Abstract
Acute-on-chronic liver failure (ACLF) is a recently described entity in chronic liver disease defined by acute hepatic decompensation, organ failure and a high risk of short-term mortality (usually less than 4 weeks). This condition is distinct from acute liver failure and stable progression of cirrhosis in numerous ways, including triggering precipitant factors, systemic inflammation, rapid progression and a potential for recovery. While a clear definition of ACLF has been forwarded from a large European Consortium study, some heterogeneity remains in how patients present and the types of organ failure, depending on whether they are described in Asian or European studies. Active alcoholism, acute alcoholic hepatitis and infections are the most frequent precipitants for ACLF. Underpinning the pathophysiology of ACLF is a state of persistent inflammation and immune dysfunction, collectively driving a systematic inflammatory response syndrome and an increased propensity to sepsis. Prevention and early treatment of organ failure are key in influencing survival. Given increasing organ shortage and more marginal grafts, liver transplantation is a limited resource and emphasises the need for new therapies to improve ACLF outcomes. Recent data indicate that liver transplantation has encouraging outcomes even in patients with advanced ACLF if patients are carefully selected during the permissive window of clinical presentation. ACLF remains a significant challenge in the field of hepatology, with considerable research and resource being channelled to improve upon the definition, prognostication, treatment and unravelling of mechanistic drivers. This Review discusses updates in ACLF definition, prognosis and management.
Keywords: cirrhosis, inflammation, liver failure, liver transplantation
Introduction
Liver cirrhosis is the result of progressive fibrosis in patients with chronic liver disease of any aetiology, and is associated with a poor prognosis, once hepatic decompensation starts.1 Cirrhosis has two main phases: the compensated phase, where patients maintain preserved liver synthetic function and have no significant extrahepatic organ impairment; this is to be compared with a decompensated phase, where increasing ascites and loss of liver synthetic function, together with presentation with other organ impairment, are common clinical presentations. Renal failure, hepatic encephalopathy (HE), recurrent infections and upper gastrointestinal bleeding from worsening portal hypertension are considered end-stage complications of decompensated cirrhosis. The term acute-on-chronic liver failure (ACLF) is used to describe the clinical syndrome where acute hepatic decompensation leads to organ failures in the setting of liver cirrhosis. ACLF was first described in 20022 where extrahepatic organ failure and systemic inflammatory response were thought to be critical factors underpinning its evolution. A decade later, a clear definition of the syndrome has emerged,3 and recognizes ACLF as a dynamic syndrome associated with high short-term mortality. Gauging the true prevalence of ACLF is problematic given historical variability in definitions across continents4 but probably occurs in about 20%–30% of hospitalised patients with cirrhosis.3 5
Definitions
There are currently three proposed definitions of ACLF, all in agreement over high early mortality but differing in aspects related to precipitating factors, nature of underlying state of liver disease and the definition of organ failures.
In a large European Consortium of Chronic Liver Failure Research (EF-CLIF) study initiative, referred to as the CANONIC study,3 with data derived from data on 1343 patients among 29 partnering institutions, ACLF was defined as ‘an acute deterioration of pre-existing chronic liver disease, sometimes related to a clear precipitating event, and associated with increased mortality at 28 days, reaching up to 76% in advanced ACLF grades due to multi-system organ failure’.3
In comparison, the Asian Pacific Association for the Study of the Liver (APASL) generated a consensus ACLF definition6 in 2009 by analysing data from 200 patients. This was further revised in 2014,6 based on a prospective follow-up of over 1300 patients. The revised APASL consensus defines ACLF as an acute hepatic insult, manifesting as jaundice (defined as serum bilirubin ≥5 mg/dL (≥85 µmol/L)) and coagulopathy (defined as international normalised ratio ≥1.5 or prothrombin activity <40%), complicated within 4 weeks by clinically detected ascites and/or encephalopathy, in a patient with or without a previous diagnosis of chronic liver disease or cirrhosis, and associated with a high 28-day mortality.6
Recently, the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) proposed another definition of ACLF that defines it as ‘a condition in patients with underlying chronic liver disease with or without cirrhosis that is associated with mortality within 3 months in the absence of treatment of the underlying liver disease, liver support, or liver transplantation’.7 The NACSELD definition is broad, and the APASL definition is most relevant to the predominance of viral hepatitis as a driver of the ‘hepatic hit’ seen in Asia. There is currently ongoing research to further validate the existing definition of ACLF. The ACLARA study seeks to assess the applicability of the CANONIC definition in Latin America. Key differences between the European (CANONIC), Asian (APASL) and North American (NACSELD) definitions are highlighted in table 1.
Table 1.
Major differences between EASL, APASL and NACSELD definitions of ACLF
| EASL | APASL | NACSELD | |
| Definition | An acute deterioration of pre-existing chronic liver disease usually related to a precipitating event and associated with increased mortality at 4 weeks due to multisystem organ failure | Acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease associated with a high 4-week mortality | A condition in patients with underlying chronic liver disease with or without cirrhosis that is associated with mortality within 3 months in the absence of treatment of the underlying liver disease, liver support or liver transplantation |
| Liver failure cut-off values | A cut-off of bilirubin >12 mg/dL | A cut-off value of bilirubin >5 mg/dL and INR>1.5 or prothrombin activity of <40% to define liver failure | None |
| Extrahepatic failure definitions |
|
West Haven HE grades 3–4 |
|
| Type of acute insult | Mainly alcohol and bacterial infections | Mainly viral infections | Not specified but mainly bacterial infections |
| Duration between acute insult and ACLF | No duration specified | 4 weeks | 3 months |
| Disease severity score | CLIF-SOFA score | No defined score to assess severity |
Modified from that published by Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426–37.
ACLF, acute-on-chronic liver failure; APASL, Asian Pacific Association for the Study of the Liver;CLIF-SOFA, Chronic Liver Failure-Sequential Organ Failure Assessment; EASL, European Association for the Study of the Liver;HE, hepatic encephalopathy; INR, international normalised ratio; NACSELD, North American Consortium for the Study of End-Stage Liver Disease.
Diagnostic features and precipitating factors
Although there is not a universal agreement about the definition of ACLF, there is a wide agreement that ACLF is a distinct syndrome that is different from chronic progressive hepatic decompensation. In most cases of ACLF, patients present initially with clinical manifestations of a decompensating event, usually renal impairment, worsening of abdominal ascites, jaundice or HE and often precipitated by bacterial infection. Precipitating factors for the development of ACLF vary depending on the geographical area and the patient populations. Eastern countries suffer from exacerbations of hepatitis B virus (HBV) as the main precipitating factor, followed by alcohol binge and bacterial infections. In Western countries, alcohol followed by bacterial infections are the main key precipitants for acute decompensation (AD).8 9 Importantly, up to 40% of patients with ACLF have no recognisable precipitating event,3 and in these circumstances, ACLF development may be explained by the presence of undetected infections, unidentified toxic liver injury or bacterial translocation.10
Organ failure in the presence of AD of chronic liver disease is the hallmark for diagnosing ACLF, and in the CANONIC study, ACLF was graded based on the relation between organ failures and short-term mortality. A classification of ACLF grades using a modified Sequential Organ Failure Assessment (SOFA) score (table 2), which evaluates liver, kidney, brain, circulation and respiratory functions, grading ACLF from 1 to 3, is shown in table 2: ACLF grade 1, where there is either isolated renal failure or a single non-renal organ failure, which is associated with either renal impairment (defined as a creatinine of 1.5–1.9 mg/dL) or isolated grade I–II HE.3 Based on the CANONIC study data, the 28-day mortality rate in ACLF-1 is 23%. Grade 2 ACLF is defined as two organ failures with a 28-day mortality rate of 32%, while grade 3 ACLF is defined by three or more organ failures and a mortality rate of >75%.3
Table 2.
Classification and grading of ACLF according to EASL definition42
| ACLF grade | Clinical presentation |
| No ACLF | No organ failure, or single non-kidney organ failure, creatinine <1.5 mg/dL, no HE |
| ACLF grade 1 | Single renal failure OR Single non-kidney organ failure, creatinine 1.5–1.9 mg/dL and/or HE grades 1–2 |
| ACLF grade 2 | Two organ failures |
| ACLF grade 4 | Three or more organ failures |
ACLF, acute-on-chronic liver failure; EASL, European Association for the Study of the Liver; HE, hepatic encephalopathy.
Pathogenesis
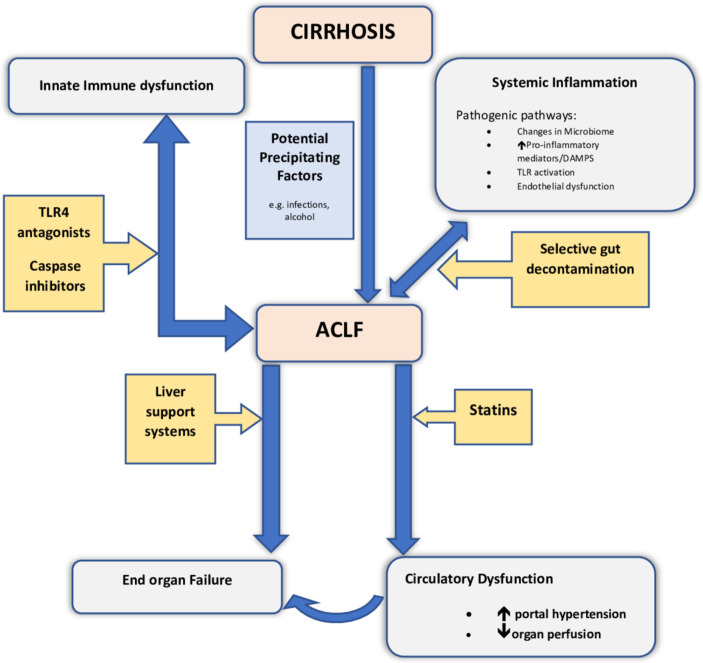
Following a potential precipitating event, patients present with AD and manifest features of systemic inflammation with cytokine release and release of damage-associated molecular patterns (DAMPs), with consequent Toll-like receptor activation.11 These features are distinct from patients with decompensation of cirrhosis alone.12 Excess systemic inflammation leads to severe circulatory dysfunction, aggravation of portal hypertension and subsequent impaired tissue perfusion and end-organ failure.13 It has been suggested that systemic inflammation can drive progression to ACLF independent of circulatory disturbance12 and this may in part be through an imbalance in immune function, whereby proinflammatory factors are unchecked by a state of relative immunoparesis. The most studied pathophysiological factors underpinning the development of ACLF are shown in the schematic in figure 1.
Figure 1.
Schematic figure showing pathogenesis of ACLF and potential therapeutic targets. This figure illustrates various contributory factors in the pathogenesis and progression of cirrhosis to ACLF, and potential targets for therapy. Precipitating events in patients with established liver cirrhosis can induce ACLF through various mechanisms including augmentation of immune dysfunction and aggravating several pathogenic pathways including endothelial dysfunction and generation of proinflammatory cytokines. These collectively promote evolution to organ failure and the development of ACLF. Also highlighted are potential targets for therapy, some under consideration in current clinical trial settings. ACLF, acute-on-chronic liver failure; DAMP, damage-associated molecular pattern; TLR4, toll-like receptor 4.
The most common precipitating factors for developing ACLF are bacterial infection and alcohol. Bacterial infection can trigger inflammation through pathogen-associated molecular patterns, resulting in production of inflammatory mediators that cause tissue damage. Tissue damage in turn can lead to the release of DAMPs, which further accentuate the inflammatory process. In this way, inflamed/necrotic hepatocytes also contribute to the inflammatory milieu and give rise to metabolites that may further contribute to multiorgan failure and immune dysfunction.14 Alcohol also contributes directly by impairing immune responses and enabling gut bacterial translocation, which further drives inflammation and perturbed immune function.
Immunosuppression, for instance, when applied in cases of alcoholic hepatitis, may also contribute to the pathogenesis of ACLF, largely through augmentation of immune paresis, and thereby further facilitate development of bacterial infections.15 16 Overexpression of a protein from monocyte subsets known as surface tyrosine-protein kinase Mer (encoded by MERTK) is found in patients with ACLF and along with prostaglandin E2, they have been suggested to play a role in immune modulation in ACLF.17 Furthermore, numerous lines of investigation suggest patients with cirrhosis incur perturbations in their gut microbiota and their intestinal barrier defence mechanisms, and these correlate with the severity of liver disease.18 This is likely to be exacerbated by excess alcohol and/or immunosuppression, further compounding the development of ACLF in such patients.
The role of cell death resulting from hepatocellular inflammation has also been investigated by studying biomarkers such as the caspase-cleaved neoepitope of cytokeratin-18, known as M30, and intact cytokeratin-18 variant (M65) antigens. Cao et al 19 showed that the ratio of M30:M65 was a good indicator of apoptosis severity, which was noticeably higher in patients with ACLF when compared with chronic HBV cirrhosis. Interestingly, a dynamic measurement of M30/M65 was increased in survivors, supporting the importance of apoptotic pathways influencing recovery in HBV-induced ACLF. A more recent study has shown that caspase-cleaved keratin-18 (cK18) can predict the progression of AD to ACLF.11
Prognostication in ACLF
The presence of ACLF alters the course of cirrhosis decompensation. By example, the CANONIC study shows that the presence of ACLF changes the phenotype of HE and is coupled with a worse outcome when compared with patients with cirrhosis who develop HE without ACLF.20 Similarly, while there are limited data on the impact of ACLF on the natural history and presentation of variceal bleeding, Mehta et al have shown that the development of ACLF and its associated inflammatory response markedly changes intrahepatic haemodynamics with subsequent increase in hepatic venous pressure gradient and decrease in hepatic blood flow.21 This compounding of ACLF on portal hypertension has been recapitulated by others, including the demonstration of ACLF as an independent predictor of mortality in patients presenting with acute gastric variceal bleeding.22 23 When considering the impact of ACLF on organ function, it is noteworthy that chronic liver failure (CLIF) Organ Failure scores provide greater prognostic accuracy in predicting 28 and 90-day mortality in patients with renal failure, compared with traditional acute kidney injury (AKI) classification.24 In fact, development of renal failure is deemed a key factor in determining outcome of patients with ACLF.
Both Child-Pugh and Model for End-Stage Liver Disease (MELD) scores were initially used as the only available tools to predict outcomes in patients with ACLF. The use of these scores has limitations as they do not include measures of extrahepatic organ failure, nor markers of systemic inflammation, both key features of ACLF, while the Child-Pugh score also incorporates subjective variables such as grading of ascites.25
A defined output of the CANONIC study was to provide a classification system that was able to describe specific ACLF phenotypes and validate a method to prognosticate outcomes in these patients.26 Thus, different grades of ACLF ranging from 1 to 3 were introduced using a modified SOFA score. For each organ system, a score was attributed leading to a linear scale of severity ranging up to 100. The resultant ACLF grading was found to improve prognostication in this patient cohort. Moreover, it was suggested that the response to treatment of ACLF could be monitored daily by calculating the CLIF Consortium ACLF (CLIF-C ACLF) score, which includes the CLIF-C Organ Failure score, age and white cell count, and serves as a dynamic prognostic tool, as detailed in table 3. Further validation studies27 have shown that CLIF-C ACLF score can be used independently to prognosticate in ACLF and with better accuracy, when compared with pre-existent scoring systems like the MELD and Child-Pugh scores. Indeed, the CLIF-C ACLF score may guide treatment options, especially when considering liver transplantation in those with the highest mortality risk but also greatest potential benefit (ACLF grade 3), or when defining ceilings of care based on very poor predicted outcome, as shown for CLIF-C ACLF score >70.28 Importantly, the resolution of ACLF is the key determinant of short and medium-term mortality, as indicated by changes in CLIF-C ACLF grade. Table 4 demonstrates the dynamic course of ACLF, and the importance a given grade imparts to transplant-free survival. Assessment of ACLF grade at days 3–7 after diagnosis may represent the best time point to define the clinical course and outcome in patients with ACLF, including considerations such as liver transplantation.27 The initial ACLF grade at presentation was found to be highly predictive of mortality, as was the change in ACLF grade over the first 7 days of evolution.
Table 3.
Derivitisation of the Chronic Liver Failure Consortium Organ Failure (CLIF-C OF) score
| Organ/system | Variable | Score=1 | Score=2 | Score=3 |
| Liver | Bilirubin (mg/dL) | <6 | ≥6 to <12 | ≥12 |
| Coagulation | INR | <2 | ≥2 to <2.5 | ≥2.5 |
| Kidney | Creatinine (mg/dL) | <2 | ≥2 to <3.5 | ≥3.5 or renal replacement therapy |
| Brain | Encephalopathy grade by West Haven Criteria | 0 | 1–2 | 3–4 |
| Circulation | MAP (mm Hg) | ≥70 | <70 | Vasopressors |
| Respiratory | PaO2/FiO2 | >300 | >200 and ≤300 | ≤200 |
| SpO2/FiO2 | >357 | >214 and ≤357 | ≤214 |
Table 3 is reproduced from Jalan R et al, J Hepatol 2014; 61: 1038-47.
FiO2, fraction of inspired oxygen; INR, international normalised ratio; MAP, mean arterial pressure; PaO2, arterial oxygen tension; SpO2, pulse oximetry saturation.
Table 4.
Clinical course and grades of ACLF with associated 28 and 90-day transplant-free mortality
| Initial ACLF grade | Final grade | |||
| No ACLF | ACLF-1 | ACLF-2 | ACLF-3 | |
| ACLF-1 | ||||
| 28-day transplant-free mortality (%) | 6.7 | 21.3 | 53.3 | 87.5 |
| 90-day transplant-free mortality (%) | 20 | 41.5 | 76.9 | 100 |
| ACLF-2 | ||||
| 28-day transplant-free mortality (%) | 2.4 | 11.8 | 29.6 | 90.63 |
| 90-day transplant-free mortality (%) | 12.8 | 31.3 | 78.3 | 100 |
| ACLF-3 | ||||
| 28-day transplant-free mortality (%) | 12.5 | 0.0 | 66.7 | 96.6 |
| 90-day transplant-free mortality (%) | 12.5 | 50.0 | 66.7 | 96.6 |
This table was reproduced from Gustot T, Fernandez J, Garcia E, et al. Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015;62:243–52.
ACLF, acute-on-chronic liver failure.
Despite the prognostic benefits of such scoring systems, there are some disadvantages. First, they are 75% accurate at best, and it remains unclear whether they can be complemented by other ‘biomarkers’ to improve predictive utility. Second, it remains to be proven if they are uniformly applicable across aetiologies of chronic liver disease such as viral hepatitis B-induced ACLF.29 Given these potential limitations, attention has evolved to address relevant pathophysiological indicators in ACLF as new biomarkers, including indicators of cell death, oxidative stress, immune dysfunction and gut dysbiosis.
Some lines of investigation have emphasised the role of inflammatory mediators and hepatic oxidative stress in ACLF outcomes, mainly in patients with hepatitis B.30 These suggest that increased oxidation products are more likely to activate innate immune responses to produce inflammatory cytokines, further promoting organ injury and failure.31 Indeed, oxidation of albumin-generating ischaemia-modified albumin may act as a prognostic marker in ACLF32 33 and could be used to rationalise the value of albumin infusion in patients with ACLF. However, at present, data on such biomarkers are limited to small clinical studies or preclinical evaluation, and as such, further clinical validation is warranted to determine how they may serve to augment current clinical scores and stratify prognosis in ACLF.
Management
Initial management
There is no specific licensed treatment for ACLF currently. Management includes an early focus on assessing any precipitant factors that can be readily treated, vigilance for latent infection and providing organ support as needed.
Preventing the development of ACLF should be a priority when assessing patients presenting with AD of cirrhosis. Thus, treating an underlying condition such as exacerbation of hepatitis B with antiviral therapy should be a consideration, and recent evidence suggests a role for antiviral therapy in improving outcomes in patients with ACLF precipitated by acute exacerbation of HBV,34 although this might not be effective in all patients. Similarly, appropriate and timely management of bacterial infections, including spontaneous bacterial peritonitis (SBP) with systemic antibiotics in accordance with local guidelines and resistance patterns, and in the case of SBP, with intravenous albumin and subsequent antibiotic secondary prophylaxis, is likely to prevent evolution to organ failure. Indeed, being alert to latent infection, including nosocomial and fungal infections, and treating them expediently and adequately (sensitivities and with reference to local resistance patterns) is key, as they are important considerations in the patient with decompensated cirrhosis that may drive progression of ACLF, and also precipitate hospital readmission in those recovering from a primary event.35 36
The main infections encountered in ACLF are SBP, urinary tract infections and pneumonia. The pattern of antibiotic response and multidrug-resistant organisms were investigated by Fernández et al, and it was found that the incidence of multidrug-resistant (MDR) bacteria has increased from 29% to 38% in culture-positive infections between 2011 and 2017, with extended-spectrum beta-lactamase producing Enterobacteriaceae as the most frequent bacterial type isolated.37 Antibiotic resistance is foreseen as a negative prognostic factor in these patients and is associated with higher mortality rates.37 Nosocomial infections, intensive care unit (ICU) admission and recent hospital admission were found to be the main independent predictors of MDR infections. More strict interventions are urgently needed to prevent spread of MDR infections in patients with cirrhosis. The use of empirical MDR antibiotic covering strategies was found to be statistically more effective than the classical antibiotic covering strategies in improving outcomes especially in nosocomial infections and patients with severe sepsis, which suggests that broader spectrum multi-drug resistance (MDR) coverage is essential in these patients.37
Moreover, early control of variceal haemorrhage and portal hypertension is likely to be beneficial in reducing progression to ACLF by maintaining organ perfusion. Interestingly, usage of low-dose (≤80 mg propranolol) beta-blockade in those with clinically significant portal hypertension and ACLF was noted to reduce inflammation (leucocyte count) and to be associated with lower ACLF grade at presentation and better outcomes than patients not on beta-blocker prophylaxsis.38
Management of individual organ failures
As described above, pathognomonic of ACLF is the development of organ failure.3 5 39 Impaired organ perfusion is common due to several factors including failed cardiac compensation to further increase cardiac output in accordance with demand, coinciding with cirrhotic cardiomyopathy (present in 40%–50% of patients), and increased organ vascular resistance.27 40 Reduced tissue perfusion in conjunction with arterial hypotension usually necessitates haemodynamic support, while assessment of fluid status in these patients is challenging, usually necessitating invasive haemodynamic monitoring to guide inotropic support.41 Patients who have respiratory failure should be supported by oxygen therapy and assisted ventilation as required.42
Renal failure is the most common organ failure in ACLF and is associated with high mortality, and in some cases, renal replacement therapy (RRT) is required. AKI in patients with ACLF is pathophysiologically linked with systemic inflammation and gut bacterial translocation.12 43 The standard of care clinical management of such patients is to exclude other causes of AKI, institute volume expansion with human albumin solution and then to consider vasopressors, if there is a failure of response to volume expansion alone.43 44 In a retrospective analysis of data from four cohorts of patients treated for hepatorenal syndrome-AKI, ACLF grade was noted as a major factor in determining the response to terlipressin and albumin, with decreased response associated with higher ACLF grades.45 RRT should be started for clinical indications such as oligoanuria, volume overload, hyperkalaemia, metabolic acidosis and refractory hyponatraemia, and continuous RRT is better than intermittent haemodialysis.41
Brain failure in ACLF is defined as HE of West Haven Criteria grades 3 and 4,46 and the outcomes of HE are worse with the development of ACLF.20 47 The main management includes standard of care for the unconscious patients with grade 3 and 4 HE, including airway support, maintenance of mean arterial pressure and ammonia lowering as feasible.48
Extracorporeal liver support systems
Extracorporeal liver support systems, with the principle of albumin dialysis with or without plasma exchange, have been suggested as potential therapies that can be considered as a bridge to liver transplantation or recovery in patients with ACLF, by enhancing clinical and biological improvement,49 50 but the overall ability of these devices to reduce inflammation in patients with liver failure remains unclear. Kribben et al investigated 28 and 90-day survival in patients with ACLF with the Prometheus device in the ‘HELIOS’ randomised clinical trial, and failed to show any survival benefit in this patient population.49
The extracorporeal liver assist device (ELAD) system is a bioartificial liver support device that is based on a platform of human-derived hepatocytes. It was assessed in patients with acute alcoholic hepatitis and high MELD score versus standard of care but did not show any significant benefit in survival, although the ELAD device arm showed a trend towards improved survival at 90 days in patients with MELD scores less than 28.51
In summary, the clinical effectiveness of such devices in the context of ACLF management remain unclear, in part due to the heterogeneity of patient populations studied and lack of definition for ACLF at the time that many of these studies were conducted, and also failure to show survival benefit and any clarity on the duration of treatment required.52 New devices are being considered and these include a European Consortium randomised study—the ALIVER trial53—which is currently recruiting, with the hope that it may show more efficacious benefit than prior devices, since it also encompasses a strategy to address circulating bacterial endotoxin. Further data are awaited.
Plasma exchange has been considered as a therapeutic option in treating patients with ACLF. A randomised study in patients with acute liver failure showed improved survival in patients treated with plasma exchange compared with a control group receiving standard therapy54 but data are still very scarce regarding outcomes in ACLF, and more studies are needed to assess its benefit.
Emerging role of liver transplantation
The role of liver transplantation in ACLF remains a topic of debate. While liver transplantation is potentially the only curative intervention in such advanced patients, it is also associated with higher postoperative complications and longer ICU and hospital stays compared with other cirrhosis indications.
Historically, there has been no priority given to patients with ACLF for organ allocation, and patient selection in centres that have adopted a policy to consider these patients for transplantation is based on the number of organ failures, absence of sepsis, systemic comorbidity, age, psychological profile and alcohol/drug dependence. Increasingly, it is recognised that there is a narrow window of opportunity to be seized, when patients with ACLF grade 3, who have the highest mortality and thereby greatest utility for an organ, are sufficiently stable to undergo liver transplantation.
Conventional scoring systems used for organ allocation such as the MELD score fail to capture the dynamic response in ACLF, and are relatively blunt tools for predicting outcome to supportive management in ACLF.55 However, there are data to support the use of the CLIF-C ACLF score to help stratify those patients with ACLF grade 3 who may benefit most from liver transplantation, especially those with a CLIF-C ACLF score >70.25 56 57
Among the CANONIC cohort, 4.9% and 15% of patients, respectively, received liver transplantation for ACLF within 28 and 90 days of admission. ACLF-2 and ACLF-3 survival was almost 20% without liver transplantation and 80% with transplantation.3 Three European studies have shown a 1-year post-transplant survival rate in patients with ACLF extending from 75% to 87%.27 58 59 Recent evidence shows that patients with ACLF grade 3, who had previously been declined from consideration for liver transplantation based on futility, achieved 1-year survival rates greater than 80% when accepted for liver transplantation.58 Moreover, extracting data from the United Network for Organ Sharing (UNOS), Sundaram et al showed that liver transplant could increase odds for survival if performed early (within 4 weeks of presentation) in patients with ACLF-3, who otherwise had poor outcomes even if they had only modest MELD-sodium scores below 25.56
Artru et al have investigated 73 patients with ACLF who had liver transplantation, and found that 1-year survival in patients with ACLF-3 with transplantation was 83.6%, compared with 90% in patients with no ACLF, but with greater incidence of complications and prolonged hospital stay.58 By comparison, O’leary et al have shown that there were no differences in survival between patients who had liver transplantation with or without ACLF at 6 months.60
Thuluvath et al have examined patients on the UNOS database who were transplanted between 2002 and 2016, and that were stratified based on timing of transplant and number of organ failures. The data suggest that there was a 9% difference in graft viability and 10% difference in patient survival between patients with no organ failure and five to six organ failures at 1 year.61 One-year survival was 90% for no organ failure, 84% for three organ failures and 81% for five to six organ failures (who in accordance with the CLIF-C ACLF score one would have a predicted very high mortality), and respiratory failure was the only organ failure associated with a lower 1-year survival. There was also no difference between the two groups at 5 years regarding graft and patient survival. The analysis has shown that the main negative predictors of patient survival are age, presence of hepatocellular carcinoma, hepatitis C infection, donor risk index and number of organ failures. According to this analysis, the main predictors of patients’ poor outcome were age and need for mechanical ventilation.61 Engelmann et al have defined a threshold for very poor survival by studying 202 patients who were admitted to intensive care unit (ITU), respectively, and they concluded that a CLIF-C ACLF score of ≥70 after 48 hours is associated with 100% mortality at 28 days.28
The increasing body of data suggests a promising role for liver transplantation in patients with grade 3 ACLF, whilst also raising further questions regarding the thresholds of severity in ACLF when transplantation and current management may be deemed ‘futile’, as well as criteria of patient selection, organ allocation and the ethical considerations of liver transplantation in this patient population.
New therapies
Several interventions are receiving attention as potential treatment for ACLF. Granulocyte colony-stimulating factor has recently been investigated as it facilitates the mobilisation of bone marrow stem cells, with the proliferation of hepatic progenitor cells,62 as well as increasing the peripheral and intrahepatic T lymphocytes.63 Some small studies have observed a decrease in infection, resolution of liver function and also a potential survival benefit, when compared with standard medical care.64 However, such observations require validation in larger randomised trials before any recommendations can be made.65
Faecal microbiota transplantation has also been explored in a few pilot studies and has been shown to have beneficial effects on the outcomes of patients with alcohol-related ACLF.66 67 A retrospective study on 23 patients with alcoholic hepatitis and ACLF, ineligible for either corticosteroid treatment or liver transplantation, received faecal microbiota transplantation. This study showed survival rates of 73% for patients with ACLF-1 and 58% for ACLF-2 or ACLF-3 after 548 days.67 Again, these results are insufficient to make specific recommendations regarding this intervention for treating ACLF, however, they are encouraging and point to more pathophysiologically relevant therapeutic targets. The LIVERHOPE trial is an ongoing multicentre randomised European Consortium clinical trial that seeks to investigate the safety and efficacy of the use of simvastatin and rifaximin in preventing evolution to ACLF in patients with decompensated cirrhosis. A safety phase of the study has been completed and the efficacy study is ongoing.68 Such combinatorial therapies may become the source of future investigation, given the complex pathophysiology driving ACLF.
Conclusion
ACLF is one of the most challenging fields in hepatology today, with a growth of research seeking to discover the mechanisms underpinning the progression to liver failure and with promise of effective therapeutic strategies. More efforts are still required to develop better prognostic scores that can accommodate various aetiologies and presentations across a wide geographical landscape. Gut microbiota and immune modulation are being explored as potential prognostic markers and therapeutic options. To date, there is no specific definitive treatment for ACLF; however, liver transplantation has an increasing swell of data and may yet prove to be an option to reduce the high early mortality that is present in those with advanced grades.
Footnotes
Contributors: RM planned the work, supervised the writing, and edited and submitted the manuscript. AA conducted the literature search and the scientific writing. RM is responsible for the overall content as a guarantor.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Zipprich A, Garcia-Tsao G, Rogowski S, et al. . Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int 2012;32:1407–14. 10.1111/j.1478-3231.2012.02830.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jalan R, Williams R. Acute-On-Chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif 2002;20:252–61. 10.1159/000047017 [DOI] [PubMed] [Google Scholar]
- 3. Moreau R, Jalan R, Gines P, et al. . Acute-On-Chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426–37. 10.1053/j.gastro.2013.02.042 [DOI] [PubMed] [Google Scholar]
- 4. Jalan R, Yurdaydin C, Bajaj JS, et al. . Toward an improved definition of acute-on-chronic liver failure. Gastroenterology 2014;147:4–10. 10.1053/j.gastro.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 5. Bajaj JS, O'Leary JG, Reddy KR, et al. . Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014;60:250–6. 10.1002/hep.27077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarin SK, Kumar A, Almeida JA, et al. . Acute-On-Chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL). Hepatol Int 2009;3:269–82. 10.1007/s12072-008-9106-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bajaj JS, Moreau R, Kamath PS, et al. . Acute-On-Chronic liver failure: getting ready for prime time? Hepatology 2018;68:1621–32. 10.1002/hep.30056 [DOI] [PubMed] [Google Scholar]
- 8. Shalimar B, Kumar D, Vadiraja PK, et al. . Acute on chronic liver failure because of acute hepatic insults: etiologies, course, extrahepatic organ failure and predictors of mortality. J Gastroenterol Hepatol 2016;31:856–64. 10.1111/jgh.13213 [DOI] [PubMed] [Google Scholar]
- 9. Li H, Chen L-Y, Zhang N-nan, et al. . Characteristics, diagnosis and prognosis of acute-on-chronic liver failure in cirrhosis associated to hepatitis B. Sci Rep 2016;6:25487 10.1038/srep25487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arroyo V, Moreau R, Kamath PS, et al. . Acute-On-Chronic liver failure in cirrhosis. Nat Rev Dis Primers 2016;2 10.1038/nrdp.2016.41 [DOI] [PubMed] [Google Scholar]
- 11. Macdonald S, Andreola F, Bachtiger P, et al. . Cell death markers in patients with cirrhosis and acute decompensation. Hepatology 2018;67:989–1002. 10.1002/hep.29581 [DOI] [PubMed] [Google Scholar]
- 12. Clària J, Stauber RE, Coenraad MJ, et al. . Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology 2016;64:1249–64. 10.1002/hep.28740 [DOI] [PubMed] [Google Scholar]
- 13. Bernardi M, Moreau R, Angeli P, et al. . Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol 2015;63:1272–84. 10.1016/j.jhep.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 14. Clària J, Moreau R, Fenaille F, et al. . Orchestration of Tryptophan‐Kynurenine pathway, acute decompensation, and Acute‐on‐Chronic liver failure in cirrhosis. Hepatology 2019;69:1686–701. 10.1002/hep.30363 [DOI] [PubMed] [Google Scholar]
- 15. Louvet A, Wartel F, Castel H, et al. . Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology 2009;137:541–8. 10.1053/j.gastro.2009.04.062 [DOI] [PubMed] [Google Scholar]
- 16. Fernández J, Acevedo J, Wiest R, et al. . Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut 2018;67:1870–80. 10.1136/gutjnl-2017-314240 [DOI] [PubMed] [Google Scholar]
- 17. Bernsmeier C, Pop OT, Singanayagam A, et al. . Patients with acute-on-chronic liver failure have increased numbers of regulatory immune cells expressing the receptor tyrosine kinase MERTK. Gastroenterology 2015;148:603–15. 10.1053/j.gastro.2014.11.045 [DOI] [PubMed] [Google Scholar]
- 18. Bajaj JS, Heuman DM, Hylemon PB, et al. . Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014;60:940–7. 10.1016/j.jhep.2013.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao Z, Li F, Xiang X, et al. . Circulating cell death biomarker: good candidates of prognostic indicator for patients with hepatitis B virus related acute-on-chronic liver failure. Sci Rep 2015;5:14240 10.1038/srep14240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cordoba J, Ventura-Cots M, Simón-Talero M, et al. . Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). J Hepatol 2014;60:275–81. 10.1016/j.jhep.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 21. Mehta G, Mookerjee RP, Sharma V, et al. . Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver Int 2015;35:724–34. 10.1111/liv.12559 [DOI] [PubMed] [Google Scholar]
- 22. Garg H, Kumar A, Garg V, et al. . Hepatic and systemic hemodynamic derangements predict early mortality and recovery in patients with acute-on-chronic liver failure. J Gastroenterol Hepatol 2013;28:1361–7. 10.1111/jgh.12191 [DOI] [PubMed] [Google Scholar]
- 23. Teng W, Chen W-T, Ho Y-P, et al. . Predictors of mortality within 6 weeks after treatment of gastric variceal bleeding in cirrhotic patients. Medicine 2014;93:e321 10.1097/MD.0000000000000321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Angeli P, Rodríguez E, Piano S, et al. . Acute kidney injury and acute-on-chronic liver failure classifications in prognosis assessment of patients with acute decompensation of cirrhosis. Gut 2015;64:1616–22. 10.1136/gutjnl-2014-307526 [DOI] [PubMed] [Google Scholar]
- 25. Mookerjee R. Prognosis and biomarkers in acute-on-chronic liver failure. Semin Liver Dis 2016;36:127–32. 10.1055/s-0036-1583200 [DOI] [PubMed] [Google Scholar]
- 26. Jalan R, Pavesi M, Saliba F, et al. . The CLIF Consortium acute decompensation score (CLIF-C ads) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol 2015;62:831–40. 10.1016/j.jhep.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 27. Gustot T, Fernandez J, Garcia E, et al. . Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015;62:243–52. 10.1002/hep.27849 [DOI] [PubMed] [Google Scholar]
- 28. Engelmann C, Thomsen KL, Zakeri N, et al. . Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care 2018;22 10.1186/s13054-018-2156-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu T, Li J, Shao L, et al. . Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut 2018;67:2181–91. 10.1136/gutjnl-2017-314641 [DOI] [PubMed] [Google Scholar]
- 30. Piano S, Singh V, Caraceni P, et al. . Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology 2019;156:1368–80. 10.1053/j.gastro.2018.12.005 [DOI] [PubMed] [Google Scholar]
- 31. Sha Y, Zmijewski J, Xu Z, et al. . Hmgb1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol 2008;180:2531–7. 10.4049/jimmunol.180.4.2531 [DOI] [PubMed] [Google Scholar]
- 32. Jalan R, Schnurr K, Mookerjee RP, et al. . Alterations in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. Hepatology 2009;50:555–64. 10.1002/hep.22913 [DOI] [PubMed] [Google Scholar]
- 33. Alcaraz‐Quiles J, Casulleras M, Oettl K, et al. . Oxidized albumin triggers a cytokine storm in leukocytes through p38 Mitogen‐Activated protein kinase: role in systemic inflammation in decompensated cirrhosis. Hepatology 2018;68:1937–52. 10.1002/hep.30135 [DOI] [PubMed] [Google Scholar]
- 34. Philips CA, Sarin SK. Potent antiviral therapy improves survival in acute on chronic liver failure due to hepatitis B virus reactivation. WJG 2014;20 10.3748/wjg.v20.i43.16037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Piano S, Morando F, Carretta G, et al. . Predictors of early readmission in patients with cirrhosis after the resolution of bacterial infections. Am J Gastroenterol 2017;112:1575–83. 10.1038/ajg.2017.253 [DOI] [PubMed] [Google Scholar]
- 36. Bajaj JS, Reddy RK, Tandon P, et al. . Prediction of fungal infection development and their impact on survival using the NACSELD cohort. Am J Gastroenterol 2018;113:556–63. 10.1038/ajg.2017.471 [DOI] [PubMed] [Google Scholar]
- 37. Fernández J, Prado V, Trebicka J, et al. . Multidrug-Resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol 2019;70:398–411. 10.1016/j.jhep.2018.10.027 [DOI] [PubMed] [Google Scholar]
- 38. Mookerjee RP, Pavesi M, Thomsen KL, et al. . Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol 2016;64:574–82. 10.1016/j.jhep.2015.10.018 [DOI] [PubMed] [Google Scholar]
- 39. O'Leary JG, Reddy KR, Garcia-Tsao G, et al. . NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology 2018;67:2367–74. 10.1002/hep.29773 [DOI] [PubMed] [Google Scholar]
- 40. Møller S, Hove JD, Dixen U, et al. . New insights into cirrhotic cardiomyopathy. Int J Cardiol 2013;167:1101–8. 10.1016/j.ijcard.2012.09.089 [DOI] [PubMed] [Google Scholar]
- 41. Nadim MK, Durand F, Kellum JA, et al. . Management of the critically ill patient with cirrhosis: a multidisciplinary perspective. J Hepatol 2016;64:717–35. 10.1016/j.jhep.2015.10.019 [DOI] [PubMed] [Google Scholar]
- 42. Angeli P, Bernardi M, Villanueva C, et al. . EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–60. 10.1016/j.jhep.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 43. Amin AA, Alabsawy EI, Jalan R, et al. . Epidemiology, pathophysiology, and management of hepatorenal syndrome. Semin Nephrol 2019;39:17–30. 10.1016/j.semnephrol.2018.10.002 [DOI] [PubMed] [Google Scholar]
- 44. Cavallin M, Kamath PS, Merli M, et al. . Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology 2015;62:567–74. 10.1002/hep.27709 [DOI] [PubMed] [Google Scholar]
- 45. Piano S, Schmidt HH, Ariza X, et al. . Association between grade of acute on chronic liver failure and response to Terlipressin and albumin in patients with hepatorenal syndrome. Clin Gastroenterol Hepatol 2018. [DOI] [PubMed] [Google Scholar]
- 46. Atterbury CE, Maddrey WC, Conn HO. Neomycin-sorbitol and lactulose in the treatment of acute portal-systemic encephalopathy. Am J Dig Dis 1978;23:398–406. 10.1007/BF01072921 [DOI] [PubMed] [Google Scholar]
- 47. Bajaj JS, O'Leary JG, Tandon P, et al. . Hepatic encephalopathy is associated with mortality in patients with cirrhosis independent of other extrahepatic organ failures. Clin Gastroenterol Hepatol 2017;15:565–74. 10.1016/j.cgh.2016.09.157 [DOI] [PubMed] [Google Scholar]
- 48. Vilstrup H, Amodio P, Bajaj J, et al. . Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American association for the study of liver diseases and the European association for the study of the liver. Hepatology 2014;60:715–35. 10.1002/hep.27210 [DOI] [PubMed] [Google Scholar]
- 49. Kribben A, Gerken G, Haag S, et al. . Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology 2012;142:782–9. 10.1053/j.gastro.2011.12.056 [DOI] [PubMed] [Google Scholar]
- 50. Bañares R, Nevens F, Larsen FS, et al. . Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the relief trial. Hepatology 2013;57:1153–62. 10.1002/hep.26185 [DOI] [PubMed] [Google Scholar]
- 51. Thompson J, Subramanian R, Al-Khafaji A, et al. . Lewis Teperman and JS. The effect of extracorporeal C3a cellular therapy in severe alcoholic hepatitis-the Elad trial. Hepatology 2015;62. [Google Scholar]
- 52. Hernaez R, Solà E, Moreau R, et al. . Acute-On-Chronic liver failure: an update. Gut 2017;66:541–53. 10.1136/gutjnl-2016-312670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gustot T, Jalan R. Acute-On-Chronic liver failure in patients with alcohol-related liver disease. J Hepatol 2019;70:319–27. 10.1016/j.jhep.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 54. Larsen FS, Schmidt LE, Bernsmeier C, et al. . High-Volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol 2016;64:69–78. 10.1016/j.jhep.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 55. Duan B-W, Lu S-C, Wu J-S, et al. . Model for end-stage liver disease (MELD) score does not predict outcomes of hepatitis B-induced acute-on-chronic liver failure in transplant recipients. Transplant Proc 2014;46:3502–6. 10.1016/j.transproceed.2014.07.075 [DOI] [PubMed] [Google Scholar]
- 56. Sundaram V, Jalan R, Wu T, et al. . Factors Associated with Survival of Patients With Severe Acute-On-Chronic Liver Failure Before and After Liver Transplantation. Gastroenterology 2019;156:1381–91. 10.1053/j.gastro.2018.12.007 [DOI] [PubMed] [Google Scholar]
- 57. Gustot T, Agarwal B. Selected patients with acute-on-chronic liver failure grade 3 are not too sick to be considered for liver transplantation. J Hepatol 2017;67:667–8. 10.1016/j.jhep.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 58. Artru F, Louvet A, Ruiz I, et al. . Liver transplantation in the most severely ill cirrhotic patients: a multicenter study in acute-on-chronic liver failure grade 3. J Hepatol 2017;67:708–15. 10.1016/j.jhep.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 59. Finkenstedt A, Nachbaur K, Zoller H, et al. . Acute-On-Chronic liver failure: excellent outcomes after liver transplantation but high mortality on the wait list. Liver Transpl 2013;19:879–86. 10.1002/lt.23678 [DOI] [PubMed] [Google Scholar]
- 60. O’Leary JG, Bajaj JS, Tandon P, et al. . Outcomes after listing for liver transplant in patients with Acute‐on‐Chronic liver failure: the multicenter North American Consortium for the study of End‐Stage liver disease experience. Liver Transpl 2019;25:571–9. 10.1002/lt.25426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Thuluvath PJ, Thuluvath AJ, Hanish S, et al. . Liver transplantation in patients with multiple organ failures: feasibility and outcomes. J Hepatol 2018;69:1047–56. 10.1016/j.jhep.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 62. Spahr L, Lambert J-F, Rubbia-Brandt L, et al. . Granulocyte-Colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology 2008;48:221–9. 10.1002/hep.22317 [DOI] [PubMed] [Google Scholar]
- 63. Khanam A, Trehanpati N, Garg V, et al. . Altered frequencies of dendritic cells and IFN-γ-secreting T cells with granulocyte colony-stimulating factor (G-CSF) therapy in acute-on-chronic liver failure. Liver Int 2014;34:505–13. 10.1111/liv.12415 [DOI] [PubMed] [Google Scholar]
- 64. Singh V, Keisham A, Bhalla A, et al. . Efficacy of Granulocyte Colony-Stimulating Factor and N-Acetylcysteine Therapies in Patients With Severe Alcoholic Hepatitis. Clin Gastroenterol Hepatol 2018;16:1650–6. 10.1016/j.cgh.2018.01.040 [DOI] [PubMed] [Google Scholar]
- 65. Chavez-Tapia NC, Mendiola-Pastrana I, Ornelas-Arroyo VJ, et al. . Granulocyte-Colony stimulating factor for acute-on-chronic liver failure: systematic review and meta-analysis. Ann Hepatol 2015;14:631–41. 10.1016/S1665-2681(19)30757-4 [DOI] [PubMed] [Google Scholar]
- 66. Philips CA, Pande A, Shasthry SM, et al. . Healthy donor fecal microbiota transplantation in Steroid-Ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol 2017;15:600–2. 10.1016/j.cgh.2016.10.029 [DOI] [PubMed] [Google Scholar]
- 67. Philips CA, Augustine P, Padsalgi G, et al. . Only in the darkness can you see the stars: severe alcoholic hepatitis and higher grades of acute-on-chronic liver failure. J Hepatol 2019;70:550–1. 10.1016/j.jhep.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 68. Pose E, Napoleone L, Ahmed Amin D, et al. . Oral Abstracts (Abstracts 1-299). Hepatology 2018;68. [Google Scholar]