Highlights
-
•
Neuropsychiatric symptoms (NPS) in dementia includes aberrant behavior and mood changes.
-
•
The buffering role of cognitive reserve has been unclear in NPS.
-
•
Open Access Series of Imaging Studies (OASIS-3) dataset was utilized.
-
•
Canonical correlation analysis identified multivariate patterns of NPS correlate.
-
•
Older adults with more educational attainment showed minimal NPS.
Keywords: Neuropsychiatric symptoms, Dementia, Gray matter volume, Education, Cognitive reserve
Abstract
Neuropsychiatric symptoms are commonly observed as brain pathology progresses with dementia. Behavioral and affective disturbances underly the distinct neuroanatomical basis of typical symptoms of cognitive impairment; however it remains unclear whether enriched intellectual experience, such as educational attainment, can mitigate the effect of brain structural patterns on neuropsychiatric symptom severity. We utilized the Open Access Series of Imaging Studies (OASIS-3) dataset, which includes brain structural MRI and behavioral symptom evaluation. We included 904 older adults who were mostly cognitively normal, clinically diagnosed with very mild to moderate Alzheimer’s disease, or other types of dementia. Canonical correlation analysis was used to identify the patterns of multivariate association between the gray matter structure and neuropsychiatric symptom severity. First, we identified two canonical modes capturing the distinct neuroanatomical basis of common and mood-specific factors of neuropsychiatric symptoms. The first common pattern reflected a smaller volume in the amygdala and adjacent temporal regional thickness. The second mood-specific pattern reflected patterns in lateral and orbital prefrontal regional thickness. In the external correlational analysis, the two canonical correlations reflected global brain volume and white matter lesions; however, the second pattern was not associated with functional impairments or cognitive function. Moreover, older adults with higher education showed an attenuated severity of behavioral symptoms, even with the presence of a brain structural pattern. Our findings suggest that educational attainment, as a proxy of cognitive reserve, can mitigate the severity of behavioral and affective symptoms of dementia.
1. Introduction
The clinical hallmarks of late-life neurodegenerative diseases include significant changes in memory, executive, and other functions across cognitive domains. Neuropsychiatric symptoms (NPS), on the other hand, are also commonly observed in individuals with mild cognitive impairment (MCI) or dementia (Lyketsos et al., 2002). The behavioral and psychological symptoms of dementia typically include a wide range of aberrant behavior, mood, affect, or thoughts (Finkel, 2000). Although NPS is not a core diagnostic feature of dementia, its presence and severity typically leads to profound changes in patients’ functional abilities and caregiver burdens (Wadsworth et al., 2012).
Initially, NPS was viewed as a non-specific consequence of global cognitive impairment; however, accumulating studies have suggested that a wide variability of behavioral abnormalities characterize the distinct clinical trajectories of dementia and neuropathological correlates (Bruen et al., 2008, Teng et al., 2007). Researches have targeted the amygdala and corticolimbic network as a neural basis of NPS, which are the core structures in maintaining socioemotional functioning, to account for the variability of NPS beyond typical cognitive impairment (Bickart et al., 2014a, Bickart et al., 2014b). Previous results have shown an unclear association between the amygdala structure and NPS in Alzheimer’s disease (AD) (Horinek et al., 2006, Hu et al., 2015, Poulin et al., 2011). Whole-brain voxel-wise exploration via repetitive univariate testing with a highly lenient threshold can result in inconsistent brain correlates that are less likely to be replicated in a new dataset (Habeck et al., 2008, Masouleh et al., 2019). It is less likely that individual differences in these symptoms are based on a focal regional morphology; therefore, a more neuroscientifically valid determination of neuroimaging markers is required (Woo et al., 2017). It is an important task to examine the neural basis of NPS with a more reliable multivariate pattern of neurodegeneration.
Currently, it is unknown whether there is a resilience factor that makes an individual tolerant to the NPS onset in the presence of a neuropathological burden. The cognitive reserve hypothesis states that enriched neural resources gained from lifespan psychosocial and intellectual experiences can buffer the deleterious effect of neuropathology on the clinical manifestation (Stern et al., 2018). Numerous studies have confirmed that educational attainment, as a proxy of cognitive reserve, attenuates or delays the progression of the cognitive impairment; however, few studies have examined the role of cognitive reserve on the affective and behavioral symptoms of dementia (Premi et al., 2013, Spreng et al., 2011). It is possible that the neuroanatomical basis of behavioral symptoms in AD differs from that of typical impairment in memory, executive, and language domain. Thus, the role of education on the NPS remains largely unexplored.
In this study, we examined the neuroanatomical correlates of NPS in an open-access dataset that includes older adults who were cognitively normal or diagnosed with dementia. We aimed to identify a multivariate association between the patterns of NPS items and regional gray matter thickness and volume. We utilized canonical correlation analysis to characterize distinctive modes of correlation between multiple measures of clinical and brain features (Drysdale et al., 2017, Moser et al., 2018, Wang et al., 2018, Xia et al., 2018). This multivariate approach may reliably capture the distributed nature of neuroanatomical correlates that have been detected in the previous studies. Moreover, we examined whether educational attainment, as a proxy of cognitive reserve, moderates the effect of brain structural patterns that are associated with NPS severity. We hypothesized that older adults with higher education will show fewer clinical manifestations in NPS even with increased brain structural risk patterns.
2. Methods
2.1. Participants
The dataset, Open Access Series of Imaging Studies (OASIS-3), was used in this study. OASIS-3 includes participants enrolled into several ongoing studies through the Charles F. and Joanne Knight Alzheimer Disease Research Center (Knight ADRC) at Washington University in St. Louis spanning over 15 years and several research studies, including Memory and Aging Project, Adult Children Study, and Healthy Aging and Senile Dementia (LaMontagne et al., 2019). OASIS-3 includes the clinical, neuropsychological, neuroimaging, and biomarker data of 1098 participants (age: 42–95 years; www.oasis-brain.org). We analyzed the initial visit session data of 904 participants who completed informant-rated behavioral symptoms and MRI scans. Participants with incomplete T1 structural scans (n = 111), informant ratings (n = 82), and cognitive tests (n = 25) at baseline were excluded from the analysis.
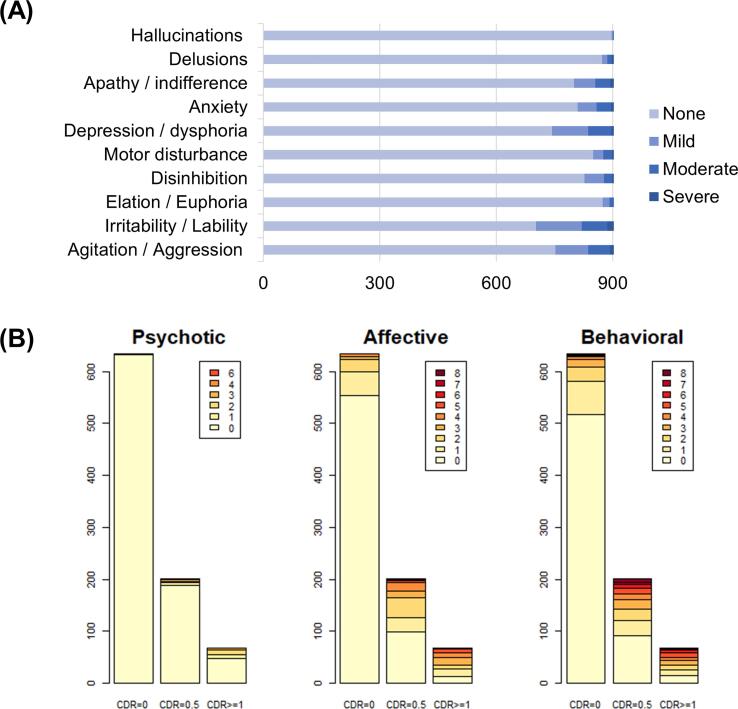
The standardized collection of OASIS-3 was designed at the Alzheimer’s Disease Centers (ADC) program of the National Institute on Aging (NIA) as a component of the Uniform Data Set (UDS) (Beekly et al., 2007, Morris et al., 2006). Clinical characteristics of dementia severity were provided based on the Clinical Dementia Rating (CDR). The CDR is a semi-structured interview developed to provide a global dementia severity rating, which is useful for staging and tracking decline in AD (Fillenbaum et al., 1996, Morris, 1997). Each CDR score represents levels of functional impairment (0 = no impairment; 0.5 = questionable or very mild impairment; 1 = mild impairment; 2 = moderate impairment) and summarizes the estimate of dementia severity (Marcus et al., 2007, Morris, 1993). Participants meeting the criteria for dementia mainly included AD followed by questionable, uncertain, or other non-AD disorders (Table 1). Our analyses were conducted across all participants available ranging from cognitively normal to moderate dementia (n = 904). In addition, same analysis was used when the target population was confined to participants with less severe symptoms (CDR ≤ 0.5, n = 836).
Table 1.
Descriptive characteristics of the participants.
| Mean ± SD / Frequency | |
|---|---|
| Age | 68.56 ± 9.45 |
| Education | 15.60 ± 2.77 |
| 7–12 | 182 (20.1%) |
| 13–15 | 175 (19.3%) |
| 16–17 | 259 (28.6%) |
| 18–29 | 288 (31.8) |
| Gender | |
| Male (0) | 409 (45.2%) |
| Female (1) | 495 (54.8%) |
| MMSE | 28.07 ± 2.75 |
| CDR | |
| 0 (No impairment) | 635 (70.2%) |
| 0.5 (Very mild) | 201 (22.2%) |
| 1 (Mild) | 65 (7.2%) |
| 2 (Moderate) | 3 (0.3%) |
| Diagnosis | |
| Cognitively normal | 636 (70.4%) |
| AD dementia | 174 (19.2%) |
| AD dementia with other comorbidities | 43 (4.8%) |
| Non-AD / Uncertain | 69 (7.6%) |
| Questionable impairment | 25 (2.8%) |
2.2. Neuropsychiatric Inventory
Behavioral and psychological symptoms were evaluated using the Neuropsychiatric Inventory, short-form (NPI-Q) (Cummings et al., 1994, Kaufer et al., 2000). The NPI is a structured interview administered to the patients’ caregivers. It consists of 12 separate items assessing neuropsychiatric disturbances, including delusion, hallucination, agitation/aggression, depression/dysphoria, anxiety, elation/euphoria, apathy/indifference, disinhibition, irritability/lability, and aberrant motor behavior (Table 2). In this study, eating abnormalities and nighttime behavior symptoms were excluded from the analyses because they are not part of the core NPI questionnaire (Cajanus et al., 2019). The NPI items coded symptoms as four levels: absent (0, no symptoms), mild (1, noticeable, but not a significant change), moderate (2, significant, but not a dramatic change), and severe (3, very marked or prominent; a dramatic change). The participants showed a relatively high prevalence in irritability (22%), agitation (17%), depression (17%), which were followed by apathy (12%), anxiety (11%), disinhibition (9%), motor (6%), elation (3%), delusion (3%), and hallucination (1%).
Table 2.
Neuropsychiatric Inventory (NPI-Q) questionnaires.
| Agitation / Aggression | Is the patient stubborn and resistive to help from others? |
| Irritability / Lability | Is the patient impatient or cranky? Does he or she have difficulty coping with delays or waiting for planned activities? |
| Elation / Euphoria | Does the patient appear to feel too good or act excessively happy? |
| Disinhibition | Does the patient seem to act impulsively? For example, does the patient talk to strangers as if he or she knows them, or does the patient say things that may hurt people’s feelings? |
| Motor disturbance | Does the patient engage in repetitive activities, such as pacing around the house, handling buttons, wrapping string, or doing other things repeatedly? |
| Depression / dysphoria | Does the patient act as if he or she is sad or in low spirits? Does he or she cry? |
| Anxiety | Does the patient become upset when separated from you? Does he or she have any other signs of nervousness, such as shortness of breath, sighing, being unable to relax, or feeling excessively tense? |
| Apathy / indifference | Does the patient seem less interested in his or her usual activities and in the activities and plans of others? |
| Delusions | Does the patient believe that others are stealing from him or her, or planning to harm him or her in some way? |
| Hallucinations | Does the patient act as if he or she hears voices? Does he or she talk to people who are not there? |
The interview informants were mostly spouses (n = 499) and children (n = 199), followed by other relationships (n = 221: sibling, neighbor, relative, and paid caregiver). The reliability of the informant was binary-coded (questionable = 1, not questionable = 0) and included as a covariate in the subsequent multiple regression model.
2.3. Neuroimaging acquisition and preprocessing
The neuroimaging data in OASIS-3 were collected in a 16-channel head coil from different scanners (Siemens TIM Trio 3T, Siemens BioGraph mMR PET-MR 3T, Siemens BioGraph mMR PET-MR 3T, Siemens Sonata 1.5T, and Siemens Vision 1.5T). High-resolution T1-weighted structural image were used for this analysis this study (TR = 2.4 s, TE = 3.08 ms, FOV = 256 × 256 mm, FA = 8°, voxel size 1 × 1 × 1 mm3). We used a fully automated preprocessing procedure implemented in CAT12 r1450 (Computational Anatomy Toolbox, Structural Brain Mapping Group, Departments of Psychiatry and Neurology, Jena University Hospital, http://dbm.neuro.uni-jena.de/cat/) to apply a standardized analysis pipeline. First, a spatial-adaptive non-local means (SANLM) denoising filter (Manjón et al., 2010) was employed. Segmentation algorithms based on the adaptive maximum a posterior (AMAP) technique implemented in CAT12, were used to classify brain tissue into gray matter (GM), white matter (WM), cerebrospinal fluid (CSF), and white matter hypointensities (WMH). Additionally, partial volume estimation was used to create a more accurate segmentation for the two mixed classes: GM–WM and GM–CSF. Projection-based estimation of cortical thickness was conducted in the segmented images (Dahnke et al., 2013, Dahnke et al., 2012), which showed a comparable accuracy with other surface-based tools (Righart et al., 2017). In total, 78 values were extracted from the CAT12 region of interest (ROI) analysis pipeline, including 68 cortical thickness and mean GM density values in ten bilateral subcortical structures (accumbens, caudate, putamen, amygdala, and hippocampus). Cortical areas were defined based on the automatic parcellation of gyri (Desikan et al., 2006). Subcortical volumes were defined using the Neuromorphometric atlas (http://Neuromorphometrics.com). The overall summary measure for the whole brain was calculated with an inverse measure of normalized whole brain volume (nWBV = 1 − (GM + WM)/Total Intracranial Volume). White matter lesions were calculated with the total amount of WMH volume and log-transformed. The 78 cortical thickness and volumetric measures were introduced into main analysis of dimension reduction and CCA. On the other hand, normalized WBV and white matter lesion volume were used in the external correlation analysis.
2.4. Dimension reduction
Principal component analysis (PCA) was conducted to reduce the high dimensionality of the brain morphometry and strong comorbidity of NPI measures. While NPI is typically composed of diverse construct of symptoms, a strong correlation between NPI-sum and NPI-item severity was observed, similar to that of the previous study (mean r = 0.57) (Kaufer et al., 2000). The valid NPI domains unit remains largely unknown in the current population; therefore, this data-driven approach was utilized to determine the optimal unit of symptom patterns.
Before conducting PCA, the original measures were scaled to have unit variance. In addition, a parallel analysis was used to determine the optimal number of components to be summarized in the further analysis. This analysis compares the scree of eigenvalues of the observed data with that of a random data matrix of the same size as the original. The random data matrix was generated with 50 iteration. Components with higher eigenvalues than the randomly generated data were considered as a meaningful unit of the principal components. This parallel analysis was conducted using the psych package (Revelle, 2018). The parallel analysis on the brain structural measures (904*78; cortical thickness and GM volume), identified 7 principal components as optimal which explained 65% of the total brain morphometry measures. The parallel analysis on the NPI measures (904*10; item scores) identified 2 principal components as optimal which explained 47% of the total items. These dimension reduction procedures prevented the subsequent analysis from capturing minute patterns of association. In sum, brain structure and NPI measures are reduced into 7 and 2 principal component scores, respectively, and used in the subsequent CCA.
2.5. Canonical correlation analysis
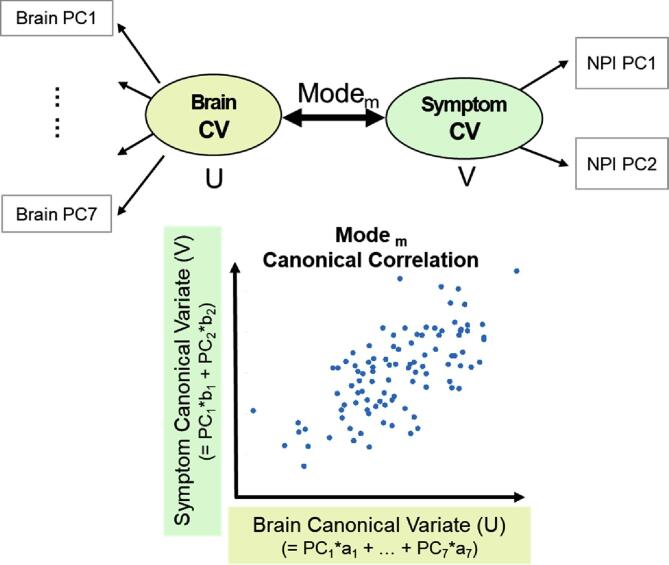
Canonical correlation analysis was conducted using the CCA package in R (González et al., 2012). The CCA finds several modes of the linear combination that produce the highest correlation between two canonical variates (Fig. 1; U and V). Each side of the variate is composed of vector weights, which indicate the relative contribution of the input variables. The canonical variate (CV) is calculated using the weighted sum of the principal component score of either brain measures or NPI scores. The number of canonical modes is limited to the minimum dimensions of the input variables; therefore, two canonical modes were produced.
Fig. 1.
Schematic figure of Canonical Correlation Analysis (CCA). Mth pairs of mode represent a multivariate association between canonical variates of brain canonical variate (U, top left) and NPI canonical variate (V, top right). Bottom: Each canonical variate represents a linear combination of the brain and NPI’s principal component (PC) scores, and identifies two highest canonical modes.
CCA identifies the linear combination that produces the highest canonical correlation; therefore, the permutation test confirmed whether the canonical correlations between two sets of variables are statistically meaningful relative to the null distribution. The subject measures of NPI were randomly shuffled 10,000 times and produced the null distribution. The p-value was calculated by p-value = (cases that were higher canonical correlation)/10,000.
We conducted 5-fold cross-validation to assess the generalizability and rate of overfitting in CCA. The canonical variates of each fold were predicted based on the CCA coefficients estimated within the independently separated folds. Next, we calculated the correlation between the two predicted canonical variates. This procedure was iterated 1000 times to assess the stability of the group partitioning. The mean and standard deviation of the iterated results are reported.
2.6. Statistical analysis
After identifying statistically meaningful canonical modes, the composition and characteristics of each canonical variate were described using Pearson’s correlation between the variate score and the original NPI or the brain measures before being reduced into principal components. Furthermore, correlations between the brain canonical variate (U) and other external measures were examined in age, sex, total intracranial volume (TIV), whole brain volume (1 − GM + WM/TIV), white matter lesion, Mini-Mental Status Examination (MMSE), and CDR.
Multiple regression analysis was conducted to examine the moderating effect of education on the relationship between brain structural pattern (U) and NPI symptom severity (V) by adding the interaction terms (Brain CV1 × Education and Brain CV2 × Education). A separate regression analysis was conducted on the distinct canonical modes that included regressors of no interest (demographics, informant reliability, TIV, CDR, and MMSE).
The freesurfer_statsurf_display function was used to visualize the canonical weight on cortical surface areas (Murdoch Childrens Research Institute Developmental, Imaging Group, 2017, https://chrisadamsonmcri.github.io/freesurfer_statsurf_display).
3. Results
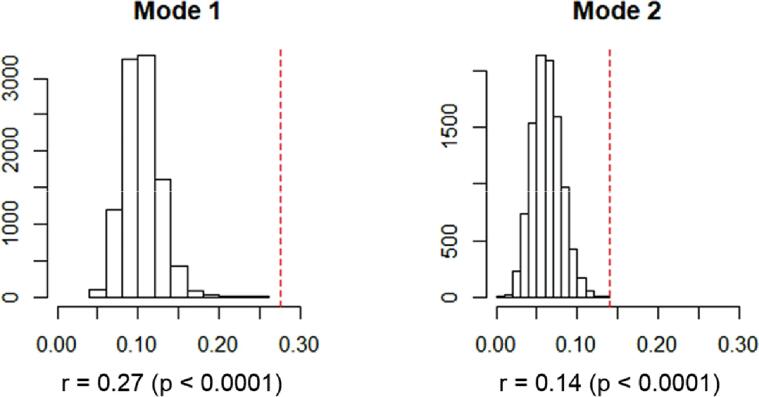
After reducing NPI and brain measures into principal component scores, the canonical correlation analysis was conducted and produced two canonical correlation modes (first mode: r = 0.275, second mode: r = 0.140). The permutation test confirmed that the true canonical correlation values were not included in the randomly generated null distributions (first mode: p < 10−5, second mode: p = 9 × 10−5; Fig. 2). To test the generalizability of the canonical correlation, 5-fold cross-validation was iterated 1000 times. We identified a canonical correlation when the coefficients were estimated based on the separately trained sample (80% of the total sample). This identified a stable canonical correlation, although the strength of association generally decreased (first mode: mean r = 0.241, SD = 0.011; second mode: mean r = 0.097, SD = 0.017). The partial correlation between two canonical scores, after adjusting for the effect of demographic characteristics, clinical severity, and cognitive function, showed a reduction especially in the first canonical mode (first mode: r = 0.11, second mode: r = 0.12).
Fig. 2.
The permutation test result of CCA. The null distribution of canonical correlation with randomly shuffled data (histogram) and the true canonical correlation (red dashed line). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
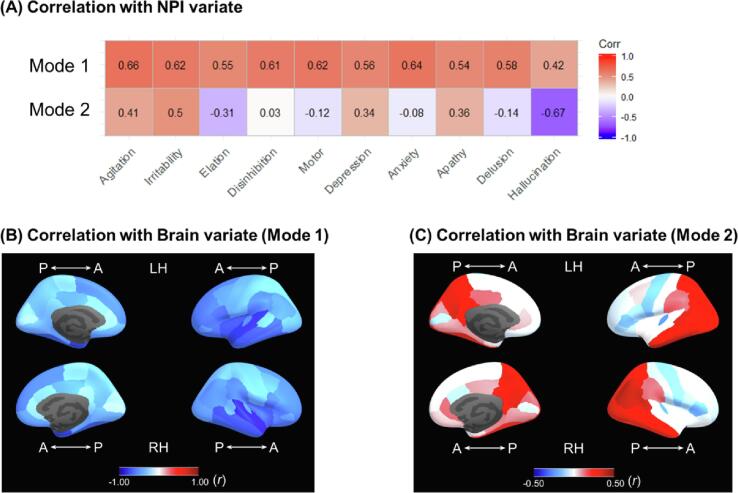
Parallel analysis on NPI identified two principal components as optimal. NPI CV showed that the first canonical mode were associated with the first PC that captured overall effect of 10 items of NPI, whereas the second mode were associated with the second PC that captured the behavior- and mood-specific items (agitation, irritability, depression, and apathy; Supplementary Table 2,3). The CV score weights were described with their correlation to original measures before being transformed into the principal component axis (Fig. 4A).
Fig. 4.
(A) Correlation between the two NPI canonical variate score (mode 1, mode 2) and each item-level symptom severity. The first mode of canonical variate was comprised of overall presence of symptoms, whereas the second mode of canonical variate was comprised of the presence of mood dysregulation symptoms. (B, C) Correlation between the two brain canonical variate score and each regional cortical thickness.
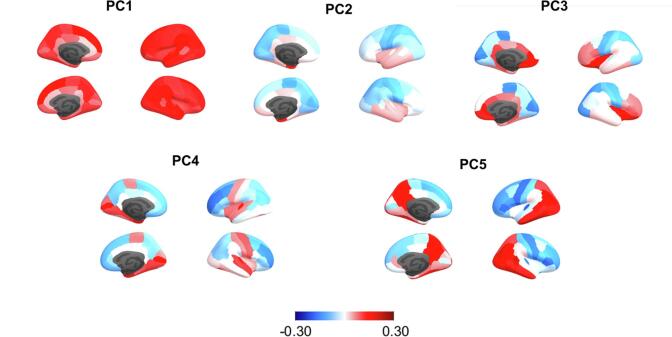
Parallel analysis on brain measures identified seven principal components of structural pattern as optimal. The PCA identified distinct constitutions of brain structural patterns in the first mode of CV, which included global (PC1), subcortical (PC2), orbital and medial prefrontal (PC3), and medial temporal (PC4) (Table 3, Fig. 3). The lateral prefrontal (PC5) and other additional components (PC4, PC7) were associated with second CV mode of CV. Overall, the brain measures (cortical thickness and subcortical volume) showed that the first canonical mode was primarily comprised of the amygdala and lateral temporal lobe, whereas the second mode was comprised of the thinner lateral, medial, and orbital prefrontal cortex and thicker temporo-parietal cortex (Fig. 4B, C, Supplementary Table 4). For the post-hoc interpretation, we also depicted the characteristic of the brain CV with the correlation between the CV score and the other external measures (Table 4). The first and second CV were overall correlated with higher age, whole brain volume, and white matter lesion volume; however, only the first canonical mode was strongly correlated with clinical impairment (CDR) and cognitive function (MMSE) (ps < 0.001).
Table 3.
Canonical weights of brain canonical variate (CV). Pairwise correlation coefficients (r) between the composite score of brain CV and principal component (PC) scores of brain measures.
| Brain regions | Correlation Between Brain Canonical Variate (CV) and PC score |
||
|---|---|---|---|
| CV1 | CV2 | ||
| PC1 | (+) superior frontal, middle frontal, supramarginal, superior temporal, inferior parietal, middle temporal | −0.790 | 0.114 |
| PC2 | (+) accumbens, amygdala, hippocampus, putamen, caudate, entorhinal | −0.417 | −0.004 |
| PC3 | (+) anterior cingulate cortex, insula, orbitofrontal (−) caudate, putamen, pericalcarine |
−0.301 | −0.067 |
| PC4 | (+) entorhinal, temporal pole, pericalcarine, hippocampus, amygdala (−) caudate, middle frontal, putamen, |
−0.279 | −0.175 |
| PC5 | (+) inferior temporal, inferior parietal (−) precentral, pars triangularis, middle frontal |
0.029 | 0.966 |
| PC6 | (+) posterior cingulate, anterior cingulate, orbitofrontal | 0.152 | 0.023 |
| PC7 | (+) posterior cingulate, hippocampus (−) lingual, pericalcarine, orbitofrontal |
−0.093 | 0.132 |
Fig. 3.
Principal component loadings (rotation matrix) of cortical thickness.
Table 4.
External correlates of brain canonical variate (CV). Pairwise correlation coefficients (r) between the composite score of brain CV and the other subject measures (demographic, clinical, and overall volumetrics) are listed.
| Mode 1 Brain CV | Mode 2 Brain CV | |
|---|---|---|
| Age | 0.36* | 0.22* |
| Sex | 0.01 | −0.14* |
| Education | −0.18* | −0.02 |
| Total Intracranial Volume | −0.02 | 0.28* |
| Informant Reliability | 0.03 | −0.01 |
| Clinical Dementia Rating (CDR) | 0.38* | 0.05 |
| MMSE | −0.36* | −0.02 |
| White matter lesion | 0.47* | 0.28* |
| Normalized Whole brain volume | 0.62* | 0.32* |
* p < 0.0001
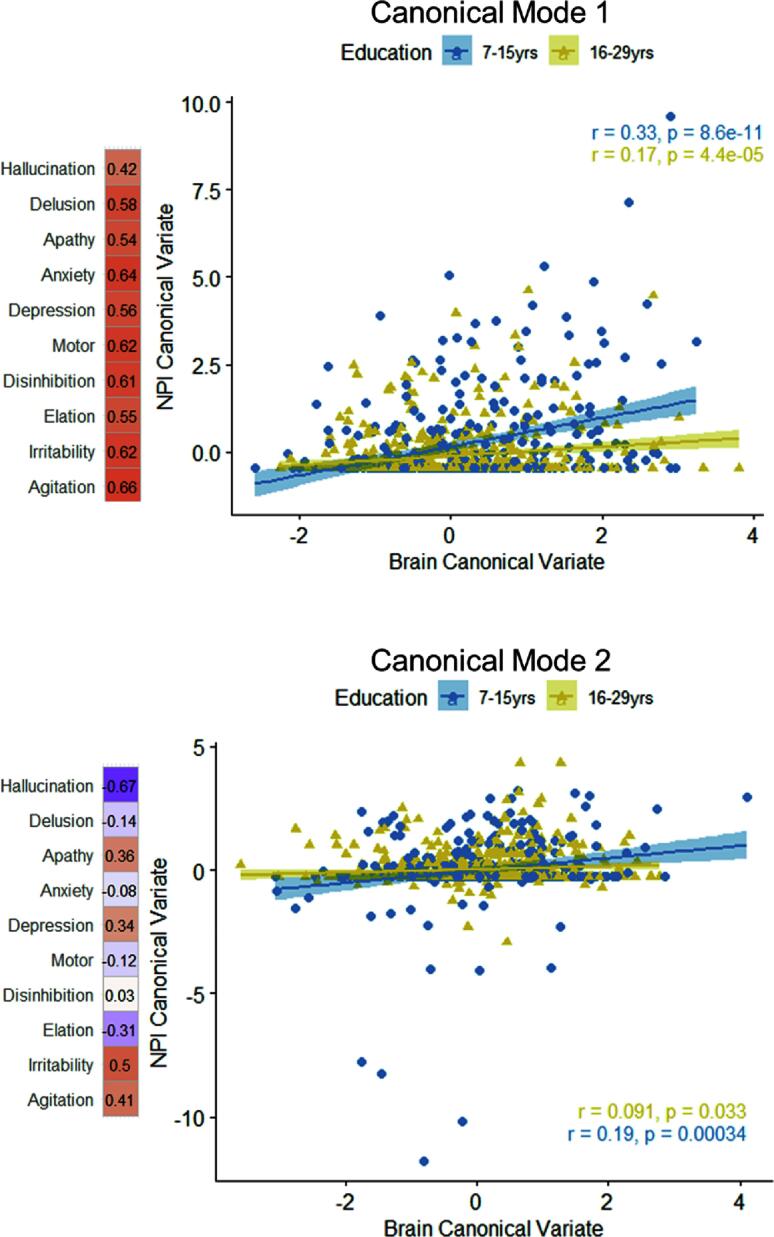
Next, we examined the moderation effect of education on the relationship between brain and NPI CV. We found that years of education moderated the effect of both first and second brain CV on NPI CV (Table 5). Older adults with higher education showed an attenuated association between brain CV and NPI CV (Fig. 5). This interaction effect remained unchanged when an alternative interaction effect (MMSE × Education) of the cognitive function was included (ps < 0.05). However, the moderating effect showed a decreasing pattern when the interaction term of general impairment (CDR × Education) was added in the model especially in the first brain variate (CV1 × Education: b = −0.012, p = 0.237; CV2 × Education: b = −0.036, p = 0.002).
Table 5.
Multiple regression model that predicts NPI canonical variate (CV). The interaction term between Brain CV and education is mainly tested while including the covariates of no interest.
| Mode 1 (DV: NPI CV1) |
Mode 2 (DV: NPI CV2) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | t | p-value | Beta | SE | T | p-value | ||
| Age | −0.01 | 0.00 | −2.10 | 0.036 | Age | 0.00 | 0.00 | −0.05 | 0.964 |
| Sex | −0.11 | 0.06 | −1.68 | 0.093 | Sex | −0.06 | 0.08 | −0.79 | 0.428 |
| Informant Reliability | 0.18 | 0.12 | 1.50 | 0.133 | Informant Reliability | −0.07 | 0.14 | −0.49 | 0.626 |
| Total Intracranial Volume | 0.00 | 0.00 | 1.09 | 0.274 | Total Intracranial Volume | 0.00 | 0.00 | 0.79 | 0.427 |
| CDR | 1.75 | 0.12 | 14.40 | <2 × 10−16 | CDR | 0.17 | 0.14 | 1.19 | 0.234 |
| MMSE | 0.05 | 0.01 | 3.18 | 0.002 | MMSE | −0.03 | 0.02 | −1.68 | 0.094 |
| Brain CV1 | 0.40 | 0.15 | 2.77 | 0.006 | Brain CV2 | 0.64 | 0.18 | 3.55 | <0.001 |
| Education | −0.03 | 0.01 | −3.27 | 0.001 | Education | 0.01 | 0.01 | 0.85 | 0.397 |
| Brain CV1 × Education | −0.02 | 0.01 | −2.06 | 0.039 | Brain CV2 × Education | −0.03 | 0.01 | −2.90 | 0.004 |
Results with p-values < 0.05 are shown in bold.
Fig. 5.
Visualization of the interaction effect between brain canonical variate and years of education. The strength of the canonical correlation was attenuated in the higher-educated older adults (yellow dots). The symptom weights of NPI items (correlation coefficient with the original measures) are depicted beside the axis.
To examine the outweighed effect of participants with severe symptoms, the same analysis was conducted within the clinically mild population (CDR ≤ 0.5, n = 836). These results showed a decreasing trend in the moderation effect (CV1 × Education: b = − 0.013, SE = 0.011, p = 0.220; CV2 × Education: b = − 0.030, SE = 0.013, p = 0.018).
4. Discussion
In the current study, we identified the neuroanatomical correlates of the behavioral and psychological symptoms of dementia and the protective role of education in the relationship between brain structural patterns and symptom severity. We identified two distinct canonical modes that map the multivariate relationship between the NPS and brain morphological patterns. The first canonical correlation captured a brain structural pattern of the medial and lateral temporal structural volumes that reflected the overall symptoms. The second pattern captured the regional components of the lateral and medial prefrontal cortex that correlated with behavior- and mood-specific symptoms. Furthermore, individuals’ educational attainment attenuated the effect of brain structural patterns on NPS severity in both models. Older adults with higher education showed minimal NPS even with a high risk of brain structural pattern.
Previous studies have examined the unique and distinct neural correlates of NPS that may explain its heterogeneous clinical manifestations. We confirmed previous findings that the amygdala is a prominent structure that accounts for the overall neuropsychiatric symptoms in AD. Amygdala, as a hub within the social brain, anchors the functional networks of multiple cortical areas, and the morphological covariance pattern of the amygdala may play a critical role in socioemotional functioning (Bickart et al., 2014b, Wei et al., 2018). In this study, the canonical variate included the entorhinal, fusiform, temporal pole, lateral orbitofrontal, and middle and superior temporal cortex thickness, which coincide with the functional network pattern of dorsal and ventrolateral amygdala (Bickart et al., 2012). Greater atrophy in these regions may lead to a selective lack of awareness or understanding of others’ social and emotional behavior, indicating its unique relevance in the onset of NPS (Bickart et al., 2014a).
It is notable that the canonical variate of NPS largely shared its variance with the general pattern of AD pathological progression. The first canonical variate was highly correlated with the levels of dementia severity (CDR) and cognitive function (MMSE). When adjusting for this shared effect, there was a large decrease in the canonical correlation with NPS. The entorhinal cortex and hippocampal structures typically undergo prominent atrophy in patients with AD; therefore, the current analysis may have captured the major source of global AD pathology. It should be noted that previous studies did not affirm the role of the amygdala structure in NPS, possibly due to insufficient sample size or exclusion from the analysis target; however, our study illustrated that the amygdala-centered multivariate pattern was a significant correlate even after adjusting global dementia severity (Cajanus et al., 2019, Horinek et al., 2006, Poulin et al., 2011).
The second canonical correlation mode showed that lateral, medial, and orbital prefrontal regional atrophy was associated with the mood-specific components of NPS. Interestingly, the second canonical variate did not correlate with global dementia impairment or cognitive function, in contrast to its association with white matter lesions and whole brain volume. Prefrontal atrophy and white matter changes may have increased the risk of mood-related NPS. This result is consistent with previous studies showing that patients with dysexecutive and behavioral variant AD show additional changes in the prefrontal volumes and white matter pathology relative to temporo-parietal changes (Ossenkoppele et al., 2015, Park et al., 2011). Due to the earlier manifestation of frontal variant neuropathology, it may be less reflective of the age effect as observed in the current study.
Consistent with previous studies, we confirmed that the lateral and orbital regions of the prefrontal cortex may play a critical role in withstanding late-life socioemotional impairment (Cajanus et al., 2019, Hu et al., 2015, Peters et al., 2006). Specifically, the left inferior gyrus is a critical region in reappraising and regulating negative emotions while attenuating excessive amygdala activity (Berna et al., 2010, Goldin et al., 2008). Moreover, the amygdala-orbitofrontal network is crucial in representing the hierarchical structures of reward values allotted in an environment (Jung et al., 2018, Stalnaker et al., 2015). Thus, the maintenance of these structures may be correlated with a larger capacity to cope with complex social situations (Kwak et al., 2018, Powell et al., 2010).
In this study, we extended the previous findings that show educational attainment can exert a resilience effect in the relationship between neurodegeneration and clinical impairment. Years of education, as a proxy of cognitive reserve, may mitigate and delay the onset of behavioral and psychological symptoms as neurodegeneration progresses. Previous studies have shown that older adults with higher education had a minimal decline in their emotional intelligence (Cabello et al., 2014). This intellectual achievement typically represents the cognitive and motivational capacity that is inherited and accumulated with lifespan development (Bartrés-Faz et al., 2018, Richards and Deary, 2005). This accumulation of resources may confer a larger capacity to maintain socioemotional functioning in the presence of neuropathology.
Previous literature has been focused on the reserve effect in cognitive tasks. Our study further suggests that education plays role in broad psychosocial functioning (Barnett et al., 2006, Watson and Joyce, 2015). In this study, the mood dysregulation component of NPS was associated with thinner cortices in the left inferior and orbitofrontal cortex. The functional neural correlate of education is ubiquitous across brain regions; however, previous studies have indicated that older adults with higher education may have developed a larger brain structure or efficient functional network in the left inferior frontal, medial frontal and orbitofrontal cortex (Arenaza-Urquijo et al., 2013, Foubert-Samier et al., 2012, Franzmeier et al., 2017, Marques et al., 2015, Premi et al., 2013). Such efficient connectedness of the control network may facilitate additional effort to compensate for the deleterious progression of dementia pathology (Arenaza-Urquijo et al., 2017).
One notable observation was that the moderating effect of education on the neuroanatomical correlate largely overlaps with its effect on global clinical impairment (i.e., the interaction between CDR and education), whereas typical cognitive impairment did not (i.e., the interaction between MMSE and education). This result indicates that the cognitive reserve mechanism is comprised of a distinct aspect of both cognitive and socioemotional functioning in explaining overall clinical impairment. Further research is required to describe the full constituents of independent daily functioning and role of early-life intellectual experience in regulating socioemotional behavior.
We note some limitations in the current study. The whole target population was taken from in the OASIS dataset; however, the majority of the individuals inculded in the brain scanning dataset did not show NPS. This leads to an unstable effect when adjusting for the effect of clinical status. Future studies are required to clarify the distinct effect of NPI subdomains and the dementia subtypes. Secondly, although NPI is a widely used instrument in assessing behavioral symptoms, the major source of information is based on the subjective assessment of real-world behaviors. Therefore, NPI is susceptible to the type and quality of the relationship between the informant and patient. Even when clinicians discerningly adjust for any informant bias, the detection and severity depends highly on caregiving context. A future study that utilizes the longitudinally fluctuating patterns of NPI or assessment of performance-based socioemotional ability test will clarify its validity (Boublay et al., 2020, Poulin et al., 2017).
Acknowledgments
Acknowledgements
Data were provided in part by OASIS-3 Principal Investigators: T. Benzinger, D. Marcus, J. Morris; NIH P50AG00561, P30NS09857781, P01AG026276, P01AG003991, R01AG043434, UL1TR000448, R01EB009352. This research is supported by the National Research Foundation of Korea (NRF-2017S1A3A2067165), funded by the Ministry of Education, Science and Technology.
Funding
None.
Declaration of Competing Interest
We declare we have no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102452.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- Arenaza-Urquijo E.M., Landeau B., La Joie R., Mevel K., Mézenge F., Perrotin A., Desgranges B., Bartrés-Faz D., Eustache F., Chételat G. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage. 2013;83:450–457. doi: 10.1016/j.neuroimage.2013.06.053. [DOI] [PubMed] [Google Scholar]
- Arenaza-Urquijo E.M., Bejanin A., Gonneaud J., Wirth M., La Joie R., Mutlu J., Gaubert M., Landeau B., de la Sayette V., Eustache F., Chételat G. Association between educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: neuroimaging evidence for protection and compensation. Neurobiol. Aging. 2017 doi: 10.1016/j.neurobiolaging.2017.06.016. [DOI] [PubMed] [Google Scholar]
- Barnett J.H., Salmond C.H., Jones P.B., Sahakian B.J. Cognitive reserve in neuropsychiatry. Psychol. Med. 2006;36:1053–1064. doi: 10.1017/S0033291706007501. [DOI] [PubMed] [Google Scholar]
- Bartrés-Faz D., Cattaneo G., Solana J., Tormos J.M., Pascual-Leone A. Meaning in life: resilience beyond reserve. Alzheimer’s Res. Ther. 2018 doi: 10.1186/s13195-018-0381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekly D.L., Ramos E.M., Lee W.W., Deitrich W.D., Jacka M.E., Wu J., Hubbard J.L., Koepsell T.D., Morris J.C., Kukull W.A. The National Alzheimer??s Coordinating Center (NACC) Database: the uniform data set. Alzheimer Dis. Assoc. Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- Berna C., Leknes S., Holmes E.A., Edwards R.R., Goodwin G.M., Tracey I. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol. Psychiatry. 2010;67:1083–1090. doi: 10.1016/j.biopsych.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Bickart K.C., Brickhouse M., Negreira A., Sapolsky D., Barrett L.F., Dickerson B.C. Atrophy in distinct corticolimbic networks in frontotemporal dementia relates to social impairments measured using the Social Impairment Rating Scale. J. Neurol. Neurosurg. Psychiatry. 2014;85:438–448. doi: 10.1136/jnnp-2012-304656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart K.C., Dickerson B.C., Feldman Barrett L. The amygdala as a hub in brain networks that support social life. Neuropsychologia. 2014;63:235–248. doi: 10.1016/j.neuropsychologia.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart, K.C., Hollenbeck, M.C., Barrett, L.F., Dickerson, B.C., 2012. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans 32, 14729–14741. DOI:10.1523/JNEUROSCI.1599-12.2012. [DOI] [PMC free article] [PubMed]
- Boublay N., Bouet R., Dorey J.-M., Padovan C., Makaroff Z., Fédérico D., Gallice I., Barrellon M.-O., Robert P., Moreaud O., Rouch I., Krolak-Salmon P., Neuroimaging Initiative D. Brain Volume predicts behavioral and psychological symptoms in Alzheimer’s disease. J. Alzheimer’s Dis. 2020;73:1343–1353. doi: 10.3233/JAD-190612. [DOI] [PubMed] [Google Scholar]
- Bruen P.D., McGeown W.J., Shanks M.F., Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain. 2008 doi: 10.1093/brain/awn151. [DOI] [PubMed] [Google Scholar]
- Cabello R., Navarro Bravo B., Latorre J.M., Fernández-Berrocal P. Ability of university-level education to prevent age-related decline in emotional intelligence. Front. Aging Neurosci. 2014;6:7. doi: 10.3389/fnagi.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajanus A., Solje E., Koikkalainen J., Lötjönen J., Suhonen N.M., Hallikainen I., Vanninen R., Hartikainen P., de Marco M., Venneri A., Soininen H., Remes A.M., Hall A. The association between distinct frontal brain volumes and behavioral symptoms in mild cognitive impairment, Alzheimer’s disease, and frontotemporal dementia. Front. Neurol. 2019 doi: 10.3389/fneur.2019.01059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J.L., Mega M., Gray K., Rosenberg-Thompson S., Carusi D.A., Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994 doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Dahnke, R., Ziegler, G., Gaser, C., Jena, F., 2012. Local Adaptive Segmentation 2012.
- Dahnke R., Yotter R.A., Gaser C. Cortical thickness and central surface estimation. Neuroimage. 2013;65:336–348. doi: 10.1016/j.neuroimage.2012.09.050. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Drysdale A.T., Grosenick L., Downar J., Dunlop K., Mansouri F., Meng Y., Fetcho R.N., Zebley B., Oathes D.J., Etkin A., Schatzberg A.F., Sudheimer K., Keller J., Mayberg H.S., Gunning F.M., Alexopoulos G.S., Fox M.D., Pascual-Leone A., Voss H.U., Casey B., Dubin M.J., Liston C. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillenbaum G.G., Peterson B., Morris J. Estimating the validity of the clinical Dementia Rating Scale: the CERAD experience. Consortium to Establish a Registry for Alzheimer’s Disease. Aging (Milano) 1996 doi: 10.1007/BF03339599. [DOI] [PubMed] [Google Scholar]
- Finkel S. Introduction to behavioural and psychological symptoms of dementia (BPSD) Int. J. Geriatr. Psychiatry. 2000;15 doi: 10.1002/(sici)1099-1166(200004)15:1+<s2::aid-gps159>3.0.co;2-3. Suppl 1S2-4. [DOI] [PubMed] [Google Scholar]
- Foubert-Samier A., Catheline G., Amieva H., Dilharreguy B., Helmer C., Allard M., Dartigues J.-F. Education, occupation, leisure activities, and brain reserve: a population-based study. Neurobiol. Aging. 2012;33:423.e15–423.e25. doi: 10.1016/j.neurobiolaging.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Franzmeier N., Hartmann J.C., Taylor A.N.W., Caballero M.Á.A., Simon-Vermot L., Buerger K., Kambeitz-Ilankovic L.M., Ertl-Wagner B., Mueller C., Catak C., Janowitz D., Stahl R., Dichgans M., Duering M., Ewers M. Left frontal hub connectivity during memory performance supports reserve in aging and mild cognitive impairment. J. Alzheimer’s Dis. 2017;59:1381–1392. doi: 10.3233/JAD-170360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González I., Déjean S., Martin P.G.P., Baccini A. CCA: an R package to extend canonical correlation analysis. J. Stat. Softw. 2012 doi: 10.18637/jss.v023.i12. [DOI] [Google Scholar]
- Habeck C., Foster N.L., Perneczky R., Kurz A., Alexopoulos P., Koeppe R.A., Drzezga A., Stern Y. Multivariate and univariate neuroimaging biomarkers of Alzheimer’s disease. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinek D., Petrovicky P., Hort J., Krasensky J., Brabec J., Bojar M., Vaneckova M., Seidl Z. Amygdalar volume and psychiatric symptoms in Alzheimer’s disease: an MRI analysis. Acta Neurol. Scand. 2006;113:40–45. doi: 10.1111/j.1600-0404.2006.00540.x. [DOI] [PubMed] [Google Scholar]
- Hu X., Meiberth D., Newport B., Jessen F. Anatomical correlates of the neuropsychiatric symptoms in Alzheimer’s disease. Curr. Alzheimer Res. 2015;12:266–277. doi: 10.2174/1567205012666150302154914. [DOI] [PubMed] [Google Scholar]
- Jung W.H., Lee S., Lerman C., Kable J.W. Amygdala functional and structural connectivity predicts individual risk tolerance. Neuron. 2018;98:394–404.e4. doi: 10.1016/j.neuron.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer D.I., Cummings J.L., Ketchel P., Smith V., MacMillan A., Shelley T., Lopez O.L., DeKosky S.T. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J. Neuropsychiatry Clin. Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- Kwak S., Joo W., Youm Y., Chey J. Social brain volume is associated with in-degree social network size among older adults. Proc. R. Soc. B Biol. Sci. 2018;285:20172708. doi: 10.1098/rspb.2017.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMontagne P.J., Benzinger T.L.S., Morris J.C., Keefe S., Hornbeck R., Xiong C., Grant E., Hassenstab J., Moulder K., Vlassenko A., Raichle M.E., Cruchaga C., Marcus D. OASIS-3: Longitudinal neuroimaging, clinical, and cognitive dataset for normal aging and Alzheimer disease. medRxiv. 2019 doi: 10.1101/2019.12.13.19014902. [DOI] [Google Scholar]
- Lyketsos C.G., Lopez O., Jones B., Fitzpatrick A.L., Breitner J., Dekosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. J. Am. Med. Assoc. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- Manjón J.V., Coupé P., Martí-Bonmatí L., Collins D.L., Robles M. Adaptive non-local means denoising of MR images with spatially varying noise levels. J. Magn. Reson. Imaging. 2010;31:192–203. doi: 10.1002/jmri.22003. [DOI] [PubMed] [Google Scholar]
- Marcus D.S., Wang T.H., Parker J., Csernansky J.G., Morris J.C., Buckner R.L. Open Access Series of Imaging Studies (OASIS): cross-sectional MRI data in young, middle aged, nondemented, and demented older adults. J. Cogn. Neurosci. 2007;19:1498–1507. doi: 10.1162/jocn.2007.19.9.1498. [DOI] [PubMed] [Google Scholar]
- Marques P., Soares J.M., Magalhães R., Santos N.C., Sousa N. The bounds of education in the human brain connectome. Sci. Rep. 2015;5:12812. doi: 10.1038/srep12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masouleh K.S., Eickhoff S.B., Hoffstaedter F., Genon S. Empirical examination of the replicability of associations between brain structure and psychological variables. Elife. 2019;8:1–25. doi: 10.7554/elife.43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris J.C. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int. Psychogeriatrics. 1997;9:173–176. doi: 10.1017/S1041610297004870. [DOI] [PubMed] [Google Scholar]
- Morris J.C., Weintraub S., Chui H.C., Cummings J., DeCarli C., Ferris S., Foster N.L., Galasko D., Graff-Radford N., Peskind E.R., Beekly D., Ramos E.M., Kukull W.A. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer disease centers. Alzheimer Dis. Assoc. Disord. 2006 doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Moser D.A., Doucet G.E., Lee W.H., Rasgon A., Krinsky H., Leibu E., Ing A., Schumann G., Rasgon N., Frangou S. Multivariate associations among behavioral, clinical, and multimodal imaging phenotypes in patients with psychosis. JAMA Psychiatry. 2018;75:386–395. doi: 10.1001/jamapsychiatry.2017.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossenkoppele R., Pijnenburg Y.A.L., Perry D.C., Cohn-Sheehy B.I., Scheltens N.M.E., Vogel J.W., Kramer J.H., Van Der Vlies A.E., Joie R.L., Rosen H.J., Van Der Flier W.M., Grinberg L.T., Rozemuller A.J., Huang E.J., Van Berckel B.N.M., Miller B.L., Barkhof F., Jagust W.J., Scheltens P., Seeley W.W., Rabinovici G.D. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain. 2015;138:2732–2749. doi: 10.1093/brain/awv191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.H., Lee J.Y., Na D.L., Kim S.Y., Cheong H.K., Moon S.Y., Shim Y.S., Park K.W., Ku B.D., Choi S.H., Joo H., Lee J.S., Go S.M., Kim S.H., Kim S., Cha K.R., Lee J., Seo S.W. Different associations of periventricular and deep white matter lesions with cognition, neuropsychiatric symptoms, and daily activities in dementia. J. Geriatr. Psychiatry Neurol. 2011 doi: 10.1177/0891988711402351. [DOI] [PubMed] [Google Scholar]
- Peters F., Perani D., Herholz K., Holthoff V., Beuthien-Baumann B., Sorbi S., Pupi A., Degueldre C., Lemaire C., Collette F., Salmon E. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 2006 doi: 10.1159/000091898. [DOI] [PubMed] [Google Scholar]
- Poulin S.P., Dautoff R., Morris J.C., Barrett L.F., Dickerson B.C. Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry Res. - Neuroimaging. 2011 doi: 10.1016/j.pscychresns.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin S.P., Bergeron D., Dickerson B.C. Risk factors, neuroanatomical correlates, and outcome of neuropsychiatric symptoms in Alzheimer’s disease. J. Alzheimer’s Dis. 2017;60:483–493. doi: 10.3233/JAD-160767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.L., Lewis P.A., Dunbar R.I.M., García-Fiñana M., Roberts N. Orbital prefrontal cortex volume correlates with social cognitive competence. Neuropsychologia. 2010;48:3554–3562. doi: 10.1016/j.neuropsychologia.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Premi E., Garibotto V., Gazzina S., Grassi M., Cosseddu M., Paghera B., Turla M., Padovani A., Borroni B. Beyond cognitive reserve: behavioural reserve hypothesis in Frontotemporal Dementia. Behav. Brain Res. 2013;245:58–62. doi: 10.1016/j.bbr.2013.01.030. [DOI] [PubMed] [Google Scholar]
- Revelle W. Northwest. Univ.; Evanston, Illinois: 2018. psych: Procedures for Personality and Psychological Research. [Google Scholar]
- Richards M., Deary I.J. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann. Neurol. 2005;58:617–622. doi: 10.1002/ana.20637. [DOI] [PubMed] [Google Scholar]
- Righart R., Schmidt P., Dahnke R., Biberacher V., Beer A., Buck D., Hemmer B., Kirschke J.S., Zimmer C., Gaser C., Mühlau M. Volume versus surface-based cortical thickness measurements: a comparative study with healthy controls and multiple sclerosis patients. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R.N., Drzezga A., Diehl-Schmid J., Kurz A., Levine B., Perneczky R. Relationship between occupation attributes and brain metabolism in frontotemporal dementia. Neuropsychologia. 2011;49:3699–3703. doi: 10.1016/j.neuropsychologia.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Stalnaker T.A., Cooch N.K., Schoenbaum G. What the orbitofrontal cortex does not do. Nat. Neurosci. 2015;18:620–627. doi: 10.1038/nn.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., Arenaza-Urquijo E.M., Bartrés-Faz D., Belleville S., Cantilon M., Chetelat G., Ewers M., Franzmeier N., Kempermann G., Kremen W.S., Okonkwo O., Scarmeas N., Soldan A., Udeh-Momoh C., Valenzuela M., Vemuri P., Vuoksimaa E., Arenaza Urquiljo E.M., Bartrés-Faz D., Belleville S., Cantillon M., Chetelat G., Clouston S.A.P., Estanga A., Ewers M., Franzmeier N., Gold B., Habeck C., Jones R., Kempermann G., Kochhann R., Kremen W., Lim Y.Y., Martínez-Lage P., Morbelli S., Okonkwo O., Ossenkoppele R., Pettigrew C., Rosen A.C., Scarmeas N., Soldan A., Song X., Udeh-Momoh C., Stern Y., Valenzuela M., Van Loenhoud A.C., Vemuri P., Vuoksimaa E. Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement. 2018;1–7 doi: 10.1016/j.jmarsys.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E., Lu P.H., Cummings J.L. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2007;24:253–259. doi: 10.1159/000107100. [DOI] [PubMed] [Google Scholar]
- Wadsworth L.P., Lorius N., Donovan N.J., Locascio J.J., Rentz D.M., Johnson K.A., Sperling R.A., Marshall G.A. Neuropsychiatric symptoms and global functional impairment along the Alzheimer’s continuum. Dement. Geriatr. Cogn. Disord. 2012 doi: 10.1159/000342119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H.T., Smallwood, J., Mourao-Miranda, J., Xia, C.H., Satterthwaite, T.D., Bassett, D.S., Bzdok, D., 2018. Finding the needle in high-dimensional haystack: A tutorial on canonical correlation analysis. arXiv Prepr. arXiv 1812.02598. [DOI] [PubMed]
- Watson A., Joyce E. Cognitive reserve and neuropsychiatric disorders. Curr. Opin. Behav. Sci. 2015;4:142–146. doi: 10.1016/j.cobeha.2015.05.003. [DOI] [Google Scholar]
- Wei L., Chen H., Wu G.-R. Structural covariance of the prefrontal-amygdala pathways associated with heart rate variability. Front. Hum. Neurosci. 2018;12 doi: 10.3389/fnhum.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C.-W., Chang L.J., Lindquist M.A., Wager T.D. Building better biomarkers: brain models in translational neuroimaging. Nat. Neurosci. Rev. 2017;20:365–377. doi: 10.1038/nn.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C.H., Ma Z., Ciric R., Gu S., Betzel R.F., Kaczkurkin A.N., Calkins M.E., Cook P.A., García de la Garza A., Vandekar S.N., Cui Z., Moore T.M., Roalf D.R., Ruparel K., Wolf D.H., Davatzikos C., Gur R.C., Gur R.E., Shinohara R.T., Bassett D.S., Satterthwaite T.D. Linked dimensions of psychopathology and connectivity in functional brain networks. Nat. Commun. 2018;9:3003. doi: 10.1038/s41467-018-05317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.