Abstract
Background:
Patients with cardiac sarcoidosis (CS) are at increased risk of atrioventricular blocks, ventricular arrhythmias, and sudden cardiac death. Objectives We aimed to investigate the characteristics associated with appropriate therapy in implantable cardiac defibrillator (ICD) -implanted CS patients.
Methods:
We performed a PubMed and Web of Science search for studies reporting patients with CS who underwent an ICD implantation. The primary criterion was an appropriate therapy.
Results:
We screened 705 studies, of which 5 were included in the final analysis. We conducted a meta-analysis including 464 patients (mean age 55 years, 282 males (60%)). The mean follow-up was 3.5 years. Among the 464 patients, 180 received an appropriate therapy (39%). Patients who received an appropriate therapy were younger (-3.33, 95% confidence interval (CI) -6.42 to -0.23, p=0.004), were more likely to be male (OR 2.06, 95% CI 1.37-3.09, p=0.0005), had a lower left ventricular ejection fraction (LVEF) (-10.5, 95% CI -18.23 to -2.78, p=0.008), had a higher rate of complete heart block (OR 2.19, 95% CI 1.20 to 3.99, p=0.01), and more frequently had ventricular pacing (OR 6.44 95% CI 2.57 to 16.16, p<0.0001).
Conclusions:
Appropriate ICD therapy during CS is associated with young age, male sex, low LVEF, history of complete heart block, and ventricular pacing. (Sarcoidosis Vasc Diffuse Lung Dis 2020; 37 (1): 17-23)
Keywords: cardiac sarcoidosis, sudden death, implantable cardioverter defibrillator, complete heart block, meta-analysis
PRISMA diagram
Forest plot of trials that analyzed appropriate versus no appropriate therapy in patients with cardiac sarcoidosis and implantable cardioverter defibrillator. Impact of syncope, primary prevention (PP) versus secondary prevention (SP), left (LBBB) and right (RBBB) bundle branch block, and cardiac magnetic resonance (CMR).
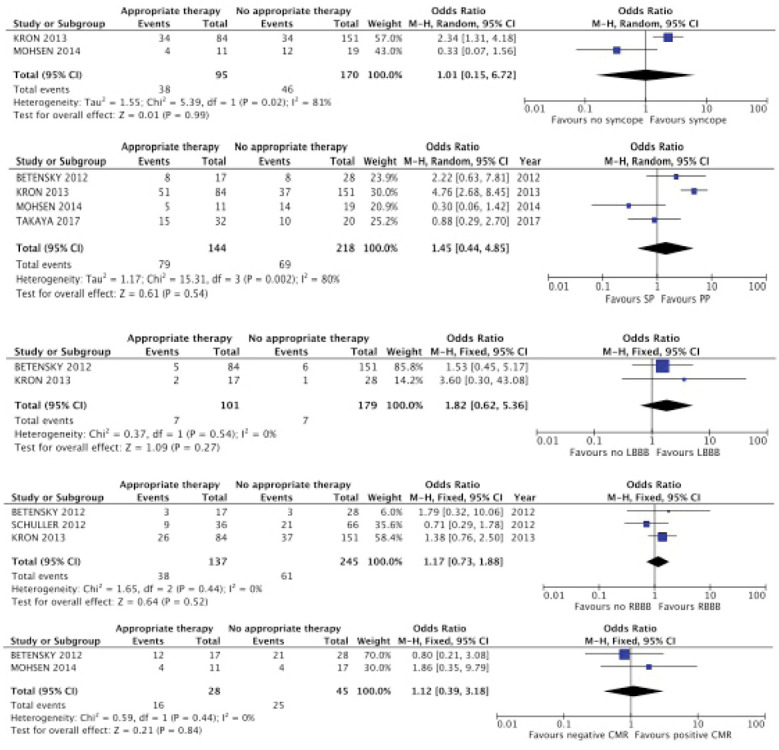
Table S1.
Search strategy
| The specific search strategy for PubMed was: ((“Sarcoidosis”[Mesh]) OR (“Sarcoidosis”[all fields]) OR (“Cardiac sarcoidosis”[all fields])) AND ((“Defibrillators, Implantable”[Mesh]) OR (“Defibrillators, Implantable”[all fields]) OR (“Death, Sudden, Cardiac”[Mesh]) OR (“Death, Sudden, Cardiac”[all fields]) OR (“Tachycardia, Ventricular”[Mesh]) OR (“Tachycardia, Ventricular”[all fields]) OR (“Ventricular Fibrillation”[Mesh]) OR (“Ventricular Fibrillation”[all fields]) OR (“Arrhythmias, Cardiac”[Mesh]) OR (“Arrhythmias, Cardiac”[all fields])) |
| The specific search strategy for Web of Science was: TS=((sarcoidosis OR cardiac sarcoidosis) AND (implantable cardiac defibrillators OR cardiac sudden death OR ventricular tachychardia OR ventricular fibrillation OR cardiac arrhythmias)) OR TI=((sarcoidosis OR cardiac sarcoidosis) AND (implantable cardiac defibrillators OR cardiac sudden death OR ventricular tachychardia OR ventricular fibrillation OR cardiac arrhythmias)) |
Sarcoidosis is a multisystemic granulomatous disease of unknown cause that occurs in young adults (1). Clinical cardiac involvement occurs in 5% of patients with sarcoidosis (2). However, autopsy studies suggest that cardiac involvement might concern more than 25% of patients with sarcoidosis (3-5). Patients with cardiac sarcoidosis (CS) are at increased risk for atrioventricular blocks, ventricular arrhythmias, and sudden cardiac death (6-8). Implantable cardioverter defibrillator (ICD) implantation is a high cost procedure and may lead to severe complications, especially in young patients who have been exposed to immunosuppressive drugs (9). Recent guidelines suggest that ICD implantation is recommended for patients with spontaneous sustained ventricular arrhythmias or left ventricular ejection fraction (LVEF) <35% (class I); can be useful for patients with an indication for permanent pacemaker implantation, unexplained syncope or inducible sustained ventricular arrhythmias (class IIa recommendation); and may be considered for patients with LVEF 36% to 40%, right ventricular ejection fraction <49%, or both (class IIb recommendation).
However, the efficacy and safety of ICDs in CS is debated. The subpopulation that benefits the most from an ICD implantation remains unknown. Appropriate ICD therapies refer to shock or antitachycardia pacing for ventricular tachycardia (VT) or ventricular fibrillation (VF). Appropriate therapy has been investigated in nonischemic cardiomyopathy and is a reliable surrogate criterion for sudden cardiac death (10).
We conducted a meta-analysis to identify the factors associated with appropriate ICD therapy in patients with CS.
Methods
Literature search
Two main investigators (L.-D.A. and F.C.-A.) searched MEDLINE (1966-July 2018) and Web of Science (1900-2018) for original articles without language restrictions. The search strategy combined free text search, advanced research, exploded MESH/EMTREE terms, and all synonyms of the following Medical Subject Headings terms: sarcoidosis, CS, ICD, cardiac sudden death, VT, VF, and cardiac arrhythmias. (The search strategy is detailed in Supplemental Table S1.) We also searched for additional articles from the reference lists of relevant papers obtained from the electronic search.
Selection criteria were determined before data collection. Inclusion criteria were as follows: (1) studies reporting on ICD (2) in patients with definite or suspected CS as defined in the Heart Rhythm Society (HRS) consensus statement in 2014 or the Japanese Ministry of Health and Welfare criteria (11) (3), in which patients who received an appropriate therapy were compared with patients who did not. Studies reporting reviews, case reports, editorials and guidelines were excluded. Studies reporting on CS patients with insufficient available information or nonextractable data were excluded. Whenever disagreement occurred, it was resolved by discussion between the 2 investigators and the advice of a third one (Z.A.) until a consensus was reached. A flow chart of reasons for rejection of citations identified from the searches was performed and is shown in Figure 1.
Fig. 1.
Flow diagram of the assessment of studies identified in the meta-analysis
Data extraction
The data were simultaneously and independently extracted by 2 investigators (L.-D.A. and F.C.-A.). For each study, the recorded information included the number of patients, inclusion criteria, CS diagnosis criteria, definition of appropriate therapy, number of patients in the appropriate therapy group, age, sex, LVEF, history of syncope or complete heart block (CHB), left or right bundle branch block (LBBB or RBBB), ventricular pacing, cardiac magnetic resonance imaging (MRI) results, and mean follow-up.
Risk of bias assessment
A subjective assessment of the methodological quality of the observational studies was evaluated by two investigators using the Newcastle-Ottawa Scale (NOS) (12), which is a quality assessment tool for nonrandomized studies. NOS uses a “star system” based on three major perspectives: the selection of the study groups (0 to 4 stars), the comparability of the groups by controlling for important and additional relevant factors (0 to 2 stars), and the ascertainment of outcome of interest or exposure (0 to 3 stars). A total score of 3 or less indicates poor quality, 4-6 indicates moderate quality, and 7-9 indicates high quality. We also used the thresholds for converting the NOS to the Agency for Healthcare Research and Quality (AHRQ) standards. Discrepant opinions between the investigators were resolved by consensus.
Data analysis
As previously indicated, appropriate therapy referred to shock or antitachycardia pacing for VT or VF. Percentages were assessed for dichotomous variables; means and standard deviations were assessed for continuous variables. We compared the characteristics of the patients in the “appropriate therapy” group versus the “no appropriate therapy” group. All included articles were analyzed using Cochrane Collaboration Review Manager statistical software (version 5.3; Cochrane Collaboration, Oxford, UK). The I2 statistic was used to measure consistency. The fixed-model effect was used, and a random-effects model was used whenever I2 exceeded 0% (suggesting a mild, moderate or important heterogeneity). A p<0.05 was considered significant. A Mantel-Haenszel test was used for dichotomous variables, and an inverse variance test was used for continuous variables. Odds ratio were estimated for dichotomous variables comparisons. The PRISMA checklist is detailed in Supplemental Figure S1.
Results
We extracted 705 articles. According to the selection criteria, 68 articles were assessed for eligibility (Figure 1). The main reason that studies were excluded was the type of articles; many of them were case reports. Five retrospective cohorts, published between 2011 and 2018, were included in the final analysis (13-17). The main results of these studies are detailed in Table 1. These 5 studies were categorized as poor or fair quality studies, according to the NOS or AHRQ tools, respectively.
Table 1.
Characteristics of the design and population in the 5 studies included in the meta-analysis
| Ref | Schuller (2012) | Betensky (2012) | Kron (2013) | Mohsen (2014) | Takaya (2017) |
| n* | 102 | 45 | 235 | 30 | 52 (27 definite CS, 25 suspected CS) |
| Design | Single-center retrospective cohort | Single-center retrospective cohort | Single-center retrospective cohort | Single-center retrospective cohort | Single-center retrospective cohort |
| Inclusion criteria | CS receiving an ICD | CS receiving an ICD | CS receiving an ICD | CS receiving an ICD | CS receiving an ICD |
| Exclusion criteria | - | Coronary heart disease More plausible explanation for their heart disease |
- | - | - |
| Cardiac sarcoidosis definition | Modification of the JMHW | JMHW or extracardiac sarcoidosis associated with a positive CMR, PET imaging, heart biopsy or explant pathology | Biopsy-proven CS or suggestive CMR or extracardiac sarcoidosis and a presumptive cardiac involvement | Biopsy proven CS or biopsy proven extracardiac sarcoidosis associated with 2 or more of the following criteria: clinical abnormality, low LVEF, suggestive CMR, suggestive ECG or EPS, suggestive PET scan | Definite CS or suspected CS according to the JMHW |
| Age (years) mean (SD) |
53.1 (11.2) | 53.5 (11.2) | 55.6 (11.1) | 53 (11) | 60.1 (15.1) Definite CS 63 (13) Suspected CS 57 (17) |
| Male, n (%) | 60 (59%) | 27 (60%) | 152 (65%) | 16 (53%) | 27 (52%) |
| LVEF (%) mean (SD) | 44.1 (15) | 45.4 (16.4) | 45 (15.7) | 41 (18) | 36 (12) Definite CS 35 (14) Suspected CS 37 (10) |
| Syncope, n (%) | NA | NA | 68 (29%) | 16 (53%) | NA |
| RBBB, n (%) | 30 (29%) | 6 (13%) | 63 (28%) | NA | NA |
| LBBB, n (%) | NA | 3 (7%) | NA | NA | NA |
| Ventricular pacing | NA | 13 (29%) | 16 (7%) | NA | NA |
| Positive CMR definition | - | Focal or diffuse areas of delayed gadolinium enhancement occurrence in a distribution inconsistent with scar due to prior infarction | Delayed contrast enhancement, wall motion abnormalities, LV dysfunction, chamber dilation | Patchy late gadolinium enhancement of the mid-myocardium and/or epicardium | - |
| Positive CMR | NA | 4 (9%) | 99 (86%) | 8 (27%) | NA |
| Primary prevention | NA | 29 (64%) | 147 (63%) | 11 (37%) | 27 (52%) |
| Follow-up from ICD implantation (years) mean (SD) | 2.4 | 2.6 (2.7) | 4.2 (4.0) | 3.8 (4.0) | NA (Median: 2) |
| Appropriate therapy definition | ATP or shocks for VT or VF | 1 or more ATP or shock for sustained monomorphic or polymorphic VT or VF; electrical storms were tabulated as a single “event” |
ATP or shocks (for VT or VF) | ATP or shocks | ATP or shocks for ventricular tachyarrhythmias |
| Appropriate therapy | |||||
| Yes No |
36 (35%) 66 (65%) |
17 (38%) 28 (62%) |
84 (36%) 151 (64%) |
11 (37%) 19 (63%) |
32 (61%) 20 (39%) |
| NOS (stars) | Selection: 3 Comparability: Outcome: 2 |
Selection: 3 Comparability: 0 Outcome: 2 |
Selection: 3 Comparability: 0 Outcome: 2 |
Selection: 3 Comparability: 0 Outcome: 2 |
Selection: 3 Comparability: 0 Outcome: 2 |
| Quality assessment | Fair quality | Fair quality | Fair quality | Fair quality | Fair quality |
| AHRQ standards | Poor quality | Poor quality | Poor quality | Poor quality | Poor quality |
* number of patients included in the study
CS cardiac sarcoidosis; EPS electrophysiologic study; JMHW Japanese Ministry of Health and Welfare; ATP Antitachycardia pacing; VT Ventricular tachycardia; VF Ventricular fibrillation; AHRQ Agency for Healthcare Research and Quality; CMR Cardiac magnetic resonance; LVEF Left ventricular ejection fraction
NA Not available; ECG Electrocardiogram; RBBB Right bundle branch block; LBBB Left bundle branch block; ICD Implantable cardioverter-defibrillator; PET Positron emission tomography; NOS Newcastle-Ottawa Scale
A total of 464 participants were included. Across the 5 studies, the mean age was 55 years (11.6). Among these 464 patients, 282 (60%) were male. The estimated mean follow-up was 3.5 years. One hundred and eighty patients (39%) received an appropriate therapy.
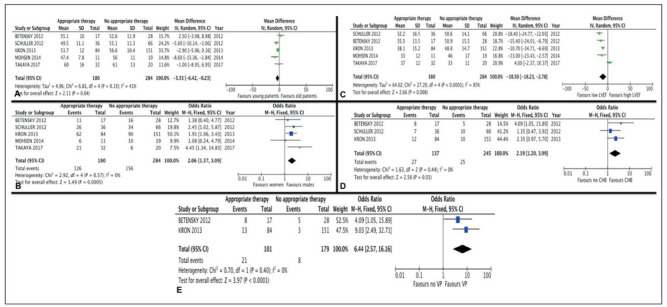
Five factors were significantly associated with receiving an appropriate therapy among the cardiac sarcoidosis patients who were implanted with an ICD: age, sex, LVEF, CHB, and ventricular pacing. These results are shown in Figure 2.
Fig. 2.
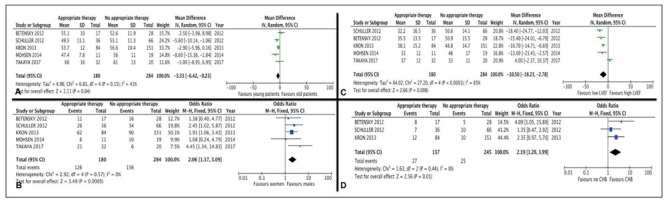
Forest plot of trials that analyzed appropriate versus no appropriate therapy in patients with cardiac sarcoidosis and an implantable cardioverter defibrillator. Impact of age (A), sex (B), left ventricular ejection fraction (LVEF) (C), complete heart block (CHB) (D), and ventricular pacing (E)
Patients who received an appropriate therapy were younger (- 3.33, 95% Confidence interval (CI) -6.42 to -0.23, p=0.004, I2=41%), more frequently male (OR 2.06, 95% CI 1.37 to 3.09, p=0.0005, I2=0%), and had a lower LVEF (-10.5, 95% CI -18.23 to -2.78, p=0.008, I2=85%). For LVEF, patients of the appropriate therapy group had a mean LVEF of 36.1% (14.8) versus 47.5% (14.4) in the no appropriate therapy group. Mean age in the appropriate therapy group was 53.9 years (11.8) versus 56 years (11.0) in the no appropriate therapy group.
For age: AT group 53.9 years (11.8); NAT group 56 years (11.0)
Three studies mentioned the presence of CHB, and the patients who received an appropriate therapy had a significant higher rate of CHB (OR 2.19, 95% CI 1.2 to 3.99, p=0.01, I2=0%) (13-15). Two studies mentioned the presence of ventricular pacing (14, 15). Patients who received an appropriate therapy were significantly more frequently paced (OR 6.44 95% CI 2.57 to 16.16, p<0.0001, I2=0%).
Conversely, LBBB, RBBB, positive CMR, syncope were not associated with receiving an appropriate therapy. The data included in such analyses were obtained from 2 or 3 articles (Supplemental Figure 2).
Finally, receiving an appropriate therapy was not associated with being implanted with an ICD for secondary prevention. Four studies were included in this analysis (14-17). This result is probably explained by a marked heterogeneity (I2=80%).
Discussion
Based on the results of 5 retrospective cohorts, this meta-analysis suggests that patients with cardiac sarcoidosis who were implanted with an ICD and received an appropriate therapy are more likely to be young males with a low LVEF, ventricular pacing, and a history of CHB.
In our meta-analysis, we found a higher rate (39%) of appropriate therapy than in previous studies reporting on other heart disease, such as ischemic heart disease or dilated cardiomyopathy, suggesting that patients with cardiac sarcoidosis are at high risk for arrhythmias. For example, 13.4% of patients received appropriate therapy after 1.9 years of follow up in a cohort of patients with ischemic heart disease and low LVEF (18). In a cohort of patients with low LVEF who had been implanted for primary prevention of cardiac sudden death, 18% had appropriate therapy during a 35-month follow up (19).
In this meta-analysis, we used appropriate therapy as the primary criterion. Appropriate shock has been investigated as a surrogate marker for sudden cardiac death in nonischemic cardiomyopathy in a randomized controlled trial including 458 patients. This study suggested that a 2:1 ratio was relevant for defining appropriate therapy as a surrogate for sudden cardiac death (10).
In addition to a LVEF <35% and spontaneous sustained ventricular arrhythmias, specific indications for ICD implantation in CS are not supported with a high level of evidence. HRS guidelines suggest an ICD implantation in patients with LVEF <35% (class I recommendation) or an LVEF between 36 and 49% or right ventricular ejection fraction (RVEF) <40% (class IIb recommendation). The impact of LVEF on the incidence of ventricular arrhythmias was recently investigated in CS (20). In this study, echocardiography recorded a LVEF <40% in 30 of 62 patients (48.4%); in the univariate analysis, these patients experienced worse survival compared with those patients with LVEF ≥40% (p=0.017). No patient with an LVEF between 41 and 50% died or had a transplant. All the studies included in our meta-analysis reported a mean LVEF lower than 40% (32-38%) in the “appropriate therapy” group, whereas all of the LVEF means in the “no appropriate therapy” group were 46-51% (except the series by Takaya, which reports a mean LVEF of 33% in the “no appropriate therapy” group), supporting the finding that LVEF is a main determinant of arrhythmia occurrence.
Cardiac sarcoidosis is a class IIa (level of evidence C) recommendation for an ICD when a pacemaker implantation is required (high-degree atrioventricular block). We found that patients who received an appropriate therapy were more likely to have CHB. These results are consistent with recent data: in a recent study, a high rate of ventricular arrhythmias and sudden cardiac death was found in patients with CS presenting with Mobitz II or CHB (21). The 5-year incidence of sudden cardiac death was 9% in the high atrioventricular block group (no VT and LVEF >50%), 14% if atrioventricular block was associated with an altered LVEF (between 35 and 50%), and up to 34% in the subgroup with VT or severe left ventricular dysfunction (<35%).
Our study has several limitations. First, the quality of all selected studies was poor to fair according to the NOS. All five were retrospective studies. Some patients were included in several studies: we could not determine which patients were included twice because it was not possible to obtain individual data. Thus, this could represent a substantial bias. However, cardiac sarcoidosis is rare and evidence level is low for its management. Thus, despite this bias, the results of this meta-analysis could help supporting low-grade recommendations (11). In this meta-analysis, we did not use data from randomized or controlled trials, because they were not available. Only retrospective studies were available. The primary endpoint was a surrogate marker of sudden cardiac death. Finally, 17 studies were excluded because the data could not be properly extracted.
Patients with CS are at high risk for sudden cardiac death, and ICD implantation should be considered in selected patients. In this meta-analysis, we identified 5 factors associated with appropriate ICD therapy in CS: age, male sex, LVEF, CHB, and ventricular pacing. These results suggest that in CS patients, low LVEF and a history of CHB should indicate ICD implantation, especially in young male patients. Ventricular pacing in a patient who was previously implanted with a pacemaker might be an indication for an ICD upgrade or subcutaneous ICD implantation.
Biography
L.-D.A., X.W., and F.C.-A. designed the study; L.-D.A., X.W., and F.C.-A. collected the data; L.D.-A. and F.C.A. conducted the statistical analysis; L.-D.A., X.W., J.H., Z.A., and F.C.-A. analyzed and interpreted the data; L.-D.A. and F.C.-A. wrote the manuscript.
References
- 1.Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Muller-Quernheim J. Sarcoidosis. Lancet. 2014;383:1155–67. doi: 10.1016/S0140-6736(13)60680-7. [DOI] [PubMed] [Google Scholar]
- 2.Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac Sarcoidosis. Journal of the American College of Cardiology. 2016;68:411–21. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 3.Iwai K, Tachibana T, Takemura T, Matsui Y, Kitaichi M, Kawabata Y. Pathological studies on sarcoidosis autopsy. I. Epidemiological features of 320 cases in Japan. Acta Pathol Jpn. 1993;43:372–6. doi: 10.1111/j.1440-1827.1993.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 4.Perry A, Vuitch F. Causes of death in patients with sarcoidosis. A morphologic study of 38 autopsies with clinicopathologic correlations. Arch Pathol Lab Med. 1995;119:167–72. [PubMed] [Google Scholar]
- 5.Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. The American journal of cardiology. 2001;88:1006–10. doi: 10.1016/s0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 6.Takaya Y, Kusano KF, Nakamura K, Ito H. Outcomes in patients with high-degree atrioventricular block as the initial manifestation of cardiac sarcoidosis. The American journal of cardiology. 2015;115:505–9. doi: 10.1016/j.amjcard.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Cohen Aubart F, Nunes H, Mathian A, Haroche J, Hie M, Le-Thi Huong Boutin D, et al. Cardiac sarcoidosis: Diagnosis and therapeutic challenges. Rev Med Interne. 2017;38:28–35. doi: 10.1016/j.revmed.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–32. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 9.Olde Nordkamp LR, Postema PG, Knops RE, van Dijk N, Limpens J, Wilde AA, et al. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: A systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm. 2016;13:443–54. doi: 10.1016/j.hrthm.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, et al. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006;113:776–82. doi: 10.1161/CIRCULATIONAHA.105.561571. [DOI] [PubMed] [Google Scholar]
- 11.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart rhythm : the official journal of the Heart Rhythm Society. 2014;11:1305–23. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 12.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 13.Schuller JL, Zipse M, Crawford T, Bogun F, Beshai J, Patel AR, et al. Implantable cardioverter defibrillator therapy in patients with cardiac sarcoidosis. Journal of cardiovascular electrophysiology. 2012;23:925–9. doi: 10.1111/j.1540-8167.2012.02350.x. [DOI] [PubMed] [Google Scholar]
- 14.Betensky BP, Tschabrunn CM, Zado ES, Goldberg LR, Marchlinski FE, Garcia FC, et al. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9:884–91. doi: 10.1016/j.hrthm.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Kron J, Sauer W, Schuller J, Bogun F, Crawford T, Sarsam S, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2013;15:347–54. doi: 10.1093/europace/eus316. [DOI] [PubMed] [Google Scholar]
- 16.Mohsen A, Jimenez A, Hood RE, Dickfeld T, Saliaris A, Shorofsky S, et al. Cardiac sarcoidosis: electrophysiological outcomes on long-term follow-up and the role of the implantable cardioverter-defibrillator. Journal of cardiovascular electrophysiology. 2014;25:171–6. doi: 10.1111/jce.12302. [DOI] [PubMed] [Google Scholar]
- 17.Takaya Y, Kusano K, Nishii N, Nakamura K, Ito H. Early and frequent defibrillator discharge in patients with cardiac sarcoidosis compared with patients with idiopathic dilated cardiomyopathy. International journal of cardiology. 2017;240:302–6. doi: 10.1016/j.ijcard.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 18.Weeke P, Johansen JB, Jorgensen OD, Nielsen JC, Moller M, Videbaek R, et al. Mortality and appropriate and inappropriate therapy in patients with ischaemic heart disease and implanted cardioverter-defibrillators for primary prevention: data from the Danish ICD Register. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2013;15:1150–7. doi: 10.1093/europace/eut017. [DOI] [PubMed] [Google Scholar]
- 19.Sjoblom J, Kalm T, Gadler F, Ljung L, Frykman V, Rosenqvist M, et al. Efficacy of primary preventive ICD therapy in an unselected population of patients with reduced left ventricular ejection fraction. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2015;17:255–61. doi: 10.1093/europace/euu219. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Lower EE, Li HP, Costea A, Attari M, Baughman RP. Cardiac Sarcoidosis: The Impact of Age and Implanted Devices on Survival. Chest. 2017;151:139–48. doi: 10.1016/j.chest.2016.08.1457. [DOI] [PubMed] [Google Scholar]
- 21.Nordenswan H-K, Lehtonen J, Ekström K, Kandolin R, Simonen P, Mäyränpää M, et al. Outcome of Cardiac Sarcoidosis Presenting With High-Grade Atrioventricular Block. Circulation: Arrhythmia and Electrophysiology. 2018;11 doi: 10.1161/CIRCEP.117.006145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA diagram

Forest plot of trials that analyzed appropriate versus no appropriate therapy in patients with cardiac sarcoidosis and implantable cardioverter defibrillator. Impact of syncope, primary prevention (PP) versus secondary prevention (SP), left (LBBB) and right (RBBB) bundle branch block, and cardiac magnetic resonance (CMR).

Table S1.
Search strategy
| The specific search strategy for PubMed was: ((“Sarcoidosis”[Mesh]) OR (“Sarcoidosis”[all fields]) OR (“Cardiac sarcoidosis”[all fields])) AND ((“Defibrillators, Implantable”[Mesh]) OR (“Defibrillators, Implantable”[all fields]) OR (“Death, Sudden, Cardiac”[Mesh]) OR (“Death, Sudden, Cardiac”[all fields]) OR (“Tachycardia, Ventricular”[Mesh]) OR (“Tachycardia, Ventricular”[all fields]) OR (“Ventricular Fibrillation”[Mesh]) OR (“Ventricular Fibrillation”[all fields]) OR (“Arrhythmias, Cardiac”[Mesh]) OR (“Arrhythmias, Cardiac”[all fields])) |
| The specific search strategy for Web of Science was: TS=((sarcoidosis OR cardiac sarcoidosis) AND (implantable cardiac defibrillators OR cardiac sudden death OR ventricular tachychardia OR ventricular fibrillation OR cardiac arrhythmias)) OR TI=((sarcoidosis OR cardiac sarcoidosis) AND (implantable cardiac defibrillators OR cardiac sudden death OR ventricular tachychardia OR ventricular fibrillation OR cardiac arrhythmias)) |


