3-dihydro-1,4-benzodiazepine-2-thione7-chloro-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepine-2-thione; 7-chloro-5-phenyl-1,3-dihydro-1,4-benzodiazepine-2-thione, 7-chloro-1,3-dihydro-5-phenyl-2H-1,4-benzodiazepine-2-thione; Thionordiazepam; Thionordiaze-pam |
1963 (2017, Sweden) |
Pale yellow powder | No published reports (NA) |
|||||||||||||||
| Methyl-clonazepam (5527-71-9) |
C16H12ClN3O3 (329.7) |
 |
5-(2-chlorophenyl)-1-methyl-7-nitro-3H-1,4-benzodiazepin-2-one Other/Street Names: 5-(2-chlorophenyl)-1,3-dihydro-1-methyl-7-nitro-2H-1,4-benzodiazepin-2-one5-(2-chlorophenyl)-1-methyl-7-nitro-(1,4)-benzodiazepin-2-one5-(o-chlorophenyl)-1-methyl-7-nitro-(1,4)-benzodiazepin-2-one5-(2-chlorophenyl)-1,3-dihydro-7-nitro-1,4-benzodiazepin-2-one; ID 690; ID-690; Ro 05-4082; R 5-4082 |
1974 (2017, Sweden) |
Pale yellow powder | Anxiolytic effect more potent than lorazepam (1-6) |
|||||||||||||||
| Fluclotizolam (54123-15-8) |
C15H10ClFN4S (332.8) |  |
2-chloro-4-(2-fluorophenyl)-9-methyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine Other/Street Names: 4-(2-fluorophenyl)-2-chloro-9-methyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine |
1974 (2017, Sweden and Denmark) |
Pale green tablets Blotters |
Potent hypnotic (0.25-0.5) |
|||||||||||||||
| Tofisopam (22345-47-7) |
C22H26N2O4 (382.5) |  |
1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5H-2,3-benzodiazepine Other/Street Names: 1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7,8-dimethoxy-5H-2,3-benzodiazepine; 7,8-dimethoxy-1-(3,4-dimethoxyphenyl)-5-ethyl-4-methyl-5H-2,3-benzodiazepine; Grandaxin®; Emandaxin®; EGYT 341; Nodeprine; Sériel; TF |
1978 (2017, Sweden) |
White tablet labelled ‘GRANDAX’ in blister packs | Anxiolytic effects with increased concentration, memory and attention, no withdrawal effects (NA) |
|||||||||||||||
| Name of BZD (CAS Registry Number) | Chemical Formula (Molecular Weight, g/mol) | Molecular Structure |
Systematical Chemical Name (IUPAC Name) Other or Street Names |
Year Patented (Year Notified to the EMCDDA) |
Aspect and Characteristics |
User Reports of Effects (Typical Recreational dose, mg) |
|||||||||||||||
| Flualprazolam (28910-91-0) |
C17H12ClFN4 (326.8) |
 |
8-chloro-6-(2-fluorophenyl)-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine Other/Street Names: 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine; 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-benzo[f][1,2,4]triazolo[4,3-a][1,4]diazepine; 12-chloro-9-(2-fluorophenyl)-3-methyl-2,4,5,8-tetraazatricyclo[8.4.0.02,6]tetradeca-1(14),3,5,8,10,12-hexaene; Ro 11-5073/000 |
1971 (2017, Sweden) |
Pale beige and yellow powder Tablet Pellet |
No published reports (NA) |
|||||||||||||||
| Clobromazolam (87213-50-1) |
C17H12BrClN4 (387.7) |
 |
8-bromo-6-(2-chlorophenyl)-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine Other/Street Names: 8-bromo-6-(2-chlorophenyl)-1-methyl-4H-s-triazolo[4,3-a][1,4]benzodiazepine; Phenazolam; BRN 4550445; DM-II-90 |
1983 (2016, Sweden) |
White capsule containing white powder | No published reports (NA) |
|||||||||||||||
| Bentazepam (Free base: 29462-18-8; Hydrochloride salt: 29462-19-9) |
C17H16N2OS (296.3) |
 |
5-phenyl-1,3,6,7,8,9-hexahydro-2H-[1]benzothieno[2,3-e][1,4]diazepin-2-one Other/Street Names: 5-phenyl-1,3,6,7,8,9-hexahydrobenzothiopheno[2,3-e][1,4]diazepin-2-one; CI 718; QM 6008; Thiadipone; Tiadipone; 608-362-7 (EC No); 29349990 (EU Customs Code CN) |
1986 (2014, Sweden) |
White round tablets | Limited published reports (30-50) |
|||||||||||||||
(Chemical structures performed by using ChemDraw for professionals version 16.0).
by investigating and collecting data coming from the literature so far published and the web as well, from pharmacological, toxicological and clinical points of view.
Acknowledgements
This paper was supported in part by grants of the European Commission (Drug Prevention and Information Programme 2014-16; contract no. JUST/2013/DPIP/AG/4823; EU-MADNESS project). Further financial support was provided by the EU Commission-targeted call on cross border law enforcement cooperation in the field of drug trafficking - DG Justice/DG Migrations and Home Affairs (JUST/2013/ISEC/DRUGS/AG/6429) Project EPS/NPS (Enhancing Police Skills concerning Novel Psychoactive Substances; NPS).
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Stahl S. Stahl’s Essential Psychopharmacology: Neuroscientific Basis & Practical Applications. 4th ed. Cambridge University Press; 2013. [Google Scholar]
- 2.Tan K.R., Rudolph U., Lüscher C. Hooked on benzodiazepines: GABAA receptor subtypes and addiction. Trends Neurosci. 2011;34(4):188–197. doi: 10.1016/j.tins.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellantuono C., Balestrieri M. Psicofarmacoterapia. Roma: Il Pensiero Scientifico Editore; 2014. [Google Scholar]
- 4.Greenblatt H.K., Greenblatt D.J. Designer Benzodiazepines: A Review of Published Data and Public Health Significance. Clin. Pharmacol. Drug Dev. 2019;8(3):266–269. doi: 10.1002/cpdd.667. [DOI] [PubMed] [Google Scholar]
- 5.Fukasawa T., Suzuki A., Otani K. Effects of genetic polymorphism of cytochrome P450 enzymes on the pharmacokinetics of benzodiazepines. J. Clin. Pharm. Ther. 2007;32(4):333–341. doi: 10.1111/j.1365-2710.2007.00829.x. [DOI] [PubMed] [Google Scholar]
- 6.Dear J.W., Bateman D.N. Benzodiazepines. Medicine (Baltimore) 2016;44:145. doi: 10.1016/j.mpmed.2015.12.025. [DOI] [Google Scholar]
- 7.Moosmann B., Bisel P., Auwärter V. Characterization of the designer benzodiazepine diclazepam and preliminary data on its metabolism and pharmacokinetics. Drug Test. Anal. 2014;6(7-8):757–763. doi: 10.1002/dta.1628. [DOI] [PubMed] [Google Scholar]
- 8.Moosmann B., King L.A., Auwärter V. Designer benzodiazepines: A new challenge. World Psychiatry. 2015;14(2):248. doi: 10.1002/wps.20236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.United Nations Office on Drugs and Crimes (UNODC) 2019 Https://www.unodc.org/documents/scientific/Global_SMART_Update_2017_Vol_18.pdf
- 10.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) 2019 Http://www.emcdda.europa.eu/system/files/publications/11364/20191724_TDAT19001ENN_PDF.pdf
- 11.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) The misuse of benzodiazepines among high-risk opioid users in Europe. 2015 Http://www.emcdda.europa.eu/system/files/publications/2733/Misuse%20of%20benzos_POD2015.pdf
- 12.World Health Organization (WHO) Critical Revie Report: flualprazolam. 2019 Https://www.who.int/medicines/access/controlled-substances/Final_Flualprazolam.pdf?ua=1
- 13.Darbinian T.M., Papin A.A., Vagina M.A., Petrov O.V. 1979.
- 14.Zakusov V.V. New psychotropic phenazepam preparation. Pharm Chem J (Ussr) 1979;13:1094–1097. doi: 10.1007/BF00772264. [DOI] [Google Scholar]
- 15.Sozinov V.A., Rudenko O.P., Zinkovskii V.G., Golovenko N.Y., Lisitsyna A.L. Synthesis of hydrogenated analog of phenazepam. Pharm Chem J (Ussr) 1984;18:721–725. doi: 10.1007/BF00773022. [DOI] [Google Scholar]
- 16.Rafstedt K., Hultén P., Brusiu K. Phenazepam as a drug of abuse-high frequency of prolonged symptoms. Clin. Toxicol. (Phila.) 2009;47:436–510. [Google Scholar]
- 17.Johnson W. Phenazepam in Wisconsin drivers.; Annual meeting of the Society of Forensic Toxicologist; Richmond, Virginia, USA. 2010. [Google Scholar]
- 18.Baselt R.C. Disposition of toxic drugs and chemicals in man. 5th ed. Seal Beach, California: Biomedical Publications; 2011. pp. 1320–1321. [Google Scholar]
- 19.Maskell P.D., Paoli G.D., Seetohul L.N., Pounder D.J. Phenazepam is currently being misused in the UK. BMJ. 2011;343:d4207. doi: 10.1136/bmj.d4207. [DOI] [PubMed] [Google Scholar]
- 20.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) 2009 Http://www.emcdda.europa.eu/publications/country-overviews/md
- 21.European Monitoring Centre for Drugs and Drug Addiction European Legal Database on Drugs. 2011 http:// www.emcdda.europa.eu/eldd
- 22.Drugs-Forum Legal status of phenazepam around the world. 2019 Https://drugs-forum.com/forum/showwiki.php?title1
- 23.Advisory Council on the Misuse of Drugs (ACMD) Phenazepam advice. 2011 Http://www.homeoffice.gov.uk/publications/agencies-public-bodies/acmd1/acmd-
- 24.Home Office 2011 Https://www.gov.uk/government/news/import-ban-of-new-legal-
- 25.Home Office 2012 Https://www.gov.uk/government/publications/a-change-to-the-misuse-of-drugs-act-1971-
- 26.Lillsunde P., Gunnar T. Drugs and driving: the Finnish perspective. 2005. [PubMed]
- 27.Gjerde H., Christophersen A.S., Normann P.T., Mørland J. Toxicological investigations of drivers killed in road traffic accidents in Norway during 2006-2008. Forensic Sci. Int. 2011;212(1-3):102–109. doi: 10.1016/j.forsciint.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Kriikku P., Wilhelm L., Rintatalo J., Hurme J., Kramer J., Ojanperä I. Phenazepam abuse in Finland: findings from apprehended drivers, post-mortem cases and police confiscations. Forensic Sci. Int. 2012;220(1-3):111–117. doi: 10.1016/j.forsciint.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Zherdev V.P., Caccia S., Garattini S., Ekonomov A.L. Species differences in phenazepam kinetics and metabolism. Eur. J. Drug Metab. Pharmacokinet. 1982;7(3):191–196. doi: 10.1007/BF03189565. [DOI] [PubMed] [Google Scholar]
- 30.Mykkanen S., Gunnar T., Ariniem K., Lillsunde P., Krasnova R. 2004. [Google Scholar]
- 31.Mrozkowska J., Vinge E., Boma C. Missbruk av fenazepam - ny företeelse i Sverige Abuse of phenazepam - new phenomenon in Sweden. Larkartidningen. 2009;106:516–517. [PubMed] [Google Scholar]
- 32.Bailey K., Richards-Waugh L., Clay D., Gebhardt M., Mahmoud H., Kraner J.C. Fatality involving the ingestion of phenazepam and poppy seed tea. J. Anal. Toxicol. 2010;34(8):527–532. doi: 10.1093/jat/34.8.527. [DOI] [PubMed] [Google Scholar]
- 33.Volgram J., Khdasevitsch T. A fatal case due to phenazepam. Bull Int Assoc Forensic Toxicologists. 1999;29(4):13. [Google Scholar]
- 34.Boone C. 2010 Http://www.ajc.com/news/news/local/exotic-drug-leaves-cherokee-teen-dead-3-friends- ho/nQkNR/
- 35.Corkery J.M., Schifano F., Ghodse H. UK fatality involving phenazepam. BMJ. 2011;343:d4207. [Google Scholar]
- 36.Yip T., Martin A., Scott K.S. 2011. [Google Scholar]
- 37.Corkery J.M., Schifano F., Ghodse A.H. Phenazepam abuse in the UK: an emerging problem causing serious adverse health problems, including death. Hum. Psychopharmacol. 2012;27(3):254–261. doi: 10.1002/hup.2222. [DOI] [PubMed] [Google Scholar]
- 38.Corkery J., Claridge H., Loi B., Goodair C., Schifano F. Drug-related deaths in the UK: January-December 2012. Annual Report 2013, National Programme on Substance Abuse Deaths (NPSAD). St George's: University of London; 2013. Http://www.sgul.ac.uk/research/population-health/our-projects/national-programme-on-substance-abuse-deaths [Google Scholar]
- 39.Dargan P.I., Davies S., Puchnarewicz M., Johnston A., Wood D.M. First reported case in the UK of acute prolonged neuropsychiatric toxicity associated with analytically confirmed recreational use of phenazepam. Eur. J. Clin. Pharmacol. 2013;69(3):361–363. doi: 10.1007/s00228-012-1361-z. [DOI] [PubMed] [Google Scholar]
- 40.National Records of Scotland 2014 http://www.gro-scotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/vital-events/deaths/drug-related-deaths-in-scotland/2013
- 41.Barnsdale L., Gordon R., McAuley A. 2015 Http://www.isdscotland.org/Health-Topics/Drugs-and-Alcohol-Misuse/Publications/2015- 04-28/2015-04-28-NDRDD-Report.pdf
- 42.McAuley A., Hecht G., Barnsdale L., Thomson C.S., Graham L., Priyadarshi S., Robertson J.R. Mortality related to novel psychoactive substances in Scotland, 2012: an exploratory study. Int. J. Drug Policy. 2015;26(5):461–467. doi: 10.1016/j.drugpo.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Shearer K., Bryce C., Parsons M., Torrance H. Phenazepam: A review of medico-legal deaths in South Scotland between 2010 and 2014. Forensic Sci. Int. 2015;254:197–204. doi: 10.1016/j.forsciint.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 44.Luzhnikov E.A., Sukhodolova G.N., Ostapenko Y.N., Kovalenko L.A., Dolginov D.M. Clinical toxicometry of acute poisonings by fenazepam in older children. Clin. Toxicol. (Phila.) 2010;48:282. [Google Scholar]
- 45.Aleksandrovskiy Y.A., Kraznov V.N., Neznanov L.V., Romasenko L.V. Efficacy of etifoxine versus phenazepam in treatment of patients with adjustment disorders (open-label randomized controlled trial). Russian J Psychiatry. 2010;1:74–78. [Google Scholar]
- 46.Ali A., Jerry J.M., Khawam E.A. Delirium induced by a new synthetic legal intoxicating drug: phenazepam. Psychosomatics. 2015;56(4):414–418. doi: 10.1016/j.psym.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 47.Bogatskiĭ A.V., Golovenko N.Ia., Zin’kovskiĭ V.G. Biull. Eksp. Biol. Med. 1980;89(1):27–29. doi: 10.1007/BF00835494. [Intrahepatic circulation of 14C-phenazepam and its metabolites in albino rats]. [DOI] [PubMed] [Google Scholar]
- 48.Kopanitsa M.V., Zbarska S.M., Boychuk Y., Krishtal O.A. Modulation of GABA-activated currents by phenazepam and its metabolites in isolated rat Purkinje neurons. Neurophysiology. 2000;32(3):192. doi: 10.1007/BF02506568. [DOI] [Google Scholar]
- 49.Dorofeyeva O.A., Syunyakov S.A., Neznamov G.G. Comparative analysis of different phenazepam formulations.Regional congress of the World Psychiatric Association. Petersburg; 2010. p. 83. [Google Scholar]
- 50.Maskell P.D., De Paoli G., Nitin Seetohul L., Pounder D.J. Phenazepam: the drug that came in from the cold. J. Forensic Leg. Med. 2012;19(3):122–125. doi: 10.1016/j.jflm.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 51. Https://www.bluelight.org
- 52. Https://www.erowid.org
- 53.Golovenko N.Y., Kravchenko I.A., Zin’kovskii V.G., Andronati S.A., Aleksandrova A.I., Ovcharenko N.V., Larionov V.B. Biokinetics of transdermal therapeutic medicinal form of phenazepam. Bull. Exp. Biol. Med. 2000;130(12):1153–1155. doi: 10.1023/A:1017519731950. [DOI] [PubMed] [Google Scholar]
- 54. Https://drugs-forum.com
- 55.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) 2007 Http://www.emcdda.europa.eu/system/files/publications/503/2007_Implementation_report_281403.pdf
- 56.Couch R.A., Madhavaram H. Phenazepam and cannabinomimetics sold as herbal highs in New Zealand. Drug Test. Anal. 2012;4(6):409–414. doi: 10.1002/dta.349. [DOI] [PubMed] [Google Scholar]
- 57.Lee C., Lee H., Pyo J., Jo J., Lee J., Choi H., Kim S., Seon R., Yonghoon H., Bang P., Hwang H., Choe S., Jung J.H. Identification of a new synthetic cannabinoid in a herbal mixture: 1-butyl-3-(2-methoxybenzoyl)indole. Forensic Toxicol. 2013;31(2):187–196. doi: 10.1007/s11419-012-0173-2. [DOI] [Google Scholar]
- 58.Tripsit Http drugs.tripsit.me/
- 59.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) 2011 Http://www.emcdda.europa.eu/system/files/publications/689/EMCDDA-Europol_Annual_Report_2011_2012_final_335568.pdf
- 60.Fracasso C., Confalonieri S., Garattini S., Caccia S. Single and multiple dose pharmacokinetics of etizolam in healthy subjects. Eur. J. Clin. Pharmacol. 1991;40(2):181–185. doi: 10.1007/BF00280074. [DOI] [PubMed] [Google Scholar]
- 61.Nakamae T., Shinozuka T., Sasaki C., Ogamo A., Murakami-Hashimoto C., Irie W., Terada M., Nakamura S., Furukawa M., Kurihara K. Case report: Etizolam and its major metabolites in two unnatural death cases. Forensic Sci. Int. 2008;182(1-3):e1–e6. doi: 10.1016/j.forsciint.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 62.National Records of Scotland 2019 https://www.nrscotland.gov.uk/files//statistics/drug-related-deaths/2018/drug-related-deaths-sub2-18.xlsx
- 63.Moosmann B., Hutter M., Huppertz L.M., Ferlaino S., Redlingshöfer L., Auwärter V. Characterization of the designer benzodiazepine pyrazolam and its detectability in human serum and urine. Forensic Toxicol. 2013;31(2):263–271. doi: 10.1007/s11419-013-0187-4. [DOI] [Google Scholar]
- 64.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) 2012 Http://www.emcdda.europa.eu/system/files/publications/734/EMCDDA-Europol_2012_Annual_Report_final_439477.pdf
- 65.Sternbach L.H., Fryer R.Y., Metlesics W., Reeder E., Sach G., Saucy G., Stempel G. Quinazolines and 1,4-Benzodiazepines. VI.1a Halo-, Methyl-, and Methoxy-substituted 1,3-Dihydro-5-phenyl-2H-1,4- benzodiazepin-2-ones1b,c. J. Org. Chem. 1962;27:3788. doi: 10.1021/jo01058a010. [DOI] [Google Scholar]
- 66.Carter M.C., Alber D.G., Baxter R.C., Bithell S.K., Budworth J., Chubb A., Cockerill G.S., Dowdell V.C.L., Henderson E.A., Keegan S.J., Kelsey R.D., Lockyer M.J., Stables J.N., Wilson L.J., Powell K.L. 1,4-Benzodiazepines as inhibitors of respiratory syncytial virus. J. Med. Chem. 2006;49(7):2311–2319. doi: 10.1021/jm051185t. [DOI] [PubMed] [Google Scholar]
- 67.Koch K., Auwärter V., Hermanns-Clausen M., Wilde M., Neukamm M.A. Mixed intoxication by the synthetic opioid U-47700 and the benzodiazepine flubromazepam with lethal outcome: Pharmacokinetic data. Drug Test. Anal. 2018;••• doi: 10.1002/dta.2391. [DOI] [PubMed] [Google Scholar]
- 68.Pettersson Bergstrand M., Helander A., Hansson T., Beck O. Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays. Drug Test. Anal. 2017;9(4):640–645. doi: 10.1002/dta.2003. [DOI] [PubMed] [Google Scholar]
- 69.Bäckberg M., Pettersson Bergstrand M., Beck O., Helander A. Occurrence and time course of NPS benzodiazepines in Sweden - results from intoxication cases in the STRIDA project. Clin. Toxicol. (Phila.) 2019;57(3):203–212. doi: 10.1080/15563650.2018.1506130. [DOI] [PubMed] [Google Scholar]
- 70.Carpenter J.E., Murray B.P., Dunkley C., Kazzi Z.N., Gittinger M.H. Designer benzodiazepines: a report of exposures recorded in the National Poison Data System, 2014-2017. Clin. Toxicol. (Phila.) 2019;57(4):282–286. doi: 10.1080/15563650.2018.1510502. [DOI] [PubMed] [Google Scholar]
- 71.Ligon E.S., Nawyn J., Jones L.V., Allred B.M., Reinhardt D.V., Jr, France S. Synthesis of Flubromazepam Positional Isomers for Forensic Analysis. J. Org. Chem. 2019;84(16):10280–10291. doi: 10.1021/acs.joc.9b01433. [DOI] [PubMed] [Google Scholar]
- 72.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) 2013 Http://www.emcdda.europa.eu/system/files/publications/814/TDAN14001ENN_475519.pdf
- 73.Manchester K.R., Maskell P.D., Waters L. Experimental versus theoretical log D7.4, pKa and plasma protein binding values for benzodiazepines appearing as new psychoactive substances. Drug Test. Anal. 2018;••• doi: 10.1002/dta.2387. [DOI] [PubMed] [Google Scholar]
- 74.Sternbach L.H., Fryer R.I., Metlesics W., Sach G., Stempel A. Quinazolines and 1,4-Benzodiazepines. V. o-Aminobenzophenones. J. Org. Chem. 1962;27(11):3781–3788. doi: 10.1021/jo01058a009. [DOI] [Google Scholar]
- 75.Babbini M., Gaiardi M., Bartoletti M. Anxiolytic versus sedative properties in the benzodiazepines series: differencies in structure-activity relationships. 1979. [DOI] [PubMed]
- 76.Sternbach L.H., Randall L.O., Banziger R., Lehr H. Structure-activity relationships in the 1,3-benzodiazepine series. In: Burger A., editor. Drugs Affecting the Central Nervous System. London: Edward Arnold; 1968. [Google Scholar]
- 77.Bradley C.M., Nicholson A.N. Activity of the chloro- and triazolo-benzodiazepines. Behavioural studies in the monkey (Macaca mulatta). Neuropharmacology. 1984;23(3):327–331. doi: 10.1016/0028-3908(84)90195-3. [DOI] [PubMed] [Google Scholar]
- 78.Cho M.J., Sethy V.H., Haynes L.C. Sequentially labile water-soluble prodrugs of alprazolam. J. Med. Chem. 1986;29(8):1346–1350. doi: 10.1021/jm00158a004. [DOI] [PubMed] [Google Scholar]
- 79.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) 2014 Http://www.emcdda.europa.eu/system/files/publications/1018/TDAN15001ENN.pdf
- 80.Thibaut J.P., Monteiro L.M., Leite L.C., Menezes C.M., Lima L.M., Noël F. The effects of 3-methylclonazepam on Schistosoma mansoni musculature are not mediated by benzodiazepine receptors. Eur. J. Pharmacol. 2009;606(1-3):9–16. doi: 10.1016/j.ejphar.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 81.Vikingsson S., Wohlfarth A., Andersson M., Gréen H., Roman M., Josefsson M., Kugelberg F.C., Kronstrand R. Identifying Metabolites of meclonazepam by high-resolution mass spectrometry using human liver microsomes, hepatocytes, a mouse model, and authentic urine samples. AAPS J. 2017;19(3):736–742. doi: 10.1208/s12248-016-0040-x. [DOI] [PubMed] [Google Scholar]
- 82.Baard A.P., Sommers D.K., Honiball P.J., Fourie E.D., du Toit L.E. Preliminary results in human schistosomiasis with Ro 11-3128. S. Afr. Med. J. 1979;55(16):617–618. [PubMed] [Google Scholar]
- 83.Ansseau M., Doumont A., Thiry D., von Frenckell R., Collard J. Initial study of methylclonazepam in generalized anxiety disorder. Evidence for greater power in the cross-over design. Psychopharmacology (Berl.) 1985;87(2):130–135. doi: 10.1007/BF00431795. [DOI] [PubMed] [Google Scholar]
- 84.Abdul-Ghani R.A., Loutfy N., Hassan A. Experimentally promising antischistosomal drugs: a review of some drug candidates not reaching the clinical use. Parasitol. Res. 2009;105(4):899–906. doi: 10.1007/s00436-009-1546-2. [DOI] [PubMed] [Google Scholar]
- 85.O’Boyle C., Lambe R., Darragh A. Central effects in man of the novel schistosomicidal benzodiazepine meclonazepam. Eur. J. Clin. Pharmacol. 1985;29(1):105–108. doi: 10.1007/BF00547377. [DOI] [PubMed] [Google Scholar]
- 86.Coassolo P., Aubert C., Cano J.P. Plasma determination of 3-methylclonazepam by capillary gas chromatography. J. Chromatogr. A. 1985;338(2):347–355. doi: 10.1016/0378-4347(85)80105-5. [DOI] [PubMed] [Google Scholar]
- 87.Ameline A., Richeval C., Gaulier J.M., Raul J.S., Kintz P. Characterization of flunitrazolam, a new designer benzodiazepine, in oral fluid after a controlled single administration. J. Anal. Toxicol. 2018;42(6):e58–e60. doi: 10.1093/jat/bky012. [DOI] [PubMed] [Google Scholar]
- 88.Huppertz L.M., Bisel P., Westphal F., Franz F., Auwärter V., Moosmann B. Characterization of the four designer benzodiazepines clonazolam, deschloroetizolam, flubromazolam, and meclonazepam, and identification of their in vitro metabolites. Forensic Toxicol. 2015;33:388–395. doi: 10.1007/s11419-015-0277-6. [DOI] [Google Scholar]
- 89.Abe M., Mikashima H., Moriwaki M., Tahara T. 1988.
- 90.Huppertz L.M., Moosmann B., Auwärter V. Flubromazolam - Basic pharmacokinetic evaluation of a highly potent designer benzodiazepine. Drug Test. Anal. 2018;10(1):206–211. doi: 10.1002/dta.2203. [DOI] [PubMed] [Google Scholar]
- 91.Noble C., Mardal M., Bjerre H.N., Stybe J.S., Linnet K. In vitro studies on flubromazolam metabolism and detection of its metabolites in authentic forensic samples. Drug Test. Anal. 2017;9(8):1182–1191. doi: 10.1002/dta.2146. [DOI] [PubMed] [Google Scholar]
- 92.Wohlfarth A., Vikingsson S., Roman M., Andersson M., Kugelberg F.C., Green H., Kronstrand R. Looking at flubromazolam metabolism from four different angles: Metabolite profiling in human liver microsomes, human hepatocytes, mice and authentic human urine samples with liquid chromatography high-resolution mass spectrometry. Forensic Sci. Int. 2017;274:55–63. doi: 10.1016/j.forsciint.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 93.Bohnenberger K., Liu M.T. Flubromazolam overdose: A review of a new designer benzodiazepine and the role of flumazenil. Ment Health Clin. 2019;9(3):133–137. doi: 10.9740/mhc.2019.05.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Łukasik-Głębocka M., Sommerfeld K., Teżyk A., Zielińska-Psuja B., Panieński P., Żaba C. Flubromazolam--A new life-threatening designer benzodiazepine. Clin. Toxicol. (Phila.) 2016;54(1):66–68. doi: 10.3109/15563650.2015.1112907. [DOI] [PubMed] [Google Scholar]
- 95.Katselou M., Papoutsis I., Nikolaou P., Spiliopoulou C., Athanaselis S. Metabolites replace the parent drug in the drug arena. The cases of fonazepam and nifoxipam. Forensic Toxicol. 2017;35(1):1–10. doi: 10.1007/s11419-016-0338-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hester J.B., Jr, Rudzik A.D., Kamdar B.V. 6-phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepines which have central nervous system depressant activity. J. Med. Chem. 1971;14(11):1078–1081. doi: 10.1021/jm00293a015. [DOI] [PubMed] [Google Scholar]
- 97.Advisory Council on the Misuse of Drugs (ACMD) 2017 Https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/616781/misuse_of_drugs_act_circular_008_2017.pdf
- 98.Shapiro A.P., Krew T.S., Vazirian M., Jerry J., Sola C. Novel ways to acquire designer benzodiazepines: a case report and discussion of the changing role of the internet. 2019. [DOI] [PubMed]
- 99.Robertson M., Raymon L. In: Rohypnol VR and other benzodiazepines. Drug-facilitated sexual assault: a forensic handbook; LeBeau, M.A. Mozayani A., editor. London: Academic Press; 2001. pp. 89–106. [Google Scholar]
- 100.DeVane C.L., Ware M.R., Lydiard R.B. Pharmacokinetics, pharmacodynamics, and treatment issues of benzodiazepines: alprazolam, adinazolam, and clonazepam. Psychopharmacol. Bull. 1991;27(4):463–473. [PubMed] [Google Scholar]
- 101.Bird M. Abuse potential of adinazolam: A comparison with diazepam, lorazepam and placebo.; 1987. [Google Scholar]
- 102.Sethy V.H., Collins R.J., Daniels E.G. Determination of biological activity of adinazolam and its metabolites. J. Pharm. Pharmacol. 1984;36(8):546–548. doi: 10.1111/j.2042-7158.1984.tb04449.x. [DOI] [PubMed] [Google Scholar]
- 103.Fraser A.D., Isner A.F., Bryan W. Urinary screening for adinazolam and its major metabolites by the Emit d.a.u. and FPIA benzodiazepine assays with confirmation by HPLC. J. Anal. Toxicol. 1993;17(7):427–431. doi: 10.1093/jat/17.7.427. [DOI] [PubMed] [Google Scholar]
- 104.Lahti R.A., Sethy V.H., Barsuhn C., Hester J.B. Pharmacological profile of the antidepressant adinazolam, a triazolobenzodiazepine. Neuropharmacology. 1983;22(11):1277–1282. doi: 10.1016/0028-3908(83)90200-9. [DOI] [PubMed] [Google Scholar]
- 105.File S.E., Pellow S. Triazolobenzodiazepines antagonize the effects of anxiogenic drugs mediated at three different central nervous system sites. Neurosci. Lett. 1985;61(1-2):115–119. doi: 10.1016/0304-3940(85)90410-0. [DOI] [PubMed] [Google Scholar]
- 106.Venkatakrishnan K., Culm K.E., Ehrenberg B.L., Harmatz J.S., Corbett K.E., Fleishaker J.C., Greenblatt D.J. Kinetics and dynamics of intravenous adinazolam, N-desmethyl adinazolam, and alprazolam in healthy volunteers. J. Clin. Pharmacol. 2005;45(5):529–537. doi: 10.1177/0091270004269105. [DOI] [PubMed] [Google Scholar]
- 107.Dunner D., Myers J., Khan A., Avery D., Ishiki D., Pyke R. Adinazolam--a new antidepressant: findings of a placebo-controlled, double-blind study in outpatients with major depression. J. Clin. Psychopharmacol. 1987;7(3):170–172. doi: 10.1097/00004714-198706000-00010. [DOI] [PubMed] [Google Scholar]
- 108.Venkatakrishnan K., von Moltke L.L., Duan S.X., Fleishaker J.C., Shader R.I., Greenblatt D.J. Kinetic characterization and identification of the enzymes responsible for the hepatic biotransformation of adinazolam and N-desmethyladinazolam in man. J. Pharm. Pharmacol. 1998;50(3):265–274. doi: 10.1111/j.2042-7158.1998.tb06859.x. [DOI] [PubMed] [Google Scholar]
- 109.Cornett E.M., Novitch M.B., Brunk A.J.M., Davidson K.S., Menard B.L., Urman R.D., Kaye A.D. New benzodiazepines for sedation. Best Pract. Res. Clin. Anaesthesiol. 2018;32(2):149–164. doi: 10.1016/j.bpa.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 110.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) 2016 Http://www.emcdda.europa.eu/system/files/publications/1018/TDAN15001ENN.pdf
- 111.Dowling G., Kavanagh P.V., Eckhardt H.G., Twamley B., Hessman G., McLaughlin G., O’Brien J., Brandt S.D. 2018. [DOI] [PubMed]
- 112.Hester J.B. Patent US3987052 - 6-Phenyl-4H-s-triazolo[4,3a][1,4]benzodiazepines. 1976 Https://patents.google.com/patent/US3987052A/en
- 113.Chaslot M., El Balkhi S., Robin T., Morichon J., Picard N., Saint‐Marcoux F. Exploration des métabolites de 8 benzodiazépines de synthèse. Toxicol Anal Clin. 2016;28(2):S32. doi: 10.1016/j.toxac.2016.03.053. [DOI] [Google Scholar]
- 114.Høiseth G., Tuv S.S., Karinen R. Blood concentrations of new designer benzodiazepines in forensic cases. Forensic Sci. Int. 2016;268:35–38. doi: 10.1016/j.forsciint.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 115.Meyer M.R., Bergstrand M.P., Helander A., Beck O. Identification of main human urinary metabolites of the designer nitrobenzodiazepines clonazolam, meclonazepam, and nifoxipam by nano-liquid chromatography-high-resolution mass spectrometry for drug testing purposes. Anal. Bioanal. Chem. 2016;408(13):3571–3591. doi: 10.1007/s00216-016-9439-6. [DOI] [PubMed] [Google Scholar]
- 116. Https://www.reddit.com
- 117.Kintz P., Richeval C., Jamey C., Ameline A., Allorge D., Gaulier J.M., Raul J.S. Detection of the designer benzodiazepine metizolam in urine and preliminary data on its metabolism. Drug Test. Anal. 2017;9(7):1026–1033. doi: 10.1002/dta.2099. [DOI] [PubMed] [Google Scholar]
- 118.Moosmann B., Bisel P., Franz F., Huppertz L.M., Auwärter V. Characterization and in vitro phase I microsomal metabolism of designer benzodiazepines - an update comprising adinazolam, cloniprazepam, fonazepam, 3-hydroxyphenazepam, metizolam and nitrazolam. J. Mass Spectrom. 2016;51(11):1080–1089. doi: 10.1002/jms.3840. [DOI] [PubMed] [Google Scholar]
- 119.Mortelé O., Vervliet P., Gys C., Degreef M., Cuykx M., Maudens K., Covaci A., van Nuijs A.L.N., Lai F.Y. In vitro Phase I and Phase II metabolism of the new designer benzodiazepine cloniprazepam using liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2018;153:158–167. doi: 10.1016/j.jpba.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 120.Schukin S.I., Zinkovsky V.G., Zhuk O.V. Elimination kinetics of the novel prodrug cinazepam possessing psychotropic activity in mice. Pharmacol. Rep. 2011;63(5):1093–1100. doi: 10.1016/S1734-1140(11)70628-4. [DOI] [PubMed] [Google Scholar]
- 121.Kerrigan S., Mellon M.B., Hinners P. Detection of phenazepam in impaired driving. J. Anal. Toxicol. 2013;37(8):605–610. doi: 10.1093/jat/bkt075. [DOI] [PubMed] [Google Scholar]
- 122.Crichton M.L., Shenton C.F., Drummond G., Beer L.J., Seetohul L.N., Maskell P.D. Analysis of phenazepam and 3-hydroxyphenazepam in post-mortem fluids and tissues. Drug Test. Anal. 2015;7(10):926–936. doi: 10.1002/dta.1790. [DOI] [PubMed] [Google Scholar]
- 123.Ekonomov A.L., Zherdev V.P. Method of quantitative gas-chromatographic determination of phenazepam and its metabolite 3-hydroxyphenazepam in plasma. Pharm. Chem. J. 1980;14:579–582. doi: 10.1007/BF00765846. [DOI] [Google Scholar]
- 124.Golovenko N.Y., Larionov V.B., Kravchenko I.A., Ovcharenko N.V., Aleksandrova A.I. Biokinetics of transdermal 3-hydroxyphenazepam. Bull. Exp. Biol. Med. 2002;134(3):254–256. doi: 10.1023/A:1021503417490. [DOI] [PubMed] [Google Scholar]
- 125.Voronina T.A., Larionov V.B., Golovenko N.Ia., Nerobkova L.N. Peculiarities of hypnotic effect and pharmacokinetics of hemisuccinate 3-hydroxyphenazepam (levana). Eksp. Klin. Farmakol. 2014;77(1):3–6. [PubMed] [Google Scholar]
- 126.2015 Http://www.who.int/medicines/access/controlled-substances/5.8_Phenazepam_PreRev.pdf(Accessed Oct 7, 2019)
- 127.Lomas E.C., Maskell P.D. Phenazepam: More information coming in from the cold. J. Forensic Leg. Med. 2015;36:61–62. doi: 10.1016/j.jflm.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 128.Mei V., Concheiro M., Pardi J., Cooper G. Validation of an LC-MS/MS Method for the Quantification of 13 Designer Benzodiazepines in Blood. J. Anal. Toxicol. 2019;43(9):688–695. doi: 10.1093/jat/bkz063. [DOI] [PubMed] [Google Scholar]
- 129.Golovenko N.Y., Larionov V.B. Pharmacodynamical and neuroreceptor analysis of the permeability of the blood-brain barrier for Derivatives of 1, 4-Benzodiazepine. Neurophysiology. 2014;46(3):199–205. doi: 10.1007/s11062-014-9429-2. [DOI] [Google Scholar]
- 130.Hallifax D., Galetin A., Houston J.B. Prediction of metabolic clearance using fresh human hepatocytes: comparison with cryopreserved hepatocytes and hepatic microsomes for five benzodiazepines. Xenobiotica. 2008;38(4):353–367. doi: 10.1080/00498250701834665. [DOI] [PubMed] [Google Scholar]
- 131.Sternbach L.H., Fryer R.I., Keller O., Metlesics W., Sach G., Steiger N. Quinazolines and 1, 4-Benzodiazepines. X. 1 Nitro-Substituted 5-Phenyl-1, 4-benzodiazepine Derivatives2. J. Med. Chem. 1963;6(3):261–265. doi: 10.1021/jm00339a010. [DOI] [PubMed] [Google Scholar]
- 132.de Silva J.A., Bekersky I., Puglisi C.V., Brooks M.A., Weinfeld R.E. Determination of 1,4-benzodiazepines and -diazepin-2-ones in blood by electron-capture gas-liquid chromatography. Anal. Chem. 1976;48(1):10–19. doi: 10.1021/ac60365a037. [DOI] [PubMed] [Google Scholar]
- 133.Maddalena D.J., Johnston G.A. Prediction of receptor properties and binding affinity of ligands to benzodiazepine/GABAA receptors using artificial neural networks. J. Med. Chem. 1995;38(4):715–724. doi: 10.1021/jm00004a017. [DOI] [PubMed] [Google Scholar]
- 134.So S.S., Karplus M. Genetic neural networks for quantitative structure-activity relationships: improvements and application of benzodiazepine affinity for benzodiazepine/GABAA receptors. J. Med. Chem. 1996;39(26):5246–5256. doi: 10.1021/jm960536o. [DOI] [PubMed] [Google Scholar]
- 135.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) 2017 Http://www.emcdda.europa.eu/system/files/publications/9282/20183924_TDAN18001ENN_PDF.pdf
- 136.Moosmann B., Auwärter V. Designer Benzodiazepines: Another Class of New Psychoactive Substances. Handb. Exp. Pharmacol. 2018;252:383–410. doi: 10.1007/164_2018_154. [DOI] [PubMed] [Google Scholar]
- 137.Earl R., Henryk S.L.
- 138.Skerritt J.H., Werz M.A., McLean M.J., Macdonald R.L. Diazepam and its anomalous p-chloro-derivative Ro 5-4864: comparative effects on mouse neurons in cell culture. Brain Res. 1984;310(1):99–105. doi: 10.1016/0006-8993(84)90013-1. [DOI] [PubMed] [Google Scholar]
- 139.Schoemaker H., Boles R.G., Horst W.D., Yamamura H.I. Specific high-affinity binding sites for [3H]Ro 5-4864 in rat brain and kidney. J. Pharmacol. Exp. Ther. 1983;225(1):61–69. [PubMed] [Google Scholar]
- 140.Marangos P.J., Patel J., Boulenger J.P., Clark-Rosenberg R. Characterization of peripheral-type benzodiazepine binding sites in brain using [3H]Ro 5-4864. Mol. Pharmacol. 1982;22(1):26–32. [PubMed] [Google Scholar]
- 141.Gavioli E.C., Duarte F.S., Bressan E., Ferrara P., Farges R.C., De Lima T.C. Antidepressant-like effect of Ro5-4864, a peripheral-type benzodiazepine receptor ligand, in forced swimming test. Eur. J. Pharmacol. 2003;471(1):21–26. doi: 10.1016/S0014-2999(03)01789-8. [DOI] [PubMed] [Google Scholar]
- 142.DalBó S., Nardi G.M., Ferrara P., Ribeiro-do-Valle R.M., Farges R.C. Antinociceptive effects of peripheral benzodiazepine receptors. Pharmacology. 2004;70(4):188–194. doi: 10.1159/000075547. [DOI] [PubMed] [Google Scholar]
- 143.Leonelli E., Yague J.G., Ballabio M., Azcoitia I., Magnaghi V., Schumacher M., Garcia-Segura L.M., Melcangi R.C. Ro5-4864, a synthetic ligand of peripheral benzodiazepine receptor, reduces aging-associated myelin degeneration in the sciatic nerve of male rats. Mech. Ageing Dev. 2005;126(11):1159–1163. doi: 10.1016/j.mad.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 144.Solhjoo S., O’Rourke B. Mitochondrial instability during regional ischemia-reperfusion underlies arrhythmias in monolayers of cardiomyocytes. J. Mol. Cell. Cardiol. 2015;78:90–99. doi: 10.1016/j.yjmcc.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Veiga S., Azcoitia I., Garcia-Segura L.M. Ro5-4864, a peripheral benzodiazepine receptor ligand, reduces reactive gliosis and protects hippocampal hilar neurons from kainic acid excitotoxicity. J. Neurosci. Res. 2005;80(1):129–137. doi: 10.1002/jnr.20430. [DOI] [PubMed] [Google Scholar]
- 146.Mills C., Makwana M., Wallace A., Benn S., Schmidt H., Tegeder I., Costigan M., Brown R.H., Jr, Raivich G., Woolf C.J. Ro5-4864 promotes neonatal motor neuron survival and nerve regeneration in adult rats. Eur. J. Neurosci. 2008;27(4):937–946. doi: 10.1111/j.1460-9568.2008.06065.x. [DOI] [PubMed] [Google Scholar]
- 147.Soustiel J.F., Zaaroor M., Vlodavsky E., Veenman L., Weizman A., Gavish M. Neuroprotective effect of Ro5-4864 following brain injury. Exp. Neurol. 2008;214(2):201–208. doi: 10.1016/j.expneurol.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 148.Arbo B.D., Hoppe J.B., Rodrigues K., Garcia-Segura L.M., Salbego C.G., Ribeiro M.F. 4′-Chlorodiazepam is neuroprotective against amyloid-beta in organotypic hippocampal cultures. J. Steroid Biochem. Mol. Biol. 2017;171:281–287. doi: 10.1016/j.jsbmb.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 149.Ma B., Liu X., Huang X., Ji Y., Jin T., Ma K. Translocator protein agonist Ro5-4864 alleviates neuropathic pain and promotes remyelination in the sciatic nerve. Mol. Pain. 2018;14:1744806917748019. doi: 10.1177/1744806917748019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ameline A., Arbouche N., Raul J.S., Kintz P. Documentation of a little-studied designer benzodiazepine after a controlled single administration: ii. concentration profile of deschloroetizolam in saliva. Ther. Drug Monit. 2018;40(6):759–761. doi: 10.1097/FTD.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 151.Moosmann B., Bisel P., Westphal F., Wilde M., Kempf J., Angerer V., Auwärter V. Characterization and in vitro phase I microsomal metabolism of designer benzodiazepines: An update comprising flunitrazolam, norflurazepam, and 4′-chlorodiazepam (Ro5-4864). Drug Test. Anal. 2019;11(3):541–549. doi: 10.1002/dta.2561. [DOI] [PubMed] [Google Scholar]
- 152.Manchester K.R., Lomas E.C., Waters L., Dempsey F.C., Maskell P.D. The emergence of new psychoactive substance (NPS) benzodiazepines: A review. Drug Test. Anal. 2017;•••:1–17. doi: 10.1002/dta.2211. [DOI] [Google Scholar]
- 153.Hester J.B., Jr, Von Voigtlander P. 1979.
- 154.Vogt S., Kempf J., Buttler J., Auwärter V., Weinmann W. Desalkylflurazepam found in patients’ samples after high-dose midazolam treatment. Drug Test. Anal. 2013;5(9-10):745–747. doi: 10.1002/dta.1484. [DOI] [PubMed] [Google Scholar]
- 155.Maskell P.D., Wilson N.E. Designer benzodiazepines: new challenges and treatment Options.Roman-Urrestarazu. In: Corazza O., editor. Handbook of Novel Psychoactive Substances: What Clinicians Should Know about NPS. Taylor & Francis; 2019. [Google Scholar]
- 156.Akatsu M. 1972.
- 157.Winkler A.D., Burden F.R., Watkins A.J.R. Atomistic topological indices applied to benzodiazepines using various regression methods. Quantitative Structure-Activity Relationships. 1998;17(1):14–19. doi: 10.1002/(SICI)1521-3838(199801)17:01<14:AID-QSAR14>3.0.CO;2-U. [DOI] [Google Scholar]
- 158.Reeder E. 1963.
- 159.Anjaneyulu Y., Marayya D., Linga R.D., Krishna R.P. High performance thin layer chromatographic determination of the related substances in alprazolam drug. Asian J. Chem. 2007;19(5):3375. [Google Scholar]
- 160.Baumann M., Baxendale I.R., Ley S.V., Nikbin N. An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Beilstein J. Org. Chem. 2011;7:442–495. doi: 10.3762/bjoc.7.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Borrey D., Meyer E., Lambert W., Van Calenbergh S., Van Peteghem C., De Leenheer A.P. Sensitive gas chromatographic--mass spectrometric screening of acetylated benzodiazepines. J. Chromatogr. A. 2001;910(1):105–118. doi: 10.1016/S0021-9673(00)01177-8. [DOI] [PubMed] [Google Scholar]
- 162.Wilhelm M., Battista H.J., Obendorf D. HPLC with simultaneous UV and reductive electrochemical detection at the hanging mercury drop electrode: a highly sensitive and selective tool for the determination of benzodiazepines in forensic samples. J. Anal. Toxicol. 2001;25(4):250–257. doi: 10.1093/jat/25.4.250. [DOI] [PubMed] [Google Scholar]
- 163.Bugey A., Rudaz S., Staub C. A fast LC-APCI/MS method for analyzing benzodiazepines in whole blood using monolithic support. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006;832(2):249–255. doi: 10.1016/j.jchromb.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 164.Ueki S., Gomita Y., Ataki Y., Yamada K., Yoshimura H. Behavioral effects of 5-(o-chlorophenyl)-1-methyl-7-nitro-1,3-dihydro-2H-1,4-benzodiazepin-2-one (ID-690). Nippon Yakurigaku Zasshi. 1977;73(2):243–255. doi: 10.1254/fpj.73.243. [DOI] [PubMed] [Google Scholar]
- 165.Fukuda H., Kudo Y., Ono H., Togari A., Tanaka Y. Pharmacological study on 5-(o-chlorophenyl)-1-methyl-7-nitro-1,3-dihydro-2H-1,4-benzodiazepin-2-one (ID-690), with special reference to the effects on motor systems. Nippon Yakurigaku Zasshi. 1977;73(1):123–134. doi: 10.1254/fpj.73.123. [DOI] [PubMed] [Google Scholar]
- 166.Fukuda H., Kudo Y., Takeuchi E. Effects of benzodiazepine derivatives on γ-motor system in rats (author’s transl). Nippon Yakurigaku Zasshi. 1979;75(4):315–319. doi: 10.1254/fpj.75.315. [DOI] [PubMed] [Google Scholar]
- 167.Watanabe S., Kawasaki H., Nishi H., Ueki S. Electroencephalographic effects of ID-690 [5-(o-chlorophenyl)-1-methyl-7-nitro-1,3-dihydro-2H-1,4-benzodiazepin-2-one] in rabbits with chronic electrode implants. Nippon Yakurigaku Zasshi. 1974;70(4):531–542. doi: 10.1254/fpj.70.531. [DOI] [PubMed] [Google Scholar]
- 168.Tsuchiya T., Fukushima H. Effects of 5-(o-chlorophenyl)-1-methyl-7-nitro-1, 3-dihydro-2HO1, 4-benzodiazepin-2-one (ID-390) as compared to nitrazepam on the sleep-wakefulness cycle in cats (author’s transl). Nippon Yakurigaku Zasshi. 1977;73(4):437–447. doi: 10.1254/fpj.73.437. [DOI] [PubMed] [Google Scholar]
- 169.Hellerbach J. 1974 Https://worldwide.espacenet.com/publicationDetails/originalDocument?CC=DE&NR=2405682A1&KC=A1&FT=D&ND=3&date=19740815&DB=EPODOC&locale=en_EP#
- 170.Zawilska J.B., Wojcieszak J. An expanding world of new psychoactive substances-designer benzodiazepines. Neurotoxicology. 2019;73:8–16. doi: 10.1016/j.neuro.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 171.ukchemicalresearch.org Https://www.ukchemicalresearch.org
- 172.Zsila F., Gergely A., Horvath P., Szasz G. Separation and identification of tofisopam stereoisomers by hyphenated HPLC-CD technique. J Liq Chrom Relat Tech. 1999;22(5):713–719. doi: 10.1081/JLC-100101693. [DOI] [Google Scholar]
- 173.Foroughbakhshfasaei M., Szabó Z.I., Mirzahosseini A., Horváth P., Tóth G. Enantiomeric quality control of R-Tofisopam by HPLC using polysaccharide-type chiral stationary phases in polar organic mode. Electrophoresis. 2018;39(20):2566–2574. doi: 10.1002/elps.201800220. [DOI] [PubMed] [Google Scholar]
- 174.Bond A., Lader M. A comparison of the psychotropic profiles of tofisopam and diazepam. Eur. J. Clin. Pharmacol. 1982;22(2):137–142. doi: 10.1007/BF00542458. [DOI] [PubMed] [Google Scholar]
- 175.Bernard P., Dufresne-Favetta C., Favetta P., Do Q.T., Himbert F., Zubrzycki S., Scior T., Lugnier C. Application of drug repositioning strategy to TOFISOPAM. Curr. Med. Chem. 2008;15(30):3196–3203. doi: 10.2174/092986708786848488. [DOI] [PubMed] [Google Scholar]
- 176.Murthy V.S., Mangot A.G. Psychiatric aspects of phosphodiesterases: An overview. Indian J. Pharmacol. 2015;47(6):594–599. doi: 10.4103/0253-7613.169593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tominaga K., Fujikawa Y., Tsumoto C., Kadouchi K., Tanaka F., Kamata N., Yamagami H., Tanigawa T., Watanabe T., Fujiwara Y., Arakawa T. Disorder of autonomic nervous system and its vulnerability to external stimulation in functional dyspepsia. J. Clin. Biochem. Nutr. 2016;58(2):161–165. doi: 10.3164/jcbn.15-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pellow S., File S.E. Is tofisopam an atypical anxiolytic? Neurosci. Biobehav. Rev. 1986;10(2):221–227. doi: 10.1016/0149-7634(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 179.Petócz L. Pharmacologic effects of tofizopam (Grandaxin). Acta Pharm. Hung. 1993;63(2):79–82. [PubMed] [Google Scholar]
- 180.Srivastava S., Bhatia M.S., Gupta K., Rajdev K. Current update on evidence based literature of tofisopam. Delhi Psychiatry J. 2014;17:154–159. [Google Scholar]
- 181.Chopin P., Stenger A., Couzinier J.P., Briley M. Indirect dopaminergic effects of tofisopam, a 2,3-benzodiazepine, and their inhibition by lithium. J. Pharm. Pharmacol. 1985;37(12):917–919. doi: 10.1111/j.2042-7158.1985.tb05003.x. [DOI] [PubMed] [Google Scholar]
- 182.Szegó J., Somogyi M., Papp E. Excerpts from the clinical-pharmacologic and clinical studies of Grandaxin. Acta Pharm. Hung. 1993;63(2):91–98. [PubMed] [Google Scholar]
- 183.Rundfeldt C., Socała K., Wlaź P. The atypical anxiolytic drug, tofisopam, selectively blocks phosphodiesterase isoenzymes and is active in the mouse model of negative symptoms of psychosis. J. Neural Transm. (Vienna) 2010;117(11):1319–1325. doi: 10.1007/s00702-010-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bihel F.J., Justiniano H., Schmitt M., Hellal M., Ibrahim M.A., Lugnier C., Bourguignon J.J. New PDE4 inhibitors based on pharmacophoric similarity between papaverine and tofisopam. Bioorg. Med. Chem. Lett. 2011;21(21):6567–6572. doi: 10.1016/j.bmcl.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 185.Vakhrushev IaM., Belova E.V., Efremova L.I. Use of grandaxine in the treatment of patients with erosive-ulcerative stomach and gastroduodenal lesions. Eksp. Klin. Gastroenterol. 2004;(4):21–24. [PubMed] [Google Scholar]
- 186.Plotnikova E.Iu., Beloborodova E.I. Use of grandaxin in hypermotor dysfunction of the biliary ducts in young people. 2005. [PubMed]
- 187.Leventer S.M., Raudibaugh K., Frissora C.L., Kassem N., Keogh J.C., Phillips J., Mangel A.W. Clinical trial: dextofisopam in the treatment of patients with diarrhoea-predominant or alternating irritable bowel syndrome. Aliment. Pharmacol. Ther. 2008;27(2):197–206. doi: 10.1111/j.1365-2036.2007.03566.x. [DOI] [PubMed] [Google Scholar]
- 188.Manabe N., Rao A.S., Wong B.S., Camilleri M. Emerging pharmacologic therapies for irritable bowel syndrome. Curr. Gastroenterol. Rep. 2010;12(5):408–416. doi: 10.1007/s11894-010-0124-1. [DOI] [PubMed] [Google Scholar]
- 189.Klebovich I., Abermann M. Pharmacokinetics and metabolism of tofizopam (Grandaxin). Acta Pharm. Hung. 1993;63(2):83–90. [PubMed] [Google Scholar]
- 190.Tomori E., Elekes I., Láng T., Horváth G. Investigation of metabolites of tofizopam in man and animals. Pol. J. Pharmacol. Pharm. 1984;36(4):423–430. [PubMed] [Google Scholar]
- 191.Hatayama M., Sumida C., Kurajoh M., Shiraishi J., Okazaki H., Shoji T., Koyama H., Tsutsumi Z., Moriwaki Y., Namba M., Yamamoto T. Acute effects of oral tofisopam on plasma concentration and urinary excretion of uric acid and oxypurinol “preliminary communication”. Curr. Clin. Pharmacol. 2015;10(2):160–164. doi: 10.2174/1574884710666150126143421. [DOI] [PubMed] [Google Scholar]
- 192.Veĭn A.V., Artemenko A.R., Oknin V.Iu., Pomortseva I.V. Effectiveness of grandaxin in the treatment of somatoform disorders. Klin. Med. (Mosk.) 1999;77(6):41–45. [PubMed] [Google Scholar]
- 193.Osipenko M.F., Khramov IuA., Makarova T.A., Vdovenko E.G. Role of grandaxin in the treatment of functional gastrointestinal diseases. Ter. Arkh. 2000;72(10):23–27. [PubMed] [Google Scholar]
- 194.Kato R., Ooi K., Ueno K. A case in which tofisopam was effective for treatment of paroxysmal supraventricular tachycardia. Yakugaku Zasshi. 2003;123(5):365–368. doi: 10.1248/yakushi.123.365. [DOI] [PubMed] [Google Scholar]
- 195.Fustero S., González J., del Pozo C. 1,4-Benzodiazepine N-nitrosoamidines: useful intermediates in the synthesis of tricyclic benzodiazepines. Molecules. 2006;11(8):583–588. doi: 10.3390/11080583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kaliszan R., Kaliszan A., Noctor T.A., Purcell W.P., Wainer I.W. Mechanism of retention of benzodiazepines in affinity, reversed-phase and adsorption high-performance liquid chromatography in view of quantitative structure retention relationships. J. Chromatogr. A. 1992;609(1-2):69–81. doi: 10.1016/0021-9673(92)80150-S. [DOI] [Google Scholar]
- 197.Garzone P.D., Kroboth P.D. Pharmacokinetics of the newer benzodiazepines. Clin. Pharmacokinet. 1989;16(6):337–364. doi: 10.2165/00003088-198916060-00002. [DOI] [PubMed] [Google Scholar]
- 198.Qian Z., Liu C., Huang J., Deng Q., Hua Z. Identification of the designer benzodiazepine 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine (flualprazolam) in an anesthesia robbery case. Forensic Toxicol. 2019;••• doi: 10.1007/s11419-019-00501-1. [DOI] [Google Scholar]
- 199.2019
- 200.RCshare Https rceshare.blogspot.com
- 201.Vejdelek Z., Protiva M. Synthesis of two 1-substituted 8-bromo-6-(2-chlorophenyl)-4H-s-triazolo[4,3-a]-1,4-benzodiazepines. Collect. Czech. Chem. Commun. 1983;48(5):1477–1482. doi: 10.1135/cccc19831477. [DOI] [Google Scholar]
- 202.Gonzalez López F., Mariño E.L., Dominguez-Gil A. Pharmacokinetics of tiadipone: a new anxiolytic. Int. J. Clin. Pharmacol. Ther. Toxicol. 1986;24(9):482–484. [PubMed] [Google Scholar]
- 203.CIMA Https://hes32-ctp.trendmicro.com:443/wis/clicktime/v1/query?url=https%3a%2f%2fcima.aemps.es%2fcima%2fpublico%2fhome.html&umid=7cec72cc-7e38-4174-a2ab-f34a2ce0e3fe&auth=768f192bba830b801fed4f40fb360f4d1374fa7c-400a686fb11df4fef2d673976f3bd27783f37b2d
- 204. Https://hes32-ctp.trendmicro.com:443/wis/clicktime/v1/query?url=https%3a%2f%2fcima.aemps.es%2fcima%2fpdfs%2fes%2fft%2f55464%2fFT%5f55464.html.pdf&umid=7cec72cc-7e38-4174-a2ab-f34a2ce0e3fe&auth=768f192bba830b801fed4f40fb360f4d1374fa7c-a12cdbf593fc8f257f0923c787e8e41839adbeb2(Accessed Oct 8, 2019)
- 205.Andrade R.J., Lucena M.I., Alcantara R., Fraile J.M. Bentazepam-associated chronic liver disease. Lancet. 1994;343(8901):860. doi: 10.1016/S0140-6736(94)92065-6. [DOI] [PubMed] [Google Scholar]
- 206.de-la-Serna C., Gil-Grande L.A., Sanromán A.L., Gonzalez M., Ruiz-del-Arbol L., Garcia Plaza A. Bentazepam-induced hepatic bridging necrosis. J. Clin. Gastroenterol. 1997;25(4):710–711. doi: 10.1097/00004836-199712000-00042. [DOI] [PubMed] [Google Scholar]
- 207.Andrade R.J., Lucena M.I., Aguilar J., Lazo M.D., Camargo R., Moreno P., García-Escaño M.D., Marquez A., Alcántara R., Alcáin G. Chronic liver injury related to use of bentazepam: an unusual instance of benzodiazepine hepatotoxicity. Dig. Dis. Sci. 2000;45(7):1400–1404. doi: 10.1023/A:1005520523502. [DOI] [PubMed] [Google Scholar]
- 208.Andrade R.J., López-Torres E., Lucena M.I. Fernández, Mdel.C. Acute hepatitis due to bentazepam. Med. Clin. (Barc.) 2003;120(17):678–679. doi: 10.1157/13047311. [DOI] [PubMed] [Google Scholar]
- 209.Andrade R.J., Lucena M.I., Kaplowitz N., García-Muņoz B., Borraz Y., Pachkoria K., García-Cortés M., Fernández M.C., Pelaez G., Rodrigo L., Durán J.A., Costa J., Planas R., Barriocanal A., Guarner C., Romero-Gomez M., Muņoz-Yagüe T., Salmerón J., Hidalgo R. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44(6):1581–1588. doi: 10.1002/hep.21424. [DOI] [PubMed] [Google Scholar]
- 210.Baumeister D., Tojo L.M., Tracy D.K. Legal highs: staying on top of the flood of novel psychoactive substances. Ther. Adv. Psychopharmacol. 2015;5(2):97–132. doi: 10.1177/2045125314559539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Schifano F., Orsolini L., Duccio P.G., Corkery J.M. Novel psychoactive substances of interest for psychiatry. World Psychiatry. 2015;14(1):15–26. doi: 10.1002/wps.20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pope J.D., Choy K.W., Drummer O.H., Schneider H.G. Novel benzodiazepines (clonazolam and flubromazolam) identified in candy-like pills. J. Appl. Lab. Med. 2018;3(1):48–55. doi: 10.1373/jalm.2017.025387. [DOI] [PubMed] [Google Scholar]
- 213.Griffin C.E., III, Kaye A.M., Bueno F.R., Kaye A.D. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214–223. [PMC free article] [PubMed] [Google Scholar]
- 214.Broséus J., Gentile N., Esseiva P. 2016.
- 215.El Balkhi S., Chaslot M., Picard N., Dulaurent S., Delage M., Mathieu O., Saint-Marcoux F. Characterization and identification of eight designer benzodiazepine metabolites by incubation with human liver microsomes and analysis by a triple quadrupole mass spectrometer. Int. J. Legal Med. 2017;131(4):979–988. doi: 10.1007/s00414-017-1541-6. [DOI] [PubMed] [Google Scholar]
- 216.Orsolini L., Papanti G.D., Francesconi G., Schifano F. Mind navigators of chemicals’ experimenters? A web-based description of e-psychonauts. Cyberpsychol. Behav. Soc. Netw. 2015;18(5):296–300. doi: 10.1089/cyber.2014.0486. [DOI] [PubMed] [Google Scholar]
- 217.Corkery J.M., Orsolini L., Papanti D., Schifano F. From concept(ion) to life after death/the grave: The ‘natural’ history and life cycle(s) of novel psychoactive substances (NPS). Hum. Psychopharmacol., 2017, 32(3). RE:view. doi: 10.1002/hup.2566. [DOI] [PubMed] [Google Scholar]
- 218.Orsolini L., Papanti D., Corkery J., Schifano F. An insight into the deep web; why it matters for addiction psychiatry? Hum. Psychopharmacol. 2017;32(3) doi: 10.1002/hup.2573. [DOI] [PubMed] [Google Scholar]
- 219.Vinkers C.H., Tijdink J.K., Luykx J.J., Vis R. Choosing the correct benzodiazepine: mechanism of action and pharmacokinetics. Ned. Tijdschr. Geneeskd. 2012;155(35):A4900. [PubMed] [Google Scholar]
- 220.Turner S., Mota N., Bolton J., Sareen J. Self-medication with alcohol or drugs for mood and anxiety disorders: A narrative review of the epidemiological literature. Depress. Anxiety. 2018;35(9):851–860. doi: 10.1002/da.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jones J.D., Mogali S., Comer S.D. Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 2012;125(1-2):8–18. doi: 10.1016/j.drugalcdep.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


