Abstract
Background:
Patients with idiopathic pulmonary fibrosis (IPF) often do not tolerate pirfenidone in the recommended dose of 2400 mg/day. The proportion of patients requiring dose reduction and its impact on survival in the real-world remain unclear.
Methods:
Consecutive subjects with IPF were enrolled between March 2017 and June 2019. The maximum tolerated dose of pirfenidone (primary outcome) and adverse drug reactions (ADRs) were recorded. A post hoc logistic regression analysis was performed to evaluate the predictors of drug discontinuation due to ADRs. We also compared survival between the full-dose (2400 mg/day), reduced-dose (< 2400 mg/day), and the no-pirfenidone groups, with age and percentage of the predicted forced vital capacity (%pred FVC) as covariates.
Results:
Of the 128 subjects (mean age, 67.4 years; 77.3% men) included, 115 were initiated on pirfenidone. Forty-nine (42.6%) and 51 (44.3%) subjects tolerated the full dose and reduced doses, respectively. Ninety-six (83.5%) subjects developed at least one ADR; anorexia dyspepsia, and nausea being the most common. Twenty-two subjects discontinued the drug; 15 of them due to ADRs. Body mass index < 20 kg/m2 was the only predictor of drug discontinuation due to ADRs. Among subjects newly initiated on treatment during the study period (n = 80), survival was longer (hazard ratio [interquartile range], 0.19 [0.04-0.96]; p = 0.045) in the full-dose but not the reduced-dose group (p = 0.08) compared with the no-pirfenidone group, after adjusting for covariates.
Conclusion:
Pirfenidone was tolerated in the full dose in a minority of patients with IPF and appears to improve survival only with the full dose. (Sarcoidosis Vasc Diffuse Lung Dis 2020; 37 (2): 148-157)
Keywords: interstitial lung disease, interstitial pneumonia, diffuse lung disease, lung fibrosis, drug safety, antifibrotic
Comparison of baseline characteristics between subjects in different pirfenidone groups in the prospective cohort (n = 80)
Cox regression for survival among the prospective cohort (n = 80) of study subjects with the inclusion of additional covariates
Introduction
Idiopathic pulmonary fibrosis (IPF) is a diffuse lung disease associated with a reduced survival.(1, 2) The disease advances inexorably with deteriorating lung function due to progressive lung fibrosis. The antifibrotic agents pirfenidone and nintedanib are the only drugs that benefit patients with IPF.(3, 4) Pirfenidone was found to reduce the rate of decline in the forced vital capacity (FVC) in two of the three phase 3 multinational, randomized trials (CAPACITY 1, CAPACITY 2, and ASCEND).(3, 5) In a pooled analysis of these studies, pirfenidone was also found to reduce the risk of death at one year.(3) The dose of pirfenidone associated with a survival advantage was 2403 mg/day.
Several studies have reported the use of pirfenidone in IPF in clinical practice.(6-11) These studies indicate that the full dose of pirfenidone is poorly tolerated in a large number of patients and frequently requires dose reduction.(9) The discontinuation rates are also high in the real-world like that in the clinical trial setting.(3, 6, 9) The effect of pirfenidone on survival is inconsistent among real-world studies.(12-17) One reason for this inconsistency could be the varying doses of pirfenidone that patients tolerate in clinical practice. In an analysis of the pooled data from the CAPACITY and ASCEND trials, as compared to placebo, pirfenidone did not change the outcome of progression or death at one year when used in reduced doses.(18) Unfortunately, there is a dearth of prospective real-world data on the effect of reduced doses of pirfenidone on survival in IPF.
Herein, we describe our experience with the dosing and tolerability of pirfenidone in patients with IPF. We analyze the factors associated with the discontinuation of pirfenidone due to adverse drug reactions (ADRs). We also compare the survival of subjects receiving the full dose or reduced doses of the drug.
Material and Methods
This prospective, observational study was performed between March 2017 and June 2019 at the Chest Clinic of this Institute. The Institutional Ethics Committee approved the study protocol, and all subjects provided a written informed consent.
Study subjects
We included consecutive subjects presenting to the Chest Clinic if they (1) were diagnosed to have IPF; and, (2) consented to participate in the study. Subjects newly diagnosed during the study period formed the prospective cohort. We also included patients diagnosed before the study period and already following up in the clinic (whether or not taking pirfenidone), whom we designated as the retrospective cohort. The following subjects were excluded: (1) subjects who opted for nintedanib; and, (2) subjects who refused consent for the study. A diagnosis of IPF was made based on the 2011 American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association criteria, as described previously.(1, 19) A lung biopsy (either a surgical lung biopsy [SLB] or a transbronchial lung cryobiopsy [TBLC]) was considered, wherever the clinician, in consultation with the radiologist, considered the diagnosis of IPF to be in doubt after reviewing the clinical and radiologic data. The biopsy was performed if the subject was willing and fit for the procedure. The degree of confidence in the diagnosis (confident, or provisional with high or low confidence) was assigned in each case by the multidisciplinary discussion (MDD) team, according to a proposed classification.(20)
Study procedure
We recorded the demographic details and co-morbid illnesses of the included subjects, the spirometric findings (FVC, percentage of the predicted FVC [% predicted FVC], forced expiratory volume in one second [FEV1], % predicted FEV1, FEV1/FVC ratio, and the type of spirometric abnormality), diffusion capacity of the lung for carbon monoxide (DLCO), resting oxygen saturation, and the six-minute walk distance. Subjects were started on pirfenidone, after being explained the risks and benefits of the medication.(2) We initiated pirfenidone at a dose of 600 mg/day in three divided doses and gradually escalated the daily dose to 2400 mg. The ADRs were recorded. In the case of intolerable ADRs, titration to the maximum tolerated dose was performed. If a subject did not tolerate a minimum dose of 600 mg/day, the drug was stopped.(21) We offered the option of switching to nintedanib to subjects who discontinued pirfenidone due to ADRs, did not tolerate the full-dose, or had disease progression on pirfenidone. We followed the subjects longitudinally during the study period at planned intervals of six months between visits. An acute exacerbation of IPF (AE-IPF) was defined as an acute (over < 1 month) worsening of respiratory symptoms and/or lung function in the absence of an alternate diagnosis (such as respiratory tract infection, aspiration, drug toxicity, heart failure, pulmonary embolism, and other such conditions).(22) We recorded the outcomes of all study subjects. For the survival analysis, we first divided the study population into two groups: pirfenidone group (those who were started on pirfenidone and continued the drug until death or last follow up) and the no-pirfenidone group (those who did not receive pirfenidone or discontinued it within six months). For the second analysis, the pirfenidone group was divided into the full-dose group (those receiving a stable dose of 2400 mg/day of pirfenidone) and reduced-dose group (those receiving less than 2400 mg/day), and was compared with the third group (the no-pirfenidone group).
Study Outcomes
The primary outcome of the study was to identify the actual tolerated doses of pirfenidone in subjects with IPF in a real-world situation. The secondary outcomes included the assessment of the adverse effects of pirfenidone and the efficacy of the drug (lung function decline). Post hoc exploratory analyses of the predictors of discontinuation of pirfenidone due to ADRs and of survival in the different pirfenidone dosing groups were performed.
Statistical analysis
Data were analyzed using the statistical package SPSS (version 23.0, IBM Inc., Armonk, NY, United States). Statistical significance was assumed at a p-value < 0.05. A sample size calculation was not performed at study initiation as the study was planned as an observational study. The data are expressed as number with percentage, mean with standard deviation, or median with interquartile range (IQR), as appropriate. The differences between categorical variables were analyzed using the chi-square test (or the Fisher’s exact test) while the differences between continuous variables were analyzed using the one-way analysis of variance or the Kruskal-Wallis test. The annualized decline in % predicted FVC was calculated by dividing the decrease in the absolute value of % predicted FVC by the interval (in years) between the measurements. A missing-value analysis was not performed. A logistic regression analysis was performed to identify the predictors for the discontinuation of pirfenidone due to ADRs. A multivariate Cox regression analysis was performed to study the factors associated with survival in the study groups in the prospective cohort. Age, % predicted FVC (at the start of the maximum tolerated dose), and the study group were entered as covariates. Percentage predicted FVC was assumed as 25% as a worst-case scenario (only for the survival analysis), for subjects who could not perform an acceptable spirometric maneuver at the initiation of the maximum tolerated dose. The hazard ratio (HR) with 95% confidence intervals (CI) were calculated. We also performed secondary analyses for survival for the entire cohort and a survival analysis with additional covariates for the prospective cohort.
Results
Of the 139 subjects screened, 128 (mean age, 67.4 years; 77.3% men) were enrolled (Table 1). Nine refused consent, while two opted for nintedanib. The definite, probable, and indeterminate patterns for UIP were present in 93 (72.7%), 26 (20.3%), and 9 (7.0%) subjects, respectively on the HRCT of the chest. A confident diagnosis on MDD was made in 108 (84.4%) subjects, while 20 received a provisional diagnosis (16 [12.5%] with high confidence, and 4 [3.1%] with low confidence). Only four subjects underwent a lung biopsy (three TBLC, one SLB). Among the nine subjects with an indeterminate pattern on HRCT, two underwent TBLC and received a confident and provisional (with high confidence) MDD diagnosis, respectively. In the remaining seven subjects (median age, 74 years), a provisional ‘clinical best-fit diagnosis’ of IPF (three high confidence and four low confidence) was made by the MDD team in view of age, clinical presentation, and the absence of any history of connective tissue disorders, and exposure to offending drugs or environmental dusts. Four subjects were lost to follow up. Of the included subjects, 80 constituted the prospective cohort, with a median (IQR) duration of follow up of 15 (6-20) months. There were 48 subjects in the retrospective cohort with a median (IQR) follow up of 40 (25-53) months from their initial clinic visit. They had been receiving pirfenidone for a median (IQR) duration of 18 (11-34) months before enrolment into the study.
Table 1.
Baseline characteristics of study subjects (n = 128)
| Parameter | Value |
| Age, years | 67.4 ± 7.8 |
| Men, Number (%) | 99 (77.3) |
| Body mass index, kg/m2 | 24.2 ± 4.5 |
| Any smoke exposure | 71 (55.5) |
| Tobacco smoking | 59 (46.1) |
| Biomass smoke exposure | 13 (10.2) |
| Comorbid illnesses | |
| Hypertension | 45 (35.2) |
| Diabetes mellitus | 25 (19.5) |
| Coronary artery disease | 17 (13.3) |
| Chronic obstructive pulmonary disease | 8 (6.3) |
| Hypothyroidism | 7 (5.5) |
| Chronic liver disease | 3 (2.3) |
| Cerebrovascular disease | 1 (0.8) |
| Gastroesophageal reflux | 43 (33.6) |
| Duration of symptoms, months | 10.5 (6-24) |
| Oxygen saturation, % | 95 (92-97) |
| Spirometric abnormality (n = 115) | |
| Obstructive defect | 8 (6.3) |
| Restrictive defect | 80 (62.5) |
| Normal | 27 (21.1) |
| Could not perform | 13 (10.2) |
| Spirometric parameters (n = 115) | |
| FVC, litres | 2.14 ± 0.65 |
| FVC, % predicted | 70.0 ± 17.5 |
| FEV1, litres | 1.75 ± 0.49 |
| FEV1, % predicted | 74.6 ± 17.8 |
| DLCO, % predicted (n = 81) | 48.8 ± 19.3 |
| Six-minute walk distance, meters (n = 103) | 372 ± 79 |
| Presence of emphysema on HRCT chest | 25 (19.5) |
| Presence of pulmonary hypertension | 29 (22.7) |
| Use of domiciliary oxygen during the clinical course | 29 (22.7) |
All values represent mean ± standard deviation, median (interquartile range), or number (percentage). DLCO-diffusion capacity of the lung for carbon monoxide, FEV1-forced expiratory volume in one second, FVC-forced vital capacity, HRCT-high resolution computed tomography
Pirfenidone was initiated in 115 (89.8%) subjects; the reasons for not starting the drug in the remaining were patient’s choice (n = 7), advanced disease (n = 3), financial constraints (n = 2), and decompensated cirrhosis (n = 1), respectively. Forty-nine (42.6%) subjects tolerated the full dose and 51 (44.3%) tolerated a reduced-dose (primary outcome; Table 2). The drug was discontinued in 22 (19.1%) subjects; in 12, it was within six months of initiation. Among the 22 subjects who discontinued the drug, 15 were due to ADRs, four due to patient’s choice, two had progression and were switched over to nintedanib; one subject discontinued due to financial constraint. Table 3 depicts the ADRs due to the drug; 96 (83.5%) subjects experienced at least one ADR. In the univariate and multivariate logistic regression analyses, body mass index (BMI) < 20 kg/m2 was the only factor that predicted drug discontinuation due to ADRs, amongst other factors including age, gender, % predicted FVC, presence of any comorbidity, and presence of gastroesophageal reflux (Table 4).
Table 2.
Tolerated dose/dose range of pirfenidone (primary outcome) and reasons for discontinuation among study subjects started on pirfenidone (n = 115)
| Tolerated dose/dose range | Number (percentage) |
| 2400 mg | 49 (42.6) |
| 1800 mg to < 2400 mg | 34 (29.6) |
| 1200 mg to < 1800 mg | 12 (10.4) |
| 600 mg to < 1200 mg | 5 (4.3) |
| Reasons for discontinuation (n = 22) | Number (percentage) |
| Discontinued due to an adverse drug reaction | 15 (68.2) |
| Patient’s choice | 4 (18.2) |
| Progression prompting a switch to nintedanib | 2 (9.1) |
| Financial constraint | 1 (4.5) |
Table 3.
Adverse drug reactions on pirfenidone treatment (n = 115)
| Adverse drug reaction | Number (percentage) |
| Any adverse drug reaction | 96 (83.5) |
| Anorexia | 48 (41.7) |
| Dyspepsia | 34 (29.6) |
| Nausea | 22 (19.1) |
| Uneasiness | 20 (17.4) |
| Rash | 20 (17.4) |
| Weight loss | 17 (14.8) |
| Itching | 16 (13.9) |
| Insomnia | 16 (13.9) |
| Giddiness | 13 (11.3) |
| Flushing | 9 (7.8) |
| Raised liver transaminases | 8 (7.0) |
| Vomiting | 8 (7.0) |
| Dry mouth | 7 (6.1) |
| Others* | 25 (21.7) |
*Other adverse drug reactions each with a frequency of < 5% included abdominal pain, chest congestion, constipation, diarrhea, drowsiness, fatigue, forgetfulness, headache, hoarseness of voice, increased cough, irritability, mucositis, nasopharyngitis, numbness, paraesthesia, slurred speech, somnolence, and vertigo.
Table 4.
Univariate and multivariate logistic regression analyses of factors predicting discontinuation of pirfenidone due to adverse drug reactions
| OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
| Age | 1.03 (0.96-1.10) | 0.46 | 1.05 (0.97-1.14) | 0.23 |
| Female gender | 0.84 (0.22-3.22) | 0.79 | 0.53 (0.10-2.76) | 0.45 |
| Body mass index < 20 kg/m2 | 4.05 (1.17-14.02) | 0.03 | 5.29 (1.22-23.06) | 0.03 |
| % predicted FVC | 1.00 (0.97-1.04) | 0.83 | 1.01 (0.98-1.04) | 0.63 |
| Any comorbidity* | 1.33 (0.44-1.02) | 0.61 | 1.63 (0.45-5.87) | 0.46 |
| Gastroesophageal reflux | 1.86 (0.62-5.57) | 0.27 | 1.82 (0.51-6.47) | 0.35 |
*The comorbidities considered were as listed in Table 1. CI-confidence intervals, FVC-forced vital capacity, OR-odds ratio
The median (IQR) annualized fall in % predicted FVC was not significantly different (p = 0.32) between the full-dose (4.5 [-1.3, 8.7]) and reduced-dose (5.5 [3.0, 10.1]) groups (secondary outcome). Twenty-nine (22.7%) subjects had at least one AE-IPF; 11 (22.0%), 13 (24.5%) and 5 (20.0%) subjects in the full-dose, reduced-dose, and no-pirfenidone groups, respectively. The occurrence of AE-IPF was not significantly different between the study groups (p = 0.89). Overall, 40 (31.3%) subjects died during the study period; 23 in the prospective cohort. A comparison of baseline characteristics of the subjects in the full-dose, reduced-dose, and the no-pirfenidone groups in the prospective cohort is provided in Supplementary Table 1. In the prospective cohort, subjects in the pirfenidone group survived longer (HR [IQR], 0.33 [0.12-0.88]; p = 0.03) than in the no-pirfenidone group (Table 5; Figure 1A). When analyzed separately for the full-dose and reduced-dose groups, the hazards for death were reduced (HR [IQR], 0.19 [0.04-0.96]; p = 0.045) only with the use of the full dose (Table 5; Figure 1B). With the use of additional covariates, the % predicted FVC and the number of comorbidities were also found to be associated with lower hazards for death apart from the use of full-dose pirfenidone (Supplementary Table 2). When analyzed for the entire population (prospective and retrospective cohorts), the survival was significantly improved in both the full-dose and reduced-dose groups compared to the no-pirfenidone group (Table 5).
| Parameter | Full-dose group (n = 24) | Reduced-dose group (n = 35) | No-pirfenidone group (n = 21) | p-value |
| Age, years | 65.5 ± 7.9 | 67.9 ± 8.5 | 70.4 ± 7.3 | 0.13 |
| Men, Number (%) | 20 (83.3) | 24 (68.6) | 17 (81.0) | 0.36 |
| Body mass index, kg/m2 | 24.4 ± 5.2 | 24.3 ± 4.1 | 23.7 ± 6.1 | 0.89 |
| Any smoke exposure | 16 (66.7) | 20 (57.1) | 12 (57.1) | 0.73 |
| Tobacco smoking | 15 (62.5) | 16 (45.7) | 10 (47.6) | 0.42 |
| Biomass smoke exposure | 1 (4.2) | 5 (14.3) | 2 (9.5) | 0.44 |
| Comorbid illnesses | ||||
| Any comorbidity | 13 (54.2) | 23 (65.7) | 14 (66.7) | 0.60 |
| Hypertension | 5 (20.8) | 15 (42.9) | 12 (57.1) | 0.04 |
| Diabetes mellitus | 8 (33.3) | 6 (17.1) | 3 (14.3) | 0.22 |
| Coronary artery disease | 3 (12.5) | 5 (14.3) | 3 (14.3) | 0.98 |
| Chronic obstructive pulmonary disease | 2 (8.3) | 3 (8.6) | 1 (4.8) | 0.86 |
| Hypothyroidism | 4 (16.7) | 0 | 0 | 0.007 |
| Chronic liver disease | 1 (4.2) | 1 (2.9) | 1 (4.8) | 0.93 |
| Cerebrovascular disease | 0 | 0 | 0 | 0.46 |
| Gastroesophageal reflux | 13 (54.2) | 12 (34.3) | 9 (42.9) | 0.32 |
| Duration of symptoms, months | 12 (5-23) | 10 (6-24) | 10 (6-30) | 0.93 |
| Pattern on HRCT chest | ||||
| Definite UIP | 18 (75.0) | 24 (68.6) | 19 (90.5) | 0.35 |
| Probable UIP | 4 (16.7) | 6 (17.1) | 2 (9.5) | |
| Indeterminate for UIP | 2 (8.3) | 5 (14.3) | 0 | |
| Presence of emphysema on HRCT chest | 8 (33.3) | 7 (10.0) | 6 (28.6) | 0.50 |
| Presence of pulmonary hypertension | 5 (20.8) | 7 (20.0) | 3 (14.3) | 0.83 |
| Level of confidence of MDD diagnosis | ||||
| Confident | 20 (83.3) | 25 (71.4) | 19 (90.5) | 0.25 |
| Provisional with high confidence | 4 (16.7) | 7 (20.0) | 2 (9.5) | |
| Provisional with low confidence | 0 | 3 (8.6) | 0 | |
| Use of domiciliary oxygen | 4 (16.7) | 8 (22.9) | 5 (23.8) | 0.80 |
| Baseline oxygen saturation, % | 95 (92-97) | 95 (93-97) | 94 (91-97) | 0.69 |
| Spirometric parameters | (n = 21) | (n = 31) | (n = 16) | |
| Type of spirometric abnormality | ||||
| Obstructive defect | 2 (9.5) | 3 (9.7) | 1 (6.3) | 0.93 |
| Restrictive defect | 16 (76.2) | 23 (74.2) | 11 (68.8) | |
| Normal | 3 (14.3) | 5 (16.2) | 4 (25.0) | |
| FVC, litres | 1.96 ± 0.44 | 1.99 ± 0.61 | 2.04 ± 0.64 | 0.92 |
| FVC, % predicted | 70.6 ± 19.2 | 71.7 ± 16.9 | 72.6 ± 16.2 | 0.67 |
| FEV1, litres | 1.69 ± 0.36 | 1.63 ± 0.49 | 1.67 ± 0.49 | 0.91 |
| FEV1, % predicted | 70.6 ± 19.2 | 71.7 ± 16.9 | 72.6 ± 16.2 | 0.94 |
| DLCO | (n = 19) | (n = 18) | (n = 10) | |
| % predicted | 48.7 ± 21.7 | 46.2 ± 18.4 | 58.3 ± 23.5 | 0.34 |
| Six-minute walk test | (n = 21) | (n = 30) | (n = 12) | |
| Distance, meters | 360 ± 85 | 345 ± 52 | 377 ± 80 | 0.39 |
All values represent mean ± standard deviation, median (interquartile range), or number (percentage). DLCO-diffusion capacity of the lung for carbon monoxide, FEV1-forced expiratory volume in one second, FVC-forced vital capacity, HRCT-high resolution computed tomography
Table 5.
Cox regression for survival among study subjects
| Covariates | HR (95% CI) | p-value |
| Prospective cohort (n = 80) | ||
| Pirfenidone group | 0.33 (0.12-0.88) | 0.03 |
| Age | 1.02 (0.96-1.08) | 0.55 |
| % predicted FVC at start of dose | 0.98 (0.95-1.00) | 0.05 |
| Prospective cohort (n = 80) | ||
| Full-dose group | 0.19 (0.04-0.96) | 0.045 |
| Reduced-dose group | 0.40 (0.14-1.12) | 0.08 |
| Age | 1.01 (0.95-1.08) | 0.74 |
| % predicted FVC at start of dose | 0.98 (0.95-1.00) | 0.06 |
| Entire cohort (n = 126)* | ||
| Pirfenidone group | 0.31 (0.13-0.70) | 0.01 |
| Age | 1.01 (0.96-1.05) | 0.79 |
| % predicted FVC at start of dose | 0.97 (0.95-0.99) | < 0.001 |
| Entire cohort (n = 126)* | ||
| Full-dose group | 0.29 (0.12-0.74) | 0.01 |
| Reduced-dose group | 0.32 (0.13-0.78) | 0.01 |
| Age | 1.01 (0.96-1.05) | 0.81 |
| % predicted FVC at start of dose | 0.97 (0.95-0.99) | < 0.001 |
*Two subjects, who switched to nintedanib were excluded from the analysis. CI-confidence intervals, FVC-forced vital capacity, HR-hazard ratio
Fig. 1.
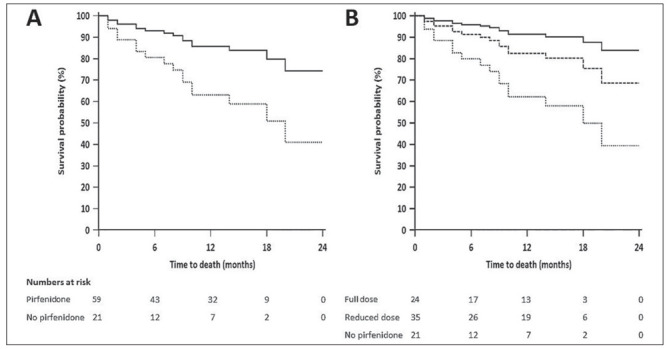
Cox regression analysis for survival in the prospective cohort. A. Two-group analysis (pirfenidone vs. no-pirfenidone); B. Three group analysis (full-dose pirfenidone, and reduced-dose pirfenidone vs. no-pirfenidone). The covariates were age, and % predicted forced vital capacity.
| Covariates | HR (95% CI) | p-value |
| Full-dose group | 0.16 (0.03-0.85) | 0.03 |
| Reduced-dose group | 0.41 (0.14-1.21) | 0.11 |
| Age | 1.01 (0.94-1.07) | 0.87 |
| Male Gender | 1.76 (0.37-8.31) | 0.47 |
| Smoke exposure | 0.92 (0.33-2.58) | 0.88 |
| Number of comorbidities | 0.45 (0.21-0.95) | 0.04 |
| Presence of pulmonary hypertension | 0.37 (0.07-1.88) | 0.23 |
| % predicted FVC at start of dose | 0.97 (0.94-0.99) | 0.03 |
Discussion
The results of this study suggest that a large proportion of our patients with IPF did not tolerate the full dose of pirfenidone. Pirfenidone improved survival, compared to those who did not receive the drug, only when used in the maximum approved dose of 2400 mg/day. A lower BMI (< 20 kg/m2) was associated with discontinuation of the drug due to ADRs.
We found that only about 42% of the study subjects tolerated the full dose of pirfenidone like in previous studies, wherein 41-45% subjects required dose reduction.(9, 23) In fact, in real-world studies from Japan, the usual targeted and tolerated dose was only 1800 mg.(6, 24) In contrast, in studies from Italy, Germany and the United States, 83-89% of the patients were compliant with a dose of 2403 mg/day.(7, 8, 10) Thus, ethnicity and body weight (with Europeans generally having a higher body weight than Asians) might be potential factors affecting the tolerated dose. The most common ADRs in our study were anorexia, dyspepsia and nausea, again mimicking the observations in previous real-world studies.(9, 25) A significant proportion of our subjects also complained of a feeling of marked ‘uneasiness’ encountered due to the drug, which could not be characterized any further.
We found that the hazards for death were significantly reduced with the use of pirfenidone in the real-world, similar to the results of the phase 3 trials.(3, 5) Other real-world studies have been more equivocal about the survival advantage with pirfenidone use in IPF (Table 6).(12-17) In the Czech EMPIRE registry, pirfenidone increased the overall survival of patients with IPF at five years over no-antifibrotic treatment.(17) In the Spanish SEPAR registry, however, the median survival of subjects receiving pirfenidone was similar to the entire cohort.(14) While Margaritopoulos et al. found improved survival with pirfenidone compared to a retrospective cohort, in the Finnish IPF registry analysis, the survival advantage with pirfenidone disappeared after adjustment for age.(13, 16) In our study, the hazards for death were lower, even after adjusting for age and % predicted FVC. Importantly, when analyzed separately for the full-dose and reduced-dose groups, we found improved survival only in the full-dose group. These findings are similar to a post hoc analysis of the pooled data from the CAPACITY and ASCEND trials.(18) In that study, the outcome of progression or death at one year was decreased for subjects who received the full dose of pirfenidone vs. placebo, but not so, with a reduced dose intensity (≤ 90% of the full dose). Moreover, in an earlier phase 3 trial from Japan, Taniguchi et al. also found a significantly better survival (p = 0.03) with a higher dose (1800 mg/day) of pirfenidone compared to a lower dose (1200 mg/day).(26) None of the previous real-world studies have separately analyzed the survival of subjects receiving full-dose or reduced-dose pirfenidone.
Table 6.
Real-world studies of survival with the use of pirfenidone in subjects with IPF
| Authors (Year) | Number | Number treated with pirfenidone | Dose of pirfenidone used | Comparator | Findings |
| Natarajan, et al. (2015)(12) | 46 | 17 | 1200-1800 mg/day | Triple therapy | No significant difference in survival |
| Margaritopoulos, et al. (2018)(13) | 294 | 82 | 2403 mg/day | Historical cohort not receiving pirfenidone | Survival improved with pirfenidone; HR: 0.32 (95% CI, 0.19–0.53; p < 0.0001) |
| Fernández-Fabrellas, et al. (2019)(14) | 608 | 231 | NA | Entire cohort | Median survival of subjects receiving pirfenidone similar to the entire cohort (5.8 years) |
| Jouneau, et al. (2019)(15) | 192 | 192 | 2403 mg/day (32.3% subjects had a dose reduction) | None | Median progression-free survival: 18.4 months |
| Kaunisto, et al. (2019)(16) | 453 | 82 (13 received nintedanib) | NA | Subjects not receiving any antifibrotic | Survival not different; HR: 0.67 (95% CI, 0.43–1.05; p = 0.078) |
| Zurkova, et al. (2019)(17) | 841 | 383 | 2403 mg/day (dose reduction NA) | Subjects not receiving any antifibrotic | Pirfenidone increased five year overall survival over no-antifibrotic treatment (55.9% vs 31.5% alive, p = 0.002) |
| Current study | 128 | 100 | Full-dose group: 2400 mg/day Reduced-dose group: < 2400 mg/day |
Subjects not receiving any antifibrotic | Full-dose group HR: 0.19 (95% CI, 0.04-0.96; p = 0.045) Reduced-dose group HR: 0.40 (95% CI, 0.14-1.12; p = 0.08) |
CI-confidence intervals, HR-hazard ratio, NA-not available
In contrast to the findings in the prospective cohort alone, in the entire population (comprising of both the prospective and retrospective cohorts), the subjects in both the full-dose and the reduced-dose groups had better survival than the no-pirfenidone group (Table 5). One explanation for this phenomenon may be the longer duration of follow up in the full cohort compared to the prospective cohort alone. It is possible that the survival advantage is similar with a reduced dose of pirfenidone to that with a full dose, when used over a longer-term. However, a more likely explanation is the attrition bias. Retrospective cohorts are susceptible to include subjects having a favorable response to the therapy of interest. Thus, patients with a good response to pirfenidone are more likely to remain under follow up compared to those who deteriorate.
We also performed a Cox proportional hazards analysis in the prospective cohort using additional covariates (pirfenidone dose group, age, male gender, smoke exposure, number of comorbidities, presence of pulmonary hypertension, and % predicted FVC at initiation of dose) (Supplementary Table 2). The essential finding of a survival advantage in the full-dose pirfenidone group remained unchanged. Additionally, we found that a higher % predicted FVC was associated with significantly lower hazards for death. Interestingly, a higher number of comorbidities was associated with reduced hazards for death. This counterintuitive finding is likely due to chance and also because adjusting for the ‘number’ of comorbidities does not permit an adjustment for the severity of such comorbidities.
We found that BMI < 20 kg/m2 was a significant predictor of drug discontinuation due to ADRs in a multivariate logistic regression analysis. Age was not a predictive factor in our study, in contrast to the findings of Galli, et al. who found age ≥70 years old to be a predictor apart from a history of congestive heart failure. (10) Our findings are, however, in conformity with the study by Uehara, et al., who found that subjects who received a higher dose of pirfenidone per kilogram of body weight had a higher incidence of adverse events.(27) Interestingly, a BMI < 22 kg/m2 has also been found to be associated with a higher incidence of hepatotoxicity with the use of nintedanib in IPF.(28)
What are the clinical implications of our study? It is not uncommon for physicians to prescribe lower doses of pirfenidone in clinical practice.(29, 30) As pirfenidone offers survival advantage mainly with the full dose, it is imperative that an attempt be made to achieve this dose by slow dose escalation. In case, a patient is unable to tolerate the full dose, the option of switching to nintedanib may be discussed. In case, a patient does not opt for or tolerate nintedanib, one can still administer a lower dose of pirfenidone as the survival in the reduced-dose group also showed a trend towards improvement in our study (though statistically non-significant). Importantly, titration to the maximum tolerated dose should be performed, even in this scenario.
Our study has a few limitations. It is a single-center study with a small sample size and a relatively short duration of follow up. Therefore, the findings of the regression analysis should be interpreted cautiously. We did not plan an imputation analysis due to a large number of randomly missing values. This is because, in the real-world, patients present for follow up at variable intervals. Further, several of our subjects with advanced disease were unable to perform an acceptable spirometric maneuver. The survival analysis was a post hoc exploratory analysis in a cohort with varying follow-up durations and thus may suffer from an inadvertent immortal time bias. It is also possible that the small group of study subjects who did not receive pirfenidone showed a worse survival independent of the lack of treatment but rather due to a selection bias. Thus, this study offers only a preliminary insight into the effects of dose reduction of pirfenidone on survival in IPF, especially from the developing world. Larger, prospective, and preferably multicenter real-world studies, with longer duration of follow up, are required to confirm our findings. Studies of dosing are also needed for other potential indications of using pirfenidone, such as other fibrosing interstitial lung diseases.(31, 32)
In conclusion, the results of this study suggest that in actual clinical practice, pirfenidone is not tolerated in the full recommended dose of 2400 mg/day in a large proportion of patients. However, it appears that it offers a significant survival advantage, only when used in the full dose.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhooria S, Agarwal R, Dhar R, Jindal A, Madan K, Aggarwal AN, et al. Expert Consensus Statement for the Diagnosis and Treatment of Idiopathic Pulmonary Fibrosis in resource constrained settings. Indian J Chest Dis Allied Sci. 2018;60:91–119. [Google Scholar]
- 3.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–92. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 4.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–82. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 5.Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377(9779):1760–9. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 6.Okuda R, Hagiwara E, Baba T, Kitamura H, Kato T, Ogura T. Safety and efficacy of pirfenidone in idiopathic pulmonary fibrosis in clinical practice. Respir Med. 2013;107(9):1431–7. doi: 10.1016/j.rmed.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Oltmanns U, Kahn N, Palmowski K, Trager A, Wenz H, Heussel CP, et al. Pirfenidone in idiopathic pulmonary fibrosis: real-life experience from a German tertiary referral center for interstitial lung diseases. Respiration. 2014;88(3):199–207. doi: 10.1159/000363064. [DOI] [PubMed] [Google Scholar]
- 8.Harari S, Caminati A, Albera C, Vancheri C, Poletti V, Pesci A, et al. Efficacy of pirfenidone for idiopathic pulmonary fibrosis: An Italian real life study. Respir Med. 2015;109(7):904–13. doi: 10.1016/j.rmed.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Salih GN, Shaker SB, Madsen HD, Bendstrup E. Pirfenidone treatment in idiopathic pulmonary fibrosis: nationwide Danish results. Eur Clin Respir J. 2016;3:32608. doi: 10.3402/ecrj.v3.32608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galli JA, Pandya A, Vega-Olivo M, Dass C, Zhao H, Criner GJ. Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: Tolerability and adverse drug reactions. Respirology. 2017;22(6):1171–8. doi: 10.1111/resp.13024. [DOI] [PubMed] [Google Scholar]
- 11.Alhamad EH. Pirfenidone treatment in idiopathic pulmonary fibrosis: A Saudi experience. Ann Thorac Med. 2015;10(1):38–43. doi: 10.4103/1817-1737.146866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Natarajan S, Subramanian P. Idiopathic Pulmonary Fibrosis: A Study of 46 Patients from Western India: Clinical Presentations and Survival. Turk Thorac J. 2015;16(3):114–20. doi: 10.5152/ttd.2015.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margaritopoulos GA, Trachalaki A, Wells AU, Vasarmidi E, Bibaki E, Papastratigakis G, et al. Pirfenidone improves survival in IPF: results from a real-life study. BMC Pulm Med. 2018;18(1):177. doi: 10.1186/s12890-018-0736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez-Fabrellas E, Molina-Molina M, Soriano JB, Portal JAR, Ancochea J, Valenzuela C, et al. Demographic and clinical profile of idiopathic pulmonary fibrosis patients in Spain: the SEPAR National Registry. Respir Res. 2019;20(1):127. doi: 10.1186/s12931-019-1084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jouneau S, Gamez AS, Traclet J, Nunes H, Marchand-Adam S, Kessler R, et al. A 2-Year Observational Study in Patients Suffering from Idiopathic Pulmonary Fibrosis and Treated with Pirfenidone: A French Ancillary Study of PASSPORT. Respiration. 2019;98(1):19–28. doi: 10.1159/000496735. [DOI] [PubMed] [Google Scholar]
- 16.Kaunisto J, Salomaa E-R, Hodgson U, Kaarteenaho R, Kankaanranta H, Koli K, et al. Demographics and survival of patients with idiopathic pulmonary fibrosis in the FinnishIPF registry. European Respiratory Journal Open Research. 2019;5:00170–2018. doi: 10.1183/23120541.00170-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zurkova M, Kriegova E, Kolek V, Lostakova V, Sterclova M, Bartos V, et al. Effect of pirfenidone on lung function decline and survival: 5-yr experience from a real-life IPF cohort from the Czech EMPIRE registry. Respir Res. 2019;20(1):16. doi: 10.1186/s12931-019-0977-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan SD, Lancaster LH, Albera C, Glassberg MK, Swigris JJ, Gilberg F, et al. Dose modification and dose intensity during treatment with pirfenidone: analysis of pooled data from three multinational phase III trials. BMJ Open Respir Res. 2018;5(1):e000323. doi: 10.1136/bmjresp-2018-000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhooria S, Agarwal R, Sehgal IS, Prasad KT, Garg M, Bal A, et al. Spectrum of interstitial lung diseases at a tertiary center in a developing country: A study of 803 subjects. PLoS One. 2018;13(2):e0191938. doi: 10.1371/journal.pone.0191938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryerson CJ, Corte TJ, Lee JS, Richeldi L, Walsh SLF, Myers JL, et al. A Standardized Diagnostic Ontology for Fibrotic Interstitial Lung Disease. An International Working Group Perspective. Am J Respir Crit Care Med. 2017;196(10):1249–54. doi: 10.1164/rccm.201702-0400PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040–7. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 22.Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med. 2016;194(3):265–75. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 23.Wijsenbeek MS, Grutters JC, Wuyts WA. Early Experience of Pirfenidone in Daily Clinical Practice in Belgium and The Netherlands: a Retrospective Cohort Analysis. Adv Ther. 2015;32(7):691–704. doi: 10.1007/s12325-015-0225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bando M, Yamauchi H, Ogura T, Taniguchi H, Watanabe K, Azuma A, et al. Clinical Experience of the Long-term Use of Pirfenidone for Idiopathic Pulmonary Fibrosis. Intern Med. 2016;55(5):443–8. doi: 10.2169/internalmedicine.55.5272. [DOI] [PubMed] [Google Scholar]
- 25.Hughes G, Toellner H, Morris H, Leonard C, Chaudhuri N. Real World Experiences: Pirfenidone and Nintedanib are Effective and Well Tolerated Treatments for Idiopathic Pulmonary Fibrosis. J Clin Med. 2016;5(9) doi: 10.3390/jcm5090078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35(4):821–9. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 27.Uehara M, Enomoto N, Oyama Y, Suzuki Y, Kono M, Furuhashi K, et al. Body size-adjusted dose analysis of pirfenidone in patients with interstitial pneumonia. Respirology. 2018;23(3):318–24. doi: 10.1111/resp.13145. [DOI] [PubMed] [Google Scholar]
- 28.Ikeda S, Sekine A, Baba T, Yamanaka Y, Sadoyama S, Yamakawa H, et al. Low body surface area predicts hepatotoxicity of nintedanib in patients with idiopathic pulmonary fibrosis. Sci Rep. 2017;7(1):10811. doi: 10.1038/s41598-017-11321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhooria S, Agarwal R. Idiopathic pulmonary fibrosis in India. Chest India. 2015;6(5):1–3. doi: 10.4103/0970-2113.148396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhooria S, Sehgal IS, Agrawal R, Aggarwal AN, Behera D. Knowledge, Attitudes, Beliefs and Practices of Physicians Regarding Idiopathic Pulmonary Fibrosis and the Impact of a Continuing Medical Education Program. J Assoc Physicians India. 2017;65(11):30–6. [PubMed] [Google Scholar]
- 31.Maher TM, Corte TJ, Fischer A, Kreuter M, Lederer DJ, Molina-Molina M, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2020;8(2):147–157. doi: 10.1016/S2213-2600(19)30341-8. [DOI] [PubMed] [Google Scholar]
- 32.Acharya N, Sharma SK, Mishra D, Dhooria S, Dhir V, Jain S. Efficacy and safety of pirfenidone in systemic sclerosis-related interstitial lung disease-a randomised controlled trial. Rheumatol Int. 2020;40(5):703–710. doi: 10.1007/s00296-020-04565-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of baseline characteristics between subjects in different pirfenidone groups in the prospective cohort (n = 80)
Cox regression for survival among the prospective cohort (n = 80) of study subjects with the inclusion of additional covariates


