Abstract
Background
Routing patients directly to endovascular capable centers (ECCs) would decrease time to mechanical thrombectomy (MT), but may delay intravenous thrombolysis (IVT).
Objective
To study the clinical outcomes of patients with a stroke transferred directly to ECCs compared with those transferred to ECCs from non-endovascular capable centers (nECCs).
Methods
Data from the STRATIS registry were analyzed to evaluate process and clinical outcomes under five routing policies: (1) transport to nearest nECC; (2) transport to STRATIS ECC over any distance or (3) within 20 miles; (4) transport to ideal ECC (iECC), over any distance or (5) within 20 miles.
Results
Among 236 patients, 117 (49.6%) were transferred by ground, of whom 62 (53%) were transferred within 20 miles. Median MT start time was accelerated in all direct transport models. IVT start was prolonged with direct transport across all distances, but accelerated with direct transport to iECC ≤20 miles. With bypass limited to ≤20 miles, the median modeled EMS arrival to IVT interval decreased for both iECCs and ECCs (by 12 min and 6 min, respectively), and median EMS arrival to puncture time decreased by up to 94 min. In this cohort, no patient would have become ineligible for IVT. Bypass to iECC modeling under 20 miles showed a significant reduction in the level of disability at 3 months, with freedom from disability (modified Rankin Scale score 0–1) at 3 months increased by 12%.
Conclusions
Direct routing of patients with a large vessel occlusion to ECCs, especially when within 20 miles, may lead to better clinical outcomes by accelerating the start of MT without any delay of IVT.
Clinical trial registration number
http://www.clinicaltrials.gov. Unique identifier: NCT02239640.
Keywords: stroke, thrombectomy
Introduction
Mechanical thrombectomy (MT) for acute ischemic stroke (AIS) provides better clinical outcomes in patients already treated with, or ineligible for, intravenous tissue plasminogen activator (IV tPA).1 2 However, the therapeutic effect of MT rapidly declines as the onset-to-reperfusion time increases.3 4 The benefit of intravenous thrombolysis (IVT) is similarly time-dependent, though the magnitude of benefit of IVT is not as great as MT.5 6 When the nearest endovascular capable stroke center (ECC) is further than the nearest non-ECC (nECC) stroke center, routing patients to the nearer nECC may result in faster start of IVT, but slower start of MT. Likewise, bypassing the nECC to bring the patient directly to the ECC would theoretically delay IVT but accelerate MT. Observational series have found that patients who arrive directly at an ECC have a higher likelihood of receiving MT,7 shorter onset-to-puncture,8 9 better functional outcomes,8 and lower mortality.10 A meta-analysis of over 2000 patients reported improved clinical outcome with direct arrival compared with secondary transfer (adjusted relative risk 0.87; 95% CI 0.77 to 0.98).11 However, these analyses predominantly compared patients for whom the nECC was the nearest hospital with patients for whom the ECC was the nearest hospital, and so did not examine the trade-offs associated with bypassing nECCs.
Modeling studies of the impact of different routing strategies incorporating actual traffic conditions have been undertaken, but have not been based on times and locations of actual stroke incidents.12–15 National guidelines recommend bypassing nECCs for ECCs in select circumstances, but differ regarding details of when bypass would be appropriate and are widely recognized as provisional due to limited evidence.16
The Systematic Evaluation of Patients Treated with Stroke Devices for Acute Ischemic Stroke (STRATIS) registry was the first large US registry to prospectively record emergency medical service (EMS) time intervals and geographic information related to the stroke system of care.3 We used patient, geographic, and temporal information from registry participants who were transferred, and also time-dependent traffic information from a Google Maps application program interface (API), to compare actual outcomes for patients who were directed to a nECC with hypothetical modeled outcomes with EMS bypass directly to the nearest STRATIS-participating ECC and nearest ECC of any type.
Methods
Study design and patient population
STRATIS is a multicenter, non-randomized, observational registry for evaluating the use of the Solitaire revascularization device (Medtronic Inc., Irvine, California, USA) and Mindframe Capture low profile revascularization device (Medtronic) in patients with AIS due to intracranial large vessel occlusion (LVO). Informed consent was provided by patients or patients' legally authorized representatives, and the study was approved by the review boards of all participating institutions. All patients underwent stent retriever MT within 8 hours of the onset of stroke symptoms. The methodology and primary results have been published previously.3 For this study, STRATIS patients were included if they: (1) had stroke onset at a location other than a hospital; (2) were transported by EMS first to a nECC and then transferred to a STRATIS-participating ECC; and (3) had study data available for their geographic location at the time of stroke onset and EMS field response. Patients transferred by ground ambulance (ground cohort) or air ambulance (aerial cohort) were included.
Hypothetical bypass scenarios
Two hypothetical bypass policies were analyzed: (1) transport from the stroke location directly to the STRATIS hospital where the patient underwent MT (STRATIS hospital group); and (2) transport from the stroke location directly to the ECC with the shortest travel time, which might or might not be a STRATIS site (ideal hospital group; iECC). These policies were explored in situations permitting direct transport for unlimited distances and confined to ≤20 miles. The 20-mile cut-off point was chosen a priori to reflect a general preference of US systems to limit transport distances to ≤20 miles in order not to deprive the originating ambulance catchment area of an immediately responding vehicle for prolonged periods
A database established by Definitive Healthcare, which included billing data from Medicare and all other payers in 2015, was used to identify ECCs.15 17 The database was queried for hospitals that performed more than 10 MT procedures in 2015.
To calculate the travel distance and time from the field stroke location to the ECC, a mapping application was developed using the Google Maps distance matrix API (online supplementary methods). For each patient, a map showing the nECC, STRATIS ECC, and other ECCs near the field stroke location was generated (online supplementary figure I). The accuracy of the travel times generated by the API was tested against the two known actual travel times for each patient: (1) from the scene to the nECC, and (2) from the nECC to the ECC.
neurintsurg-2019-015593supp003.pdf (221.7KB, pdf)
neurintsurg-2019-015593supp001.pdf (1.2MB, pdf)
Process and clinical outcome measures
To evaluate workflow, EMS transport records and in-hospital care process times were prospectively collected, including time that the emergency call was received, time of EMS arrival at the stroke scene, time of EMS arrival at the nECC, time of ambulance departure from the nECC, and time of arrival at a STRATIS hospital. These data enabled analysis of process outcomes, including time intervals from onset (last known well) to IV tPA, onset to arterial puncture for MT, EMS arrival on-scene to IV tPA, and EMS arrival on-scene to arterial puncture. Clinical outcomes at 90 days included freedom from disability (modified Rankin Scale (mRS) score 0–1), functional independence (mRS score 0–2), degree of disability across all seven mRS levels, and mortality. Actual process and clinical outcomes under the policy of transport to nECC first, and interfacility transfer to ECC were compared with patient-specific modeled outcomes under the two bypass strategies. Subgroup analyses were performed according to the mode of interfacility ambulance transportation, evaluating the ground cohort and aerial cohort patients separately.
Statistical analysis
Student t-tests were used for between-group comparisons of continuous variables. For categorical variables, Pearson chi-square tests were performed for multiple-group comparisons and Fisher’s exact tests for two-group comparisons. Predictive modeling was used to determine modeled bypass transports and the effect of bypass on mRS scores at 90 days (online supplemental methods and online supplementary figure II). A two-sided shift test was used to analyze the distribution of 90 day mRS outcomes across groups. For all statistical analyses, two-tailed p values are presented, with p<0.05 considered statistically significant. Analyses were conducted in SAS version 9.4 (SAS Institute, Cary, North Carolina, USA) and R version 3.2 (R Foundation for Statistical Computing, Vienna, Austria).
neurintsurg-2019-015593supp002.pdf (149.7KB, pdf)
Results
Of 984 patients enrolled in STRATIS, 539 arrived directly to the ECC, 42 had in-hospital stroke onset, 123 used private vehicles or were not documented as using EMS to reach the initial nECC hospital, and 44 did not have scene geographic coordinates documented, resulting in 236 patients meeting study entry criteria (figure 1). Baseline characteristics are presented in online supplementary table I.
Figure 1.
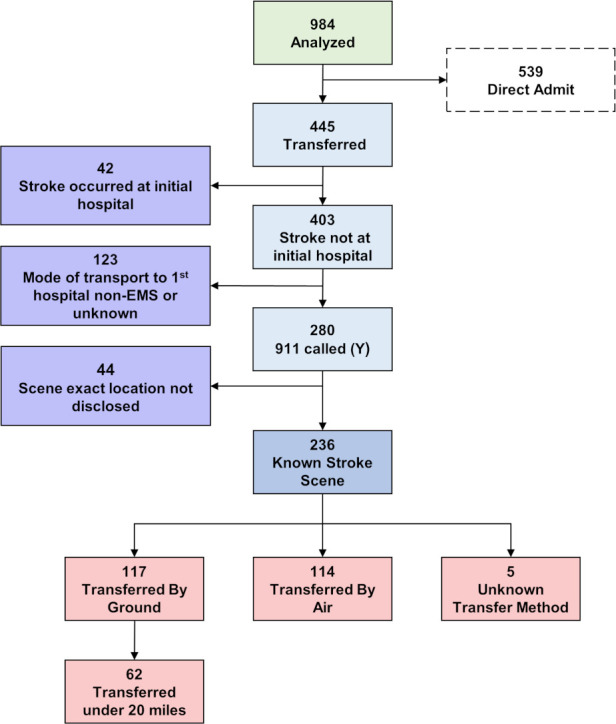
Patient selection flowchart. Flowchart shows the process and outcomes of identification of patients eligible for this study, based on whether they were first brought to the non-endovascularcapable center (nECC) and then transferred by ground or air to STRATIS ECC hospitals.
Median distances from the scene to the actual and potential receiving facilities were six miles for the actual initial nECC hospital, 34.1 miles for the eventual STRATIS ECC, and 28.1 miles for the iECC (table 1 and online supplementary figure III). For the 117 ground cohort patients, median distances from the scene to the actual initial nECC was 4.0 miles, compared with 21.6 miles to the eventual STRATIS ECC, and 18.6 miles to the iECC (online supplementary table II).
Table 1.
Distances and time intervals (in minutes) for all patients (n=236)
| Interval | Actual Mean±SD (N) Median (IQR) |
Bypass to STRATIS hospital Mean±SD (N) Median (IQR) |
P value | Bypass to ideal hospital Mean±SD (N) Median (IQR) |
P value |
| Distance: scene to initial hospital (miles) | 11.3±23.1 (230) 6.0 (3.0–12.8) |
– | – | – | – |
| Distance: initial hospital to endovascular hospital (miles) | 48.1±47.3 (232) 30.5 (15.0–70.3) |
– | – | – | – |
| Distance: scene to endovascular hospital (miles) | – | 52.4±47.5 (236) 34.1 (18.6–71.7) |
– | 43.6±41.1 (236) 28.1 (15.8–62.6) |
– |
| Onset to initial hospital | 77.0±59.0 (209) 58.0 (38.0–97.0) |
– | – | – | – |
| Onset to endovascular door | 229.6±79.6 (224) 217.0 (173.5–283.5) |
125.4±70.6 (236) 106.0 (70.0–172.3) |
<0.001 | 115.3±67.2 (236) 96.5 (65.0–151.3) |
<0.001 |
| Onset to IV tPA | 124.2±51.9 (149) 110.0 (86.0–148.0) |
154.2±64.5 (152) 135.5 (104.0–194.3) |
<0.001 | 143.2±59.9 (152) 124.0 (99.8–179.5) |
<0.001 |
| Onset to arterial puncture | 287.0±84.3 (232) 273.5 (225.0–339.3) |
214.9±70.9 (232) 195.0 (159.0–262.0) |
<0.001 | 204.7±67.5 (232) 184.5 (154.0–241.5) |
<0.001 |
| EMS arrival to initial hospital | 28.6±20.6 (152) 25.0 (19.0–32.3) |
– | – | – | – |
| EMS arrival to endovascular door | 182.6±61.4 (222) 173.5 (144.0–210.0) |
75.5±45.2 (236) 60.0 (41.8–100.0) |
<0.001 | 65.4±39.2 (236) 53.0 (39.0–83.3) |
<0.001 |
| EMS arrival to IV tPA | 84.9±33.6 (149) 77.0 (65.0–94.0) |
115.0±47.6 (152) 98.0 (82.0–137.0) |
<0.001 | 103.9±41.5 (152) 90.5 (76.0–121.3) |
<0.001 |
| EMS arrival to arterial puncture | 236.7±69.9 (232) 228.5 (186.8–272.3) |
164.6±45.4 (232) 148.5 (130.8–189.0) |
<0.001 | 154.3±39.5 (232) 141.5 (128.0–172.3) |
<0.001 |
EMS, emergency medical services; IV tPA, intravenous tissue plasminogen activator.
neurintsurg-2019-015593supp004.pdf (941.9KB, pdf)
Among the ground cohort, 62 (53.0%) patients had hypothetical bypass distances to the STRATIS and ideal hospitals of ≤20 miles. In this cohort, median distance from scene to the actual initial nECC was 5.5 miles, compared with 13.3 miles to the eventual STRATIS hospital, and 8.7 miles to the iECC (table 2). For the aerial cohort, median distance from scene to the actual initial nECC was 7.0 miles (online supplementary table III), compared with 60.2 miles to the eventual STRATIS ECC, and 51.5 miles to the iECC.
Table 2.
Within 20 miles distance and time intervals (in minutes)
| Interval | Actual Mean±SD (N) Median (IQR) |
Bypass to STRATIS hospital Mean±SD (N) Median (IQR) |
P value | Bypass to ideal hospital Mean±SD (N) Median (IQR) |
P value |
| Distance: scene to initial hospital (miles) | 5.5±5.4 (62) 4.0 (2.0–8.0) |
– | – | – | – |
| Distance: initial to endovascular hospital (miles) | 11.3±6.4 (60) 10.5 (6.8–15.3) |
– | – | – | – |
| Distance: scene to endovascular hospital (miles) | – | 12.5±5.0 (62) 13.3 (8.5–16.8) |
– | 10.4±5.1 (62) 8.7 (6.2–15.1) |
– |
| Onset to initial hospital | 74.1±58.2 (60) 52.0 (31.0–87.3) |
– | – | – | – |
| Onset to endovascular door | 222.4±77.5 (59) 208.0 (167.5–284.0) |
87.6±57.1 (62) 61.0 (50.0–107.5) |
<0.001 | 83.1±56.6 (62) 56.0 (46.3–102.3) |
<0.001 |
| Onset to IV tPA | 119.3±47.7 (38) 108.0 (89.3–139.8) |
111.1±45.0 (39) 93.0 (84.5–118.0) |
0.079 | 107.1±45.4 (39) 88.0 (80.0–117.5) |
0.003 |
| Onset to arterial puncture | 270.8±77.0 (61) 265.0 (224.0–328.0) |
177.4±57.3 (61) 151.0 (139.0–197.0) |
<0.001 | 172.8±56.8 (61) 145.0 (136.0–195.0) |
<0.001 |
| EMS arrival to initial hospital | 23.5±8.6 (42) 21.5 (18.0–31.0) |
– | – | – | – |
| EMS arrival to endovascular door | 172.2±61.1 (59) 165.0 (130.0–198.5) |
38.0±7.5 (62) 38.0 (33.3–41.0) |
<0.001 | 33.5±6.6 (62) 32.0 (28.0–38.8) |
<0.001 |
| EMS arrival to IV tPA | 83.9±23.6 (38) 81.0 (72.3–89.5) |
75.1±7.8 (39) 75.0 (69.0–79.0) |
0.079 | 71.1±7.1 (39) 69.0 (66.0–75.0) |
0.003 |
| EMS arrival to arterial puncture | 220.5±61.2 (61) 215.0 (183.0–255.0) |
127.1±7.6 (61) 127.0 (123.0–130.0) |
<0.001 | 122.5±6.7 (61) 121.0 (117.0–128.0) |
<0.001 |
EMS, emergency medical services; IV tPA, intravenous tissue plasminogen activator.
There was a high correlation for the projected versus actual times from arrival on scene to arrival at the nECC (r=0.70), while absolute values were slightly higher for the projected versus actual arrival on scene-to-door times (30.0 vs 26.0 min; p=0.001). There was a high correlation for projected versus actual travel times for interfacility transfer from the nECC to the STRATIS ECC (r=0.75), and absolute values were well-matched (37.8 vs 39.0 min; p=0.70).
Door-to-needle times were longer among patients arriving at the nECC than among the STRATIS patients brought directly to ECCs and receiving IV tPA there (median 54 vs 37 min; p<0.001).8 Median door-to-puncture times at the ECC were shorter among patients arriving by transfer than patients arriving directly (49 vs 89 min; p<0.001). For the 63.1% (149/236) of patients who received IV tPA at the nECC before transfer to the STRATIS ECC, the median time from EMS arrival on-scene to IV tPA was 77 min (table 1). In the bypass model with no limitation on distance to the receiving ECC, seven patients would not have received IV tPA owing to later arrival. Average scene-to-needle time was increased to 98 min (p<0.001) for transport directly to the STRATIS ECC, and to 91 min (p<0.001) for transport directly to the iECC. In contrast, median actual time from EMS arrival on-scene to arterial puncture after first transport to the nECC and interfacility transfer to the STRATIS ECC was 229 min, but decreased in the modeled bypass scenarios to 149 min (p<0.001) for direct transport to STRATIS ECC, and to 142 min (p<0.001) for direct transport to the iECC.
Among the 62 patients transferred ≤20 miles to an ECC, the median actual time from EMS arrival on scene to IV tPA at the nECC was 81 min (table 2). Under the modeled bypass policies, all patients still would have received IV tPA, and scene-to-needle time decreased to 75 min (p=0.08) for transport direct to the STRATIS ECC and to 69 min (p=0.003) for transport direct to the iECC. Seventy-five percent of eligible patients would have had a shorter time from EMS arrival on scene to IV tPA start with direct transport to the iECC than with transport to the nECC (online online supplementary figure 4). The decrease was due to shorter ECC door-to-needle times outweighing longer scene-to-door times.8
neurintsurg-2019-015593supp005.pdf (904.8KB, pdf)
The median actual time from EMS arrival on scene to arterial puncture, after first transport to the nECC and interfacility transfer to the STRATIS ECC, was 215 min. In the modeled bypass scenarios, scene-to-puncture time was decreased to 127 min (p<0.001) for transport direct to the STRATIS ECC, and to 121 min (p<0.001) for transport direct to the iECC.
Among all 236 transfer patients, clinical outcomes at 3 months (actual and modeled with no limitation on ECC travel distance) are shown in figure 2A. Bypass directly to an ECC was associated with reduced disability at 3 months, for direct to STRATIS ECC versus nECC first (common OR (cOR) for a lower mRS disability grade of 1.36 (95% CI 1.02 to 1.80); p=0.017); and for direct to iECC versus nECC first (cOR 1.41 (95% CI 1.06 to 1.86); p=0.009). Considering dichotomized outcomes, policies of bypass direct to ECC were associated with a significant increase in freedom from disability (mRS score 0–1) at 3 months for both direct to STRATIS ECC versus nECC first (42.8% vs 31.8%; p=0.022), and for direct to iECC versus nECC first (43.4% vs 31.8%; p=0.015); as well as trends for increased functional independence (mRS score 0–2) for both direct to STRATIS ECC versus nECC first (56.0% vs 48.4%; p=0.12), and for direct to iECC first versus nECC first (56.6% vs 48.4%; p=0.10).
Figure 2.
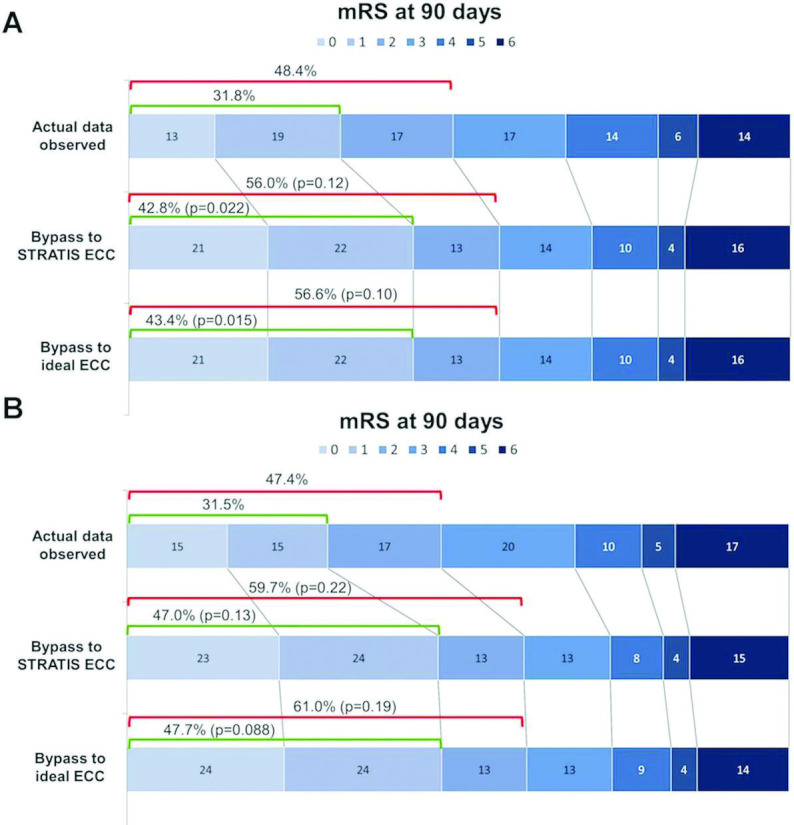
Distribution of modeled mRS outcomes at 3 months. Rows indicate actual outcomes with first ground transport to a nECC and then transfer to an ECC, modeled outcomes with first transport direct to STRATIS ECC, and modeled outcomes with first transport direct to an iECC. (A) Among the 236 patients transferred with no limitation of transfer distance. (B) Among the 62 patients transferred by ground within 20 miles of the stroke scene to an ECC (cOR 1.67 (95% CI 1.04 to 2.68]; two-sided shift test, p=0.034). ECC, endovascular capable center; iECC, ideal endovascular capable center; mRS, modified Rankin Scale; nECC, non-endovascular capable center
Clinical outcomes at 3 months for the 62 patients with ground transfer within 20 miles of an ECC (actual and modeled with direct transport to ECC) are shown in figure 2B. Overall outcomes in this cohort would have been significantly improved with bypass direct to an ECC versus a nECC first (cOR 1.67 95% CI 1.04 to 2.68); two-sided shift test, p=0.034). For dichotomized outcomes, policies of bypass direct to an ECC demonstrated favorable but non-significant outcomes for freedom from disability (mRS score 0–1) at 3 months for both direct to STRATIS ECC versus nECC first (47.0% vs 31.5%; p=0.13) and to an iECC versus nECC first (47.7% vs 31.5%; p=0.088), as well as in functional independence (mRS score 0–2) for both direct to STRATIS ECC first versus a nECC (59.7% vs 47.4%; p=0.22), and direct to an iECC versus nECC first (61.0% vs 47.4%; p=0.19). Mortality was reduced, but not significantly, with direct routing for both direct to STRATIS ECC first versus nECC first (14.8% vs 16.9%; p=0.68) or for direct to iECC vs nECC first (13.6% vs 16.9%; p=0.47).
Modeled clinical outcomes were improved. For every 100 patients treated with MT who were routed directly to ECCs instead of nearer nECCs, 17 would be less disabled at 3 months (number needed to treat (NNT) 6), including eight more patients who would be functionally independent (NNT 12). Direct routing limited to the nECC ≤20 miles reduced EMS arrival-to-puncture time by 98 min with a decrease of EMS scene-to-needle time by 13 min. For every 100 patients bypassed to an iECC ≤20 miles, 25 would be less disabled at 3 months (NNT 4), including 14 more patients who would be functionally independent (NNT 7). See online supplementary figure 5.
neurintsurg-2019-015593supp006.pdf (722KB, pdf)
Discussion
Analysis of patients from the STRATIS Registry treated with MT suggests that bypass from the stroke location to an ECC affords better process metrics and clinical outcomes than for those transferred secondarily from a nECC. Direct routing to the nearest ECC, irrespective of mode of transport or distance, was associated with a reduction of EMS arrival-to-puncture time, an increase of EMS arrival to IV tPA needle time, and a reduction in functional disability at 3 months. Several studies modeling different routing strategies have suggested improved outcomes for patients with a stroke who are bypassed to ECCs.12–15 Our study, however, used data from patients actually undergoing MT with known outcomes under one treatment strategy, and with known exact time and location of stroke scene, using chronologically specific individualized traffic flow conditions. A particular strength of this study was the availability of transport and hospital records for each patient, allowing granular analysis of the effect of routing policies on key processes of care time intervals.
The effect of bypass on treatment times varied with aspects of the bypass policy. In patients who were routed to the nearest ECC irrespective of the mode of transport and distance, times from EMS arrival to puncture were reduced substantially while times from EMS arrival to IV tPA were increased only minimally. These results are in agreement with a recent systematic review and meta-analysis demonstrating that patients who were transported directly to the nearest ECC had significantly shorter onset-to-needle time and onset-to-puncture time than patients for whom IVT was started at the nearest primary stroke center and then transported to the ECC.18 In our study, when bypass distances were limited to ECCs ≤20 miles from the stroke scene, both EMS arrival to puncture and EMS arrival to IV tPA were reduced. Time to IV tPA with bypass reflected the influence of longer scene-to-door times but shorter door-to-needle times. Actual door-to-needle times at ECC hospitals for patients in the STRATIS registry were 18 min shorter than at non-ECC hospitals.8
The added benefit of IV tPA in patients treated with MT is uncertain; a recent meta-analysis demonstrated that endovascular therapy alone has clinical outcomes similar to those of endovascular therapy plus IV-tPA in acute anterior circulation strokes.19 Trials evaluating the efficacy of MT for AIS have revealed an MT-related NNT of 3–7.20 21 In contrast, for patients receiving IV tPA for AIS within 0–3 hours after stroke onset, the NNT was 8.22 A systematic review and pooled analysis showed that endovascular therapy in combination with IV tPA improves AIS outcomes in comparison with patients treated with IV tPA alone.23 In that study, the NNT when treated with endovascular therapy plus IV tPA versus IV tPA alone was 5.3, and for patients eligible for endovascular treatment who received only IV-tPA, the number needed to harm was 9. Randomized trials of direct MT without IV tPA are underway (http://www.clinicaltrials.gov. Unique identifier: NCT01657461 and NCT03469206; http://controlled-trials.com. Unique identifier: ISRCTN80619088).
Our findings show that even if IV tPA provides additional benefits, the penalty of later IVT start in patients transported directly to ECC hospitals is negligible because of greater care efficiency.8 None of the patients who were bypassed to an ECC≤20 miles from the stroke location would have become ineligible for IV tPA, and median time to IV tPA would have been shorter with three-quarters of the patients also benefiting from accelerated IV thrombolysis with bypass. Similar findings of improved treatment efficiency in ECC vs nECC for IV tPA have been published,24 25 corroborating the shorter mean door-to-needle time for ECC versus nECC. The ongoing prospective clinical trial, RACECAT, will provide insights into the benefits of direct ECC access for patients with AIS with LVO, compared patients transferred to the nearest stroke center in geographic areas with larger transport distances (http://www.clinicaltrials.gov. Unique identifier: NCT02795962). One small single-center series calculated a 2.5% decrease in MT eligibility for every minute of delay,26 and thus bypass directly to an ECC would expand access to MT. Patient selection for MT in ECCs performed by a neurointerventionalist, especially in short-range bypass situations, can increase MT treatment rates: in South Florida, where bypass to an ECC has been phased in since 2015, up to a fourfold higher use MT has been documented in comparison with other regions of the state without systematic bypass.27 28
The benefit of routing directly to an ECC was higher when the bypass distance was ≤20 miles, reflecting the lesser impact of scene-to-thrombolysis start times. While implementation of bypass must be tailored to the local EMS and hospital capabilities, our data suggest that improved outcomes can be expected where short-range bypass is feasible. Our real-world data, added to prior modeling studies, support national policies recommending direct routing to an ECC.29 Our model did not require use of a prehospital screening tool, which might have varying sensitivities or specificities,30 but rather assumes that all patients with clinical suspicion or stroke would be directly bypassed to an ECC for the highest level of care, where all treatment options are available. It is unclear whether the potential increase in patients (with and without a true LVO) treated at ECCs under this policy would affect ECC centers. The increased volume could allow for economies of scale and justification for in-house stroke resources and in-house 24/7 interventionalists to increase efficiency; however, more research is necessary to determine the effect of bypass on the administrative and clinical practices of ECCs. Nonetheless, the faster door-to-needle time at ECCs outweighs the increased travel time for most patients within 20 miles, which would also apply to patients requiring only thrombolysis.
Limitations
Our study represents a modeled analysis. The benefits of direct routing estimated by our model are conservative as it included only patients from the STRATIS registry who had a confirmed LVO, received MT, and survived the procedure within 8 hours, and thus may not be generalizable. A limitation of our model is that it does not include patients who were delivered to an ECC but were found to be ineligible for MT. STRATIS also did not collect information about patients who received only IV tPA at a nECC and did not receive MT. As STRATIS enrolled only patients with LVO, this analysis cannot provide insights into the effect of a universal bypass policy beyond 20 miles for those patients who ultimately receive only thrombolysis when the travel time to bypass to an ECC beyond 20 miles might delay thrombolysis.
In addition, patients who were taken to a nECC and then transported to an ECC may have different severity than those transferred directly to an ECC. Furthermore, process times for IV tPA and MT of STRATIS centers and ECCs overall have since probably been further reduced, as may have IV tPA process times of referring nECCs. Randomized trials are needed to confirm our hypothesis by tracking all patients with a stroke, including those who become ineligible for either IV tPA or MT by bypass or transfer, respectively.
Deficiencies in prehospital time intervals, such as time of EMS departure from incident locations and exclusion of patients with incomplete data, could have influenced summary statistics, possibly skewing our analyses. However, our analysis used scene arrival/first medical provider to nECC versus ECC intervals, which were calculated from the time points for scene arrival and hospital arrival, obviating the need to know exact scene departure times. Nevertheless, STRATIS was the largest cohort to report the clinical outcomes of stent retriever MT, thereby providing sufficient statistical power for estimated comparisons.
Summary
The STRATIS registry documented better process times and functional outcomes in patients with a stroke who arrived directly at an ECC than for patients who underwent transfer to ECCs. Our models indicate that bypass to an ECC shortens the time from EMS arrival at the scene to puncture, especially for patients transferred ≤20 miles, without delay of the EMS arrival to IV tPA interval. Moreover, all ground transported patients, especially those who were bypassed ≤20 miles to an ECC, were modeled to experience significant improvements in clinical outcomes. Further trials are warranted for urban geographic areas with short transport distances that have not yet implemented EMS bypass to ECCs.
Acknowledgments
The authors acknowledge Medtronic for editing assistance.
Footnotes
Collaborators: Mohammad Ali Aziz-Sultan, Richard P. Klucznik, Frank R. Hellinger Jr, Dileep R. Yavagal, Tom L. Yao, Ashutosh P. Jadhav, Rishi Gupta, Ameer E. Hassan, Coleman O. Martin, Hormozd Bozorgchami, Ritesh Kaushal, Raul G. Nogueira, Ravi H. Gandhi, Eric C. Peterson, Shervin R. Dashti, Curtis A. Given II, Brijesh P. Mehta, Vivek Deshmukh, Sidney Starkman, Italo Linfante, Scott H. McPherson, Peter Kvamme, Thomas J. Grobelny, Muhammad S. Hussain, Ike Thacker, Nirav Vora, Peng Roc Chen, Stephen J. Monteith, Robert D. Ecker, Clemens M. Schirmer, Eric Sauvageau, Alex Abou-Chebl, Colin P. Derdeyn, Lucian Maidan, Aamir Badruddin, Adnan H. Siddiqui, Travis M. Dumont, Abdulnasser Alhajeri, M. Asif Taqi, Khaled Asi, Jeffrey Carpenter, Alan Boulos, Gaurav Jindal, Ajit S. Puri, Rohan Chitale, Eric M. Deshaies, David H. Robinson, David F. Kallmes, Blaise W. Baxter, Mouhammad A. Jumaa, Peter Sunenshine, Aniel Majjhoo, Joey D. English, Shuichi Suzuki, Richard D. Fessler, Josser E. Delgado Almandoz, Jerry C. Martin, Diogo C. Haussen
Contributors: All authors made substantial contributions to the conception and design, analysis, and interpretation of data; drafted or critically revised the article; and gave final approval of the version to be published.
Funding: This study was sponsored by Medtronic, Inc.
Competing interests: NM-K and OZ serve as scientific consultants regarding trial design and conduct to Medtronic, who sponsored this study. MTF serves as a scientific consultant to Medtronic, Stryker, Balt, Viz.ai, NeurVana, and Genentech, and has received research funding from the National Institutes of Health (NIH), Stryker, Medtronic, Microvention, and Endophys. The University of California, Regents receives funding for Dr Jahan’s services as a scientific consultant regarding trial design and conduct to Medtronic/Covidien; RJ is an employee of the University of California, which holds a patent on retriever devices for stroke. The University of California, Regents receives funding for Dr Saver’s services as a scientific consultant regarding trial design and conduct to Covidien and Stryker; JLS is an employee of the University of California, which holds a patent on retriever devices for stroke. DL serves as an imaging core laboratory consultant for Cerenovus, Genentech, Medtronic, Stryker, and Vesalio.
Patient consent for publication: Not required.
Ethics approval: The study was performed in compliance with the World Medical Association's Declaration of Helsinki. The study protocol was approved by the local ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Goyal M, Menon BK, van Zwam WH, et al. . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–31. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2. Nogueira RG, Jadhav AP, Haussen DC, et al. . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 3. Mueller-Kronast NH, Zaidat OO, Froehler MT, et al. . Systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke: primary results of the STRATIS registry. Stroke 2017;48:2760–8. 10.1161/STROKEAHA.117.016456 [DOI] [PubMed] [Google Scholar]
- 4. Saver JL, Goyal M, van der Lugt A, et al. . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279–88. 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 5. Emberson J, Lees KR, Lyden P, et al. . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929–35. 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saver JL, Fonarow GC, Smith EE, et al. . Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013;309:2480–8. 10.1001/jama.2013.6959 [DOI] [PubMed] [Google Scholar]
- 7. Fuentes B, Alonso de Leciñana M, Ximénez-Carrillo A, et al. . Futile interhospital transfer for endovascular treatment in acute ischemic stroke: the Madrid stroke network experience. Stroke 2015;46:2156–61. 10.1161/STROKEAHA.115.009282 [DOI] [PubMed] [Google Scholar]
- 8. Froehler MT, Saver JL, Zaidat OO, et al. . Interhospital transfer before thrombectomy is associated with delayed treatment and worse outcome in the STRATIS registry (systematic evaluation of patients treated with neurothrombectomy devices for acute ischemic stroke). Circulation 2017;136:2311–21. 10.1161/CIRCULATIONAHA.117.028920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gerschenfeld G, Muresan I-P, Blanc R, et al. . Two paradigms for endovascular thrombectomy after intravenous thrombolysis for acute ischemic stroke. JAMA Neurol 2017;74:549–56. 10.1001/jamaneurol.2016.5823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rinaldo L, Brinjikji W, Rabinstein AA. Transfer to high-volume centers associated with reduced mortality after endovascular treatment of acute stroke. Stroke 2017;48:1316–21. 10.1161/STROKEAHA.116.016360 [DOI] [PubMed] [Google Scholar]
- 11. Ismail M, Armoiry X, Tau N, et al. . Mothership versus drip and ship for thrombectomy in patients who had an acute stroke: a systematic review and meta-analysis. J Neurointerv Surg;2018 https://www.ncbi.nlm.nih.gov/pubmed/30297541 [DOI] [PubMed] [Google Scholar]
- 12. Ali A, Zachrison KS, Eschenfeldt PC, et al. . Optimization of prehospital triage of patients with suspected ischemic stroke. Stroke 2018;49:2532–5. 10.1161/STROKEAHA.118.022041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holodinsky JK, Williamson TS, Demchuk AM, et al. . Modeling stroke patient transport for all patients with suspected large-vessel occlusion. JAMA Neurol 2018;75:1477 10.1001/jamaneurol.2018.2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Milne MSW, Holodinsky JK, Hill MD, et al. . Drip 'n ship versus mothership for endovascular treatment: modeling the best transportation options for optimal outcomes. Stroke 2017;48:791–4. 10.1161/STROKEAHA.116.015321 [DOI] [PubMed] [Google Scholar]
- 15. Phan TG, Beare R, Chen J, et al. . Googling service boundaries for endovascular clot retrieval hub hospitals in a metropolitan setting: proof-of-concept study. Stroke 2017;48:1353–61. 10.1161/STROKEAHA.116.015323 [DOI] [PubMed] [Google Scholar]
- 16. Powers WJ, Rabinstein AA, Ackerson T, et al. . 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018;49:e46–110. 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 17. DeMets D, Tabak L. Advisory Committee to the director data and informatics Working group. National Institutes of Health, 2012. [Google Scholar]
- 18. Ciccone A, Berge E, Fischer U. Systematic review of organizational models for intra-arterial treatment of acute ischemic stroke. Int J Stroke 2019;14:12–22. 10.1177/1747493018806157 [DOI] [PubMed] [Google Scholar]
- 19. Kim CH, Jeon JP, Kim S-E, et al. . Endovascular treatment with intravenous thrombolysis versus endovascular treatment alone for acute anterior circulation stroke: a meta-analysis of observational studies. J Korean Neurosurg Soc 2018;61:467–73. 10.3340/jkns.2017.0505.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saver JL, Goyal M, Bonafe A, et al. . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95. 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 21. Berkhemer OA, Fransen PSS, Beumer D, et al. . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 22. Saver JL, Gornbein J, Grotta J, et al. . Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3- to 4.5-hour window: joint outcome table analysis of the ECASS 3 trial. Stroke 2009;40:2433–7. 10.1161/STROKEAHA.108.543561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hussain M, Moussavi M, Korya D, et al. . Systematic review and pooled analyses of recent neurointerventional randomized controlled trials: setting a new standard of care for acute ischemic stroke treatment after 20 years. Interv Neurol 2016;5:39–50. 10.1159/000442355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Man S, Zhao X, Uchino K, et al. . Comparison of acute ischemic stroke care and outcomes between comprehensive stroke centers and primary stroke centers in the United States. Circ Cardiovasc Qual Outcomes 2018;11:e004512 10.1161/CIRCOUTCOMES.117.004512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. International stroke conference The impact of EMS directly transporting patients with suspected acute ischemic stroke to comprehensive stroke centers in South Florida :. American Heart Association/American Stroke Association, 2017. [Google Scholar]
- 26. Prabhakaran S, Ward E, John S, et al. . Transfer delay is a major factor limiting the use of intra-arterial treatment in acute ischemic stroke. Stroke 2011;42:1626–30. 10.1161/STROKEAHA.110.609750 [DOI] [PubMed] [Google Scholar]
- 27. Mueller-Kronast N, Marulanda-Londono ET, Wang K, et al. . Abstract WP229: the impact of EMS directly transporting patients with suspected acute ischemic stroke to comprehensive stroke centers in South Florida. Stroke 2018;49:AWP229–AWP29. 10.1161/str.49.suppl_1.WP229 [DOI] [Google Scholar]
- 28. Asdaghi N, Wang K, Ciliberti-Vargas MA, et al. . Predictors of thrombolysis administration in mild stroke: Florida-Puerto Rico collaboration to reduce stroke disparities. Stroke 2018;49:638–45. 10.1161/STROKEAHA.117.019341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Severity-Based Stroke Triage Algorithm for EMS: Mission:Lifeline, American Heart Association/American Stroke Association, 2019. Available: https://mlnetwork.heart.org/resources/364
- 30. Zhelev Z, Walker G, Henschke N, et al. . Prehospital stroke scales as screening tools for early identification of stroke and transient ischemic attack. Cochrane Database Syst Rev 2019;4:CD011427 10.1002/14651858.CD011427.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
neurintsurg-2019-015593supp003.pdf (221.7KB, pdf)
neurintsurg-2019-015593supp001.pdf (1.2MB, pdf)
neurintsurg-2019-015593supp002.pdf (149.7KB, pdf)
neurintsurg-2019-015593supp004.pdf (941.9KB, pdf)
neurintsurg-2019-015593supp005.pdf (904.8KB, pdf)
neurintsurg-2019-015593supp006.pdf (722KB, pdf)


