Abstract
The endometrium, composed of epithelial and stromal cell compartments, is tightly regulated by the ovarian steroid hormones estrogen (E2) and progesterone (P4) during early pregnancy. Through the progesterone receptor (PGR), steroid receptor coactivators, and other transcriptional coregulators, progesterone inhibits E2-induced cell proliferation and induces the differentiation of stromal cells in a process called decidualization to promote endometrial receptivity. Although interleukin-13 receptor subunit alpha-2 (Il13ra2) is expressed in the human and mouse endometrium, its potential role in the steroid hormone regulation of the endometrium has not been thoroughly examined. In this study, we employed PGR knockout mice and steroid receptor coactivator-1 knockout mice (SRC-1−/−) to profile the expression of Il13ra2 in the murine endometrium and determine the role of these transcriptional regulators in the hormone-responsiveness of Il13ra2 expression. Furthermore, we utilized a well-established decidualization-inducing steroidogenic cocktail and a siRNA-based knockdown of IL13RA2 to determine the importance of IL13RA2 in the decidualization of primary human endometrial stromal cells. Our findings demonstrate that Il13ra2 is expressed in the subepithelial stroma of the murine endometrium in response to ovarian steroid hormones and during early pregnancy in a PGR- and SRC-1-dependent manner. Furthermore, we show that knockdown of IL13RA2 before in vitro decidualization of primary human endometrial stromal cells partially compromises the full decidualization response. We conclude that Il13ra2 is a downstream target of progesterone through PGR and SRC-1 and plays a role in mediating the stromal action of ovarian steroid hormones.
Keywords: endometrium, progesterone receptor, steroid hormones, Il13ra2
Expression of Il13ra2 is induced by progesterone in the murine uterine stroma through the progesterone receptor and steroid receptor coactivator-1, and it is involved in human stromal cell decidualization.
Introduction
The uterus is a complex organ, which is made up of unique, heterogeneous cell types with specialized roles that work together to establish and sustain pregnancy. The myometrium, the outer layer composed primarily of smooth muscle, gives the uterus structure and contracts during parturition [1], while the endometrium comprises the inner lining that is the primary player in uterine receptivity to embryo implantation [2]. Within the endometrium, epithelial cells and surrounding stromal cells must coordinate seamlessly to provide a window of receptivity where the epithelium ceases proliferation to allow blastocyst invasion, at which time stromal cells transition into decidual cells to support and nourish the embryo development [3–5].
Each step of this complex process is temporally and spatially governed by the ovarian steroid hormones estrogen (E2) and progesterone (P4) acting through their cognate receptors (estrogen receptor and progesterone receptor [PGR]) and downstream signaling pathways that induce target gene transcription [6, 7]. Dysregulation of the tightly regulated balance of E2 and P4 commonly results in uterine disorders such as infertility, endometriosis, and endometrial cancer [8–10]. E2 dominates the proliferative phase of the menstrual cycle to allow thickening of the endometrium until P4 causes the transition into the secretory phase, when proliferation ceases, decidualization occurs, and the window of receptivity opens [10]. Decidualization, a key piece of this process, involves the differentiation of endometrial stromal cells to an epithelioid, secretory phenotype, and these supportive cells surround an implanting embryo to facilitate normal pregnancy establishment [7].
P4 is often labeled the “hormone of pregnancy” in part because of how integral expression of its target genes is to successful implantation [6]. Like other steroid hormones, P4’s genomic effects are primarily enacted through binding its cognate nuclear receptor, PGR, which induces transcription of its target genes [3, 11, 12]. Analysis of PGR knockout (PRKO) mice revealed the critical nature of PGR in the female reproductive tract because PRKO females had many reproductive abnormalities, including anovulation and a defect of decidualization [12].
When P4 binds PGR, the complex does not carry out its functions independently but instead recruits coregulators that are necessary for proper modification of chromatin and assembling of transcriptional machinery [13]. One family of such coregulators intimately connected with P4 signaling is the steroid receptor coactivator (SRC) family, consisting of SRC-1, SRC-2, and SRC-3 [7, 14]. In the endometrium, SRC-3 dysregulation is primarily associated with increased cancer risk [15, 16], but SRC-1 and SRC-2 are expressed in both the endometrial epithelium and stroma and are critical mediators of P4 action in the decidualization response [7, 14, 17]. SRC-1 loss alone does not render mice infertile [18], but knocking out SRC-2 in the uterus causes fertility defects that are exacerbated by the additional loss of SRC-1, firmly establishing the need for these two coregulators in proper P4 regulation of the endometrium [14, 19].
One role of P4 signaling in the uterus is to downregulate inflammation, which involves discouraging high levels of proinflammatory cytokines [8, 20]. Interleukin-13 (IL-13) is a cytokine linked primarily to immune dysregulation in various autoimmune and allergic diseases [21], but it is also expressed in the uterus throughout the menstrual cycle [22] and responds to ovarian steroid hormones in endometrial stromal and epithelial cells [23]. IL-13 is generally considered anti-inflammatory in that it inhibits the production of proinflammatory cytokines such as IL-1β, IL-6, IL-8, tumor necrosis factor-alpha, granulocyte colony-stimulating factor, and interferon-alpha [24, 25]. In the endometrium, IL-13 is found in all cellular compartments, but its expression is strongest in the luminal epithelium [22]. IL-13 appears to act primarily through binding a heterodimer composed of IL-13 receptor subunit alpha-1 (IL13RA1) and IL-4 receptor alpha (IL4R), but it also recognizes IL-13 receptor subunit alpha-2 (IL13RA2) [26–28]. IL13RA2 was first thought to only act as a decoy receptor to compete for and sequester IL-13; however, more recent findings indicate an active signaling role for IL13RA2 [27, 28] in extracellular signal-regulated kinase (ERK)/activator protein 1 (AP-1)-mediated proliferation [29] and transforming growth factor-beta-mediated fibrosis [30].
The binding of IL-13 by IL13RA2 amplifies the invasion and metastasis of pancreatic [31] and ovarian cancers [29] and has been suggested as a biomarker for ovarian cancer [32]. Increased matrix metalloproteinase expression in response to IL13RA2 binding activates the AP-1 transcription factor pathway and ERKs, which, in turn, encourage proliferative action in cancer cells [29]. Research on the role of IL13RA2 in the endometrium is incomplete. Though IL-13 expression in the endometrium appears to influence inflammatory conditions [23] and is upregulated in women with recurrent spontaneous abortion [22], its biological significance is unclear, and any importance of IL13RA2 in this role of IL-13 is unknown. However, IL13RA2 was identified by microarray analysis both in human endometrial stromal cells (hESCs), where it was found downregulated in response to P4 treatment [33], and in mice, where it was found downregulated at the implantation site (IS) in one study [34] and upregulated in response to P4 in another study [35]. Furthermore, one study found that Il13ra2 expression is induced by steroid hormones in the mouse uterus dependent on the presence of SRC-1 [36]. Together, these data indicate a potential role for IL13RA2 in steroid hormone regulation of the endometrium. Within the present study, we examined the spatiotemporal expression of Il13ra2 in the mouse uterus during early pregnancy and the regulation of Il13ra2 in response to P4 and E2 in the uterus. Furthermore, we characterized PGR- and SRC-1-dependent regulation of Il13ra2 expression in the mouse uterus and investigated the importance of IL13RA2 in the in vitro decidualization of primary hESCs.
Materials and methods
Animals and tissue collection
Mice were maintained in the designated animal care facility at Michigan State University according to the institutional guidelines for the care and use of laboratory animals. For hormone response tests, mice at 6 weeks of age were ovariectomized. Two weeks later, ovariectomized wild type, PRKO [12], and SRC-1−/− (SRC-1 knockout) [18] mice were injected with one of the following: vehicle (sesame oil), P4 (1 mg/mouse), E2 (0.1 μg/mouse), or a combination of P4 and E2 at the same doses as administered individually. Mouse uteri were collected at 4, 16, or 40 h of treatment (n = 3/group). The injections were repeated every 12 h for the 16 and 40 h samples. Uterine samples from specific stages of pregnancy were obtained by the mating of wild type mice, and the morning of a vaginal plug was designated as gestation day (GD) 0.5. Uterine samples for in situ hybridization at GD 0.5, GD 2.5, and GD 4.5 were taken from pseudopregnant mice after mating with vasectomized males. Samples at GD 5.5 and GD 7.5 were taken from IS regions of normal pregnant uteri. Samples for quantitative real-time polymerase chain reaction (RT-qPCR) were taken from pregnant mice, with GD 5.5 and GD 7.5 samples separated into IS and interimplantation site (I-IS) regions. Uterine tissues were flash frozen at the time of dissection and stored at −80 °C for later RNA extraction or fixed with 10% (v/v) formalin for in situ hybridization.
Quantitative real-time PCR
RNA was extracted from the uterine tissues or cultured cells using the RNeasy total RNA isolation kit (Qiagen, Valencia, CA). Expression levels of mouse Il13ra2 mRNA and human stromal cell IL13RA2 mRNA were measured by RT-qPCR TaqMan analysis using the ABI Prism 7700 Sequence Detector System according to the manufacturer’s instructions (PE Applied Biosystems, Foster City, CA). Prevalidated probes and primers were purchased from Applied Biosystems for Il13ra2 (00515166), IL13RA2 (00152924), RPL7 (02596927), Gapdh (99999915), and 18S RNA (99999901). cDNA was produced from 1 μg of total RNA using random hexamers and MMLV Reverse Transcriptase (Invitrogen, Carlsbad, CA). RT-qPCR was performed using RT-qPCR Universal Master Mix reagent (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. All RT-qPCR was done by using three independent RNA sets. Target gene mRNA quantities were normalized against 18S (mouse), Gapdh (mouse) or RPL7 (human).
In situ hybridization
The protocol for in situ hybridization was essentially as described previously [37, 38]. Briefly, uterine tissues were fixed at room temperature in 10% (v/v) formalin then dehydrated using a graded ethanol series before embedding in paraffin. Tissue sections were mounted onto poly-l-lysine-coated slides (VWR Scientific Products, West Chester, PA) before use in in situ hybridization. Generation of riboprobes was accomplished by in vitro transcription of amplified DNA products containing the T7 polymerase promoter sequence flanking the Il13ra2 nucleotide primer sequence using [35S] UTP (Promega, Madison, WI). Slides were incubated in a buffer containing Proteinase K, Tris, and EDTA, acetylated with acetic anhydride, dehydrated, and exposed to denatured antisense or sense probes in hybridization buffer. Hybridization was performed overnight before exposure to RNase A and washing in a mixture of formamide, saline sodium citrate, and 2-mercaptoethanol, followed by dehydration in a graded series of ethanol in ammonium acetate. The slides were then exposed to Biomax MR film (Kodak, Rochester, NY) overnight before being dipped in autoradiography emulsion (Amersham Biosciences, Piscataway, NJ) and being stored for several days in a refrigerated light proof box. At the completion of the developing process, slides were counterstained with hematoxylin.
Primary hESC cultures and in vitro decidualization
Human stromal cell isolation, culture, siRNA knockdown, and in vitro decidualization were performed based on previously described protocols [39, 40]. Human primary endometrial stromal cells (hESCs) were obtained from Michigan State University’s Center for Women’s Health Research Female Reproductive Tract Biorepository. Samples were obtained with written informed consent from premenopausal women undergoing hysterectomy for benign indications in the absence of all hormonal therapies for at least 90 days before surgery. Isolation of hESCs from pure endometrial tissue by collagenase digestion and filtration was performed as previously described [41]. Isolated cells were cultured and grown to confluency in phenol red–free RPMI-1640 medium (Gibco, Grand Island, NY) containing 10% fetal bovine serum (FBS; Gibco), 0.1 mM sodium pyruvate (Gibco), and 1% penicillin streptomycin (P/S; Gibco). For induction of in vitro decidualization, cells were transferred to OPTI-MEM medium (Gibco) with 2% FBS depleted of steroids by pretreatment with dextran-coated charcoal (CS-FBS; Gibco), 10 nM estradiol (E2, Sigma-Aldrich, St. Louis, MO), 1 μM medroxyprogesterone acetate (Sigma-Aldrich), 50 μM cAMP (Sigma-Aldrich), and 1% P/S. Growth media were replaced every 2 days for the course of the 6-day treatment. Transfection with IL13RA2 siRNA (L-004598-00-0020, Dharmacon, Lafayette, CO) to achieve mRNA knockdown and with nontargeting scrambled siRNA (NC1486135, Dharmacon) as a control was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) based on the manufacturer’s instructions before in vitro decidualization. Briefly, siRNAs were diluted in Opti-MEM to 50 nM then mixed with diluted transfection agent. After complex formation, complexes were diluted in Opti-MEM with 2% CS-FBS to make transfection media and added to confluent hESCs. Cells were incubated with transfection media for 6 h before replacing with growth media and incubating for 2 days before in vitro decidualization induction.
Statistical analysis
Statistical analyses were performed using Student’s t-test for data with only two groups. For tests involving multiple comparisons, we performed one-way ANOVA analysis followed by Tukey’s post hoc test using the Instat package from GraphPad (GraphPad Software, Inc., San Diego, CA). A value of P < 0.05 was considered statistically significant.
Results
Il13ra2 is a target of SRC-1 and PGR in the mouse uterus
To examine whether Il13ra2 expression is induced by P4, we first analyzed the levels of Il13ra2 mRNA in ovariectomized wild type mouse uteri 4 h after vehicle or P4 treatment using RT-qPCR. Il13ra2 expression was significantly upregulated in the P4-treated group compared to the vehicle-treated group (Figure 1A and B). To test whether this induction is dependent on SRC-1 and PGR, we utilized ovariectomized SRC-1−/− [18] and PRKO [12] mice and again treated with P4 or vehicle. We observed a significant decrease in Il13ra2 mRNA expression in both SRC-1−/− and PRKO mice compared to wild type mice treated with P4 (Figure 1A and B).
Figure 1.
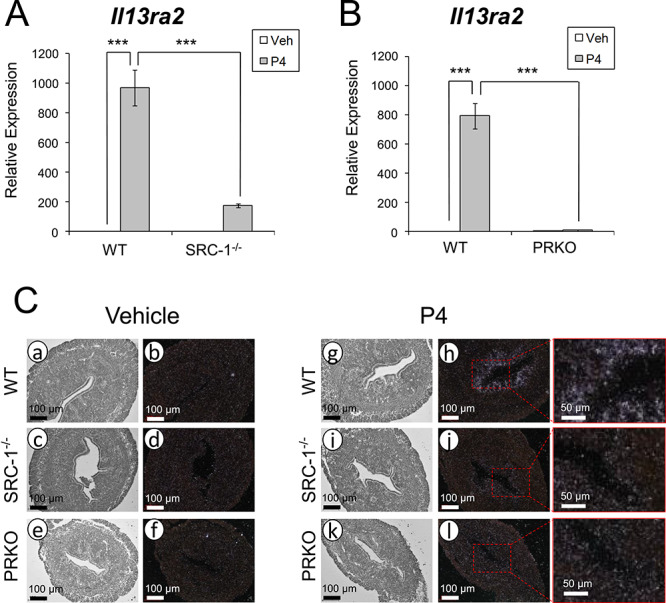
Uterine Il13ra2 expression in response to P4 treatment. (A and B) Relative Il13ra2 mRNA expression 4 h after administration of vehicle or P4 as measured by RT-qPCR in whole uterine tissue preparations from ovariectomized wild type (WT), SRC-1−/−, and PRKO mice. Graphs represent the mean ± SEM (n = 3; ***P < 0.001). (C) Bright-field (a, c, e, g, i, k) and dark-field (b, d, f, h, j, l) photomicrographs of WT, SRC-1−/−, and PRKO mouse uterine sections after in situ hybridization probing for Il13ra2 mRNA 4 h after administration of vehicle (a–f) or P4 (g–l; n = 3).
To gain an understanding of the spatial localization of Il13ra2 mRNA, we performed in situ hybridization on uterine cross-sections from wild type, SRC-1−/−, and PRKO mice treated with either vehicle or P4. We observed signal only in the wild type P4-treated group, as expected, but the expression was notably limited to the subepithelial stromal cells (Figure 1C). As shown by these findings, Il13ra2 expression is induced in a localized region of the murine uterus in response to P4 and mediated by SRC-1 and PGR.
Il13ra2 expression is induced by E2 and P4 in a synergistic and time-dependent manner
In order to observe the broader effect of steroid hormone signaling on Il13ra2 expression, we treated ovariectomized wild type mice with vehicle, E2, P4, or E2 + P4 and assessed the relative expression of Il13ra2 in the murine uterus at 16 and 40 h of treatment. Using RT-qPCR, we found that the combination treatment of E2 + P4 synergistically upregulated Il13ra2 expression significantly higher than any other treatment at both 16 h and 40 h, with the 40-h treatment group’s mRNA expression level being the highest (Figure 2A).
Figure 2.
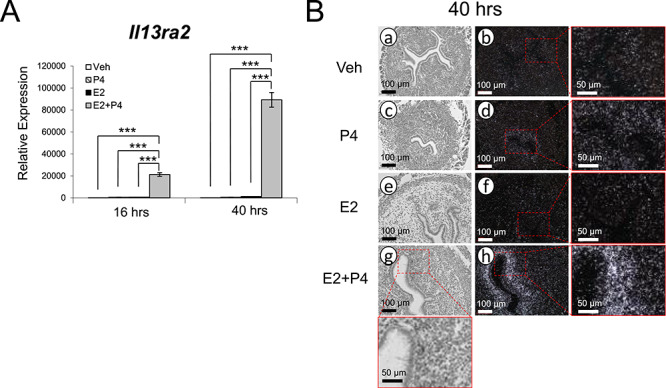
Time course of uterine Il13ra2 expression in response to ovarian steroid hormones. (A) Relative Il13ra2 mRNA expression after administration of vehicle, P4, E2, or combined E2 + P4 as measured by RT-qPCR after 16 h and 40 h in whole uterine tissue preparations from ovariectomized WT mice. Graphs represent the mean ± SEM (n = 3; ***P < 0.001). (B) Bright-field (a, c, e, g) and dark-field (b, d, f, h) photomicrographs of WT mouse uterine sections after in situ hybridization probing for Il13ra2 mRNA after administration of vehicle, P4, E2, or combined E2 + P4 at 40 h after treatment (n = 3).
Corroborating these results, in situ hybridization performed on uterine tissue sections from the 40-h treatment group revealed slightly increased Il13ra2 mRNA expression in the P4 only treated group and intensely increased expression in the E2 + P4-treated group (Figure 2B). Expression was again localized to the subepithelial stromal cells of the uterus. Overall, these results indicate a time-dependent induction of Il13ra2 by cooperative action of E2 and P4.
Il13ra2 expression in the uterus during early pregnancy
Steroid hormone signaling is tightly regulated during early pregnancy and critical for pregnancy success, so the expression pattern of Il13ra2 during this time is of great interest. To characterize its expression over the course of early pregnancy, we performed in situ hybridization and RT-qPCR on mouse uterine tissue collected during early pregnancy or pseudopregnancy from GD 0.5 to GD 7.5. With in situ hybridization, we observed notable signal at GD 4.5 but at no other stage (Figure 3A). With RT-qPCR, we detected significant increases in Il13ra2 expression at GD 4.5 and at GD 5.5 I-IS regions (Figure 3B). This finding indicates Il13ra2 is most highly expressed in the uterus at the early implantation stage of early pregnancy and away from the IS postimplantation [4].
Figure 3.
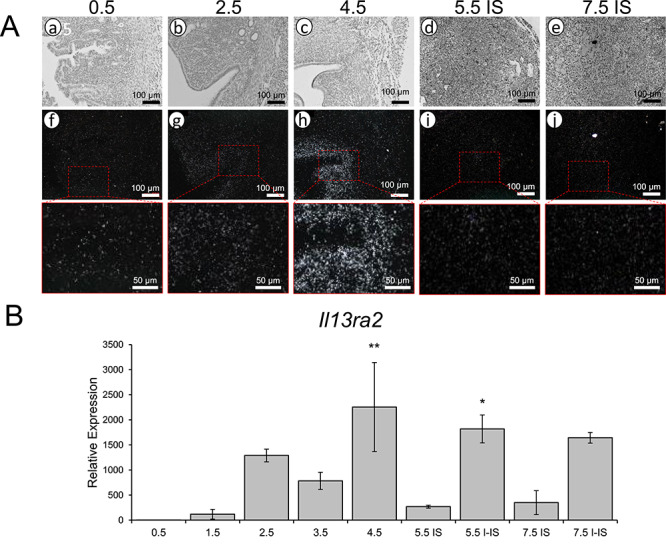
Il13ra2 expression during early pregnancy. (A) Bright-field (a–e) and dark-field (f–j) photomicrographs of WT mouse uterine sections from GD 0.5 to GD 7.5 showing Il13ra2 mRNA expression by in situ hybridization (n = 3). (B) Il13ra2 mRNA expression by RT-qPCR in mouse uterine samples from GD 0.5 to GD 7.5, with GD 0.5 and GD 7.5 samples separated into implantation site (IS) and interimplantation site (I-IS) regions. Graphs represent the mean ± SEM (n = 3; *P < 0.05, **P < 0.01, compared to GD 0.5).
IL13RA2 in in vitro decidualization of hESCs
Given the hormonal control and temporal and spatial expression pattern of Il13ra2 in mice, we asked whether it may be functionally involved in the decidualization process of hESCs. To determine the basal expression level of in IL13RA2 in hESCs, we utilized the IGROV-1 human ovarian adenocarcinoma cell line [42, 43] as a positive control because ovarian cancer cells are known to consistently express IL13RA2 [32, 44]. Using RT-qPCR, we found that our cultured hESCs at 38% the level of IGROV-1 cells (Figure 4A). To determine if IL13RA2 is involved in the differentiation of hESCs to a decidual character, we utilized a common protocol for in vitro decidualization induction using a combination of E2, medroxyprogesterone acetate (a P4 analog), and cAMP, together comprising EPC media [39, 41]. Confirming the successful induction of in vitro decidualization, decidualization marker genes PRL and IGFBP1 were significantly upregulated by day 6 of EPC treatment compared to untreated controls according to RT-qPCR analysis (Figure 4B). IL13RA2 was also significantly upregulated on day 6 of in vitro decidualization (Figure 4B).
Figure 4.
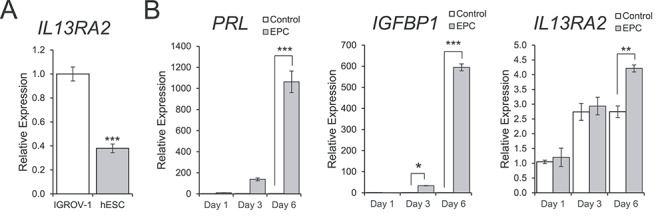
Expression of IL13RA2 during in vitro decidualization of primary human endometrial stromal cells. (A) RT-qPCR showing the basal expression of IL13RA2 mRNA in primary human endometrial stromal cells (hESC) relative to the positive control IGROV-1 Human Ovarian Cancer Cell Line. The graph represents the mean ± SEM (n = 3; ***P < 0.001). (B) RT-qPCR showing the mRNA expression levels of PRL, IGFBP1, and IL13RA2 in primary human endometrial stromal cells on days 1, 3, and 6 of treatment with control or in vitro decidualization induction (EPC) media. Graphs represent the mean ± SEM (n = 3; *P < 0.05; **P < 0.01, ***P < 0.001).
To determine if IL13RA2 is functionally important for in vitro decidualization of hESCs, we transfected the cells with IL13RA2 siRNA or with nontargeting control siRNA prior to inducing decidualization. Efficient knockdown of IL13RA2 mRNA levels was confirmed with RT-qPCR before inducing decidualization (−2.60-fold; P < 0.001; Figure 5A). Knockdown of IL13RA2 prior to EPC treatment significantly diminished the induction of PRL and IGFBP1 on day 6 of in vitro decidualization, indicating a functional role for IL13RA2 in enabling a full decidualization response in hESCs (Figure 5B).
Figure 5.
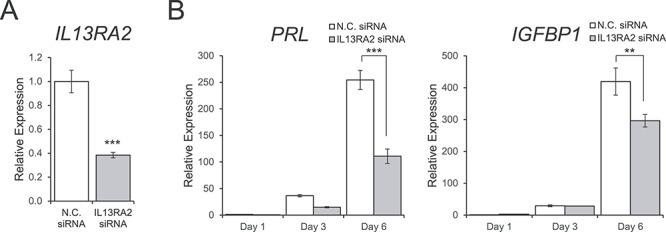
Effect of Il13ra2 knockdown on in vitro decidualization of primary human endometrial stromal cells. (A) RT-qPCR showing knockdown efficiency of Il13ra2 mRNA expression after transfection with IL13RA2 siRNA compared to nontargeting control siRNA. The graph represents the mean ± SEM (n = 3; ***P < 0.001). (B) RT-qPCR showing the expression of the decidualization marker genes PRL and IGFBP1 on days 1, 3, and 6 of in vitro decidualization induction after transfection with IL13RA2 siRNA or nontargeting control siRNA. Graphs represent the mean ± SEM (n = 3; **P < 0.01, ***P < 0.001).
Discussion
A thorough knowledge of the complex mechanisms of E2 and P4 signaling interplay in the uterus is critical to understanding both healthy uterine function and the pathophysiology of uterine dysfunction [2, 6–9]. P4 action through PGR, in particular, has been repeatedly shown to induce many factors necessary for maintenance of uterine function such as Ihh [45, 46], Hand2 [47], Areg [48], Gata2 [49, 50], and Sox17 [49, 51]. While much progress has been made in recent years unraveling the complex networks regulated by P4 and PGR in the uterus, many players downstream of PGR still need to be explored, particularly because of the complexity of P4 signaling regulation in epithelial–stromal cross-talk [3]. In this study, we have identified Il13ra2 as a uterine stromal target of PGR and SRC-1.
Previous reports of Il13ra2 expression in the endometrium have been contradictory and limited in detail. The first noted mention of Il13ra2 in the uterus by Reese et al. was in a large microarray experiment designed to find markers of uterine receptivity in mice, where Il13ra2 expression was found downregulated at newly forming ISs compared to I-IS regions [34]. Our contrasting finding that Il13ra2 expression increases in the subepithelial stroma at peri-implantation might be explained by the fact that the previous experiment included all layers of the uterus as well as the blastocyst in the IS analysis. Indeed, uterine epithelial-specific ablation of PGR does not significantly alter Il13ra2 expression after P4 treatment, highlighting the importance of uterine compartment-specific analysis [52]. Two past studies identified Il13ra2 expression changes in uterine cells after P4 treatment via microarray analysis [33, 35]. In cultured hESCs, P4 treatment decreased IL13RA2 expression, although this microarray result was not validated [33]. However, in mouse uteri, P4 treatment induced Il13ra2 in a PGR-dependent manner [35, 52]. Our data corroborate the previous mouse findings by showing that Il13ra2 is upregulated in the subepithelial stroma after P4 treatment.
It is important to note the role of immune cells in the endometrial stroma. Specifically, there have been reports of important functions for uterine macrophages [53] and dendritic cells [54] during early pregnancy in mice. While the expression and function of Il13ra2 in immune cells has not been well characterized, its expression has been reported in the immune cell-rich spleen [55] and specifically in macrophages [30]. Thus, it is possible that Il13ra2 may be expressed and function in the immune cells of the endometrial stromal bed during early pregnancy.
Our results demonstrate that uterine SRC-1 is necessary for full induction of Il13ra2 expression in response to P4 treatment, which is consistent with a previous report showing the induction of Il13ra2 expression by E2 and P4 treatment is abrogated in SRC-1−/− mice [36]. However, we found that a low level of Il13ra2 induction still occurs in P4-treated SRC-1−/− mice, which may be due to compensatory or redundant action of SRC-2 and/or SRC-3. Indeed, previous studies have shown that uterine deletion of both SRC-1 and SRC-2 in mice causes uterine defects beyond those seen in mice with individual deletions of SRC-1 or SRC-2 [14, 18, 19]. P4 and PGR action in uterine stromal cells during early pregnancy has been repeatedly shown to be necessary for implantation and decidualization success, and both critical events are in progress at GD 4.5 and GD 5.5 in mice [56]. Our finding that Il13ra2 expression is strongest at GD 4.5 suggests that it may be involved in the regulation of the early stages of decidualization in mice. However, strong expression in I-IS regions but not IS regions at postimplantation time points indicates Il13ra2 is not involved throughout the decidualization process but only the early priming of stromal cells. Rather, Il13ra2 may be downregulated directly at the IS by factors involved in the later stages of implantation.
Though we found that Il13ra2 expression is induced moderately by short-term P4 treatment alone, it is induced much more strongly by combined E2 and P4 treatment over a longer period. The temporal and spatial interplay between E2 and P4 signaling during early pregnancy is very intricate and complex, but both hormones are necessary in proper proportion [6, 7]. One possible explanation for our observation is that E2 drives PGR expression in the stroma, but P4 downregulates PGR overall through negative feedback [57]. Since we showed PGR expression to be necessary for induction of Il13ra2 in the stroma using PRKO mice, the addition of E2 to the P4 treatment may have increased Il13ra2 expression by maintaining stronger PGR activity in the stroma.
Our findings that IL13RA2 expression is significantly increased in hESCs in vitro decidualization conditions and that knockdown of IL13RA2 by siRNA transfection before treatment partially compromises the decidualization response reveal a new steroid hormone-regulated gene involved in decidualization. This finding parallels the uterine Il13ra2 expression pattern during early pregnancy in mice but not perfectly, since GD 4.5 marks the beginning of the decidualization response [4]. Il13ra2 appears to then be downregulated as decidual cells mature in mice, whereas it is slightly upregulated in decidualizing hESCs later in the process. This discrepancy could be due to species differences or to in vivo versus in vitro conditions. Interestingly, SRC-1 was previously shown to be necessary for a full decidualization response [18], and our finding that Il13ra2 is downstream of SRC-1 implicates it as an effector of SRC-1’s role in decidualization. Additional support for Il13ra2 as an important gene in decidualization comes from the known role of Il13ra2 in facilitating ERK-mediated proliferation through phosphorylation of ERK1/2 [29]. ERK1/2 activation by phosphorylation is necessary for decidualization, and pERK1/2 is upregulated in the subepithelial stroma of mice at GD 4.5, mirroring the Il13ra2 expression profile that we report here [39]. Thus, IL13RA2 may function in decidualization upstream of ERK1/2 activation. The fact that decidualization was only moderately affected by knockdown of IL13RA2 may imply a more complex regulation of decidualization by IL13RA2 possibly involving IL-13 and a compensatory or competing role for IL13RA1/IL4R. It is known that IL-13 secretion by cultured stromal cells increases during decidualization, but no functional role has been established for IL-13 in decidual cells [58]. Thus, more detailed functional studies will be necessary to investigate these possibilities.
Notably, IL-13 levels decrease in normal endometrium from fertile women during the mid-secretory phase, which contains the implantation window, but they are significantly increased in women with recurrent spontaneous abortion at this time, leading to the speculation that appropriate levels of IL-13 and its downstream signaling consequences may be important to govern the immune and inflammatory responses necessary for endometrial function and pregnancy establishment [22]. Additionally, both epithelial and stromal cells of the endometrium secrete heightened levels of IL-13 in response to treatment with E2, P4, or E2 + P4 in culture [23]. In the context of our study, this may implicate IL13RA2 as a hormone-responsive modulator of IL-13 signaling during implantation, either to sequester IL-13 or to activate ERK/AP-1 or another signaling pathway. At this point, however, more experiments are necessary to determine if IL13RA2 plays a role modulating IL-13 signaling during implantation and if it is important for decidualization or immune regulation. Overall, our study indicates that Il13ra2 is induced synergistically by E2 and P4 through PGR and SRC-1 during the early stages of decidualization in the mouse and is functionally involved in complete decidualization of human stromal cells.
Author contributions
K.L., F.J.D., and J.W.J designed experiments; R.M.M., T.H.K., and J.W.J performed experiments; R.M.M., K.L., F.J.D., and J.W.J analyzed and interpreted data; R.M.M., T.H.K., B.L., and J.W.J wrote the manuscript.
Acknowledgments
PRKO and SRC-1−/− mice were provided by Drs John P. Lydon and Jianming Xu at Baylor College of Medicine, respectively. IGROV-1 human ovarian adenocarcinoma cell cDNA was provided by Dr John Risinger at Michigan State University.
Footnotes
† Grant Support: Research reported in this publication was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R01HD084478 to J.W.J. and T32HD087166 to R.M.M., MSU AgBio Research, and Michigan State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Wu SP, DeMayo FJ. Progesterone receptor signaling in uterine myometrial physiology and preterm birth. Curr Top Dev Biol 2017; 125:171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vasquez YM, DeMayo FJ. Role of nuclear receptors in blastocyst implantation. Semin Cell Dev Biol 2013; 24:724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang X, Wu SP, DeMayo FJ. Hormone dependent uterine epithelial-stromal communication for pregnancy support. Placenta 2017; 60(Suppl 1):S20–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hantak AM, Bagchi IC, Bagchi MK. Role of uterine stromal-epithelial crosstalk in embryo implantation. Int J Dev Biol 2014; 58:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rubel CA, Jeong JW, Tsai SY, Lydon JP, Demayo FJ. Epithelial-stromal interaction and progesterone receptors in the mouse uterus. Semin Reprod Med 2010; 28:27–35. [DOI] [PubMed] [Google Scholar]
- 6. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med 2012; 18:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol 2012; 358:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand 2017; 96:623–632. [DOI] [PubMed] [Google Scholar]
- 9. Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Semin Reprod Med 2010; 28:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marquardt RM, Kim TH, Shin JH, Jeong JW. Progesterone and estrogen signaling in the endometrium: what goes wrong in endometriosis? Int J Mol Sci 2019; 20: 3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology 1999; 140:5310–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 1995; 9:2266–2278. [DOI] [PubMed] [Google Scholar]
- 13. Millard CJ, Watson PJ, Fairall L, Schwabe JW. An evolving understanding of nuclear receptor coregulator proteins. J Mol Endocrinol 2013; 51:T23–T36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Szwarc MM, Lydon JP, O'Malley BW. Steroid receptor coactivators as therapeutic targets in the female reproductive system. J Steroid Biochem Mol Biol 2015; 154:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balmer NN, Richer JK, Spoelstra NS, Torkko KC, Lyle PL, Singh M. Steroid receptor coactivator AIB1 in endometrial carcinoma, hyperplasia and normal endometrium: Correlation with clinicopathologic parameters and biomarkers. Mod Pathol 2006; 19:1593–1605. [DOI] [PubMed] [Google Scholar]
- 16. Sakaguchi H, Fujimoto J, Sun WS, Tamaya T. Clinical implications of steroid receptor coactivator (SRC)-3 in uterine endometrial cancers. J Steroid Biochem Mol Biol 2007; 104:237–240. [DOI] [PubMed] [Google Scholar]
- 17. Jeong JW, Lee KY, Han SJ, Aronow BJ, Lydon JP, O'Malley BW, DeMayo FJ. The p160 steroid receptor coactivator 2, SRC-2, regulates murine endometrial function and regulates progesterone-independent and -dependent gene expression. Endocrinology 2007; 148:4238–4250. [DOI] [PubMed] [Google Scholar]
- 18. Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 1998; 279:1922–1925. [DOI] [PubMed] [Google Scholar]
- 19. Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, Demayo FJ, Lydon JP, O'Malley BW. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol 2006; 26:6571–6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum Reprod Update 2015; 21:155–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mao YM, Zhao CN, Leng J, Leng RX, Ye DQ, Zheng SG, Pan HF. Interleukin-13: a promising therapeutic target for autoimmune disease. Cytokine Growth Factor Rev 2018; 45: 9–23. [DOI] [PubMed] [Google Scholar]
- 22. Chegini N, Ma C, Roberts M, Williams RS, Ripps BA. Differential expression of interleukins (IL) IL-13 and IL-15 throughout the menstrual cycle in endometrium of normal fertile women and women with recurrent spontaneous abortion. J Reprod Immunol 2002; 56:93–110. [DOI] [PubMed] [Google Scholar]
- 23. Roberts M, Luo X, Chegini N. Differential regulation of interleukins IL-13 and IL-15 by ovarian steroids, TNF-alpha and TGF-beta in human endometrial epithelial and stromal cells. Mol Hum Reprod 2005; 11:751–760. [DOI] [PubMed] [Google Scholar]
- 24. Dembic Z. The Cytokines of the Immune System the Role of Cytokines in Disease Related to Immune Response. London: Academic Press; 2015: 310. [Google Scholar]
- 25. Chomarat P, Banchereau J. Interleukin-4 and interleukin-13: their similarities and discrepancies. Int Rev Immunol 1998; 17:1–52. [DOI] [PubMed] [Google Scholar]
- 26. Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, Willson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci U S A 1996; 93:497–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andrews AL, Nordgren IK, Kirby I, Holloway JW, Holgate ST, Davies DE, Tavassoli A. Cytoplasmic tail of IL-13Ralpha2 regulates IL-4 signal transduction. Biochem Soc Trans 2009; 37:873–876. [DOI] [PubMed] [Google Scholar]
- 28. Chen W, Sivaprasad U, Tabata Y, Gibson AM, Stier MT, Finkelman FD, Hershey GK. IL-13R alpha 2 membrane and soluble isoforms differ in humans and mice. J Immunol 2009; 183:7870–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fujisawa T, Joshi BH, Puri RK. IL-13 regulates cancer invasion and metastasis through IL-13Ralpha2 via ERK/AP-1 pathway in mouse model of human ovarian cancer. Int J Cancer 2012; 131:344–356. [DOI] [PubMed] [Google Scholar]
- 30. Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med 2006; 12:99–106. [DOI] [PubMed] [Google Scholar]
- 31. Fujisawa T, Joshi B, Nakajima A, Puri RK. A novel role of interleukin-13 receptor alpha2 in pancreatic cancer invasion and metastasis. Cancer Res 2009; 69:8678–8685. [DOI] [PubMed] [Google Scholar]
- 32. Kioi M, Kawakami M, Shimamura T, Husain SR, Puri RK. Interleukin-13 receptor alpha2 chain: a potential biomarker and molecular target for ovarian cancer therapy. Cancer 2006; 107:1407–1418. [DOI] [PubMed] [Google Scholar]
- 33. Okada H, Nakajima T, Yoshimura T, Yasuda K, Kanzaki H. Microarray analysis of genes controlled by progesterone in human endometrial stromal cells in vitro. Gynecol Endocrinol 2003; 17:271–280. [DOI] [PubMed] [Google Scholar]
- 34. Reese J, Das SK, Paria BC, Lim H, Song H, Matsumoto H, Knudtson KL, DuBois RN, Dey SK. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J Biol Chem 2001; 276:44137–44145. [DOI] [PubMed] [Google Scholar]
- 35. Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, DeMayo FJ. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology 2005; 146:3490–3505. [DOI] [PubMed] [Google Scholar]
- 36. Han SJ, Jeong J, Demayo FJ, Xu J, Tsai SY, Tsai MJ, O'Malley BW. Dynamic cell type specificity of SRC-1 coactivator in modulating uterine progesterone receptor function in mice. Mol Cell Biol 2005; 25:8150–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oh SJ, Kim TH, Lim JM, Jeong JW. Progesterone induces expression of Lrp2 in the murine uterus. Biochem Biophys Res Commun 2013; 441:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim TH, Lee DK, Franco HL, Lydon JP, Jeong JW. ERBB receptor feedback inhibitor 1 regulation of estrogen receptor activity is critical for uterine implantation in mice. Biol Reprod 2010; 82:706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee CH, Kim TH, Lee JH, Oh SJ, Yoo JY, Kwon HS, Kim YI, Ferguson SD, Ahn JY, Ku BJ, Fazleabas AT, Lim JM et al. Extracellular signal-regulated kinase 1/2 signaling pathway is required for endometrial decidualization in mice and human. PLoS One 2013; 8:e75282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim TH, Yoo JY, Choi KC, Shin JH, Leach RE, Fazleabas AT, Young SL, Lessey BA, Yoon HG, Jeong JW. Loss of HDAC3 results in nonreceptive endometrium and female infertility. Sci Transl Med 2019; 11:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim JJ, Jaffe RC, Fazleabas AT. Comparative studies on the in vitro decidualization process in the baboon (Papio anubis) and human. Biol Reprod 1998; 59:160–168. [DOI] [PubMed] [Google Scholar]
- 42. Benard J, Da Silva J, De Blois MC, Boyer P, Duvillard P, Chiric E, Riou G. Characterization of a human ovarian adenocarcinoma line, IGROV1, in tissue culture and in nude mice. Cancer Res 1985; 45:4970–4979. [PubMed] [Google Scholar]
- 43. Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun 2013; 4:2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murata T, Obiri NI, Debinski W, Puri RK. Structure of IL-13 receptor: Analysis of subunit composition in cancer and immune cells. Biochem Biophys Res Commun 1997; 238:90–94. [DOI] [PubMed] [Google Scholar]
- 45. Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol 2002; 16:2338–2348. [DOI] [PubMed] [Google Scholar]
- 46. Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol 2002; 245:280–290. [DOI] [PubMed] [Google Scholar]
- 47. Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 2011; 331:912–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol 1995; 9:691–705. [DOI] [PubMed] [Google Scholar]
- 49. Rubel CA, Lanz RB, Kommagani R, Franco HL, Lydon JP, DeMayo FJ. Research resource: genome-wide profiling of progesterone receptor binding in the mouse uterus. Mol Endocrinol 2012; 26:1428–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rubel CA, Wu SP, Lin L, Wang T, Lanz RB, Li X, Kommagani R, Franco HL, Camper SA, Tong Q, Jeong JW, Lydon JP et al. A Gata2-dependent transcription network regulates uterine progesterone responsiveness and endometrial function. Cell Rep 2016; 17:1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang X, Li X, Wang T, Wu SP, Jeong JW, Kim TH, Young SL, Lessey BA, Lanz RB, Lydon JP, DeMayo FJ. SOX17 regulates uterine epithelial-stromal cross-talk acting via a distal enhancer upstream of Ihh. Nat Commun 2018; 9:4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, Jeong JW, Spencer TE, Behringer RR, Lydon JP, Demayo FJ. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J 2012; 26:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Care AS, Diener KR, Jasper MJ, Brown HM, Ingman WV, Robertson SA. Macrophages regulate corpus luteum development during embryo implantation in mice. J Clin Invest 2013; 123:3472–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, Jung S. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest 2008; 118:3954–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Donaldson DD, Whitters MJ, Fitz LJ, Neben TY, Finnerty H, Henderson SL, O'Hara RM Jr, Beier DR, Turner KJ, Wood CR, Collins M. The murine IL-13 receptor alpha 2: molecular cloning, characterization, and comparison with murine IL-13 receptor alpha 1. J Immunol 1998; 161:2317–2324. [PubMed] [Google Scholar]
- 56. Wetendorf M, DeMayo FJ. Progesterone receptor signaling in the initiation of pregnancy and preservation of a healthy uterus. Int J Dev Biol 2014; 58:95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tibbetts TA, Mendoza-Meneses M, O'Malley BW, Conneely OM. Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol Reprod 1998; 59:1143–1152. [DOI] [PubMed] [Google Scholar]
- 58. Sharma S, Godbole G, Modi D. Decidual control of trophoblast invasion. Am J Reprod Immunol 2016; 75:341–350. [DOI] [PubMed] [Google Scholar]


