Abstract
Background:
Pulmonary hypertension (PH) is a known complication of pulmonary sarcoidosis and is associated with higher morbidity and mortality. Currently, there are no approved PH-targeted therapies for sarcoidosis-associated pulmonary hypertension (SAPH). Macitentan is frequently used as treatment for pulmonary arterial hypertension, but no results are known in the SAPH population.
Objective:
We investigated the safety and effect of macitentan as treatment for SAPH.
Methods:
We retrospectively reviewed our patient database for all SAPH patients receiving macitentan as treatment, with a minimum follow-up of twelve months for monitoring safety. Safety outcomes included reported side-effects, hospitalisations and mortality. Furthermore, six-minutes walking distance, New York Heart Association functional class and NT-proBNP levels were collected.
Results:
Six cases (three men) with a median age of 64 years (range 52-74 years) were identified. During macitentan treatment, one patient experienced side effects and aborted therapy after five days of treatment and died 16 months later. Three patients were hospitalised during treatment for congestive heart failure. Four patients showed improvement of their functional class and three patients in exercise capacity after 12 months of therapy.
Conclusion:
Macitentan was well tolerated in five out of six cases with severe pulmonary sarcoidosis and PH. Functional capacity improved in four cases. Prospective controlled trials are warranted before therapeutic recommendations can be made. (Sarcoidosis Vasc Diffuse Lung Dis 2020; 37 (1): 74-78)
Keywords: sarcoidosis, macitentan, pulmonary hypertension, endothelin receptor antagonist
Introduction
Pulmonary hypertension (PH) is a known complication of pulmonary sarcoidosis. Prevalence numbers range from 3% in early stage pulmonary sarcoidosis, up to 70% in patients awaiting lung transplantation.(1,2) Sarcoidosis-associated pulmonary hypertension (SAPH) is associated with increased morbidity and mortality.(3,4) The underlying pathophysiological mechanism of SAPH remains unclear. Hypothesised mechanisms include destruction of the pulmonary vascular bed by pulmonary fibrosis, granulomatous vasculopathy, extrinsic compression from thoracic lymphadenopathy, mediastinal fibrosis and cardiac involvement. (4–6) Currently, there are no approved PH-targeted treatments for SAPH. Although endothelin receptor antagonists are well used in pulmonary arterial hypertension, studies have shown mixed results in SAPH.(7,8) To our best knowledge, the safety and effect of the endothelin receptor antagnost macitentan in SAPH patients has not been evaluated. We report the results of a single centre case-series.
Methods
The St. Antonius hospital is a tertiary referral centre for sarcoidosis and PH. We retrospectively reviewed our patient database between 2014-2018 to include all patients aged ≥18 years, diagnosed with both sarcoidosis and PH, received macitentan as treatment (mono- or dual therapy), and at least 12 months of follow-up for monitoring safety outcomes. The diagnosis of sarcoidosis was based on current clinical diagnostic guidelines.(9) PH was confirmed by right heart catheterization (RHC) and defined as a resting mean pulmonary artery pressure (mPAP) of ≥25mmHg. The decision to start treatment was made by a multidisciplinary team. Safety outcomes included side effects leading to (temporarily) aborting therapy, hospitalisation for heart failure or dyspnoea, and death.
Baseline was defined as start of PH-targeted treatment. At baseline, N-terminal pro-brain natriuretic peptide (NT-proBNP) levels, forced vital capacity (FVC), New York Heart Association (NYHA) functional class were measured and a six-minute walking distance (6-MWD) was obtained. Macitentan was administered at a dose of 10mg/day. If applicable, sildenafil was dosed 20mg three times daily. All outcome parameters were obtained by chart review. Written informed consent was obtained in all cases. The study was approved by the local institutional review board.
Results
Figure 1 shows the flowchart of case selection. In total, 27 patients with SAPH were identified between 2014-2018. Of these patients, 8 were treated with macitentan. Of these, 6 patients had a follow-up of at least twelve months, while the other two patients were recently started on macitentan. The baseline characteristics and outcome parameters of all cases are shown in table 1. Six patients (three men) with a median age of 64 years (range 52-74 years) were identified. All cases were Caucasian patients with biopsy confirmed sarcoidosis. RHC showed a median mPAP of 49 (27 – 66) mmHg and the pulmonary vascular resistance (PVR) was > 3 Wood Units (WU) in all cases.
Fig. 1.
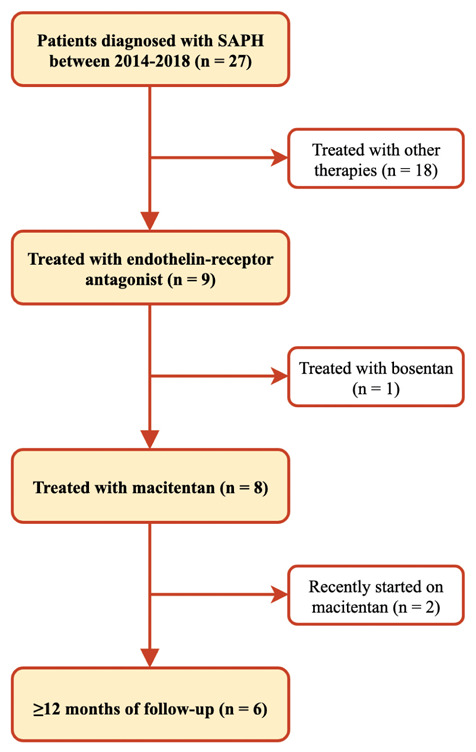
Table 1.
Case characteristics
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
| Demographics | ||||||
| Age (years) | 60 | 74 | 64 | 52 | 69 | 65 |
| Male / female | Female | Male | Male | Female | Female | Male |
| Time since sarcoidosis diagnosis (years) | 8,3 | 4,3 | 20 | 0,2 | 22,5 | 12,2 |
| Pulmonary function | ||||||
| FEV1 (% predicted) | 75.9 | 90.0 | 49.0 | 54.9 | 36.9 | 25.6 |
| FVC (% predicted) | 104.1 | 88.0 | 69.0 | 62.5 | 50.4 | 75.0 |
| DLCO SB (% predicted) | 76.4 | 25.0 | - | 40.3 | 13.9 | 57.1 |
| Fibrosis on HRCT | Yes | Yes | No | No | Yes | Yes |
| Heamodynamics | ||||||
| sPAP / dPAP (mmHg) | 110/40 | 60/32 | 43/19 | 96/49 | 85/35 | 76/30 |
| mPAP (mmHg) | 63 | 37 | 27 | 66 | 55 | 43 |
| PAWP (mmHg) | 6 | 11 | 10 | 18 | 5 | 12 |
| PVR (Wood Units) | 10.3 | 10.4 | 3.2 | 11.3 | 13.9 | 7.2 |
| Cardiac output (L/min) | 5.6 | 2.5 | 5.3 | 4.3 | 3.6 | 4.6 |
| Sarcoidosis treatment | ||||||
| Supplemental oxygen use | No | Yes | Yes | Yes | Yes | No |
| Immunosuppressive treatment | No | Yes | Yes | Yes | No | Yes |
| Escalation of immunosuppressive treatment during follow-up | Yes | No | No | No | Yes | No |
| PH treatment | ||||||
| Initial PH-targeted therapy | Macitentan | Macitentan | Dual | Macitentan | Dual | Sildenafil |
| Time before start dual treatment (months) | 3 | 1 | - | 10 | - | 15 |
| Follow-up duration (months) | 42 | 12 | 12 | 42 | 36 | 18 |
| Outcome parameters | ||||||
| NYHA functional class at baseline | III | III | III | III | IV | III |
| NYHA functional class at 12 months | II | III | II | II | III | III |
| NYHA functional class at 24 months | II | III | II | II | III | IV |
| 6-MWD at baseline (meters) | 327 | 365 | 445 | 364 | 145 | 341 |
| 6-MWD at 12 months (meters) | 439 | - | 457 | 367 | 340 | 244 |
| 6-MWD at 24 months (meters) | 456 | - | - | 422 | 243 | - |
| NT-proBNP at baseline (pg/mL) | 136 | 959 | 52 | 3382 | 5875 | 346 |
| NT-proBNP at 12 months (pg/mL) | 110 | 1516 | 45 | 3512 | 343 | 688 |
| NT-proBNP at 24 months (pg/mL) | 38 | - | - | 1543 | 210 | - |
FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; DLCO SB: diffusing capacity of the lung for carbon monoxide single breath; HRCT: high resolution chest tomography; sPAP: systolic pulmonary artery pressure; dPAP: diastolic pulmonary artery pressure; mPAP: mean pulmonary artery pressure; PAWP: pulmonary arterial wedge pressure; PVR: pulmonary vascular resistance; PH: pulmonary hypertension; NYHA: New York Heart Association; 6-MWD: six minute walking distance
Case 1 was 60-year old female with suspected fibrosing pulmonary sarcoidosis and severe PH. Macitentan was started after PH diagnosis. After two months, sarcoidosis was proven on biopsy and immunosuppressive therapy with methotrexate 15mg/week was initiated due to active disease on FDG-PET (fluodeoxyglucose-positron emission tomography) with compression of the pulmonary artery. At three months, echocardiography showed improved right ventricular function and sildenafil was added. At 7 months, immunosuppressive therapy was switched to azathioprine 100mg/day due to side effects (depressive thoughts). At one year, there was an improvement of mPAP (47mmHg), PVR (5.0WU), 6-MWD, and NYHA functional class. NT-proBNP levels and the FVC remained stable. During her 3.5 years follow-up, macitentan was well tolerated with no reported side effects.
Case 2 was a 74-year old male with fibrosing pulmonary sarcoidosis. RHC showed severe PH and macitentan was started. After four weeks, he was admitted for pneumonia and congestive heart failure. After recovery, sildenafil was added. At 12 months, no side effects were reported. NT-proBNP levels had increased, while NYHA functional class and FVC remained stable. A 6-MWD was not performed during follow-up due to persisting disabilities after a cerebrovascular event.
Case 3 was 64-year old male with pulmonary sarcoidosis. After PH diagnosis, macitentan and sildenafil were started with good effect on NYHA functional class at 12 months while other outcome parameters remained stable or showed mild improvement. Macitentan was well tolerated.
Case 4 was a 52-year old female, with recently diagnosed pulmonary sarcoidosis and severe PH. Macitentan treatment was started with initial good effect on functional capacity. At 10 months, she was hospitalized due to congestive heart failure. After recovery, RHC was repeated which showed a mPAP of 58mmHg and PVR 12.5WU. Sildenafil was added and after 24 months all outcome parameters improved, while FVC remained stable. During 3.5 years follow-up, macitentan was well tolerated and the patient remained clinically stable.
Case 5 was 69-year old female patient with fibrosing pulmonary sarcoidosis and severely reduced exercise capacity. Dual treatment with macitentan and sildenafil was started. The FDG-PET showed enhanced inflammatory activity, and high-dosage prednisone was started at 6 weeks. At 12 months, there was an improvement in 6-MWD (340 vs 145m), NT-proBNP and NYHA functional class. FVC also improved during treatment (74.0% vs 50.4%). Echocardiography showed improvement in right ventricular function after 12 months. No side effects were reported during follow-up.
Case 6 was a 65-year old male patient with fibrosing pulmonary sarcoidosis. After PH diagnosis, initial treatment with sildenafil was started. After 12 months of treatment, RHC showed a mPAP of 47mmHg with no subjective improvement. FDG-PET revealed no signs of inflammatory activity and macitentan was initiated. Macitentan was not well tolerated and aborted after five days due to severe muscle aches and fatigue. Several days later, this patient was hospitalised for increasing dyspnoea and sildenafil was aborted due to no clinical improvement. The patient was discharged home with oxygen therapy and diuretics and died sixteen months later due to right ventricular failure.
Discussion
To the best of our knowledge, this is the first case series describing the safety and effect of macitentan, either as monotherapy or as dual treatment with sildenafil, as treatment for SAPH in predominantly patients with fibrosing pulmonary sarcoidosis. We found that macitentan was well tolerated in five patients, but one patient aborted macitentan therapy due to side effects and died sixteen months later.
Judson et al. investigated the role of the endothelin receptor antagonist ambrisentan as treatment for SAPH. They found that 11 out of 21 patients aborted ambrisentan therapy, mostly due to increasing dyspnoea.(7) In our case series, no patients aborted therapy due to dyspnoea. However three patients were hospitalised for dyspnoea due to congestive heart failure, but recovered with diuretic treatment. A known side-effect of a pulmonary vasodilator in parenchymal lung disease is the possible worsening of ventilation/perfusion mismatch, which could lead to increasing dyspnoea.(10,11) Unfortunately, we were not able to evaluate the effect of macitentan on gas exchange before and during treatment due to missing data for arterial blood gas analyses. The found rate of adverse events are in line with the MUSIC trial. This study showed that 12 months of macitentan therapy was well tolerated in patients with idiopathic pulmonary fibrosis, with 12.6% of patients aborting therapy due to adverse events.(12)
Furthermore, four patients showed improvement of their functional class and three patients in exercise capacity after 12 months of therapy. A possible confounder for this improvement is the escalating immunosuppressive treatment for increased sarcoidosis activity. It is known In pulmonary sarcoidosis that immunosuppressive treatment can improve FVC.(13) This could explain the functional improvement in case 5 as this was the only case with an improved FVC during follow-up compared to baseline. In all other cases the FVC remained stable.
In conclusion, this is the first case-series describing the safety and effect of macitentan therapy in SAPH. Macitentan was well tolerated in five out of six cases with severe pulmonary sarcoidosis and PH. Functional capacity improved in four out of six cases. However, results of this case series need to be interpreted with caution. Prospective controlled trials are warranted before therapeutic recommendations can be made.
Abbreviation list:
- 6-MWD:
six-minute walking distance
- FDG-PET:
fluodeoxyglucose-positron emission tomography
- FVC:
forced vital capacity
- mPAP:
mean pulmonary artery pressure
- NT-proBNP:
N-terminal pro-brain natriuretic peptide
- NYHA:
New York Heart Association
- PH:
pulmonary hypertension
- PVR:
pulmonary vascular resistance
- SAPH:
sarcoidosis-associated pulmonary hypertension
- RHC:
right heart catheterization
- WU:
Wood Units
References
- 1.Huitema MP, Bakker ALM, Mager JJ, Rensing BJWM, Smits F, Snijder RJ, et al. Prevalence of pulmonary hypertension in pulmonary sarcoidosis; the first large European prospective study. Eur Respir J [Internet] 2019 Jul 18 doi: 10.1183/13993003.00897-2019. 1900897. Available from: http://erj.ersjournals.com/lookup/doi/10.1183/13993003.00897-2019 . [DOI] [PubMed] [Google Scholar]
- 2.Shorr AF, Helman DL, Davies DB, Nathan SD. Pulmonary hypertension in advanced sarcoidosis: Epidemiology and clinical characteristics. Eur Respir J. 2005 May;25(5):783–8. [Google Scholar]
- 3.Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis-associated pulmonary hypertension: The importance of hemodynamic evaluation. Chest. 2010 Nov 1;138(5):1078–1085. doi: 10.1378/chest.09-2002. [DOI] [PubMed] [Google Scholar]
- 4.Sulica R, Teirstein AS, Kakarla S, Nemani N, Behnegar A, Padilla ML. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest. 2005;128(3):1483–1489. doi: 10.1378/chest.128.3.1483. [DOI] [PubMed] [Google Scholar]
- 5.Takemura T, Matsui Y, Saiki S, Mikami R. Pulmonary vascular involvement in sarcoidosis: A report of 40 autopsy cases. Hum Pathol. 1992;23(11):1216–1223. doi: 10.1016/0046-8177(92)90288-e. [DOI] [PubMed] [Google Scholar]
- 6.Nunes H, Humbert M, Capron F, Brauner M, Sitbon O, Battesti JP, et al. Pulmonary hypertension associated with sarcoidosis: Mechanisms, haemodynamics and prognosis. Thorax. 2006 Jan;61(1):68–74. doi: 10.1136/thx.2005.042838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Judson MA, Highland KB, Kwon S, Donohue JF, Aris R, Craft N, et al. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffus Lung Dis. 2011;28(2):139–145. [PubMed] [Google Scholar]
- 8.Baughman RP, Culver DA, Cordova FC, Padilla M, Gibson KF, Lower EE, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: A double-blind placebo controlled randomized trial. Chest. 2014;145(4):810–817. doi: 10.1378/chest.13-1766. [DOI] [PubMed] [Google Scholar]
- 9.Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14(4):735–737. doi: 10.1034/j.1399-3003.1999.14d02.x. [DOI] [PubMed] [Google Scholar]
- 10.Corte TJ, Keir GJ, Dimopoulos K, Howard L, Corris PA, Parfitt L, et al. Bosentan in pulmonary hypertension associated with fibrotic idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2014;190:208–217. doi: 10.1164/rccm.201403-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghu G. Treatment of Idiopathic Pulmonary Fibrosis With Ambrisentan. Ann Intern Med. 2013;158:641–649. doi: 10.7326/0003-4819-158-9-201305070-00003. [DOI] [PubMed] [Google Scholar]
- 12.Raghu G, Million-Rousseau R, Morganti A, Perchenet L, Behr J. Macitentan for the treatment of idiopathic pulmonary fibrosis: the randomised controlled MUSIC trial. Eur Respir J. 2013 Dec;42(6):1622–1632. doi: 10.1183/09031936.00104612. [DOI] [PubMed] [Google Scholar]
- 13.Nunes H, Jeny F, Bouvry D, Uzunhan Y, Valeyre D. Indications for treatment of sarcoidosis. Curr Opin Pulm Med. 2019;25(5):505–518. doi: 10.1097/MCP.0000000000000604. [DOI] [PubMed] [Google Scholar]


