Abstract
Background and Objectives:
Sarcoidosis typically presents with peribronchovascular and perilymphatic nodules on high-resolution computed tomography (HRCT); a miliary pattern is reported but not well described.
Design, setting:
We describe four patients with miliary sarcoidosis and results of a systematic review of all previously reported cases from 1985 onwards.
Results:
We identified only 27 cases of “miliary” sarcoidosis in the HRCT era. These patients were older (85.2% older than 40 years), had more co-morbidities (72.7%) and were symptomatic compared to “typical” sarcoidosis. Respiratory symptoms were present in 61.9% at diagnosis. Hypercalcemia was seen in 28.5%. On review of HRCT images, only 34.6% (9/26) had a “true miliary” pattern without fissural nodules. In our series, prominent perivascular granulomas were seen on histopathology in all. 44.4% (12/27) had tuberculosis preceding or concurrent to miliary sarcoidosis. Of the eight true associations, tuberculosis preceded sarcoidosis by 52 (median, IQR 36) weeks in six and occurred concurrently in another two. The diagnosis of tuberculosis was clinical in all with concurrent diagnosis of tuberculosis and sarcoidosis. Treatment with steroids had 100% response and 14.2% relapse.
Conclusions:
A true miliary pattern in the HRCT era is very rare in sarcoidosis and subtle perilymphatic pattern is nearly always seen; this should be labeled “pseudo-miliary”. Prominent perivascular granulomas are associated with true miliary pattern. Miliary sarcoidosis patients are older and symptomatic, needing treatment at diagnosis. “Miliary” sarcoidosis may follow treatment for tuberculosis; concurrent cases possibly indicate the difficulty in differentiating both or a “tuberculo-sarcoid” presentation. (Sarcoidosis Vasc Diffuse Lung Dis 2020; 37 (1): 53-65)
Keywords: sarcoidosis, miliary, micronodules, miliary tuberculosis
Summary of all reported cases of thoracic sarcoidosis with miliary nodules on High-resolution Computed Tomography$
$Poor quality HRCT images precluded assessment of type of nodules; **In one miliary sarcoidosis followed miliary tuberculosis; %Concurrent tuberculosis and miliary sarcoidosis with organomegaly, neurologic and bone involvement; #Tuberculosis was diagnosed based on pathology, radiology and clinical response; ATT stopped at 5 months due to liver injury; ##Tb was diagnosed microbiologically based on mycobacterial cultures
Abbreviations: M Male, F Female, U.S United States, NA Not available, BLTx Bilateral lung transplantation, HBLTx Heart and lung transplantation, HRCT high resolution computed tomography, UL Upper lobe, PL Peri-lymphatic pattern, IL Interlobular nodules, ILo, Intralobular septal thickening, CAD coronary artery disease, MI Myocardial infarction, TB tuberculosis, DM Diabetes mellitus, HTN hypertension, COPD Chronic obstructive pulmonary disease, AKI acute kidney injury, INH isoniazid, ML Mediastinal, BHL bilateral Hilar lymphadenopathy, RPT Right paratracheal, LNE lymphadenopathy, Y Yes, Kgs kilograms, Bx biopsy, TBLB Transbronchial lung biopsy, SLBx surgical lung biopsy, EBUS-FNA endobronchial ultrasound guided fine needle aspiration, ATT Anti-tuberculosis treatment, MTX-Methotrexate
Introduction
Miliary sarcoidosis has been described as a rare presentation of sarcoidosis and was reported in <1% of all cases in the pre-computed tomography (CT) era on chest radiography (1). With the advent of high-resolution-CT (HRCT), peribronchovascular and perilymphatic nodules in relation to the secondary pulmonary lobule are recognized as classic of sarcoidosis (2). However, random micronodules (miliary pattern) have been described and sarcoidosis is included in the differential diagnosis of a “miliary” pattern on HRCT (3, 4). There are no well-described series or systematic reviews of “miliary” sarcoidosis in the high-resolution CT (HRCT) era. On the other hand, tuberculosis continues to be a global pandemic with an estimated 10 million cases worldwide in 2016 (5). Miliary tuberculosis accounts for 1-2% of all cases of new cases of tuberculosis and 8% of all extra-pulmonary tuberculosis (6). The relationship between tuberculosis and sarcoidosis continues to be enigmatic; tuberculosis can precede, follow or present concurrently with sarcoidosis (7). In patients with miliary tuberculosis, the clinical symptoms of night sweats, weight loss and fatigue are non-specific and overlap markedly with sarcoidosis. Respiratory symptoms are often minimal in either forms of miliary disease and microbiological evidence of M.tuberculosis is only present in 20-30% of miliary tuberculosis (8). Sarcoidosis has a characteristic perilymphatic pattern on histopathology and radiology (2); the co-relation of random nodules seen on radiology with pathology in “miliary” sarcoidosis is unclear. We describe a series of patients with miliary sarcoidosis and the results of our systematic review on miliary sarcoidosis in the HRCT era.
Materials and Methods
We describe four patients with miliary nodules and proven sarcoidosis that was managed in the authors’ institution over three years and performed a systematic search of literature for miliary sarcoidosis.
Systematic search of literature
Two of the authors (R.S and R.K) conducted a systematic search of literature in PUBMED independently using the MeSH term “miliary” AND “sarcoidosis” limited to title/abstract and restricted the search from the year 1985 onwards (9). The choice of the year was defined by the first description of the current HRCT protocols and detailed description. This was further supplemented by search of IndMed using the same terms, the references of retrieved articles, our personal records and the Internet search engine GOOGLE. These articles were screened without blinding, by title and abstract review to identify relevant studies. Any discrepancy was resolved by consensus. Full texts or abstracts were then retrieved to identify the type of lung involvement in reported cases with miliary sarcoidosis. Only those articles that included patients with HRCT according to established protocols (9), included representative images or detailed descriptions and had a histological diagnosis compatible with sarcoidosis (10) were included for analysis. For the purpose of this systematic review, we included all descriptions that were accepted as “miliary” in published cases but re-analyzed the images independently. The diagnosis of sarcoidosis was according to previous published guidelines (10).
Data extraction
Data was abstracted from selected cases in a pre-defined data extraction form (Supplementary Table).
Statistical analysis
Continuous data are presented as mean (standard deviation (SD), median [range or Interquartile Range (IQR)] and categorical data as percentages and proportions. All statistical analysis was done using the Statistical software SPSS Version 14.
Ethics
The Human Ethics Committee of PSG IMS&R, Coimbatore, approved the study. All data was anonymized to maintain patient confidentiality.
Case reports
Case 1
A 65-year-old lady presented with cough, night sweats and exertional breathlessness of two months’ duration. There was no history of weight loss or fever. Her past history was significant for hypertension and non-productive cough two years before that was evaluated at another center. CT had shown right paratracheal and bilateral hilar lymphadenopathy along with upper lobe-predominant micronodules. Tuberculin skin testing was negative and endobronchial ultrasound-guided needle aspiration (EBUS-FNA) and transbronchial lung biopsy (TBLB) were performed. EBUS-FNA showed few non-caseating granulomas and TBLB was non-contributory. A presumptive diagnosis of tuberculosis was made and four-drug anti-tuberculosis treatment (ATT) was administered for six months with improvement in cough. Examination was unremarkable during current evaluation. There was no exposure to pets, drugs or occupational dusts. Chest radiography was normal. Spirometry was normal and diffusion capacity showed mild reduction. HRCT showed profuse random micronodules without lymphadenopathy and an upper-lobe and peripheral predominance that was visualized better with maximum intensity projection (MIP, Figure 1). Renal function tests, serum calcium and angiotensin-converting enzyme (ACE) levels were normal. Fibreoptic bronchoscopy (FOB) was done with bronchoalveolar lavage (BAL) and TBLB; BAL Fluid was negative for acid-fast bacilli and Xpert MTB/RIF and TBLB showed interstitial inflammation only. Surgical lung biopsy was performed and showed extensive perivascular non-caseating granulomas (Figure 2). BAL mycobacterial cultures were subsequently reported as negative. She was started on steroids with resolution in cough and breathlessness by second month of treatment. She remains asymptomatic and off steroids after one year of treatment.
Fig. 1.
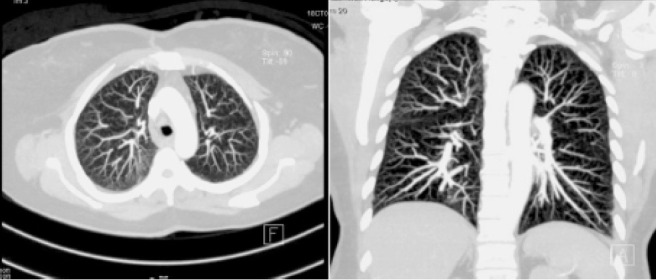
Composite image of Maximal Intensity Projection images of high-resolution computed tomography (HRCT) of the thorax from patient 1 showing upper-lobe and peripheral-predominant miliary micronodules
Fig. 2.
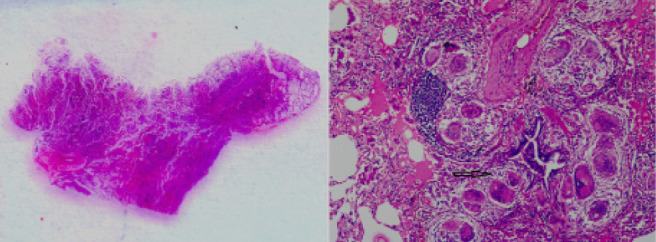
Composite image of photomicrographs of surgical lung biopsy specimen of Patient 1 with left [Gross Pathological specimen, transverse plane] showing subpleural sparing and (right, Hematoxylin and Eosin stain (H & E), x 4) showing peribronchial and perivascular granulomas (arrow)
Case 2
A 40-year old gentleman presented with polyuria, fatigue and nausea of 20 days duration. There was also history of unquantified weight loss and exertional dyspnea. He did not smoke and did not raise pets, abuse drugs or have exposure to occupational dusts. Physical examination showed raised papulonodular skin lesions over hands and legs. Renal function tests showed serum creatinine of 2.65 mg/dL (normal <0.8 mg/dL); Urine microscopy showed 2+ albumin with bland sediments. Hemoglobin was 10.9 g/dL with normal leukocyte and platelet counts. Ultrasound showed normal kidney size with preserved corticomedullary distinction. Chest radiographs showed miliary nodules. Serum ionized calcium was 1.8 mg/dL (normal 1.1-1.3 mg/dL) with suppressed parathyroid hormone (1.9 pg/mL, normal 15-65) and normal vitamin D levels (23.6 ng/mL). ACE levels were elevated (98 U/mL, normal 8-53). Tuberculin skin testing was negative. CT-Chest showed random micronodules without zonal preponderance and bilateral hilar lymphadenopathy (Figure 3, left). FOB was done with BAL and TBLB; BAL was negative for acid-fast bacilli and Xpert MTB/RIF. TBLB showed perivascular non-caseating granulomas with Schaumann and asteroid bodies (Figure 4). Punch biopsy of the skin also showed non-caseating granulomas in the dermis. He was started on steroids with resolution of hypercalcemia and normalization of renal function. His subsequent course was complicated by steroid-aggravated diabetes presenting with hyperosmolar coma; methotrexate was added and steroids were tapered. He remained asymptomatic with near-complete radiologic clearing (Figure 3, right) after one year of stopping methotrexate but relapsed by 18 months and remains on methotrexate.
Fig. 3.
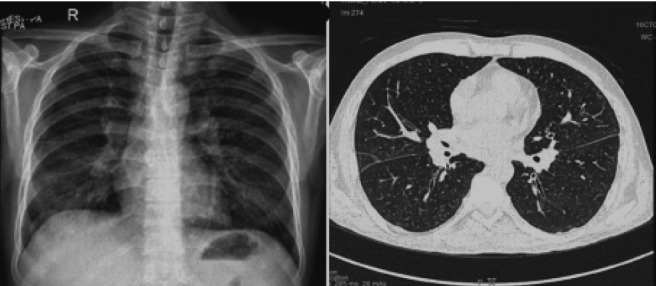
Composite images of chest radiography of patient 2 showing miliary nodules (left) and HRCT images (major fissure level) showing upper lobe-predominant random nodules. No involvement of the fissure or “beading” of the fissure is seen
Fig. 4.
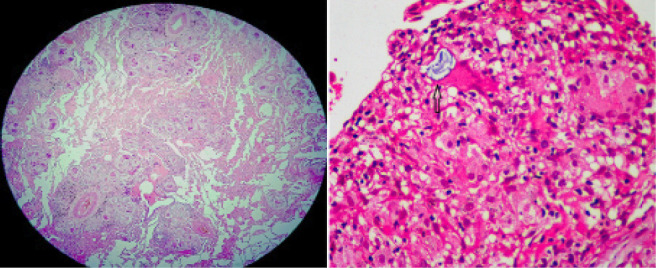
Composite image of Photomicrographs of lung biopsy sample of Patient 2 with left, [Hematoxylin and Eosin stain (H & E), x scanner power] showing multiple non-necrotizing perivascular granulomas, and right, [H & E stain, x 40) showing a Schaumann body within a giant cell (arrow)
Case 3
A 55-year old gentleman with moderately controlled diabetes mellitus presented with exertional breathlessness, cough, right-sided shoulder pain and weight loss of one month’s duration. There was no anorexia or expectoration. He was a farmer by occupation and did not have exposure to poultry or occupational dusts and chemicals. Physical examination showed fine bilateral crepitations without clubbing. Renal function tests, serum calcium, urine microscopy, ACE levels and hemogram were normal. Chest radiographs showed miliary nodules (Figure 5, left). Human Immunodeficiency virus was negative by enzyme-linked sorbent assay. Tuberculin skin testing was strongly positive (18 mm, 1 TU). CT-Chest showed random micronodules without lymphadenopathy (Figure 6, left). FOB was done with BAL and TBLB; BAL was negative for acid-fast bacilli and Xpert MTB/RIF. TBLB showed well-formed perivascular granulomas with Langhans’ giant cells, occasional fibrinoid necrosis and lymphocytes rimming (Figure 6, right). A diagnosis of miliary tuberculosis was made and four-drug weight-based ATT was started. However, his symptoms continued to worsen with worsening nodules and development of hypoxemia needing oxygen at four weeks (Figure 5, right). BAL mycobacterial cultures were negative in the meantime. Steroids were added with a presumptive diagnosis of immune reconstitution syndrome, with resolution of hypoxemia and symptoms. Symptoms recurred after stopping steroids at four weeks; right-sided vision loss due to uveitis occurred. After a clinico-pathological review, a possibility of concurrent miliary tuberculosis with sarcoidosis was considered. ATT was continued and low-dose tapering steroids and methotrexate (in view of poorly controlled diabetes) were added, with complete resolution of symptoms by eight weeks. He remains asymptomatic after two years of cessation of ATT and methotrexate.
Fig. 5.
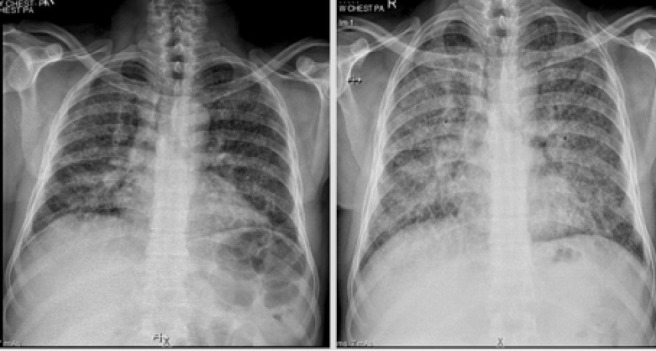
Composite image of the Chest Radiographs of Patient 3 at initial evaluation (left) showing extensive miliary nodules. Subsequent Chest radiograph (right) after six weeks of anti-tuberculosis treatment alone show profusion of nodules with diffuse ground-glass opacification. Worsening hypoxemia was clinically noted
Fig. 6.
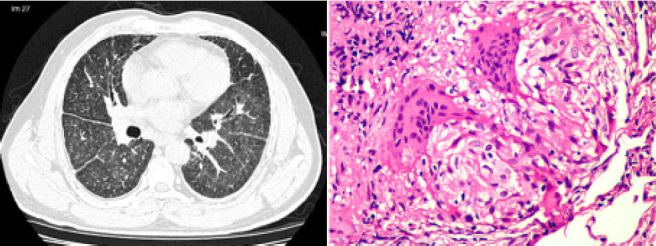
Composite image of the HRCT image of Patient 3 at the level of major fissure during worsening hypoxemia corresponding to right-side of Figure 5 (left), showing random nodules with fissural prominence, with beading in both oblique fissures; some interlobular septal thickening and diffuse ground glass opacity can be seen and right, (Hematoxylin and Eosin stain, x 40) showing a non-necrotizing granuloma with peripheral lymphocyte cuffing
Case 4
A 48-year-old gentleman presented with fatigue for two weeks. There was no history of night sweats, weight loss or fever. He did not smoke, did not raise pets, abuse alcohol or drugs and did not report exposure to occupational dusts. Physical examination was normal. Renal function tests, urine microscopy and hemogram were normal. Serum calcium was elevated (1.43 mg/dL, normal 1.1-1.3). Chest radiographs showed miliary nodules (Figure 7, left). ACE levels were elevated (76 U/mL, normal 8-53). Tuberculin skin testing was negative. CT-Chest showed upper lobe predominant random micronodules and lower-lobe interlobular septal thickening (Figure 8, left & right). FOB was done with BAL and TBLB; BAL was negative for mycobacterial cultures and Xpert MTB/RIF. TBLB showed prominent perivascular non-necrotizing epitheloid granulomas with asteroid bodies (Figure 7, right). A diagnosis of miliary sarcoidosis was made and steroids were initiated. His symptoms and hypercalcemia resolved by four weeks and steroids were tapered over the next nine months. He remains asymptomatic on follow-up.
Fig. 7.
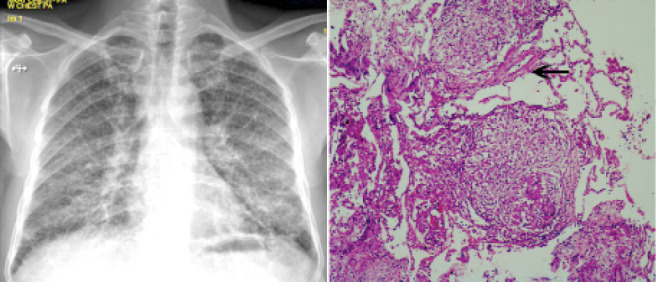
Composite image of chest radiography images (left) of Patient 4 showing miliary nodules at diagnosis and photomicrographs of the lung biopsy specimen [right, Hematoxylin and Eosin stain (H & E) x 4] showing multiple perivascular granulomas (arrow)
Fig. 8.
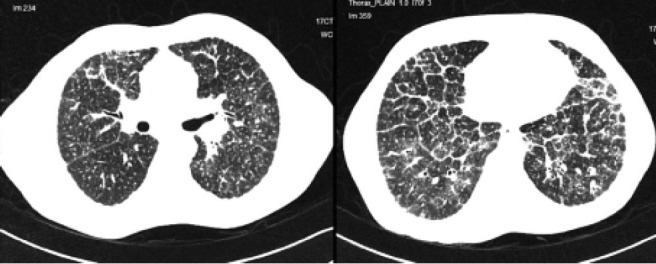
Composite image of the HRCT image of Patient 4 showing (left, upper lobes) random nodules with perilymphatic predominance, beading of the right oblique fissure and perivascular nodules and (right, lower lobes) marked interlobular septal thickening in the lower lobes associated with random nodules
Results of the systematic search
Our systematic search identified 27 reports of miliary sarcoidosis in 21 reports (3, 11-30); this included 16 men and 9 women (Table 1, supplementary Table). This cohort of “miliary sarcoidosis” was older, was mostly symptomatic and had high co-morbidity burden. The mean age of the included patients was 47.5±13.2 years; 85.2% were older than 40 years. 72.7% reported at least one associated co-morbidity (11, 12, 16, 18, 21, 27, 30).
Table 1.
Summary of clinical features of all reported cases of thoracic sarcoidosis with miliary nodules on High-resolution Computed Tomography (N=27) $
| Characteristic | Description |
| Age, Mean ± S.D, N=24 | 47.5±13.2 years; Age >40 years 85.2% |
| Gender (Male: Female), N=25 | 16:9 |
| In those >40 years, Male: Females O.R 0.26, p=1.00 (61.9% vs 75%) | |
| Country of description | India (10/26), United States (7/26), Greece (5/26), Japan (3/26), Germany (1/26) |
| Symptoms at presentation | |
| Any symptoms, N=22 | None, asymptomatic (9.1%, 2/22) |
| Symptom duration prior to presentation, Median, IQR N=19 | 8, (12) weeks |
| Symptoms | Any respiratory symptom (63.6%, 14/22) |
| Dyspnea (45.4%, 10/22), cough (45.4%, 10/22) | |
| Fatigue (30.4%, 7/23), fever (27.3%, 6/22), weight loss (22.7%, 5/22), night sweats (11.1%, 3/22) | |
| Acute kidney injury | 9.1%, 2/22 |
| Hypercalcemia | 27.3%, 6/22 |
| Co-morbidities, N=22 | Any 72.7% (16/22) |
| Smoking (18.2%, 4/22), diabetes (13.6%, 3/22), hypertension (18.2%, 4/22), lung transplantation (9.1%, 2/22), dust exposure (13.6%, 3/22) | |
| Symptomatic disseminated sarcoidosis | 8/22 (36.4%) |
| Other site involvement | Ocular (13.6%, 3/22), pleural effusion (9.1%, 2/22), bone (4.5%, 1/22), sicca syndrome (4.5%, 1/22), pancreas (4.5%, 1/22) |
| Radiological findings (N=26) | |
| True miliary pattern on HRCT | 17/26 (65.4%) only |
| Perilymphatic nodules on HRCT in miliary CT | 8/17 (47.1%); Truly random nodules total 9/26 (34.6%) |
| Zonal predominance seen | Upper & middle (7/26, 26.9%), central (7.7%, 2/26), peripheral (19.2%, 5/26) |
| Fissural nodules | 65.4% (17/26) |
| Peripheral subpleural predominance | 69.2% (18/26) |
| Interlobular septal thickening | 23.1% (6/26) |
| Intralobular septal thickening | 11.5% (3/26) |
| Ground glass opacity | 11.5% (3/26) |
| Mediastinal adenopathy | Yes (50%, 13/26); Right paratracheal (23.1%, 6/23), bilateral hilar (23.1%, 9/23), others (8.7%, 2/23) |
| Generalized lymphadenopathy | 8.7% (2/23) |
| Steroid treatment | Yes 21/22 (95.2%), Response 100% (21/21) |
| Relapse | 14.2% (3/21) |
| Tuberculosis and miliary sarcoidosis | |
| Both tuberculosis and sarcoidosis | Both tuberculosis and sarcoidosis 44.4% (12/27) |
| Definite or probable tuberculosis followed by sarcoidosis 29.7% (8/27) | |
| Probable misdiagnosis of tuberculosis 33.3%, (4/12) | |
| Microbiologically proven 37.5% (3/8) of all cases only | |
| Sequential (87.5%, 7/8), concurrent (25%, 2/8)@@ | |
| Time to sequential miliary sarcoidosis in those with tuberculosis preceding sarcoidosis, (Median, IQR) N=7 | 52 (36) weeks |
| Site of tuberculosis | Pulmonary 87.5% (7/8), spinal 12.5% (1/8) |
| Yield of TBLB, N=21 | 85.7% (18/21) |
| Miliary tuberculosis preceding or concurrent with miliary sarcoidosis | 5/8 (62.5%), 2/5 with miliary TB preceding miliary sarcoidosis |
| ATT-induced hepatitis | 2/8 (25%) |
Abbreviations: S.D standard deviation, IQR Inter-quartile range, HRCT High-resolution Computed Tomography, TBLB transbronchial lung biopsy, ATT Anti-tuberculosis treatment
@@One patient had both sequential and concurrent diagnosis of tuberculosis and sarcoidosis
The median time to presentation was 8 (IQR 12) weeks. The majority had symptoms at the time of presentation (90.9%, 20/22); only two patients recognized by surveillance transbronchial lung biopsies post-lung transplantation were asymptomatic at diagnosis (11, 12). Respiratory symptoms, however, were present in 63.6% only at diagnosis (13, 14, 16, 20, 21, 23-25, 28-30). In those with respiratory symptoms, breathlessness and cough were present in less than 50% (45.4%). Fever (27.3%) (13, 21-23, 29), night sweats (11.1%) (22, 27) and weight loss (22.7%)(18, 22, 27) were all seen in a significant percentage at diagnosis.
Symptomatic multi-systemic disease in association with miliary sarcoidosis was seen in 36.4%.(17, 19, 20, 23, 30) Hypercalcemia was seen in 27.3% (19, 20, 23, 27) and acute kidney injury was seen in 9.1% (19) at diagnosis. None needed dialysis but chronic kidney disease occurred in association with nephrocalcinosis (19). Ocular (13.6%, 3/22) involvement (25), bone (4.5%, 1/22) disease (17), sicca syndrome (4.5%, 1/22) (20) and pancreatic mass (4.5%, 1/22) (28) were the other manifestations seen. Pleural effusion was seen in association in 9.1% (2/22) (24, 28).
On review of the HRCT images, a “true miliary” pattern was seen in 65.4% (17/26) of the reported cases only. In these cases, fissural nodules and prominence could still be seen in 47.1% (8/17); only 34.6% (9/26) of reported cases could be classified as random micronodules on radiologic review (12-14, 17, 18, 24, 26, 28, 30). An upper and middle lobe distribution of nodules (11-13, 15, 16, 25-27) was seen in 26.9% (7/26) and a peripheral distribution was seen in 69.2% (11, 12, 15, 24, 28). Perilymphatic clustering of nodules (3, 11-18, 22, 24-26, 28) or a perivascular dominance (3, 13, 15, 19, 22, 26-28) of nodules was seen in 65.4% and 42.3% respectively. Interlobular septal thickening, often lower-lobe in distribution, was seen in 23.1% (Table 1) (12, 14, 15, 18). Mediastinal adenopathy was observed in 50% in association with micronodules and generalized systemic lymphadenopathy was seen in 8.7% of patients (19, 20).
44.4% (12/26) of the reported patients had tuberculosis preceding or concurrent to the diagnosis of miliary sarcoidosis (13-17, 20-22, 29). In four of these patients, this was likely a misdiagnosis (13, 14, 30). In eight patients, tuberculosis occurred preceding (15-17, 21, 22) sarcoidosis in six (62.5%), concurrently in one (37.5%) (29) and both in one (23). In those with tuberculosis preceding sarcoidosis, a microbiological confirmation was made in 42.9% (15-17, 22); Others were diagnosed based on pathology and clinical response (21). In patients with concurrent diagnosis of tuberculosis and sarcoidosis, the diagnosis of tuberculosis was clinical in all; in all these patients, clinical and pathological findings, including caseous necrosis, were consistent with tuberculosis but clinical resolution occurred on initiation of steroids only (23, 29). The time to subsequent diagnosis of miliary sarcoidosis after treatment for tuberculosis was a median of 52 (IQR, 36) weeks. Miliary tuberculosis followed or concurrent with miliary sarcoidosis was reported in four patients (Table 2) (15, 16, 23, 29).
Table 2.
Reported causes of miliary nodules on High-resolution Computed Tomography
| Classification | Cause of miliary Nodules | Site of micronodules in SPL |
| Infectious | Mycobacterium tuberculosis | Random |
| Disseminated Bacillus-Calmette-Guerin infection | Random | |
| Bacterial: Mycoplasma pneumoniae, Hemophilus influenzae | Centrilobular | |
| Fungal: Histoplasmosis, Coccidioidomycosis, hematogenous Candida infection | Random | |
| Viral: Varicella-zoster, cytomegalovirus | Centrilobular | |
| Parasitic: Tropical pulmonary eosinophilia | Centrilobular | |
| Immunologic | Pneumoconiosis: Silicosis | Perilymphatic |
| Pneumoconiosis: Coal worker’s pneumoconiosis | Perilymphatic | |
| Hypersensitivity pneumonitis | Centrilobular | |
| Sarcoidosis | Perilymphatic | |
| Idiopathic pulmonary hemosiderosis | Centrilobular | |
| Pulmonary alveolar microlithiasis | Centrilobular | |
| Diffuse panbronchiolitis | Centrilobular | |
| Allergic bronchopulmonary aspergillosis | Centrilobular | |
| Malignancy | Secondaries from bronchogenic carcinoma | Perilymphatic |
| Others: melanoma, papillary thyroid carcinoma, renal, breast, Trophoblastic tumor, osteosarcoma | Random | |
| Pulmonary Langerhans’ cell histiocytosis | Random | |
| Metastasizing leimyoma | Random | |
| Lymphoma | Perilymphatic | |
| Substance abuse | Excipient lung disease | Centrilobular |
Abbreviations: SPL secondary pulmonary lobule
TBLB was the most common modality used for diagnosis and was reported as diagnostic in 85.7%. As most patients were symptomatic, treatment with steroids was offered in 95.2%, with 100% response. Methotrexate was administered in two patients for steroid-related severe adverse effects, with good response in both. Three patients (14.2%) relapsed after steroid taper; prompt response was noted on re-institution without further relapse.(21)
Discussion
The description of “miliary” refers to small, well-defined, 1 to 3 mm nodules on chest radiography and is derived from Latin miliarius, meaning millet seed (8). This pattern implies hematogenous dissemination of disease and is classically associated with hematogenous spread of tuberculosis, fungal infections and malignancy (31). However, more than 40 diseases have been associated with this pattern on chest radiography (Table 2). The importance of quickly establishing the diagnosis, especially in high prevalence areas of tuberculosis, is that miliary tuberculosis is the most common cause, has 25-30% mortality in adults and is invariably fatal if untreated (8).
The finding of a miliary pattern on chest radiographs was noted in early roentgenologic series of thoracic sarcoidosis; 1.3% (2/150) had “miliary” disease (1). Thoracic sarcoidosis has been reported to have several typical and atypical findings on HRCT; miliary is classified as an atypical finding (Table 3) (3). The description of micronodules by their distribution in relation to the “secondary pulmonary lobule” on HRCT along with the clarity of margins and attenuation further helps in narrowing the differential diagnosis of diffuse pulmonary micronodules. Micronodules can be accurately classified as having a centrilobular, perilymphatic or random distribution with their characteristic disease associations (Table 2) (4). Miliary nodules have a random distribution and can be seen in relation to pleural surfaces, small vessels and interlobular septa but do not have a consistent or predominant relationship to any of these (31). Given the reported prevalence of sarcoidosis varies from 4.5-36 per 100,000, the very small number of cases of “miliary” sarcoidosis reported in literature in the HRCT era (Table 1) suggests that this is a very rare occurrence. Most series of computed tomographic findings in sarcoidosis and a review on atypical findings in thoracic sarcoidosis did not report “miliary” sarcoidosis (32, 33).
Table 3.
High-resolution computed tomography findings in Thoracic Sarcoidosis (Modified from Criado et al)
| Typical features | |
| Lymphadenopathy | Site (hilar, right paratracheal), bilateral, symmetrical, well defined. May show “cluster of black pearls” sign. If calcified, patchy or egg-shell |
| Nodules | Micronodules 2-4 mm, bilateral, well-defined, upper and middle zone and peri-hilar predominant. Peri-bronchovascular, subpleural, interlobular septal (peri-lymphangitic pattern) |
| Fibrotic changes | Interlobular septal thickening, Reticular opacities, architectural distortion, traction bronchiectasis |
| Atypical features | |
| Lymphadenopathy | Unilateral, isolated, anterior or posterior mediastinal |
| Nodules | Miliary |
| Lower-lobe predominant, unilateral, “Halo” sign or “Atoll” sign | |
| Consolidation | Mass-like opacities, conglomerate masses, solitary pulmonary nodules, confluent alveolar opacities and ground-glass opacities |
| Linear opacities | Intra-lobular septal thickening |
| Airway | Mosaic attenuation, trachea-bronchial abnormalities, atelectasis |
| Pleural | Effusion, chylothorax, pneumothorax, pleural thickening, calcification, plaque-like lesions |
Our systematic review suggests that this small reported number is an overestimate as a true miliary pattern was seen only in 34.6% (9/26) of the published cases of “miliary” sarcoidosis; and subtle perilymphatic pattern is nearly always seen and the term “pseudo-miliary” may be more appropriate. Distinct perilymphatic clustering of nodules or a perivascular dominance of nodules was seen in 65.4% and 42.3%; fissural nodules leading to the “beaded fissure sign” was prominent and seen in 65.4% of cases. Further, unlike true miliary nodules that lack any zonal predominance, an upper lobe and peripheral clustering was seen in 30.8% and 19.2% respectively. In all our patients with random micronodules, we observed a prominent perivascular distribution of micronodules on histopathology (Figures 2, 4, 6 and 8); two of these had associated “beaded fissure sign” on HRCT. Pathologically, sarcoidosis causes perilymphatic clustering of granulomas; hematogenous or airway spread has not been postulated (34). Profuse clustering of micronodules along the centrilobular and perilymphatic compartments gives rise to an appearance of random nodules in the majority with a “pseudo-miliary” appearance. In the few patients with a true random pattern, prominent perivascular granulomas or drainage into the pulmonary lymphatics and subsequent dissemination by hematogenous route could be postulated.
The link between mycobacteria and sarcoidosis continues to be an enigma (7, 35). In the original descriptions, about 5% of sarcoidosis had tuberculosis preceding them. Polymerase chain reaction analyses of lung biopsies of sarcoidosis have shown mycobacterial DNA in 26.4%.(35) Tuberculosis may present concurrently and confound the diagnosis and management of these patients (23, 36, 37). It has been hypothesized that the immunological responses in sarcoidosis are analogous to “tuberculoid” or “pauci-bacillary” leprosy (38); persisting immunological responses to mycobacterial antigen can explain the extraordinarily high rates of tuberculosis preceding the diagnosis of pseudo-miliary sarcoidosis in the present systematic review.
The diagnosis of sarcoidosis rests on a constellation of clinical, radiological, histopathological and laboratory findings (39). Constitutional features including significant weight loss can occur in up to 25% of patients (Table 2). Fibrinoid necrosis and rarely, caseation can occur in sarcoidosis on histopathology (40, 41). Caseation may sometimes be absent in tuberculosis (41). Radiology can help in the differentiation of sarcoidosis from tuberculosis (26). The presence of hypercalcemia, skin involvement, negative tuberculin skin-testing, upper and middle-lobe distribution of nodules, a peripheral distribution of nodules when present and interlobular septal thickening all point to the diagnosis of “pseudo-miliary” sarcoidosis alone (42). However, sarcoidosis can precede, occur concurrent with or follow tuberculosis and differentiating tuberculosis from sarcoidosis remains a major challenge, especially in high prevalence areas of tuberculosis (43, 44). When the constellation of findings are non-conclusive, a trial of anti-tuberculosis treatment is appropriate in high prevalence areas given the very small number of true miliary sarcoidosis reported in literature. Steroids could be added when there is no clear response at 4-6 weeks (39, 43).
Conclusion
A true miliary pattern in the HRCT era is very rare in sarcoidosis and subtle perilymphatic pattern is nearly always seen; this pattern should be labeled “pseudo-miliary”. Prominent perivascular granulomas are associated with true miliary pattern. Miliary sarcoidosis patients are older and symptomatic and mostly need treatment at diagnosis. “Miliary” sarcoidosis may follow treatment for tuberculosis; concurrent cases possibly indicate difficulty in diagnosis or a “tuberculo-sarcoid” presentation. Transbronchial lung biopsy had a good yield for diagnosis. Most patients were symptomatic and treated with steroids, with 100% response and 14.4% relapse.
Acknowledgements
Dr. R.S, Dr. RM.PL and Dr. R.K were responsible for patient care and initiated the manuscript preparation and performed the systematic review. Dr. S.S reported the histopathologic findings of these patients and provided valuable inputs to the manuscript. Dr.B.D reviewed all the radiology images and the images retrieved from the systematic review for re-reporting.
Drs. R.S and Dr. S.S take responsibility for the content of the manuscript, including the data and analysis
The authors would like to thank Senior Librarian, PSGIMS&R, for his help in procuring manuscripts for the systematic review. No funding was utilized for this manuscript
References
- 1.Kirks DR, McCormick VD, Greenspan RH. Pulmonary sarcoidosis. Roentgenologic analysis of 150 patients. Am J Roentgenol Radium Ther Nucl Med. 1973;117(4):777–86. doi: 10.2214/ajr.117.4.777. PubMed PMID: 4266919. [DOI] [PubMed] [Google Scholar]
- 2.Koyama T, Ueda H, Togashi K, Umeoka S, Kataoka M, Nagai S. Radiologic manifestations of sarcoidosis in various organs. Radiographics : a review publication of the Radiological Society of North America, Inc. 2004;24(1):87–104. doi: 10.1148/rg.241035076. doi: 10.1148/rg.241035076. PubMed PMID: 14730039. [DOI] [PubMed] [Google Scholar]
- 3.Criado E, Sanchez M, Ramirez J, Arguis P, de Caralt TM, Perea RJ, et al. Pulmonary sarcoidosis: typical and atypical manifestations at high-resolution CT with pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2010;30(6):1567–86. doi: 10.1148/rg.306105512. Epub 2010/11/13. doi: 10.1148/rg.306105512. PubMed PMID: 21071376. [DOI] [PubMed] [Google Scholar]
- 4.Raoof S, Amchentsev A, Vlahos I, Goud A, Naidich DP. Pictorial essay: multinodular disease: a high-resolution CT scan diagnostic algorithm. Chest. 2006;129(3):805–15. doi: 10.1378/chest.129.3.805. doi: 10.1378/chest.129.3.805. PubMed PMID: 16537886. [DOI] [PubMed] [Google Scholar]
- 5.Global tuberculosis report 2018. Geneva: World Health Organization. 2018 Licence: CC BY-NC-SA 3.0 IGO2018 10/11/2018. [Google Scholar]
- 6.Sharma SK, Mohan A, Sharma A. Challenges in the diagnosis & treatment of miliary tuberculosis. Indian J Med Res. 2012;135(5):703–30. PubMed PMID: 22771605; PubMed Central PMCID: PMCPMC3401706. [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Sarcoidosis and tuberculosis: the same disease with different manifestations or similar manifestations of different disorders. Curr Opin Pulm Med. 2012;18(5):506–16. doi: 10.1097/MCP.0b013e3283560809. doi: 10.1097/MCP.0b013e3283560809. PubMed PMID: 22759770. [DOI] [PubMed] [Google Scholar]
- 8.Sharma SK, Mohan A, Sharma A, Mitra DK. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis. 2005;5(7):415–30. doi: 10.1016/S1473-3099(05)70163-8. doi: 10.1016/S1473-3099(05)70163-8. PubMed PMID: 15978528. [DOI] [PubMed] [Google Scholar]
- 9.Zerhouni EA, Naidich DP, Stitik FP, Khouri NF, Siegelman SS. Computed tomography of the pulmonary parenchyma. Part 2: Interstitial disease. Journal of thoracic imaging. 1985;1(1):54–64. doi: 10.1097/00005382-198512000-00008. PubMed PMID: 3843414. [DOI] [PubMed] [Google Scholar]
- 10.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 1999;16(2):149–73. PubMed PMID: 10560120. [PubMed] [Google Scholar]
- 11.Kazerooni EA, Jackson C, Cascade PN. Sarcoidosis: recurrence of primary disease in transplanted lungs. Radiology. 1994;192(2):461–4. doi: 10.1148/radiology.192.2.8029415. Epub 1994/08/01. doi: 10.1148/radiology.192.2.8029415. PubMed PMID: 8029415. [DOI] [PubMed] [Google Scholar]
- 12.Kazerooni EA, Cascade PN. Recurrent miliary sarcoidosis after lung transplantation. Radiology. 1995;194(3):913. doi: 10.1148/radiology.194.3.7863002. Epub 1995/03/01. doi: 10.1148/radiology.194.3.7863002. PubMed PMID: 7863002. [DOI] [PubMed] [Google Scholar]
- 13.Gupta D, Kumar S, Jindal SK. Bilateral miliary mottling without hilar lymphadenopathy: A rare presentation of sarcoidosis. Lung India. 1996;14(2):87–8. [Google Scholar]
- 14.Chugh IM, Agarwal AK, Arora VK, Shah A. Bilateral miliary pattern in sarcoidosis. The Indian journal of chest diseases & allied sciences. 1997;39(4):245–9. PubMed PMID: 9654821. [PubMed] [Google Scholar]
- 15.Voloudaki AE, Tritou IN, Magkanas EG, Chalkiadakis GE, Siafakas NM, Gourtsoyiannis NC. HRCT in miliary lung disease. Acta radiologica (Stockholm, Sweden : 1987) 1999;40(4):451–6. doi: 10.3109/02841859909177764. Epub 1999/07/08. PubMed PMID: 10394878. [DOI] [PubMed] [Google Scholar]
- 16.Hatzakis K, Siafakas NM, Bouros D. Miliary sarcoidosis following miliary tuberculosis. Respiration; international review of thoracic diseases. 2000;67(2):219–22. doi: 10.1159/000029492. Epub 2000/04/25. doi: 10.1159/000029492. PubMed PMID: 10773799. [DOI] [PubMed] [Google Scholar]
- 17.Kline MY, Leibert EM. A case of pulmonary tuberculosis and miliary sarcoidosis. Chest. 2005:480S–1S. [Google Scholar]
- 18.Stroebe K, Haddadin H. Bilateral miliary sarcoidosis. Chest. 2012:1036A. [Google Scholar]
- 19.Hodges HK, Lee PY, Hausmann JS, Teot LA, Sanford EL, Levin KW. Hypercalcemia and miliary sarcoidosis in a 15-year-old boy. Arthritis and rheumatism. 2013;65(8):2112. doi: 10.1002/art.38019. Epub 2013/05/21. doi: 10.1002/art.38019. PubMed PMID: 23686651. [DOI] [PubMed] [Google Scholar]
- 20.Kumar P, Jaco MJ, Pandit AG, Shanmughanandan K, Jain A, Rajee V, et al. Miliary sarcoidosis with secondary Sjogren’s syndrome. The Journal of the Association of Physicians of India. 2013;61(7):505–7. Epub 2014/04/30. PubMed PMID: 24772762. [PubMed] [Google Scholar]
- 21.Bostantzoglou C, Samitas K, Gkogkou C, Zervas E, Gaga M. Mediastinal widening and miliary chest radiograph pattern in a middle aged man: could it be sarcoidosis? BMJ case reports. 2014;2014 doi: 10.1136/bcr-2014-204884. Epub 2014/07/19. doi: 10.1136/bcr-2014-204884. PubMed PMID: 25035447; PubMed Central PMCID: PMCPMC4112298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luetkens JA, Zoghi S, Rockstroh JK, Naehle CP. Pulmonary sarcoidosis shortly after spinal tuberculosis infection: a diagnostic challenge. BMJ case reports. 2014;2014 doi: 10.1136/bcr-2013-203333. Epub 2014/04/15. doi: 10.1136/bcr-2013-203333. PubMed PMID: 24728896; PubMed Central PMCID: PMCPMC3987564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandal SK, Ghosh S, Mondal SS, Chatterjee S. Coexistence of pulmonary tuberculosis and sarcoidosis: a diagnostic dilemma. BMJ case reports. 2014;2014 doi: 10.1136/bcr-2014-206016. Epub 2014/12/21. doi: 10.1136/bcr-2014-206016. PubMed PMID: 25527682; PubMed Central PMCID: PMCPMC4275725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enomoto Y, Yokomura K, Suda T. Bilateral pleural effusion associated with miliary sarcoidosis. American journal of respiratory and critical care medicine. 2015;191(4):474–5. doi: 10.1164/rccm.201411-2022IM. Epub 2015/02/14. doi: 10.1164/rccm.201411-2022IM. PubMed PMID: 25679105. [DOI] [PubMed] [Google Scholar]
- 25.Taki M, Ikegami N, Konishi C, Nakao S, Funazou T, Ariyasu R, et al. Pulmonary Sarcoidosis Presenting with Miliary Opacities. Internal medicine (Tokyo, Japan) 2015;54(19):2483–6. doi: 10.2169/internalmedicine.54.4681. Epub 2015/10/02. doi: 10.2169/internalmedicine.54.4681. PubMed PMID: 26424308. [DOI] [PubMed] [Google Scholar]
- 26.Bhalla AS, Das A, Naranje P, Goyal A, Guleria R, Khilnani GC. Dilemma of diagnosing thoracic sarcoidosis in tuberculosis endemic regions: An imaging-based approach. Part 1. Indian J Radiol Imaging. 2017;27(4):369–79. doi: 10.4103/ijri.IJRI_200_17. doi: 10.4103/ijri.IJRI_200_17. PubMed PMID: 29379230; PubMed Central PMCID: PMCPMC5761162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaddha U, English R, Daniels J, Walia R, Mehta AC, Panchabhai TS. A 58-Year-Old Man With Fatigue, Weight Loss, and Diffuse Miliary Pulmonary Opacities. Chest. 2017;151(6):e131–e4. doi: 10.1016/j.chest.2016.11.015. Epub 2017/06/11. doi: 10.1016/j.chest.2016.11.015. PubMed PMID: 28599946. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura S, Mochizuka Y, Oishi K, Miyashita K, Naoi H, Mochizuki E, et al. Sarcoidosis with Pancreatic Mass, Endobronchial Nodules, and Miliary Opacities in the Lung. Internal medicine (Tokyo, Japan) 2017;56(22):3083–7. doi: 10.2169/internalmedicine.8916-17. Epub 2017/09/26. doi: 10.2169/internalmedicine.8916-17. PubMed PMID: 28943576; PubMed Central PMCID: PMCPMC5725865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arar O, Boni F, Meschi T, Tana C. Pulmonary sarcoidosis presenting with miliary opacities. Current Medical Imaging Reviews. 2018 doi: 10.2174/1573405614666180806141415. doi: 10.2174/1573405614666180806141415. [DOI] [PubMed] [Google Scholar]
- 30.Joshi V, Jain S, Sharma V, Khippal N, Chaturvedi A. Granulomatous Hepatitis with Miliary Mottling: A Rare Cause. Journal of The Association of Physicians of India. 2018;66:97–8. [PubMed] [Google Scholar]
- 31.Andreu J, Mauleon S, Pallisa E, Majo J, Martinez-Rodriguez M, Caceres J. Miliary lung disease revisited. Current problems in diagnostic radiology. 2002;31(5):189–97. PubMed PMID: 12419999. [PubMed] [Google Scholar]
- 32.Kuhlman JE, Fishman EK, Hamper UM, Knowles M, Siegelman SS. The computed tomographic spectrum of thoracic sarcoidosis. Radiographics : a review publication of the Radiological Society of North America, Inc. 1989;9(3):449–66. doi: 10.1148/radiographics.9.3.2727355. doi: 10.1148/radiographics.9.3.2727355. PubMed PMID: 2727355. [DOI] [PubMed] [Google Scholar]
- 33.Cozzi D, Bargagli E, Calabro AG, Torricelli E, Giannelli F, Cavigli E, et al. Atypical HRCT manifestations of pulmonary sarcoidosis. La Radiologia medica. 2018;123(3):174–84. doi: 10.1007/s11547-017-0830-y. doi: 10.1007/s11547-017-0830-y. PubMed PMID: 29124658. [DOI] [PubMed] [Google Scholar]
- 34.Spagnolo P, Rossi G, Trisolini R, Sverzellati N, Baughman RP, Wells AU. Pulmonary sarcoidosis. Lancet Respir Med. 2018;6(5):389–402. doi: 10.1016/S2213-2600(18)30064-X. doi: 10.1016/S2213-2600(18)30064-X. PubMed PMID: 29625772. [DOI] [PubMed] [Google Scholar]
- 35.Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis. Eur Respir J. 2007;30(3):508–16. doi: 10.1183/09031936.00002607. doi: 10.1183/09031936.00002607. PubMed PMID: 17537780. [DOI] [PubMed] [Google Scholar]
- 36.Smith-Rohrberg D, Sharma SK. Tuberculin skin test among pulmonary sarcoidosis patients with and without tuberculosis: its utility for the screening of the two conditions in tuberculosis-endemic regions. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2006;23(2):130–4. PubMed PMID: 17937109. [PubMed] [Google Scholar]
- 37.Shah JR, Hede J, Mathur RS. Diagnostic criteria of tuberculous sarcoidosis. Lung India. 2009;26(3):86–8. doi: 10.4103/0970-2113.53232. doi: 10.4103/0970-2113.53232. PubMed PMID: 20442843; PubMed Central PMCID: PMCPMC2862513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal R, Gupta D. Tuberculous sarcoidosis: Is it a separate entity? Lung India. 2009;26(3):61–2. doi: 10.4103/0970-2113.53225. doi: 10.4103/0970-2113.53225. PubMed PMID: 20442837; PubMed Central PMCID: PMCPMC2862507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jindal SK, Gupta D, Aggarwal AN. Sarcoidosis in India: practical issues and difficulties in diagnosis and management. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2002;19(3):176–84. PubMed PMID: 12405486. [PubMed] [Google Scholar]
- 40.Karkhanis V, Joshi JM. All that caseate is not TB. Lung India. 2007;24:100–1. [Google Scholar]
- 41.Danila E, Zurauskas E. Diagnostic value of epithelioid cell granulomas in bronchoscopic biopsies. Internal medicine (Tokyo, Japan) 2008;47(24):2121–6. doi: 10.2169/internalmedicine.47.1452. doi: 10.2169/internalmedicine.47.1452. PubMed PMID: 19075536. [DOI] [PubMed] [Google Scholar]
- 42.Bhalla AS, Das A, Naranje P, Goyal A, Guleria R, Khilnani GC. Dilemma of diagnosing thoracic sarcoidosis in tuberculosis-endemic regions: An imaging-based approach. Part 2. Indian J Radiol Imaging. 2017;27(4):380–8. doi: 10.4103/ijri.IJRI_201_17. doi: 10.4103/ijri.IJRI_201_17. PubMed PMID: 29379231; PubMed Central PMCID: PMCPMC5761163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guleria R, Mahashur A, Ghoshal AG, Thomas PK, Raghu G, Baughman RP. Challenges in diagnosing Sarcoidosis in tuberculosis endemic regions: Clinical scenario in India. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2016;33(4):381–4. PubMed PMID: 28079850. [PubMed] [Google Scholar]
- 44.Jain R, Mohan A, Guleria R. Sarcoidosis vs tuberculosis: Diagnostic mystery still unresolved. Indian J Tuberc. 2017;64(4):243–5. doi: 10.1016/j.ijtb.2017.09.001. doi: 10.1016/j.ijtb.2017.09.001. PubMed PMID: 28941846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of all reported cases of thoracic sarcoidosis with miliary nodules on High-resolution Computed Tomography$
$Poor quality HRCT images precluded assessment of type of nodules; **In one miliary sarcoidosis followed miliary tuberculosis; %Concurrent tuberculosis and miliary sarcoidosis with organomegaly, neurologic and bone involvement; #Tuberculosis was diagnosed based on pathology, radiology and clinical response; ATT stopped at 5 months due to liver injury; ##Tb was diagnosed microbiologically based on mycobacterial cultures
Abbreviations: M Male, F Female, U.S United States, NA Not available, BLTx Bilateral lung transplantation, HBLTx Heart and lung transplantation, HRCT high resolution computed tomography, UL Upper lobe, PL Peri-lymphatic pattern, IL Interlobular nodules, ILo, Intralobular septal thickening, CAD coronary artery disease, MI Myocardial infarction, TB tuberculosis, DM Diabetes mellitus, HTN hypertension, COPD Chronic obstructive pulmonary disease, AKI acute kidney injury, INH isoniazid, ML Mediastinal, BHL bilateral Hilar lymphadenopathy, RPT Right paratracheal, LNE lymphadenopathy, Y Yes, Kgs kilograms, Bx biopsy, TBLB Transbronchial lung biopsy, SLBx surgical lung biopsy, EBUS-FNA endobronchial ultrasound guided fine needle aspiration, ATT Anti-tuberculosis treatment, MTX-Methotrexate