Abstract
Background
Cognitive impairment is an adverse reaction of cancer chemotherapy and is likely to affect up to 75% of patients during the treatment and 35% of patients experience it for several months after the chemotherapy. Patients manifest symptoms like alteration in working ability, awareness, concentration, visual-verbal memory, attention, executive functions, processing speed, fatigue and behavioural dysfunctions. Post-chemotherapy, cancer survivors have a reduced quality of life due to the symptoms of chemobrain. Apart from this, there are clinical reports which also associate mood disorders, vascular complications, and seizures in some cases. Therefore, the quality of lifestyle of cancer patients/ survivors is severely affected and only worsens due to the absence of any efficacious treatments. With the increase in survivorship, it’s vital to identify effective strategies, until then only symptomatic relief for chemobrain can be provided. The depressive symptoms were causally linked to the pathophysiological imbalance between the pro and anti-inflammatory cytokines.
Conclusion
The common causative factor, cytokines can be targeted for the amelioration of an associated symptom of both depression and chemotherapy. Thus, antidepressants can have a beneficial effect on chemotherapy-induced inflammation and cognitive dysfunction via cytokine balance. Also, neurogenesis property of certain antidepressant drugs rationalises their evaluation against CICI. This review briefly glances upon chemotherapy-induced cognitive impairment (CICI), and the modulatory effect of antidepressants on CICI pathomechanisms.
Keywords: Antidepressants, chemobrain, chemotherapy, cognitive impairment, cytokines, neuroinflammation
1. INTRODUCTION
The discovery and development of a wide range of chemotherapeutic drugs in recent times have significantly increased cancer survivorship. The five-year survival rates for all the cancers combined is a little over 60% with the highest rate for prostate cancer, followed by skin melanoma and breast cancer [1]. Unfortunately, every treatment has its drawbacks. Chemotherapy-induced cognitive impairment (CICI) /Chemobrain/Chemofog is a very common and debilitating side effect of cancer chemotherapy [2]. CICI is the combined effect of neuronal damage, abnormality in repair and brain remodelling leading to cognitive deficits [3]. Approximately, out of 15 million survivors in the US, 5 million are affected by chemofog [4]. Cognitive deficit is experienced by the majority of cancer patients while undergoing chemotherapy. These symptoms can persist for a longer period of time even after the termination of a chemotherapy regimen. A similar scenario is observed for Chemotherapy-induced neurological dysfunction as well [5]. Longitudinal [6] and cross-sectional [7, 8] reports state that these side effects induced by anticancer agents may persist 1-2 and 4-10 years, respectively after cessation of treatment.
Twenty per cent of cancer patients, self-report cognitive difficulties even before the start of chemotherapy suggesting that the systemic tumorigenic growth-induced inflammatory responses contribute to the impairment of cognition. Stress, anxiety and depression due to the diagnosis of cancer also play an important role in this. Several other confounding factors may contribute to neurological disorders such as age-related complications, physiological and psychological stress and concomitant medications to treat multiple underlying disease conditions [9]. Neuropsychiatric parameter assessments reveal that self-reported cognitive decline before and after chemotherapy are widely different from each other [10].
Aside from the fact that such disorders may be the result of tumorigenic development, there is evidence of complaints after the onset of treatment, indicating that chemotherapy may be directly related to the problem. Apart from these, genetic detriments also confer the susceptibility of cancer survivors for CICI. In breast cancer and lymphoma survivors, epsilon 4 allele apolipoprotein E (APOE) gene was potentially correlated with CICI [11]. In another study, breast cancer survivors with a valine allele catechol-O-methyltransferase (COMT‐Val) genotype experienced greater cognitive impairment and are susceptible to negative effects on their cognitive health [12]. The present review focuses on the issue of cognitive dysfunction due to chemotherapy per se.
2. PRINCIPLE SITES OF CICI
Brain neuroimaging has helped to locate the sites which are principally affected in CICI. In breast cancer patients, neuroimaging scans have revealed decreased grey matter density in frontal, temporal, cerebellar and right thalamus regions, primarily one-month post-chemotherapy [13]. Later, the major location of neuronal damage was found to be prefrontal cortex of breast cancer patients who received chemotherapy [14]. A recent preclinical report validates the involvement of hippocampal dysfunction in precipitating CICI in rats [15]. Prefrontal cortex and hippocampus areas are primary centres for learning and memory therefore any damage can manifest as cognitive dysfunction. Therefore, defective neuronal functioning in frontal cortex and hippocampus are linked with altered behaviour and memory [14, 16, 17]. Further, the frontal cortex region showed depletion of white/grey matter integrity, volume and density. The individual structural deficits severely affect the associated behavioural and neurocognitive functions [7, 18]. Advanced MRI scans revealed that breast cancer patients receiving adjuvant chemotherapy for a one-year period exhibited not only white and grey matter volume depletion in core functional centres of the brain but also depleted attentive and cognitive processes associated to these specific areas [19].
3. COGNITIVE IMPAIRMENT MECHANISMS OF CHEMOTHERAPEUTIC DRUGS
Chemotherapy poses threat to brain structural composition via depletion of white matter integrity in the prefrontal cortex. The harmful effects on neuronal morphology include abnormal deformities in dendritic spines, arborisation, anatomical structure and density. Accumulated evidence shows that cytokine dysregulation impairs synaptic network, hippocampal volume and brain metabolism in prefrontal and temporal cortex [20]. Depletion of white matter and oligodendrocyte cause hindrance in neurotransmission affecting the processing speed in patients undergoing chemotherapy [21]. Mitochondrial damage and decreased cellular activity add to the progression of these neurotoxic effects. Neurodegeneration and subsequent cognitive disturbances follow [22].
In vitro reports claim that some chemotherapeutic drugs either cause Neuronal Progenitor Cell (NPC) apoptosis or simply reduce NPC count. Regardless of this, all chemotherapy drugs disrupt neurogenic proliferation, thus considered neurotoxic [23]. Although chemotherapy might cause transient impairment in rodent models, progressive neurodegenerative symptoms were documented [24]. Chemotherapy reduces blood flow, negatively affecting brain glucose metabolism and cognition. Chemotherapeutic agents, such as methotrexate mediate vascular toxicity via its anti-angiogenic effect [25]. Overall the complex nature of anticancer treatment coupled with the diverse mechanism of action may provoke multi-layered cognitive disorder symptoms and behavioural toxicities. Doxorubicin commonly used in breast cancer has shown to increase peripheral ROS production thereby develop brain mitochondrial dysfunction, structural deformity and cognitive impairment in both clinical and preclinical settings [26]. Doxorubicin enhances pro-apoptotic mitochondrial protein expression and damage brain complex formation. In rodent brains, intra-mitochondrial calcium infiltration and oxidative stress were observed [27]. Doxorubicin’s ability to cross the membrane barrier increased TNF-α levels both peripherally and centrally. As a result, TNF-α accumulated in choroid plexus impairing mitochondrial function [28]. In an early-stage breast cancer clinical trial, patients underwent a chemotherapeutic regimen of doxorubicin/docetaxel along with cyclophosphamide that elevated plasma IL-1β and IL-6 along with increased cognitive abnormalities and decreased responsiveness respectively while an increase in IL-4 caused improvement of the aforementioned problems [29].
Behavioural defects were observed with both doxorubicin and cyclophosphamide, though the latter was associated with microglia induced inflammation. Activated microglial cells in hippocampus caused hippocampal-dependent memory deficiency in rats. Both drugs showed almost 90% decline in neurogenesis, as assessed by immunofluorescence techniques [30]. Cyclophosphamide has been explored to impair passive avoidance learning in animals either alone or with doxorubicin [31]. Thiotepa also inhibits neurogenesis and brings down memory-related performance [32]. Adriamycin unexpectedly elevated peripheral and central TNF-α levels questioning its ability to cross the vascular barrier [33]. Etoposide acts through p38 MAPK (Mitogen-activated protein kinase) pathway to induce fatigue and elevate serum IL-6 levels in mice [34].
Platinum-based agents (cisplatin, oxaliplatin, carboplatin etc.) disrupt protein synthesis by cross-linking the vulnerable mitochondrial DNA to irreversible mutation than nuclear DNA. These agents are neurotoxic to NPC and oligodendrocytes through excitotoxic alterations and apoptotic pathway [35, 36]. The mechanism in cisplatin-induced cognitive decline has been reported by deep brain penetration and neuronal stem cell proliferation inhibition in a mice model [37]. Further, cognitive abnormalities occur through mitochondrial dysfunction, disruptions of cerebral white matter and NPC depletion in the dentate gyrus [38]. Cisplatin enhances lipid peroxidation by inhibiting mitochondrial respiration and ATP synthesis. It is also increasingly deposited in peripheral dorsal root ganglions (DRG) [39]. Oxaliplatin gravely affects mitochondrial structures and functions and reduces anti-inflammatory cytokines in the spinal dorsal horn [40]. Oxaliplatin sensitizes nociceptors by up-regulation of pro-inflammatory cytokines and chemokines [41]. Oxaliplatin upregulates glial fibrillary acidic protein (GFAP) immunodensity, TNF-α, IL-1β, Monocyte Chemoattractant Protein-1 (MCP-1) and Macrophage Inflammatory Protein-1 α (MIP-1α) expression [5]. Melatonin has pain-relieving anti-nociceptive activity on oxaliplatin-induced spinal dorsal horn neuroinflammation. Other platinum-based chemo drugs disrupt the electron transport chain, harming mitochondrial antioxidation system severely [42].
Taxanes (Paclitaxel, docetaxel) cause a structural compromise in vital areas of the cerebral cortex and peripheral neurons. Mitochondria is prone to taxanes-induced damage as established in a neuropathic rodent model [38]. Taxanes depolarize mitochondrial membrane by intracellular calcium influx resulting in compromised structural composition and cytochrome-c activated apoptosis [43]. Paclitaxel is a microtubule inhibitor and antimitotic drug producing a variety of depressive and neuropathic symptoms affecting the quality of life in survivors. It induces endoplasmic reticulum (ER) stress promoting neurotoxic effects [44]. Paclitaxel heightens peripheral neuropathy through Toll-like receptor-1 (TLR-1) leading NF-κB mediated upregulation of various pro-inflammatory cytokines in the target site [45].
Anti-metabolite methotrexate is highly toxic to neural cells especially the glial progenitor cells responsible for white matter integrity [46]. Methotrexate severely impairs cognitive functions and precipitate depressive symptoms in a breast cancer cell line mouse model. In addition to this, there is a significant reduction in hippocampal NPC in both tumour-bearing and non-tumor-bearing samples, with the former stimulating high levels of COX-2 and iNOS [47]. Contrary to popular belief, methotrexate may be capable of crossing the blood-brain barrier (BBB) to exert cytostatic effects on hippocampal cell proliferation [24]. In this particular preclinical study, low intravenous dose of methotrexate inhibited NPC growth and a high dose of methotrexate disrupted hippocampal-dependent spatial memory and comparator function. A high intraperitoneal dose of methotrexate precipitated seizures and long-term memory deficits in behavioural assessment tasks. Methotrexate also has plasma corticosterone lowering properties leading to cognitive toxicity in rodent models [48]. In a separate preclinical study, although neuroimaging results suggested activation of microglia cells however, this activation did not elevate cytokines measured in the hippocampus. This is another aspect which explains the neuroprotective action of microglial cells without elevating excess of cytokines in the brain [25].
5-fluorouracil another potent anti-metabolite, curtailed dentate gyrus (DG) neurogenesis, two weeks post-treatment with a rebound after about 2 months. 5-fluorouracil has also shown short term deficits in Sub-ventricular zone (SVZ) along with the emergence of cell suppression post 6 months of treatment in both DG and SVZ. 5-fluorouracil impairs cognition by decreasing NPC proliferation in SVZ of the dentate gyrus. 5-fluorouracil crossed BBB via passive diffusion to exert its actions [49, 50]. 5-fluorouracil alone with other drugs demyelinate neurons, decrease oligodendrocyte expression and precursor synthesis in rat corpus callosum [51]. Methotrexate and 5-fluorouracil inhibit adult hippocampal cell proliferation and working memory [30]. In some cases, they elevate rodent exploratory behaviour and anxiety while lowering glucose metabolism and cerebral blood flow [52].
In some cancers, chemotherapeutic agents are prescribed in combination at lower doses with an intention of maximum effectiveness and lower risk of toxicity [5]. Triple chemotherapy of cyclophosphamide, methotrexate and 5-fluorouracil for breast cancer patients result in neuroinflammation and neurodegeneration in the prefrontal cortex and hippocampus. Loss of neurons in the major centres for learning and memory lead to severe cognitive difficulties and dementia [37]. Four weeks cycle of the same combination of drugs resulted in IL-1β and TNF-α expression in rat corpus callosum with the opposite effect on IL-10 levels and affected working memory performance. Further, the combination therapy caused a variety of neurological side effects related to memory, cognition, attention and processing speed. COX-2 inhibitor was able to alleviate the inflammatory effects of the combined chemotherapy [39, 53].
Another combination therapy of cyclophosphamide, doxorubicin, 5-fluorouracil increased fatigue and proinflammatory cytokines in a murine model [39, 54]. Cyclophosphamide and doxorubicin together are responsible for cognitive impairment and decreased neurogenesis in rodents with the latter showing increased anxiety response in a number of neurological assessment tasks. However, information on the exact mechanism of action is still limited. Docetaxel-adriamycin-cyclophosphamide triple therapy was seen to elevate both central and peripheral pro-inflammatory cytokine levels and decrease anti-inflammatory cytokines producing a cytokine system imbalance causing cognitive impairment by disrupted neuronal plasticity. Neuroimages also showed reduced dendritic spine formation in this model [55].
Therefore, to summarize, cognition is adversely affected by chemotherapy. Available reports on breast cancer survivors indicate towards CICI. Different chemotherapy regimens at different doses are associated with different levels of cytokine concentrations and varying degrees of cognitive impairment. The onset of cognitive disturbance also remains to be investigated. The inflammatory or neurotoxic adverse effect in CNS very much dependant on the cellular targets, the chemical nature of drugs, dosing regimen and various other confounding factors. Peripheral inflammation might also lead to cognitive disturbances. Therefore, it’s essential to carefully monitor each individual therapy and its observational effects in both preclinical and clinical investigations.
3.1. Chemotherapy-induced Neuroinflammation
Amongst the majorly accepted mechanism of chemobrain is cytokine dysregulation. Previous preclinical and clinical investigations have shown elevation of cytokine levels following chemotherapy and play an important role in neuroinflammation, cancer progression, peripheral neuropathy and cognitive disabilities. Heterogeneous dysregulation of cytokine levels in the CNS occurs via multiple mechanisms. Either due to cancerous tumour itself which increases circulating cytokines or by acute and chronic stressor mechanisms. Though tumorigenic developments influence the cytokine concentrations in the brain, the majority of reports indicate inflammatory symptoms during and after the chemotherapeutic intervention. Cancer metastasis and/or anticancer drugs cause an outburst of peripheral cytokines from tumour cells, WBC and stimulated macrophages [20], leading to cognitive decline. The exact mechanism is still unknown. Clinical results of different doses of chemotherapy may vary from patient to patient.
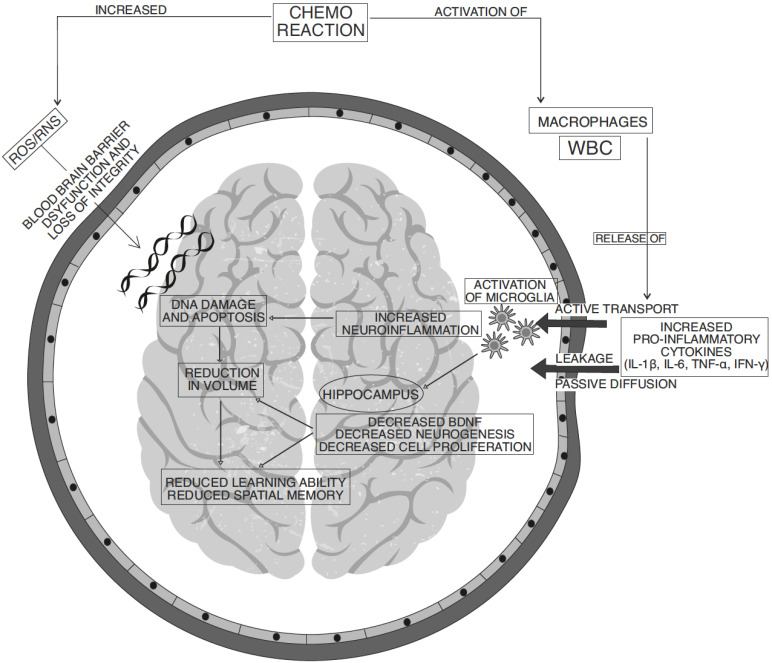
Cytokines are small pleiotropic protein molecules produced mostly from activated T helper (Th) cells, peripheral antigen-presenting cells and macrophages in a cascade triggering central as well as peripheral inflammation in pathological conditions [56]. They are considered as potential biomarkers for dementia and memory deficiency [20]. Cytokines in the brain are mainly released by microglial cells while astrocytes and oligodendrocytes assist in the process. Microglia, the regulators of cytokine signalling, are in resting state under normal physiological conditions performing neurogenesis, neurotrophin-mediated neuronal branching and proliferation. Any physical or chemical insult on the brain cause disruption in the homeostasis, resulting in microglial activation to release cytokines [57]. However, the chronic imbalance between pro- and anti-inflammatory cytokines results in serious complications on neuronal integrity in the brain [58]. Similarly, intercommunication between peripheral and central cytokines cause cytokine elevation in the brain. Peripheral cytokine triggered central cytokine cascade is the reason for neural plasticity defects (Fig. 1).
Fig. (1).
Different pathological mechanisms involved in anticancer chemotherapy-induced cognitive impairment. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Cytotoxic effects of chemotherapies or even pro-inflammatory cytokines can alter the endothelial structural integrity and make a passage for cytokines to crossover into the brain [59]. The crossover of drugs and inflammatory mediators occur through membrane disintegration resulting in pore formation in the densely interconnected network of capillaries [5]. The peripheral cytokines then can either actively transport through the membrane barrier via saturated efflux transporters (P-glycoprotein transporters and ABC transporters) or passively transport via diffusion through the leaks in the endothelial junction at circumventricular organs [31]. Moreover, receptor-mediated cytosis and local inflammatory network also contribute towards central cytokine elevation [58]. Overflow of peripheral cytokines and chemotherapeutic drugs through the damaged BBB possess neurotoxic insult to the vulnerable brain regions [55].
Cytokines can modulate neuroendocrine actions, neuronal cell functions, repair and regeneration along with neurotransmitter metabolism. Accordingly, chemotherapy-induced cytokine dysregulation would cause a chain of events that ultimately decline in neurocognitive abilities. Various key events in this process are NMDA receptor-mediated excitotoxicity, monoaminergic, GABAergic and cholinergic neurotransmitter system irregularities. Cytokines are also involved in neuropeptide synthesis alterations and increased oxidative stress-induced cellular dysfunctions and apoptosis [20]. The cytokine-mediated alterations of neurotransmitters levels in the CNS may wreck the neuronal synaptic plasticity and eventually cause neurodegeneration. The combined ability of uncontrolled pro-inflammatory cytokines proliferation along with altered emotional and neuroendocrine levels can result in disruption of hippocampal functions [47].
Further, the evidence clearly states that mitochondrial complexes are susceptible to inflammatory responses. Chronic stress leads to mitochondrial p53 protein accumulation in the membrane and subsequent activation of the apoptotic mechanism [28]. Extracellular stress and mitochondrial stress amplify the glutamate accumulation and activate NMDA receptors. An increased intracellular influx of calcium through this receptor exceeds the normal physiological limits resulting in apoptosis. Blockade of calcium excitotoxicity is a potential target for preventing neurodegeneration, however, clinical trials have not yet provided conclusive results [37].
Cytokine-mediated depression and clinical depression have converging evidence in their symptomatic similarities [60]. The administration of cytokine specific antibody in animal models of chemobrain inhibits the cytokine cascade release, confirming that these cytokines are directly responsible for chemotherapy-induced cognitive impairment [58]. The inability of antibodies to cross the blood-brain barrier has limited their use. Once the upstream mechanisms of cognitive dysfunction have set in permanently, the antibodies aren’t helpful [61].
Contrasting theories suggest that inflammatory mediators may not always cause neurocognitive symptoms, so alternate inflammatory processes should be considered. Genetic polymorphisms like SNP of genes expressing pro-inflammatory cytokines have been identified [38]. Clinical trials for patients with genetic alterations have given inconclusive results. Further research is recommended in this area for a better understanding of the relationships between gene expression and susceptibility to inflammation and neurotoxicity. These evidence suggests that for the management of CICI, it is essential to investigate different pathophysiological patterns of cytokines.
3.2. Role of Cytokines in CICI
Elevated levels of pro-inflammatory cytokines and oxidative stress in the brain are well-known indicators of neuroinflammatory response. Multifactorial involvement of both in CICI is discussed below.
Treatment with chemotherapeutic agents accentuates the production as well as the release of pro-inflammatory cytokines such as IL-6, IL-1β, TNF-α from stimulated peripheral macrophages [62]. Doxorubicin initiates cytokine-mediated inflammatory cascade via MAPK (p38/JNK) as well as NLRP3 inflammasome pathway activation to release active IL-1β from proIL-1β. IL-1β localization in the hippocampus alters the site-specific function of memory, cognition and mood [34]. Excessive IL-1β localisation is known to alter neuronal proliferation. The loss of proliferating cells in the hippocampus instigates neurotoxic symptoms [56]. Recent evidence suggests that IL-1β affect neurogenesis by the kynurenine pathway via upregulation of key enzymes like IDO (Indoleamine 2,3-dioxygenase), KMO (kynurenine-3-monooxygenase) and kynureninase [63]. IL-1β enzymatically converts tryptophan to end product quinolinic acid (QA), an agonist of glutamate (NMDA) receptors, in the microglial cells. Increased levels of QA combined with NMDA receptor activity enhance neurotoxic events. Some studies with respect to depressive conditions associate the decline in kynurenic acid, an NMDA antagonist, due to loss of astrocytes. In another study, inhibition of KMO enzyme reversed the effects of IL-1β induced neuronal impairment and motor functions in a few neuropsychiatric disorders (Alzheimer’s, Huntington’s etc.) [63]. Since these conditions have overlapping pathomechanisms with CICI, assessing the role of this pathway for IL-1β-mediated cognitive decline in CICI can be a scope of future studies.
IFN-ϒ, a pro-inflammatory cytokine, is produced by activated T-lymphocytes and natural killer cells that further stimulates IL-1b and IL-6 release from immune cells [64]. IFN-ϒ was found to stimulate IDO activity resulting in a significant reduction of tryptophan and serotonin levels in the brain. The over-activation of IDO enzyme induces depressive symptoms through excitotoxicity and free radical damage [65]. IFN-ϒ causes macrophage-mediated ROS generation. Although the pathological role of IFN-gamma has been studied in depressive conditions, its role in CICI needs to be elucidated.
Another important pro-inflammatory cytokine is IL-6. Transgenic studies using knockout animals, IL-6 deficient genotypes, pro-inflammatory cytokine receptor antibodies (IL-6Ra) and inhibitors asserted that IL-6 has a role in cognitive impairment [55, 58]. Just like IL-1β, IL-6 also acts through a p38-MAPK pathway, established in vitro studies. Peripheral nerve injuries have expressed IL-6 cytokines [66]. Further in vitro analysis confirms that the concentrations of IL-6 and IL-1β in the blood is directly proportional to the sickness severity and neuroendocrine stimulation in the brain [65]. In the clinical trial of breast cancer survivors, IL-6 and TNF-α decreased hippocampal-dependent learning function and its volume [67].
TNF-α mediates its apoptotic and pro-inflammatory actions through cell surface TNFR-1 and TNFR-2 receptors present on both neurons and glial cells [56]. TNF-α enters the brain via receptor-mediated endocytosis and triggers the activation of the nuclear transcription factor (NF-κB) through downstream signalling to increase the levels of TNF-α and iNOS metabolites. Nitric oxide (NO) produced from the iNOS pathway activates NF-κB in an endless loop of cell signalling processes [20]. The intracellular death domain of TNFR-1 inhibits hippocampal neurogenesis whereas TNFR-2 promotes it. Clinical trials of breast cancer patients showed elevation of soluble TNFR-2 in blood plasma of chemotherapy-treated groups, associated with reduced frontal cortex metabolism and increased memory loss. Patients who didn’t have elevated plasma TNFR-2 were devoid of such complications which could be attributed to membrane-bound TNFR-2 mediated neurogenesis indicating that TNF-α has a major role in CICI [67]. This can be an interesting aspect to study TNF-α mediated cognitive impairment in CICI.
Despite the detrimental effects on the nervous system, adequate amounts of pro-inflammatory mediators are essential for normal neuronal function. Proven through preclinical investigations, in cytokine knockout transgenic rodent models, the absence of IL-6 and TNF-α can impair learning and memory, neurogenesis and hippocampal function [58]. Hence, a balance between pro- and anti-inflammatory cytokines is essential for normal neuronal functioning.
Reduced pro-inflammatory cytokines and elevated anti-inflammatory cytokines reverse the neurotoxic effects and elicit the neuroprotective action. Major types of anti-inflammatory cytokines include IL-4, IL-10, IL-11, IL-13 and IL-1RA. Others like IFN-α, TGF-β, leukaemia inhibitory factor (LIF) rides the line between pro and anti-inflammatory based on the situation [56]. IL-4 cytokine has neuroprotective properties, where it neutralizes pro-inflammatory action of other cytokines [55]. Another anti-inflammatory cytokine IL-10 is produced by helper T-lymphocytes, B-lymphocytes and monocytes. It blocks pro-inflammatory cytokine release as well as tips the balance of pro and anti-inflammatory cytokines towards the neuroprotective side.
Since a large portion of pathophysiological mechanisms involved in neuroinflammatory cognitive decline remains unexplained and unknown, a number of strategies have been suggested to look forward into this matter. These strategies include PET scanning of activated microglia and CSF cytokine levels. Preclinical animal studies are very much preferred due to the ease of control in producing inflammatory disorders without any patient-related complications or concern. Whole tissue analysis for easy identification of biomarkers and different targets for therapeutic intervention [39]. Furthermore, targeting the specific effector molecules, cell signalling pathways and cellular receptors either by transgenic gene knockout animal models or molecular inhibitors may help researchers to come up with novel therapeutic strategies for the treatment of these disorders [65].
4. ROLE OF OXIDATIVE STRESS IN CICI
The brain relies on aerobic respiration to supply the body’s energy needs (ATP) and therefore the mass utilization of oxygen in the brain makes it prone to get affected by oxidative and nitrosative damage in comparison to other organs [58]. Imbalance in the pro- and -antioxidant system result in overproduction of oxidative stress markers (ROS and RNS). ROS and RNS are originated from the metabolic reaction in the electron transport chain of the mitochondrial matrix and rarely in the endoplasmic reticulum [68].
Local cytokine-mediated NOS pathway activation causes oxidative stress leading to DNA damage. When the toxic free radicals exceed the neutralizing capacity of the antioxidant system, it starts damaging the tissues, lipid membrane, denatures, and degrades proteins. The free radicals attack unsaturated PUFA in the brain to cause lipid peroxidation. In addition to this, it attacks the mature adult neurons which have no regenerative capacity (except NSC), causing permanent damage [68].
Damage to mitochondrial integrity causes further free radical damage and cellular energy deficiency [69]. Structural and functional alteration in mitochondria and other neurons occurs due to ineffective free radical scavenging mechanisms and augmented oxidative stress. Together these elevated endogenous biomolecules initiate a chain of events which eventually terminates in neurotoxicity, dysfunction and neuronal apoptosis. Loss of neurons in the prefrontal cortex and hippocampus leads to the development of cognitive impairment [68].
Nearly 40% of clinically available cancer chemotherapeutic drugs produce oxidative stress-induced damages. Chemotherapy-induced mitochondrial dysfunction through oxidative stress is a major component of neuronal and cognitive impairment in CICI. Neuronal cells in the cerebral cortex and oligodendrocytes are susceptible targets for such toxic events [70]. Oxidative stress in the hippocampal NPC results in not only decreased expression of BDNF neurotrophin but also cause apoptosis via the SOD releasing properties [71]. Calcium exocytosis and ionic imbalance occur in the mitochondria causing loss of neuronal and synaptic plasticity [70]. Calcium influx induced membrane depolarization and DNA disruption lead to caspase-3 activated apoptotic mechanisms in oxidative stress [72]. Cytosolic accumulation of ROS leads to NLRP3 inflammasome mediated caspase-1 activation. It also affects the mitochondrial pathway of apoptosis [73].
It can rightfully be concluded that oxidative stress coupled with neuroinflammation causes neurotoxicity in the structural and functional areas of the brain causing cognitive decline [70].
5. REDUCED NEUROGENESIS AND COGNITIVE IMPAIRMENT
Brain-derived neurotrophic factor (BDNF) is majorly distributed as well as characterized neurotrophin class of growth factors found in the brain having functional specificity among other proteins [74]. BDNF binds to Tyrosine kinase B (TrkB) receptors and mediates neural progenitor cell (NPC) survival, proliferation, differentiation and maturation into adult neurons [75]. The BDNF-TrkB signal activity has been associated with the reversal of age-related cognitive decline.
Process of neurogenic synthesis involving BDNF is essential for forming the neural network, dendritic arborisation, synaptic plasticity and connectivity in the CNS [75]. Evidence has revealed that hippocampal synaptic plasticity and its interaction with prefrontal cortex contribute to spatial memory formation [60]. BDNF also has memory formation and anti-depressive properties mediated through its neurogenic action. Severely depressed patients show depletion of serum BDNF levels from their storage sites [30].
The reduction of BDNF levels in the brain either by age-related complications or polymorphic encoding genes. These all attribute to spatial and episodic memory defects, hippocampal structural alterations, feeding abnormalities and overall cognitive decline in both animals and humans alike. Even other neurodegenerative/neuropsychiatric disorders like Alzheimer’s schizophrenia, anxiety and depression have shown suppressed BDNF concentrations in the brain [75].
The inability of astrocytes to clear hypersecretion of glutamate in severe stressful conditions can pose a harmful neurotoxic threat since it is associated with a reduction of BDNF in the brain. Antidepressants through NMDA receptor blockade is able to reverse the toxic symptoms. NMDA antagonist ketamine potentiates its effectiveness [73]. Evidence from animal models of depression has reported the significant loss of BDNF and VEGF in CNS leading to reduction of brain volume. In the conventional treatment for depression, antidepressants possess properties to enhance the neurotrophin release as well as promote neuronal cell proliferation. Therefore, these classes of drugs may be capable of reversing the damage left behind by neurotoxic stimulus [73]. Another hypothesis states that decreased vasculature and metabolic energy production in the brain also results in the reduction of endothelial BDNF release thereby hampering neurogenic proliferation in the brain functional areas [76].
Positive regulators of neurogenesis include physical exercise and hippocampal-dependent learning tasks. Physical exercise is a natural intervention applied to boost hippocampal neurogenesis and cognitive skills. While the negative regulators are chemotherapy mediated insult, irradiation, neuroinflammation, glutamate release and oxidative damage among others [77]. Preclinical data establishes that chemotherapy plus routine daily exercise improves significant neurogenic loss and cognitive impairment when compared to chemotherapy alone [78, 79]. There are two theories that link cognitive memory to either synaptic formation irrespective of neurogenesis or differential neural cells’ contribution in synapse formation. Mood is dependent on neuronal remodelling. Hence in some cases, newly differentiated mature cells may be detected but not the improvement of cognitive memory functions and vice-versa [56]. Proliferation deficits occur within a short period after chemotherapy with a small time frame for assessment, as shown in preclinical studies, it takes six weeks for NPC to reach full developmental and functional maturity in the dentate gyrus. Therefore, cognitive deformities can be attributed to chemotherapy-induce toxicity rather than NPC growth.
Recent scientific theories have deduced that the inhibition of post-natal neurogenesis and gliogenesis might be the primary cause of localized cellular dysfunction in the white matter of patients. Clinical methods of proliferating neuronal progenitor stem cells might be the viable therapy against chemotherapy-induced cognitive deficits in the future. While assessment in rodent models is easily achieved. Any significant influence on human neurogenesis by the same chemotherapy and radiation is still under examination. Further research in understanding the mechanism of neurogenesis and their functions should be able to find therapeutic solutions to prevent chemotherapy-related neurodegeneration and restore their normal physiological cognitive roles in the nervous system [77].
6. POSSIBLE BENEFICIAL ROLE OF ANTI-DEPRESSANT DRUGS IN CICI
The depressive symptoms are linked to the pathophysiological imbalance between the pro- and anti-inflammatory cytokines [58]. Cytokines, being common causative factors in depressive conditions and CICI, can be targeted for amelioration of associated depression and CICI symptoms. This speculates the possibility of antidepressants to be of benefit in CICI [80].
The literature on in vitro and ex vivo studies state the anti-inflammatory actions of MAO inhibitor antidepressants [34]. Studies provide evidence that Selective Serotonin Reuptake Inhibitors (SSRIs) and Tri-cyclic Antidepressants (TCAs) were able to inhibit pro-inflammatory cytokines like IL-1β, TNF-α, IFN-ϒ and lymphocyte proliferation [81, 82]. High levels of pro-inflammatory cytokines lower the BDNF and BDNF-TrkB receptor activity in the brain. Therefore, suggesting a correlation between inflammatory and neurogenic hypothesises in CNS. Thus, with knowledge of antidepressants possessing anti-inflammatory potential, their effect can be correlated to neurogenic activity as well [73]. A few theories are hypothesized for antidepressants to possess anti-neuroinflammatory activity.
The first theory states that high binding affinity of SSRIs to serotonin transporter (5-HTT) may launch a cascade of anti-inflammatory pathways. Further, both TCA and SSRI are able to inhibit microglial translocation of activated NF-κB and its pro-inflammatory cell signalling pathways. While conflicting research disregards this as drugs did not have any effect on in vitro IL-10 modulation. Another minor theory states that antidepressants might influence anti-inflammatory activity by microglial β-adrenergic receptors interactions [83].
Finally, researchers found that SSRI like fluoxetine has an inhibitory role in pro-inflammatory cytokine gene expression and their mRNA transcription with supplementary benefits in the inhibition of MAPK pathway phosphorylation [84]. Antidepressants act through cAMP-mediated protein kinase activation to reduce microglia and macrophage inflammation. Evidence confirm this theory via an association between elevated cAMP levels and reduced cytokine release and vice versa. Moreover, antidepressants may be able to alleviate symptoms of oxidative stress by inhibition of cytokine cascade and free radical production along with elevation of antioxidant enzymes [68]. The anti-inflammatory effects of SSRIs have been reported to therapeutically benefit in a number of autoimmune and inflammatory diseases (multiple sclerosis, stroke, rheumatoid arthritis). Therefore, they are bound to exert similar therapeutic properties in CICI due to overlapping pathomechanisms [73].
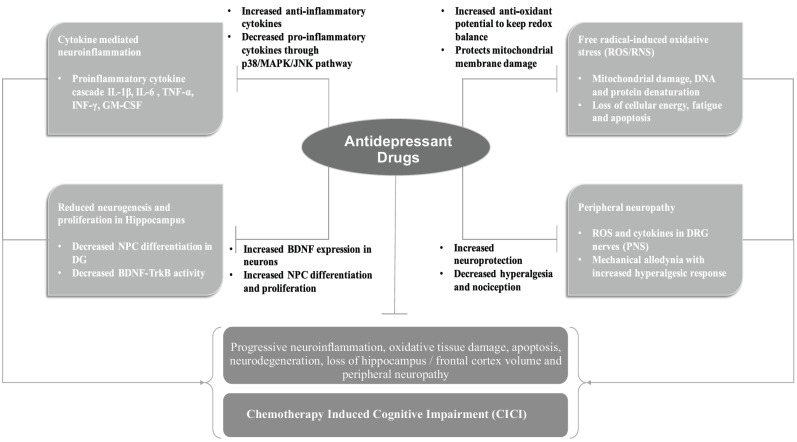
Different antidepressants from all categories of antidepressants and their respective pharmacological effects (Fig. 2) are discussed below. These known pharmacological effects make antidepressants to be a good candidate for testing against the symptoms of CICI.
Fig. (2).
Brief summary of pathophysiological mechanisms involved in the development of CICI and the different pathways through which antidepressant treatment targets these mechanisms to provide a neuroprotective effect. [BDNF (Brain-Derived Neurotrophic Factor); DG (Dentate Gyrus); DRG (Dorsal Root Ganglion); GM-CSF (Granulocyte Monocyte Colony Stimulating Factor); IL (Interleukins); INF (Interferon); JNK (Janus Kinase); MAPK (Mitogen-Activated Protein Kinase); NPC (Neuronal Progenitor Cell); PNS (Peripheral Nervous System); RNS (Reactive Nitrogen Species); ROS (Reactive Oxygen Species); TNF (Tumour Necrosis Factor); Trk-B (Tyrosine Receptor Kinase B)]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
6.1. Fluoxetine
Fluoxetine, a selective serotonin reuptake inhibitor (SSRI), is one of the most well-known and clinically prescribed antidepressant drugs. Fluoxetine has been investigated in different preclinical models for its anti-inflammatory and anti-neuropathic activities [64]. Fluoxetine also improved cognition in a number of neurological disorders through a positive effect on neurogenesis by upregulation of BDNF. Fluoxetine is also reported to stimulate gliogenesis in the extra-hippocampal prefrontal cortex [10]. Fluoxetine blocks serotonin reuptake transporters (SERT) thereby increases the 5-HT concentration in the synaptic cleft. Thus elevated 5-HT interacts with the postsynaptic serotonin receptors for synthesizing BDNF by various cellular pathways [85].
Fluoxetine prevented 5-fluorouracil mediated reduced proliferation of hippocampal cells and corresponding cognitive impairment in rats. However, it did not reverse the deficits when it was given after termination of chemotherapy [50]. In another rat model of 5-fluorouracil-induced cognitive decline, fluoxetine treatment enhanced hippocampal-dependent spatial memory function and eliminated neural cell depletion in the hippocampus. The effects were not only limited to hippocampus but also observed in temporal lobes, anterior cingulate cortex and frontal cortex. Fluoxetine even ameliorated methotrexate-induced cognitive impairment and deficit in hippocampal proliferation in rats [49]. Fluoxetine causes neurogenic stimulation in the hippocampus, stress-responsive periventricular areas of the brain exhibiting neurogenic proliferation in tamoxifen administered transgenic mice [86].
In addition to the above effects, fluoxetine exerts anti-inflammatory and anti-oxidative actions. These actions are reported in the studies other than CICI condition. Fluoxetine alleviates CNS neuroinflammation and microgliosis [73] with downregulation of IL-6, TNF-α, NO levels in LPS-activated microglial cells [64]. Therefore, fluoxetine, when given along with chemotherapy, can improve cognition, behavioural learning deficits as well as elevate neuronal cell proliferation, survival and dendritic branching. Fluoxetine may be preferred in combination therapy as it has a significant synergistic action with NSAIDs, melatonin and even other antidepressant classes of drugs [64]. For example, it’s a better anti-inflammatory when given along with celecoxib [87], or provides a faster onset of action in depressive models with aspirin [73]. On the contrary, investigations have also shown that chronic administration of fluoxetine in mice for 9 weeks significantly decreased neurogenesis and cell proliferation in the subventricular zone, thereby suggesting suppression of neurogenesis [88]. There is a dearth of evidence for the direct effects of fluoxetine on inflammatory markers in CICI.
6.2. Other SSRIs
Other SSRIs such as fluvoxamine, reboxetine, paroxetine, citalopram, escitalopram etc. also been tested in various preclinical models. Their effects on oxidative stress, inflammatory markers and neurotoxicity are discussed below. Fluvoxamine demonstrated inhibition of ER stress through sigma-1 receptor agonism to alleviate paclitaxel-induced neurotoxicity in neuronal cell cultures [44]. Along with imipramine and fluvoxamine, reboxetine was able to lower IL-6 production in IFN-ϒ stimulated microglial cells. Paroxetine, when given along with sertraline inhibited on IFN-ϒ, induced TNF-α and NO elevation in vitro [89]. It prevented TNF- α release from stimulated T cells [73]. In a preclinical study of antidepressants, results showed that citalopram was pro-inflammatory by elevating cytokine levels (IL-1Β, TNF- α, IL-22) while escitalopram was anti-inflammatory by lowering IL-17 [80]. Further, escitalopram is a better antioxidant than fluoxetine in MDD and can elevate Vitamin-C levels as well [68].
Sertraline manifests its activity independent of 5HT receptor by significantly improving GSH levels, cognitive performance, inhibiting lipid peroxidation along with elevated nitric oxide in nitrosative stress models [68]. Sertraline inhibited IFN-ϒ induced TNF-α and nitric oxide elevation in vitro [89] and promoted IL-10 and IFN-ϒ ratio towards the neuroprotective side in healthy human blood samples [64]. Sertraline in combination with naringin act through serotonin modulation and exerted mitochondrial protective activity in doxorubicin-challenged rats.
Vortioxetine is a relatively newer investigational antidepressant drug which acts through different serotonergic receptors. High dose of vortioxetine exhibited a stimulatory effect on dendritic morphology, enhanced granule cell proliferation, maturation and survival in the hippocampal dentate gyrus of mice [90]. Clinical trials reports reveal that major drugs belonging to the SSRI class of antidepressants like fluoxetine, fluvoxamine, citalopram, sertraline etc. reverse the peripheral oxidative stress markers and convert antioxidant enzyme levels and antioxidant profile back to normal [91].
6.3. Serotonin Norepinephrine Reuptake Inhibitors (SNRIs)
Duloxetine is a common SNRI class of antidepressants with clinical analgesic properties [92]. A clinical trial of chemotherapy-related peripheral neuropathy showed daily duloxetine caused pain reduction in 59% of patients when compared to the placebo control group while only 10% patients reported increased pain throughout the time period [93]. Venlafaxine is also an SNRI antidepressant [92] whose treatment in the mouse model of chronic hippocampal oxidative stress showed potent protective action against DNA damage [68]. Chronic preclinical venlafaxine dosing provides antioxidative protection against nuclear acid damage by maintaining redox balance in the brain [91]. Venlafaxine exhibits anti-inflammatory properties through lowering of cytokine ratio [89]. Low dose of venlafaxine and amitriptyline in particular raised the BDNF and Bcl2 immunostaining intensity in rat hippocampal pyramidal neurones and hippocampal mossy fibres respectively [91]. Both these drugs have substantial clinical evidence for their anti-nociceptive activity against chemotherapy-related peripheral neuropathy. Venlafaxine was particularly effective against oxaliplatin-induced acute and chronic neuronal toxicity in patients [87].
6.4. Tricyclic Antidepressants (TCAs)
Imipramine, a 5HT-1A receptor agonist belongs to the TCA class of drugs. Both short and long-term treatments have improved neurogenesis in the dentate gyrus of a controlled cortical impact model in rats. Furthermore, it increased the BDNF mRNA expression in these rats [66]. In traumatic brain injury murine model, imipramine improved neurogenesis and cognitive performance without effective improvement in motor functions. Besides, histological analysis using immunofluorescence method showed preservation of NPC proliferation and maturation in the dentate gyrus [51]. Reduced CDK inhibitor p21 expression and depressive symptoms with a consequent increase in dentate gyrus neurogenesis of immature NPC were observed in chronic administration, in an in vivo model [94]. Acute and chronic effects of imipramine on rodent prefrontal cortex and hippocampus significantly reduced lipid peroxidation, protein damage and enhanced antioxidant enzymes (SOD and catalase) levels. Imipramine together with clomipramine lowered NOS concentration. TCA has an inhibitory effect on in vitro IFN-ϒ levels, IFN-ϒ activated microglial cells [91] and IL-1β and TNF-α [73].
Amitriptyline, another TCA [92] when administered intrathecally, stimulated microglial IL-10 expression [73] while reducing IL-1β and TNF-α levels. Amitriptyline attenuated hydrogen peroxide-induced cell degeneration in rat pheochromocytoma PC12 cell lines along with increased superoxide dismutase (SOD) enzyme levels and inhibited apoptosis [91].
6.5. Atypical Antidepressant Drugs
Among the atypical antidepressant, mirtazapine is an adrenergic (α2) and serotonergic (5-HT2A and 5-HT3) antagonist [92] with anti-oxidative effects. Mirtazapine yields this beneficiary results in the cisplatin-induced neurotoxic rodent model. The drug lowers several oxidative stress markers significantly, demonstrating its neuroprotective effects [95]. Long-term use of mirtazapine with other antidepressants have a stimulatory effect on antioxidant enzyme mRNA level expression in peripheral monocytes when compared to short-term use that decreased levels. This may imply that the efficacies of these treatments are dependent on their usage duration. Another drug i.e. bupropion has nitric oxide inhibitory property [91]. However, no further significant data is available on other atypical antidepressants related to oxidative stress, inflammation and neurotoxicity.
Contradictory evidence is gathered against the effectiveness of antidepressants in oxidative stress mechanisms. For example, sertraline, venlafaxine, reboxetine antidepressant therapy for a period of 6 weeks didn’t show any peripheral oxidative protection against plasma MDA or modification in oxidation susceptive erythrocytes in MDD affected patients. Another contrasting study elucidates that amitriptyline caused plasma coenzyme Q deficiency-induced stress [68]. Other clinical trial reports state that antidepressants treatment was unable to influence the reduced blood plasma coenzyme Q10 levels in depressed patients despite the fact that the enzyme was not directly associated with the occurrence and severity of depressive episodes [91]. Further investigations are highly recommended to elucidate active co-relationship between antioxidant enzymes, free radicals and associated symptoms.
CONCLUSION
This review gives a brief overview of the chemotherapy-induced cognitive impairment and its causative mechanisms such as neuroinflammation, oxidative stress, and related molecular pathways. It also attempts to summarize the unexplored and potential antidepressants to be repurposed and develop as a future therapy to counteract CICI. However, there is a scope for translational research to understand the correlation between cognitive impairment with respect to i) type of cancer, ii) impact of radiotherapy and/or chemotherapy, iii) assessing the association of CICI with depression and other pre-existing neurological conditions which may affect cognition, iv) potential therapeutic strategies, v) non-pharmacological approaches, and vi) cognitive-behavioural herapy (CBT). To conclude, substantial evidence has demonstrated that in addition to monoaminergic functions, antidepressants are also capable of inhibiting progressive neuroinflammation, neurotoxicity and can improve long term neurogenesis. Other evidence also shows that the anti-inflammatory agents work synergistically with antidepressants, and even enhance their actions. Maximization of the therapeutic potential of antidepressants would be possible with a better understanding of their unexplored mechanisms against multifactorial cause and symptoms of CICI. Future clinical and preclinical prospective studies are needed for a better understanding of CICI to unearth the neuropharmacology of potential treatments against CICI.
Acknowledgements
We would like to thank Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, India for providing infrastructural support and School of Pharmacy and Pharmacology, MHIQ, QUM Network, Griffith University, Gold Coast, Australia for collaborative efforts in developing this review.
LIST OF ABBREVIATIONS
- BBB
Blood Brain Barrier
- BDNF
Brain-Derived Neurotrophic Factor
- CICI
Chemotherapy-Induced Cognitive Impairment
- DG
Dentate Gyrus
- DRG
Dorsal Root Ganglion
- ER
Endoplasmic Reticulum
- GM-CSF
Granulocyte Monocyte Colony Stimulating Factor
- IL
Interleukins
- INF
Interferon
- JNK
Janus Kinase
- MAPK
Mitogen-Activated Protein Kinase
- MDA
Malondialdehyde
- MDD
Major Depressive Disorder
- NPC
Neuronal Progenitor Cell
- PNS
Peripheral Nervous System
- QA
Quinolinic Acid
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- SNRI
Serotonin Norepinephrine Reuptake Inhibitors
- SSRI
Selective Serotonin Reuptake Inhibitors
- TCA
Tricyclic Antidepressants
- TLR
Toll-like Receptor
- TNF
Tumour Necrosis Factor
- TNFR
Tumour Necrosis Factor Receptor
- Trk-B
Tyrosine Receptor Kinase B
Consent for Publication
Not applicable.
Funding
This study was supported by “Griffith University and The Hong Kong Polytechnic University Collaborative Research Grant” to DA.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Henderson F.M., Cross A.J., Baraniak A.R. ‘A new normal with chemobrain’: Experiences of the impact of chemotherapy-related cognitive deficits in long-term breast cancer survivors. Health Psychol. Open. 2019;6(1):2055102919832234. doi: 10.1177/2055102919832234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staat K., Segatore M. The phenomenon of chemo brain. Clin. J. Oncol. Nurs. 2005;9(6):713–721. doi: 10.1188/05.CJON.713-721. [DOI] [PubMed] [Google Scholar]
- 4.DeSantis C., Siegel R., Jemal A. Cancer treatment and survivorship: facts and figures 2014-2015. Am Cancer Soc; 2014. pp. 3–6. [Google Scholar]
- 5.Wang X-M., Walitt B., Saligan L., Tiwari A.F., Cheung C.W., Zhang Z-J. Chemobrain: a critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine. 2015;72(1):86–96. doi: 10.1016/j.cyto.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahles T.A., Saykin A.J., McDonald B.C., Li Y., Furstenberg C.T., Hanscom B.S., Mulrooney T.J., Schwartz G.N., Kaufman P.A. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J. Clin. Oncol. 2010;28(29):4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ruiter M.B., Reneman L., Boogerd W., Veltman D.J., Caan M., Douaud G., Lavini C., Linn S.C., Boven E., van Dam F.S., Schagen S.B. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multimodal magnetic resonance imaging. Hum. Brain Mapp. 2012;33(12):2971–2983. doi: 10.1002/hbm.21422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreukels B.P., Schagen S.B., Ridderinkhof K.R., Boogerd W., Hamburger H.L., Muller M.J., van Dam F.S. Effects of high-dose and conventional-dose adjuvant chemotherapy on long-term cognitive sequelae in patients with breast cancer: an electrophysiologic study. Clin. Breast Cancer. 2006;7(1):67–78. doi: 10.3816/CBC.2006.n.015. [DOI] [PubMed] [Google Scholar]
- 9.Winocur G., Berman H., Nguyen M., Binns M.A., Henkelman M., van Eede M., Piquette-Miller M., Sekeres M.J., Wojtowicz J.M., Yu J., Zhang H., Tannock I.F. Neurobiological mechanisms of chemotherapy-induced cognitive impairment in a transgenic model of breast cancer. Neuroscience. 2018;369:51–65. doi: 10.1016/j.neuroscience.2017.10.048. [DOI] [PubMed] [Google Scholar]
- 10.Fardell J.E., Vardy J., Johnston I.N., Winocur G. Chemotherapy and cognitive impairment: treatment options. Clin. Pharmacol. Ther. 2011;90(3):366–376. doi: 10.1038/clpt.2011.112. [DOI] [PubMed] [Google Scholar]
- 11.Ahles T.A., Saykin A.J., Noll W.W., Furstenberg C.T., Guerin S., Cole B., Mott L.A. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12(6):612–619. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 12.Small B.J., Rawson K.S., Walsh E., Jim H.S., Hughes T.F., Iser L., Andrykowski M.A., Jacobsen P.B. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117(7):1369–1376. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]
- 13.McDonald B.C., Conroy S.K., Ahles T.A., West J.D., Saykin A.J. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res. Treat. 2010;123(3):819–828. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald B.C., Conroy S.K., Ahles T.A., West J.D., Saykin A.J. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J. Clin. Oncol. 2012;30(20):2500–2508. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bagnall-Moreau C., Chaudhry S., Salas-Ramirez K., Ahles T., Hubbard K. Chemotherapy-induced cognitive impairment is associated with increased inflammation and oxidative damage in the hippocampus. Mol. Neurobiol. 2019;56(10):7159–7172. doi: 10.1007/s12035-019-1589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruno J., Hosseini S.M., Kesler S. Altered resting state functional brain network topology in chemotherapy-treated breast cancer survivors. Neurobiol. Dis. 2012;48(3):329–338. doi: 10.1016/j.nbd.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deprez S., Amant F., Yigit R., Porke K., Verhoeven J., Van den Stock J., Smeets A., Christiaens M.R., Leemans A., Van Hecke W., Vandenberghe J., Vandenbulcke M., Sunaert S. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum. Brain Mapp. 2011;32(3):480–493. doi: 10.1002/hbm.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stemmer S.M., Stears J.C., Burton B.S., Jones R.B., Simon J.H. White matter changes in patients with breast cancer treated with high-dose chemotherapy and autologous bone marrow support. AJNR Am. J. Neuroradiol. 1994;15(7):1267–1273. [PMC free article] [PubMed] [Google Scholar]
- 19.Inagaki M., Yoshikawa E., Matsuoka Y., Sugawara Y., Nakano T., Akechi T., Wada N., Imoto S., Murakami K., Uchitomi Y. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109(1):146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 20.Ren X., St Clair D.K., Butterfield D.A. Dysregulation of cytokine mediated chemotherapy induced cognitive impairment. Pharmacol. Res. 2017;117:267–273. doi: 10.1016/j.phrs.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Gibson E.M., Nagaraja S., Ocampo A., Tam L.T., Wood L.S., Pallegar P.N., Greene J.J., Geraghty A.C., Goldstein A.K., Ni L. Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. 2019. [DOI] [PMC free article] [PubMed]
- 22.Parikh S. The neurologic manifestations of mitochondrial disease. Dev. Disabil. Res. Rev. 2010;16(2):120–128. doi: 10.1002/ddrr.110. [DOI] [PubMed] [Google Scholar]
- 23.Briones T.L., Woods J. Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 2011;12(1):124. doi: 10.1186/1471-2202-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seigers R., Fardell J.E. Neurobiological basis of chemotherapy-induced cognitive impairment: a review of rodent research. Neurosci. Biobehav. Rev. 2011;35(3):729–741. doi: 10.1016/j.neubiorev.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Seigers R., Timmermans J., van der Horn H.J., de Vries E.F., Dierckx R.A., Visser L., Schagen S.B., van Dam F.S., Koolhaas J.M., Buwalda B. Methotrexate reduces hippocampal blood vessel density and activates microglia in rats but does not elevate central cytokine release. Behav. Brain Res. 2010;207(2):265–272. doi: 10.1016/j.bbr.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Keeney J.T.R., Ren X., Warrier G., Noel T., Powell D.K., Brelsfoard J.M., Sultana R., Saatman K.E., Clair D.K.S., Butterfield D.A. Doxorubicin-induced elevated oxidative stress and neurochemical alterations in brain and cognitive decline: protection by MESNA and insights into mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”). Oncotarget. 2018;9(54):30324–30339. doi: 10.18632/oncotarget.25718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cardoso S., Santos R.X., Carvalho C., Correia S., Pereira G.C., Pereira S.S., Oliveira P.J., Santos M.S., Proença T., Moreira P.I. Doxorubicin increases the susceptibility of brain mitochondria to Ca(2+)-induced permeability transition and oxidative damage. Free Radic. Biol. Med. 2008;45(10):1395–1402. doi: 10.1016/j.freeradbiomed.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Tangpong J., Cole M.P., Sultana R., Joshi G., Estus S., Vore M., St Clair W., Ratanachaiyavong S., St Clair D.K., Butterfield D.A. Adriamycin-induced, TNF-α-mediated central nervous system toxicity. Neurobiol. Dis. 2006;23(1):127–139. doi: 10.1016/j.nbd.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Cheung Y.T., Ng T., Shwe M., Ho H.K., Foo K.M., Cham M.T., Lee J.A., Fan G., Tan Y.P., Yong W.S., Madhukumar P., Loo S.K., Ang S.F., Wong M., Chay W.Y., Ooi W.S., Dent R.A., Yap Y.S., Ng R., Chan A. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann. Oncol. 2015;26(7):1446–1451. doi: 10.1093/annonc/mdv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitamura Y., Hattori S., Yoneda S., Watanabe S., Kanemoto E., Sugimoto M., Kawai T., Machida A., Kanzaki H., Miyazaki I., Asanuma M., Sendo T. Doxorubicin and cyclophosphamide treatment produces anxiety-like behavior and spatial cognition impairment in rats: Possible involvement of hippocampal neurogenesis via brain-derived neurotrophic factor and cyclin D1 regulation. Behav. Brain Res. 2015;292:184–193. doi: 10.1016/j.bbr.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Konat G.W., Kraszpulski M., James I., Zhang H-T., Abraham J. Cognitive dysfunction induced by chronic administration of common cancer chemotherapeutics in rats. Metab. Brain Dis. 2008;23(3):325–333. doi: 10.1007/s11011-008-9100-y. [DOI] [PubMed] [Google Scholar]
- 32.Mignone R.G., Weber E.T. Potent inhibition of cell proliferation in the hippocampal dentate gyrus of mice by the chemotherapeutic drug thioTEPA. Brain Res. 2006;1111(1):26–29. doi: 10.1016/j.brainres.2006.06.093. [DOI] [PubMed] [Google Scholar]
- 33.Tangpong J., Cole M.P., Sultana R., Estus S., Vore M., St Clair W., Ratanachaiyavong S., St Clair D.K., Butterfield D.A. Adriamycin-mediated nitration of manganese superoxide dismutase in the central nervous system: insight into the mechanism of chemobrain. J. Neurochem. 2007;100(1):191–201. doi: 10.1111/j.1471-4159.2006.04179.x. [DOI] [PubMed] [Google Scholar]
- 34.Wood L.J., Nail L.M., Perrin N.A., Elsea C.R., Fischer A., Druker B.J. The cancer chemotherapy drug etoposide (VP-16) induces proinflammatory cytokine production and sickness behavior-like symptoms in a mouse model of cancer chemotherapy-related symptoms. Biol. Res. Nurs. 2006;8(2):157–169. doi: 10.1177/1099800406290932. [DOI] [PubMed] [Google Scholar]
- 35.Chovanec M., Vasilkova L., Setteyova L., Obertova J., Palacka P., Rejlekova K., Sycova‐Mila Z., Kalavska K., Svetlovska D., Cingelova S. Long‐term cognitive functioning in testicular germ‐cell tumor survivors. Oncologist. 2018;23(5):617–623. doi: 10.1634/theoncologist.2017-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.John T., Lomeli N., Bota D.A. Systemic cisplatin exposure during infancy and adolescence causes impaired cognitive function in adulthood. Behav. Brain Res. 2017;319:200–206. doi: 10.1016/j.bbr.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou W., Kavelaars A., Heijnen C.J. Metformin prevents cisplatin-induced cognitive impairment and brain damage in mice. PLoS One. 2016;11(3):e0151890. doi: 10.1371/journal.pone.0151890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lacourt T.E., Heijnen C.J. Mechanisms of neurotoxic symptoms as a result of breast cancer and its treatment: considerations on the contribution of stress, inflammation, and cellular bioenergetics. Curr. Breast Cancer Rep. 2017;9(2):70–81. doi: 10.1007/s12609-017-0245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vichaya E.G., Chiu G.S., Krukowski K., Lacourt T.E., Kavelaars A., Dantzer R., Heijnen C.J., Walker A.K. Mechanisms of chemotherapy-induced behavioral toxicities. Front. Neurosci. 2015;9:131. doi: 10.3389/fnins.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janes K., Esposito E., Doyle T., Cuzzocrea S., Tosh D.K., Jacobson K.A., Salvemini D. A3 adenosine receptor agonist prevents the development of paclitaxel-induced neuropathic pain by modulating spinal glial-restricted redox-dependent signaling pathways. Pain. 2014;155(12):2560–2567. doi: 10.1016/j.pain.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makker P.G., Duffy S.S., Lees J.G., Perera C.J., Tonkin R.S., Butovsky O., Park S.B., Goldstein D., Moalem-Taylor G. Characterisation of immune and neuroinflammatory changes associated with chemotherapy-induced peripheral neuropathy. PLoS One. 2017;12(1):e0170814. doi: 10.1371/journal.pone.0170814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melli G., Taiana M., Camozzi F., Triolo D., Podini P., Quattrini A., Taroni F., Lauria G. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Exp. Neurol. 2008;214(2):276–284. doi: 10.1016/j.expneurol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Flatters S.J., Bennett G.J. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122(3):245–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanimukai H., Kudo T. Fluvoxamine alleviates paclitaxel-induced neurotoxicity. Biochem. Biophys. Rep. 2015;4:202–206. doi: 10.1016/j.bbrep.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y., Zhang H., Kosturakis A.K., Cassidy R.M., Zhang H., Kennamer-Chapman R.M., Jawad A.B., Colomand C.M., Harrison D.S., Dougherty P.M. MAPK signaling downstream to TLR4 contributes to paclitaxel-induced peripheral neuropathy. Brain Behav. Immun. 2015;49:255–266. doi: 10.1016/j.bbi.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris G.M., Hopewell J.W., Morris A.D. A comparison of the effects of methotrexate and misonidazole on the germinal cells of the subependymal plate of the rat. Br. J. Radiol. 1995;68(808):406–412. doi: 10.1259/0007-1285-68-808-406. [DOI] [PubMed] [Google Scholar]
- 47.Yang M., Kim J-S., Kim J., Jang S., Kim S-H., Kim J-C., Shin T., Wang H., Moon C. Acute treatment with methotrexate induces hippocampal dysfunction in a mouse model of breast cancer. Brain Res. Bull. 2012;89(1-2):50–56. doi: 10.1016/j.brainresbull.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 48.English J., Aherne G.W., Arendt J., Marks V. The effect of abolition of the endogenous corticosteroid rhythm on the circadian variation in methotrexate toxicity in the rat. Cancer Chemother. Pharmacol. 1987;19(4):287–290. doi: 10.1007/BF00261474. [DOI] [PubMed] [Google Scholar]
- 49.ElBeltagy M., Mustafa S., Umka J., Lyons L., Salman A., Chur-yoe G.T., Bhalla N., Bennett G., Wigmore P.M. Fluoxetine improves the memory deficits caused by the chemotherapy agent 5-fluorouracil. Behav. Brain Res. 2010;208(1):112–117. doi: 10.1016/j.bbr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Lyons L., ElBeltagy M., Bennett G., Wigmore P. Fluoxetine counteracts the cognitive and cellular effects of 5-fluorouracil in the rat hippocampus by a mechanism of prevention rather than recovery. PLoS One. 2012;7(1):e30010. doi: 10.1371/journal.pone.0030010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han R., Yang Y.M., Dietrich J., Luebke A., Mayer-Pröschel M., Noble M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J. Biol. 2008;7(4):12. doi: 10.1186/jbiol69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mizusawa S., Kondoh Y., Murakami M., Nakamichi H., Sasaki H., Komatsu K., Takahashi A., Kudoh Y., Watanabe K., Ono Y. Effect of methotrexate on local cerebral blood flow in conscious rats. Jpn. J. Pharmacol. 1988;48(4):499–501. doi: 10.1254/jjp.48.499. [DOI] [PubMed] [Google Scholar]
- 53.Briones T.L., Woods J. Dysregulation in myelination mediated by persistent neuroinflammation: possible mechanisms in chemotherapy-related cognitive impairment. Brain Behav. Immun. 2014;35:23–32. doi: 10.1016/j.bbi.2013.07.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weymann K.B., Wood L.J., Zhu X., Marks D.L. A role for orexin in cytotoxic chemotherapy-induced fatigue. Brain Behav. Immun. 2014;37:84–94. doi: 10.1016/j.bbi.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi D-D., Huang Y-H., Lai C.S.W., Dong C.M., Ho L.C., Wu E.X., Li Q., Wang X-M., Chung S.K., Sham P.C., Zhang Z.J. Chemotherapy-induced cognitive impairment is associated with cytokine dysregulation and disruptions in neuroplasticity. Mol. Neurobiol. 2019;56(3):2234–2243. doi: 10.1007/s12035-018-1224-4. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J-M., An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kraft A.D., Harry G.J. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int. J. Environ. Res. Public Health. 2011;8(7):2980–3018. doi: 10.3390/ijerph8072980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim Y-K., Na K-S., Myint A-M., Leonard B.E. The role of pro-inflammatory cytokines in neuroinflammation, neurogenesis and the neuroendocrine system in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:277–284. doi: 10.1016/j.pnpbp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 59.Myers J.S., Pierce J., Pazdernik T. Neurotoxicology of chemotherapy in relation to cytokine release, the blood-brain barrier, and cognitive impairment. Oncol. Nurs. Forum. 2008;35(6):916–920. doi: 10.1188/08.ONF.916-920. [DOI] [PubMed] [Google Scholar]
- 60.Borsini A., Alboni S., Horowitz M.A., Tojo L.M., Cannazza G., Su K-P., Pariante C.M., Zunszain P.A. Rescue of IL-1β-induced reduction of human neurogenesis by omega-3 fatty acids and antidepressants. Brain Behav. Immun. 2017;65:230–238. doi: 10.1016/j.bbi.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barry M.A., Behnke C.A., Eastman A. Activation of programmed cell death (apoptosis) by cisplatin, other anticancer drugs, toxins and hyperthermia. Biochem. Pharmacol. 1990;40(10):2353–2362. doi: 10.1016/0006-2952(90)90733-2. [DOI] [PubMed] [Google Scholar]
- 62.Seruga B., Zhang H., Bernstein L.J., Tannock I.F. Cytokines and their relationship to the symptoms and outcome of cancer. Nat. Rev. Cancer. 2008;8(11):887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 63.Zunszain P.A., Anacker C., Cattaneo A., Choudhury S., Musaelyan K., Myint A.M., Thuret S., Price J., Pariante C.M. Interleukin-1β: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37(4):939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Caiaffo V., Oliveira B.D., de Sá F.B., Evêncio Neto J. Anti-inflammatory, antiapoptotic, and antioxidant activity of fluoxetine. Pharmacol. Res. Perspect. 2016;4(3):e00231. doi: 10.1002/prp2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loftis J.M., Huckans M., Morasco B.J. Neuroimmune mechanisms of cytokine-induced depression: current theories and novel treatment strategies. Neurobiol. Dis. 2010;37(3):519–533. doi: 10.1016/j.nbd.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J., Groff R.F., Dayawansa S. Imipramine treatment increases cell proliferation following fluid percussion brain injury in rats. Neurol. Res. 2013;35(3):247–254. doi: 10.1179/1743132813Y.0000000164. [DOI] [PubMed] [Google Scholar]
- 67.Chen Z., Palmer T.D. Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain Behav. Immun. 2013;30:45–53. doi: 10.1016/j.bbi.2013.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee S-Y., Lee S-J., Han C., Patkar A.A., Masand P.S., Pae C-U. Oxidative/nitrosative stress and antidepressants: targets for novel antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;46:224–235. doi: 10.1016/j.pnpbp.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 69.Ma J., Kavelaars A., Dougherty P.M., Heijnen C.J. Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source. Cancer. 2018;124(11):2289–2298. doi: 10.1002/cncr.31248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dietrich J., Han R., Yang Y., Mayer-Pröschel M., Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J. Biol. 2006;5(7):22. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Circu M.L., Aw T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010;48(6):749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akpinar A., Uğuz A.C., Nazıroğlu M. Agomelatine and duloxetine synergistically modulates apoptotic pathway by inhibiting oxidative stress triggered intracellular calcium entry in neuronal PC12 cells: role of TRPM2 and voltage-gated calcium channels. J. Membr. Biol. 2014;247(5):451–459. doi: 10.1007/s00232-014-9652-1. [DOI] [PubMed] [Google Scholar]
- 73.Walker F.R. A critical review of the mechanism of action for the selective serotonin reuptake inhibitors: do these drugs possess anti-inflammatory properties and how relevant is this in the treatment of depression? Neuropharmacology. 2013;67:304–317. doi: 10.1016/j.neuropharm.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Greenberg M.E., Xu B., Lu B., Hempstead B.L. New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci. 2009;29(41):12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei Z., Liao J., Qi F., Meng Z., Pan S. Evidence for the contribution of BDNF-TrkB signal strength in neurogenesis: An organotypic study. Neurosci. Lett. 2015;606:48–52. doi: 10.1016/j.neulet.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 76.Seigers R., Schagen S.B., Beerling W., Boogerd W., van Tellingen O., van Dam F.S., Koolhaas J.M., Buwalda B. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav. Brain Res. 2008;186(2):168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Monje M., Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav. Brain Res. 2012;227(2):376–379. doi: 10.1016/j.bbr.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Salerno E.A., Rowland K., Kramer A.F., McAuley E. Acute aerobic exercise effects on cognitive function in breast cancer survivors: a randomized crossover trial. BMC Cancer. 2019;19(1):371. doi: 10.1186/s12885-019-5589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winocur G., Wojtowicz J.M., Huang J., Tannock I.F. Physical exercise prevents suppression of hippocampal neurogenesis and reduces cognitive impairment in chemotherapy-treated rats. Psychopharmacology (Berl.) 2014;231(11):2311–2320. doi: 10.1007/s00213-013-3394-0. [DOI] [PubMed] [Google Scholar]
- 80.Munzer A., Sack U., Mergl R., Schönherr J., Petersein C., Bartsch S., Kirkby K.C., Bauer K., Himmerich H. Impact of antidepressants on cytokine production of depressed patients in vitro. Toxins (Basel) 2013;5(11):2227–2240. doi: 10.3390/toxins5112227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Diamond M., Kelly J.P., Connor T.J. Antidepressants suppress production of the Th1 cytokine interferon-γ, independent of monoamine transporter blockade. Eur. Neuropsychopharmacol. 2006;16(7):481–490. doi: 10.1016/j.euroneuro.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 82.Hashioka S., Klegeris A., Monji A., Kato T., Sawada M., McGeer P.L., Kanba S. Antidepressants inhibit interferon-γ-induced microglial production of IL-6 and nitric oxide. Exp. Neurol. 2007;206(1):33–42. doi: 10.1016/j.expneurol.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 83.Dello Russo C., Boullerne A.I., Gavrilyuk V., Feinstein D.L. Inhibition of microglial inflammatory responses by norepinephrine: effects on nitric oxide and interleukin-1β production. J. Neuroinflammation. 2004;1(1):9. doi: 10.1186/1742-2094-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu D., Wang Z., Liu S., Wang F., Zhao S., Hao A. Anti-inflammatory effects of fluoxetine in lipopolysaccharide(LPS)-stimulated microglial cells. Neuropharmacology. 2011;61(4):592–599. doi: 10.1016/j.neuropharm.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 85.Karthik L., Kumar G., Keswani T., Bhattacharyya A., Chandar S.S., Bhaskara Rao K.V. Protease inhibitors from marine actinobacteria as a potential source for antimalarial compound. PLoS One. 2014;9(3):e90972. doi: 10.1371/journal.pone.0090972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sachs B.D., Caron M.G. Chronic fluoxetine increases extra-hippocampal neurogenesis in adult mice. Int. J. Neuropsychopharmacol. 2014;18(4):pyu029. doi: 10.1093/ijnp/pyu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pachman D.R., Linquist B.M., Barton D.L., Fee-Schroeder K.C., Smith T.J., Lachance D.H., Liu H., Seisler D.K., Loprinzi C.L. Pilot study of Scrambler therapy for the treatment of chemotherapy-induced peripheral neuropathy. American Society of Clinical Oncology; 2012. [Google Scholar]
- 88.Ohira K., Miyakawa T. Chronic treatment with fluoxetine for more than 6 weeks decreases neurogenesis in the subventricular zone of adult mice. Mol. Brain. 2011;4(1):10. doi: 10.1186/1756-6606-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohgi Y., Futamura T., Kikuchi T., Hashimoto K. Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2013;103(4):853–859. doi: 10.1016/j.pbb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Guilloux J-P., Mendez-David I., Pehrson A., Guiard B.P., Repérant C., Orvoën S., Gardier A.M., Hen R., Ebert B., Miller S., Sanchez C., David D.J. Antidepressant and anxiolytic potential of the multimodal antidepressant vortioxetine (Lu AA21004) assessed by behavioural and neurogenesis outcomes in mice. Neuropharmacology. 2013;73:147–159. doi: 10.1016/j.neuropharm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 91.Behr G.A., Moreira J.C., Frey B.N. Preclinical and clinical evidence of antioxidant effects of antidepressant agents: implications for the pathophysiology of major depressive disorder. Oxid. Med. Cell. Longev. 2012;2012:609421. doi: 10.1155/2012/609421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Konieczyńska M.J. Quality of life in schizophrenia: Impact of psychopathology, patients’ gender and antipsychotic treatment. Int. J. Psychiatry Clin. Pract. 2001;5(1):19–26. doi: 10.1080/136515001300224854. [DOI] [PubMed] [Google Scholar]
- 93.Smith E.M.L., Bridges C.M., Kanzawa G., Knoerl R., Kelly J.P., IV, Berezovsky A., Woo C. Cancer treatment-related neuropathic pain syndromes--epidemiology and treatment: an update. Curr. Pain Headache Rep. 2014;18(11):459. doi: 10.1007/s11916-014-0459-7. [DOI] [PubMed] [Google Scholar]
- 94.Pechnick R.N., Zonis S., Wawrowsky K., Cosgayon R., Farrokhi C., Lacayo L., Chesnokova V. Antidepressants stimulate hippocampal neurogenesis by inhibiting p21 expression in the subgranular zone of the hipppocampus. PLoS One. 2011;6(11):e27290. doi: 10.1371/journal.pone.0027290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gulec M., Oral E., Dursun O.B., Yucel A., Hacimuftuoglu A., Akcay F., Suleyman H. Mirtazapine protects against cisplatin-induced oxidative stress and DNA damage in the rat brain. Psychiatry Clin. Neurosci. 2013;67(1):50–58. doi: 10.1111/j.1440-1819.2012.02395.x. [DOI] [PubMed] [Google Scholar]