Abstract
Objective
We performed a systematic review of the literature to synthesize the data on EEG findings in COVID-19. Frontal EEG patterns are reported to be a characteristic finding in COVID-19 encephalopathy. Although several reports of EEG abnormalities are available, there is lack of clarity about typical findings.
Methods
Research databases were queried with the terms “COVID” OR “coronavirus” OR “SARS” AND “EEG”. Available data was analyzed from 617 patients with EEG findings reported in 84 studies.
Results
The median age was 61.3 years (IQR 45−69, 33.3 % female). Common EEG indications were altered mental status (61.7 %), seizure-like events (31.2 %), and cardiac arrest (3.5 %). Abnormal EEG findings (n = 543, 88.0 %) were sub-classified into three groups: (1) Background abnormalities: diffuse slowing (n = 423, 68.6 %), focal slowing (n = 105, 17.0 %), and absent posterior dominant rhythm (n = 63, 10.2 %). (2) Periodic and rhythmic EEG patterns: generalized periodic discharges (n = 35, 5.7 %), lateralized/multifocal periodic discharges (n = 24, 3.9 %), generalized rhythmic activity (n = 32, 5.2 %). (3) Epileptiform changes: focal (n = 35, 5.7 %), generalized (n = 27, 4.4 %), seizures/status epilepticus (n = 34, 5.5 %). Frontal EEG patterns comprised of approximately a third of all findings. In studies that utilized continuous EEG, 96.8 % (n = 243) of the 251 patients were reported to have abnormalities compared to 85.0 % (n = 311) patients who did not undergo continuous EEG monitoring (χ2 = 22.8, p =< 0.001).
Significance
EEG abnormalities are common in COVID-19 related encephalopathy and correlates with disease severity, preexisting neurological conditions including epilepsy and prolonged EEG monitoring. Frontal findings are frequent and have been proposed as a biomarker for COVID-19 encephalopathy.
Keywords: COVID-19, EEG, Seizure, Viral encephalitis, Encephalopathy, SARS CoV-2
1. Introduction
The COVID-19 pandemic continues to test the resilience of health systems and the limits of our understanding of viral infections of the nervous system. There has been interest in the EEG findings in COVID-19 related viral encephalitis/encephalopathy.
Neurological complications have been reported in 34.6 % patients with severe COVID-19 and include stroke, headache, and seizures. [1] Over the past few months, a plethora of studies have reported on the EEG profile of patients with COVID-19. The EEG reports in COVID-19 patients range from single case reports [2,3] to larger studies of up to 111 EEGs [4]. Findings from these studies are varied and included mostly normal EEGs [5] to diffuse slowing in all [[6], [7], [8]].
Individual studies stress a specific population demographic or peculiar aspect of the EEG, but together provide a mosaic of EEG findings in varied groups from children to elderly, and asymptomatic patients to those with severe encephalopathy and status epilepticus. Some studies have noted a preponderance of frontal findings, putatively linked to the mode of entry of the virus into the brain. [6,[9], [10], [11], [12]] In summary, there is no common EEG pattern that has evolved in spite of numerous publications in the short period of time since COVID-19 emerged.
Here, we perform a systematic study of the EEG findings in patients with COVID-19 to synthesize the available data and to elucidate common patterns.
2. Methods
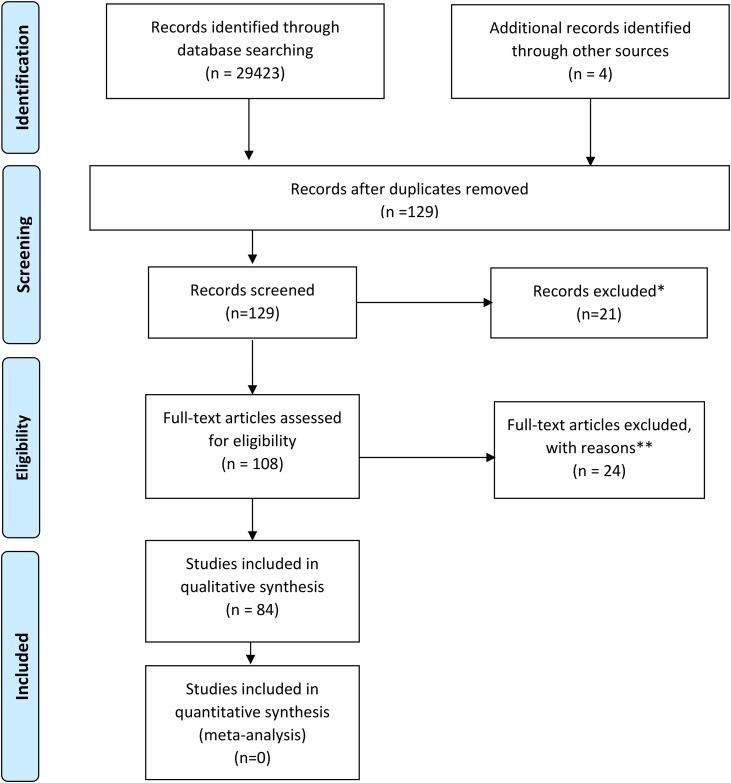
We searched Pubmed, Google Scholar, medRxiV/bioRxiV preprint servers for reports of EEG in patients with COVID-19. The following terms were used in the search- “COVID” OR “coronavirus” OR “SARS” AND “EEG”. Records identified by screening included PubMed (n = 96), Google scholar (n = 29,200), MedRxiv (n = 72), BioRxiv (n = 51), and Research Square (n = 4). Full text of 108 studies were accessed after excluding studies not relevant to the current project and excluding duplicates. A total of 84 manuscripts were selected for analysis after review by two board-certified epilepsy specialists (AA and ZH). We were unable to use part of the data from one study which reported that nine patients had seizures or encephalopathy and three other studies due to lack of specific details. [5,[13], [14], [15]] Findings from two studies with quantitative EEG analysis were included [14,16]. The process of selection of manuscripts for analysis is detailed as per PRISMA recommendations in Fig. 1 [17].
Fig. 1.
PRISMA diagram detailing the selection process of studies in the systematic review. * Reasons for exclusion were absence of description of EEG findings (n = 14) and review articles (n = 7). ** Reasons for exclusion are review articles (n = 7), absence of EEG reports (n = 8), articles related to other corona viruses (n = 6) and articles detailing EEG recording techniques and safety during COVID-19 pandemic (n = 3).
EEG findings of 617 patients reported across 84 selected retrospective studies were reviewed. A systematic analysis was performed for the following data: age, sex, EEG type (e.g. routine, continuous), indication for EEG, co-morbid neurological conditions, and imaging abnormalities. Detailed data were not available for some parameters in all studies and the analysis details reflect availability- for example, data regarding sex were available in 75 studies and data regarding indications for EEG were available in 423 patients. The EEG type was considered as routine EEG, unless specified as continuous EEG or rapid response 8-channel EEG (Ceribell), which was reported in in three studies. Details of available data are noted in Table1 .
Table 1.
Demographic features of patients.
| Demographics | Total number (n) | 617 |
| Median age (IQR)1 | 61.3 years (45−69) | |
| Gender M:F2 | 288:144 | |
| Children (age<18y) | 14 | |
| EEG type | Routine EEG (n, %) | 60, 71.4 % |
| Continuous EEG (n, %) | 24, 28.6 % | |
| Rapid-response 8-channel EEG (Ceribell) (n, %) | 3, 3.6 % | |
| EEG Indications3 | Altered mental status (n, %) | 261, 61.7 % |
| Seizure-like events (n, %) | 132, 31.2 % | |
| Cardiac arrest (n, %) | 15, 3.5 % | |
| Speech issues (n, %) | 7, 1.7 % | |
| Unspecified (n, %) | 8, 1.9 % |
Superscripts indicate the number of studies the data is available from: 1: 73 studies; 2: 75 studies, 3: 423 patients.
EEG abnormalities were classified into the following three broad categories to highlight similar findings and further sub-analyzed as follows: (1) background abnormalities, (2) Periodic and rhythmic EEG patterns, and (3) other epileptiform changes and seizures/status epilepticus. EEG background abnormalities were sub-categorized as follows: diffuse slowing, focal slowing, slow posterior dominant rhythm, absent posterior dominant rhythm, background attenuation/ suppression, and discontinuous EEG/burst suppression, lateralized asymmetry, and decreased reactivity. Periodic and rhythmic activity [18] were sub-categorized by location into generalized, lateralized/multifocal, and unspecified localization.
Frontal lobe findings were re-analyzed separately since several studies reported EEG findings involving the frontal lobe as being a characteristic finding of COVID-19 encephalopathy. [6,9,12] EEGs performed on children with COVID-19 infection were also assessed separate from adult EEG for unique findings.
Wherever possible, the currently accepted EEG nomenclature was substituted in place of older terminology reported. [18] Some of the older terminology was retained when it was unclear what the most appropriate current terminology would be. For example, with SIRPIDS, it was unclear if the activity was ictal, rhythmic or periodic discharges specifically. EEG under sedation and theta slowing were counted as diffuse slowing.
All statistical analyses were performed using MatlabR2018b (MathWorks Inc, Natick, Massachusetts). Proportions, medians, and inter-quartile ranges (IQR) were calculated for descriptive analyses. Proportions were compared using Pearson Chi-square (χ2) test with a significance level of p<0.05.
3. Results
The demographic characteristics, EEG type, and EEG indications of the subjects are summarized in Table 1. Data regarding sex was available in 432 subjects, of whom 144 were females (33.3 %) and 14 were children (2.3 %). The median age of the study participants was 61.3 (IQR 45−69) years. The most common reason for ordering EEGs was altered mental status (n = 261, 61.7 %) followed by seizure-like events (n = 132, 31.2 %). Other indications included cardiac arrest (n = 15, 3.5 %), speech issues (n = 7, 1.7 %), others (n = 8, 1.9 %). The EEG type included routine EEGs in 60 (71.4 %), and continuous EEG monitoring in 24 studies (28.6 %).
Neurological conditions reported that could affect EEG included dementia (n = 18), stroke (n = 47), parkinsonism and related disorders (n = 4), subdural hematoma (n = 7), epilepsy (n = 38), hydrocephalus (n = 1), cognitive delay/ mental retardation (n = 10), encephalitis (n = 5), traumatic brain injury (n = 6), brain tumors (n = 5), anoxia (n = 2), Creutzfeldt-Jakob disease (n = 1), and unspecified “others” (n = 18). When reviewing reported neurological history that can alter EEG, infective, limbic and autoimmune (NMDA) encephalitis were considered together. While sedation can affect the EEG background, most studies did not report whether sedation was present. Two studies that provided this information reported that sedation was used during or immediately prior to EEG in 25 % [12] and 73 % [9] of pateints. Neuroimaging was abnormal in 226 patients (36.6 %) showing small vessel disease, strokes, tumors, encephalomalacia, and T2 hyperintensities. Patient level details of EEG findings and MRI abnormalities were not reported in most studies preventing further EEG-MRI correlation.
While analyzing indications for ordering EEG, delirium, coma, inability to wake up after discontinuation of sedation and confusion were grouped together as “altered mental status”. Transient loss of consciousness and gaze deviation were included with “seizure-like events”. Cognitive delay and mental retardation were counted as a single category.
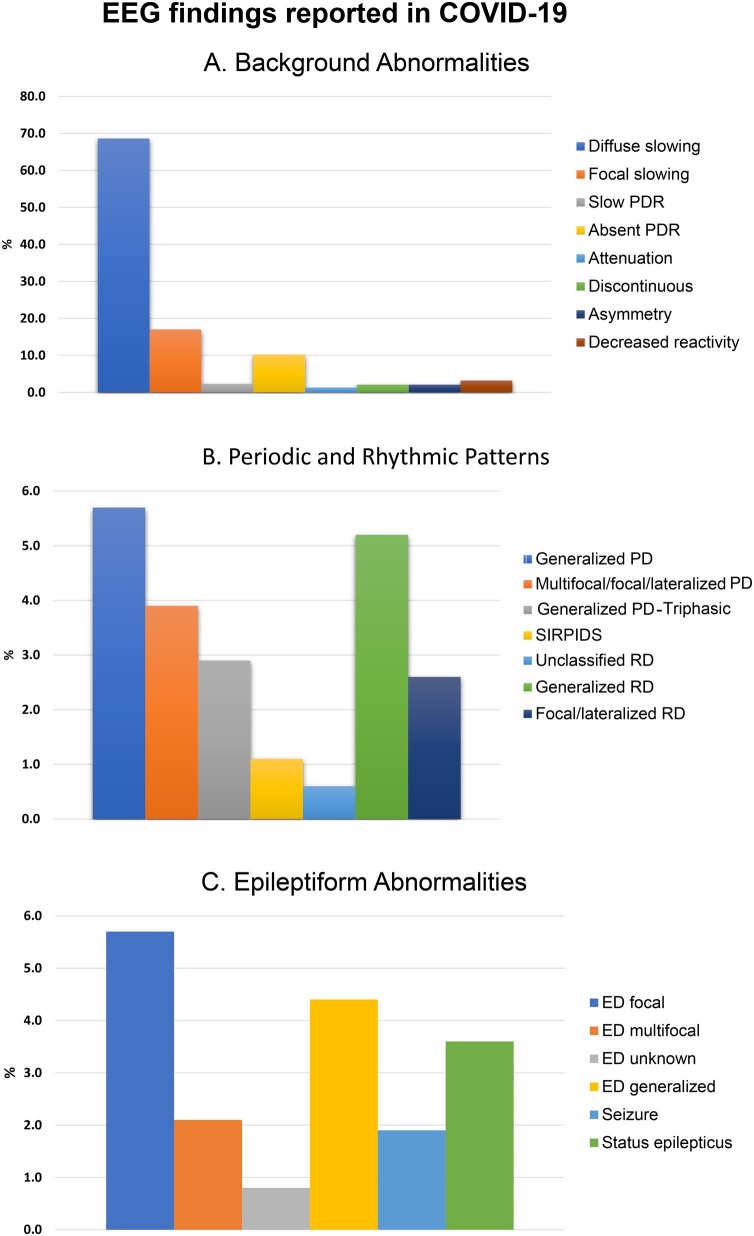
The EEG abnormalities reported are summarized in Fig. 2 and Table 2 . EEG was abnormal in 543 (88.0 %) patients. Nonspecific/ unclear changes were noted in 31 (5.0 %) patients.
Fig. 2.
EEG findings reported in COVID-19 classified by A: Background abnormalities B: Periodic and Rhythmic EEG patterns C: Epileptiform Abnormalities.
Table 2.
EEG features classified by background abnormalities, periodic and rhythmic activity, sharp waves, seizures, and status epilepticus. Note that the number of EEG findings may be higher than the number of patients as a given patient may have more than one finding.
| EEG Abnormality | Number of patients | Percentage of total patients (n = 617) | Percentage of abnormal EEGs in each category |
|---|---|---|---|
| Background Abnormalities (n = 659) | |||
| Diffuse slowing | 423 | 68.6 % | 64.2 % |
| Focal slowing | 105 | 17.0 % | 16.0 % |
| Slowing of PDR | 14 | 2.3 % | 2.1 % |
| Absent PDR | 63 | 10.2 % | 9.6 % |
| Attenuation | 8 | 1.3 % | 1.2 % |
| Discontinuous EEG | 13 | 2.1 % | 2.0 % |
| Asymmetry | 13 | 2.1 % | 2.0 % |
| Decreased reactivity | 20 | 3.2 % | 3.0 % |
| Periodic and Rhythmic patterns (n = 138) | |||
| Generalized PD | 35 | 5.7 % | 25.4 % |
| Lateralized/ multi-focal PD | 24 | 3.9 % | 17.4 % |
| Generalized PD with triphasic morphology. | 18 | 2.9 % | 13.0 % |
| SIRPIDS | 7 | 1.1 % | 5.1 % |
| Unclassified PD | 4 | 0.6 % | 2.9 % |
| Generalized RD | 32 | 5.2 % | 23.2 % |
| Lateralized/ multifocal RD | 16 | 2.6 % | 11.6 % |
| Unclassified RD | 2 | 0.3 % | 1.4 % |
| Epileptiform changes (n = 114) | |||
| Focal ED | 35 | 5.7 % | 30.7 % |
| Generalized ED | 27 | 4.4 % | 23.7 % |
| Multifocal ED | 13 | 2.1 % | 11.4 % |
| Unspecified ED | 5 | 0.8 % | 4.4 % |
| Seizures | 12 | 1.9 % | 10.5 % |
| Status epilepticus | 22 | 3.6 % | 19.2 % |
[PDR- posterior dominant rhythm, PD- periodic discharges, RD- rhythmic discharges, ED- epileptiform discharges].
(1) Background abnormalities: The most common background abnormality noted was diffuse slowing (n = 423, 68.6 %). Other background abnormalities included focal slowing (n = 105,17 %), slow posterior dominant rhythm (n = 14, 2.3 %), absent posterior dominant rhythm (n = 63, 10.2 %), background attenuation/ suppression (n = 8,1.3 %), and discontinuous EEG/burst suppression (n = 13,2.1 %), asymmetry (n = 13, 2.1 %), and decreased reactivity (n = 20, 3.2 %).
(2) Periodic and rhythmic patterns: Periodic discharges were classified as generalized (n = 35, 5.7 %), lateralized/ multifocal (n = 24, 3.9 %), generalized periodic discharges with triphasic morphology (n = 18, 2.9 %), SIRPIDs (n = 7, 1.1 %), and unclassified (n = 4, 0.6 %). Rhythmic activity was sub-categorized into generalized rhythmic delta activity (GRDA) (n = 32, 5.2 %), lateralized/ multifocal rhythmic discharges (n = 16, 2.6 %) and unclassified (n = 2, 0.3 %).
(3) Other epileptiform changes and seizures: Epileptiform discharges were categorized as focal (n = 35, 5.7 %), multifocal (n = 13, 2.1 %), unspecified localization (n = 5, 0.8 %) and generalized (n = 27, 4.4 %). Twelve seizures and 22 cases of status epilepticus were reported.
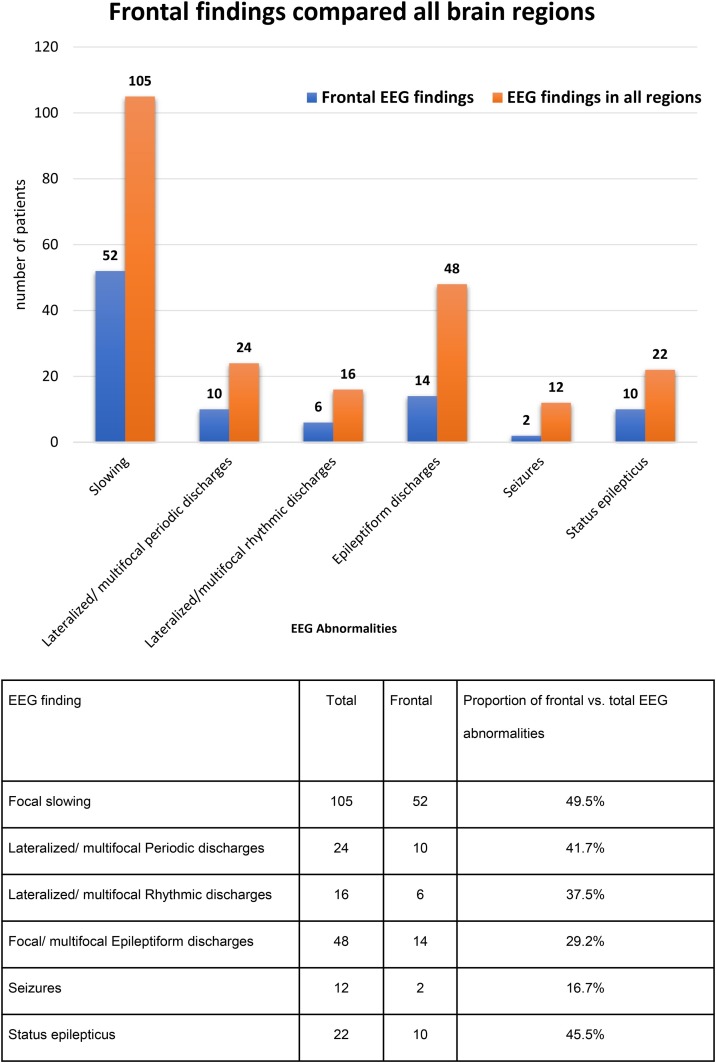
Frontal findings including slowing, periodic and rhythmic discharges, and epileptiform changes were re-analyzed. Several focal EEG abnormalities were more common in the frontal region (Fig. 3 ). Of interest, half of all reported focal slowing (52 out of 105) and status epilepticus (10 out of 22) involved the frontal regions.
Fig. 3.
Frontal EEG findings compared to all brain regions.
We found only 14 EEG reports of children (28 days to 16 years), suggesting a lower incidence of COVID-19 and neurological complications in this population. [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29]] EEGs were ordered for analysis of seizure-like events in all and none had a prior history of neurological disorders. Neuroimaging was abnormal in one child. Slowing was diffuse in five children and frontal in one. Focal IEDs were noted in two children and status epilepticus was reported in one [19]. Follow-up EEGs were available in 37 (6.0 %) patients- EEG improvement was noted in 21, worsening in four, and no significant changes in 12 patients.
The timing of EEG in relation to the duration of COVID-19 infection was not available in most studies. However, a large study of 111 patients reported that EEG studies were performed after a median of 10.8 days after hospitalization. [4] No significant correlation was noted between the timing of EEG and epileptiform abnormalities in relation to the duration of illness. It was noted that EEGs performed early during hospitalization were for cardiac arrest and seizure while the indication for later EEGs was unexplained encephalopathy [4].
In our review, 82.5 % (n = 66) of epileptiform discharges and 67.6 % (n = 23) of seizures/status epilepticus were reported in the 24 studies that utilized continuous EEG monitoring. In addition, 96.8 % (n = 243) of the 251 patients were reported to have abnormalities in studies that utilized continuous EEG, compared to 85.0 % (n = 311) patients who did not undergo continuous EEG monitoring (χ2 = 22.8, p = 0.00001).
4. Discussion
We present a large systematic review of EEG abnormalities in patients with COVID-19. In our review of 617 patients from 84 reports, we found that EEG abnormalities are common and encompass a wide variety of findings such as background abnormalities, periodic and rhythmic activity and other epileptiform abnormalities. The most common reason for obtaining EEG was altered mentation, and the most common EEG finding was diffuse slowing. Of interest, frontal lobe findings were common and included focal slowing, periodic discharges and rhythmic delta activity. Half of all status epilepticus and focal slowing originated in the frontal lobes.
Diffuse background slowing was the most common EEG finding reported in two-thirds (68.6 %) of patients indicating that a diffuse non-specific encephalopathy was the most common brain abnormality in this condition. Other EEG features suggesting diffuse encephalopathy included generalized rhythmic delta activity and generalized periodic discharges with triphasic morphology. Lateralized periodic and rhythmic abnormalities were also seen suggesting a co-existent focal dysfunction in some patients. Epileptiform discharges were common (13.0 %) indicating underlying cortical irritability predisposing to seizures. In fact, 5.5 % had seizures or status epilepticus.
Several studies noted characteristic abnormalities in the frontal region hypothesizing that this correlates with the purported entry of COVID-19 into the brain. [6,[9], [10], [11], [12]] Early clinical manifestations of COVID-19 like anosmia and ageusia are thought to be due to viral entry in the nasal and oral mucosa facilitated by ACE-2 receptors [30]. Subsequent spread to the orbitofrontal region [30,31] via afferent nerves leads to preferential involvement of the olfactory bulb and orbitofrontal/frontal regions and can explain the preponderance of frontal EEG findings. This theory is also corroborated by frontal hypometabolism seen in PET scans in these patients [32]. This viral spread hypothesis and anatomo-clinico-electrophysiological correlation is analogous to the temporal/frontal EEG findings in Herpes simplex encephalitis corresponding with its pattern of anatomic involvement [30].
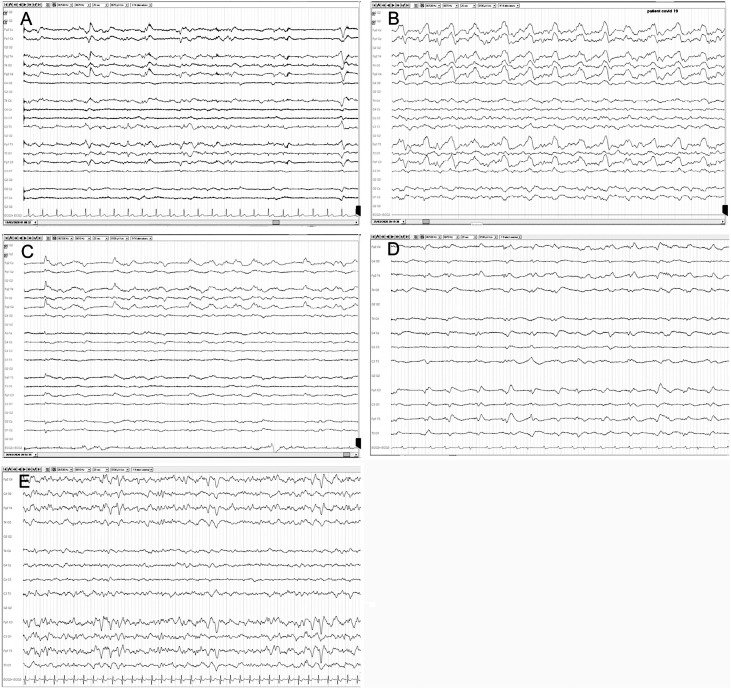
Frontal epileptiform discharges have been proposed as biomarkers for COVID-19. [2,3,6,33,34] Examples of frontal monomorphic biphasic slow waves and periodic discharges are shown in Fig. 4 [35]. Approximately half of all reported status epilepticus (10 out of 22) arose in the frontal regions, compared to 18 % (4 out of 22) involving the occipital lobe. In addition to the frontal lobe, the piriform cortex, limbic structures, thalamus, hypothalamus, brainstem and autonomic structures can also be involved by COVID-19. [36] The more extensive EEG finding seen in other patients could be due to greater viral spread or other factors like pulmonary involvement/hypoxia, cardiac arrest, other metabolic changes, sedation, systemic inflammatory response syndrome, hypercoagulability, vasculitis, and strokes. In support of systemic causes, one report found the severity of EEG abnormalities to correlate with oxygen saturation at admission, but not with neuroimaging [37]. EEG changes could also be due to pre-existing neurological diseases like epilepsy [4,9].
Fig. 4.
Frontal EEG findings in patients with COVID-19.
A: Generalized rhythmic delta activity (GRDA) with intermittent biphasic delta waves in bilateral frontal regions that are symmetric and monomorphic with low-voltage rhythmic background activity. B: GRDA with frequent high-amplitude biphasic delta waves in bilateral frontal regions that are symmetric and polymorphic with low-voltage rhythmic theta background activity. C: Lateralized periodic discharges of high-amplitude monomorphic delta activity with right frontal region predominance and low-voltage rhythmic theta background activity. D: GRDA with intermittent low-amplitude slow biphasic delta waves in bilateral frontal regions that are slightly asymmetric and monomorphic with low-voltage continuous background activity. E: GRDA with intermittent high-amplitude biphasic delta waves in bilateral frontal regions that are symmetric and monomorphic with intermittent low-voltage rhythmic theta background activity (10–20 system, referential montage, 20 s epoch).
Reprinted with permission from Vespignani H, Colas D, Lavin BS, Soufflet C, Maillard L, Pourcher V, et al. Report of EEG Finding on Critically Ill Patients with COVID ‐19. Ann Neurol. 2020 Jun.
In our review, approximately one-third of the patients with EEGs had abnormal neuroimaging. The higher incidence of abnormal neuroimaging in our review could be due to the older age group, larger proportion of pre-existing neurological conditions and the possibility of sicker patients being triaged for neuroimaging. Several studies have shown a higher sensitivity for EEG compared to brain CT or MRI for COVID-19 related encephalopathy. Background abnormalities, with or without epileptiform findings, were frequently seen in patients with neurological symptoms when neuroimaging was unremarkable. [8,37,38] In one study, 59 % of patients with neurological symptoms had an abnormal MRI, while EEG was abnormal in 83 % [13]. In another study, 13 patients with abnormal EEGs suggesting encephalopathy had normal neuroimaging [39].
In general, the extent of EEG abnormalities correlated with the clinical status of patients and pre-existing neurological diseases. [6,8,9] We included only COVID-19 positive patients in our analysis- however, when those who tested negative were reported, it was found that a higher proportion of positive patients had epileptiform abnormalities (40.9 %) compared to those who were negative (16.7 %) [6]. EEG abnormalities may be correlated with the severity of COVID-19 infection. [12,38] A history of CNS pathology was reported in 43 % of patients with epileptiform findings compared to 9 % in those without epileptiform findings [9]. Interestingly, in another study the absence of the posterior dominant rhythm was more common in patients who were on sedation on the day of EEG or the prior to the test compared those who were not on it [9]. A significantly higher proportion of EEG abnormalities- diffuse slowing in 100 %, generalized periodic discharges in 31.8 %, sharp waves in 13.6 % and seizures in 9.1 % were reported in one study, possibly due to greater use of continuous EEG monitoring [8]. In our systematic review, continuous EEG studies reported significantly more abnormalities compared to routine EEGs suggesting higher sensitivity of prolonged EEG monitoring.
In our review, 56.8 % of the follow up EEG studies reported improvement. In a study involving 26 patients, two had iso-electric EEG suggesting brain death and three of the five patients who had periodic discharges on EEG died a few days later. [34] In quantitative EEG of patients with COVID-19, greater delta-theta band spectral power, higher temporal variance, diversity in frequency band and spatial extent at baseline were associated with a better outcome [14].
Previous coronavirus epidemics are also known to cause neurological changes including seizures. The earliest available report of neurological complication of SARS−COV infection was a patient with respiratory failure and seizures, although EEG was not performed. [40] Not many reports of EEG findings are available - a normal EEG in a patient with generalized tonic clinic seizures due to SARS-CoV-1 virus [41] and diffuse slowing in a patient with MERS [42] have been reported. EEG reports are considerably more in COVID-19 compared to these previous coronavirus infections, maybe due to the larger extent of the current pandemic, and more frequent use of EEG including continuous EEG monitoring currently than in the past. [8,41,42]
This study has the shortcomings of other systematic reviews including lack of access to original data such as EEG waveforms. We understand that many normal EEGs will not be reported, and EEGs were possibly performed disproportionately on patients with neurological symptoms. Many patients with suspicion of clinical seizure-like events were placed on anti-seizure medications prior to EEG, which may have reduced the likelihood of detecting epileptiform changes. The diversity of findings reported could partly reflect different terminologies and reading styles used world-wide- for example generalized periodic discharges may be worded as “generalized discharges” or as “periodic discharges” in different papers. The term “triphasic waves” seemed to be used by authors, especially outside the US where the ACNS terminology may not be as widely adopted. However, we used the term “generalized periodic discharges with triphasic morphology” to be consistent with the currently accepted terminology. Even though background abnormalities like slow posterior rhythm, discontinuous background and decreased reactivity were detailed in some studies, several authors did not list them separately.
Several studies discussed labs and MRIs but failed to specify the imaging results of the patients who had EEG. Only some studies specifically reported on EEGs without sedation. [43] While we attempted to quantify specific abnormalities such as frontal changes, many reports including large studies [4] did not have this level of detail, which could have led to under-estimating their relevance. It is difficult to find the total number of patients with EEG abnormalities because several studies do not specifically state the number of subjects with overlap of the reported abnormalities. Despite these limitations, several authors commented that greater use of EEG would help characterize more patients with COVID-19 related encephalopathy. [2,38,44] We would like to add that MedRxiv, BioRxiv and Research Square publish preprints references are preliminary reports of work that have not been peer reviewed. However, they are still important sources of information that speeds up dissemination of scientific information, which is especially important in a rapidly evolving situation like COVID-19.
EEG remains a crucial tool in the management of patients with neurological manifestations of COVID-19 especially with encephalopathy, seizures, and status epilepticus. Abnormalities when present include slowing, periodic discharges, epileptiform discharges, seizures and status epilepticus, indicating the presence of a localized dysfunction, nonspecific encephalopathy and cortical irritability in this condition. The extent of EEG abnormalities correlates with the diagnosis of COVID-19, duration of monitoring, pre-existing neurological conditions like epilepsy and severity of the disease. EEG abnormalities affecting the frontal lobe seems to be common in COVID-19 encephalopathy and has been proposed as a potential biomarker if recorded consistently. We encourage clinical alertness to identify such findings. It has been speculated that frontal EEG findings result from direct brain involvement in COVID-19, while more diffuse changes may result from either systemic involvement or more diffuse viral involvement of the brain.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors report no declarations of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.seizure.2020.10.014.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flamand M., Perron A., Buron Y., Szurhaj W. Pay more attention to EEG in COVID-19 pandemic. Clin Neurophysiol. 2020;131:2062–2064. doi: 10.1016/j.clinph.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balloy G., Leclair-Visonneau L., Péréon Y., Magot A., Peyre A., Mahé P.-J., et al. Non-lesional status epilepticus in a patient with coronavirus disease 2019. Clin Neurophysiol. 2020;131:2059–2061. doi: 10.1016/j.clinph.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellinen J., Carroll E., Friedman D., Boffa M., Dugan P., Friedman D.E., et al. Continuous EEG findings in patients with COVID-19 infection admitted to a New York academic hospital system. Epilepsia. 2020 doi: 10.1111/epi.16667. [DOI] [PubMed] [Google Scholar]
- 5.Parra A., Juanes A., Losada C.P., Álvarez-Sesmero S., Santana V.D., Martí I., et al. Psychotic symptoms in COVID-19 patients. A retrospective descriptive study. Psychiatry Res. 2020;291 doi: 10.1016/j.psychres.2020.113254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galanopoulou A.S., Ferastraoaru V., Correa D.J., Cherian K., Duberstein S., Gursky J., et al. EEG findings in acutely ill patients investigated for SARS-CoV-2/COVID-19: a small case series preliminary report. Epilepsia Open. 2020;5:314–324. doi: 10.1002/epi4.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis S., Dhawan A., Newey C., Nair D., Jehi L., Hantus S., et al. Continuous electroencephalography characteristics and acute symptomatic seizures in COVID-19 patients. Clin Neurophysiol. 2020 doi: 10.1016/j.clinph.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayub N., Cohen J., Jing J., Jain A., Tesh R., Mukerji S.S., et al. Clinical electroencephalography findings and considerations in hospitalized patients with coronavirus SARS-CoV-2. MedRxiv. 2020 doi: 10.1101/2020.07.13.20152207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Stefano P., Nencha U., De Stefano L., Mégevand P., Seeck M. Focal EEG changes indicating critical illness associated cerebral microbleeds in a Covid-19 patient. Clin Neurophysiol Pract. 2020;5:125–129. doi: 10.1016/j.cnp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Guennec L., Devianne J., Jalin L., Cao A., Galanaud D., Navarro V., et al. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia. 2020 doi: 10.1111/epi.16612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrescu A.-M., Taussig D., Bouilleret V. Electroencephalogram (EEG) in COVID-19: a systematic retrospective study. Neurophysiol Clin. 2020;50:155–165. doi: 10.1016/j.neucli.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chougar L., Shor N., Weiss N., Galanaud D., Leclercq D., Mathon B., et al. Retrospective observational study of brain magnetic resonance imaging findings in patients with acute SARS-CoV-2 infection and neurological manifestations. Radiology. 2020 doi: 10.1148/radiol.2020202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pati S., Toth E., Chaitanya G. Quantitative EEG markers to prognosticate critically ill patients with COVID-19: a retrospective cohort study. Clin Neurophysiol. 2020;131:1824–1826. doi: 10.1016/j.clinph.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butt I., Sawlani V., Geberhiwot T. Prolonged Confusional state as first manifestation of COVID‐19. Ann Clin Transl Neurol. 2020 doi: 10.1002/acn3.51067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pastor J., Vega-Zelaya L., Martín Abad E. Specific EEG encephalopathy pattern in SARS-CoV-2 patients. J Clin Med. 2020:9. doi: 10.3390/jcm9051545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch L.J., LaRoche S.M., Gaspard N., Gerard E., Svoronos A., Herman S.T., et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 19.Agha R., Kojaoghlanian T., Avner J.R. Initial observations of COVID-19 in US children. Hosp Pediatr. 2020 doi: 10.1542/hpeds.2020-000257. [DOI] [PubMed] [Google Scholar]
- 20.Cai X., Ma Y., Li S., Chen Y., Rong Z., Li W. Clinical characteristics of 5 COVID-19 cases with non-respiratory symptoms as the first manifestation in children. Front Pediatr. 2020;8:258. doi: 10.3389/fped.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chacón-Aguilar R., Osorio-Cámara J.M., Sanjurjo-Jimenez I., González-González C., López-Carnero J. Pérez-Moneo B. COVID-19: fever syndrome and neurological symptoms in a neonate. An Pediatr (Engl Ed) 2020;92:373–374. doi: 10.1016/j.anpede.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silvagni D., Soloni P., Darra F., Biban P. Self-limited focal epilepsy in a young child with SARS-CoV-2: serendipity or causal association? Research Square Preprint. 2020 doi: 10.21203/rs.3.rs-30907/v1. [DOI] [PubMed] [Google Scholar]
- 23.Dugue R., Cay-Martínez K.C., Thakur K.T., Garcia J.A., Chauhan L.V., Williams S.H., et al. Neurologic manifestations in an infant with COVID-19. Neurology. 2020;94:1100–1102. doi: 10.1212/WNL.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAbee G.N., Brosgol Y., Pavlakis S., Agha R., Gaffoor M. Encephalitis associated with COVID-19 infection in an 11-Year-Old child. Pediatr Neurol. 2020;109:94. doi: 10.1016/j.pediatrneurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farley M., Zuberi J. COVID-19 precipitating status epilepticus in a pediatric patient. Am J Case Rep. 2020;21:e925776-1–e925776-4. doi: 10.12659/AJCR.925776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhavsar S.M., Agarwal S., Lewis R., Ganta A., Roshchina Y.S., Clouser K.N., et al. COVID-19 infection associated with encephalitis in an adolescent. Neurol Clin Pract. 2020;10 doi: 10.1212/CPJ.0000000000000911. 1212/CPJ.0000000000000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.García-Howard M., Herranz-Aguirre M., Moreno-Galarraga L., Urretavizcaya-Martínez M., Alegría-Echauri J., Gorría-Redondo N., et al. Case report: benign infantile seizures temporally associated with COVID-19. Front Pediatr. 2020:8. doi: 10.3389/fped.2020.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swarz J.A., Daily S., Niemi E., Hilbert S.G., Ibrahim H.A., Gaitanis J.N. COVID-19 infection presenting as acute-onset focal status epilepticus. Pediatr Neurol. 2020;112:7. doi: 10.1016/j.pediatrneurol.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DosSantos M.F., Devalle S., Aran V., Capra D., Roque N.R., Coelho-Aguiar J de M., et al. Neuromechanisms of SARS-CoV-2: a review. Front Neuroanat. 2020;14:37. doi: 10.3389/fnana.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohmwald K., Gálvez N.M.S., Ríos M., Kalergis A.M. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haseli S., Karimi-Galougahi M. Reply to “MRI Evaluation of the Olfactory Clefts in Patients with SARS-CoV-2 Infection Revealed an Unexpected Mechanism for Olfactory Function Loss. Acad Radiol. 2020;27:1192. doi: 10.1016/j.acra.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy-Gash F., DE Mesmay M., Devys J.-M., Vespignani H., Blanc R., Engrand N. COVID-19-associated acute cerebral venous thrombosis: clinical, CT, MRI and EEG features. Crit Care. 2020;24:419. doi: 10.1186/s13054-020-03131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vespignani H., Colas D., Lavin B.S., Soufflet C., Maillard L., Pourcher V., et al. Report on electroencephalographic findings in critically ill patients with COVID-19. Ann Neurol. 2020 doi: 10.1002/ana.25814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vellieux G., Rouvel-Tallec A., Jaquet P., Grinea A., Sonneville R., d’Ortho M.-P. COVID-19 associated encephalopathy: Is there a specific EEG pattern? Clin Neurophysiol. 2020;131:1928–1930. doi: 10.1016/j.clinph.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manganelli F., Vargas M., Iovino A., Iacovazzo C., Santoro L., Servillo G. Brainstem involvement and respiratory failure in COVID-19. Neurol Sci. 2020;41:1663–1665. doi: 10.1007/s10072-020-04487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cecchetti G., Vabanesi M., Chieffo R., Fanelli G., Minicucci F., Agosta F., et al. Cerebral involvement in COVID-19 is associated with metabolic and coagulation derangements: an EEG study. J Neurol. 2020 doi: 10.1007/s00415-020-09958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasini E., Bisulli F., Volpi L., Minardi I., Tappatà M., Muccioli L., et al. EEG findings in COVID-19 related encephalopathy. Clin Neurophysiol. 2020;131:2265–2267. doi: 10.1016/j.clinph.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scullen T., Keen J., Mathkour M., Dumont A.S., Coronavirus Kahn L. (COVID-19)-Associated encephalopathies and cerebrovascular disease: the new orleans experience. World Neurosurg. 2019;2020 doi: 10.1016/j.wneu.2020.05.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung Ecw, Chim Ssc, Chan Pks, Tong Yk, Ng Eko, Chiu Rwk, et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. 2003;49:2108–2109. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau K.-K., Yu W.-C., Chu C.-M., Lau S.-T., Sheng B., Yuen K.-Y. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10:342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H., et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kremer S., Lersy F., de Sèze J., Ferré J.-C., Maamar A., Carsin-Nicol B., et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020 doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020 doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.