Abstract
Allergic asthma is defined as asthma associated with sensitization to aeroallergens, which leads to asthma symptoms and airway inflammation. Allergic asthma is the most common asthma phenotype. The onset of allergic asthma is most often in childhood and is usually accompanied by other comorbidities including atopic dermatitis and allergic rhinitis. It is often persistent although there is a wide variation in disease severity. It is a TH2-driven process. Biomarkers have been identified to distinguish patients with allergic asthma, particularly serum IgE levels, tests to indicate sensitization to aeroallergens such as specific IgE or skin prick test positivity, blood and sputum eosinophil levels, fraction of exhaled nitric oxide, and periostin. Treatments for allergic asthma include environmental control measures, allergen immunotherapy, and glucocorticoids. Biologics, targeting the TH2 pathway, have been shown to be effective in the treatment of allergic asthma.
Keywords: Asthma, Allergic asthma, Allergic phenotype, Allergic endotypes, Biomarkers
DEFINING ALLERGIC ASTHMA
Allergic asthma is usually defined as asthma associated with sensitization to aeroallergens. Sensitization to aeroallergens significantly contributes to asthma symptoms and airway inflammation.1 Inhalation of allergen leads to acute bronchoconstriction, followed by inflammatory cell influx, which triggers a late asthmatic response.2 Allergic asthma is the most common asthma phenotype.3 It is estimated that up to 80% of childhood asthma and more than 50% of adult asthma cases may have an allergic component.4 The Third National Health and Nutrition Examination Survey estimated that 56% of asthma cases in the United States were associated with atopy.5 Allergic asthma is a TH2-driven process.2 The main cytokines involved include IL-4, IL-5, and IL-13. IL-4 and IL-13 are critical for IgE class switching.6 In a population-based cohort of young adults, cytokine profiles of participants with an allergic asthma phenotype were compared with those with a nonallergic asthma phenotype. Patients with allergic asthma had elevated IL-5 secretion as well as a TH2-skewed cytokine response with a lower IFN-γ to TH2 cytokine ratio.7 Other important inflammatory cells in the TH2 pathway include mast cells, CD4+ T cells, and type 2 innate lymphoid cells.8
CLINICAL CHARACTERISTICS OF ALLERGIC ASTHMA
Allergic asthma is often accompanied by a history of eczema2 and allergic rhinitis.6 It is more common in males than in females.9 Allergic asthma typically starts in childhood.10,11 In a cross-sectional analysis of subjects with severe asthma, those with early onset asthma, before age 12 years, had significantly more allergen sensitization and allergic symptoms compared with those with late-onset asthma: 98% versus 76%.10 In this study, 40% of those with early onset asthma had eczema or a history of eczema.10 In a study of adolescents and adults with and without asthma, history of allergic rhinitis and seasonal exacerbations of asthma were more likely in allergic asthma, although the prevalence of rhinitis symptoms was the same in both groups.9 Patients with allergic asthma are more likely to have a family history of asthma.12
Allergic asthma is not common in the first year of life given that sensitization to aeroallergens usually does not occur before age 2 years. However, the prevalence of allergic asthma increases throughout childhood and adolescence.1 Sensitization to allergens is thought to have a significant role in the development of asthma. The Asthma Predictive Index is used to determine the likelihood that a child with a history of wheezing will develop asthma. The Asthma Predictive Index is considered positive if a child has more than 3 episodes of wheezing in the first 3 years of life and at least 1 major or 2 minor criteria. The major criteria are physician-diagnosed atopic dermatitis, parental history of asthma, and sensitization to an aeroallergen. The minor criteria are peripheral eosinophils more than 4%, wheezing unrelated to the common cold, and sensitization to a food allergen.13 Holt et al14 hypothesized that host response to viral antigens and aeroallergens is responsible for airway inflammation early in life. A low level of TH1 immunity and a skewed TH2 response lead to airway inflammation and subsequent damage to airway mucosal tissues. If a patient is exposed to both infection and sensitization to allergens, there is a high risk for persistent asthma.14 This is supported by a prospective cohort study of 2601 children, followed from birth, which found that lower respiratory tract illness with wheezing in the first year of life and atopy were independently associated with an increased risk for asthma at the age of 6 years. This risk was increased even more in children with both respiratory infections in the first year of life and atopy.15 A study of subjects at risk for atopic disorders demonstrated a strong association between early sensitization, wheezing past age 5 years, and bronchial hyperresponsiveness.16
Allergic asthma is often persistent2 and typically continues into adulthood.14 The German Multicentre Allergy Study followed 1314 children from birth to 13 years and found that children with early onset wheeze who were also sensitized to perennial allergens were more likely to develop persistent asthma.17 There is a large variation in disease severity.2 In the Epidemiological Study on the Genetics and Environment of Asthma (EGEA), atopic children were found to have significantly milder asthma compared with nonatopic children.18 However, a study by Fitzpatrick et al19 that followed school-age children with asthma longitudinally over 6 months found that children with severe asthma had more allergic sensitization than those with mild to moderate asthma. In adults, a cross-sectional observation study comparing subjects with severe asthma to subjects with asthma controlled by low-dose inhaled corticosteroids (ICSs) found that subjects in the severe asthma group had less sensitization to allergens.20
Clustering analyses have been done in adults and children to determine distinct asthma groups, each identifying at least 1 cluster with allergic asthma.11,21-24 (Table I). One study performed latent class analysis in adults from EGEA and the European Community Respiratory Health Survey. In each sample, there was an active treated, allergic, childhood-onset asthma cluster.22 Hierarchical cluster analysis using subjects from the Severe Asthma Research Program cohort identified 5 clusters, 3 of which had atopic asthma. Most of these subjects had early onset asthma. The 3 clusters with atopic asthma varied with regard to asthma severity, baseline lung function, response to bronchodilators, medication requirements, asthma symptoms, and health care utilization.11 A second Severe Asthma Research Program analysis performed on subjects with bronchoalveolar lavage, blood, and fractional exhaled nitric oxide (Feno) data identified an early onset allergic asthma cluster. Patients in this cluster had low lung function and eosinophilic inflammation.26 A k-means cluster analysis was performed in a population with mild to moderate asthma managed by primary care and a population with refractory asthma managed in secondary care. Clusters of early onset atopic asthma were identified in both populations. There was 1 atopic cluster in the primary care group and 2 atopic clusters in the secondary care cohort. Those with atopic asthma in the primary care group had a greater number of hospitalizations and asthma exacerbations when compared with other clusters within the group.21 Those with atopic asthma within the secondary care population had greater airway dysfunction and more asthma symptoms and eosinophilic airway inflammation on higher doses of corticosteroids when compared with the atopic asthma group with more mild disease. Overall, the difference between the groups was in the severity of disease.21 Another study performed latent class analysis in 3001 adults with a history of asthma from 3 large epidemiological studies (EGEA, European Community Respiratory Health Survey, and the Swiss Cohort Study on Air Pollution and Lung and Heart Diseases in Adults). Four classes were identified, 2 with allergic asthma.25 Latent transition analysis was applied to longitudinal data from 3320 adults with asthma from the same 3 studies. Seven groups were identified, 4 of which were identified as “allergic.” The clusters were observed at 2 time points, 10 years apart. Allergic status remained stable over time. Subjects with an allergic phenotype were more likely to demonstrate improvement over time compared with those with a nonallergic phenotype.24
TABLE I.
Clustering analyses
| Study | Analysis | Ages of subjects |
Study population | Clusters identified | Distinguishing features of atopic clusters |
|---|---|---|---|---|---|
| Moore et al11 | Hierarchical cluster analysis | ≥12 y | Severe Asthma Research Program—subjects with persistent asthma of all severity levels | Cluster 1: Younger, F > M, childhood onset, atopic, normal lung function Cluster 2: F > M, childhood onset, atopic, relatively normal lung function, more medication use Cluster 3: F > M, older, highest BMI, late-onset asthma, less atopy, decreased lung function, more medicines Cluster 4: F = M, childhood onset, atopy, decreased lung function Cluster 5: F > M, later onset, less atopy, low pre- and postbronchodilator FEV1 |
Clusters 1, 2, and 4 most consistent with allergic phenotype: atopic, highest serum total IgE levels, childhood onset Differences in clusters 1, 2, and 4: baseline lung function, response to bronchodilators, medication requirements, asthma symptoms, and health care utilization |
| Haldar et al21 | k-means cluster analysis | Adults | Subjects from 2 populations: Mild to moderate disease managed in primary care and refractory asthma managed in secondary care |
Mild to moderate disease: Cluster 1: Early onset, atopic, eosinophilic airway inflammation Cluster 2: Obese, F > M, noneosinophilic Cluster 3: “Benign asthma,” middle-aged, well-controlled symptoms and inflammation Refractory disease: Cluster 1: Early onset, atopic Cluster 2: Obese, noneosinophilic Cluster 3: Early onset, atopic, symptom predominant, minimal eosinophilic disease Cluster 4: Late-onset, M > F, eosinophilic inflammation, few symptoms |
Cluster 1 from Mild to moderate disease and clusters 1 and 3 from refractory disease with characteristics consistent with allergic phenotype: atopic, Early onset Cluster 1 from both groups with eosinophilic inflammation (elevated sputum eosinophil count and Feno) but cluster 3 with minimal eosinophilic disease |
| Siroux et al22 | Latent class analysis | Adults | ECRHSII and EGEA2 | ECRHSII: Phenotype A: Atopic, childhood-onset, active disease Phenotype B: Older, adult-onset, F > M, active disease Phenotype C: “Inactive/mild untreated allergic asthma” Phenotype D: “Inactive/Mild untreated nonallergic asthma” EGEA2: Phenotype E: Atopic, childhood-onset, active disease Phenotype F: Older, adult-onset, active disease Phenotype G: “Inactive/Mild untreated allergic, childhood-onset asthma” Phenotype H: “Inactive/Mild untreated adult-onset asthma” |
Phenotypes A, C, E, and G consistent with allergic asthma phenotype: atopy, earlier onset, greater % with IgE ≥ 100 IU/mL Phenotypes A and E with active disease Phenotype E with highest eosinophil count |
| Fitzpatrick et al23 | Cluster analysis | Children aged 6-17 y | Severe Asthma Research Program—Mild to moderate asthma and severe asthma |
Cluster 1: Late-onset, symptomatic, normal lung function, less atopy Cluster 2: Early onset, atopic, normal lung function Cluster 3: Early onset, atopic, Mild airflow limitation Cluster 4: Early onset, atopic asthma, advanced airflow limitation |
Clusters 2, 3, and 4 with characteristics consistent with allergic asthma: atopy, Early onset, higher serum IgE |
| Boudier et al24 | Latent transition analysis | Adults | Subjects from 3 studies: ECRHS, SAPALDIA, and EGEA |
Phenotype A: “Allergic, few symptoms, no treatment” Phenotype B: “Nonallergic, few symptoms, no treatment” Phenotype C: “Nonallergic, high symptoms, treatment” Phenotype D: “Allergic, high symptoms, treatment, BHR” Phenotype E: “Allergic, moderate symptoms, BHR” Phenotype F: “Allergic, moderate symptoms, normal lung function” Phenotype G: “Nonallergic, moderate symptoms, no treatment” |
Phenotypes A, D, E, and F with characteristics consistent with allergic phenotype: atopy, greater % with total IgE ≥ 100 IU/mL A, D, E, and F differed on the basis of symptom frequency and lung function |
| Siroux et al25 | Latent class analysis | Adults | Subjects from 3 studies: ECRHS, SAPALDIA, EGEA |
Phenotype A: “Inactive/mild nonallergic asthma” Phenotype B: “Inactive/mild allergic asthma” Phenotype C: “Active allergic asthma” Phenotype D: “Active adult-onset, nonallergic asthma” |
Phenotypes B and C with characteristics consistent with allergic phenotype: atopy, childhood-onset, higher likelihood of IgE ≥100 IU/mL |
| Wu et al26 | Unsupervised clustering methods | Adults | Severe Asthma Research Program |
Cluster 1: Normal controls Cluster 2: Mild asthma, early onset Cluster 3: F > M, frequent symptoms, allergic sensitization Cluster 4: F > M, high BMI, frequent symptoms, early onset Cluster 5: Older, later onset, poor lung function, nasal polyps and sinusitis Cluster 6: Early onset, frequent symptoms, poorest lung function |
Cluster 4 identified as early onset, allergic cluster; however, clusters 2, 4, and 6 had early onset asthma and most clusters had multiple allergen skin reactions and symptoms caused by animal exposure Cluster 6 had the highest Feno; Clusters 2, 4, and 5 had higher blood eosinophil numbers than cluster 1 |
| Howrylak et al27 | Spectral clustering | Children | Mild to moderate aged 5-12 y persistent asthma |
Cluster 1: “Mild asthma with low atopy, obstruction, and exacerbation rate” Cluster 2: “Atopic asthma with low levels of obstruction and medium rates of exacerbation” Cluster 3: “Atopic asthma with high levels of obstruction and medium rates of exacerbation” Cluster 4: “Moderately atopic asthma with high levels of obstruction and high exacerbation rates” Cluster 5: “Highly atopic asthma with high levels of obstruction and high exacerbation rates” |
Clusters 2, 3, 4, and 5 with atopy, differing in regard to level of obstruction and exacerbation rates Clusters 2 and 5 universally report AD Clusters 2 and 3 with high prevalence of allergic rhinitis and skin test reactivity Cluster 5 with highest skin test reactivity prevalence and highest IgE levels, intermediate prevalence of allergic rhinitis Cluster 4 with no history of AD, intermediate prevalence of allergic rhinitis, and lower IgE levels |
AD, Atopic dermatitis; BHR, bronchial hyperresponsiveness; BMI, body mass index; ECRHS, European Community Respiratory Health Survey; F, female; M, male; SAPALDIA, Swiss Cohort Study on Air Pollution and Lung and Heart Diseases in Adults; SC, subcutaneous.
A study by Fitzpatrick et al23 performed cluster analysis aimed at identifying phenotypes in school-age children with severe asthma. Three of the 4 clusters identified had early onset atopic asthma. The clusters varied on the basis of degree of airflow limitation.23 Spectral clustering was performed in 1041 children with mild to moderate persistent asthma, and 5 patient clusters were identified. Of these 5 clusters, 4 had atopic asthma, differing on the basis of levels of obstruction and exacerbation rates. There was consistency in phenotypic distributions over time.27
HISTOLOGY IN ALLERGIC ASTHMA
The airways of patients with allergic asthma have increased reticular basement membrane thickness6,28 as well as smooth muscle hypertrophy and epithelial damage.6 There is mucosal infiltration by eosinophils, mast cells, and CD4+ T cells as well as expression of high-affinity IgE receptors on inflammatory and resident cells. One study found increased expression of the gelforming mucins, MUC5AC and MUC2, and repression of MUC5B expression in airway epithelial cells in TH2-high asthma when compared with TH2-low asthma and healthy controls.28
LUNG FUNCTION IN ALLERGIC ASTHMA
The association between allergic asthma and lung function is unclear. Some studies have found that patients with allergic asthma have better lung function than do those with nonallergic asthma,29 whereas others have suggested that patients with allergic asthma have decreased lung function.17,30 A cross-sectional analysis using survey data from children and adolescents suggested that asthma, sensitization to house-dust mite, and increasing severity of allergic rhinitis were associated with a lower FEV1.31 At the same time, other studies demonstrated that FEV1 was higher in patients with allergic asthma than in those with nonallergic asthma.9,10
BIOMARKERS IN ALLERGIC ASTHMA
Multiple biomarkers have been established to identify patients with allergic asthma including total IgE and tests for atopy such as specific IgE and skin prick tests for allergens. Allergic asthma often presents with TH2-high biomarkers including elevated blood and sputum eosinophil counts and Feno, as well as periostin in adults.32 A study classified subjects with asthma into TH2-high or TH2-low asthma, on the basis of high or low expression of IL-13—inducible genes, respectively. Patients with TH2-high asthma had increased allergen skin prick test reactivity, higher serum IgE levels, and higher peripheral blood and bronchoalveolar lavage eosinophil counts relative to those with TH2-low asthma.28
An association between allergic asthma and elevated total IgE has been shown. In the EGEA study, total IgE was positively associated with history of hospitalization for asthma and ICS treatment in the past 12 months; however, it was not associated with clinical severity score.18 On the other hand, another study found that children with severe asthma had significantly higher serum IgE compared with subjects with mild to moderate asthma.19
Specific IgE antibodies to environmental allergens suggest sensitization and are associated with allergic asthma because these allergens are often asthma triggers.33 It is important to note that sensitization alone may not be clinically relevant because the interpretation of skin prick tests and specific IgE to whole allergen extracts relies on arbitrary cutoffs, which do not distinguish between pathologic and benign sensitizations.34 Pathologic sensitization is sensitization associated with clinical symptoms, whereas benign sensitization is sensitization without symptoms. Component-resolved diagnostics measure levels of specific IgE to a large number of allergenic molecules and may help identify clinically relevant sensitization.35 Latent variable modeling with a number of allergen components demonstrated 3 patterns of IgE responses. One group with sensitization to 27 components of plant, animal, and fungal origin had a strong association with asthma.36 Another study examined the relationship between IgE responses to multiple allergen components and their associations with asthma. This study found that the pattern of interaction between component-specific IgEs was the major predictor of asthma.37 It is still to be determined how this can be integrated into clinical use. Allergen-specific IgG/IgE antibody ratios may help distinguish benign and pathologic sensitization, although not currently used in clinical practice.38 Among sensitized children from 2 birth cohorts in the United Kingdom and Australia, IgG/IgE antibody ratios for dust mite were lower among children with asthma than among asymptomatic children. This suggests that allergen-specific IgG antibodies may protect against expression of symptoms.39
Feno is generated by inducible nitric oxide synthase, which is induced by IL-13.40 It has been used for the assessment of eosinophilic airway inflammation in asthma41 and has the advantage of being noninvasive and easily measured.42 Per the American Thoracic Society guidelines, a Feno greater than 50 parts per billion in adults or 35 parts per billion in children is an indicator of eosinophilic inflammation and likely response to corticosteroids.42 Online Feno levels were measured in 495 individuals enrolled in the Severe Asthma Research Program cohort. Patients with high Feno were more likely to have early onset asthma, more positive skin test results, and higher serum IgE and blood and sputum eosinophils.41 Elevated Feno levels must be considered cautiously because they can be elevated in relation to allergic rhinitis, eosinophilic bronchitis, and diet, as well as allergen or viral exposure.42 A single-blind, placebo-controlled trial of inhaled fluticasone in patients with undiagnosed respiratory symptoms found greater steroid response in subjects with higher Feno.43 A systematic review investigating the utility of a Feno-based strategy to guide asthma management found a reduction in asthma exacerbation in both children and adults; however, the benefit was inconsistent and did not extend to other asthma outcomes such as asthma symptoms or rescue medication use.44
Sputum eosinophils can be a useful biomarker of allergic asthma.45 A systematic review found that using sputum eosinophilia to guide asthma management resulted in reduced exacerbations in adults but not a significant difference in children when compared with guideline-based management.44 Sputum in children with atopic and nonatopic asthma and healthy controls was compared. In sputum, median eosinophil counts as well as concentrations of IL-4, IL-5, IFN-γ, IL-2, and IL-12p70 were higher in children with atopic asthma than in those with nonatopic asthma or healthy controls.46 However, there is limitation in using sputum eosinophils in clinical practice due to technical difficulties in obtaining sputum eosinophils.47 A systematic review investigating the accuracy of Feno, IgE, and blood eosinophils as single markers for airway eosinophilia in patients with asthma found that these markers had only moderate sensitivities and specificities.48
Eosinophil peroxidase (EPX) has been suggested as a proxy for lower airway eosinophilia. EPX is a protein secreted by eosinophils that can be detected by ELISA. One study demonstrated correlation between sputum eosinophil percentage and nasal and pharyngeal EPX levels. Using EPX levels is minimally invasive and easier to obtain than sputum eosinophils49; however, clinical use is limited given that diagnostic cutoff levels have not been established.50
Serum periostin is a ligand for integrin receptors and is produced by airway epithelial cells and lung fibroblasts in response to IL-13 and IL-4.51 It has been considered as a biomarker of TH2-high asthma because of its association with increased airway hyperresponsiveness, elevated total IgE, and eosinophilic inflammation.51 In an observational study of subjects with uncontrolled severe asthma on high-dose ICS, serum periostin was shown to be a biomarker of persistent airway eosinophilia.52 However, another study in adults with poorly controlled asthma demonstrated that although periostin levels were significantly associated with airway eosinophilia and were higher in patients with eosinophilic asthma compared with noneosinophilic asthma, periostin could not predict the presence of an eosinophilic inflammatory subtype.47
Studies have attempted to identify nasal biomarkers that are representative of the lower airways. One study found moderate correlations between IL-24 levels in the upper and lower airways. Nasal IL-24 levels showed a strong negative correlation to the Global Initiative for Asthma score, whereas sputum IL-24 levels were increased in patients with asthma in season.53 Another study, in children, found increased expression of vascular endothelial growth factor, TGF-β2, and periostin in airway epithelial cells of atopic children with asthma compared with atopic children without asthma and healthy controls. There was good correlation between bronchial and nasal epithelial expression of these factors.54
Biomarkers that have been associated with the allergic phenotype of asthma include elevated IgE levels, elevated sputum eosinophils, periostin, and Feno. These biomarkers are reflective of the pathophysiologic mechanisms underlying allergic asthma. Investigation for novel biomarkers is currently ongoing.
GENETICS OF ALLERGIC ASTHMA
Interactions between genes and environmental factors influence the development of allergic asthma.14 Variants of the Filaggrin, ORMDL3, and thymic stromal lymphopoietin (TSLP) promoter genes as well as the chromosome 17q12-21 locus have been associated with allergic asthma.6,55 Single nucleotide polymorphisms (SNPs) have been associated with allergic asthma. In a latent class analysis that identified 2 phenotypes with allergic asthma, inactive and active, specific SNPs were associated with each phenotype. SNPs in IL1RL1, IL18R1, DPP10, TSLP, TAD50-IL13, HLA-DQ, IL33, RORA, ORMDL3/GSDMB, and IL12RB were associated with active allergic asthma, whereas SNPs in IL1RL1, HLA-DQ, IL33, and SMAD3 were significantly associated with inactive allergic asthma.25 In pooled genome-wide association studies in pediatric allergic asthma, SNPs have been identified in the coding sequence of C5 as well as loci regulating mediation of immune or inflammatory mechanisms and airway smooth muscle contraction.6 Polymorphisms in the coding region of the IL-4 receptor alpha chain have been associated with susceptibility to atopic asthma.56 DNA methylation changes have been identified in the nasal epithelia of inner-city children with allergic asthma.57
TREATMENT OF ALLERGIC ASTHMA
There are multiple treatment considerations for allergic asthma, including environmental control measures, immunotherapy, glucocorticoids, and biologics. Environmental control measures can have a significant impact in allergic asthma. A study of 937 children with atopic asthma in 7 cities in the United States demonstrated that an intervention to decrease cockroach and dust mite allergens resulted in decreased asthma symptom days.58 A randomized controlled trial (RCT) found that the use of dust mite—impermeable bedding effectively decreased the number of mite-sensitized children with asthma attending the hospital with asthma exacerbations.59 However, it is often difficult to achieve significant remediation leading to clinical improvements in a nonclinical study environment.
Allergen immunotherapy (AIT) has been shown to be effective in the treatment of allergic asthma. Multiple guidelines recommend consideration of AIT for patients with allergic asthma.60-62 Although AIT can be used in patients with well-controlled asthma, it is not routinely used in severe asthma given that uncontrolled asthma is the major risk factor for severe AIT-related adverse events.63 Use of omalizumab in combination with AIT can increase the effectiveness and safety of subcutaneous immunotherapy (SCIT).64,65 Studies have demonstrated significant improvement in asthma symptoms in children with allergic asthma after AIT.64 AIT has the potential to decrease the risk of progression from allergic rhinitis to asthma and prevent new allergen sensitizations.66 AIT can be administered either subcutaneously (SCIT) or sublingually (sublingual immunotherapy [SLIT]).67 Patients can receive single or multiallergen SCIT; however, there are only 4 Food and Drug Administration (FDA)-approved SLIT products (1 for ragweed, 1 for dust mite, and 2 for grass). SCIT has been shown to reduce asthma symptoms, airway hyperresponsiveness, and medication requirements68-70 although its effect on lung function is unclear.71 SLIT is recommended as add-on therapy for asthma in adults and adolescents with allergic rhinitis, asthma exacerbations despite ICS with FEV1 greater than or equal to 70% predicted, and sensitizations to house-dust mite.61 Both a systematic review and a meta-analysis concluded that SLIT decreased asthma symptoms and medication use.70,72 Although both SCIT and SLIT have been shown to improve asthma symptoms, SLIT may have a more favorable safety profile.73 AIT may also help prevent asthma in patients at risk for allergic asthma. The Preventive Allergy Treatment Study was a 10-year follow-up study of SCIT in children with seasonal rhinoconjunctivitis that demonstrated that treatment with AIT had a preventive effect on the development of asthma.74 Similarly, the 5-year grass SLIT asthma prevention trial in children with grass pollen allergy found that treatment with grass SLIT decreased the risk of asthma symptoms or using asthma medication during the 5-year trial period, although there was no effect on the time to asthma onset.75
Patients with allergic asthma are typically responsive to corticosteroids. A multicenter, randomized, double-blind, double-dummy clinical trial in children with asthma found that subjects with best response to daily ICS were those with aeroallergen sensitization and blood eosinophilia.76 Another study comparing ICS to montelukast in children with asthma found that children with Feno greater than 25 ppb, blood eosinophil count greater than 350 cells/mm3 and serum IgE greater than 200 kU/L responded better to ICSs.77 Both of these studies support the use of blood eosinophilia and allergic sensitization in predicting response to ICS in children. In a study in adults, subjects with TH2-high asthma treated with inhaled fluticasone had significant improvements in FEV1 at 4 and 8 weeks compared with subjects treated with a placebo whereas those with TH2-low asthma did not.28
In patients with poorly controlled allergic or eosinophilic asthma on high-dose ICS-long-acting β-agonists or maintenance oral corticosteroids, treatment with a biologic should be considered.61 Multiple biologics targeting the TH2 pathway underlying allergic asthma are currently approved or in clinical trials. These targets include IgE, IL-5, IL-4 receptor alpha (receptor for both IL-4 and IL-13), IL-13, and TSLP.78 There are 5 FDA-approved biologics for the treatment of asthma: omalizumab, mepolizumab, reslizumab, benralizumab, and dupilumab (Table II).
TABLE II.
Biologics approved for treatment of asthma
| Medication | Mechanism of action | Indications | Mode of administration | Biomarkers predicting response to therapy |
Biomarkers modulated by therapy |
|---|---|---|---|---|---|
| Omalizumab | mAb to IgE: Recognizes IgE at FcεRI binding site leading to decreased free IgE, decreased expression of FcεRI, and decreased mediator release | Moderate to severe persistent asthma in patients ≥6 y with evidence of sensitization to a perennial aeroallergen and symptoms not controlled with ICS | 75-375 mg SC every 2 or 4 wk. Dose and dosing frequency determined by serum total IgE and body weight | Feno (≥19.5 ppb), blood eosinophils (≥260 cells/μL), periostin (≥50 ng/mL) associated with greater effects in reductions in exacerbations79 Feno (≥25 ppb) and blood eosinophils (≥300 cells/μL) associated with ACT scores and lung function improvement but not clinically significant80 |
IgE Feno81 |
| Dupilumab | mAb to IL-4 receptor alpha | Moderate to severe asthma in patients ≥12 y with an eosinophilic phenotype or oral corticosteroid–dependent asthma | Administered subcutaneously. Initial dose of 400 mg followed by 200 mg every other week. For patients requiring oral corticosteroids or with moderate to severe atopic dermatitis, initial dose of 600 mg followed by 300 mg every other week | For exacerbations: Feno and blood eosinophils For FEV1: Feno, blood eosinophils, periostin, total IgE82 |
Feno IgE83 |
| Mepolizumab | mAb against IL-5 | Add-on maintenance treatment for patients with severe asthma ≥6 y with an eosinophilic phenotype | Patients ≥ 12 years: 100 mg SC every 4 weeks; 6-11 years: 40 mg SC every 4 weeks | Blood eosinophils ≥150 cells/μL84 | Blood eosinophils Sputum eosinophils85 |
| Reslizumab | mAb against IL-5 | Add-on maintenance treatment for patients with severe asthma ≥18 y with an eosinophilic phenotype | 3 mg/kg IV every 4 wk | Blood eosinophils ≥400 cells/μL86-88 | Blood eosinophils |
| Benralizumab | mAb against IL-5 receptor alpha | Add-on maintenance treatment for patients with severe asthma ≥12 y, with an eosinophilic phenotype | 30 mg SC every 4 weeks for the first 3 doses, followed by once every 8 weeks thereafter | Blood eosinophils ≥300 cells/μL89-91 | Blood eosinophils |
ACT, Asthma Control Test; mAb, monoclonal antibody; SC, subcutaneous.
Omalizumab is a recombinant humanized anti-IgE monoclonal antibody (mAb) that binds IgE at the FcεRI binding site, the same site as the high-affinity IgE receptor. Treatment with omalizumab reduces levels of free IgE and downregulates the expression of FcεRI receptors on mast cells, basophils, and dendritic cells. Decreased expression of FcεRI receptors on mast cells causes reduced mast cell activation and sensitivity, leading to a reduction in the influx and activation of eosinophils.92 Reduction of FcεRI receptors on dendritic cells restores anti-viral activity important in mitigating viral-induced asthma exacerbations. Omalizumab is FDA approved for patients 6 years or older with uncontrolled moderate to severe persistent allergic asthma despite high-dose ICS. It has been shown to be effective in decreasing asthma symptoms and exacerbations in a number of double-blind RCTs.93,94 Omalizumab has been shown to have an ICS-sparing effect when compared with placebo in RCTs in children and adolescents with moderate to severe allergic asthma95-97; however, the same benefit has not been shown with oral corticosteroids.98 A Cochrane review concluded that omalizumab treatment resulted in fewer asthma exacerbations, a reduction in ICS and rescue medication use, and an improvement in asthma symptom scores and quality of life.98 Omalizumab has only a modest effect on lung functions. Data from RCTs have shown inconsistent effects of omalizumab on lung function93,96,99 in patients with allergic asthma. However, 2 recent studies, 1 prospective and 1 retrospective, demonstrated a clinically meaningful improvement in lung function in adolescents treated with omalizumab.80,100
Dupilumab is a human mAb to the IL-4 receptor alpha that inhibits IL-4 and IL-13 signaling.83 It is FDA approved for the treatment of moderate to severe asthma in patients 12 years or older. In RCTs, patients treated with dupilumab had decreased rates of asthma exacerbations as well as a significant increase in FEV1 compared with placebo.101,102
Mepolizumab is a humanized mAb against IL-5, a cytokine involved in eosinophilic inflammation.103 It should therefore be considered in patients with peripheral eosinophilia. Mepolizumab is approved for patients 6 years or older with eosinophilic asthma. Mepolizumab has been shown to improve asthma control and decrease asthma exacerbations, particularly in patients with blood eosinophil counts greater than or equal to 150 cells/μL. DREAM (Dose Ranging Efficacy and Safety with Mepolizumab in severe asthma) and MENSA (Mepolizumab as Adjunctive Therapy in Patients with Severe Asthma) were both RCTs that demonstrated that mepolizumab significantly reduces the rate of asthma exacerbations when compared with placebo.103,104
Benralizumab is an anti—IL-5 receptor alpha mAb that leads to antibody-dependent cell-mediated cytotoxicity of cells bearing this receptor including eosinophils and basophils, ultimately causing depletion of eosinophils.89 It is approved for patients 12 years or older with severe eosinophilic asthma. In a double-blind RCT, treatment with benralizumab decreased asthma exacerbations in adults with uncontrolled asthma and eosinophil counts of greater than or equal to 300 cells/μL.90 Two other RCTs, in patients aged 12 to 75 years, demonstrated that treatment with benralizumab significantly lowered annual exacerbation rates and improved prebronchodilator FEV1 and asthma symptom scores.89,91
Dupilumab, mepolizumab, and benralizumab have been shown to have oral corticosteroid—sparing effects.
Reslizumab is a humanized mAb against IL-5. It is approved for adults with severe eosinophilic asthma. It is administered intravenously every 4 weeks. In RCTs, it has been shown to lead to improvements in FEV1, asthma exacerbations, rescue inhaler use, asthma control, asthma symptoms, and quality of life in patients with poorly controlled asthma and blood eosinophilia.86,87 However, reslizumab has not been shown to be as effective in patients with eosinophil counts of less than 400 cells/μL,88 nor has it been effective when dosed subcutaneously.
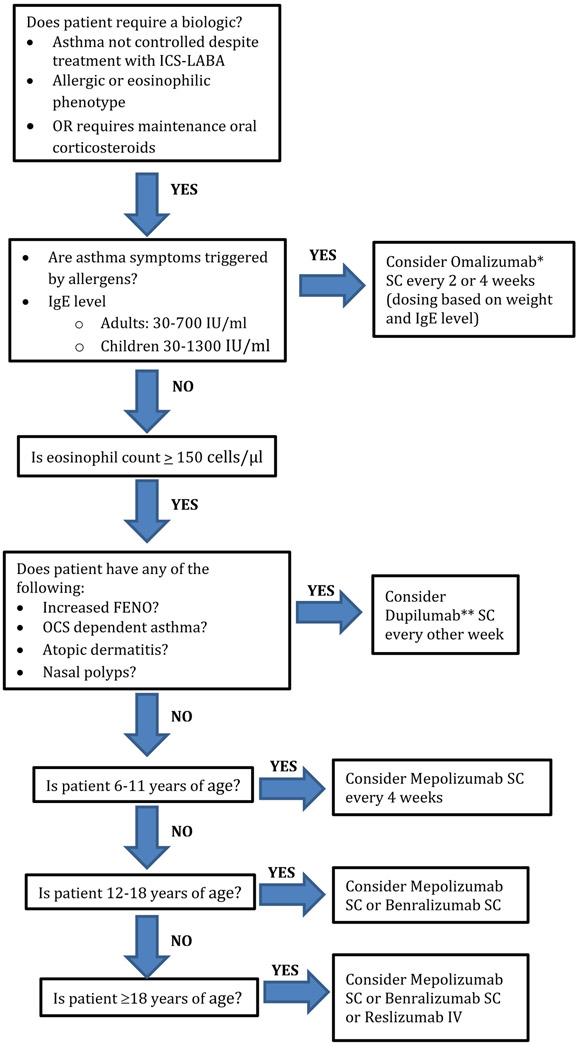
Selecting the most appropriate biologic is based on a number of factors including blood eosinophil count, evidence of allergic sensitization, age, and comorbid conditions (Figure 1).61 In patients with the classic allergic phenotype, including symptoms triggered by allergens, evidence of sensitization based on skin prick testing or specific IgE, or onset in childhood, omalizumab is likely the most appropriate biologic.61 Omalizumab and now mepolizumab are the only biologics approved for children aged 6 to 12 years. Omalizumab is approved for patients aged 6 to less than 12 years with IgE levels 30 to 1300 IU/mL and for patients 12 years or older with total IgE level 30 to 700 IU/mL105; however, benefit has been shown even with levels outside of these parameters. In patients with eosinophilic asthma as well as evidence of TH2-high asthma such as elevated Feno, dupilumab may be the most appropriate biologic to select. Dupilumab is FDA approved for the treatment of patients with oral corticosteroid dependence. It is FDA approved for the treatment of atopic dermatitis and nasal polyps and so it may be most appropriate for patients with asthma and these comorbid conditions.106 Per the most recent Global Initiative for Asthma guidelines, patients with eosinophilic asthma and more exacerbations in the previous year may have the best response to an anti—IL-5 or anti—IL-5 receptor alpha such as mepolizumab, reslizumab, or benralizumab.61 In this case, patient preference for mode of administration may play a role because mepolizumab and benralizumab are both administered subcutaneously whereas reslizumab is administered intravenously. Mepolizumab, as mentioned above, is approved for patients 6 years or older, benralizumab for patients 12 years or older, and reslizumab for patients 18 years or older.
Figure 1.
Choosing a biologic. IV, Intravenous; LABA, long-acting β-agonist; OCS, oral corticosteroid; SC, subcutaneous. *Omalizumab is approved for patients ≥ 6 years of age. **Dupilumab is approved for patients ≥ 12 years of age with asthma and ≥ 6 years with atopic dermatitis.
New potential targets for allergic asthma are being investigated. GATA3 is a transcription factor of the TH2 pathway, and SB010 is an inhaled DNAzyme that inactivates expression of GATA3. In a double-blind RCT, SB010 decreased early and late responses to allergen challenge compared with placebo in patients with allergic asthma and sputum eosinophilia.107 Other therapies currently under investigation include anti—IL-33 antibodies,108 CRTH2 antagonists,109 anti-TSLP mAbs,108 and chemokine receptor 4 antagonists.110,111
CONCLUSIONS
Effective treatments for allergic asthma exist, including those targeting the allergic trigger such as environmental control measures or immunotherapy, and those targeting the underlying TH2 pathway such as the mAbs.
Despite overlap with other asthma phenotypes, allergic asthma can be classified as a distinct phenotype. It is a TH2-driven process that can be distinguished on the basis of its clinical presentation with asthma symptoms following exposure to allergic triggers as well as specific biomarkers. Multiple therapeutic options for allergic asthma exist and understanding of the underlying mechanisms in allergic asthma can help guide effective treatment.
Acknowledgments
Funding was provided by the National Institutes of Health (grant nos. K24 AI 106822, U01 AI110397, U01 AI126614, and T32 AI007512) and Allergy and Asthma Awareness Initiative Inc.
Abbreviations used
- AIT
Allergen immunotherapy
- EGEA
Epidemiological Study on the Genetics and Environment of Asthma
- EPX
eosinophil peroxidase
- FDA
Food and Drug Administration
- Feno
Fractional exhaled nitric oxide
- ICS
Inhaled corticosteroid
- mAb
Monoclonal antibody
- RCT
Randomized controlled trial
- SCIT
Subcutaneous immunotherapy
- SLIT
Sublingual immunotherapy
- SNP
Single nucleotide polymorphism
- TSLP
Thymic stromal lymphopoietin
Footnotes
Conflicts of interest: N. Akar-Ghibril declares no relevant conflicts of interest. T. Casale is a consultant for Genentech, Novartis, Sanofi, Regeneron, and GlaxoSmithKline; is a speaker for Genentech; and has been an investigator on grants to his institution from Genentech, Novartis Sanofi, and Regeneron. A. Custovic reports personal fees from Novartis, Regeneron/Sanofi, Thermo Fisher Scientific, Boehringer Ingelheim, Novartis, and Philips, outside the submitted work. W. Phipatanakul is a consultant/speaker for Regeneron, Sanofi, Teva, GlaxoSmithKline, Genentech, and Novartis; and has received funding or grant support to her institution from the National Institutes of Health, Novartis, Genentech, Regeneron, Sanofi, Thermo Fisher, Monaghen, Lincoln Diagnostics, and Alk Abello.
REFERENCES
- 1.Lemanske RF Jr, Busse WW. Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol 2010;125:S95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol 2011;127:355–60. [DOI] [PubMed] [Google Scholar]
- 3.Schatz M, Rosenwasser L. The allergic asthma phenotype. J Allergy Clin Immunol Pract 2014;2:645–8. [DOI] [PubMed] [Google Scholar]
- 4.Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 2001;56:813–24. [DOI] [PubMed] [Google Scholar]
- 5.Arbes SJ Jr, Gergen PJ, Vaughn B, Zeldin DC. Asthma cases attributable to atopy: results from the Third National Health and Nutrition Examination Survey. J Allergy Clin Immunol 2007;120:1139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agache I, Akdis C, Jutel M, Virchow JC. Untangling asthma phenotypes and endotypes. Allergy 2012;67:835–46. [DOI] [PubMed] [Google Scholar]
- 7.Zoratti E, Havstad S, Wegienka G, Nicholas C, Bobbitt KR, Woodcroft KJ, et al. Differentiating asthma phenotypes in young adults through polyclonal cytokine profiles. Ann Allergy Asthma Immunol 2014;113:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stokes JR, Casale TB. Characterization of asthma endotypes: implications for therapy. Ann Allergy Asthma Immunol 2016;117:121–5. [DOI] [PubMed] [Google Scholar]
- 9.Romanet-Manent S, Charpin D, Magnan A, Lanteaume A, Vervloet D, EGEA Cooperative Group. Allergic vs nonallergic asthma: what makes the difference? Allergy 2002;57:607–13. [DOI] [PubMed] [Google Scholar]
- 10.Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol 2004;113:101–8. [DOI] [PubMed] [Google Scholar]
- 11.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med 2010;181:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet 2006; 368:804–13. [DOI] [PubMed] [Google Scholar]
- 13.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med 2000;162:1403–6. [DOI] [PubMed] [Google Scholar]
- 14.Holt PG, Upham JW, Sly PD. Contemporaneous maturation of immunologic and respiratory functions during early childhood: implications for development of asthma prevention strategies. J Allergy Clin Immunol 2005;116:16–24. [DOI] [PubMed] [Google Scholar]
- 15.Oddy WH, de Klerk NH, Sly PD, Holt PG. The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. Eur Respir J 2002;19:899–905. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes HL, Thomas P, Sporik R, Holgate ST, Cogswell JJ. A birth cohort study of subjects at risk of atopy: twenty-two-year follow-up of wheeze and atopic status. Am J Respir Crit Care Med 2002;165:176–80. [DOI] [PubMed] [Google Scholar]
- 17.Illi S, von Mutius E, Lau S, Niggemann B, Gruber C, Wahn U, et al. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet 2006;368:763–70. [DOI] [PubMed] [Google Scholar]
- 18.Siroux V, Oryszczyn MP, Paty E, Kauffmann F, Pison C, Vervloet D, et al. Relationships of allergic sensitization, total immunoglobulin E and blood eosinophils to asthma severity in children of the EGEA Study. Clin Exp Allergy 2003;33:746–51. [DOI] [PubMed] [Google Scholar]
- 19.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG. National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. Features of severe asthma in school-age children: atopy and increased exhaled nitric oxide. J Allergy Clin Immunol 2006;118:1218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J 2003;22:470–7. [DOI] [PubMed] [Google Scholar]
- 21.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med 2008;178:218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siroux V, Basagana X, Boudier A, Pin I, Garcia-Aymerich J, Vesin A, et al. Identifying adult asthma phenotypes using a clustering approach. Eur Respir J 2011;38:310–7. [DOI] [PubMed] [Google Scholar]
- 23.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol 2011;127: 382–389.e1-389.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boudier A, Curjuric I, Basagana X, Hazgui H, Anto JM, Bousquet J, et al. Ten-year follow-up of cluster-based asthma phenotypes in adults: a pooled analysis of three cohorts. Am J Respir Crit Care Med 2013;188:550–60. [DOI] [PubMed] [Google Scholar]
- 25.Siroux V, Gonzalez JR, Bouzigon E, Curjuric I, Boudier A, Imboden M, et al. Genetic heterogeneity of asthma phenotypes identified by a clustering approach. Eur Respir J 2014;43:439–52. [DOI] [PubMed] [Google Scholar]
- 26.Wu W, Bleecker E, Moore W, Busse WW, Castro M, Chung KF, et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol 2014;133: 1280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howrylak JA, Fuhlbrigge AL, Strunk RC, Zeiger RS, Weiss ST, Raby BA, et al. Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications. J Allergy Clin Immunol 2014;133:1289–300. 1300.e1-1300.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009;180:388–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lampalo M, Jukic I, Bingulac-Popovic J, Safic H, Ferara N, Popovic-Grle S. The role of Pai-1 gene 4g/5g polymorphism and diagnostic value of biomarkers in allergic and non-allergic asthma phenotype. Acta Clin Croat 2018; 57:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995;332:133–8. [DOI] [PubMed] [Google Scholar]
- 31.Ulrik CS, Backer V. Markers of impaired growth of pulmonary function in children and adolescents. Am J Respir Crit Care Med 1999;160:40–4. [DOI] [PubMed] [Google Scholar]
- 32.Casale TB. Biologics and biomarkers for asthma, urticaria, and nasal polyposis. J Allergy Clin Immunol 2017;139:1411–21. [DOI] [PubMed] [Google Scholar]
- 33.Rath N, Raje N, Rosenwasser L. Immunoglobulin E as a biomarker in asthma. Immunol Allergy Clin North Am 2018;38:587–97. [DOI] [PubMed] [Google Scholar]
- 34.Custovic A, Lazic N, Simpson A. Pediatric asthma and development of atopy. Curr Opin Allergy Clin Immunol 2013;13:173–80. [DOI] [PubMed] [Google Scholar]
- 35.Roberts G, Ollert M, Aalberse R, Austin M, Custovic A, DunnGalvin A, et al. A new framework for the interpretation of IgE sensitization tests. Allergy 2016;71:1540–51. [DOI] [PubMed] [Google Scholar]
- 36.Simpson A, Lazic N, Belgrave DC, Johnson P, Bishop C, Mills C, et al. Patterns of IgE responses to multiple allergen components and clinical symptoms at age 11 years. J Allergy Clin Immunol 2015;136:1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fontanella S, Frainay C, Murray CS, Simpson A, Custovic A. Machine learning to identify pairwise interactions between specific IgE antibodies and their association with asthma: q cross-sectional analysis within a population-based birth cohort. PLoS Med 2018;15:e1002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonntag HJ, Filippi S, Pipis S, Custovic A. Blood biomarkers of sensitization and asthma. Front Pediatr 2019;7:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holt PG, Strickland D, Bosco A, Belgrave D, Hales B, Simpson A, et al. Distinguishing benign from pathologic TH2 immunity in atopic children. J Allergy Clin Immunol 2016;137:379–87. [DOI] [PubMed] [Google Scholar]
- 40.Chibana K, Trudeau JB, Mustovich AT, Hu H, Zhao J, Balzar S, et al. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin Exp Allergy 2008;38:936–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dweik RA, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair SA, et al. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med 2010;181:1033–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011;184:602–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith AD, Cowan JO, Brassett KP, Filsell S, McLachlan C, Monti-Sheehan G, et al. Exhaled nitric oxide: a predictor of steroid response. Am J Respir Crit Care Med 2005;172:453–9. [DOI] [PubMed] [Google Scholar]
- 44.Petsky HL, Cates CJ, Kew KM, Chang AB. Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils): a systematic review and meta-analysis. Thorax 2018;73:1110–9. [DOI] [PubMed] [Google Scholar]
- 45.Diamant Z, Vijverberg S, Alving K, Bakirtas A, Bjermer L, Custovic A, et al. Towards clinically applicable biomarkers for asthma—an EAACI position paper. Allergy 2019;74:1835–51. [DOI] [PubMed] [Google Scholar]
- 46.Vizmanos-Lamotte G, Moreno-Galdo A, Munoz X, Gomez-Olles S, Gartner S, Cruz MJ. Induced sputum cell count and cytokine profile in atopic and nonatopic children with asthma. Pediatr Pulmonol 2013;48:1062–9. [DOI] [PubMed] [Google Scholar]
- 47.Simpson JL, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Periostin levels and eosinophilic inflammation in poorly-controlled asthma. BMC Pulm Med 2016;16:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korevaar DA, Westerhof GA, Wang J, Cohen JF, Spijker R, Sterk PJ, et al. Diagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: a systematic review and meta-analysis. Lancet Respir Med 2015;3:290–300. [DOI] [PubMed] [Google Scholar]
- 49.Rank MA, Ochkur SI, Lewis JC, Teaford HG III, Wesselius LJ, Helmers RA, et al. Nasal and pharyngeal eosinophil peroxidase levels in adults with poorly controlled asthma correlate with sputum eosinophilia. Allergy 2016;71:567–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi Y, Jeon H, Yang EA, Yoon JS, Kim HH. Nasal eosinophilia and eosinophil peroxidase in children and adolescents with rhinitis. Korean J Pediatr 2019;62:353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiappori A, De Ferrari L, Folli C, Mauri P, Riccio AM, Canonica GW. Biomarkers and severe asthma: a critical appraisal. Clin Mol Allergy 2015;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol 2012;130:647–654.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zissler UM, Ulrich M, Jakwerth CA, Rothkirch S, Guerth F, Weckmann M, et al. Biomatrix for upper and lower airway biomarkers in patients with allergic asthma. J Allergy Clin Immunol 2018;142:1980–3. [DOI] [PubMed] [Google Scholar]
- 54.Lopez-Guisa JM, Powers C, File D, Cochrane E, Jimenez N, Debley JS. Airway epithelial cells from asthmatic children differentially express pro-remodeling factors. J Allergy Clin Immunol 2012;129:990–997.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andiappan AK, Sio YY, Lee B, Suri BK, Matta SA, Lum J, et al. Functional variants of 17q12-21 are associated with allergic asthma but not allergic rhinitis. J Allergy Clin Immunol 2016;137:758–766.e3. [DOI] [PubMed] [Google Scholar]
- 56.Beghe B, Barton S, Rorke S, Peng Q, Sayers I, Gaunt T, et al. Polymorphisms in the interleukin-4 and interleukin-4 receptor alpha chain genes confer susceptibility to asthma and atopy in a Caucasian population. Clin Exp Allergy 2003;33:1111–7. [DOI] [PubMed] [Google Scholar]
- 57.Yang IV, Pedersen BS, Liu AH, O’Connor GT, Pillai D, Kattan M, et al. The nasal methylome and childhood atopic asthma. J Allergy Clin Immunol 2017; 139:1478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R III, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med 2004;351:1068–80. [DOI] [PubMed] [Google Scholar]
- 59.Murray CS, Foden P, Sumner H, Shepley E, Custovic A, Simpson A. Preventing severe asthma exacerbations in children: a randomized trial of mite-impermeable bedcovers. Am J Respir Crit Care Med 2017;196:150–8. [DOI] [PubMed] [Google Scholar]
- 60.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol 2007;120:S94–138. [DOI] [PubMed] [Google Scholar]
- 61.Global Initiative for Asthma. Global strategy for asthma management and prevention, 2019. Available from: www.ginasthma.org. Accessed December 11, 2019.
- 62.Brozek JL, Bousquet J, Baena-Cagnani CE, Bonini S, Canonica GW, Casale TB, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol 2010;126:466–76. [DOI] [PubMed] [Google Scholar]
- 63.Pitsios C, Demoly P, Bilo MB, Gerth van Wijk R, Pfaar O, Sturm GJ, et al. Clinical contraindications to allergen immunotherapy: an EAACI position paper. Allergy 2015;70:897–909. [DOI] [PubMed] [Google Scholar]
- 64.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol 2011;127:S1–55. [DOI] [PubMed] [Google Scholar]
- 65.Abrams EM, Szefler SJ, Becker AB. Effect of asthma therapies on the natural course of asthma. Ann Allergy Asthma Immunol 2016;117:627–33. [DOI] [PubMed] [Google Scholar]
- 66.Tosca MA, Licari A, Olcese R, Marseglia G, Sacco O, Ciprandi G. Immunotherapy and asthma in children. Front Pediatr 2018;6:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol 2015;136:556–68. [DOI] [PubMed] [Google Scholar]
- 68.Papadopoulos NG, Arakawa H, Carlsen KH, Custovic A, Gern J, Lemanske R, et al. International consensus on (ICON) pediatric asthma. Allergy 2012;67: 976–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev 2010:CD001186. [DOI] [PubMed] [Google Scholar]
- 70.Lin SY, Azar A, Suarez-Cuervo C, Diette GB, Brigham E, Rice J, et al. The role of immunotherapy in the treatment of asthma. Rockville, MD: AHRQ Comparative Effectiveness Reviews; 2018. Agency for Healthcare Research and Quality. [PubMed] [Google Scholar]
- 71.Pifferi M, Baldini G, Marrazzini G, Baldini M, Ragazzo V, Pietrobelli A, et al. Benefits of immunotherapy with a standardized Dermatophagoides pteronyssinus extract in asthmatic children: a three-year prospective study. Allergy 2002;57:785–90. [DOI] [PubMed] [Google Scholar]
- 72.Penagos M, Compalati E, Tarantini F, Baena-Cagnani R, Huerta J, Passalacqua G, et al. Efficacy of sublingual immunotherapy in the treatment of allergic rhinitis in pediatric patients 3 to 18 years of age: a meta-analysis of randomized, placebo-controlled, double-blind trials. Ann Allergy Asthma Immunol 2006;97:141–8. [DOI] [PubMed] [Google Scholar]
- 73.Asamoah F, Kakourou A, Dhami S, Lau S, Agache I, Muraro A, et al. Allergen immunotherapy for allergic asthma: a systematic overview of systematic reviews. Clin Transl Allergy 2017;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobsen L Preventive aspects of immunotherapy: prevention for children at risk of developing asthma. Ann Allergy Asthma Immunol 2001;87:43–6. [DOI] [PubMed] [Google Scholar]
- 75.Valovirta E, Petersen TH, Piotrowska T, Laursen MK, Andersen JS, Sorensen HF, et al. Results from the 5-year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol 2018;141:529–538.e13. [DOI] [PubMed] [Google Scholar]
- 76.Fitzpatrick AM, Jackson DJ, Mauger DT, Boehmer SJ, Phipatanakul W, Sheehan WJ, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol 2016;138:1608–1618.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol 2005;115: 233–42. [DOI] [PubMed] [Google Scholar]
- 78.Pepper AN, Renz H, Casale TB, Garn H. Biologic therapy and novel molecular targets of severe asthma. J Allergy Clin Immunol Pract 2017;5:909–16. [DOI] [PubMed] [Google Scholar]
- 79.Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013;187:804–11. [DOI] [PubMed] [Google Scholar]
- 80.Casale TB, Luskin AT, Busse W, Zeiger RS, Trzaskoma B, Yang M, et al. Omalizumab effectiveness by biomarker status in patients with asthma: evidence from PROSPERO, a prospective real-world study. J Allergy Clin Immunol Pract 2019;7:156–164.e1. [DOI] [PubMed] [Google Scholar]
- 81.Silkoff PE, Romero FA, Gupta N, Townley RG, Milgrom H. Exhaled nitric oxide in children with asthma receiving Xolair (omalizumab), a monoclonal anti-immunoglobulin E antibody. Pediatrics 2004;113:e308–12. [DOI] [PubMed] [Google Scholar]
- 82.Wenzel SE, Pavord I, Zhang B, Maroni J, Rowe P, Hamilton JD, et al. Type 2 biomarkers associated with dupilumab efficacy in patients with uncontrolled, moderate-to-severe asthma enrolled in the phase 3 study LIBERTY ASTHMA QUEST. Am J Respir Crit Care Med 2018;197:A5949. [Google Scholar]
- 83.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med 2013;368:2455–66. [DOI] [PubMed] [Google Scholar]
- 84.Ortega HG, Yancey SW, Mayer B, Gunsoy NB, Keene ON, Bleecker ER, et al. Severe eosinophilic asthma treated with mepolizumab stratified by baseline eosinophil thresholds: a secondary analysis of the DREAM and MENSA studies. Lancet Respir Med 2016;4:549–56. [DOI] [PubMed] [Google Scholar]
- 85.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med 2009;360:973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest 2016;150:789–98. [DOI] [PubMed] [Google Scholar]
- 87.Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015;3:355–66. [DOI] [PubMed] [Google Scholar]
- 88.Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest 2016;150:799–810. [DOI] [PubMed] [Google Scholar]
- 89.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting beta2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016;388:2115–27. [DOI] [PubMed] [Google Scholar]
- 90.Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al. Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med 2014;2:879–90. [DOI] [PubMed] [Google Scholar]
- 91.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016;388:2128–41. [DOI] [PubMed] [Google Scholar]
- 92.Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol 2005;115:459–65. [DOI] [PubMed] [Google Scholar]
- 93.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol 2001;108: 184–90. [DOI] [PubMed] [Google Scholar]
- 94.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 2011;364:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, et al. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab). Pediatrics 2001;108:E36. [DOI] [PubMed] [Google Scholar]
- 96.Soler M, Matz J, Townley R, Buhl R, O’Brien J, Fox H, et al. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J 2001;18:254–61. [DOI] [PubMed] [Google Scholar]
- 97.Holgate ST, Chuchalin AG, Hebert J, Lotvall J, Persson GB, Chung KF, et al. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy 2004;34:632–8. [DOI] [PubMed] [Google Scholar]
- 98.Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 2014:CD003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bardelas J, Figliomeni M, Kianifard F, Meng X. A 26-week, randomized, double-blind, placebo-controlled, multicenter study to evaluate the effect of omalizumab on asthma control in patients with persistent allergic asthma. J Asthma 2012;49:144–52. [DOI] [PubMed] [Google Scholar]
- 100.Busse WW, Stephenson P, Iqbal A, Trzaskoma BL, Conde LG, Hepburn J, et al. The effect of omalizumab on lung function in adolescents with moderate-to-severe allergic asthma. Presented at: ATS Conference Washington, DC; May 23, 2017 Available from: https://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2017.195.1_MeetingAbstracts.A5105. Accessed October 14, 2019. [Google Scholar]
- 101.Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016;388:31–44. [DOI] [PubMed] [Google Scholar]
- 102.Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018;378:2486–96. [DOI] [PubMed] [Google Scholar]
- 103.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012;380:651–9. [DOI] [PubMed] [Google Scholar]
- 104.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014;371:1198–207. [DOI] [PubMed] [Google Scholar]
- 105.Xolair (omalizumab) [package insert]. San Francisco, CA: Genentech, Inc; 2018. [Google Scholar]
- 106.Dupixent (dupilumab) [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; 2019. [Google Scholar]
- 107.Krug N, Hohlfeld JM, Kirsten AM, Kornmann O, Beeh KM, Kappeler D, et al. Allergen-induced asthmatic responses modified by a GATA3-specific DNAzyme. N Engl J Med 2015;372:1987–95. [DOI] [PubMed] [Google Scholar]
- 108.Zhu L, Ciaccio CE, Casale TB. Potential new targets for drug development in severe asthma. World Allergy Organ J 2018;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Erpenbeck VJ, Popov TA, Miller D, Weinstein SF, Spector S, Magnusson B, et al. The oral CRTh2 antagonist QAW039 (fevipiprant): a phase II study in uncontrolled allergic asthma. Pulm Pharmacol Ther 2016;39:54–63. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Wu Y, Qi H, Xiao J, Gong H, Zhang Y, et al. A new antagonist for CCR4 attenuates allergic lung inflammation in a mouse model of asthma. Sci Rep 2017;7:15038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakagami Y, Kawase Y, Yonekubo K, Nosaka E, Etori M, Takahashi S, et al. RS-1748, a novel CC chemokine receptor 4 antagonist, inhibits ovalbumin-induced airway inflammation in guinea pigs. Biol Pharm Bull 2010;33:1067–9. [DOI] [PubMed] [Google Scholar]