Abstract
As the incidence of inflammatory bowel disease (IBD) rises and the global population ages, the number of older people living with these conditions will inevitably increase. The challenges posed by comorbid conditions, polypharmacy, the unintended consequences of long-term treatment and the real but often underestimated mismatch between chronological and biological ages underpin management. Significantly, there may be differences in disease characteristics, presentation and management of an older patient with IBD, together with other unique challenges. Importantly, clinical trials often exclude older patients, so treatment decisions are frequently pragmatic, extrapolated from a number of sources of evidence and perhaps primarily dictated by concerns around adverse effects. This review aimed to discuss the epidemiology, clinical features and considerations with management in older patients with IBD.
Keywords: IBD, elderly, ulcerative colitis, crohn's disease
Introduction
As the incidence of inflammatory bowel disease (IBD) rises and the global population ages, the number of older people living with these conditions will inevitably increase.1 2 Clinicians will see two groups of patients: those diagnosed with IBD at a younger age who have attained an older age and individuals who are diagnosed at or after the age of 60 years (the accepted defining age threshold for this group).2 The challenges posed by comorbid conditions, polypharmacy, the unintended consequences of long-term treatment and the real but often underestimated mismatch between chronological and biological ages underpin management. Significantly, there is often a disconnect between chronological age and biological age, and therefore, in the ‘elderly’, one must distinguish between the fit and the frail.3 Clinical trials often exclude older patients, so treatment decisions are frequently pragmatic, extrapolated from a number of sources of evidence and perhaps primarily dictated by concerns around adverse effects. This review aims to discuss the epidemiology, clinical features and considerations with management in older patients with IBD. Where the term elderly or ‘elderly onset’ has been used in the literature, we have indicated this in quotation marks, while we suggest the term ‘older’ be used in preference.
Epidemiology
The majority (65%) of older persons diagnosed with IBD are diagnosed in their 60s, 25% in their 70s and as many as 10% in the ninth decade of life.4
The incidence of older-onset ulcerative colitis (UC) and Crohn’s disease (CD) varies from 1.1/100 000 to 16.5/100 000 and from 0/100 000 to 18.9/100 000, respectively.5 In the large French population-based (EPIMAD) registry, the incidence of UC and CD in the general population was 3.1/10 000 and 2.6/100 000, respectively.4
The incidence of UC is higher than CD in older individuals and, in a study from Hungary, the incidence of UC in this group also increased from 1.09 between 1977 and 1981 to 10.8 per 100 000 from 2002 to 2007.6 Similarly, the incidence of CD in older individuals increased from being virtually unknown until the 1990s to 3.04/100 000 from 2002 to 2007.6 A Dutch population-based study reported that the incidence of ‘elderly-onset’ IBD increased from 11.71/100 000 persons in 1991 to 23.66/100 000 persons in 2010.7
Disease characteristics and natural history
The differential diagnosis of IBD in older individuals is wide (table 1), which may lead to a misdiagnosis (in up to 60%) or delayed diagnosis (by up to 6 years).2
Table 1.
Differential diagnosis in older individuals
| Differential diagnosis | Symptoms that may mimic | Points to aid differential |
| Infectious | Diarrhoea, blood, weight loss | Recent travel; new medication, especially antibiotics |
| Ischaemic colitis | Bloody diarrhoea, abdominal pain usually sudden onset, pain associated after food | Cardiovascular disease Peripheral vascular disease Diabetes, hypertension, hypercholesterolaemia Smoker, arrhythmias |
| Diverticular disease | Left-sided abdominal pain, bloody diarrhoea | Classic left-sided pain Colonoscopic/radiological evidence Segmental colitis associated with diverticulitis Segmental peridiverticular distribution with rectum and proximal colon spared |
| Microscopic colitis | Diarrhoea | Drugs that may contribute non-steroidal anti-inflammatory drugs, lansoprazole, SSRIs Normal macroscopic findings |
| Radiation-induced colitis | Bloody diarrhoea | Previous radiotherapy |
| Solitary rectal ulcer | Rectal bleeding, tenesmus | History of chronic constipation with straining |
| Colorectal cancer | Change in bowel habit, rectal bleeding, weight loss | Systemic features, weight loss |
| Pancreatic insufficiency | Diarrhoea, weight loss | Floating, pale, foul-smelling stools |
SSRI, selective serotonin reuptake inhibitors.
After diagnosis, while older-onset IBD is broadly comparable to that seen in younger patients with IBD, there are clear and significant differences8 (tables 2 and 3). It has been noted that abdominal pain and systemic features of IBD are less commonly reported in older patients.4 9 For CD, colonic disease location is most common (range 30%–65%) followed by ileal (range 10%–50%) and ileocolonic (range 10%–38%), with inflammatory behaviour being the most common (68%–96%), followed by stricturing and penetrating disease.7 Perianal and upper gastrointestinal involvement is less common.
Table 2.
Crohn’s disease in older individuals (compared with younger)
| Location | Colonic disease more common than ileocolonic |
| Bleeding | Less than younger patients |
| Penetration | Less penetration and less stricturing |
| Extraintestinal manifestations | Less common |
| Cancer risk | More common |
Table 3.
Ulcerative colitis in older individuals (compared with younger)
| Location | More likely left-sided or extensive than proctitis, less disease extension |
| Symptoms | Less diarrhoea |
| Flares | Less flares |
| Extraintestinal manifestations | Less common |
| Cancer | More common |
For UC, left-sided disease appears most common (45%, 95% CI 40% to 52%), followed by pancolitis (31%) and proctitis (22%).10 Proximal extension also appears less common.11
A recent meta-analysis showed overall rates of surgery, immunomodulator use and biologic use in patients with UC of 9%, 17% and 4%, respectively.10 Patients with ‘elderly-onset’ UC were less likely to receive immunomodulators (OR 0.60, 95% CI 0.45 to 0.79) or biologic therapies (OR 0.41, 95% CI 0.27 to 0.62) and were more likely to undergo surgery (OR 1.36, 95% CI 1.18 to 1.57; p<0.001), suggesting that it is perhaps physician hesitancy with prescribing biologics rather than a truly indolent disease course.10 11
In the same meta-analysis, overall rates of surgery, immunomodulator use and biologic use in ‘elderly-onset’ CD patients were 32%, 32% and 15%, respectively.10
The rates of disease progression in patients with ‘elderly-onset’ CD have been lower than those in patients with non-elderly-onset CD.4 7 12
Specific challenges in older individuals
Healthcare use
Older patients with IBD are at a higher risk of hospitalisation. A recent US study reported that 25% of all IBD-related hospital admissions in the USA were in patients aged over 65 years. These patients were more likely to be malnourished, anaemic and hypovolaemic, with higher transfusion requirements, requiring longer postoperative hospital stays.13 Venous thromboembolic complications are also more common.2 Higher healthcare use as an outpatient and higher rates for admission to hospital is a common feature across other studies, where a longer average length of stay has also been demonstrated.14 Furthermore, patients with ‘elderly-onset’ IBD have a 1.5 times greater risk of all-cause mortality when compared with the background population (figure 1).15
Figure 1.
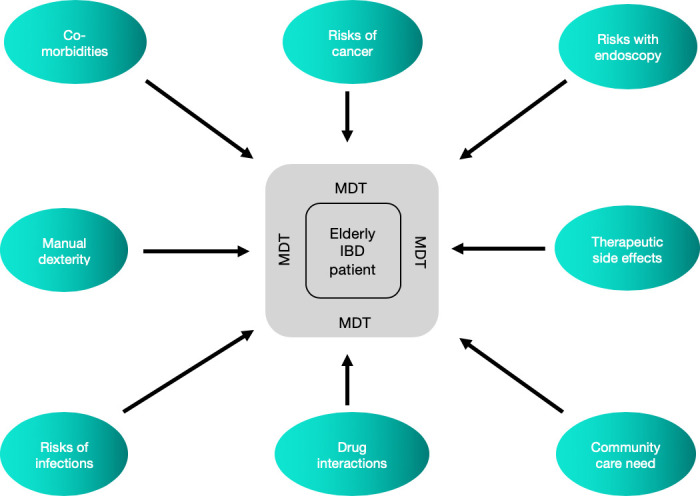
Considerations for treatment of an elderly patient with IBD. IBD, inflammatory bowel disease; MDT, multi-disciplinary team.
Mental and neurodegenerative illnesses
Evidence suggests that in older patients with CD, depression and perceived stress are more common and therefore should be screened and treated early.16 IBD itself may be a risk factor for the development of other cognitive conditions; for example, patients with IBD appear to have a 22% increased risk of Parkinson’s disease when compared with the background population, implicating gut inflammation and the psychoneuroimmunological axis.17
Metabolic bone disease
Bone health is a specific concern. A large population study showed that elderly patients with IBD were 1.3 times more likely to develop a fracture when compared with younger patients, underpinning the need to exercise particular caution when prescribing corticosteroids in this vulnerable group of patients.18 We recommend that older patients with IBD should be risk assessed using a bone mineral density scan and the FRAX score19 to determine their risk of bone disease. Those patients considered at high risk should be considered for bone-protective therapy and repeated bone mineral density scans annually or if stable at least every 3 years.20
Comorbidities
Conditions unrelated to IBD, but coexistent in older patients, will influence treatment efficacy and adverse effects. For example, patients with diabetes and hypertension may not be ideal candidates for steroids.21 In patients with congestive heart failure New York Heart Association (NYHA) class III/IV), anti-tumour necrosis factor (TNF) therapy is contraindicated, and patients with a history of recent malignancy (<2 years) may not be suitable candidates for thiopurines where the risk of lymphoproliferative disorders is higher.22
In one study, elderly patients with IBD were prescribed up to 10 medications, wherein a third had potential drug interactions with IBD medications.23 Furthermore, medication adherence with such a high pill burden cannot be underestimated. Importantly, natural decline in renal, cognitive and cardiovascular function may impact on the pharmacokinetics and pharmacodynamics of IBD medications. Specific medication challenges are discussed in the next section.
Specific medications
Aspirin and non-steroidal anti-inflammatory drugs (NSAIDs)
Many older patients will be treated with NSAIDs and aspirin for a variety of comorbidities. Data are conflicting regarding the safety of NSAIDs in IBD. A long-term follow-up study suggested that they are probably safe in short courses but may be responsible for 20% of clinical relapses, with certain cyclooxygenase-2 (COX-2) inhibitors having a better safety profile in IBD.24 Conversely, aspirin use appears to have no direct impact on IBD course in older persons and should be used as necessary in the context of coexisting medical conditions.25 We advise to try and avoid NSAID use in the elderly with IBD and to continue aspirin if clinically indicated for a comorbidity.
Antibiotics
Antibiotics have been used in some patients with mild–moderate colonic CD, infectious complications of fistulising CD, pouchitis and as an adjunct to surgical drainage of CD-related abscesses.26 While useful, the risk of adverse effects, particularly Clostridium difficile, must be considered. The condition is associated with a significant increase in hospital mortality in older patients,27 28 and antibiotic classes often used in IBD (such as fluoroquinolones) are particular risk factors for C. difficile in the elderly.28
Aminosalicyclates (5-ASAs)
5-ASAs, such as sulfasalazine and mesalazine, are a foundational therapy for UC in elderly patients. Despite their controversial role in CD, in the EPIMAD study, 90% of older CD patients were on 5-ASAs.29 This appears to be similar in other population-based cohorts,4 6 possibly due to prevalent colonic disease location, but could also reflect a reluctance for escalating therapy (physician or patient).
Although it has been established that the combination of oral and topical 5-ASAs in UC is more effective than oral therapy alone, fine motor issues with the use of topical therapy may be a limitation.30 Furthermore, anorectal dysfunction in the elderly ranges between 10% and 25% in hospitalised patients and 4% in outpatients,31 32 an obvious limitation for topical medication requiring retention. This effect is compounded by the presence of active inflammation, but selecting preparations with a lower volume of delivery or foam may be of benefit.
Therefore, a once daily preparation and foam-based preparation with an easy applicator may be more appropriate. 5-ASAs are relatively safe overall, but the British National Formulary guidance still advises ‘caution’ in older patients. Rare side effects can still occur and may be more relevant in an older group with comorbid conditions. These include diarrhoea, especially with olsalazine, pancreatitis and glomerulonephritis.33 As chronic kidney disease is common in older patients, care must be taken when initiating 5-ASA, and regular biochemical monitoring must be adhered to as recommended by the British Society of Gastroenterology (BSG) guidelines.20 5-ASAs interact with six thioguanine, increasing the risk of myelosuppression, and can increase the anticoagulant effects of coumarin-based anticoagulants such as warfarin.34
Corticosteroids
Corticosteroids are the cornerstone for the decisive induction of remission in both CD and UC but well-recognised to be associated with undesirable side effects, and in this is only magnified in older patients.35 Steroids may worsen fluid retention in heart failure, glycaemic control in diabetes, hypertension and osteoporosis, increasing the risks of fragility fractures, and may increase risk of cataracts and glaucoma, all conditions associated with advancing age.36 It is also important to note that corticosteroids interact with phenytoin, phenobarbital, rifampicin and warfarin, reducing their drug activity.2
Corticosteroids also increase the risk of gastrointestinal haemorrhage especially in elderly patients with antiplatelets or anticoagulation agents.34 In addition, they may also be associated with an altered mental states such as delirium, psychosis and hallucinations (perhaps more frequently) in elderly patients.37 These important considerations and the sheer futility of prolonged steroid exposure underpin the need for astute steroid ‘stewardship’ with careful contingency planning with next steps in treatment.38
Budesonide is used for mild to moderate distal small bowel and right colonic CD,37 and its newer modified-released multimatrix system has been licensed for mild to moderate UC. It has a high first-pass metabolism and is therefore reported to have less systemic side effects.35 This may be favourable in the older age group, but more concrete data are needed to position these agents in treatment paradigms for older patients.39
Immunomodulators
A retrospective study by Juneja et al showed that 6% of elderly patients are on thiopurine and 1% are on methotrexate.40 Thiopurines are associated with leucopenia, opportunistic infections, transaminitis and pancreatitis.41 Therefore, regular biochemical monitoring is essential.41 Thiopurines reduce the availability of warfarin, and regular INR monitoring is required while initiating thiopurines.34 The increased risk of non-melanoma skin cancers necessitates annual dermatological examination.42 Of major concern is the risk from lymphoproliferative disorders with a meta-analysis suggesting that the risk was four times greater than the general population.43 Furthermore, like colorectal cancer (CRC), longer duration of IBD and thiopurine exposure were independent risk factors for lymphoproliferative diseases. As such, thiopurines should be avoided if at all possible in older patients, and their use, if considered, should be balanced against other comorbidities and risk of adverse events.20
Methotrexate may be used in CD, but monotherapy is not recommended in UC. It is associated with cytopaenia, transaminitis and gastrointestinal adverse effects such as nausea, diarrhoea and stomatitis.44 In older patients, the side effects are more pronounced, leading to a higher discontinuation rate.45 There are significant pharmacological interactions that should be carefully considered. Ciclosporin (CsA) can be used as a rescue therapy in acute severe UC. However, there are a number of considerations. CsA can worsen hypertension, which is common in the elderly. It can also induce nephrotoxicity when used with antibiotics such as gentamicin, trimethoprim, ciprofloxacin, histamin-2 receptor antagonists such as cimetidine and NSAIDs. CsA has a narrow therapeutic window and its level can be affected by P-450 cytochrome inhibitors and inducers. Therefore, the use of CsA should be carefully weighted against potential risks in older patients with multiple comorbidities and polypharmacy.
Anti-TNFs
Anti-TNFs have a pivotal role in the induction and maintenance of moderate to severely active IBD. However, the evidence supporting efficacy in elderly patients is conflicting. Desai et al demonstrated a lower response rate in the elderly than in younger adults (61% vs 83% p=0.01),46 but this was not apparent in a Leuven cohort (79% vs 83%, p=0.64).47
There is, however, clear evidence for more frequent anti-TNF-related adverse events. An Italian study showed that patients over 60 years old on anti-TNFs are at greater risk of severe infections and mortality compared with younger patients or age-matched patients without anti-TNFs.48 The risk of infection is yet higher in older patients on combination therapy with anti-TNF and an immunomodulator, leading to higher discontinuation rates.46 As mentioned previously, anti-TNF therapy is contraindicated in patients with congestive heart failure (NYHA class III/IV).49 As such, the European Crohn's and Colitis Organisation (ECCO) recommends that when used, monotherapy is preferred over bimodal immunosuppressive therapy in older persons.2 Although there are no specific studies looking at higher trough levels in older patients, it appears that, in general, when necessary, optimising their use to attain adequate (or even higher) trough levels is not associated with an increase in significant adverse effects.50 51
Anti-integrins
Anti-integrins such as vedolizumab are a relatively new addition to the evolving treatment paradigm in IBD. From the landmark GEMINI trials, efficacy and safety appear similar across all age groups. Patients over the age of 55 who were on vedolizumab have the lowest rate of severe infections and side effect-related hospitalisation.52 There were also no differences in malignancy or deaths between different age groups.52 A recent retrospective study exploring the safety and effectiveness of anti-TNFs and vedozilumab in 131 patients showed that both anti-TNFs and vedolizumab had similar safety and effectiveness in the elderly population.53 Head-to-head prospective clinical trials between vedolizumab and other biologics have been completed and will begin to influence the positioning of anti-integrins in treatment algorithms54 in general, but the gut specificity and excellent adverse event profile are understandably appealing in selected older patients with IBD.
Ustekinumab
Ustekinumab is a humanised monoclonoal antibody against a common p40 subunit of interleukin 12 and 23. Following the UNITI trial,55 it was approved for induction and remission of CD in 2017 in the UK. Although there are no studies directly comparing its efficacy and side effects between age groups, long-term safety data through to week 96 from the IM-UNITI study showed that the rates of serious adverse events and serious infections are similar in the active and placebo arms.56 In a psoriasis cohort (where a lower dose of ustekinumab is used), no increased risk of adverse events and infections was demonstrated in elderly patients in two retrospective studies with a total of 46 patients.57 58 Further data will be needed to ensure its safety and effectiveness in the elderly population. The UNIFI study published recently demonstrated its efficacy in UC,59 and ustekinumab is currently under review in the UK as treatment for induction and maintenance of UC.
Janus kinase (JAK) inhibitors
Tofacitinib is an oral small molecule, JAK inhibitor approved by National Institute for Health and Care Excellence (NICE) in 2018 for moderate to severely active UC.60 At 52 weeks, patients receiving tofacitinib 5 mg BD achieved significantly higher remission rates than those receiving placebo (34% vs 11%, p<0.001).61 Again, however, efficacy data in older patients are lacking. The drug is associated with an increased rate of herpes zoster infection especially in elderly patients who are on concomitant glucocorticoid.61 62 Exacerbation of dyslipidaemia may be a concern in older patients and warrant treatment after risk stratification in line with national guidelines.63 Tofacitinib also increases cardiovascular events in older patients.62 Based on the experience in rheumatoid arthritis, the Food and Drug Administration has recently warned a safety alert for the risk of pulmonary embolism and death in patients on 10 mg BD regime, which is used in induction for UC.64 Therefore, further safety data especially in a larger older cohort is needed before specific recommendations can be made, but there are reasonable cautions and a pragmatic decision-making process is required. JAK inhibitors should be used with caution in those with previous pulmonary embolisms, deep vein thrombosis or clotting abnormalities.65
Combined immunosuppression
In the post hoc analysis of the REACT66 trial (a randomised trial comparing early immunosuppression for CD vs standard treatment), the efficacy and safety of combined immunotherapy in the elderly was no different to conventional management in older and younger patients.67
Immunosuppressive medications, however, especially when used in combination with other medications in older age, are associated with an increased risk for opportunistic infections.68–70 A study from the Mayo Clinic showed that three out of four deaths attributable to infliximab treatment were in patients aged 65 years or older. Notably, these patients had a longer disease course (15–26 years), severe disease and comorbid conditions, as well as being on concomitant immunomodulator therapy.
With vedolizumab and ustekinumab, the immunogenicity is low (4%71 and 2.3%,55 respectively), and the benefits of combination therapy with an immunomodulator are less clear. Post hoc analyses from GEMINI 1 and 2 trials did not show any additional benefits with a combination therapy in vedolizumab.72 73 The use of an immunomodulator did not increase the ustekinumab trough level in a prospective study of 62 patients,74 and there are conflicting studies for its synergistic effects.75 76 Therefore, the use of a concomitant immunomodulator is less important in vedolizumab and ustekinumab, and it is not recommended especially in an older population where the risk of side effects is much higher.
Non-disease-modifiable medications
Importantly many older patients may be on other medications for symptoms associated with their IBD which do not modify the disease itself.
Iron
Many patients may require iron replacement therapy. An awareness of important side effects to include constipation, poor tolerance and abdominal pain are particularly important to consider in the older age group.
Loperamide
Urgency and incontinence can be very problematic and a source of embarrassment and social exclusion in an older patient with IBD. It has been reported that up to 24% of patients with IBD experience incontinence, and this is likely to be higher in an older population due to weakening of the anal sphincter muscles and comorbidities.77 Antidiarrhoea agents such as loperamide may help these symptoms. Important side effects in the older patient to be aware of in IBD include dizziness and drowsiness.
Bile salt sequestrants
Bile salt sequestrants are typically used for patients with IBD with concurrent bile acid malabsorption. Specifically, these medications can bind other medications and prevent their clinical effect. Caution must therefore be applied to an older person with IBD using a bile salt sequestrant on concurrent medications. Advice should be provided to take other medications 1 hour before taking a bile salt sequestrant. As these are fat soluble vitamins, an annual check or consideration of fat-soluble vitamin status is recommended.
Diet and nutrition
The role of a dietary support is paramount in the elderly IBD population who are at increased risk of malnutrition. The European Society for Clinical Nutrition and Metabolism recommends that oral nutrition supplements should be considered especially in IBD flare.78 Not only optimising nutrition is beneficial in IBD, but also the Mediterranean and Dietary Approaches to Stop Hypertension diet may have an additional benefit in elderly patients with IBD.79 These diets limit simple carbohydrates, saturated fats and processed food while promoting plant-based food (fruits, vegetables, whole grains, legumes, seeds and nuts) and fish. In addition to an anti-inflammatory effect in CD with the Mediterranean diet (reduction in C reactive protein and normalisation of intestinal microbiota,80 81 they also improve metabolic abnormalities such as cardiovascular diseases and type 2 diabetes, which are common in the elderly. Despite difficulties in conducting robust clinical trials on dietary intervention in elderly patients with IBD, it is intuitive to promote healthy diets in elderly patients with IBD with cardiovascular risk factors.
Surgery
Around 25% of all intestinal IBD surgeries are performed in elderly patients.13 The indications for surgery are the same across all age groups, and evidence suggests that the morbidity and mortality from IBD surgery is similar when compared with a younger age group.6 82 83 As in all age groups, optimisation of nutritional status and general health to the maximal extent possible, is desirable.2 Stoma formation may present specific considerations in an older patient. These include the procedure itself, the postoperative recovery and the management of the stoma afterwards. It has been shown that overall, older patients have longer hospital stays, more postoperative complications and higher mortality rates, but it remains an acceptable option in an older patient.84 Restorative surgeries for UC in selected patients may be appropriate; however, older patients undergoing ileoanal pouch formation have higher postoperative complications and longer lengths of stay.85 These risks therefore need to be weighed up on an individual case-by-case basis, taking into account the alternatives and other comorbidities.
CRC risk and specific cancers not drug-related
Overall, the older population with IBD has a higher risk of cancer of any type than younger patients. A large nationwide study suggested that elderly patients with IBD are three times more likely than the background population to develop cancer when compared with younger patients with IBD, with the highest risks for CRC, non-Hodgkin's lymphoma and urinary tract malignancies.86 A greater risk of non-melanoma skin cancers has also been reported.87 The risks are likely to be multifactorial, including length of time on immunosuppression as well as an increasing duration of IBD (a well-known risk factor for the development of CRC as well as for lymphoproliferative diseases).22 88–91 When considering patients diagnosed with IBD at a later age, a study suggested that there was no increased risk of CRC92 when compared with patients without IBD. While this may be biassed by the lack of long-term follow-up, studies from the USA and Italy also did not find any difference in the CRC risk between the elderly and adults with IBD.87 93
In the UK, CRC screening is offered until a patient reaches 75 years of age. Currently, there are no clear guidelines when to stop screening for patients with IBD, but it has been suggested to continue bowel screening if the clinical outcome and benefits outweigh the risks of doing the procedure. It has been noted that there is an increased risk of complications following a colonoscopy in the elderly,94 and therefore the need for surveillance should be carefully discussed with patients.
Vaccinations
Older patients with IBD can be placed at higher risk of infections due to comorbidities, immunosuppressive agents such as thiopurines, methotrexate and biologics, suboptimal IBD control (leading to poor nutrition) and immunosenescence.95 Patients are considered as immunosuppressed if they are on 20 mg or more of prednisolone or other equivalent steroids, have ongoing treatment with adequate doses of thiopurines, methotrexate or biologics, or had the aforementioned medications in the last 3 months.61 Therefore, live vaccines such as intranasal influenza, BCG, yellow fever, varicella, oral typhoid, anthrax, and measles, mumps and rubella must be avoided in these patients. If live vaccines are required, they must be given at least 3 months after stopping immunosuppressive therapies or at least 3 weeks prior to these agents.95 96
Recommended vaccinations in patients with IBD are similar across all age groups. These include annual inactivated influenza vaccine, 5 yearly pneumococcal vaccine, hepatitis B and meningococcus, especially in those with splenectomy.95 96 Therefore, vaccination serology and history must be taken at the earliest opportunity at the diagnosis of IBD followed by appropriate vaccinations.
Conclusion
A myriad of considerations underpin the holistic and considered management of elderly patients with IBD. These include age-related alterations in biological function, the likely impact of clinical comorbidities and concomitant medications, and locomotor and cognitive function. Effective management must also take into account the potential disconnect between ‘chronological’ and ‘biological’ age. Pragmatism must underpin treatment considerations. Thus, a frail patient at any age with comorbid illness and limited mobility may be at a higher risk from complications of a medical or surgical intervention than an older but ‘fit’ individual, and in some instances, mild or relatively asymptomatic disease may not require aggressive therapy with symptom control perhaps taking precedence. Yet, the message is not to ‘undertreat’, but to recognise the distinction between ‘fit’ versus ‘frail’, consider the potential for more pronounced adverse effects, and thereby set realistic and suitable targets for treatment in providing optimal and personalised care for this group of patients.
Footnotes
Twitter: @jonathansegal85, @IBDdoc
Contributors: JPS, HMTH, JL and BH have all reviewed the literature and prepared the manuscript. All have helped in writing and have contributed to critical revisions of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2018;390:2769–78. 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 2. Sturm A, Maaser C, Mendall M, et al. European Crohn’s and Colitis Organisation Topical Review on IBD in the Elderly: Table 1. J. Crohn’s Colitis 2016;11:jjw188. [DOI] [PubMed] [Google Scholar]
- 3. Katz S, Pardi DS. Inflammatory bowel disease of the elderly: frequently asked questions (FAQs). Am J Gastroenterol 2011;106:1889–97. 10.1038/ajg.2011.271 [DOI] [PubMed] [Google Scholar]
- 4. Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut 2014;63:423–32. 10.1136/gutjnl-2012-303864 [DOI] [PubMed] [Google Scholar]
- 5. Stepaniuk P, Bernstein CN, Targownik LE, et al. Characterization of inflammatory bowel disease in elderly patients: a review of epidemiology, current practices and outcomes of current management strategies. Can J Gastroenterol Hepatol 2015;29:327–33. 10.1155/2015/136960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lakatos PL, David G, Pandur T, et al. Ibd in the elderly population: results from a population-based study in Western Hungary, 1977-2008. J Crohns Colitis 2011;5:5–13. 10.1016/j.crohns.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 7. Jeuring SFG, van den Heuvel TRA, Zeegers MP, et al. Epidemiology and long-term outcome of inflammatory bowel disease diagnosed at elderly Age—An increasing distinct entity? Inflamm Bowel Dis 2016;22:1425–34. 10.1097/MIB.0000000000000738 [DOI] [PubMed] [Google Scholar]
- 8. Greenwald DA, Brandt LJ. Inflammatory bowel disease after age 60. Curr Treat Options Gastroenterol 2003;6:213–25. 10.1007/s11938-003-0003-z [DOI] [PubMed] [Google Scholar]
- 9. Triantafillidis JK, Emmanouilidis A, Nicolakis D, et al. Crohn's disease in the elderly: clinical features and long-term outcome of 19 Greek patients. Dig Liver Dis 2000;32:498–503. 10.1016/S1590-8658(00)80007-9 [DOI] [PubMed] [Google Scholar]
- 10. Ananthakrishnan AN, Shi HY, Tang W, et al. Systematic review and meta-analysis: phenotype and clinical outcomes of Older-onset inflammatory bowel disease. ECCOJC 2016;10:1224–36. 10.1093/ecco-jcc/jjw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song EM, Lee H-S, Park SH, et al. Clinical characteristics and long-term prognosis of elderly onset ulcerative colitis. J Gastroenterol Hepatol 2018;33:172–9. 10.1111/jgh.13826 [DOI] [PubMed] [Google Scholar]
- 12. Limdi JK. Aminosalicylates and Elderly-onset Colonic Crohn’s Disease—More Than Meets the Eye? Crohn’s Colitis 2017;11 10.1093/ecco-jcc/jjx017 [DOI] [PubMed] [Google Scholar]
- 13. Ananthakrishnan AN, Binion DG. Treatment of ulcerative colitis in the elderly. Dig Dis 2009;27:327–34. 10.1159/000228569 [DOI] [PubMed] [Google Scholar]
- 14. Sulz MC, Siebert U, Arvandi M, et al. Predictors for hospitalization and outpatient visits in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2013;25:790–7. 10.1097/MEG.0b013e32836019b9 [DOI] [PubMed] [Google Scholar]
- 15. Olén O, Askling J, Sachs MC, et al. Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964–2014. Gut 2019:gutjnl-2018-317572 10.1136/gutjnl-2018-317572 [DOI] [PubMed] [Google Scholar]
- 16. Goodhand JR, Wahed M, Mawdsley JE, et al. Mood disorders in inflammatory bowel disease: relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm Bowel Dis 2012;18:2301–9. 10.1002/ibd.22916 [DOI] [PubMed] [Google Scholar]
- 17. Villumsen M, Aznar S, Pakkenberg B, et al. Inflammatory bowel disease increases the risk of Parkinson’s disease: a Danish nationwide cohort study 1977–2014. Gut 2019;68:18–24. 10.1136/gutjnl-2017-315666 [DOI] [PubMed] [Google Scholar]
- 18. Ludvigsson JF, Mahl M, Sachs MC, et al. Fracture risk in patients with inflammatory bowel disease: a nationwide population-based cohort study from 1964 to 2014. Am J Gastroenterol 2019;114:291–304. 10.14309/ajg.0000000000000062 [DOI] [PubMed] [Google Scholar]
- 19. Unnanuntana A, Gladnick BP, Donnelly E, et al. The assessment of fracture risk. J Bone Joint Surg Am 2010;92:743–53. 10.2106/JBJS.I.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lamb CA, Kennedy NA, Raine T, et al. British Society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019:gutjnl-2019-318484 10.1136/gutjnl-2019-318484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frauman AG. An overview of the adverse reactions to adrenal corticosteroids. Adverse Drug React Toxicol Rev 1996;15:203–6. [PubMed] [Google Scholar]
- 22. Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. The Lancet 2009;374:1617–25. 10.1016/S0140-6736(09)61302-7 [DOI] [PubMed] [Google Scholar]
- 23. Parian A, Ha CY. Older age and steroid use are associated with increasing polypharmacy and potential medication interactions among patients with inflammatory bowel disease. Inflamm Bowel Dis 2015;27:1 10.1097/MIB.0000000000000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kvasnovsky CL, Aujla U, Bjarnason I. Nonsteroidal anti-inflammatory drugs and exacerbations of inflammatory bowel disease. Scand J Gastroenterol 2015;50:255–63. 10.3109/00365521.2014.966753 [DOI] [PubMed] [Google Scholar]
- 25. Ananthakrishnan AN, Higuchi LM, Huang ES, et al. Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann Intern Med 2012;156:350–9. 10.7326/0003-4819-156-5-201203060-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khan KJ, Ullman TA, Ford AC, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2011;106:661–73. 10.1038/ajg.2011.72 [DOI] [PubMed] [Google Scholar]
- 27. Rezapour M, Galoosian A, Liu B, et al. Clostridium difficile co-infection in inflammatory bowel disease is associated with significantly increased in-hospital mortality. Eur J Gastroenterol Hepatol 2018;30:1041–6. 10.1097/MEG.0000000000001185 [DOI] [PubMed] [Google Scholar]
- 28. Louie TJ, Miller MA, Crook DW, et al. Effect of age on treatment outcomes in Clostridium difficile infection. J Am Geriatr Soc 2013;61:222–30. 10.1111/jgs.12090 [DOI] [PubMed] [Google Scholar]
- 29. Gower-Rousseau C, Vasseur F, Fumery M, et al. Epidemiology of inflammatory bowel diseases: new insights from a French population-based registry (EPIMAD). Digestive and Liver Disease 2013;45:89–94. 10.1016/j.dld.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 30. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohn’s Colitis 2017;11:649–70. 10.1093/ecco-jcc/jjx008 [DOI] [PubMed] [Google Scholar]
- 31. Nigam GB, Limdi JK, Vasant DH. Current perspectives on the diagnosis and management of functional anorectal disorders in patients with inflammatory bowel disease. Therap Adv Gastroenterol 2018;11:175628481881695 10.1177/1756284818816956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rao SSC. Diagnosis and management of fecal incontinence. American College of gastroenterology practice parameters Committee. Am J Gastroenterol 2004;99:1585–604. 10.1111/j.1572-0241.2004.40105.x [DOI] [PubMed] [Google Scholar]
- 33. Gisbert JP, González-Lama Y, Maté J. 5-Aminosalicylates and renal function in inflammatory bowel disease. Inflamm Bowel Dis 2007;13:629–38. 10.1002/ibd.20099 [DOI] [PubMed] [Google Scholar]
- 34. Prelipcean CC, Mihai C, Gogalniceanu P, et al. What is the impact of age on adult patients with inflammatory bowel disease? Clujul Med 2013;86:3–9. [PMC free article] [PubMed] [Google Scholar]
- 35. Frey BM, Frey FJ. Clinical pharmacokinetics of prednisone and prednisolone. Clin Pharmacokinet 1990;19:126–46. 10.2165/00003088-199019020-00003 [DOI] [PubMed] [Google Scholar]
- 36. Akerkar GA, Peppercorn MA, Hamel MB, et al. Corticosteroid-associated complications in elderly Crohn's disease patients. Am J Gastroenterol 1997;92:461–4. [PubMed] [Google Scholar]
- 37. Gomollón F, Dignass A, Annese V, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. ECCOJC 2017;11:3–25. 10.1093/ecco-jcc/jjw168 [DOI] [PubMed] [Google Scholar]
- 38. Selinger CP, Parkes GC, Bassi A, et al. A multi-centre audit of excess steroid use in 1176 patients with inflammatory bowel disease. Aliment Pharmacol Ther 2017;46:964–73. 10.1111/apt.14334 [DOI] [PubMed] [Google Scholar]
- 39. Travis SPL, Danese S, Kupcinskas L, et al. Once-Daily budesonide MMX in active, mild-to-moderate ulcerative colitis: results from the randomised core II study. Gut 2014;63:433–41. 10.1136/gutjnl-2012-304258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Juneja M, Baidoo L, Schwartz MB, et al. Geriatric inflammatory bowel disease: phenotypic presentation, treatment patterns, nutritional status, outcomes, and comorbidity. Dig Dis Sci 2012;57:2408–15. 10.1007/s10620-012-2083-x [DOI] [PubMed] [Google Scholar]
- 41. Present DH, Meltzer SJ, Krumholz MP, et al. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med 1989;111:641–9. 10.7326/0003-4819-111-8-641 [DOI] [PubMed] [Google Scholar]
- 42. Ariyaratnam J, Subramanian V. Association between thiopurine use and nonmelanoma skin cancers in patients with inflammatory bowel disease: a meta-analysis. Am J Gastroenterol 2014;109:163–9. 10.1038/ajg.2013.451 [DOI] [PubMed] [Google Scholar]
- 43. Siegel CA, Marden SM, Persing SM, et al. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease: a meta-analysis. Clin Gastroenterol Hepatol 2009;7:874–81. 10.1016/j.cgh.2009.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morgacheva O, Furst DE. Use of MTX in the elderly and in patients with compromised renal function. Clin Exp Rheumatol 2010;28:S85–94. [PubMed] [Google Scholar]
- 45. Tett SE, Triggs EJ. Use of methotrexate in older patients. Drugs Aging 1996;9:458–71. 10.2165/00002512-199609060-00008 [DOI] [PubMed] [Google Scholar]
- 46. Desai A, Zator ZA, de Silva P, et al. Older age is associated with higher rate of discontinuation of anti-TNF therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis 2013;19:309–15. 10.1002/ibd.23026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lobatón T, Ferrante M, Rutgeerts P, et al. Efficacy and safety of anti-TNF therapy in elderly patients with inflammatory bowel disease. Aliment Pharmacol Ther 2015;42:441–51. 10.1111/apt.13294 [DOI] [PubMed] [Google Scholar]
- 48. Cottone M, Kohn A, Daperno M, et al. Advanced age is an independent risk factor for severe infections and mortality in patients given anti-tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol 2011;9:30–5. 10.1016/j.cgh.2010.09.026 [DOI] [PubMed] [Google Scholar]
- 49. O’Meara S, Nanda KS, Moss AC. Antibodies to infliximab and risk of infusion reactions in patients with inflammatory bowel disease. Inflamm Bowel Dis 2014;20:1–6. 10.1097/01.MIB.0000436951.80898.6d [DOI] [PubMed] [Google Scholar]
- 50.Bodini G, Demarzo MG, Saracco M, et al. High anti-TNF alfa drugs trough levels are not associated with the occurrence of adverse events in patients with inflammatory bowel disease. [DOI] [PubMed]
- 51. Greener T, Kabakchiev B, Steinhart AH, et al. Higher infliximab levels are not associated with an increase in adverse events in inflammatory bowel disease. Inflamm Bowel Dis 2018;24:1808–14. 10.1093/ibd/izy066 [DOI] [PubMed] [Google Scholar]
- 52. Navaneethan U, Edminister T, Zhu X, et al. Vedolizumab is safe and effective in elderly patients with inflammatory bowel disease. Inflamm Bowel Dis 2017;23:E17 10.1097/MIB.0000000000001071 [DOI] [PubMed] [Google Scholar]
- 53. Adar T, Faleck D, Sasidharan S, et al. Comparative safety and effectiveness of tumor necrosis factor α antagonists and vedolizumab in elderly IBD patients: a multicentre study. Aliment Pharmacol Ther 2019;49:873–9. 10.1111/apt.15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sands BE, Peyrin-Biroulet L, Loftus EV, et al. 416a – Vedolizumab shows superior efficacy versus adalimumab: results of Varsity—The first head-to-head study of biologic therapy for moderate-to-severe ulcerative colitis. Gastroenterology 2019;156:S-81 10.1016/S0016-5085(19)36989-6 [DOI] [Google Scholar]
- 55. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med 2016;375:1946–60. 10.1056/NEJMoa1602773 [DOI] [PubMed] [Google Scholar]
- 56. Sandborn WJ, Rutgeerts P, Gasink C, et al. Long-Term efficacy and safety of ustekinumab for Crohn's disease through the second year of therapy. Aliment Pharmacol Ther 2018;48:65–77. 10.1111/apt.14794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hayashi M, Umezawa Y, Fukuchi O, et al. Efficacy and safety of ustekinumab treatment in elderly patients with psoriasis. J Dermatol 2014;41:974–80. 10.1111/1346-8138.12653 [DOI] [PubMed] [Google Scholar]
- 58. Megna M, Napolitano M, Balato N, et al. Efficacy and safety of ustekinumab in a group of 22 elderly patients with psoriasis over a 2-year period. Clin Exp Dermatol 2016;41:564–6. 10.1111/ced.12850 [DOI] [PubMed] [Google Scholar]
- 59. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–14. 10.1056/NEJMoa1900750 [DOI] [PubMed] [Google Scholar]
- 60. NICE Overview tofacitinib for moderately to severely active ulcerative colitis guidance.
- 61. Sandborn WJ, Su C, Sands BE, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. 10.1056/NEJMoa1606910 [DOI] [PubMed] [Google Scholar]
- 62. Cohen SB, Tanaka Y, Mariette X, et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann Rheum Dis 2017;76:1253–62. 10.1136/annrheumdis-2016-210457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. NICE Lipid modification: cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. [DOI] [PubMed]
- 64. Desai RJ, Pawar A, Weinblatt ME, et al. Comparative risk of venous thromboembolism in rheumatoid arthritis patients receiving tofacitinib versus those receiving tumor necrosis factor inhibitors: an observational cohort study. Arthritis Rheumatol 2019;71:892–900. 10.1002/art.40798 [DOI] [PubMed] [Google Scholar]
- 65. D’Amico F, Fiorino G, Furfaro F, et al. Janus kinase inhibitors for the treatment of inflammatory bowel diseases: developments from phase I and phase II clinical trials. Expert Opin Investig Drugs 2018;27:595–9. 10.1080/13543784.2018.1492547 [DOI] [PubMed] [Google Scholar]
- 66. Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn's disease (react): a cluster randomised controlled trial. The Lancet 2015;386:1825–34. 10.1016/S0140-6736(15)00068-9 [DOI] [PubMed] [Google Scholar]
- 67. Singh S, Stitt LW, Zou G, et al. Early combined immunosuppression may be effective and safe in older patients with Crohn’s disease: post hoc analysis of REACT. Aliment Pharmacol Ther 2019;49:1188–94. 10.1111/apt.15214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Toruner M, Loftus EV, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008;134:929–36. 10.1053/j.gastro.2008.01.012 [DOI] [PubMed] [Google Scholar]
- 69. Colombel J-F, Loftus EV, Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn’s disease: The Mayo Clinic experience in 500 patients. Gastroenterology 2004;126:19–31. 10.1053/j.gastro.2003.10.047 [DOI] [PubMed] [Google Scholar]
- 70. Lorenzetti R, Zullo A, Ridola L, et al. Higher risk of tuberculosis reactivation when anti-TNF is combined with immunosuppressive agents: a systematic review of randomized controlled trials. Ann Med 2014;46:547–54. 10.3109/07853890.2014.941919 [DOI] [PubMed] [Google Scholar]
- 71. Rosario M, Dirks NL, Milch C, et al. A review of the clinical pharmacokinetics, pharmacodynamics, and immunogenicity of Vedolizumab. Clin Pharmacokinet 2017;56:1287–301. 10.1007/s40262-017-0546-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Colombel J-F. Sa1270 Efficacy of Vedolizumab With Concomitant Corticosteroid or Immunomodulator Use in Patients With Crohn’s Disease From GEMINI 2. Gastroenterology 2015;148:S-277. [Google Scholar]
- 73. Colombel J-F, Loftus EV, Siegel CA, et al. Sa1271 efficacy of Vedolizumab with concomitant corticosteroid or immunomodulator use in patients with ulcerative colitis from gemini 1. Gastroenterology 2015;148:S-277–8. 10.1016/S0016-5085(15)30911-2 [DOI] [Google Scholar]
- 74. Battat R, Kopylov U, Bessissow T, et al. Association between ustekinumab Trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn's disease. Clin Gastroenterol Hepatol 2017;15:1427–34. 10.1016/j.cgh.2017.03.032 [DOI] [PubMed] [Google Scholar]
- 75. Ma C, Fedorak RN, Kaplan GG, et al. Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn's disease: real world experience from a multicentre cohort. Aliment Pharmacol Ther 2017;45:1232–43. 10.1111/apt.14016 [DOI] [PubMed] [Google Scholar]
- 76. Khorrami S, Ginard D, Marín-Jiménez I, et al. Ustekinumab for the treatment of refractory Crohnʼs disease. Inflamm Bowel Dis 2016;22:1662–9. 10.1097/MIB.0000000000000842 [DOI] [PubMed] [Google Scholar]
- 77. Norton C, Dibley LB, Bassett P. Faecal incontinence in inflammatory bowel disease: associations and effect on quality of life. Journal of Crohn's and Colitis 2013;7:e302–11. 10.1016/j.crohns.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 78. Owczarek D, Rodacki T, Domagała-Rodacka R, et al. Diet and nutritional factors in inflammatory bowel diseases. World J Gastroenterol 2016;22:895–905. 10.3748/wjg.v22.i3.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eder P, Niezgódka A, Krela-Kaźmierczak I, et al. Dietary support in elderly patients with inflammatory bowel disease. Nutrients 2019;11:1421 10.3390/nu11061421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Marlow G, Ellett S, Ferguson IR, et al. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn's disease patients. Hum Genomics 2013;7:24 10.1186/1479-7364-7-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Molendijk I, Marten JE VLE. P592 towards a food pharmacy: increased dietary quality reduces CRP and improves quality of life in IBD patients in remission. Available: https://www.ecco-ibd.eu/publications/congress-abstract-s/abstracts-2019/item/p592-towards-a-food-pharmacy-increased-dietary-quality-reduces-crp-and-improves-quality-of-life-in-ibd-patients-in-remission.html [Accessed 22 OCt 2019].
- 82. OP005. Is elderly-onset ulcerative colitis a different entity? - Natural disease course and treatment response compared to adult-onset disease in the population-based IBD-SL cohort. Available: https://www.ecco-ibd.eu/publications/congress-abstract-s/abstracts-2014/item/op005-is-elderly-onset-ulcerative-colitis-a-different-entity-natural-disease-course-and-treatment-response-compared-to-adult-onset-disease-in-the-population-based-ibd-sl-cohort.h [Accessed 23 Jun 2019].
- 83. European Crohn's and Colitis Organisation - ECCO P303. Ulcerative colitis (UC) in the elderly - Moderate at onset but then a milder course? An IG-IBD study, 2014. Available: https://www.ecco-ibd.eu/publications/congress-abstract-s/abstracts-2014/item/p303-ulcerative-colitis-uc-in-the-elderly-moderate-at-onset-but-then-a-milder-course-an-ig-ibd-study.html [Accessed 23 Jun 2019].
- 84. Bosshardt TL. Outcomes of ostomy procedures in patients aged 70 years and older. Arch Surg 2003;138:1077 10.1001/archsurg.138.10.1077 [DOI] [PubMed] [Google Scholar]
- 85. Colombo F, Sahami S, de Buck Van Overstraeten A, et al. Restorative proctocolectomy in elderly IBD patients: a multicentre comparative study on safety and efficacy. ECCOJC 2016;111:jjw209 10.1093/ecco-jcc/jjw209 [DOI] [PubMed] [Google Scholar]
- 86. Khan N, Vallarino C, Lissoos T, et al. Risk of malignancy in a nationwide cohort of elderly inflammatory bowel disease patients. Drugs Aging 2017;34:859–68. 10.1007/s40266-017-0498-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hou JK, Feagins LA, Waljee AK. Characteristics and behavior of Elderly-onset inflammatory bowel disease: a multi-center US study. Inflamm Bowel Dis 2016;22:2200–5. 10.1097/MIB.0000000000000849 [DOI] [PubMed] [Google Scholar]
- 88. Armstrong RG, West J, Card TR. Risk of cancer in inflammatory bowel disease treated with azathioprine: a UK population-based case-control study. Am J Gastroenterol 2010;105:1604–9. 10.1038/ajg.2009.745 [DOI] [PubMed] [Google Scholar]
- 89. Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666–89. 10.1136/gut.2009.179804 [DOI] [PubMed] [Google Scholar]
- 90. Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 2010;138:738–45. 10.1053/j.gastro.2009.12.037 [DOI] [PubMed] [Google Scholar]
- 91. Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. N Engl J Med Overseas Ed 1990;323:1228–33. 10.1056/NEJM199011013231802 [DOI] [PubMed] [Google Scholar]
- 92. Cheddani H, Dauchet L, Fumery M, et al. Cancer in elderly onset inflammatory bowel disease: a population-based study. Am J Gastroenterol 2016;111:1428–36. 10.1038/ajg.2016.304 [DOI] [PubMed] [Google Scholar]
- 93. Fries W, Viola A, Manetti N, et al. Disease patterns in late-onset ulcerative colitis: Results from the IG-IBD "AGED study". Dig Liver Dis 2017;49:17–23. 10.1016/j.dld.2016.09.006 [DOI] [PubMed] [Google Scholar]
- 94. Bielawska B, Day AG, Lieberman DA, et al. Risk factors for early colonoscopic perforation include non-gastroenterologist endoscopists: a multivariable analysis. Clin Gastroenterol Hepatol 2014;12:85–92. 10.1016/j.cgh.2013.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohn’s Colitis 2014;8:443–68. 10.1016/j.crohns.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 96. Wasan SK, Baker SE, Skolnik PR, et al. A practical guide to vaccinating the inflammatory bowel disease patient. Am J Gastroenterol 2010;105:1231–8. 10.1038/ajg.2009.733 [DOI] [PubMed] [Google Scholar]


