Abstract
Background:
Osteonecrosis of the femoral head (ONFH) is a frequent orthopedic disease leading to destruction of the hip joint and disabling arthritis. Several procedures have been developed to treat the joint deterioration in case of osteonecrosis, trying to avoid or delay an intervention of total hip replacement, especially in young patients. The aim of this study was to analyze the use of autologous bone micrografts derived from cancellous bone in the management of avascular ONFH. The treatment described was implemented using the Rigenera® protocol to obtain autologous micrografts: small fragments of cancellous bone collected by femoral neck, disaggregated and injected in the necrotic area using an empty screw.
Materials and methods:
Twenty adult patients affected by avascular ONFH were enrolled in this study; all patients reported a preoperative intermittent coxo-arthrosis and limited function of intra and extra rotation of the hip. Inclusion criteria were an Oxford Hip Score between (OHS) 20 and 39, a Harris hip score (HHS) showing pre-operative poor results (lower than 70 points) and a stage II-IIIA and IIIB according with the classification proposed by the Association Research Circulation Osseous (ARCO).
Results:
Using an MRI evaluation, after six months, the authors observed a complete regression of necrotic area and the restoration of osseous structure. Clinical outcome has been evaluated at 6-12 and 24 months follow-up. At the final F.U. the HHS rised from poor to good results (mean value at final F.U of 84) while the OHS improved significantly already after 21 days from micrografts injection (mean 35.4 ± 7.5) with an increasing trend until to two-year final FU (mean 37.4 ± 9.5). The full recovery of daily and mild sport activities was reached after 20 and 90 days from intervention, respectively.
Conclusion:
The results of this study are suggestive for a new approach in the treatment of avascular ONFH assuming a process of bone regeneration based on a dual mechanism of action, biological and mechanical, induced by micrografts and injected using an empty screw as vehicle. (www.actabiomedica.it)
Keywords: autologous, micrografts, bone regeneration, conservative treatment, osteonecrosis, femoral head
Introduction
Aseptic, non-traumatic osteonecrosis of the femoral head (ONFH) is a rare, but disabling orthopaedic desease that usually results in cellular necrosis of the femoral head leading to bone collapse and hip joint destruction (1).
Etiology and pathogenesis of ONFH are still unclear. Several risk factors have been proposed in its development and progression such as trauma, chronic use of corticosteroids, alcohol consumption, coagulation disorders and different pathogenic mechanisms are supposed to be related with ONFH, including ischemia due to vascular interruption, thrombosis, sickle cell occlusion, or direct cellular toxicity due to pharmacological agents or oxidative stress (2-3). Furthermore, because of lack of specific symptoms, especially in its early stage, the diagnosis of ONFH can be extremely difficult.
Standard anteroposterior and lateral frog-leg radiographs and magnetic resonance imaging (MRI) are effective diagnostic tools (4). Anteroposterior and lateral frog-leg radiographs allow to identify both necrosis around sclerotic bone as well as segmental collapse, while MRI has a high sensitivity and specificity visualizing necrotic areas also in absence of subchondral fractures (4).
Two different approaches are usually adopted in the management of ONFH: a joint-preserving strategy or a joint-replacing treatment. Conservative treatments are usually based on pharmacological agents, biophysical therapy and multiple surgical approaches, classified as femoral head sparing procedures (FHSP) as core decompression (5), osteotomies, not vascularized (6) and vascularized bone grafting (7). These are usually indicated to address a pre-collapse stage and they are also finalized to delay as much as possible the time of total hip arthroplasty (THA). Joint-replacing treatments are commonly applied managing a post-collapse stage of ONFH.
Several bone grafting procedures have been proposed in the literature and performed using not vascularized or vascularized bone grafts to fill the necrotic area (8). The objective of this study was to evaluate subjective patient satisfaction (PROMs) as well as objective clinical and radiological outcomes in a series of patients affected by ONFH and undergoing a novel FHSP procedure where a classic core decompression surgery was implemented using autologous bone micrograft. A new system (Rigenera® technology ) has been used obtaining fast and easily viable bone micrografts avoiding in vitro cells expansion or other biochemical manipulations (9). The clinical effectiveness of micrografts has already been demonstrated in different clinical areas including dentistry, dermatology or management of chronic wounds, showing the capacity to promote bone or dermal regeneration (10-11). The current authors hypothesized that autologous micrografts could play a significant role in the treatment of avascular ONFH supporting the subchondral bone and articular cartilage in the immediate post-operative stage and secondarily they could improve the function of the hip stimulating a rapid bone regeneration. None of the authors have conflict of interest in connection with this study.
Materials and methods
The study protocol used in this report complied with the Declaration of Helsinki of 1975, the European regulations and the current authors proceeded to the data collection after concession of written consent for personal data collection and biological sampling by each patient.
Twenty consecutive male patients affected by idiopathic, aseptic and avascular ONFH were enrolled in this study. The mean age was 51.5 years (range from 40 to 63 years). The inclusion criteria for the study were an Oxford Hip Score (OHS) between 20 and 39, a Harris hip score (HHS) showing a pre-operative poor (lower than 70 points) score and a stage II-IIIA and IIIB according to the classification proposed by the Association Research Circulation Osseous (ARCO) and documented by an MRI. The exclusion criteria were the following: chronic steroid therapy, previous serious traumatic hip injuries, previous surgical hip treatments, rheumatoid or other immunological arthropathies.
Clinical and radiographic assessments
All patients were clinically assessed preoperatively and at 7-14 - 21 days, 2 months, 1 year and 2 years follow-up (F.U) according to the Harris Hip Score (HHS) and the Oxford Hip Score questionnaire (OHS) (12) as patient reported outcomes measurement systems (PROMs) and as overall validated measurement instruments.
For radiographic assessment, standard antero-posterior weight-bearing and lateral frog-leg radiographs were performed in all patients preoperatively, at 6 months and final 2 years FU; all patients underwent MRI evaluation pre-operatively and at 6 months FU. All radiographs were reviewed by an orthopedic surgeon not part of the surgical team (SR), blinded to the clinical outcomes results: the radiological evaluation was performed according to ARCO classification system (13) for disease staging, necrotic lesions size and position and healing process.
All surgical procedures were performed by a single surgeon from January 2015 to December 2015 following spinal anesthesia with the patient in the lateral decubitus position. Following radiographic planning, a Kirschner wire (2,5 mm) was introduced in the femoral neck (Fig. 1A-B) in order to drive the insertion of a 6-mm cannulated cutter. Subsequently, a small piece of cancellous bone from the lateral edge of femoral neck was collected (Fig. 1C) and mechanically disaggregated according to the Rigenera® protocol using a class-I medical device (Rigeneracons. Human Brain Wave, LLC, Turin, Italy) (14).
Figure 1.
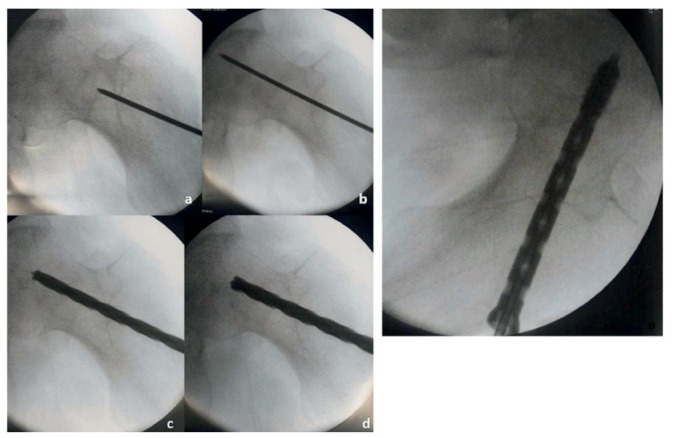
Surgical procedure: a Kirschner wire (2,5 mm) was first introduced in the femoral neck (A-B) and a small fragment of cancellous bone from the lateral edge of femoral neck was collected (C); the femoral head was then prepared with a 8 mm cutter (D); finally, a cannulated screw was implanted and the micrografts injected in the necrotic camera using the screw as a carrier (E)
The Rigeneracons is a biological disruptor of human tissues, composed by a grid made by 100 exagonal holes which are embraced by six micro-blades designed for efficient cutting of both hard and soft tissues, able to filter and select a specific cell population of 50-micron size enriched progenitor cells and expressing markers typical of mesenchymal stem cells (9).
The fragments of cancellous bone were then mixed into the rigeneracons, adding 1.5 ml of sterile saline solution and then disrupted for 5 minutes by a pure mechanical rotation process. After this, the cell suspension was collected from a needle-free syringe for a total of 10 ml of micrograft disaggregating multiple bone fragments for each patient
The femoral head was then prepared with a 8 mm cutter (Fig. 1D); at this time, an cannulated screw was implanted and used as carrier to inject the micrografts directly in the necrotic camera (Fig. 1E). Finally, the screw head was obliterated with a pin to reinforce the fixation system and creating a closed camera.
All interventions were performed as outpatient and all patients were discharged few hours after injection. Patients were maintained at toe-touch weight bearing with crutches for two days: after that, weight-bearing as tolerated was allowed.
Results
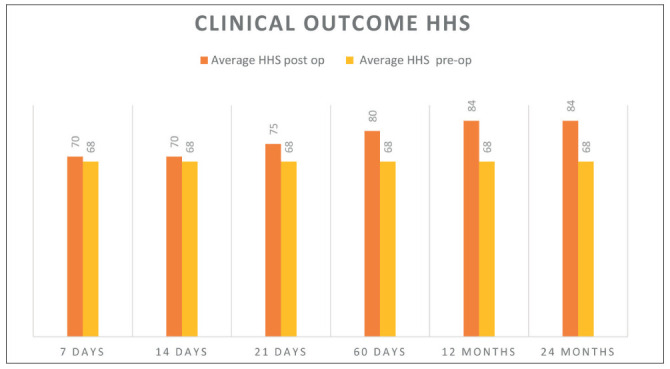
A continuous improvement in the Harris hip score in 19/20 patients was noted after 7, 14, 21, 60 days, and 1year and two years FU. HHS was calculated pre-operatively (mean value of 68) and post-operatively (table 2) showing a significant improvement in function and pain relief: at the final F.U the HHS rise from poor to good results (mean, 84 points). Micrografts application failed only in one patient: a 58 Years old gentleman who subsequently underwent THA one year after the index procedure.
Table 2.
Harris Hip Score evaluation before surgery and after 7, 14, 21, 60 days and 1, 2 years of follow up. Pre-operative evaluation showed poor results indicating a moderate to severe hip arthritis and disability. The improvement at the final F.U. was from poor to good results (mean of 84 points)
|
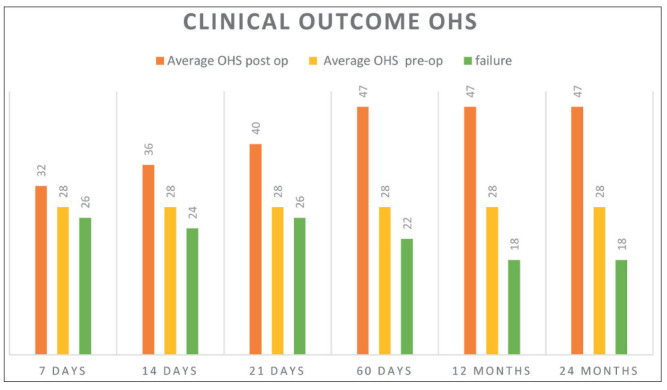
The average preoperative OHS was 28.1 ± 6.5, while the postoperative OHS improved significantly starting 21 days from micrografts injection (mean 35.4 ± 7.5), with an increasing trend to improvement after 60 days and all the way to one- and two-year FU (mean 37.4 ± 9.5). (table 1)
Table 1.
Oxford Hip Score evaluation before and after 7, 14, 21, 60 days and 1, 2 years of follow up. The results are expressed as average of all patients
|
The full recovery allowing complete activities of daily activities was reached after 20 days from intervention, while sports activities were not allowed before the 90 days landmark.
All hips underwent MRI evaluation at the six months FU, using an artefacts suppression technique: the authors observed the complete regression of the necrotic area accomplished by restoring of osseous structure suggesting an autologous micrografts-induced bone regeneration in 19 out of 20 patients (Fig. 2).
Figure 2.
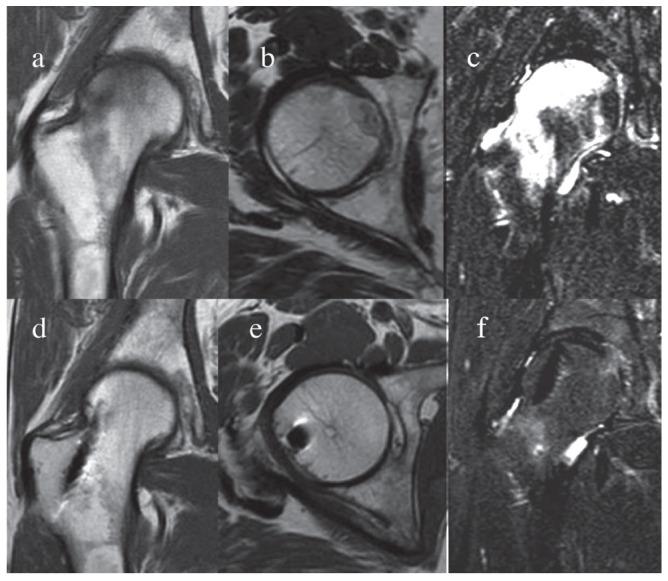
Magnetic resonance imaging (MRI) before and after micrografts treatment. The femoral head MRI was performed using a standard technique for artefact suppression. A complete regression of the necrotic area accomplished by restoring of osseous structure suggesting an autologous micrografts-induced bone regeneration
Discussion
Osteonecrosis of the femoral head is a disabling orthopedic disease which represents an important economic burden on the public health care system affecting young and middle-aged subjects. It can be asymptomatic until the progression to a symptomatic osteonecrotic phase, leading to femoral collapse. For these reasons, new approaches to manage this pathological condition become mandatory in order to preserve the joint and delay as much as possible the THA intervention.
In this study, we reported the positive role of autologous micrografts on the treatment of ONFH, confirming the hypothesis of the study. We observed a clinical improvement according to the HHS and OHS; regression of the necrotic area and new bone regeneration were both observed following MRI after six months from application.
The goal of the treatment of ONFH is to prevent rapid joint degeneration. The ideal approach should consider patients age, stage and location of the disease and the amount of bone affected and even if several options have been previously described, unfortunately there is not consensus regarding the ideal approach (15). The current authors assume that a pure and isolated mechanical action of core decompression (16) might be a limitated approach to start the healing process: a combined mechanism of action, biological and mechanical, induced respectively by the use of micrografts and a cannulated screw as a delivery method could be adopted to increase the success rate.
To support the role of micrografts in the treatment of ONFH, previous studies have reported their application in the management of chronic and complex wounds such as ulcers of different etiology (17-19), wound dehiscence (14, 20) and pathological scars (11). More recent studies have demonstrated the efficacy of micrografts also for cartilage (21-22) and cardiac regeneration (23). To date, the efficacy of autologous micrografts in bone regeneration is more evident in the dentistry applications including periodontal regeneration (24), sinus lift augmentation (10, 25) and alveolar socket preservation (26).
The use of autologous bone grafts in the treatment of osteonecrosis has been already reported but more clinical studies are mandatory to evaluate the real advantages of this approach. A recent study showed that the outcomes of autologous concentrated bone marrow grafting for advanced osteonecrosis of the humeral head are variable, concluding that further investigations are needed to determine their effectiveness (27). Other authors, analyzing the factors related to the clinical failure of bone grafting in the treatment of avascular ONFH, suggested that the advanced stage of the disease increases the risk of failure after bone graft surgery (28). According to our results, only patients belonging to ARCO stage II and IIIA showed a positive response to this treatment.
With the increasing interest for stem cell research and regenerative medicine, several authors consider the ONFH as a result of an imbalance between osteocytes formation and apoptosis suggesting that cytotherapy could represent a new approach (29-30). Preclinical studies in animal models have confirmed the positive effects of stem cells injection in the treatment of ONFH (31-32) and promising results were also obtained in few clinical reports showing as bone marrow cells (BMCs) transplantation reduces volume of necrosis in patients in the pre-collapse stage (33-34-35). These evidences are in line with the results described in our study: in fact, micrografts are able to maintain great regenerative properties similar to MSCs (36), leading to expression of several markers of mesenchymal stem cells, such as CD73, CD90 and CD105 (9, 37). Conversely, the current literature is heterogeneous in terms of the choice of cells, method of cell processing, cell characterization, quantitative and qualitative assessment of the cells used, and finally surgical methods of cell delivery (38-39). Despite cellular therapies are certainly a promising tool for restoring local cell populations after an injury or disease and encouraging data are reported also in the current study, more clinical trials, including a control group when possible, are required for a better understanding about the role of micrografts in the treatment of ONFH.
Conclusions
The results of this study confirm the authors hypothesis on the clinical efficacy of micrografts in the treatment of aseptic, avascular and non-traumatic ONFH: this technology appears to improve the quality of life of patients at least at a short F.U. and offers a valid alternative tool among those already used in the clinical practice.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Gangji V, Hauzeur JP, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. A pilot study. J Bone Joint Surg Am. 2004;86-A(6):1153–60. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop. 2015;6:590–e601. doi: 10.5312/wjo.v6.i8.590. doi.org/10.5312/wjo.v6.i8.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zalavras CG, Lieberman JR. Osteonecrosis of the femoral head: evaluation and treatment. J Am Acad Orthop Surg. 2014;22(7):455–64. doi: 10.5435/JAAOS-22-07-455. doi: 10.5435/JAAOS-22-07-455. [DOI] [PubMed] [Google Scholar]
- 4.Choi HR, Steinberg ME, Cheng E. Osteonecrosis of the femoral head: diagnosis and classification systems. Curr Rev Musculoskelet Med. 2015;8(3):210–20. doi: 10.1007/s12178-015-9278-7. doi: 10.1007/s12178-015-9278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markel DC, Miskovsky C, Sculco TP, et al. Core decompression for osteonecrosis of the femoral head. Clin Orthop Relat Res. 1996;323:226–33. doi: 10.1097/00003086-199602000-00031. [DOI] [PubMed] [Google Scholar]
- 6.Buckley PD, Gearen PF, Petty RW. Structural bone-grafting for early atraumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1991;73:1357–64. [PubMed] [Google Scholar]
- 7.Ali SA, Christy JM, Griesser MJ, et al. Treatment of avascular necrosis of the femoral head utilising free vascularised fibular graft: a systematic review. Hip Int. 2014;24:5–13. doi: 10.5301/hipint.5000076. [DOI] [PubMed] [Google Scholar]
- 8.Sorich MM, Cherian JJ, McElroy MJ, et al. Osteonecrosis of the Hip in Hematologic Disease: A Review of Conditions and Treatment Options. J Long Term Eff Med Implants. 2015;25(4):253–68. doi: 10.1615/jlongtermeffmedimplants.2015012529. [DOI] [PubMed] [Google Scholar]
- 9.Trovato L, Monti M, Del Fante C, et al. A New Medical device rigeneracons allows to obtain viable micro-grafts from mechanical disaggregation of human tissues. J Cell Physiol. 2015;230:2299–303. doi: 10.1002/jcp.24973. [DOI] [PubMed] [Google Scholar]
- 10.Brunelli G, Motroni A, Graziano A, D’Aquino R, Zollino I, Carinci F. Sinus lift tissue engineering using autologous pulp micro-grafts: A case report of bone density evaluation. J Indian Soc Periodontol. 2013;17(5):644–7. doi: 10.4103/0972-124X.119284. doi: 10.4103/0972-124X.119284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svolacchia F, De Francesco F, Trovato L, Graziano A, Ferraro GA. An innovative regenerative treatment of scars with dermal micrografts. J Cosmet Dermatol. 2016;15(3):245–53. doi: 10.1111/jocd.12212. doi: 10.1111/jocd.12212. [DOI] [PubMed] [Google Scholar]
- 12.Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement. British Journal of Bone and Joint Surgery. 1996;78-B(2):185–190. [PubMed] [Google Scholar]
- 13.Gardeniers JWM. Report of the Committee of Staging and Nomenclature. ARCO News Letter. 1993;5(2):79–82. [Google Scholar]
- 14.Marcarelli M, Trovato L, Novarese E, Riccio M, Graziano A. Rigenera protocol in the treatment of surgical wound dehiscence. Int Wound J. 2017;14(1):277–281. doi: 10.1111/iwj.12601. doi: 10.1111/iwj.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrory BJ, York SC, Iorio R, et al. Current practices of AAHKS members in the treatment of adult osteonecrosis of the femoral head. J Bone Joint Surg Am. 2007;89:1194e204. doi: 10.2106/JBJS.F.00302. doi.org/10.2106/JBJS.F.00302. [DOI] [PubMed] [Google Scholar]
- 16.Pierce TP, Jauregui JJ, Elmallah RK, Lavernia CJ, Mont MA, Nace J. A current review of core decompression in the treatment of osteonecrosis of the femoral head. Curr Rev Musculoskelet Med. 2015;8:228e32. doi: 10.1007/s12178-015-9280-0. doi.org/10.1007/s12178-015-9280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Francesco F, Graziano A, Trovato L, et al. A Regenerative Approach with Dermal Micrografts in the Treatment of Chronic Ulcers. Stem Cell Rev. 2017;13(1):149. doi: 10.1007/s12015-016-9698-9. doi: 10.1007/s12015-016-9698-9. [DOI] [PubMed] [Google Scholar]
- 18.Trovato L, Failla G, Serantoni S, Palumbo FP. Regenerative Surgery in the Management of the Leg Ulcers. J Cell Sci Ther. 2016;7:238. doi:10.4172/2157-7013.1000238. [Google Scholar]
- 19.Miranda R, Farina E, Farina MA. Micrografting chronic lower extremity ulcers with mechanically disaggregated skin using a micrograft preparation system. J Wound Care. 2018;27(2):60–65. doi: 10.12968/jowc.2018.27.2.60. [DOI] [PubMed] [Google Scholar]
- 20.Baglioni E, Trovato L, Marcarelli M, Frenello A, Bocchiotti MA. Treatment of Oncological Post-surgical Wound Dehiscence with Autologous Skin Micrografts. Anticancer Research. 2016;36(3):975–980. [PubMed] [Google Scholar]
- 21.Ceccarelli G, Gentile P, Marcarelli M, et al. In Vitro and In Vivo Studies of Alar-Nasal Cartilage Using Autologous Micro-Grafts: The Use of the Rigenera® Protocol in the Treatment of an Osteochondral Lesion of the Nose. Pharmaceuticals (Basel) 2017;10(2) doi: 10.3390/ph10020053. pii: E53. doi: 10.3390/ph10020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Reconstruction of Alar Nasal Cartilage Defects Using a Tissue Engineering Technique Based on a Combined Use of Autologous Chondrocyte Micrografts and Platelet-rich Plasma: Preliminary Clinical and Instrumental Evaluation. Plast Reconstr Surg Glob Open. 2016;4(10):e1027. doi: 10.1097/GOX.0000000000001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lampinen M, Nummi A, Nieminen T, Harjula A, Kankuri E. Intraoperative processing and epicardial transplantation of autologous atrial tissue for cardiac repair. Journal of Heart and Lung Transplantation. 2017;36(9):1020–1022. doi: 10.1016/j.healun.2017.06.002. doi.org/10.1016/j.healun.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Graziano A, Carinci F, Scolaro S, D’Aquino R. Periodontal tissue generation using autologous dental ligament micro-grafts: case report with 6 months follow-up. Annals of Oral & Maxillofacial Surgery. 2013;1(2):20. [Google Scholar]
- 25.Rodriguez y Baena, D’Aquino R, et al. Autologous periosteum-derived micrografts and PLGA/HA enhance the bone formation in sinus lift augmentation. Frontiers in cell and developmental biology, 5, 87. 2017 doi: 10.3389/fcell.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Aquino R, Trovato L, Graziano A, et al. Periosteum-derived micro-grafts for tissue regeneration of human maxillary bone. Journal of Translational Science. 2016;2(2):125–29. doi: 10.15761/JTS.1000128. [Google Scholar]
- 27.Makihara T, Yoshioka T, Sugaya H, Yamazaki M, Mishima H. Autologous Concentrated Bone Marrow Grafting for the Treatment of Osteonecrosis of the Humeral Head: A Report of Five Shoulders in Four Cases. Case Rep Orthop. 2017;2017:4898057. doi: 10.1155/2017/4898057. doi: 10.1155/2017/4898057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuo W, Sun W, Zhao D, Gao F, Su Y, Li Z. Investigating Clinical Failure of Bone Grafting through a Window at the Femoral Head Neck Junction Surgery for the Treatment of Osteonecrosis of the Femoral Head. PLoS One. 2016;11(6):e0156903. doi: 10.1371/journal.pone.0156903. doi: 10.1371/journal.pone.0156903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee K, Chan CK, Patil N, Goodman SB. Cell therapy for bone regeneration—bench to bedside. J Biomed Mater Res B Appl Biomater. 2009;89:252–263. doi: 10.1002/jbm.b.31199. [DOI] [PubMed] [Google Scholar]
- 30.Gangji V, Hauzeur JP, Schoutens A, Hinsenkamp M, Appelboom T, Egrise D. Abnormalities in the replicative capacity of osteoblastic cells in the proximal femur of patients with osteonecrosis of the femoral head. J Rheumatol. 2003;30:348–351. [PubMed] [Google Scholar]
- 31.Asada T, Kushida T, Umeda M, et al. Prevention of corticosteroid-induced osteonecrosis in rabbits by intra-bone marrow injection of autologous bone marrow cells. Rheumatology. 2008;47:591–596. doi: 10.1093/rheumatology/ken037. doi: 10.1093/rheumatology/ken037. [DOI] [PubMed] [Google Scholar]
- 32.Yan Z, Hang D, Guo C, Chen Z. Fate of mesenchymal stem cells transplanted to osteonecrosis of femoral head. J Orthop Res. 2009;27:442–446. doi: 10.1002/jor.20759. doi: 10.1002/jor.20759. [DOI] [PubMed] [Google Scholar]
- 33.Hernigou P, Beaujean F. (2002) Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23. doi: 10.1097/00003086-200212000-00003. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2008;43:40–45. doi: 10.4103/0019-5413.45322. doi: 10.4103/0019-5413.45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernigou P, Zilber S, Filippini P, Rouard H, Mathieu G, Poignard A. Bone marrow injection in hip osteonecrosis. Tech Orthop. 2008;23:18–25. doi: 10.1097/BTO.0b013e3181690814. [Google Scholar]
- 36.Monti M, Graziano A, Rizzo S, et al. In Vitro and In Vivo Differentiation of Progenitor Stem Cells Obtained after Mechanical Digestion of Human Dental Pulp. J Cell Physiol. 2017;232:548–555. doi: 10.1002/jcp.25452. doi: 10.1002/jcp.25452. [DOI] [PubMed] [Google Scholar]
- 37.Rohban R, Pieber TR. Mesenchymal Stem and Progenitor Cells in Regeneration: Tissue Specificity and Regenerative Potential. Stem Cells Int. 2017;2017:5173732. doi: 10.1155/2017/5173732. doi: 10.1155/2017/5173732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piuzzi NS, Chahla J, Jiandong H, et al. Analysis of Cell Therapies Used in Clinical Trials for the Treatment of Osteonecrosis of the Femoral Head: A Systematic Review of the Literature. J Arthroplasty. 2017;32(8):2612–2618. doi: 10.1016/j.arth.2017.02.075. doi: 10.1016/j.arth.2017.02.075. [DOI] [PubMed] [Google Scholar]
- 39.Piuzzi NS, Chahla J, Schrock J, et al. Evidence for the use of cell-based therapy for the treatment of osteonecrosis of the femoral head: a systematic review of the literature. J Arthroplasty. 2017;32(5):1698–1708. doi: 10.1016/j.arth.2016.12.049. doi: 10.1016/j.arth.2016.12.049. [DOI] [PubMed] [Google Scholar]


