Abstract
Background:
Posterior reversible encephalopathy (PRES) is a rare syndrome characterized by headache, confusion, seizures, visual changes and white matter edema at radiological imaging. Its pathophysiology is not clarified and different causes, including uncontrolled hypertension, eclampsia, chemotherapy and hypomagnesemia have been suggested.
Case report:
A woman affected by stage IV breast cancer with lower extremity deep vein thrombosis treated with low-molecular-weight-heparin, currently in therapy with Palbociclib/Fulvestrant (antiCDK4 and 6/estrogen receptor antagonist) but previously treated with several other chemotherapy lines (including VEGF inhibitor bevacizumab), was admitted to our Internal Medicine department because of ascites and abdominal pain. She was treated with diuretics (and paracentesis). Recently (six-month earlier) a pan-encephalic radiotherapy was done because of brain and skull metastasis. Among blood tests, low serum levels of hypomagnesemia were observed. She developed PRES that rapidly progressed to lethargy, unresponsiveness till coma without changes in blood pressure. Magnetic Resonance Imaging study showed bilateral parieto-occipital edema and a thrombosis of left transverse and sigmoid sinuses. Anti-edema therapy, intravenous supplementation of magnesium and decoagulation were started, with complete and rapid recovery (within 18 hours) of clinical and radiologic changes.
Conclusions:
PRES diagnosis was based on the rapid clinical recovery after antiedema treatment and magnesium supplementation. Low magnesium level related to both diuretic and Fulvestrant/Palbociclib therapies and recent radiotherapy can represent potential mechanisms favouring PRES development. The previous bevacizumab treatment may also be involved as a PRES predisposing factor. The concomitant occurrence of cerebral thrombosis can have precipitated the clinical situation. (www.actabiomedica.it)
Keywords: posterior reversible encephalopathy syndrome, hypomagnesemia, breast cancer, chemotherapy, cerebral thrombosis
Introduction
Posterior reversible encephalopathy syndrome (PRES) is a clinical syndrome characterized by headache, confusion or decreased level of consciousness, visual changes and seizures, associated with typical neuroimaging findings of posterior cerebral white matter edema, first described in 1996 (1). Despite its name, the clinical condition is not always reversible, and it represents a medical emergency, leading rapidly to permanent cerebral damage or to death (2, 3). In addition to the clinical features that can include elevated blood pressure levels, it has to be noted that the classic symmetrical white matter changes leading to neurological manifestations of PRES are visible on diffusion-weighted imaging (DWI), but not to standard magnetic Resonance Imaging (MRI) (4). PRES can occur in a variety of clinical conditions, such as in autoimmune disease (5, 6), eclampsia (7), hypertensive emergencies (8, 9), immunosuppressive therapy (10-12), red cell blood transfusions (13) or in clinical situations where electrolyte disorders are present such as hypomagnesemia (14). It is more common in women (3, 15) than in men and its exact incidence is not known. PRES pathogenic mechanisms are still unclear.
We present a clinical case involving an oncological patient with multiple factors contributing to the development of PRES focusing on a hypomagnesemia as a potential precipitating mechanism that was reversed by magnesium salt supplementation.
Case presentation
A 60 years old Caucasian woman was admitted to the Emergency Department of our Hospital because of ascites and abdominalgia. Twenty years earlier she was diagnosed with breast cancer, subjected to mastectomy and then treated with several chemotherapy lines, including the inhibitor of vascular endothelial growth factor A Bevacizumab, hormonal therapy and local radiotherapy. About 5 months before the admission, she developed seizures and sudden cognitive impairment, with loss of contact: a MRI revealed cranial and cerebral metastasis. An antiepileptic drug Levetiracetam was started as well panencephalic radiotherapy was given for five days. Chemotherapy was also administered with resolution of symptoms and regression of metastasis. One month before the hospitalization, she developed lower limb deep vein thrombosis, so she started fondaparinux as anticoagulation therapy. The patient continued its oncological follow-up schedule of a stage IV breast cancer, with multiple liver, pleural, pulmonary and bone metastasis, treated with Palbociclib, anti-CDK4 and 6 antagonist and Fulvestrant, an estrogen receptor antagonist. At two follow-up visits during the 4 weeks prior to admission to the Emergency Department because of low circulating serum magnesium levels, magnesium and potassium salts supplementation was reported and then suspended due to the appearance of abdominal pain. She was sent to the Emergency department by the oncologist because of abdominalgia associated to nausea and vomiting. She has been treated with a proton pump inhibitor for at least 6 months because of heartburn, regurgitation, and symptoms related to gastroesophageal reflux disease. An abdominal ultrasound highlighted abundant ascites. She was vigilant and collaborating, without alteration on neurological exam. Her vital parameters were normal, and laboratory tests showed mild anemia (Hemoglobin 10.6 g/dl, hematocrit 32%), no renal (creatinine 0.4 mg/dL) or hepatic (GOT 21 U/L; GPT 13 U/L; total bilirubin 0.4 mg/dL, albumin 3.2 g/dL) impairment and a moderate increase in C reactive protein (63 mg/L; normal values < 5 mg/L). Plasma sodium (135 mEq/L), potassium (3.9 mEq/L), chloride (98 mEq/L), calcium (8.2 mg/dL) and phosphate (4.0 mg/dL) concentration were normal, whereas magnesium levels were at the lower limit (1.8 mg/dL). Within the normal range was also pH and bicarbonate. Coagulation was normal, except for a significant increase of d-dimer. On physical examination, she had ascites and abdominalgia without breathing impairment. She felt nausea and vomit; symptomatic therapy with metoclopramide was given and a parenteral nutrition infusion with Olimel N4 (1500 ml/day, Glucose 112,5 g, Lipids 45 g, Amino-acids 38 g, Nitrogen 6 g; sodium 21 mEq/L, potassium 16 mEq/L, Mg 2.2 mEq/L, osmolarity 760 mOsm/L) was started. She was treated parenterally with loop of Henle diuretic therapy, furosemide associated with potassium sparing diuretic canrenoate, associated to water restriction. An abundant diuretic response and the reduction of ascites was observed in the 48 hours following, with a significant weight loss and an almost complete resolution of the abdominalgia. No changes in blood pressure or heart rate were measured. She suddenly presented confused and disoriented. She was not able to attempt a simple order and she complained headache. Her blood pressure was still normal and the neurological exam did not show any focal or lateral abnormality. Ammonium blood levels were normal (37 uM/L, normal values between 10-50). An urgent cerebral tomography was performed, showing a diffuse cerebral edema (Figure 1,ABC). Patient neurological condition rapidly got worse, till vigil coma, with loss of interaction ability, while her vital parameters (blood pressure, heart rate and peripheral oxygen saturation) remained normal. Treatment with intravenous desametasone (8 mg twice a day) and mannitol (mannitol 18%, 100 ml four times per day followed by 50 ml four times per day), associated with magnesium sulphate (40 mEq in saline solution 250 ml per day in 6-hour infusion period) was immediately started and continued for three days. A cerebral MRI was urgently performed showing diffuse increase in signal alteration of the periventricular, deep and sub-cortical white matter in parieto-occipital region of the two sides, as from leukoencephalopathy, also in relation to post-radiotherapy modifications. venous thrombosis of the distal segment of the transverse sinus and of part of the left sigmoid sinus (Figure 2A1-3).
Figure 1.

(A,B,C). Brain CT Scan performed upon the admission and showing a diffuse bilateral cerebral edema, especially in posterior cerebral lobes (parietal and occipital): radiological signs suspected for Posterior reversible encephalopathy syndrome (PRES)
Figure 2.

(A1,2,3, B1,2,3, C1,2,3). Brain MRI urgently performed upon admission to the Emergency Department (A1-3), ten days (B1-3) and one month later (C1-3). Axial FSE T2 w-i showed diffuse symmetrical hyperintensities in the periventricular, deep and subcortical white matter, consistent with leukoencephalopathy, both in relation to concomitant PRES and to post-radiotherapy modifications
She continued subcutaneous Fondaparinux at anticoagulating dose (7,5 mg/die) while Palbociclib and Fulvestrant were discontinued. Anti-edema (desametasone and mannitol) treatment as well magnesium sulphate infusion were continued. Her clinical condition rapidly improved with resolution of the acute episode.
A brain MRI was performed after 10 days, showing less but still persistent bilateral parieto-temporal-occipital vasogenic edema, partial resolution of the cerebral vein thrombosis, but evidencing the presence of few micro-haemorrhages in left temporal and parietal site (Figure 2B1-3).
Patient neurological status completely recovered and after 2 weeks, she was discharged with the diagnosis of PRES.
At a MRI brain scan, performed the following month, a reduction of the vasogenic edema but a persistent even if reduced left transverse sinus thrombosis (Fig. 2C1-3). After a month, she was admitted to the hospital because of severe respiratory distress due to pneumonia and after three days she passed away.
Discussion
PRES is an increasingly recognizable neuro-clinical syndrome where a posterior white matter brain edema represents the classic radiological feature. PRES should be distinguished from other causes of cerebral edema as depicted in Figure 3. Several mechanisms have been suggested to participate to PRES development, including endothelial dysfunction, as a result of cytotoxic therapy (chemotherapy and immunosuppressive therapies) or electrolyte disorders, altered cerebral autoregulation, often occurring during eclampsia or severe arterial hypertension or hypertensive emergencies (16,17). Recently, another fascinating hypothesis regards the increase in arginine vasopressin secretion as a pathogenic mechanism occurring in many clinical scenarios preluding PRES development; if this hypothesis will be validated the Authors suggested arginine vasopressin system as a target for possible treatment (Figure 4) (18).
Figure 3.

Posterior reversible encephalopathy syndrome differential diagnosis includes vascular and non vascular causes of cerebral edema
Figure 4.
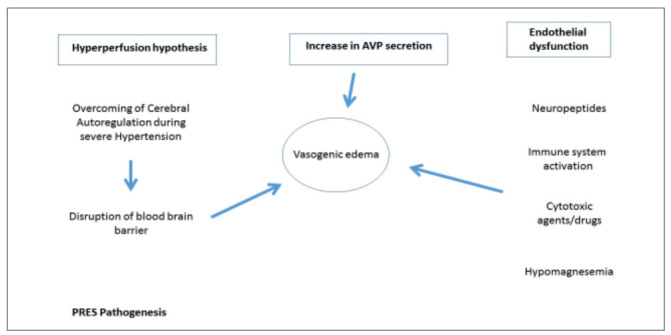
The three main hypotheses explaining the pathophysiology of posterior reversible encephalopathy and associated conditions
In the present case, a cluster of factors combined contributes to PRES development in a normotensive condition. First, our patient underwent multiple cycle of chemiotherapic drugs, including Bevacizumab, a monoclonal antibody anti-VEGF that has been associated with various cases of PRES in literature (19-24); in some cases, PRES developed months after discontinuing the therapy (21). Bevacizumab is associated to the development of hypertension (25) and proteinuria (26,27). It is hypothesized that it disrupts the blood-brain barrier through endothelial dysregulation resulting in hyperperfusion and vasogenic edema leading to failure of cerebral autoregulation. In addition to bevacizumab-induced hypertension as a mechanism of PRES development, cases of bevacizumab-induced PRES have been also reported in normotensive patients (28), as the case of our patient.
Second, a confounding element was observed in our patient that could have precipitated her clinical neurological status, and is represented by the coexistence of the thrombosis of left transverse and sigmoid sinuses, which has been associated in several case report to PRES, especially in eclampsia or in postpartum females (29-31).
What really surprised us in the clinical management of the patient, was the observation of the concomitant presence of magnesium plasma levels at lower limit of range potentially contributing to PRES development and its rapid resolution followed intravenous magnesium salt supplementation together with anti-edema therapies, desametasone and mannitol. Altered magnesium circulating levels can contribute to endothelial dysfunction and cerebral edema of the posterior circulation territories often observed in patients with PRES (14, 32) and cerebellar syndromes (33, 34). Magnesium owns a role as a regulator of endothelial integrity and function as well as vascular tone and reactivity (35). Decline in intracellular free magnesium concentration reduces ATP synthesis and influence cell membrane permeability, induces an impairment of membrane stability by promoting free radical production, cellular entry of Ca2+ and endothelial activation trough cytokines generation and intercellular leaking (36-41). The blood-brain barrier constituted by capillary endothelial cells is essential for optimal brain function. A crucial role in the pathogenesis of brain edema development is represented by Aquaporin (AQP)-4, a bidirectional transmembrane water channel expressed mainly in astrocytes (42,43); the upregulation of AQP-4 in brain injury leads to an increase in brain water content, resulting in brain edema (43). Magnesium treatment induces a down-regulation of AQP-4 (44) and attenuates brain edema in an experimental model of brain injury (45). In our case, the patient was in treatment with Fulvestrant (an estrogen receptor antagonist) and Palbociclib, (a selective inhibitor of CDK4 and CDK6, which are critical components of the cell-cycle regulatory machinery); the association of these drugs has been reported to reduce magnesium serum levels and induce hypomagnesemia in clinical and preclinical studies in 13-20% of treated patients (46). Our patient was underweight, the chemotherapy was associated to proton pump inhibitor, the latter also a potentially inducer of hypomagnesemia (47). A relation between Fulvestrant/Palbociclib and PRES development has never been described in literature.
Figure 5.

Mechanism of Brain Edema and Blood Brain Barrier Disruption linked to Magnesium depletion
Radiotherapy could have also contributed to PRES development. Our patient had radiotherapy almost six months before PRES development Radiotherapy can cause endothelial damage that lead to disruption of the blood-brain barrier and other late vascular effects, such as telangiectasias, microvascular dilatation, thickening and hyalinization of the vessel wall, microbleeds, as observed in our case. These changes as reported in literature can occur months to years after brain radiotherapy (48).
Conclusion
Our case highlights the particular attention that the clinician must have when cancer patients are treated with new molecular-targeted agents because of the potential PRES development especially when multiple factors combined can facilitate the onset of such a neurological status that represents an oncological emergency despite its rarity. Attention should be paid specifically to check electrolytes levels, especially magnesium levels, whose alterations (especially hypomagnesemia) can concurrently with several types of chemotherapies and radiotherapy lead to this severe but reversible
neurological status impairment. In this case, we cannot exclude that the resolution of the patient neurological status could be attributed only to the anti-edema treatment or to the magnesium salt supplementation but their combination proved to be effective in resolving leading to a full recovery of patient neurological condition. The concomitant presence of thrombosis could have potentially precipitated and complicated the clinical setting, but we can exclude that the subsequent anticoagulant therapy could have improved the patient clinical condition so rapidly, with complete neurological status recovery, because at the brain MRI performed after a month, the thrombosis was still present.
The concomitant presence of several factors inducing PRES should make the clinicians think about all potential multiple causes whose consideration and correction allow the best therapeutic strategy for this very serious neurological complication.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Hinchey J, Chaves C, Appignani B, et al. A Reversible Posterior Leukoencephalopathy syndrome. N Engl J Med. 1996:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 2.Stott V, Hurrell M, Anderson TJ. Reversible posterior leukoencephalopathy syndrome: a misnomer reviewed. Intern Med J. 2005;35(2):83–90. doi: 10.1111/j.1445-5994.2004.00750.x. [DOI] [PubMed] [Google Scholar]
- 3.Siebert E, Bohner G, Liebig T, Endres M, Liman TG. Factors associated with fatal outcome in posterior reversible encephalopathy syndrome: a retrospective analysis of the Berlin PRES study. J Neurol. 2017;264(2):237–242. doi: 10.1007/s00415-016-8328-4. [DOI] [PubMed] [Google Scholar]
- 4.Ay H, Buonanno F, Schaefer P, et al. Posterior leukoencephalopathy without severe hypertension: utility of diffusion-weighted MRI. Neurology. 1998;51(5):1369–1376. doi: 10.1212/wnl.51.5.1369. [DOI] [PubMed] [Google Scholar]
- 5.Mar GR. Encefalopatía reversible posterior en una niña con lupus eritematoso sistémico. Presentación de un caso. Arch Argent Pediatr. 2015;113(5):271–274. doi: 10.5546/aap.2015.e271. [DOI] [PubMed] [Google Scholar]
- 6.Gatla N, Annapureddy N, Sequeira W, Jolly M. Posterior reversible encephalopathy syndrome in systemic lupus erythematosus. J Clin Rheumatol. 2013;19(6):334–340. doi: 10.1097/RHU.0b013e3182a21ffd. [DOI] [PubMed] [Google Scholar]
- 7.Camara-Lemarroy C, Escobedo-Zúñiga N, Villarreal-Garza E, García-Valadez E, Góngora-Rivera F, Villarreal-Velázquez HJ. Posterior reversible leukoencephalopathy syndrome (PRES) associated with severe eclampsia: Clinical and biochemical features. Pregnancy Hypertens. 2017;7:44–49. doi: 10.1016/j.preghy.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Toledano M, Fugate JE. Posterior reversible encephalopathy in the intensive care unit. Handb Clin Neurol. 2017;141:467–483. doi: 10.1016/B978-0-444-63599-0.00026-0. [DOI] [PubMed] [Google Scholar]
- 9.Thompson R, Sharp B, Pothof J, Hamedani A. Posterior Reversible Encephalopathy Syndrome in the Emergency Department: Case Series and Literature Review. West J Emerg Med. 2015;16(1):5–10. doi: 10.5811/westjem.2014.12.24126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maur M, Tomasello C, Frassoldati A, Dieci M, Barbieri E, Conte P. Posterior reversible encephalopathy syndrome during ipilimumab therapy for malignant melanoma. J Clin Oncol. 2012;30(6):76–78. doi: 10.1200/JCO.2011.38.7886. [DOI] [PubMed] [Google Scholar]
- 11.Malkan UY, Gunes G, Demiroglu H, Goker H. Immunosuppression-associated posterior reversible encephalopathy syndrome in an acute leukemia case. Hematol Rep. 2018;10(4):96–97. doi: 10.4081/hr.2018.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yafour N, Krim A, Bouhass R, Bekadja MA. Cyclosporine-related brainstem atypical posterior reversible leukoencephalopathy syndrome following hematopoietic stem cell transplant. Hematol Oncol Stem Cell Ther. 2016;9(1):36–38. doi: 10.1016/j.hemonc.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Sugino M, Tsukahara A, Nakazawa H, Yamamoto N, Arawaka S. Posterior reversible encephalopathy syndrome with extensive cytotoxic edema after blood transfusion: a case report and literature review. BMC Neurol. 2018;18(1):190. doi: 10.1186/s12883-018-1194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chardain A, Mesnage V, Alamowitch S, et al. Posterior reversible encephalopathy syndrome (PRES) and hypomagnesemia: A frequent association. Rev Neurol (Paris) 2016;172:384–388. doi: 10.1016/j.neurol.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Schweitzer A, Parikh N, Askin G, et al. Imaging Characteristics Associated with Clinical Outcomes in Posterior Reversible Encephalopathy Syndrome. Neuroradiology. 2017;59(4):379–386. doi: 10.1007/s00234-017-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartynski WS. Posterior reversible encephalopathy syndrome, Part 2: Controversies surrounding pathophysiology of vasogenic edema. Am J Neuroradiol. 2008;29(6):1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fugate J, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14(9):914–925. doi: 10.1016/S1474-4422(15)00111-8. [DOI] [PubMed] [Google Scholar]
- 18.Largeau B, Le Tilly O, Sautenet B, Salmon Gandonnière C, Barin-Le Guellec C, Ehrmann S. Arginine Vasopressin and Posterior Reversible Encephalopathy Syndrome Pathophysiology: the Missing Link. Mol Neurobiol. 2019 doi: 10.1007/s12035-019-1553-y. [DOI] [PubMed] [Google Scholar]
- 19.Seet R, Rabinstein AA. Clinical features and outcomes of posterior reversible encephalopathy syndrome following bevacizumab treatment. Qjm. 2012;105(1):69–75. doi: 10.1093/qjmed/hcr139. [DOI] [PubMed] [Google Scholar]
- 20.Katada E, Mitsui A, Sasaki S, Uematsu N, Anan C. Posterior Reversible Encephalopathy Syndrome after a Variety of Combined Chemotherapies Containing Bevacizumab for Metastatic Colon Cancer. Intern Med. 2018;57(16):2403–2407. doi: 10.2169/internalmedicine.0284-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eryllmaz MK, Mutlu H, Salim DK, Musri FY, Coşkun HŞ. Fatal posterior revesible leukoencephalopathy syndrome associated coma induced by bevacizumab in metastatic colorectal cancer and review of literature. J Oncol Pharm Pract. 2016;22(6):806–810. doi: 10.1177/1078155215611048. [DOI] [PubMed] [Google Scholar]
- 22.El Maalouf G, Mitry E, Lacout A, Lièvre A, Rougier P. Isolated brainstem involvement in posterior reversible leukoencephalopathy induced by bevacizumab. J Neurol. 2008;255(2):295–296. doi: 10.1007/s00415-008-0692-2. [DOI] [PubMed] [Google Scholar]
- 23.Hamid M, Ghani A, Micaily I, Sarwar U, Lashari B, Malik F. Posterior reversible encephalopathy syndrome (PRES) after bevacizumab therapy for metastatic colorectal cancer. J Community Hosp Intern Med Perspect. 2018;8(3):130–133. doi: 10.1080/20009666.2018.1478563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munoz J, Kumar V, Hamilton J. Posterior Reversible Encephalopathy Syndrome: More Than Meets the Eye. J Clin Oncol. 2013;31(20):360–363. doi: 10.1200/JCO.2012.46.6573. [DOI] [PubMed] [Google Scholar]
- 25.Ozcan C, Wong S, Hari P. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354(9):980–982. [PubMed] [Google Scholar]
- 26.Wu S, Kim C, Baer L, Zhu X. Bevacizumab increases risk for severe proteinuria in cancer patients. J Am Soc Nephrol. 2010;21(8):1381–1389. doi: 10.1681/ASN.2010020167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lafayette RA, McCall B, Li N, et al. Incidence and Relevance of Proteinuria in Bevacizumab-Treated Patients: Pooled Analysis from Randomized Controlled Trials. Am J Nephrol. 2014;40:75–83. doi: 10.1159/000365156. [DOI] [PubMed] [Google Scholar]
- 28.Lazarus M, Amundson S, Belani R. An Association between Bevacizumab and Recurrent Posterior Reversible Encephalopathy Syndrome in a Patient Presenting with Deep Vein Thrombosis: A Case Report and Review of the Literature. Case Rep Oncol Med. 2012;2012:1–4. doi: 10.1155/2012/819546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lio CF, Lee YH, Chan HY, Yu CC, Peng NJ, Chan HP. Posterior reversible encephalopathy syndrome in a postpartum hemorrhagic woman without hypertension. Med (United States) 2017;96(16) doi: 10.1097/MD.0000000000006690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dabla S, Juneja H, Garg A, Bansal R, Kumar S. Cerebral Venous Sinus Thrombosis and Posterior Reversible Encephalopathy Syndrome Coexisting in a Woman: A Rare Coincidence. J assoc Physicians India. 2017;65(4):90–92. [PubMed] [Google Scholar]
- 31.Saran S, Bansal P, Singhal S, Malik A. Coexisting cerebral venous sinus thrombosis and posterior reversible encephalopathy syndrome in a preeclamptic female. Ann Afr Med. 2018;17(2):94–95. doi: 10.4103/aam.aam_41_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Almoussa M, Goertzen A, Brauckmann S, Fauser B, Zimmermann CW. Posterior Reversible Encephalopathy Syndrome due to Hypomagnesemia: A Case Report and Literature Review. Case Rep Med. 2018;2018:1–6. doi: 10.1155/2018/1980638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos AF, Sousa F, Rodrigues M, Ferreira C, Soares-Fernandes J, Maré R. Reversible cerebellar syndrome induced by hypomagnesemia. Neurol Clin Neurosci. 2015;3(5):190–191. [Google Scholar]
- 34.Blasco LM. Cerebellar Syndrome in Chronic Cyclic Magnesium Depletion. The Cerebellum. 2013;12(4):587–588. doi: 10.1007/s12311-012-0431-1. [DOI] [PubMed] [Google Scholar]
- 35.Laurant P, Touyz RM. Physiological and pathophysiological role of magnesium in the cardiovascular system: implications in hypertension. J Hypertens. 2000;18(9):1177–1191. doi: 10.1097/00004872-200018090-00003. [DOI] [PubMed] [Google Scholar]
- 36.Grubbs RD, Maguire ME. Magnesium as a regulatory cation: criteria and evaluation. Magnesium. 1987;6(3):113–127. [PubMed] [Google Scholar]
- 37.Ebel H, Günther T. Magnesium metabolism: a review. J Clin Chem Clin Biochem. 1980;18(5):257–270. doi: 10.1515/cclm.1980.18.5.257. [DOI] [PubMed] [Google Scholar]
- 38.Bara M, Guiet-Bara A. Potassium, magnesium and membranes. Review of present status and new findings. Magnesium. 1984;3(4-6):215–225. [PubMed] [Google Scholar]
- 39.Altura B, Gebrewold A, Zhang A, Altura B. Low extracellular magnesium ions induce lipid peroxidation and activation of nuclear factor-kappa B in canine cerebral vascular smooth muscle: possible relation to traumatic brain injury and strokes. Neurosci Lett. 2003;341(3):189–192. doi: 10.1016/s0304-3940(03)00134-4. [DOI] [PubMed] [Google Scholar]
- 40.Billard JM. Ageing, hippocampal synaptic activity and magnesium. Magnes Res. 2006;19(3):199–215. [PubMed] [Google Scholar]
- 41.Rayssiguier Y, Libako P, Nowacki W, Rock E. Magnesium deficiency and metabolic syndrome: stress and inflammation may reflect calcium activation. Magnes Res. 2010;23(2):73–80. doi: 10.1684/mrh.2010.0208. [DOI] [PubMed] [Google Scholar]
- 42.Amiry-Moghaddam M, Otsuka T, Hurn P, et al. An alfa-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci. 2003;100(4):2106–2111. doi: 10.1073/pnas.0437946100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zador Z, Stiver S, Wang V, Manley GT. Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol. 2009;190:159–170. doi: 10.1007/978-3-540-79885-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghabriel M, Thomas A, Vink R. Magnesium restores altered aquaporin-4 immunoreactivity following traumatic brain injury to a pre-injury state. Acta Neurochir Suppl. 2006;96:402–406. doi: 10.1007/3-211-30714-1_83. [DOI] [PubMed] [Google Scholar]
- 45.Okiyama K, Smith D, Gennarelli T, Simon R, Leach M, McIntosh TK. The sodium channel blocker and glutamate release inhibitor BW1003C87 and magnesium attenuate regional cerebral edema following experimental brain injury in the rat. J Neurochem. 1995;2:802–809. doi: 10.1046/j.1471-4159.1995.64020802.x. [DOI] [PubMed] [Google Scholar]
- 46.Palbociclib (PD-0332991), Investigator’s Brochure February, 2015 [Google Scholar]
- 47.Regolisti G, Cabassi A, Parenti E, Maggiore U, Fiaccadori E. Severe Hypomagnesemia During Long-term Treatment With a Proton Pump Inhibitor. Am J Kidney Dis. 2010;56(1):168–174. doi: 10.1053/j.ajkd.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 48.Roongpiboonsopit D, Kuijf HJ, Charidimou A, et al. Evolution of cerebral microbleeds after cranial irradiation in medulloblastoma patients. Neurology. 2017;88(8):789–796. doi: 10.1212/WNL.0000000000003631. [DOI] [PMC free article] [PubMed] [Google Scholar]


