Abstract
The Coronavirus disease 19 (COVID-19) outbreak has been recognized as a global threat to public health. It is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and no effective therapies currently exist against this novel viral agent. Along with extensive public health measures, an unprecedented global effort in identifying effective drugs for the treatment is being implemented. Potential drug targets are emerging as the result of a fast-evolving understanding of SARS-CoV-2 virology, host response to the infection, and clinical course of the disease. This brief review focuses on the latest and most promising pharmacological treatments against COVID-19 currently under investigation and discuss their potential use based on either documented efficacy in similar viral infections, or their activity against inflammatory syndromes. Ongoing clinical trials are also emphasized. (www.actabiomedica.it)
Keywords: COVID-19, SARS-CoV-2, drugs, therapy, antivirals, inflammation
Introduction and aim
Human coronaviruses (HCoVs) have traditionally received relatively little attention due to their mild phenotypes in humans, and are considered inconsequential pathogens causing approximately 15% of “common cold” in healthy people (1,2). However, in less than two decades, we have already encountered two highly pathogenic and large-scale epidemic HCoVs, namely SARS-CoV in 2003 and MERS-CoV in 2012 (3), with a case fatality rate of 9.5 and 35%, respectively (4). In late December of 2019, the scientific community has been informed of an emerging outbreak caused by a novel pathogen from a viral family, formerly thought to be relatively benign, but later recognized as global threat to public health and named COVID-19 by World Health Organization (WHO). In less than two months we rapidly learned that COVID-19 infection is not like the seasonal flu, with one in five patients presenting with a severe infection and requiring oxygen or even mechanical ventilation (5).
As of April 25th, 2.686.785 laboratory-confirmed cases of COVID-19 were reported involving 213 countries and resulting in more than 184.681 confirmed deaths worldwide (6). As pauci-symptomatic (and even asymptomatic) people may be fueling the coronavirus spread, COVID-19 burden is likely underestimated, thus further complicating a careful monitoring of the natural course of the disease, the rate of its spreading, as well as the development of effective epidemic prevention strategies (7).
While witnessing a rapidly increasing number of new cases, a greater understanding of the etiology and pathogenesis of this previously unknown illness has been progressively gained, from viral gene sequencing to elucidation of transmission route, from virulence mechanisms to the investigation of the wide variability of clinical spectrum observed in routine practice. Along with extensive public health measures promoting social distancing and reducing the massive strain on the healthcare infrastructure, global efforts are currently directed to the development of a vaccine and therapeutic agents while optimizing marketed medications, mainly employed in severe respiratory viral infections. Nevertheless, no effective prophylactic or post-exposure therapies are currently available. Here, we briefly review the latest and most promising pharmacological treatments against COVID-19 currently under investigation and discuss their potential use by virtue of either documented efficacy in similar viral infections, or their activity against the so-called “cytokine storm” or the newly defined sepsis-induced-coagulopathy (8). Figure 1 illustrates the main pharmacological strategies proposed so far.
Figure 1.
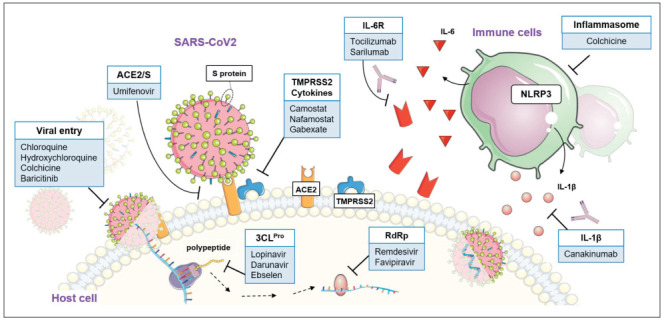
Graphic summary of the emerging drug targets for the treatment of COVID-19. The pharmacological targets (white text boxes) and the relative proposed drugs (light blue text boxes) are noted. Abbreviations: ACE2, angiotensin converting enzyme 2; TMPRSS2, type 2 transmembrane serine protease; S protein, spike protein; 3CLPro, 3-chymotripsin like protease; RdRp, RNA dependent RNA polymerase; IL-6, interleukin 6; IL-6R, interleukin 6 receptor; IL-1β, interleukin 1 beta; NLRP3, nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3. Images from Smart Servier Medical Art (https://smart.servier.com/) have been combined for creating the illustration. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
COVID-19 disease and therapeutic strategies
A fast-evolving understanding of how the SARS-CoV-2 targets cells around the body and a mounting clinical evidence of the COVID-19 disease course in hospitalized patients have been providing potential targets for drug therapy (9-11). While we are just beginning to probe the degree of lung damage, clinical reports suggest that other organs can also be injured, including heart, liver, kidney and the lower gastrointestinal tract. Emerging evidence suggests that COVID-19 may demand a multi-target approach thus involving viral entry and lifecycle, host response or even both.
SARS-CoV-2 enters host cells by taking advantage from the binding of the viral spike (S) protein to host cell angiotensin-converting enzyme 2 (ACE2) receptor and the type 2 transmembrane serine protease (TMPRSS2). Overall, coronavirus entry into susceptible cells requires the concerted action of receptor-binding and proteolytic processing of the S protein to promote virus-cell fusion (12-14). Of note, this route of entry was found being 100- to 1000-fold more efficient than the endosomal pathway and the availability of proteases in the extracellular milieu being a key factor of tropism (15). Drugs preventing viral entry by inhibiting TMPRSS2 (i.e. camostat mesylate), targeting S protein/ACE2 interaction (i.e. umifenovir), inhibiting viral entry and endocytosis (i.e. cloroquine/hydroxychloroquine) may all stand as potential therapies along with drugs targeting viral polyprotein synthesis (i.e. lopinavir, remdesivir, etc). To date, such strategies seem to hold the greatest promise when applied early in the course of the illness, but their usefulness in advanced stages is still quite uncertain (16, 17) (Fig. 1).
The effectual host immune response, including innate and adaptive immunity against SARS-CoV-2, may also serve as a therapeutic avenue with inhibition of IL-6 signaling playing a protective role if given at the time of overly elevated immune response to the virus. In this context, it has been speculated that drugs targeting immune regulation pathway (i.e. tocilizumab, sarilumab) may control the extreme cytokine reaction (termed “cytokine storm”) that is accompanied by infiltration of inflammatory monocytes/macrophages into the lung without deleterious effects on virus replication (5,18). A stage classification system has been recently proposed to improve the therapeutic strategies employed in COVID-19 patients so far, by distinguishing the phase where the viral pathogenicity dominates versus when the host inflammatory response overtakes the pathology (18). Such approach may be helpful to better correlate stage of illness severity with response to therapy and clinical outcomes, and to weight the benefit/risk ratio for the currently investigated pharmacological treatments.
The clinical spectrum of COVID-19 is very wide, ranging from unspecific symptoms (i.e. fever, dry cough), sometimes combined with mild pneumonia and dyspnea and very often associated with olfactory and gustative disorders, to severe pneumonia and altered gas exchange, resulting in approximately 5% of infected patients with severe lung dysfunction, or multiple (extra pulmonary) organ failure (19,20). While we are gaining better knowledge of the clinical features of the disease, a mounting evidence is disclosing risk factors and biochemical parameters associated with poor prognosis that could serve as therapeutic targets. Haemostatic abnormalities, including disseminated intravascular coagulation (DIC), occur in patients affected by COVID-19 along with increased levels of D-dimer. Additionally, the severe inflammatory response, critical illness, and underlying traditional risk factors may all predispose to thrombotic events (21). As a result, one of the most significant poor prognostic features in those patients is the development of coagulopathy. Of note, platelet activation is also likely to be contributing to the hypercoagulability, but the extent to which it does, and which signaling pathways are involved are a matter of debate. In this still evolving clinical scenario, the use of heparins has been investigated by virtue of their anticoagulant activity, protective action in the vascular endothelium and pleiotropic properties (8).
Overall, based on the aforementioned premises, a rising number of trials are currently authorized worldwide (902 as of April 26th) (22), thirteen of which are in Italy (23).
Antiviral drugs
Lopinavir/Ritonavir
Lopinavir/ritonavir is an orally administered, fixed dose combination approved by EMA and FDA for the treatment of HIV infections. Both the antiretrovirals are protease inhibitors, although lopinavir is the active antiviral moiety. Lopinavir inhibits the proteases of the human immunodeficiency virus type 1 and 2 (HIV-1 and HIV-2), thus preventing the cleavage of the gag-pol polyprotein, and producing an immature virus, which is not infectious. Therefore, the antiviral effect mediated by lopinavir is mainly attributable to its ability to prevent the infection of susceptible cells. However, lopinavir has low bioavailability due to a high first-pass effect mediated by the cytochrome P450 3A4 (CYP3A4) in the liver. By inhibiting the CYP3A4 isoenzyme, ritonavir acts as a pharmacokinetic “booster”, reducing the metabolism of lopinavir and increasing its plasma half-life (24). Currently, the lopinavir/ritonavir combination is used for the treatment of adult patients, adolescents and children (aged from 14 days and older) with HIV-1 infection, often associated to other antiviral drugs (25).
Several scientific evidences argued for the off-label use of lopinavir/ritonavir in patients with SARS-CoV-2 infection. In particular, in vitro and in vivo studies have shown that lopinavir may inactivate 3-chymotrypsin-like protease (3CLpro, also known as Main protease, Mpro), which represents a key target for human coronavirus replication (i.e. SARS-CoV and MERS-CoV) (26,27). Very recently, one in vitro study showed that lopinavir inhibits also SARS-CoV-2 replication, with a half-maximal effective concentration (EC50) at 26.63 μM (28).
Studies conducted both on patients and on animal models suggested that the lopinavir/ritonavir combination was able to improve the clinical and symptomatic course of respiratory syndromes caused by SARS-CoV or MERS-CoV (29-31). A clinical trial evaluating lopinavir/ritonavir potential in SARS showed some benefits, such as reduced intubation rates and mortality, particularly if treatment was started early, i.e. within the first 7-10 days of exposure (32). However, the observational retrospective nature of the study prevents for conclusive recommendations.
In February 2020, short guidelines issued by the Zhongnan hospital of Wuhan University for the diagnosis and treatment of SARS-COV-2 pneumonia were published, which recommend the use of lopinavir/ritonavir in the initial stages of disease to reduce mortality and glucocorticoid consumption based on results from a brief systematic review of the literature (33). More recently, an open-label randomized controlled trial (LOTUS China - Lopinavir Trial for Suppression of SARS-CoV-2 in China) evaluated efficacy and safety of lopinavir/ritonavir versus standard care on 199 hospitalized adult subjects with COVID-19 pneumonia (17). In the study, 99 subjects were treated with lopinavir/ritonavir (400 mg/100 mg twice daily for up to 14 days) in addition to standard therapy and 100 patients with only standard therapy. The results showed no statistically significant difference in terms of clinical improvement (hazard ratio [HR] for symptom improvement, 1.31; 95% confidence interval [CI], 0.95-1.80), viral clearance and 28-day mortality (19.2 % vs. 25.0%; absolute difference -5.8% in favor of the antiviral combination did not reach statistical significance; 95% CI, -17.3%-5.7%). Although the delayed treatment start (median time from symptom onset to randomization, 13 days, interquartile range [IQR] 11-16) may partially accounts for the ineffectiveness of treatment, a post hoc analysis by subgroups showed no benefit in patients treated within 12 days from the onset of symptoms (HR, 1.25; 95% CI, 0.77-2.05). Moreover, 13 patients (~14%) discontinued therapy early because of gastrointestinal adverse events. The safety profile of lopinavir/ritonavir is of particular concern in SARS-CoV-2 infected patients. Common lopinavir/ritonavir adverse effects include nausea, diarrhea (up to ~30%), and hepatotoxicity (2%-10%), and these conditions may be exacerbated in COVID-19 patients, since 20% to 30% of them present with transaminases elevation (34). Other detrimental factors may be drug-drug interactions (i.e. co-administration of CYP3A4 substrates/inducers) and potential severe adverse drug reactions (pancreatitis, liver damage, cardiac conduction abnormalities) (25).
Although several RCTs are ongoing, the current evidence suggests poor benefit from lopinavir/ritonavir therapy. Treatment should be limited to COVID-19 patients of lesser severity and applied in the initial stages of the disease.
In addition, the darunavir/cobicistat combination (i.e., protease inhibitor/CYP3A4 inhibitor) is being also used as an alternative to lopinavir/ritonavir because of its greater intestinal tolerability (35). An in vitro study showed also activity of darunavir against SARS-CoV-2 (36). In COVID-19 patients, it is administered as one tablet (darunavir 800 mg/cobicistat 150 mg) daily for 5 days in combination with standard treatments. A phase 3, randomized, open label clinical trial evaluating efficacy and safety of darunavir/cobicistat for the treatment of pneumonia caused by SARS-CoV-2 is ongoing, with an estimated primary completion date at 31 August 2020 (37).
Remdesivir
Remdesivir (GS-5734) is a monophosphoramidate pro-drug of the nucleoside analogue of adenine GS-441524. Inside the cells, GS-441524 is converted into the pharmacologically active form of triphosphate (GS-443902), which in turn is able to inhibit the viral RNA-dependent RNA polymerase (RdRp). Emerged during the screening of agents against RNA viruses, remdesivir proved promising against Ebola infections, inhibiting RdRp with a low EC50 and with a high selectivity towards the viral enzyme (38). Remdesivir has shown broad-spectrum antiviral activity with good in vitro and in vivo efficacy on animal models against various RNA viruses, such as SARS-CoV and MERS-CoV, including SARS-CoV-2 (EC50: 0.77 μM; EC90: 1.76 μM) (16,39,40). Hence, several in vitro studies point to remdesivir as a promising candidate for the treatment of respiratory disease COVID-19, particularly in association with chloroquine (16). Remdesivir is currently not approved for marketing in any country for any indication, and must be obtained via compassionate use or enrollment in a clinical trial. As recommended for compassionate use, treatment may begin following a worsening of the patient’s clinical status. A phase 1 clinical trial, evaluating safety and pharmacokinetics of single- and multiple-dose regimen, found that intravenous infusions of remdesivir between 3 and 250 mg were well tolerated, with no signs of liver or kidney damage (41). In this dose range, kinetics was linear, showing intracellular half-life of more than 35 hours. On a multiple-dosing regimen the treatment induces a reversible transaminase elevation.
Firstly used in clinic for the treatment of Ebola virus disease (42), remdesivir has been successfully used for treating COVID-19, as reported by two recent case reports (43,44).
Treatment schedule consists of a 200-mg loading dose on day 1, followed by 100-mg daily infusions (1 hour) for 5 or 10 days, depending on the active protocol. Several ongoing studies are evaluating remdesivir efficacy and safety in COVID-19, including two randomized open-label phase 3 trials promoted by Gilead Sciences, enrolling patients with moderate (NCT04292730, EudraCT 2020-000842-32) (45) or severe (NCT04292899, EudraCT 2020-000841-15) (46) disease. These trials are also active in Italy, where the Italian Medicines Agency (AIFA) cooperates in identifying centers with the largest number of hospitalized patients (note: “Azienda Ospedaliero-Universitaria di Parma”, Italy, is actively contributing to both studies) (47). The potential of remdesivir in COVID-19 is tested in Italy also in the context of the WHO-promoted SOLIDARITY mega-trial (32 centers involved) (48) and several compassionate use programs (49). Other clinical trials are currently testing remdesivir worldwide, such as DisCoVeRy - an open-label randomized study based on a WHO adaptive protocol to compare various treatments in hospitalized patients, promoted by the French INSERM (NCT04315948 (50), EudraCT 2020-000936-23); an international double-blind placebo-controlled study sponsored by the U.S. National Institute of Allergic and Infectious Diseases (NIAID), conducted in USA, South Korea and Singapore (NCT04280705) (51). Two randomized double-blind placebo-controlled studies in China, on patients with mild/moderate (NCT04252664) (52) and severe (NCT04257656) (53) COVID-19, are currently suspended and terminated, respectively.
Very recently, a study evaluated clinical outcomes in a cohort of 53 patients hospitalized for severe COVID-19 in USA, Europe, Canada and Japan, treated with intravenous remdesivir for compassionate use (54). Median symptom duration before receiving the treatment was 12 days (IQR, 9 to 15), 30 patients (57%) receiving mechanical ventilation and 4 (8%) being on extracorporeal membrane oxygenation (ECMO). During a median follow-up of 18 days, the need for oxygen support improved in 36 (68%) patients; 17 of 30 patient receiving mechanical ventilation were extubated; 26 patients (47%) were discharged; 7 patients died (overall mortality, 13%; 18% in those receiving mechanical ventilation; 5% in those receiving noninvasive ventilation or oxygen supplementation). The study suggests substantial benefit from remdesivir treatment in people with severe COVID-19. However, the study lacks a comparison group, thus preventing definitive conclusions and pointing to RCT results for further recommendations.
Chloroquine and hydroxychloroquine
The therapeutic use of chloroquine (CQ) and hydroxychloroquine (HCQ) dates back to the mid-twentieth century. These two drugs are usually indicated in the treatment of both malaria and some autoimmune diseases, including systemic lupus erythematosus and rheumatoid arthritis (55,56). After oral intake, both drugs are well absorbed in the gastrointestinal tract. They are metabolized by hepatic deamination with elimination half-lives that can reach 45 days and are mainly excreted via the kidneys.
These two drugs have almost identical mechanisms of action. Their pharmacological effects include stabilization of lysosomal membranes, inhibition of polymorphonuclear chemotaxis and phagocytosis, interference with pro-inflammatory cytokine production (e.g., interleukin 1 beta) by monocytes and inhibition of the release of superoxide by neutrophils (56).
CQ and HCQ showed antiviral effects against SARS-CoV-2 infection models in vitro. These agents appear to block viral entry into the cells through endosomal acidification, which mediates the virus-host cell fusion, proteolytic processing and inhibition of host receptor glycosylation (57,58). In addition, CQ has been reported to block SARS-CoV-2 replication at concentrations that can be achieved after a 600-mg oral loading dose in humans (16). In vitro, CQ and HCQ exert their antiviral activity against SARS-CoV-2 with EC50 in the low micromolar range, although HCQ shows higher potency (EC50: 6.14 μM [HCQ] vs 23.90 μM [CQ]) (59). Moreover, the immunomodulating activity exerted by these drugs may synergistically enhance their antiviral action in vivo.
Optimal dosing for CQ and HCQ in COVID-19 has not been univocally defined. Some evidences reported 500 mg orally once or twice daily on 5-10 days for CQ (60). As for HCQ, pharmacokinetic simulations recommended an oral loading dose of 400 mg twice daily on day 1, then 200 mg twice daily in the next 2-5 days (59).
Noteworthy, the use of CQ or HCQ in COVID-19 patients is not yet supported by high-quality scientific evidences. A brief letter from China reported that CQ is superior to control in improving the disease course in more than 100 patients with COVID-19 pneumonia (61). Although this could be the first evidence of clinical benefits from CQ treatment in such context, trial design and outcome data are still lacking, thus preventing any further inference. Two small clinical trials studied the therapeutic potential of HCQ in COVID-19 patients. A Chinese study of 30 patients on standard care (supportive care, antivirals), randomly (1:1) assigned to HCQ 400 mg daily, for 5 days, or standard care only, reported no improvement in viral clearance (93.3% vs 86.7%, respectively; p>0.05) (62). On the other hand, a French study on 36 patients (20 on treatment, 16 on control arm) assigned to HCQ 200 mg every 8 hours or to standard supportive care reported higher viral clearance in HCQ group (14/20, 70%) compared to control group (2/16, 12.5%). Moreover, authors claimed further improvement by adding azithromycin to HCQ vs HCQ alone (6/6, 100% vs 8/14, 57%, respectively).
Data from these studies should be interpreted with care since they present major limitations, such as small sample size and poor study design. In particular, the French study did not control for patient dropout (early cessation due to critical illness and/or toxicity) and baseline viral load. Clinical or safety outcomes were also not reported. A recent retrospective analysis of data from 368 patients hospitalized with confirmed SARS-CoV-2 infection in USA found no evidence that using HCQ in combination with or without azithromycin reduced the risk of mechanical ventilation COVID-19 patients (63). On the contrary, they found association of increased overall mortality in patients treated with HCQ alone.
These findings highlight the importance of awaiting the results of ongoing prospective RCTs (64,65) before recommending a widespread use of these drugs. Although CQ and HCQ are in general well tolerated, their use should be carefully monitored in some patient subsets. Serious adverse effects may occur, such as hemolysis (in patients with glucose 6-phosphate dehydrogenase deficiency), retinal toxicity, hearing reduction, neuropsychiatric symptoms, agranulocytosis, hypoglycaemia, hypotension and prolongation of the QT tract (66). For this reason, CQ or HCQ should not be administered in patients receiving concomitant QT-interval prolonging agents (e.g. amiodarone, azithromycin or other macrolides and fluoroquinolones). Compared to CQ, HCQ seems to determine a slightly milder toxicity profile in animal models and in humans (67,68).
Other antivirals
Several antivirals have been used since the COVID-19 outbreak in China and worldwide. However, most of them, including oseltamivir and ribavirin, have shown limited value or futility (10).
Umifenovir (also known by its trade name Arbidol) is a more promising antiviral agent, approved in Russia and China for the treatment and prophylaxis of influenza at the dose of 200 mg orally every 8 hours. In addition, umifenovir showed in vitro activity against SARS-CoV (69). Its mechanism of action is peculiar, since the drug seems to block the interaction between S protein and ACE2 receptor, thus preventing the fusion of viral envelope with cell membrane (70). Currently 8 clinical trials are studying umifenovir in COVID-19 patients (71), in combination with other therapeutics or in monotherapy (72). At moment, clinical evidence in COVID-19 is limited to observational data, though suggesting lower mortality and higher discharge rate in umifenovir-treated patients (73).
Favipiravir is an antiviral drug, approved in Japan (trade name, Avigan) for the treatment of influenza from new or re-emerging viral strains which do not respond to conventional antiviral therapies (74). Many countries have used it off-label to treat new viral infections, including Ebola and Lassa. More recently, it was approved in China for the treatment of COVID-19. Favipiravir is a prodrug analogue of a purine nucleotide, favipiravir ribofuranosyl-5’-triphosphate (favipiravir-RTP). The active metabolite is recognized as a substrate, which selectively inhibits the RNA-dependent RNA polymerase (RdRp) of RNA viruses, halting viral replication. The catalytic domain of RdRp is preserved in various types of RNA viruses, which supports the wider antiviral spectrum of favipiravir. In addition, the drug has been shown inducing a high rate of RNA mutations, thus generating a non-viable viral phenotype (75,76). The in vitro EC50 estimates of favipiravir showed much higher potency against influenza, compared to Ebola and SARS-CoV-2 (77,78). Accordingly, dosing regimens in vivo have been modified in COVID-19 studies, in which oral loading doses of 1800 mg twice daily on day 1, followed by 800 mg twice daily in the next 2-5 days have shown higher antiviral potency (79,80). After oral dosing, favipiravir showed almost complete bioavailability (97,6%) but complex nonlinear pharmacokinetics, which is also affected by differences in body weight and ethnicity (81). The elimination half-life is approximately 2-5 hours; however, lower than expected plasma levels have been observed in patients with Ebola or severe flu, raising concerns about bioavailability and/or metabolism alterations in seriously ill patients (82,83). The drug is overall well tolerated, with adverse reactions, such as hyperuricemia, diarrhea, elevated transaminases and neutropenia, occurring in a dose-dependent manner (81,84).
To date, limited clinical experience supports the use of favipiravir for COVID-19. A prospective, randomized, multicenter study compared favipiravir with umifenovir (120 patients in each arm) for treating moderate to severe COVID-19 infection (85). Favipiravir improved clinical recovery at day 7 in patients with moderate infections (71.4% favipiravir vs 55.9% umifenovir, p=0.0199). No significant differences were observed by comparing treatments in severe or severe-moderate (combined analysis) arms.
Recently, preliminary results from an open-label, controlled, non-randomized clinical trial on 80 patients with laboratory-confirmed COVID-19 and with the onset of symptoms in the 7 days prior to enrollment in the study have been published (86). Favipiravir showed a higher antiviral activity than lopinavir/ritonavir, in terms of virus elimination and rate of improvement of thoracic CT imaging. However, these results must be interpreted with caution considering the preliminary nature of the study, which has methodological limitations (open-label study, no randomization, in both groups patients took interferon alfa-1b by aerosol, exclusion of severely ill patients).
Several clinical studies testing efficacy and safety of favipiravir in COVID-19 are ongoing (87), including a multi-center, randomized, double-blind, placebo-controlled study coordinated in Italy at the “ASST Fatebenefratelli Sacco”, Milan (NCT04336904) (88).
Other investigational compounds, such as DAS181 (Fludase), have been considered promising based on their mechanism of action and preclinical data, showing broad spectrum antiviral activity (89-91). DAS181 is a novel sialidase fusion protein which enzymatically cleaves viral receptors on the host epithelial cell, preventing the binding of influenza, parainfluenza, and other viruses (91). No clinical evidence has been reported so far, but at least four clinical trials testing its potential against COVID-19 are underway (92) (NCT04324489; NCT04298060; NCT03808922; NCT04354389 - this is a multicenter, randomized, placebo-controlled, double-blind study coordinated in Italy by “Fondazione IRCCS Ca’ Granda - Ospedale Maggiore Policlinico Milano” and “Azienda Ospedaliero-Universitaria Policlinico di Modena” with estimated primary completion by June 2020 (93)).
Very recently, more than ten thousand pharmacologically active compounds, including many approved drugs, were screened as inhibitors of the SARS-CoV-2 main protease (3CLpro or Mpro) (94). Out of six agents selected, ebselen (also known as PZ-51 or DR-3305) exhibited higher potency (half-maximal inhibitory concentration: 0.67 µM) and promising antiviral activity in cell-based in vitro assays. This is of interest because ebselen has already been tested in clinical setting because of its antioxidant or lithium-mimetic properties (95). Further studies are needed to test its in vivo efficacy against SARS-CoV-2 infection.
Drugs targeting inflammation and hypercoagulability
Great expectations are currently placed on the use of drugs selectively blocking the inflammatory response triggered by SARS-CoV-2 and the associated thromboembolism. Moreover, other agents affecting crucial signaling pathways in host cells have been proposed. Since these drugs could potentially modify the course of the disease even in patients with severe infections, the topic is of great interest. However, no high-quality evidence exists and no general recommendations for their use can be made yet.
Some repurposed and investigational drugs are reviewed here below.
Tocilizumab
Interleukin 6 (IL-6) is a cytokine relevant to many inflammatory and metabolic processes in the body (96). High levels of IL-6 are implicated in the pathogenesis of various inflammatory and autoimmune disorders, including many forms of rheumatic diseases. IL-6 is also involved in the cytokine release syndrome (CRS), a systemic inflammatory response triggered by several factors such as infections and certain drugs, also known as “cytokine storm” (96,97). In CRS, plasma and tissue levels of various pro-inflammatory cytokines (e.g. TNF-α, IL-1, IL-6) and chemokines (e.g., IL-8) increase, producing long-term damage and fibrosis of the lung tissue, clotting disorders and multi-organ dysfunction.
Tocilizumab (trade name RoActemra) is a humanized monoclonal antibody that blocks the interleukin 6 receptor, thus inhibiting the downstream inflammatory cascade. Tocilizumab is indicated for the treatment of severe rheumatoid arthritis, systemic juvenile idiopathic arthritis, juvenile idiopathic polyarthritis and for the treatment of chimeric antigen receptor T-cell (CAR-T) induced CRS (98) in adults and pediatric patients (aged 2 years or older).
The rationale for its off-label use in patients with severe COVID-19 is based on the evidence that SARS-CoV-2 infection induces an excessive and aberrant host immune response (99). This response may further evolve toward an acute respiratory distress syndrome and, in most critically ill patients, triggering a “cytokine storm” (100). By analogy with the treatment of CAR-T associated CRS, tocilizumab has been used in small groups of severe COVID-19 cases, with early reports of success. A Chinese report of 21 patients with severe or critical COVID-19 pneumonia showed that treatment with tocilizumab 400 mg IV reduced oxygen demand in 15/20 (75%) patients, induced resolution of lung injury as reported by CT scan in 19/21 (91%) patients, normalization of lymphocyte count (10/19), reduction of PCR levels (16/19) and hospital discharge (19/21), with an average hospitalization of 13.5 days (101). However, the small sample size and the lack of a comparison group prevent any firm conclusion about the drug’s specific effects.
By the end of April 2020, more than twenty-five RCTs are testing the efficacy of tocilizumab, alone or in combination, in COVID-19 patients with severe disease are ongoing (102) (e.g., three studies authorized by AIFA in Italy (23): NCT04317092 – a study coordinated by “Istituto Nazionale Tumori, IRCSS, Fondazione G. Pascale”, Naples (103); NCT04346355 – another study involving also the “Azienda Ospedaliero-Universitaria di Parma” (104); NCT04320615 – a multicenter study promoted by F. Hoffmann-La Roche Ltd (105)). Treatment consists of one single intravenous infusion of tocilizumab, at the dose of 8 mg/kg, up to a maximum dose of 800 mg. Primary completion of these studies is expected between May and December of 2020.
The safety profile of tocilizumab should be monitored, since peculiar toxicity may occur. As an immunosuppressive drug, there is a risk of reactivating latent infections (i.e., mycobacteria). Other adverse reactions include neutropenia and thrombocytopenia, hepatotoxicity and severe hypersensitivity reactions. It is known that IL-6 reduces the expression and activity of specific cytochrome P450 (CYP) enzymes, particularly CYP3A4. Therefore, caution should be used for potential interactions, as tocilizumab can reverse the inhibitory effect of IL-6 and restore the CYP3A4 activity, modifying the exposure and activity of CYP3A4 substrate drugs (106,107).
Sarilumab
Sarilumab (trade name Kevzara) is a fully human monoclonal antibody that binds specifically to both soluble and membrane-bound IL-6 receptors, inhibiting IL-6 mediated signaling. Hence, it shares its mechanism of action, most of its therapeutic indications, and toxicity, with tocilizumab. Currently, high (400 mg IV) or low (200 mg IV) dosing protocols are under investigation. At least ten studies are evaluating efficacy and safety of sarilumab in monotherapy or in combination in COVID-19 patients (108) (e.g., NCT04327388 – a multicenter study promoted by Sanofi/Regeneron Pharmaceuticals, authorized in Italy by AIFA and involving the “Azienda Ospedaliero-Universitaria di Parma” (109)).
Anakinra and Epamalumab
Anakinra (trade name Kineret) is a biological, recombinant analogue of the human interleukin-1 type I receptor (IL-1RI) antagonist (IL-1RA). In particular, the drug inhibits the biological response to IL-1α and IL-1β, two important pro-inflammatory cytokines (110). In European Union (EU) countries, it is authorized for the treatment of rheumatoid arthritis (RA), Cryopyrin-Associated Periodic Syndromes (CAPS) and Still’s Disease, in monotherapy or in combination with other anti-inflammatory and disease-modifying drugs (111).
Emapalumab (trade name Gamifant) is a fully human monoclonal antibody, which binds and neutralizes interferon-γ (IFN-γ) (112,113). This cytokine is critical for innate and adaptive immunity against viral and other infections, exerting important immunomodulatory effects. Moreover, aberrant IFNγ expression has been found in some autoinflammatory syndromes and autoimmune diseases (114,115). In November 2018, emapalumab received the global approval for the treatment of pediatric and adult patients with primary hemophagocytic lymphohistiocytosis (HLH) with refractory, recurrent, or progressive disease or intolerance to HLH therapy (112,113). In EU, the drug received orphan designation and PRIority MEdicine (PRIME) status by the European Medicines Agency (EMA) (116).
The rationale for the off-label use of anakinra or emapalumab in the treatment of COVID-19 patients lies in their ability of counteracting the hyper-inflammatory response triggered at the lung level and the “cytokine storm” (see above). At the moment there is no information on these alleged positive effects, but the great interest is attested by the clinical studies launched worldwide. By the end of April 2020, at least seven phase 2 or 3 RCTs have been registered for anakinra (117), and one study evaluating the efficacy and safety of both anakinra and emapalumab versus standard of care (118). The latter is an open label, controlled, parallel group, 3-arm, multicenter study coordinated in Italy and promoted by Swedish Orphan Biovitrum (SOBI) company (NCT04324021 – authorized by AIFA (23), it also involves “Azienda Ospedaliero-Universitaria di Parma” as a research center (118)). Anakinra and epamalumab are administered according to a complex dosing scheme, consisting of intravenous infusions over several days and at different dosages. Importantly, all patients being treated with emapalumab receive also methylprednisolone, as recommended. The primary completion date is estimated on July 2020.
Colchicine
Colchicine is an old drug, initially extracted from the autumn crocus (Colchicum autumnale) and used for centuries in counteracting various inflammatory diseases, though its mechanism of action became clearer in the last decades. Colchicine disrupts microtubule dynamics, both by inhibiting tubulin polymerization and by fostering its depolymerization (119,120). The block of microtubules affects many cell pathways involved in the inflammation machinery, thus impairing the expression of cell adhesion molecules and the secretion of several cytokines and chemokines (121,122). In particular, through this mechanism colchicine counteracts the assembly of the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome, thereby reducing the intracellular processing and the release of IL-1β and other interleukins, including IL-6 (123). Moreover, it is known that colchicine concentrates in neutrophils and macrophages, prolonging its own action. Beyond its anti-inflammatory and immunomodulating activities, colchicine showed also some antiviral properties. By damaging microtubules, the drug reduced the cell entry and the intracellular trafficking of viral particles (124,125). Interestingly, microtubules seems to play a role also in the replication of SARS-CoV, by favouring the formation of double-membrane vesicles in infected cells (126). Noteworthy, other studies showed that the envelope protein E (viroporin E) of SARS-CoV, as well as the SARS-CoV accessory protein open reading frames (SARS 3a) alter ionic equilibrium in intracellular compartments and activate NLRP3 (127,128). However, whether SARS-CoV-2 may replicate the same mechanisms is still unknown.
Colchicine is currently used in the treatment of gout, pericarditis, Familiar Mediterranean Fever and Behçet’s disease (122). Its ability in counteracting the “cytokine storm” lays the ground for its use also in COVID-19 patients. The specific inhibition of NLRP3 exerted by colchicine further supports its use in this context. Colchicine is a well-known, inexpensive, well tolerated drug, with a long-standing use in clinic and this could be an advantage over newer immunomodulating drugs.
These premises prompted researchers and clinicians to test efficacy and safety of colchicine in SARS-CoV-2 infected patients. At least seven studies have been registered at the moment, including two Italian studies authorized by AIFA (EudraCT 2020-001475-33 (129) – an interventional, pilot, multicenter, randomized, open-label, phase 2 study, coordinated by the “Azienda Ospedaliero-Universitaria di Perugia”; EudraCT 2020-001258-23, NCT04322565 (130) – a multicenter, randomized, open-label, phase 2 study on COVID-19 patients at low or intermediate risk, coordinated by the “Azienda Ospedaliero-Universitaria di Parma”). Colchicine is orally dosed 1 mg daily, half dose (0.5 mg) daily in case of acute or chronic kidney failure, or 0.5 mg every other day in case of advanced liver impairment. Moreover, caution should be applied for the concomitant use of colchicine and strong inhibitors of P-glycoprotein or CYP3A4 (e.g., clarithromycin, erythromycin) for the risk of drug-drug interactions.
Heparins
Since its discovery in 1916, the efficacy of heparin for the prevention and treatment of venous thrombosis and pulmonary embolism, the prevention of mural thrombosis after myocardial infarction (MI), and the treatment of patients with unstable angina and MI has been largely documented. However, unfractionated heparin (UFH) displays some limitations, based on its pharmacokinetic, biophysical, and non-anticoagulant biological properties, that paved the way to the development of low-molecular-weight heparins (LMWHs). LMWHs, derived from heparin by chemical or enzymatic depolymerization, have shown a more predictable anticoagulant effect than UFH, are easier to administer, and may not require monitoring (131-133).
Preliminary laboratory information and earlier clinical observations indicated that the plasma of COVID-19 patients is hypercoagulable, thus suggesting that early anticoagulation may inhibit clotting formation and reduce micro-thrombi, thereby reducing the risk of major organ damage. The use of anticoagulants (such as heparin) for patients with severe COVID-19 was initially recommended by an expert consensus in China (134). Two subsequent studies evaluated the potential benefit of LMWHs in patients with COVID-19.
In a retrospective study of 449 patients with severe COVID-19, 7-day anticoagulant therapy mainly with LMWH appeared to be associated with lower mortality in the subpopulation meeting sepsis-induced coagulopathy criteria or with markedly elevated D-dimer (8). While the study suggests that there is a survival advantage for those patients with COVID-19 who receive administration of prophylactic dose of heparin agents, it did not describe to what extent heparin improves the abnormal coagulation. In the second study, which was not peer-reviewed before publication, LMWH was found improving the coagulation dysfunction of COVID-19 patients and exerting anti-inflammatory effects by reducing IL-6 and increasing lymphocyte percentages (135). Furthermore, it has been suggested that the non-anticoagulant properties of heparin, e.g. its anti-inflammatory function, may also be relevant in this setting as well as heparin ability to protect the endothelium may help impacting the microcirculatory dysfunction and possibly limiting organ damage. Finally, another interesting concept is the potential antiviral action of heparin by virtue of its poly-anionic nature that would make heparin a potential effective inhibitor of viral attachment as documented in herpes simplex virus or SARS-associated CoV infections (136-138).
Nevertheless, additional findings from larger and randomised studies are needed to determine whether heparin may be beneficial in COVID-19 patients. In this context, matters worthy of investigation are the proper timing, dosages and administration scheme of heparins, as well as the evaluation whether in vitro studies of anti-inflammatory functions, endothelial protection and viral inhibition should be considered independently of anticoagulant properties (20,139).
Earlier case reports and later clinical observations suggested that the incidence of venous thromboembolism (VTE) could be higher among COVID-19 patients with increased D-dimer levels predicting VTE risk thus raising questions on the clinical significance of increased D-dimer in severe COVID-19 and the role of anticoagulant therapy in the prophylaxis and treatment of these patients. However, it is also possible that a pulmonary embolism could occur in more severely ill COVID-19 patients before hospitalizations thus clarifying the documented ineffectiveness of prophylactic doses of heparins during their hospital stay (20).
Recently, both the International Society on Thrombosis and Haemostasis (ISTH) and the American Society of Hematology (ASH) suggested that all hospitalized COVID-19 patients should receive thromboprophylaxis, or full therapeutic-intensity anticoagulation if such an indication is present (140). However, some argue that it would be necessary to recalibrate the recommended approach to the use of heparin by considering early initiation of therapeutic anticoagulation with UFH prior to significant clinical deterioration to avoid further decline in patients without significant bleeding risks (141). Furthermore, the use of therapeutic doses of UFH or LMWH is currently not supported by evidence outside of established diagnoses of VTE or as a bridging strategy in patients on vitamin K antagonists (VKA), and cannot be recommended as a standard treatment for all COVID-19 patients (20). Overall, the hypothesis of improving the clinical outcome of COVID-19 patients by simple and inexpensive antithrombotic drugs is very attractive, but more robust evidence from properly designed clinical trials with strong endpoints are necessary.
Miscellanea
The whole scientific community and health professionals are involved in an enormous effort aimed at evaluating as many therapeutic options as possible. Thousands of compounds are indeed considered (142), and the number of those deemed promising grows on a daily basis. This brief overview of emerging drugs must be necessarily selective. Nevertheless, due to the peculiar mechanism of action proposed, the following therapeutic and investigational compounds deserve a brief mention.
Baricitinib is a small molecule inhibitor of Janus kinase subtype 1 and 2 (JAK1, JAK2), approved in EU and USA for the treatment of rheumatoid arthritis resistant to TNFα-antagonist therapy (143,144). Baricitinib has been selected as a molecule potentially useful in patients with COVID-19 because of its dual anti-inflammatory and antiviral activity in vitro. The latter has been recently reported by a study identifying baricitinib as an inhibitor of AP2-associated protein kinase 1 (AAK1), a protein playing a key role in endocytosis and viral entry in the alveolar type 2 epithelial cells (145,146). Clinical trials studying baricitinib in COVID-19 patients are ongoing (147).
In Italy, AIFA also authorized Ruxolitinib, another JAK1/2 inhibitor, and Canakinumab, a monoclonal antibody targeting IL-1β potentially counteracting the “cytokine storm”, for compassionate use in COVID-19 patients (49).
Camostat mesilate is a non peptidic, orally bioavailable serine protease inhibitor, approved in Japan for the treatment of pancreatitis. It can also inhibit TMPRSS2, the host cell serine protease which is important for viral entry (14,148), and the same mechanism has been recently shown for the mucolytic cough suppressant drug bromhexine (149). Both drugs are currently studied as a promising therapeutic agent in COVID-19 (150,151).
Gabexate mesilate is another synthetic serine protease inhibitor indicated for the anticoagulant treatment in disseminated intravascular coagulation (152). Gabexate is also able to reduce the production of inflammatory cytokines and its use has also been explored in cancer, ischemia/reperfusion injury and pancreatitis (153-155). The drug exerts potent anticoagulant actions as a direct inhibitor of kallikrein, plasmin and thrombin (156). Finally, gabexate could block the entry of the virus into lung cells by inhibiting TMPRSS2. Nafamostat mesilate, a molecule structurally related to gabexate, has been shown to inhibit TMPRSS2 (157,158) and is already under clinical testing in COVID-19 (159).
Controversies
Since ACE2 has been discovered as the cell-entry receptor for SARS-CoV-2, scientists and clinicians questioned whether ACE inhibitors or angiotensin receptor blockers may have any therapeutic or harmful role in treating COVID-19 patients (160). Data reported so far remain contradictory and inconclusive, warranting the indication of continuing therapy for patients already treated with these drugs (161,162).
Another controversial issue concerns the use of corticosteroids. As effective anti-inflammatory drugs, they may reduce lung damage associated with SARS-CoV-2 infection, thwarting the onset of ARDS. However, the risk-benefit balance is unclear, since delayed viral clearance or secondary infections may arise due to their broad immunosuppressive activity (163). Unless otherwise indicated for concomitant use (e.g., methylprednisolone and emapalumab) or comorbidities, the current evidence warns about their use in this context.
Conclusions and perspectives
The world is currently facing one of the largest public health emergencies since the 1918 influenza outbreak. From the beginning, the scientific community has devoted enormous efforts in organizing a rapid and effective response against this new viral disease. It is worth noting that the pathogenetic mechanisms of SARS-CoV-2 have been only partially unraveled. Of great practical relevance is the fact that, it is still not known if stable and effective immunological memory against SARS-CoV-2 may develop after the first infection. Data on recovered patients are still scarce and anecdotal, and the use of convalescent plasma, isolated from putatively immune subjects, as a post exposure prophylaxis led to conflicting results (164). Notwithstanding, over 45 studies using hyperimmune plasma have been launched worldwide (165), including two Italian studies coordinated in Pavia (166) and Bergamo (167), whose preliminary results seem very encouraging.
Pending further research, enormous resources have been also ploughed into vaccine development programs. Vaccine is certainly the most effective resource for preventing new coronavirus epidemics in the long term. As of this writing, more than 120 vaccines are in the pipeline, 70 of which are in preclinical evaluation and 7 already in clinical development (mainly in phase 1) (168).
In contrast, no drug has been approved so far for the treatment of COVID-19. Though promising pharmacological strategies are emerging in parallel with the growing knowledge of the disease (Fig. 1), the availability of higher quality clinical evidence in the near future remains compelling, since most of the studies completed so far showed high risk of bias or did not undergo a rigorous peer-review process.
Hundreds of phase 2 or phase 3 clinical trials are currently ongoing and even more are on the starting blocks. Old and recent drugs are being repurposed while a number of investigational compounds showed promise in preclinical and clinical studies. Expensive biological drugs, such as monoclonal antibodies, are being employed for compassionate use or in clinical studies, generating wide interest. However, it is likely that their indications will eventually be restricted to the most severe forms of the disease.
The identification of drugs with a good efficacy and safety profile, allowing a satisfactory control of COVID-19 not only in hospitals, but also at home, is a rational goal to be achieved in the near future.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Paules CI, Marston HD, Fauci AS. Coronavirus Infections-More Than Just the Common Cold. JAMA - J Am Med Assoc. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. doi:10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 2.Yi Y, Lagniton PNP, Ye S, Li E, Xu RH. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16(10):1753–1766. doi: 10.7150/ijbs.45134. doi:10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2019, 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. doi:10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.J G. Three Emerging Coronaviruses in Two Decades. Am J Clin Pathol. 2020;153(4) doi: 10.1093/ajcp/aqaa029. doi:10.1093/AJCP/AQAA029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buonaguro FM, Puzanov I, Ascierto PA. Anti-IL6R role in treatment of COVID-19-related ARDS. J Transl Med. 2020;18(1):165. doi: 10.1186/s12967-020-02333-9. doi:10.1186/s12967-020-02333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Coronavirus disease (COVID-19) Outbreak situation_WHO. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 . [Google Scholar]
- 7.Lai CC, Liu YH, Wang CY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.02.012. doi:10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. March 2020 doi: 10.1111/jth.14817. doi:10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadman M, Couzin-Frankel J, Kaiser J, Matacic C. A rampage through the body. Science. 2020;368(6489):356–360. doi: 10.1126/science.368.6489.356. doi:10.1126/science.368.6489.356. [DOI] [PubMed] [Google Scholar]
- 10.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA. April 2020 doi: 10.1001/jama.2020.6019. doi:10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 11.Lake MA. What we know so far: COVID-19 current clinical knowledge and research. Clin Med. 2020;20(2):124–127. doi: 10.7861/clinmed.2019-coron. doi:10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Millet JK, Whittaker GR. Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021. doi:10.1016/j.virusres.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.058. doi:10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. April 2020 doi: 10.1016/j.cell.2020.02.052. doi:10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. doi:10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. doi:10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao B, Wang Y, Wen D, et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. March 2020 doi: 10.1056/NEJMoa2001282. doi:10.1056/nejmoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiqi HK, Mehra MR. COVID-19 Illness in Native and Immunosuppressed States: A Clinical-Therapeutic Staging Proposal. J Hear Lung Transplant. March 2020 doi: 10.1016/j.healun.2020.03.012. doi:10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. doi:10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marietta M, Ageno W, Artoni A, et al. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET) Blood Transfus. April 2020 doi: 10.2450/2020.0083-20. doi:10.2450/2020.0083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bikdeli B, Madhavan M V, Jimenez D, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up. J Am Coll Cardiol. April 2020 doi: 10.1016/j.jacc.2020.04.031. doi:10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Search of: COVID-19 - List Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19 . Accessed April 27, 2020. [Google Scholar]
- 23. Sperimentazioni cliniche - COVID-19 | Agenzia Italiana del Farmaco. https://www.aifa.gov.it/sperimentazioni-cliniche-covid-19 . Accessed April 26, 2020. [Google Scholar]
- 24.Cvetkovic RS, Goa KL. Lopinavir/ritonavir: A review of its use in the management of HIV infection. Drugs. 2003;63(8):769–802. doi: 10.2165/00003495-200363080-00004. doi:10.2165/00003495-200363080-00004. [DOI] [PubMed] [Google Scholar]
- 25. Kaletra | European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/kaletra . Accessed April 24, 2020. [Google Scholar]
- 26.Barrila J, Bacha U, Freire E. Long-range cooperative interactions modulate dimerization in SARS 3CL pro. Biochemistry. 2006;45(50):14908–14916. doi: 10.1021/bi0616302. doi:10.1021/bi0616302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y, Liu H, Galasiti Kankanamalage AC, et al. Reversal of the Progression of Fatal Coronavirus Infection in Cats by a Broad-Spectrum Coronavirus Protease Inhibitor. PLoS Pathog. 2016;12(3) doi: 10.1371/journal.ppat.1005531. doi:10.1371/journal.ppat.1005531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choy K-T, Yin-Lam Wong A, Kaewpreedee P, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178:104786. doi: 10.1016/j.antiviral.2020.104786. doi:10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chu CM, Cheng VCC, Hung IFN, et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. doi:10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan JF-W, Yao Y, Yeung M-L, et al. Treatment With Lopinavir/Ritonavir or Interferon-β1b Improves Outcome of MERS-CoV Infection in a Nonhuman Primate Model of Common Marmoset. J Infect Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. doi:10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim UJ, Won EJ, Kee SJ, Jung SI, Jang HC. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-a for Middle East respiratory syndrome. Antivir Ther. 2016;21(5):455–459. doi: 10.3851/IMP3002. doi:10.3851/IMP3002. [DOI] [PubMed] [Google Scholar]
- 32.Chan KS, Lai ST, Chu CM, et al. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: A multicentre retrospective matched cohort study. Hong Kong Med J. 2003;9(6):399–406. [PubMed] [Google Scholar]
- 33.Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1) doi: 10.1186/s40779-020-0233-6. doi:10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. March 2020 doi: 10.1001/jamainternmed.2020.0994. doi:10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarro J, Curran A. Profile of once-daily darunavir/cobicistat fixeddose combination for the treatment of HIV/AIDS. HIV/AIDS - Res Palliat Care. 2016;8:175–182. doi: 10.2147/HIV.S56158. doi:10.2147/HIV.S56158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. doi:10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 37. Efficacy and Safety of Darunavir and Cobicistat for Treatment of COVID-19 - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04252274 . Accessed April 24, 2020. [Google Scholar]
- 38.Siegel D, Hui HC, Doerffler E, et al. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J Med Chem. 2017;60(5):1648–1661. doi: 10.1021/acs.jmedchem.6b01594. doi:10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 39.Al-Tawfiq JA, Al-Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis. 2020 doi: 10.1016/j.tmaid.2020.101615. doi:10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gordon CJ, Tchesnokov EP, Woolner E, et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020 doi: 10.1074/jbc.RA120.013679. doi:10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. EMA provides recommendations on compassionate use of remdesivir for COVID-19 | European Medicines Agency. https://www.ema.europa.eu/en/news/ema-provides-recommendations-compassionate-use-remdesivir-covid-19 . Accessed April 24, 2020. [Google Scholar]
- 42.Jacobs M, Rodger A, Bell DJ, et al. Late Ebola virus relapse causing meningoencephalitis: a case report. Lancet. 2016;388(10043):498–503. doi: 10.1016/S0140-6736(16)30386-5. doi:10.1016/S0140-6736(16)30386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. doi:10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kujawski SA, Wong KK, Collins JP, et al. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. medRxiv. doi: 10.1038/s41591-020-0877-5. 2020:2020.03.09.20032896. doi:10.1101/2020.03.09.20032896. [DOI] [PubMed] [Google Scholar]
- 45. Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734TM) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04292730 . Accessed April 24, 2020. [Google Scholar]
- 46. Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734TM) in Participants With Severe Coronavirus Disease (COVID-19) - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04292899 . Accessed April 24, 2020. [Google Scholar]
- 47. AIFA e Gilead annunciano che l’Italia è tra i Paesi che testeranno l’antivirale remdesivir per il trattamento del COVID-19. https://www.aifa.gov.it/web/guest/-/aifa-e-gilead-annunciano-che-l-italia-e-tra-i-paesi-che-testeranno-l-antivirale-remdesivir-per-il-trattamento-del-covid-19 . Accessed April 24, 2020. [Google Scholar]
- 48. notizia | Agenzia Italiana del Farmaco. https://www.aifa.gov.it/web/guest/-/covid-19-aifa-autorizza-lo-studio-solidarity-promosso-dall-oms . Accessed April 24, 2020. [Google Scholar]
- 49. Programmi di uso compassionevole – COVID-19 | Agenzia Italiana del Farmaco. https://www.aifa.gov.it/programmi-di-uso-compassionevole-covid-19 . Accessed April 24, 2020. [Google Scholar]
- 50. Trial of Treatments for COVID-19 in Hospitalized Adults - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04315948?term=NCT04315948&draw=2&rank=1 . Accessed April 24, 2020. [Google Scholar]
- 51. Adaptive COVID-19 Treatment Trial (ACTT) - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04280705?term=NCT04280705&draw=2&rank=1 . Accessed April 24, 2020. [Google Scholar]
- 52. A Trial of Remdesivir in Adults With Mild and Moderate COVID-19 - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04252664?term=NCT04252664&draw=2&rank=1 . Accessed April 24, 2020. [Google Scholar]
- 53. A Trial of Remdesivir in Adults With Severe COVID-19 - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04257656?term=NCT04257656&draw=2&rank=1 . Accessed April 24, 2020. [Google Scholar]
- 54.Grein J, Ohmagari N, Shin D, et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med. April 2020 doi: 10.1056/NEJMoa2007016. doi:10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: An old drug against today’s diseases? Lancet Infect Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. doi:10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155–166. doi: 10.1038/s41584-020-0372-x. doi:10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 57.Al-Bari MAA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect. 2017;5(1) doi: 10.1002/prp2.293. doi:10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Devaux CA, Rolain J-M, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. March 2020:105938. doi: 10.1016/j.ijantimicag.2020.105938. doi:10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao X, Ye F, Zhang M, et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin Infect Dis an Off Publ Infect Dis Soc Am. March 2020 doi: 10.1093/cid/ciaa237. doi:10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105932. doi:10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1) doi: 10.5582/bst.2020.01047. doi:10.5582/BST.2020.01047. [DOI] [PubMed] [Google Scholar]
- 62.CHEN J, LIU D, LIU L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ (Med Sci) 2020;49(February):1–10. doi:10.3785/j.issn.1008-9292.2020.03.03. [Google Scholar]
- 63.Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv. April 2020 doi: 10.1016/j.medj.2020.06.001. 2020.04.16.20065920. doi:10.1101/2020.04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chloroquine/Hydroxychloroquine Prevention of Coronavirus Disease (COVID-19) in the Healthcare Setting - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04303507?term=nct04303507&draw=2&rank=1 . Accessed April 25, 2020. [Google Scholar]
- 65. Post-exposure Prophylaxis / Preemptive Therapy for SARS-Coronavirus-2 - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04308668?term=nct04308668&draw=2&rank=1 . Accessed April 25, 2020. [Google Scholar]
- 66. COVID-19: reminder of risk of serious side effects with chloroquine and hydroxychloroquine | European Medicines Agency. https://www.ema.europa.eu/en/news/covid-19-reminder-risk-serious-side-effects-chloroquine-hydroxychloroquine . Accessed April 24, 2020. [Google Scholar]
- 67.McChesney EW. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am J Med. 1983;75(1 PART 1):11–18. doi: 10.1016/0002-9343(83)91265-2. doi:10.1016/0002-9343(83)91265-2. [DOI] [PubMed] [Google Scholar]
- 68.Bergholz R, Schroeter J, Rüther K. Evaluation of risk factors for retinal damage due to chloroquine and hydroxychloroquine. Br J Ophthalmol. 2010;94(12):1637–1642. doi: 10.1136/bjo.2009.174458. doi:10.1136/bjo.2009.174458. [DOI] [PubMed] [Google Scholar]
- 69.Khamitov RA, Loginova SI, Shchukina VN, Borisevich S V, Maksimov VA, Shuster AM. [Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures] Vopr Virusol. 2008;53(4):9–13. [PubMed] [Google Scholar]
- 70.Kadam RU, Wilson IA. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc Natl Acad Sci U S A. 2017;114(2):206–214. doi: 10.1073/pnas.1617020114. doi:10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Search of: umifenovir | COVID-19 - List Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=umifenovir&cntry=&state=&city=&dist=&Search=Search . Accessed April 25, 2020. [Google Scholar]
- 72. Clinical Study of Arbidol Hydrochloride Tablets in the Treatment of Pneumonia Caused by Novel Coronavirus - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04260594?term=umifenovir&cond=COVID-19&draw=2&rank=3 . Accessed April 25, 2020. [Google Scholar]
- 73.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. March 2020 doi: 10.1093/cid/ciaa272. doi:10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hayden FG, Shindo N. Influenza virus polymerase inhibitors in clinical development. Curr Opin Infect Dis. 2019;32(2):176–186. doi: 10.1097/QCO.0000000000000532. doi:10.1097/QCO.0000000000000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Japan Acad Ser B Phys Biol Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. doi:10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013;100(2):446–454. doi: 10.1016/j.antiviral.2013.09.015. doi:10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sissoko D, Laouenan C, Folkesson E, et al. Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea. PLoS Med. 2016;13(3) doi: 10.1371/journal.pmed.1001967. doi:10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mentré F, Taburet AM, Guedj J, et al. Dose regimen of favipiravir for Ebola virus disease. Lancet Infect Dis. 2015;15(2):150–151. doi: 10.1016/S1473-3099(14)71047-3. doi:10.1016/S1473-3099(14)71047-3. [DOI] [PubMed] [Google Scholar]
- 79.McKimm-Breschkin JL, Fry AM. Meeting report: 4th ISIRV antiviral group conference: Novel antiviral therapies for influenza and other respiratory viruses. Antiviral Res. 2016;129:21–38. doi: 10.1016/j.antiviral.2016.01.012. doi:10.1016/j.antiviral.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McKimm-Breschkin JL, Jiang S, Hui DS, Beigel JH, Govorkova EA, Lee N. Antiviral Research. Vol 149. Elsevier B.V; 2018. Prevention and treatment of respiratory viral infections: Presentations on antivirals, traditional therapies and host-directed interventions at the 5th ISIRV Antiviral Group conference; pp. 118–142. doi:10.1016/j.antiviral.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Madelain V, Nguyen THT, Olivo A, et al. Ebola Virus Infection: Review of the Pharmacokinetic and Pharmacodynamic Properties of Drugs Considered for Testing in Human Efficacy Trials. Clin Pharmacokinet. 2016;55(8):907–923. doi: 10.1007/s40262-015-0364-1. doi:10.1007/s40262-015-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nguyen THT, Guedj J, Anglaret X, et al. Favipiravir pharmacokinetics in Ebola-Infected patients of the JIKI trial reveals concentrations lower than targeted. PLoS Negl Trop Dis. 2017;11(2) doi: 10.1371/journal.pntd.0005389. doi:10.1371/journal.pntd.0005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Favié LMA, Murk JL, Meijer A, Laura Nijstad A, Van Maarseveen EM, Sikma MA. Pharmacokinetics of favipiravir during continuous venovenous haemofiltration in a critically ill patient with influenza. Antivir Ther. 2018;23(5):457–461. doi: 10.3851/IMP3210. doi:10.3851/IMP3210. [DOI] [PubMed] [Google Scholar]
- 84. Phase 3 Efficacy and Safety Study of Favipiravir for Treatment of Uncomplicated Influenza in Adults - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02008344?term=NCT02008344&draw=2&rank=1 . Accessed April 25, 2020. [Google Scholar]
- 85.Chen C, Huang J, Cheng Z, et al. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. medRxiv. 2020 2020.03.17.20037432. doi:10.1101/2020.03.17.20037432. [Google Scholar]
- 86.Cai Q, Yang M, Liu D, et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering. March 2020 doi: 10.1016/j.eng.2020.03.007. doi:10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Search of: favipiravir | COVID-19 - List Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=favipiravir&cntry=&state=&city=&dist=&Search=Search . Accessed April 25, 2020. [Google Scholar]
- 88. Clinical Study To Evaluate The Performance And Safety Of Favipiravir in COVID-19 - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04336904?term=NCT04336904&draw=2&rank=1 . Accessed April 25, 2020. [Google Scholar]
- 89.Maeurer M, Rao M, Zumla A. Host-directed therapies for antimicrobial resistant respiratory tract infections. Curr Opin Pulm Med. 2016;22(3):203–211. doi: 10.1097/MCP.0000000000000271. doi:10.1097/MCP.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 90.Salvatore M, Satlin MJ, Jacobs SE, et al. DAS181 for Treatment of Parainfluenza Virus Infections in Hematopoietic Stem Cell Transplant Recipients at a Single Center. Biol Blood Marrow Transplant. 2016;22(5):965–970. doi: 10.1016/j.bbmt.2016.02.011. doi:10.1016/j.bbmt.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 91.Dhakal B, D’Souza A, Pasquini M, et al. DAS181 Treatment of Severe Parainfluenza Virus 3 Pneumonia in Allogeneic Hematopoietic Stem Cell Transplant Recipients Requiring Mechanical Ventilation. Case Rep Med. 2016;2016:8503275. doi: 10.1155/2016/8503275. doi:10.1155/2016/8503275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Search of: das181 | COVID-19 - List Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=das181&cntry=&state=&city=&dist=&Search=Search . Accessed April 25, 2020. [Google Scholar]
- 93. DAS181 for STOP COVID-19 - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04354389?term=das181&cond=COVID-19&draw=2&rank=2 . Accessed April 25, 2020. [Google Scholar]
- 94.Jin Z, Du X, Xu Y, et al. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature. April 2020 doi: 10.1038/s41586-020-2223-y. doi:10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 95.Parnham MJ, Sies H. The early research and development of ebselen. Biochem Pharmacol. 2013;86(9):1248–1253. doi: 10.1016/j.bcp.2013.08.028. doi:10.1016/j.bcp.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 96.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121(9):3375–3383. doi: 10.1172/JCI57158. doi:10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1) doi: 10.1186/s40425-018-0343-9. doi:10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oved JH, Barrett DM, Teachey DT. Cellular therapy: Immune-related complications. Immunol Rev. 2019;290(1):114–126. doi: 10.1111/imr.12768. doi:10.1111/imr.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30566-3. doi:10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. doi:10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu , Xiaoling , Han , Mingfeng , Li , Tiantian , Sun , Wei , Wang , Dongsheng , Fu , Binqing , Zhou , Yonggang , Zheng , Xiaohu , Yang , Yun , Li , Xiuyong , Zhang , Xiaohua , Pan , Aijun , Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv. 2020 doi: 10.1073/pnas.2005615117. doi:10.12074/202003.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Search of: tocilizumab | COVID-19 - List Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=tocilizumab&cntry=&state=&city=&dist=&Search=Search . Accessed April 26, 2020. [Google Scholar]
- 103. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19) - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04317092?term=tocilizumab&cond=COVID-19&draw=2&rank=1 . Accessed April 26, 2020. [Google Scholar]
- 104. Efficacy of Early Administration of Tocilizumab in COVID-19 Patients - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04346355?term=parma&cond=COVID-19&draw=2&rank=5 . Accessed April 26, 2020. [Google Scholar]
- 105. A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04320615?term=tocilizumab&cond=COVID-19&draw=2&rank=7 . Accessed April 26, 2020. [Google Scholar]
- 106.Kim S, Östör AJK, Nisar MK. Interleukin-6 and cytochrome-P450, reason for concern? Rheumatol Int. 2012;32(9):2601–2604. doi: 10.1007/s00296-012-2423-3. doi:10.1007/s00296-012-2423-3. [DOI] [PubMed] [Google Scholar]
- 107.Zhang X, Peck R. Clinical pharmacology of tocilizumab for the treatment of patients with rheumatoid arthritis. Expert Rev Clin Pharmacol. 2011;4(5):539–558. doi: 10.1586/ecp.11.33. doi:10.1586/ecp.11.33. [DOI] [PubMed] [Google Scholar]
- 108. Search of: sarilumab | COVID-19 - List Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=sarilumab&cntry=&state=&city=&dist=&Search=Search&flds=abkuy . Accessed April 26, 2020. [Google Scholar]
- 109. Sarilumab COVID-19 - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04327388?term=sarilumab&cond=COVID-19&draw=2&rank=7 . Accessed April 26, 2020. [Google Scholar]
- 110.Dinarello CA, Simon A, Van Der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. doi:10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kineret | European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/kineret . Accessed April 26, 2020. [Google Scholar]
- 112.Al-Salama ZT. Emapalumab: First Global Approval. Drugs. 2019;79(1):99–103. doi: 10.1007/s40265-018-1046-8. doi:10.1007/s40265-018-1046-8. [DOI] [PubMed] [Google Scholar]
- 113.Lounder DT, Bin Q, De Min C, Jordan MB. Treatment of refractory hemophagocytic lymphohistiocytosis with emapalumab despite severe concurrent infections. Blood Adv. 2019;3(1):47–50. doi: 10.1182/bloodadvances.2018025858. doi:10.1182/bloodadvances.2018025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schoenborn JR, Wilson CB. Regulation of Interferon-γ During Innate and Adaptive Immune Responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. doi:10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 115.Ye L, Schnepf D, Staeheli P. Interferon-λ orchestrates innate and adaptive mucosal immune responses. Nat Rev Immunol. 2019;19(10):614–625. doi: 10.1038/s41577-019-0182-z. doi:10.1038/s41577-019-0182-z. [DOI] [PubMed] [Google Scholar]
- 116. EU/3/10/749 | European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu310749 . Accessed April 26, 2020. [Google Scholar]
- 117. Search of: anakinra | COVID-19 - List Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=anakinra&cntry=&state=&city=&dist=&Search=Search . Accessed April 26, 2020. [Google Scholar]
- 118. Efficacy and Safety of Emapalumab and Anakinra in Reducing Hyperinflammation and Respiratory Distress in Patients With COVID-19 Infection. - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04324021?term=emapalumab&cond=COVID-19&draw=2&rank=1 . Accessed April 26, 2020. [Google Scholar]
- 119.Panda D, Daijo JE, Jordan MA, Wilson L. Kinetic Stabilization of Microtubule Dynamics at Steady State in Vitro by Substoichiometric Concentrations of Tubulin-Colchicine Complex. Biochemistry. 1995;34(31):9921–9929. doi: 10.1021/bi00031a014. doi:10.1021/bi00031a014. [DOI] [PubMed] [Google Scholar]
- 120.Ravelli RBG, Gigant B, Curmi PA, et al. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428(6979):198–202. doi: 10.1038/nature02393. doi:10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 121.Perico N, Ostermann D, Bontempeill M, et al. Colchicine interferes with L-selectin and leukocyte function-associated antigen-1 expression on human T lymphocytes and inhibits T cell activation. J Am Soc Nephrol. 1996;7(4) doi: 10.1681/ASN.V74594. [DOI] [PubMed] [Google Scholar]
- 122.Stack J, Ryan J, McCarthy G. Colchicine: New Insights to an Old Drug. Am J Ther. 2015;22(5):e151–e157. doi: 10.1097/01.mjt.0000433937.07244.e1. doi:10.1097/01.mjt.0000433937.07244.e1. [DOI] [PubMed] [Google Scholar]
- 123.Martínez GJ, Celermajer DS, Patel S. The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 2018;269:262–271. doi: 10.1016/j.atherosclerosis.2017.12.027. doi:10.1016/j.atherosclerosis.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 124.Biswas K, Das Sarma J, Perlman S. Effect of Microtubule Disruption on Neuronal Spread and Replication of Demyelinating and Nondemyelinating Strains of Mouse Hepatitis Virus In Vitro. J Virol. 2014;88(5):3043–3047. doi: 10.1128/JVI.02545-13. doi:10.1128/jvi.02545-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lu N, Yang Y, Liu H, et al. Inhibition of respiratory syncytial virus replication and suppression of RSV-induced airway inflammation in neonatal rats by colchicine. 3 Biotech. 2019;9(11) doi: 10.1007/s13205-019-1917-z. doi:10.1007/s13205-019-1917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Angelini MM, Akhlaghpour M, Neuman BW, Buchmeier MJ. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. MBio. 2013;4(4) doi: 10.1128/mBio.00524-13. doi:10.1128/mBio.00524-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nieto-Torres JL, Verdiá-Báguena C, Jimenez-Guardeño JM, et al. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. doi:10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yue Y, Nabar NR, Shi CS, et al. SARS-Coronavirus Open Reading Frame-3a drives multimodal necrotic cell death. Cell Death Dis. 2018;9(9) doi: 10.1038/s41419-018-0917-y. doi:10.1038/s41419-018-0917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. COVID-19 - AIFA autorizza sperimentazione clinica con colchicina. https://www.aifa.gov.it/web/guest/-/covid-19-aifa-autorizza-sperimentazione-clinica-con-colchicina . Accessed April 26, 2020. [Google Scholar]
- 130. Colchicine Counteracting Inflammation in COVID-19 Pneumonia - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/study/NCT04322565?term=NCT04322565&cond=COVID-19&draw=2&rank=1#contacts . Accessed April 23, 2020. [Google Scholar]
- 131.Mukherjee D, Topol EJ. The role of low-molecular-weight heparin in cardiovascular diseases. Prog Cardiovasc Dis. 2002;45(2):139–156. doi: 10.1053/pcad.2002.127679. doi:10.1053/pcad.2002.127679. [DOI] [PubMed] [Google Scholar]
- 132.Yan AT, Goodman SG. Low-molecular-weight heparins in ischemic heart disease. Curr Opin Cardiol. 2004;19(4):309–316. doi: 10.1097/01.hco.0000129665.55707.f6. doi:10.1097/01.hco.0000129665.55707.f6. [DOI] [PubMed] [Google Scholar]
- 133.Hirsh J, Anand SS, Halperin JL, Fuster V. Guide to Anticoagulant Therapy: Heparin. Circulation. 2001;103(24):2994–3018. doi: 10.1161/01.cir.103.24.2994. doi:10.1161/01.CIR.103.24.2994. [DOI] [PubMed] [Google Scholar]
- 134.Li T, Lu H, Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9(1):687–690. doi: 10.1080/22221751.2020.1741327. doi:10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.SHI C, WANG C, WANG H, et al. Clinical observations of low molecular weight heparin in relieving inflammation in COVID-19 patients: A retrospective cohort study. medRxiv. April 2020 doi: 10.1111/cts.12880. 2020.03.28.20046144. doi:10.1101/2020.03.28.20046144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest. 2001;108(4):503–510. doi: 10.1172/JCI13799. doi:10.1172/jci13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ghezzi S, Cooper L, Rubio A, et al. Heparin prevents Zika virus induced-cytopathic effects in human neural progenitor cells. Antiviral Res. 2017;140:13–17. doi: 10.1016/j.antiviral.2016.12.023. doi:10.1016/j.antiviral.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vicenzi E, Canducci F, Pinna D, et al. Coronaviridae and SARS-associated Coronavirus Strain HSR1. Emerg Infect Dis. 2004;10(3):413–418. doi: 10.3201/eid1003.030683. doi:10.3201/eid1003.030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. April 2020 doi: 10.1111/jth.14821. doi:10.1111/jth.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. March 2020 doi: 10.1111/jth.14810. doi:10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.CD B, HB M, MB Y, EE M. ISTH Interim Guidance on Recognition and Management of Coagulopathy in COVID-19: A Comment. J Thromb Haemost. 2020 doi: 10.1111/jth.14860. doi:10.1111/JTH.14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lythgoe MP, Middleton P. Ongoing Clinical Trials for the Management of the COVID-19 Pandemic. Trends Pharmacol Sci. April 2020 doi: 10.1016/j.tips.2020.03.006. doi:10.1016/j.tips.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Olumiant | European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant . Accessed April 27, 2020. [Google Scholar]
- 144. Drug Approval Package: Olumiant (baricitinib). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/207924Orig1s000TOC.cfm . Accessed April 27, 2020. [Google Scholar]
- 145.Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. doi: 10.1016/S0140-6736(20)30304-4. doi:10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. doi:10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Search of: baricitinib | COVID-19 - List Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=baricitinib&cntry=&state=&city=&dist=&Search=Search . Accessed April 27, 2020. [Google Scholar]
- 148.Kawase M, Shirato K, van der Hoek L, Taguchi F, Matsuyama S. Simultaneous Treatment of Human Bronchial Epithelial Cells with Serine and Cysteine Protease Inhibitors Prevents Severe Acute Respiratory Syndrome Coronavirus Entry. J Virol. 2012;86(12):6537–6545. doi: 10.1128/JVI.00094-12. doi:10.1128/jvi.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maggio R, Corsini GU. Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection. Pharmacol Res. April 2020:104837. doi: 10.1016/j.phrs.2020.104837. doi:10.1016/j.phrs.2020.104837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Search of: camostat | COVID-19 - List Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=camostat&cntry=&state=&city=&dist=&Search=Search . Accessed April 27, 2020. [Google Scholar]
- 151. Search of: bromhexine | COVID-19 - List Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=bromhexine&cntry=&state=&city=&dist=&Search=Search . Accessed April 27, 2020. [Google Scholar]
- 152.Yuksel M, Okajima K, Uchiba M, Okabe H. Gabexate mesilate, a synthetic protease inhibitor, inhibits lipopolysaccharide-induced tumor necrosis factor-α production by inhibiting activation of both nuclear factor-κB and activator protein-1 in human monocytes. J Pharmacol Exp Ther. 2003;305(1):298–305. doi: 10.1124/jpet.102.041988. doi:10.1124/jpet.102.041988. [DOI] [PubMed] [Google Scholar]
- 153.Brandi G, Tavolari S, De Rosa F, et al. Antitumoral efficacy of the protease inhibitor gabexate mesilate in colon cancer cells harbouring KRAS, BRAF and PIK3CA mutations. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0041347. doi:10.1371/journal.pone.0041347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xie LB, Zeng DY, Wang XD, Lin T, Li YP, Lu YP. Preconditioning with gabexate is superior to inosine for ameliorating acute renal ischemia-reperfusion injury in rats. Transplant Proc. 2014;46(1):40–45. doi: 10.1016/j.transproceed.2013.10.037. doi:10.1016/j.transproceed.2013.10.037. [DOI] [PubMed] [Google Scholar]
- 155.Kim SC, Yang HR. Clinical efficacy of gabexate mesilate for acute pancreatitis in children. Eur J Pediatr. 2013;172(11):1483–1490. doi: 10.1007/s00431-013-2068-6. doi:10.1007/s00431-013-2068-6. [DOI] [PubMed] [Google Scholar]
- 156.Tamura Y, Hirado M, Okamura K, Minato Y, Fujii S. Synthetic inhibitors of trypsin, plasmin, kallikrein, thrombin, C1r, and C1 esterase. BBA - Enzymol. 1977;484(2):417–422. doi: 10.1016/0005-2744(77)90097-3. doi:10.1016/0005-2744(77)90097-3. [DOI] [PubMed] [Google Scholar]
- 157.Yamamoto M, Kiso M, Sakai-Tagawa Y, et al. The anticoagulant nafamostat potently inhibits SARS-CoV-2 infection in vitro: an existing drug with multiple possible therapeutic effects. bioRxiv. April 2020 2020.04.22.054981. doi:10.1101/2020.04.22.054981. [Google Scholar]
- 158.Yamamoto M, Matsuyama S, Li X, et al. Identification of nafamostat as a potent inhibitor of middle east respiratory syndrome Coronavirus s protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob Agents Chemother. 2016;60(11):6532–6539. doi: 10.1128/AAC.01043-16. doi:10.1128/AAC.01043-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Efficacy of Nafamostat in Covid-19 Patients (RACONA Study) - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04352400?term=nafamostat&cond=COVID-19&draw=2&rank=1 . Accessed April 27, 2020. [Google Scholar]
- 160.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 doi: 10.1002/ddr.21656. doi:10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang . Accessed April 27, 2020. [Google Scholar]
- 162. EMA advises continued use of medicines for hypertension, heart or kidney disease during COVID-19 pandemic | European Medicines Agency. https://www.ema.europa.eu/en/news/ema-advises-continued-use-medicines-hypertension-heart-kidney-disease-during-covid-19-pandemic . Accessed April 27, 2020. [Google Scholar]
- 163.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. doi:10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. doi:10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Search of: convalescent Plasma | COVID-19 - List Results - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=COVID-19&term=convalescent+Plasma&cntry=&state=&city=&dist=&Search=Search . Accessed April 28, 2020. [Google Scholar]
- 166. Hyperimmune Plasma for Critical Patients With COVID-19 - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04321421?term=hyperimmune+Plasma&cond=COVID-19&draw=2&rank=1 . Accessed April 28, 2020. [Google Scholar]
- 167. Convalescent Antibodies Infusion in Critically Ill COVID 19 Patients - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04346589?term=convalescent+Plasma&cond=COVID-19&draw=2&rank=42 . Accessed April 28, 2020. [Google Scholar]
- 168. WHO Solidarity Trial – Accelerating a safe and effective COVID-19 vaccine. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-trial-accelerating-a-safe-and-effective-covid-19-vaccine . Accessed April 27, 2020. [Google Scholar]


