Abstract
Sarcoidosis-Associated Pulmonary Hypertension (SAPH) is a common finding in patients with chronic sarcoidosis and is associated with increased mortality. The optimal treatment for SAPH is not known; however, therapies approved for Group 1 pulmonary hypertension have improved hemodynamics and functional status. Prostanoids, including epoprostenol, have been therapeutic in short-term studies of SAPH, but long-term efficacy is unknown. In this study, we evaluated the long-term effect of epoprostenol therapy in 12 patients with SAPH. Hemodynamic assessment after an average of 4.1 years of epoprostenol therapy demonstrated significant improvement in mean pulmonary arterial pressure, pulmonary vascular resistance, and cardiac output; furthermore, patients demonstrated improved NYHA functional class. To evaluate further the long-term effect of epoprostenol, we compared survival of SAPH patients to a cohort of hemodynamically matched patients from the same center treated with epoprostenol for Idiopathic Pulmonary Arterial Hypertension (IPAH). Interestingly, there was no difference in survival, despite the additional systemic disease burden of the SAPH subjects. Subgroup analysis by Scadding stage demonstrated that Scadding stages 1-3 had improved survival compared to Scadding stage 4. These observations suggest that epoprostenol is an effective long-term therapy for patients with SAPH; it improves hemodynamics, functional class, and provides survival similar to that seen in a hemodynamically-matched cohort of IPAH patients. Furthermore, we identify a subgroup of SAPH patients (nonfibrotic lung disease Scadding 1-3) who may derive significant benefit from prostanoid therapy. (Sarcoidosis Vasc Diffuse Lung Dis 2020; 37 (2): 184-191)
Keywords: Sarcoid associated pulmonary hypertension, epoprostenol, sarcoidosis, pulmonary hypertension
Introduction
Sarcoidosis-Associated Pulmonary Hypertension (SAPH) is a complication of sarcoidosis; however, the exact incidence is unknown. Using echocardiography, the largest studies have reported pulmonary hypertension (PH) in 5-50% of patients with known sarcoidosis1, 2, 3. Other series using right heart catheterization, have reported pulmonary hypertension in 49-73% of patients with symptomatic sarcoidosis4, 5. However, determining the exact prevalence of SAPH in patients with sarcoidosis is difficult because of the heterogeneity of the population and the varying severity of the underlying sarcoidosis. Yet, it is clear that the presence of SAPH confers a poor prognosis compared to sarcoidosis without SAPH2, 6, 7. SAPH occurs due to complex interactions between sarcoid involvement in the lung parenchyma and the pulmonary vasculature. Several distinct mechanisms have been suggested by which sarcoidosis can induce pulmonary hypertension, including: hypoxia, pulmonary artery vasculitis, sarcoidosis-associated heart failure, fibrotic destruction of pulmonary vasculature, occlusion of pulmonary vasculature by enlarged lymph nodes or granulomatous tissue, thromboembolic disease and sarcoidosis-induced hepatic disease and subsequent portopulmonary hypertension8. Moreover, in an individual patient, several of these mechanisms may occur simultaneously and contribute to the development of SAPH9.
Treatment of SAPH has focused separately on optimization of the treatment of the underlying sarcoidosis and management of the PH as distinct issues. Studies specifically evaluating the effect of treating sarcoidosis with immunomodulatory therapy alone have demonstrated mixed results on pulmonary hemodynamics7, 10, 11, 12. SAPH is classified as WHO Group 5 PH in part because of the multiple disease processes which affect the lung in SAPH, and because similar diseases produce PH that does not respond well to vasodilator therapy.
In idiopathic pulmonary arterial hypertension (IPAH), intravenous prostanoid therapy improves functional and clinical status, as well as survival13. Likewise, in patients with SAPH, prostanoids have been shown to be effective vasodilators, whether administered as inhaled or intravenous therapy14, 15, 16, 17, 18. Trials evaluating the effect of endothelium receptor antagonists (bosentan and ambrisentan) and phosphodiesterase-5 inhibitors (sildenafil and tadalafil) have also demonstrated improved hemodynamics in some patients with SAPH19, 20, 21. Previously, short-term benefits of epoprostenol in a small series of patients with SAPH16 have been demonstrated. However, there exist limited data regarding long-term outcomes of SAPH patients treated with epoprostenol.
In this retrospective cohort study, we report the largest group of patients with SAPH treated with epoprostenol. Furthermore, the observation period is greater than previously published cohorts. In addition, we characterize this patient population in order to better understand the long term effects of epoprostanol therapy in patients with SAPH. Also, unlike previous studies, we compared the response in this cohort of SAPH patients to a hemodynamically-matched cohort of IPAH patients treated with epoprostenol at the same institution. Finally, we compared survival between fibrotic (Scadding stage 4) and nonfibrotic (Scadding stage 1-3) subgroups of pulmonary sarcoidosis in SAPH to assess whether the presence of fibrotic lung disease affects survival.
Methods
Design and data collection
We conducted a retrospective review of all patients treated with epoprostenol from January 2000 to January 2018 in accordance with a protocol approved by the University Institutional Review Board. Inclusion criteria were PH patients treated with intravenous epoprostenol with the diagnosis of sarcoidosis or IPAH. The diagnosis was based on review of the medical record, including compatible historical information and/or pathology findings. All patients were diagnosed with SAPH by appropriate hemodynamic parameters at right heart catheterization (RHC; see Table 1). Patients were excluded if there was evidence of any of the following: connective tissue disease, portal hypertension, cardiac disease, HIV disease, history of anorexigen or illicit drug use, or thromboembolic disease. For each case, we collected patient demographics, stage of sarcoidosis, treatment of sarcoidosis, pulmonary function testing, echocardiography, baseline and post-treatment hemodynamics, and long-term clinical outcome.
Table 1.
Baseline patient characteristics. Demographics, promonary function testing, and echocardiography data obtained at diagnosis. Epoprostenol dose at initiation was 10 ± 3 ng/kg/min and titrated up as tolerated to an average dose of 64 ± 48. New York Heart Association Functional Class improved from 3.5 ± 0.5 at initiation of epoprostenol to 2.6 ± 0.8 p = 0.005.
| Demographics | |||
| Sex (Male) | 4 (33%) | ||
| Age (Years) | 48 ± 10 | ||
| BMI | 29 ± 9.5 | ||
| Smoker | 6 (50%) | 2/6 active smokers | |
| Race | |||
| AA | 8 (67%) | ||
| Caucasian | 3 (25%) | ||
| Hispanic | 1 (8%) | ||
| Pulmonary Function Testing | Liters | % Predicted | |
| FVC | 1.93 ± 0.5 | 60 ± 16 | |
| FEV1 | 1.3 ± 0.4 | 54 ± 14 | |
| FEV1/FVC | 68 ± 12 | ||
| TLC | 3.3 ± 0.6 | 62 ± 12 | |
| DLCO (mL/mmHg/min) | 6.6 ± 3.7 | 32 ± 17 | |
| Echocardiogram | |||
| EF % | 57 ± 12 | ||
| PASP mmHg | 69 ± 17 | ||
| RV Dilation: | |||
| Severe | 6 (50%) | ||
| Moderate | 3 (25%) | ||
| Mild | 1 (8%) | ||
| None | 2 (17%) | ||
| Initiation | Maintenance | P-Value | |
| Epoprostenol Dose ng/kg/min | 10 ± 3 | 64 ± 48 | 0.002 |
| NYHA FC | 3.5 ± 0.5 | 2.6 ± 0.8 | 0.005 |
Vasodilator Treatment with Epoprostenol
Long-term IV epoprostenol therapy was administered via a centrally inserted tunneled catheter. Dose adjustments were made as dictated by patient symptoms and clinical status. Patients were treated with supplemental oxygen as required to maintain oxygen saturation ≥ 90%. Patients underwent surveillance RHC to assess their response to treatment as dictated by symptoms and clinical status.
Immunosuppressive therapy for sarcoidosis
Corticosteroid or immunosuppressive therapy was initiated or continued based on standard indications for treatment of sarcoidosis (ie, hypercalcemia, pulmonary, ocular, or CNS involvement)22.
Statistical analyses
Student’s T-test in Microsoft Excel was used for inter-group comparisons. A paired T-test was used for intra-group comparisons. Means and standard deviations are reported. Time-to-death between SAPH and IPAH groups, as well as subgroup analysis between SAPH pooled as Scadding stages 1-3 and Scadding stage 4 subgroups was compared using a log-rank test with R software (R Development Core Team, 2008). Time-to-death analysis was performed only in patients treated with epoprostenol for ≥ 30 days. In the survival analysis, transplant was considered the same as death.
Results
Twelve patients with SAPH fulfilling the study criteria were identified after screening all patients treated with epoprostenol during the defined time frame and included in these analyses. (Table 1). 4 (33%) were male, 6 (50%) were smokers (2/6 active smokers), and 7 (67%) were African American. Average age was 48 ± 10 years and average BMI was 29 ± 9.5. At baseline, the FVC was 1.93 ± 0.5 L (60 ± 16% predicted), FEV1 1.3 ± 0.4 L (54 ± 14% predicted), and FEV1/FVC 68 ± 12. TLC was 3.3 ± 0.6 L (62 ± 12% predicted) and DLCO 6.6 ± 3.7 mL/mmHg/min (32 ± 17% predicted).
The diagnosis of sarcoidosis was established in 67% of subjects by clinical findings and a biopsy specimen demonstrating non-necrotizing granulomas (Table 2). In the other 33% of subjects, sarcoidosis was diagnosed by typical clinical features in the absence of biopsy. 58% of subjects had a Scadding stage of 4, the remaining 42% were classified in stages 1-3. All subjects had pulmonary involvement. Frequency of extrapulmonary organ involvement was: CNS 2 (25%), ocular 2 (25%), bone marrow 1 (8%). At initiation of epoprostenol therapy, 6 (50%) of patients were receiving prednisone, 2 (16%) plaquenil, 1 (16%) methotrexate, 1 (8%) azathioprine, and 1 (8%) infliximab (1 8%); 2 (16%) of the subjects ultimately underwent lung transplantation.
Table 2.
Description of sarcoidosis features. Eight of twelve patients had biopsy proven sarcoidosis. Seven of Twelve patients had Scadding class 4 sarcoidosis. Extrapulmonary organ involvement and sarcoidosis medications are listed.
| Sarcoid Features: | |
| Diagnosis | |
| Biopsy | 8 (67%) |
| Clinical | 4 (33%) |
| Sarcoid Stage | |
| 1-3 | 5 (42%) |
| 4 | 7 (58%) |
| Extrapulmonary Organ Involvement | |
| Ocular | 3 (25%) |
| CNS | 3 (25%) |
| Bone Marrow | 1 (8%) |
| Treatment | |
| Prednisone | 6 (50%) |
| Hydroxychloroquine | 2 (16%) |
| Methotrexate | 2 (16%) |
| Azathioprine | 1 (8%) |
| Lung Transplant | 2 (16%) |
By transthoracic echocardiogram, LVEF was estimated at 57 ± 12% and PASP 69 ± 17 mm Hg (Table 1). Right ventricular (RV) dilation was assessed as severe in 6 (50%), moderate in 3 (24%), and mild in 1 (8%). In the remaining 2 (16%), RV size was normal. Hemodynamic measurements from the diagnostic RHC are shown in Table 3: mPAP was 57 ± 8 mmHg, PVR 12.4 ± 2.8 WU, CO 3.7 ± 0.8 L/min (Thermodilution, TD), and CI 2.0 ± 0.4 L/min/m2. Initiation of epoprostenol was guided by invasive hemodynamic monitoring and side effects; at discharge (after initial titration), the dose was 10 ± 3 ng/kg/min. Acute hemodynamic changes with initiation of epoprostenol have been previously reported16; as in that study, we observed significant improvements in CO (4.1 ± 0.7 L/min pre, 6.2 ± 1.4 L/min post TD, p = 0.005) and PVR (11.8 ± 2.8 pre, 6.3 ± 2.9 WU post units, p = 0.008).
Table 3.
Diagnostic Right Heart Catheterization (N = 12)
| Diagnostic RHC | |
| RV sys (mmHg) | 87 ± 15 |
| RA dia (mmHg) | 14 ± 5.2 |
| PAP sys (mmHg) | 88 ± 13 |
| PAP dia (mmHg) | 39 ± 8 |
| mPAP (mmHg) | 57 ± 8 |
| PCWP (mmHg) | 13 ± 5 |
| CO TD (L/min) | 3.7 ± 0.8 |
| CI TD (L/min/m2) | 2 ± 0.4 |
| BSA (m2) | 2 ± 0.3 |
| PVR TD (WU) | 12.4 ± 2.8 |
| SVR TD (mmHg min mL-1) | 1922 ± 477 |
Hemodynamic measurements obtained to diagnose pulmonary hypertension.
RV (Right ventricle), PAP (Pulmonary Arterial Pressure), mPAP (Mean Pulmonary Arterial Pressure), PCWP (Pulmonary Cappillary Wedge Pressure), CO TD (Cardiac Output via thermodilution method), CI Cardiac Index), BSA (Body Surface Area), PVR (Pulmonary Vascular Resistance), SVR (Systemic Vascular Resistance).
After discharge, all patients were followed by regular clinic visits and surveillance RHC as clinically indicated. At initiation of epoprostenol, some patients developed expected manageable prostanoid-related side effects; which resolved with treatment and/or time. Several patients developed transient minor V/Q mismatch, treated conservatively, that resolved within 4-6 weeks. No patients had persistent V/Q mismatch or shunting.
The epoprostenol dose was titrated to symptoms and clinical status. Patients underwent surveillance RHC (Table 4) at a mean duration of 4.1 years after initiation of epoprostenol; at that time, mean epoprostenol dose was 64 ± 48 ng/kg/min. In addition to epoprostenol, some patients were also treated with tadalafil (4 patients; 33%), sildenafil (1 patient; 8%), and ambrisentan (1 patient; 8%). Long-term epoprostenol therapy was associated with a significant improvement in hemodynamics (Table 4): mPAP (Pre 59 ± 10 vs Post 36 ± 11 mmHg, p = 0.002), PVR (Pre 11.8 ± 2.0 vs Post 4.3 ± 1.9 WU < 0.001) and CO (Pre 4.0 ± 0.7 vs Post 5.8 ± 1.5 L/min, p = 0.002) CI (Pre 2 ± 0.3 vs Post 3 ± 0.6 L/min/m2, p = 0.008). NYHA-FC had improved on average one full classification, 3.5 ± 0.5 pre-treatment to 2.6 ± 0.8 post-treatment (0.005).
Table 4.
Surveillance Right Heart Catheterizations
| Diagnostic | Follow up | p Value | |
| RV systolic (mmHg) | 88 ± 22 | 51 ± 23 | 0.015 |
| RV diastolic (mmHg) | 13 ± 4.6 | 2.8 ± 3.7 | 0.002 |
| PAP systolic (mmHg) | 90 ± 18 | 53 ± 23 | 0.006 |
| PAP diastolic (mmHg) | 40 ± 10 | 24 ± 7 | 0.006 |
| mPAP (mmHg) | 59 ± 10 | 36 ± 11 | 0.002 |
| PCWP (mmHg) | 12 ± 3 | 11 ± 4 | 0.46 |
| CO TD (L/min) | 4.0 ± 0.7 | 5.8 ± 1.5 | 0.01 |
| CI TD (L/min/m2) | 2 ± 0.3 | 3 ± 0.6 | 0.008 |
| BSA (m2) | 1.9 ± 0.2 | 1.8 ± 0.2 | 0.43 |
| PVR TD (WU) | 11.8 ± 2.1 | 4.3 ± 1.9 | < 0.001 |
| SVR TD (mmHg min mL-1) | 1652 ± 381 | 1347 ± 512 | 0.19 |
Surveillance RHC, with mean follow up of 4.1 years, showing improvement in hemodynamics compared to pre-treatment diagnostic catheterization. N = 7 patients. See Table 3 Legend for abbreviations.
Survival of patients with SAPH treated with epoprostenol was compared to patients with hemodynamically-similar IPAH treated with epoprostenol (Table 5). At initiation of epoprostenol therapy, there was no significant difference between SAPH and IPAH groups with respect to age 48 ± 10 vs 57 ± 16 years (p = 0.07) or sex 33 vs 38% male (p = 0.75). Diagnostic hemodynamic measurements were also similar between SAPH and IAPH groups: mPAP 57 ± 8 vs 56 ± 13 mmHg (p = 0.88), PVR 12.4 ± 2.8 vs 12.2 ± 3.9 WU (p = 0.89). Mean survival for the IPAH patients was 43 ± 41 months and 54 ± 57 months for the SAPH patients (Figure 1A). Time-to-death analysis of these cohorts demonstrated that there was no difference in survival of SAPH patients compared to IPAH patients by log rank test (p = 0.28).
Table 5.
Comparison between SAPH and IPAH patients
| IPAH (N = 52) | SAPH (N = 12) | P Value | |
| Age (Years) | 57 ± 16 | 48 ± 10 | 0.07 |
| Sex (% Male) | 38 | 33 | 0.75 |
| mPAP (mmHg) | 56 ± 13 | 57 ± 8 | 0.88 |
| PVR (WU) | 12.2 ± 3.9 | 12.4 ± 2.8 | 0.89 |
IPAH and SAPH subjects are similar in demographics and disease severity.
Fig. 1a.
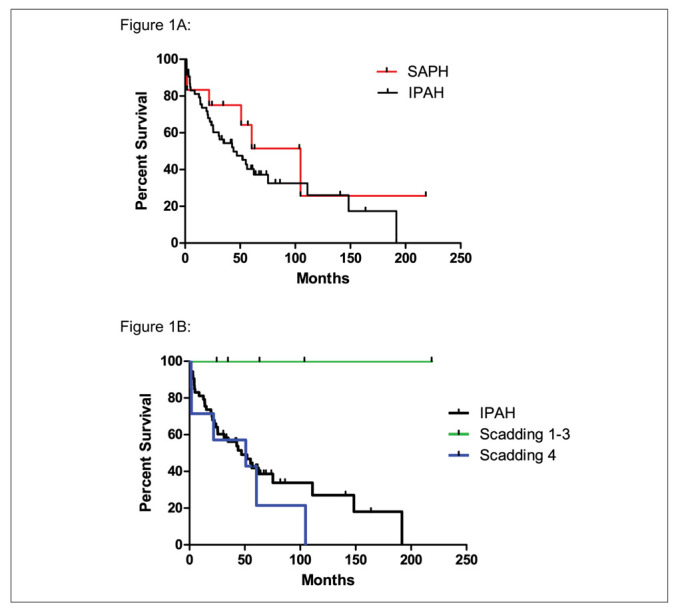
Log-rank test comparing survival of patients with SAPH vs IPAH. There is no difference in time-to-death between SAPH and IPAH. (Mean survival in months SAPH 62 ± 60 vs IPAH 46 ± 42, p = 0.28). Fig. 1b. Survival of SAPH Scadding stages 1-3 (non-fibrotic disease) is significantly greater than SAPH Scadding stage 4 (fibrotic disease) by log-rank test (Scadding stages 1-3 89 ± 79 months vs Scadding stage 4 42 ± 37 months p = 0.017). Survival of SAPH Scadding stages1-3 is also significantly greater than IPAH p = 0.02. There was no difference in survival between Scadding 4 and IPAH p = 0.43.
Analysis of the SAPH cohort by Scadding classification (Figure 1B) Scadding stages 1-3 (nonfibrotic disease) vs Scadding stage 4 (fibrotic disease) revealed significantly longer survival in the Scadding stage 1-3 group (mean 89 ± 79 months) vs the Scadding stage 4 group (42 ± 37 months), (p = 0.02). No deaths were observed in the Scadding stages 1-3 group, whereas five deaths occurred in the Scadding stage 4 group. Comparison of SAPH Scadding stages 1-3 vs IPAH demonstrated that SAPH Scadding stages 1-3 had significantly higher survival over the follow up period (p = 0.02). Then SAPH Scadding stage 4 group survival was compared separately with that of IPAH; there was no difference (p = 0.43).
Discussion
We have described a cohort of patients with SAPH treated long-term with epoprostenol. This study confirms the hemodynamic response to epoprostenol previously observed in SAPH patients16. All patients in this cohort demonstrated both improved hemodynamic and functional status. Of note, this is the largest cohort of SAPH patients treated with epoprostenol to date and builds on the findings previously reported by Fisher, et al16. In addition, this is the first study to compare survival between patients with SAPH and IPAH treated with epoprostenol, and compare survival with epoprostenol therapy by Scadding stage.
SAPH occurs via multiple pathological processes, any, or all of which, might affect a given individual. Thus, difference in outcome observed in small patient cohorts are likely due to heterogeneity of SAPH and the predominance of various pathways in the progression of the disease. That said, it is clear that survival in patients with SAPH is significantly less than in patients with sarcoidosis alone. In one study, five-year survival in patients with SAPH was 55%10. Similarly, the five-year survival of our cohort was 42%. Importantly, two of our patients have been treated with epoprostenol for greater than 17 years. Such survival suggests that prostanoid therapy can be highly effective long-term therapy for SAPH.
This study also confirms previous reports that the presence of pulmonary fibrosis confers a negative prognosis. In this cohort, there was no difference in the baseline hemodynamics of fibrotic and nonfibrotic patients; and, there was no difference in hemodynamic response to epoprostenol therapy by Scadding class. Although both groups experienced a similar improvement in functional class, there was a marked difference in survival with no mortality in the nonfibrotic subgroup. Dobarro previously described a survival difference between Scadding stages 1-3 and stage 4 groups in patients with SAPH receiving multiple different treatment regimens23. The current study demonstrates an even more dramatic survival difference based on the presence of fibrotic lung disease in patients treated with epoprostenol at a single center. The pathophysiology of SAPH is complex and further studies are needed to explore the mechanism by which fibrotic lung disease increases mortality in SAPH.
Despite classification as Group 5 PH, it is clear that some patients with SAPH exhibit similar hemodynamic profiles as patients with Group 1 PH (IPAH) and respond to PH-specific therapy in a similar manner. In fact, the existent literature does suggest that PH-specific therapy can improve SAPH in some patients. For example, in a study of 13 subjects with severe SAPH treated with either systemic epoprostenol or treprostinil, the 9 subjects who survived one year exhibited hemodynamic and functional improvements similar to those observed in this study as well as in our previous report16, 17. The effect of oral PH-specific medications has also been studied in SAPH, but has yielded mixed results12, 24, 25. In the largest study, bosentan was effective in treating patients with SAPH21. However, the PH in patients in studies of oral therapy was significantly less severe than in the patients in the current study. Thus, prostanoids may be preferable in the treatment of severe SAPH.
The current study compared survival between SAPH and IPAH and found it to be similar. In previous studies, SAPH survival has been reported at 50-59% at 5 years7, 16, 26. Indirect comparison of IPAH and SAPH suggests that 5 year survival is somewhat similar27, 28. Observations by us and others might suggest that there exists a sub-group of SAPH patients, more likely nonfibrotic patients, who respond better to prostanoid therapy. However, Bonham et al described a similar cohort demonstrating significant early mortality in a cohort with 77% of patients having Scadding stage 4 disease; yet, not surprisingly, those patients who survived to long-term evaluation demonstrated significant improvements in hemodynamics17.
This study has several limitations: 1) a relatively small sample size; 2) the retrospective nature; and 3) potential bias in the choice of patients treated with epopprostenol. However, the study does demonstrate long-term efficacy of epoprostenol therapy in patients with severe SAPH. Although patients with sarcoidosis may be a heterogeneous group in regard to the pathophysiologic mechanisms of PAH, all SAPH patients in this cohort were responsive to epoprostenol therapy, demonstrating significant improvement in hemodynamics and functional class. Surprisingly, in this cohort, patients with SAPH treated with epoprostenol had similar survival as a hemodynamically-matched IPAH cohort treated at the same center. Subgroup analysis identified Scadding stages 1-3 classification as having significantly improved survival over Scadding stage 4 and IPAH. These data suggest that epoprostenol is an effective vasodilator in patients with SAPH, should be considered in patients with severe SAPH, and identifies a SAPH subgroup which may uniquely benefit from prostanoid therapy. As such, further study is warranted in patients with SAPH to define those populations that are likely to be responsive to vasodilator therapy.
References
- 1.Handa T, Nagai S, Miki S, Fushimi Y, Ohta K, Mishima M, Izumi T. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest. 2006;129:1246–52. doi: 10.1378/chest.129.5.1246. [DOI] [PubMed] [Google Scholar]
- 2.Alhamad EH, Idrees MM, Alanezi MO, Alboukai AA, Shaik SA. Sarcoidosis-associated pulmonary hypertension: Clinical features and outcomes in Arab patients. Ann Thorac Med. 2010;5:86–91. doi: 10.4103/1817-1737.62471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sulica R, Teirstein AS, Kakarla S, Nemani N, Behnegar A, Padilla ML. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest. 2005;128:1483–9. doi: 10.1378/chest.128.3.1483. [DOI] [PubMed] [Google Scholar]
- 4.Shorr AF, Helman DL, Davies DB, Nathan SD. Pulmonary hypertension in advanced sarcoidosis: epidemiology and clinical characteristics. Eur Respir J. 2005;25:783–8. [Google Scholar]
- 5.Baughman RP, Engel PJ, Meyer CA, Barrett AB, Lower EE. Pulmonary hypertension in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2006;23:108–16. [PubMed] [Google Scholar]
- 6.Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis-associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest. 2010;138:1078–85. doi: 10.1378/chest.09-2002. [DOI] [PubMed] [Google Scholar]
- 7.Nunes H, Humbert M, Capron F, Brauner M, Sitbon O, Battesti JP, Simonneau G, Valeyre D. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax. 2006;61:68–74. doi: 10.1136/thx.2005.042838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlobin OA, Baughman RP. Sarcoidosis-Associated Pulmonary Hypertension. Semin Respir Crit Care Med. 2017;38:450–462. doi: 10.1055/s-0037-1603767. [DOI] [PubMed] [Google Scholar]
- 9.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M Group ESCSD. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 10.Boucly A, Cottin V, Nunes H, Jais X, Tazi A, Prevot G, Reynaud-Gaubert M, Dromer C, Viacroze C, Horeau-Langlard D, Pison C, Bergot E, Traclet J, Weatherald J, Simonneau G, Valeyre D, Montani D, Humbert M, Sitbon O, Savale L. Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur Respir J. 2017;50 doi: 10.1183/13993003.00465-2017. [DOI] [PubMed] [Google Scholar]
- 11.Gluskowski J, Hawrylkiewicz I, Zych D, Zielinski J. Effects of corticosteroid treatment on pulmonary haemodynamics in patients with sarcoidosis. Eur Respir J. 1990;3:403–7. [PubMed] [Google Scholar]
- 12.Ford HJ, Baughman RP, Aris R, Engel P, Donohue JF. Tadalafil therapy for sarcoidosis-associated pulmonary hypertension. Pulm Circ. 2016;6:557–562. doi: 10.1086/688775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sitbon O, Humbert M, Nunes H, Parent F, Garcia G, Herve P, Rainisio M, Simonneau G. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–8. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 14.Preston IR, Klinger JR, Landzberg MJ, Houtchens J, Nelson D, Hill NS. Vasoresponsiveness of sarcoidosis-associated pulmonary hypertension. Chest. 2001;120:866–72. doi: 10.1378/chest.120.3.866. [DOI] [PubMed] [Google Scholar]
- 15.Barst RJ, Ratner SJ. Sarcoidosis and reactive pulmonary hypertension. Arch Intern Med. 1985;145:2112–4. [PubMed] [Google Scholar]
- 16.Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130:1481–8. doi: 10.1378/chest.130.5.1481. [DOI] [PubMed] [Google Scholar]
- 17.Bonham CA, Oldham JM, Gomberg-Maitland M, Vij R. Prostacyclin and oral vasodilator therapy in sarcoidosis-associated pulmonary hypertension: a retrospective case series. Chest. 2015;148:1055–1062. doi: 10.1378/chest.14-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baughman RP, Judson MA, Lower EE, Highland K, Kwon S, Craft N, Engel PJ. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26:110–20. [PubMed] [Google Scholar]
- 19.Milman N, Burton CM, Iversen M, Videbaek R, Jensen CV, Carlsen J. Pulmonary hypertension in end-stage pulmonary sarcoidosis: therapeutic effect of sildenafil? J Heart Lung Transplant. 2008;27:329–34. doi: 10.1016/j.healun.2007.11.576. [DOI] [PubMed] [Google Scholar]
- 20.Barnett CF, Bonura EJ, Nathan SD, Ahmad S, Shlobin OA, Osei K, Zaiman AL, Hassoun PM, Moller DR, Barnett SD, Girgis RE. Treatment of sarcoidosis-associated pulmonary hypertension. A two-center experience. Chest. 2009;135:1455–1461. doi: 10.1378/chest.08-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baughman RP, Culver DA, Cordova FC, Padilla M, Gibson KF, Lower EE, Engel PJ. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest. 2014;145:810–817. doi: 10.1378/chest.13-1766. [DOI] [PubMed] [Google Scholar]
- 22.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 23.Dobarro D, Schreiber BE, Handler C, Beynon H, Denton CP, Coghlan JG. Clinical characteristics, haemodynamics and treatment of pulmonary hypertension in sarcoidosis in a single centre, and meta-analysis of the published data. Am J Cardiol. 2013;111:278–85. doi: 10.1016/j.amjcard.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 24.Judson MA, Highland KB, Kwon S, Donohue JF, Aris R, Craft N, Burt S, Ford HJ. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28:139–45. [PubMed] [Google Scholar]
- 25.Keir GJ, Walsh SL, Gatzoulis MA, Marino PS, Dimopoulos K, Alonso R, Raposeiras-Roubin S, Renzoni EA, Maher TM, Wells AU, Wort SJ. Treatment of sarcoidosis-associated pulmonary hypertension: A single centre retrospective experience using targeted therapies. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:82–90. [PubMed] [Google Scholar]
- 26.Tiosano S, Versini M, Dar Antaki L, Spitzer L, Yavne Y, Watad A, Gendelman O, Comaneshter D, Cohen AD, Amital H. The long-term prognostic significance of sarcoidosis-associated pulmonary hypertension - A cohort study. Clin Immunol. 2018 doi: 10.1016/j.clim.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Farber HW, Miller DP, Poms AD, Badesch DB, Frost AE, Muros-Le Rouzic E, Romero AJ, Benton WW, Elliott CG, McGoon MD, Benza RL. Five-Year outcomes of patients enrolled in the REVEAL Registry. Chest. 2015;148:1043–54. doi: 10.1378/chest.15-0300. [DOI] [PubMed] [Google Scholar]
- 28.Gall H, Felix JF, Schneck FK, Milger K, Sommer N, Voswinckel R, Franco OH, Hofman A, Schermuly RT, Weissmann N, Grimminger F, Seeger W, Ghofrani HA. The Giessen Pulmonary Hypertension Registry: Survival in pulmonary hypertension subgroups. J Heart Lung Transplant. 2017;36:957–967. doi: 10.1016/j.healun.2017.02.016. [DOI] [PubMed] [Google Scholar]


