Abstract
Background:
Zonal aggregates of elastic fibres (zonal elastosis) and intraalveolar collagenosis with septal elastosis are histologic components of subpleural fibroelastosis of idiopathic pleuroparenchymal fibroelastosis (IPPFE). Zonal elastosis is considered to result from alveolar collapse, but this mechanism has not been fully justified.
Methods:
We immunohistochemically attempted to identify epithelial cells in zonal elastosis of 10 patients with IPPFE. The thickness of the zonal elastosis in relation to the total thickness of the fibroelastosis was examined to estimate the influence of zonal elastosis on the occurrence and development of IPPFE.
Results:
In 9 of the 10 patients, multi-cytokeratin-positive cells were found lining the inner surface of slit-like spaces embedded in the zonal elastosis. Zonal elastosis was predominant when fibroelastosis was < 1 mm thick but less predominant when it was ≥1 mm.
Conclusion:
The zonal elastosis was proven to result from alveolar collapse, which might be an initial lesion in IPPFE. (Sarcoidosis Vasc Diffuse Lung Dis 2020; 37 (2): 212-217)
Keywords: zonal elastosis, intra-alveolar fibrosis, septal elastosis, two-layered pattern, elastic fibre
Introduction
Idiopathic pleuroparenchymal fibroelastosis (IPPFE) is a rare subtype of idiopathic interstitial pneumonias predominantly located in the upper lobes, presenting with subpleural fibroelastosis with or without collagenous fibrosis of the visceral pleura (1-6). The most striking histology of PPFE is collagen-filled alveoli (intra-alveolar fibrosis) with septal elastosis (4). Furthermore, zonal or band-like dense aggregates of elastic fibres (zonal elastosis) in the subpleural areas are also key findings in PPFE (7-10). PPFE often shows a two-layered structure of fibroelastosis, with a subpleural layer of zonal elastosis and an inner layer of intra-alveolar fibrosis with septal elastosis (7-10).
Some investigators have speculated that zonal elastosis results from alveolar collapse (7-9). However, this speculation is not entirely convincing, as zonal elastosis usually consists of dense aggregates of elastic fibres without identifiable alveolar structures. Furthermore, the pathological process of fibroelastosis in PPFE remains unclear.
The present study therefore determined whether or not zonal elastosis is caused by alveolar collapse and speculated the role of zonal elastosis in the early stage of fibroelastosis in IPPFE.
Materials and Methods
Subjects
We retrospectively reviewed our clinical records between 1995 and 2017, and selected 18 patients with IPPFE who had been clinically diagnosed and pathologically confirmed according to the recently proposed criteria (8). We excluded six patients with non-PPFE fibrosis in the lower lobes based on radiologic (n=1), histologic (n=1), or radiologic and histologic assessment (n=4). We also excluded two patients in whom only one histologic specimen remained for the evaluation. Ultimately, 10 IPPFE patients without non-PPFE fibrosis in the lower lobes were eligible for the present study (pneumonectomy for lung transplantation, n = 2; surgical lung biopsy, n = 8).
Clinical and respiratory function data
Clinical data were collected from the patients’ medical records. We evaluated the respiratory function parameters that had been measured less than one year before the histologic examinations, including the forced vital capacity (FVC), residual volume (RV), and RV/total lung capacity (TLC). The respiratory function data were expressed as absolute values (mL) and percentages of predicted values (% pred) (11).
Fibroelastosis pattern
Elastica van Gieson (EVG)-stained slides were scanned and converted to whole-slide images using NanoZoomer 2.0-RS (Hamamatsu Photonics, Hamamatsu, Japan). Each parameter was measured on the whole-slide images of all available specimens in each patient.
Zonal elastosis comprises a dense accumulation of elastic fibres without intervening collagen fibres (Fig. 1A). In lesions of intra-alveolar fibrosis with septal elastosis (IAFE), alveoli were filled with mature collagen (Fig. 1B and 1D).
Fig. 1.
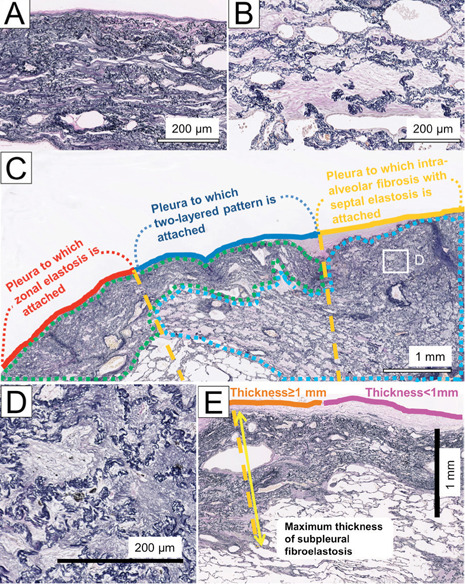
Elastica van Gieson-stained sections in a patient with IPPFE. (A) Zonal elastosis. (B) Intra-alveolar fibrosis with septal elastosis (IAFE). Fibroelastosis in PPFE was segmented into zonal elastosis (green lesion) or IAFE (blue lesion) (C). We then determined the part of the pleura associated with zonal elastosis, IAFE, or a two-layered pattern involving zonal elastosis and IAFE. Each fibroelastosis pattern was decided on the orange-dotted vertical lines originating at the pleura and extending inward from the pleura. In this example, the percentages of the pleural length with zonal elastosis, a two-layered pattern, and IAFE were calculated to be 32.9%, 38.4%, and 28.8%, respectively. Fine elastic fibres are sometimes observed in old collagen-filled alveoli with septal elastosis (D, inset of C). (E) Maximal thickness of the subpleural fibroelastosis and pleura with subpleural fibroelastosis <1 mm or ≥1 mm thick
We classified subpleural fibroelastosis into the following three patterns: (i) zonal elastosis not associated with IAFE; (ii) IAFE not associated with zonal elastosis; and (i+ii) a two-layered pattern comprising a subpleural layer of zonal elastosis and an inner layer of IAFE (Fig. 1C). The thickness of subpleural fibroelastosis was defined as the maximal distance from the outer elastic layer of the visceral pleura to the edge of the fibroelastosis, measured on a line running vertically from the pleura toward the inside (Fig. 1E). Using all specimens available, we measured the length of the pleura to which subpleural fibroelastosis (i, ii or i+ii) was attached to assess fibroelastosis quantitatively (Fig. 1C). The percentage of the total length of the pleura with each type of fibroelastosis to that with all types of fibroelastosis was calculated in each patient. After categorizing the pleura into two groups based on the thickness of subpleural fibroelastosis (pleura accompanied by fibroelastosis <1 mm or ≥1 mm thick), we compared the percentage of each type of fibroelastosis in each pleural group.
Immunohistochemistry
Immunostaining was performed on the specimens containing the zonal elastotic lesions using Multi-Cytokeratin (CK) AE1/AE3 (1:400 dilution; Leica Biosystems, Newcastle, UK) to identify cytokeratin-positive cells in the zonal elastotic lesions in each patient.
Statistical analyses
Continuous data are presented as medians and interquartile ranges in parentheses. Wilcoxon’s signed-rank test was used to examine the difference in the percentage of fibroelastosis patterns. P<0.05 was considered to indicate statistical significance. All statistical analyses were conducted using the R software program (version 3.2.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Clinical characteristics of patients with IPPFE were as follows: 6 males and 4 females; mean age: 56.1 years old; range: 23-68 years old; body mass index: 16.3 (15.6-18.1) kg/m2; Krebs von den Lungen-6: 374 (275-408) U/mL; FVC: 1980 (1350-2210) mL (65.5 [37.5-83.0]%); RV: 1690 (1028-1773) mL (94.3 [65.3-111]%) and RV/TLC: 0.41 (0.35-0.46) (118 [114-141]%).
Fibroelastosis pattern
A median of 5.2 specimens were available per patient for the histological examinations. The patterns of fibroelastosis in IPPFE patients are summarized in Table 1. The percentages of the length of the pleura with zonal elastosis, 2-layered pattern, and IAFE were 37.7% (33.0-46.6%), 40.4% (35.0-44.0%), and 19.1% (3.39-25.2%), respectively; the zonal elastosis and 2-layered patterns were more predominant than the IAFE pattern (p=0.01 and p=0.002, respectively).
Table 1.
The patterns of fibroelastosis in IPPFE patients
| Patterns of fibroelastosis Thickness of fibroelastosis |
Zonal elastosis, % | Two-layered pattern, % | Intra-alveolar fibrosis with septal elastosis, % | P value† |
| Any thickness | 37.7 (33.0-46.6) | 40.4 (35.0-44.0) | 19.1 (3.39-25.2) | 1.00 |
| <1 mm thick | 69.1 (54.7-87.9) | 14.6 (1.88-23.2) | 0 (0-18.4) | 0.002 |
| ≥1 mm thick | 33.0 (16.1-39.4) | 51.9 (44.4-59.9) | 14.0 (0-30.9) | 0.04 |
*The data are expressed as the group median (interquartile range).
†P-values were calculated for the comparison of the zonal elastosis and two-layered patterns.
The relationship between the thickness of fibroelastosis and the pattern of fibroelastosis
The average maximum thickness of subpleural fibroelastosis in each patient was 5.68 mm (range, 2.06-9.93).
In subpleural fibroelastosis <1 mm thick, the percentage of the length of the pleura with zonal elastosis (69.1% [54.7-87.9%]) was higher than that of the two-layered pattern (14.6% [1.88-23.2%]) (p=0.002) or IAFE (0% [0-18.4]) (p=0.004), whereas in subpleural fibroelastosis ≥1 mm thick, the percentage of the length of the pleura with zonal elastosis (33.0% [16.1-39.4%]) was lower than that pf the two-layered pattern (51.9% [44.4-59.9%]) (p=0.04) but higher than that of IAFE (14.0% [0-30.9%]) (p=0.002). Representative IPPFE cases are shown in Fig. 2.
Fig. 2.
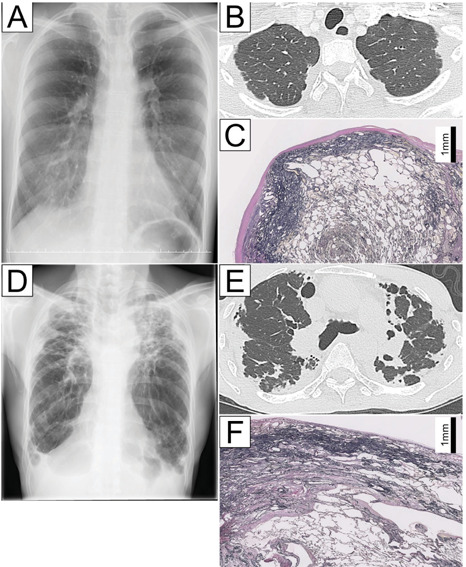
Case 1 (A-C). Chest radiography and computed tomography findings for a 23-year-old female with IPPFE demonstrating modest wedge-shaped alveolar consolidation in the subpleural region of the upper lobes (A and B). An Elastica van Gieson-stained section of the upper lobe showing a zonal elastotic lesion (C). Case 2 (D-F). Chest radiography and computed tomography findings for a 64-year-old man with IPPFE demonstrating extensive wedge-shaped alveolar consolidation in the subpleural region of the upper lobes (D and E). An Elastica van Gieson-stained section of the upper lobe showing a two-layered fibroelastosis pattern (F).
Alveolar collapse in the zonal elastosis
Zonal elastosis was identified in 10 patients with IPPFE. Thickened elastic fibres ran parallel to the pleura in these lesions (Figs. 3A and 3B). The inner surfaces of the slit-like or small cystic spaces positively reacted with CK AE1/AE3 in the zonal elastosis (Fig. 3C). Immunohistochemical positivity was identified in the zonal elastosis in 9 of the 10 patients with IPPFE.
Fig. 3.
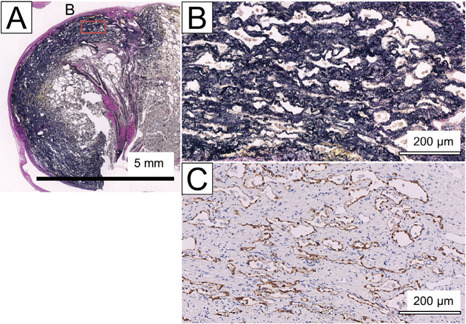
An EVG-stained section (A and B [inset of A]) and a corresponding CK AE1/AE3-immunostained section (C)
Discussion
We found that CK AE1/AE3-positive cells lined the inner surface of the slit-like spaces embedded in the zonal elastosis in 9 of our 10 patients with IPPFE. Thus, the slit-like spaces were confirmed to be alveolar lumens that had been compressed or collapsed. The zonal elastosis in IPPFE was proven to result from alveolar collapse.
Disease progression of PPFE is characterised by increased thickness of subpleural fibroelastosis with a volume loss of the upper lobes as well as caudal progression (5, 12). When the thickness of subpleural fibroelastosis was <1 mm, the percentage of the length of the pleura with zonal elastosis was much higher than that of the length of the pleura with the two-layered pattern. However, when the thickness of subpleural fibroelastosis was ≥1 mm, the percentage of the length of the pleura with a two-layered pattern was higher than that of the length of the pleura with zonal elastosis. Thus, fibroelastosis in patients with IPPFE may arise and progress in two stages (Fig. 4): the early stage, when the alveolar structure collapses and forms the zonal elastosis; and the advanced stage, when IAFE arises adjacent to the zonal elastosis, extending inward to form two-layered fibroelastosis.
Fig. 4.
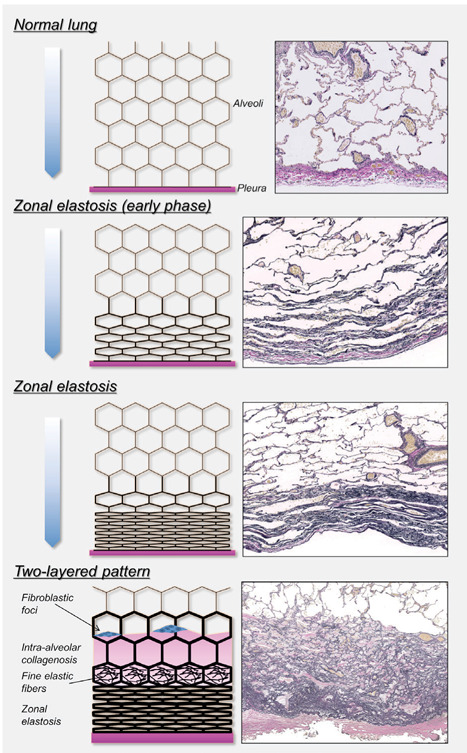
A schematic overview of the disease progression hypothesis in IPPFE. The alveolar structure collapses, forming zonal elastotic lesions in the early phase of IPPFE. Consequently, intra-alveolar fibrosis with septal elastosis arises, progressing inward and forming a two-layered fibroelastosis pattern in IPPFE. Fine elastic fibres are sometimes observed in old collagen-filled alveoli with septal elastosis
Amitani et al.(3) recognized a flattened chest cage as a constitutional characteristic of patients with idiopathic pulmonary upper lobe fibrosis that is probably included in the broad concept of IPPFE nowadays, although it is not always constitutional but sometimes acquired and progressive in patients with idiopathic PPFE. Previously, we showed that the thoracic cage becomes flattened during the deteriorated course of PPFE (13). A narrowed thoracic space in PPFE could prevent lungs from fully expanding during inspiration, possibly resulting in the compression or collapse of subpleural alveoli, which may be functionally expressed by a marked decrease in FVC.
We previously reviewed four cases of PPFE in which surgical lung biopsies were performed twice at intervals (14). In two of these cases, the pathological diagnosis of the initial lung biopsy was cellular interstitial pneumonia with granuloma and cellular and fibrotic interstitial pneumonia, respectively. In both cases, the alveoli were not collapsed, but septal elastosis of the alveolar walls was already evident at the initial biopsy. However, the second biopsy showed subpleural zonal aggregates of elastic fibres, which suggested that alveoli with septal elastosis had been gradually compressed and shifted to subpleural areas, progressing to zonal aggregates of elastic fibres by the second lung biopsy. The histological changes in those two cases may support the present results, at least in part. Furthermore, in the present study, we proposed another possibility concerning the development of PPFE: lesions of intra-alveolar collagenous fibrosis with septal elastosis can arise adjacent to the pre-existing zonal elastosis.
The pathogenetic mechanism underlying intra-alveolar collagenosis in IPPFE is largely unknown. Jonigk et al. (15) presented an evolutionary model for the alveolar fibroelastosis pattern in stem cell transplantation-related PPFE: fibrin-rich exudates in the alveolar lumen lead to unsuccessful resolution by macrophages, resulting in fully remodelled alveolar fibroelastosis. The lymphatic vasculature plays a key role in tissue homeostasis of the lung (16). The excess protein-rich fluid that extravasates from the blood vessels returns to the blood circulation via the pleural or peri-bronchiolar lymphatics (17). We previously showed that the density and number of lymphatic vessels were significantly increased in the lesions of intra-alveolar collagenosis in PPFE, and lymphatic vessels were less frequently observed in the subpleural zonal elastotic lesions than in the lesions of intra-alveolar collagenosis (10). We speculate that the zonal collapse of the alveoli inhibits the innate lymphatic drainage of the lung via the pleural lymphatics. Increases in the density and number of lymphatic vessels in the lesions of intra-alveolar collagenosis may be compensatory mechanisms against decreased drainage via the pleural lymphatics.
Several limitations associated with the present study should be mentioned. First, this was a retrospective study conducted in a single centre, and the number of patients was small. Second, we investigated the histological fibroelastosis pattern in patients with IPPFE alone. The histological pattern in secondary PPFE may be different. Third, we proposed a potential mechanism underlying the development and progression of PPFE based on the histological findings of biopsied materials obtained from 10 patients with idiopathic PPFE, but we were unable to confirm the temporal changes in the histology of each patient. It will be necessary to establish animal models of PPFE to confirm this hypothesis.
In conclusion, zonal elastosis in IPPFE patients was shown to be associated with alveolar collapse. Early lesions of fibroelastosis in IPPFE might start with zonal elastosis.
Acknowledgement
The authors thank Ms. H. Fukagawa, Department of Pathology, Fukuoka University School of Medicine and Hospital, for her skillful assistance with the preparation and staining of tissue samples.
Compliance with Ethical Standards:
The Fukuoka University Hospital Institutional Review Board approved the study protocol and waived the requirement for informed consent (approval number: 217M037).
Conflict of Interest:
The authors declare no conflicts of interest in association with the present study.
Contributions:
YK contributed to the design and concept of the study, data analysis, and writing of the manuscript. HI, HK, and MF contributed to the data analysis and final approval of the manuscript. KN contributed to the data analysis and gave advice on the design and concept of the study. KW contributed to the design and concept of the study, data analysis, and development of the manuscript.
Funding:
This study was partially supported by a grant from the Ministry of Health, Labor and Welfare of Japan awarded to the Study Group on Diffuse Pulmonary Disorders, Scientific Research/Research on Intractable Diseases.
References
- 1.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frankel SK, Cool CD, Lynch DA, Brown KK. Idiopathic pleuroparenchymal fibroelastosis: description of a novel clinicopathologic entity. Chest. 2004;126:2007–2013. doi: 10.1378/chest.126.6.2007. [DOI] [PubMed] [Google Scholar]
- 3.Amitani R, Niimi A, Kuse F. Idiopathic pulmonary upper lobe fibrosis. 1992;11:693–699. [Google Scholar]
- 4.Reddy TL, Tominaga M, Hansell DM, et al. Pleuroparenchymal fibroelastosis: A spectrum of histopathological and imaging phenotypes. Eur Respir J. 2012;40:377–385. doi: 10.1183/09031936.00165111. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe K. Pleuroparenchymal fibroelastosis: Its clinical characteristics. Curr Respir Med Rev. 2014;9:229–237. doi: 10.2174/1573398X0904140129125307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinoshita Y, Watanabe K, Ishii H, et al. Proliferation of elastic fibres in idiopathic pulmonary fibrosis: a whole-slide image analysis and comparison with pleuroparenchymal fibroelastosis. Histopathology. 2017;71:934–942. doi: 10.1111/his.13312. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe K, Nagata N, Kitasato Y, et al. Rapid decrease in forced vital capacity in patients with idiopathic pulmonary upper lobe fibrosis. Respir Investig. 2012;50:88–97. doi: 10.1016/j.resinv.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe K, Ishii H, Kiyomi F, et al. Criteria for the diagnosis of idiopathic pleuroparenchymal fibroelastosis: A proposal. Respir Investig. 2019;57:312–320. doi: 10.1016/j.resinv.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Kawabata Y, Matsuoka R. Pathology of idiopathic pulmonary upper lobe fibrosis. 2003;62:S196–S202. [Google Scholar]
- 10.Kinoshita Y, Watanabe K, Ishii H, et al. Significant increases in the density and number of lymphatic vessels in pleuroparenchymal fibroelastosis. Histopathology. 2018;73:417–427. doi: 10.1111/his.13634. [DOI] [PubMed] [Google Scholar]
- 11.Tokyo: Medical Review Publishers; 2004. Japanease Respiratory Society Guidelines for respiratory function tests. [Google Scholar]
- 12.Enomoto Y, Nakamura Y, Satake Y, et al. Clinical diagnosis of idiopathic pleuroparenchymal fibroelastosis: A retrospective multicenter study. Respir Med. 2017;133:1–5. doi: 10.1016/j.rmed.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Harada T, Yoshida Y, Kitasato Y, et al. The thoracic cage becomes flattened in the progression of pleuroparenchymal fibroelastosis. Eur Respir Rev. 2014;23:263–266. doi: 10.1183/09059180.00006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirota T, Yoshida Y, Kitasato Y, et al. Histological evolution of pleuroparenchymal fibroelastosis. Histopathology. 2015;66:545–554. doi: 10.1111/his.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonigk D, Rath B, Borchert P, et al. Comparative analysis of morphological and molecular motifs in bronchiolitis obliterans and alveolar fibroelastosis after lung and stem cell transplantation. J Pathol Clin Res. 2017;3:17–28. doi: 10.1002/cjp2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Chemaly S, Pacheco-Rodriguez G, Ikeda Y, Malide D, Moss J. Lymphatics in idiopathic pulmonary fibrosis: new insights into an old disease. Lymphat Res Biol. 2009;7:197–203. doi: 10.1089/lrb.2009.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okada Y, Ito M, Nagaishi C. Anatomical study of the pulmonary lymphatics. Lymphology. 1979;12:118–124. [PubMed] [Google Scholar]


