Abstract
Acute kidney injury (AKI) and acute kidney disease (AKD) are common complications in hospitalized patients and are associated with adverse outcomes. Although consensus guidelines have improved the care of patients with AKI and AKD, guidance regarding quality metrics in the care of patients after an episode of AKI or AKD is limited. For example, few patients receive follow-up laboratory testing of kidney function or post-AKI or AKD care through nephrology or other providers. Recently, the Acute Disease Quality Initiative developed a consensus statement regarding quality improvement goals for patients with AKI or AKD specifically highlighting efforts regarding quality and safety of care after hospital discharge after an episode of AKI or AKD. The goal is to use these measures to identify opportunities for improvement that will positively affect outcomes. We recommend that health care systems quantitate the proportion of patients who need and actually receive follow-up care after the index AKI or AKD hospitalization. The intensity and appropriateness of follow-up care should depend on patient characteristics, severity, duration, and course of AKI of AKD, and should evolve as evidence-based guidelines emerge. Quality indicators for discharged patients with dialysis requiring AKI or AKD should be distinct from end-stage renal disease measures. Besides, there should be specific quality indicators for those still requiring dialysis in the outpatient setting after AKI or AKD. Given the limited preexisting data guiding the care of patients after an episode of AKI or AKD, there is ample opportunity to establish quality measures and potentially improve patient care and outcomes. This review will provide specific evidence-based and expert opinion–based guidance for the care of patients with AKI or AKD after hospital discharge.
Keywords: acute kidney injury, outcomes, quality, recovery
Acute kidney injury (AKI) is a common clinical syndrome and remains associated with increased morbidity, mortality, and cost of care1,2 despite recent publications demonstrating that AKI rates and severity may be reduced through the use of care bundles.3, 4, 5 The Kidney Disease Improving Global Outcomes AKI work group originally introduced the concept of acute kidney disease (AKD) to underscore the importance of prolonged kidney dysfunction (in the presence or absence of AKI) that may occur before a patient meets the 90-day criteria needed for the diagnostic criteria of chronic kidney disease (CKD).6 Subsequently, the Acute Disease Quality Initiative (ADQI) proposed staging criteria for AKD (Figure 1).7 Because the care of AKI is not well-standardized, it should be unsurprising that the follow-up care of patients with AKD is even less so and no published quality and patient care guidelines exist. The Acute Disease Quality Initiative is a multiprofessional, interdisciplinary consensus organization that identifies areas of importance within the field of AKI and develops consensus statements regarding clinical care and research. Recently, ADQI XXII met to develop quality improvement goals for AKI and AKD.8 Here, we will discuss the opportunities to establish quality measures in those with AKI or AKD after hospital discharge.
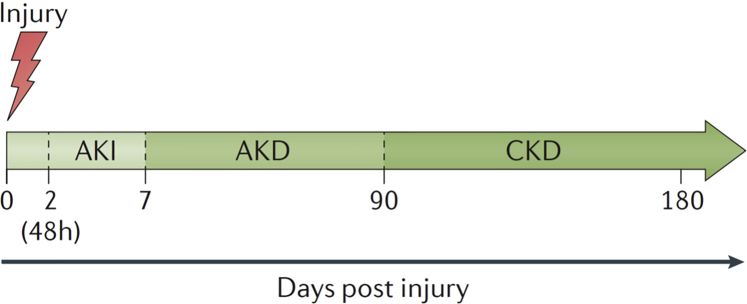
Figure 1.
The spectrum of acute kidney injury (AKI), acute kidney disease (AKD), and chronic kidney disease (CKD). Acute kidney injury, AKD, and CKD can form a spectrum of diagnoses in which the initial kidney injury can potentially lead to the development of CKD. Acute kidney injury describes a process of damage, loss of kidney function, or both for 7–90 days after exposure to an AKI initiating event. For patients with preexisting CKD, the AKI event can be superimposed on CKD, with AKD existing on a background of CKD. Patients who experience AKD with preexisting CKD are probably at high risk for kidney disease progression. Modified from Acute Disease Quality Initiative XVI (www.adqi.org).7
Statement 1: To Optimize the Care of Patients With AKI or AKD, Health Care Systems Need to Quantitate the Proportion of Patients Who Need and Do Receive Follow-Up Care Following Their Index AKI or AKD Hospitalization
Given the significant morbidity and mortality associated with AKI, a comprehensive medical follow-up is desirable, although the evidence base in terms of quality metrics is sparse.9 Even in cases of severe AKI, patients rarely receive nephrology follow-up; in a retrospective cohort study of US veterans, only 17 of 57 subjects with stage 3 AKI were referred for follow-up.10 However, despite a lack of robust data to support their beliefs, most nephrologists and health care providers believe that follow-up after an episode of AKI or AKD is important.11 As a first step, health care systems need to determine systematically the proportion of hospitalized patients who have AKI or AKD as well as the number who receive postdischarge follow-up care. Once baseline numbers are established, they can be followed as a quality indicator. This will allow health care systems to identify barriers to appropriate follow-up and how best to achieve appropriate follow-up in 100% of patients.
As discussed subsequently, the type and intensity of follow-up will depend on patient characteristics and the severity of AKI or AKD. Depending on local systems, it may be most practical to take a staged approach to quality improvement. This can be done through large-scale data collection tools to look at system-wide practices, but it may be easiest to start tracking the follow-up for patients seen by nephrology specialists who received renal replacement therapy during admission; systems must find a model that works for them and build on it. Using quality improvement approaches, barriers to follow-up care should be identified and remediated. If the proportion of those receiving proper care is already high, or as this proportion increases, subsequent quality improvement efforts can focus on less severe AKI and AKD populations. Supplementary Appendix A provides a case-based example of how this process could work.
Statement 2: Intensity and Appropriateness of Follow-up Depend on Patient Characteristics as Well as Severity, Duration, and Course of AKI
Given the increasing numbers of patients with severe AKI and AKD and the work force issues within nephrology, it is important to prioritize patients who are most in need of nephrology follow-up. Even within the group of patients with the most severe AKI, follow-up is suboptimal. In a cohort of patients with AKI after cardiac surgery, only 66 of 359 received nephrology follow-up within the first postoperative year (18%), even though 54% of the cohort had received renal replacement therapy in the early postoperative period.12 Most of this group ideally should have received follow-up in a nephrology-focused, multidisciplinary setting, such as a dedicated post-AKI or AKD clinic, where the nephrologist could collaborate with allied health care practitioners (pharmacists, dietitians, and social workers) and primary care. However, follow-up for less severe AKI or AKD (e.g., stage 1 AKI) may be through non-nephrology providers, including primary care and other medical subspecialists. Although some areas or countries may be able to provide care to all AKD patients, in other areas or countries, the number of AKI and AKD survivors may be greater than the capacity for the nephrology community to provide care. As such, these non-nephrology providers have an integral role in the long-term care of post-AKI and AKD patients. Follow-up may be to ensure that full recovery has occurred based on laboratory testing (e.g., serum creatinine [SCr] and albuminuria) or monitoring for sequelae of AKI that may be associated with the development of CKD (e.g., hypertension, development of albuminuria).
Patient Characteristics
The healthy patient with no comorbidities who experiences a short, transient, and completely reversible episode of AKI does not require nephrology follow-up. When SCr has returned to baseline at the time of discharge, patient assessment at 6–12 months would be reasonable, perhaps as a part of routine care with a primary care provider, including simple kidney blood and urine tests. When individuals have a more protracted AKI or AKD course, follow-up within 3–6 months seems appropriate. When there is stage 3 AKI or AKD with nonrecovery, follow-up should engage specialist services at appropriate times (Figure 2),8 often within days of discharge. In addition to AKI or AKD severity, patient comorbidities are a critical determinant for the type and intensity of follow-up. Patients with significant comorbidities, especially those with significant CKD, should remain under close nephrology care.
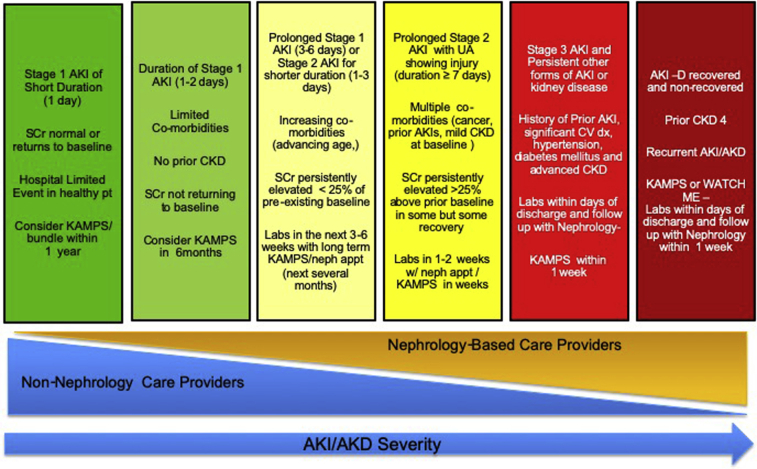
Figure 2.
Schematic for acute kidney injury (AKI) and acute kidney disease (AKD) follow-up. The figure displays a paradigm for the care of patients who experience AKI or AKD. Follow-up with nephrology and non-nephrology changes based on the duration and severity of AKI or AKD and varies along the horizontal axis. The timing and nature of follow-up are suggestions because there are limited data to inform this process. AKI-D, patients with dialysis-requiring AKI; CKD, chronic kidney disease; CV dx, cardiovascular disease; KAMPS, Kidney Function Check, Advocacy, Medications, Pressure, Sick Day Protocols; UA, urinalysis; neph appt, nephrology appointment; SCr, serum creatinine; Weight Assessment, Access, Teaching, Clearance, Hypotension, and Medications. Modified from Acute Disease Quality Initiative XXII (www.adqi.org).8
To determine which post-AKI and AKD patients were at highest risk for developing CKD, James and colleagues13 derived (Alberta) and validated (Ontario) a predictive model using data from over 12,500 Canadian admissions. Their risk score is composed of 6 graded components, including patient age, gender, baseline SCr, degree of albuminuria, peak AKI stage, and discharge SCr. The most heavily weighted variable in the score is discharge SCr; the most points are awarded for those who had an SCr of >1.3 mg/dl. Although this score was externally validated in its original publication, it has yet to be validated in other international cohorts or implemented on a wide scale.13
Although the score of James and colleagues13 provides some clues as to who needs closer follow-up for long-term complications of AKI, those with recent AKI-related acute complications such as acid-base or electrolyte disorders, volume overload, and incomplete kidney recovery will also require close nephrology follow-up.
Patients with severe AKI appear to have better outcomes with specialist follow-up, although those already under nephrology care before AKI or AKD may fare less well, probably reflecting the complications of long-standing kidney disease.14 The absence of CKD does not imply that follow-up is not needed. Readmission after AKI or AKD is common in patients with chronic obstructive pulmonary disease or urinary tract infection, and those with volume-overload or pulmonary edema.15,16 Emerging data suggest that patients with documented AKI are at increased risk for heart failure readmission.17,18
Timing of Follow-up
There is no evidence as to the optimal timing of follow-up after an episode of AKI. Figure 2 shows our proposed schema with regard to the timing of follow-up. The recommended timing cannot be viewed in isolation, but is tailored to the patient’s comorbidities and severity of AKI or AKD as well as the need for follow-up with nephrology or non-nephrology providers. As follow-up becomes more standardized, it may be appropriate for health care systems to track AKI and AKD outcomes specifically at 90 days or perhaps at 1 year, as well as to ascertain who is providing that care.
Emerging data suggest that measuring the urinary albumin to creatinine ratio (uACR) 3 months after hospital discharge after an episode of AKI may allow for the identification of patients at highest risk for kidney disease progression (defined as halving of the estimated glomerular filtration rate [GFR] or the development of end-stage kidney disease [ESRD]).19 In a multicenter, prospective, observational cohort of 1538 North Americans, uACR at 3 months was a valuable stratification tool for kidney disease progression, whereas severity and staging of AKI were not. In the future, measuring uACR after AKI may be linked to specific protocolized interventions in high-risk patients.19 Future investigations should attempt to discern the impact of early versus late follow-up as well as the ability of other biomarkers to stratify long-term patient outcomes.
Potential Interventions
Similar to timing, AKI and AKD follow-up investigations depend on patient comorbidities and the severity of AKI or AKD. Given the relative lack of evidence-based care in this population, a standardized definition of appropriate follow-up care is needed. Nephrologists need to identify patients who benefit most from follow-up. Based on the limited published evidence and current expert opinion, we recommend the following key components of a post-AKI and AKD bundle (Table 1).8 Compliance with the whole bundle or individual components can then be used as a quality indicator over time. The Kidney Function Check, Advocacy, Medications, Pressure, Sick Day Protocols care bundle includes functional kidney testing, including both GFR estimation and indices of tubular and glomerular dysfunction (e.g., albuminuria, proteinuria). Blood pressure control as well as review of medications are paramount, particularly concerning over-the-counter and herbal therapies. Communication with other health providers and the patient are critical, particularly in relation to medications that may need monitoring during episodes of acute illness (e.g., medications excreted predominantly by the kidney and nephrotoxic drugs, kidney-excreted nephrotoxic drugs [KENDS]). Keeping a close eye on KENDS, medication review and reconciliation are an essential part of AKI and AKD care and should occur at the first postdischarge and all future clinic appointments.20 Although nephrologists are ideally equipped to perform post-AKI and AKD medication review and reconciliation, this can be done by pharmacists. Many of these components are not derived from multicenter studies, but they are all grounded in the consensus care for patients with AKI.6 Adherence to such an approach will potentially provide optimal management strategies and standardize care. Monitoring of adherence and subsequent clinical outcomes will lead to the development of a more robust evidence base for the care of these patients.
Table 1.
Post–acute kidney injury and acute kidney disease kidney health care bundle
| KAMPS | Components |
|---|---|
| Kidney Function Check | Kidney function measurement by serum creatinine or cystatin C; measured or estimated glomerular filtration rate Proteinuria or albuminuria When available, consider biomarkers, imaging, and other tests as feasible and indicated |
| Advocacy | Patient and caregiver education about acute kidney injury and chronic kidney disease Communication with other care providers (i.e., general practitioners, dietitians, nurses, pharmacists, and social workers) |
| Medications | Medication reconciliation, review, and management: specifically, discuss the risk benefits of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers Review KENDs and over the counter medications |
| Pressure | Ensure patient understands blood pressure goals and targets Discuss fluid status, ideal weight and the role of diuretics |
| Sick Day protocols | Educate patients on medications that need monitoring during acute illnesses Consider protocols to withhold kidney-excreted nephrotoxic drugs |
Improving care for AKD patients requires a change from the current management paradigm as well as prioritization and implementation efforts. Appropriate risk stratification, timely and reliable pharmacologic treatments, and education of patients, their caregivers, and non-nephrologists could be strategies to optimize care. The post-AKI and AKD care process starts for an inpatient at the time of hospital-diagnosed AKI, continues in the community, and should include any and all readmissions. Each of these phases requires specific interventions, with nephrologists leading the multidisciplinary AKI and AKD process.
To start, care should focus on the AKI to CKD transition, especially with regard to the high risk for subsequent ESRD and cardiovascular disease. Because only a minority of patients with severe AKI or AKD (e.g., stage 2 or 3 AKI) actually receive dedicated nephrology follow-up, an emphasis on the need for appropriate long-term nephrology care earlier in the inpatient stay should allow for more appropriate outpatient follow-up. In a retrospective propensity score–matched cohort study, nephrology follow-up after AKI was associated with lower all-cause mortality compared with non-nephrology care (hazard ratio = 0.76, 95% confidence interval, 0.62–0.93). However, the nature of this benefit needs to be investigated further, perhaps through randomized controlled trials, to determine the benefits of follow-up in less severe forms of AKI.14
Statement 3: Post-AKI and AKD Care Should Be Evidence-Based and Evolve With Emerging Data
Tertiary prevention involves managing a disease process after it is already clinically apparent. Acute kidney injury increases the incidence of de novo CKD, 30-day readmission after discharge, long-term dialysis, remote organ injury, and death.21, 22, 23, 24 The tertiary prevention of AKI should focus on maintenance or improvement in the quality of life after AKI to mitigate long-term comorbidities or consequences.25 However, as with other areas of post-AKI and AKD care, there is no high-level clinical evidence that current follow-up care plans affect these outcomes.
Rates of kidney-related laboratory testing after hospital discharge are low. In the United States in 2013, follow-up creatinine measurements after an episode of AKI occurred within 6 months in only 54% of surviving patients.26 These data are even more concerning when one considers that after AKI or AKD, many fewer patients have quantitative proteinuria, parathyroid hormone, hemoglobin, and phosphorus measurements. Although not all AKI and AKD patients may require each of these measurements, these retrospective data demonstrates that a minority of appropriate patients receive standard nephrology follow-up.27
Multiple AKI researchers have proposed that the transition period between AKI and CKD may be an opportunity to intervene. However, currently, no proven interventions enable long-term target organ protection in AKD patients. Emerging animal models and limited retrospective human data suggest that interventions including improved blood pressure control, avoidance of nephrotoxins, and the initiation of renin–angiotensin–aldosterone system (RAAS) blockade agents28,29 or mineralocorticoid receptor antagonists30 may prevent the progression of kidney disease and its complications. However, in humans, after an episode of AKI, administration of RAAS agents may increase the risk for hyperkalemia as well as the need for nephrology-based hospitalization.29
In a retrospective cohort study of US adults, post-AKI patients had a 22% increase in the odds of developing hypertension.31 However, RAAS agents are associated with functional AKI, especially in the setting of acute hypovolemia.6,32,33 After any hospitalization, patients are at risk for unintentional medication discontinuation, and hypertension agents are often discontinued during inpatient stays in the setting of hypotension or transfer to the intensive care unit. As such, medication reconciliation is an essential part of post-AKI and AKD care. Potential discrepancies should be accounted for after hospital discharge to ensure they are meeting established targets recommended by hypertension, diabetes, lipid, and CKD practice guidelines.34
From a population-based cohort study, statin use is associated with reduced risks for 1-year and in-hospital mortality in patients with dialysis-requiring AKI (AKI-D)35 and with decreased sepsis-related mortality in patients with advanced CKD.36 Data from a retrospective cohort demonstrate that in diabetic patients with a history of AKI-D, dipeptidyl peptidase 4 inhibitors use is associated with a reduced risk for ESRD and mortality.37 Finally, clinically unwarranted receipt of medications that have been specifically implicated and associated with increased risk for AKI (e.g., proton-pump inhibitors or nonsteroidal anti-inflammatory drugs) should be avoided.38 Although there are minimal prospective data to inform these recommendations, medication reconciliation, avoidance of potential nephrotoxins, and appropriate medication dosing seem like proper steps to improve outcomes in patients after an episode of AKI or AKD
Statement 4: Quality Indicators for AKI-D and Postdischarge AKD-D Should Be Similar to Quality Indicators During Hospitalization and Distinct From ESRD Measures. In Addition, There Need To Be Specific Quality Indicators for the Outpatient Setting (e.g., Compliance With the Weight Assessment, Access, Teaching, Clearance, Hypotension, Medications Care Bundle Could Be Considered)
In contrast to the ESRD population, there are currently no established quality indicators for AKI-D care.39 An important limitation has been the lack of large-scale, prospective, clinical trials to support specific measures.40 End-stage kidney disease quality measures may not be appropriate for the AKI population, because patients with AKI will be at greater risk for complications and also have the goal of kidney function recovery. Determining factors that predict and promote kidney recovery after AKI-D may help to improve the quality of care. Premature designation of patients with AKI-D as ESRD may constitute a lost opportunity to promote kidney recovery.41
As patients with AKI-D transition from the acute period into the outpatient setting, it is critical for them to be recognized as a population with special needs. This begins with an appropriate hand off to the outpatient team and includes educating providers and patients themselves. Based on the available retrospective cohort data and expert opinion, we recently proposed key elements (the Weight Assessment, Access, Teaching, Clearance, Hypotension, and Medications care bundle) (Table 2) for high-quality AKI-D care in the outpatient setting.8
Table 2.
Acute kidney injury requiring dialysis and acute kidney disease requiring dialysis kidney health care bundle
| WATCH-ME | Components |
|---|---|
| Weight Assessment | Discuss Dry Weight monitoring and permissive hypervolemia Discuss the role of diuretics in maintaining urine output and ideal volume status |
| Access | Educate patients about the care of central venous catheters Vein preservation protocols AND awareness When appropriate, begin to plan and educate about the role of arteriovenous access and other RRT modalities |
| Teaching | Patient and Caregiver education about dialysis requiring acute kidney disease and short- and long-term risks and consequence Communication with other care providers (e.g., general practitioners, dietitians, nurses, pharmacists, and social workers) about patient needs (e.g., alterations in medication regimens in the setting of new RRT). |
| Clearance | Frequent assessments of underlying kidney function (through predialysis laboratory values or timed clearances) Frequent assessments of the quality of the RRT being provided to ensure adequate clearance |
| Hypotension | Patient education and optimization of care to avoid intradialytic about hypotension Education regarding blood pressure medication administration in peri-RRT period |
| Medications | Medication reconciliation, review, and management Specifically, discuss risk benefits of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers Review kidney-excreted and nephrotoxic drugs and over-the-counter medications |
RRT, renal replacement therapy.
Weight Assessment
In contrast to the paradigm of dry weight challenges for ESRD patients, AKI-D patients may require a mild permissive hypervolemia approach that emphasizes avoiding intradialytic hypotension (IDH). The dangers of IDH and high ultrafiltration rates (>13 ml/kg/h) in ESRD patients have been well-described.42 In AKI-D, IDH may exacerbate ischemic kidney injury and decrease the chances of recovery.43 Two separate retrospective studies found that higher ultrafiltration rates and more frequent IDH were associated with a lower likelihood of recovery in outpatients with AKI-D.43,44 At a minimum, patients with AKI-D should have a regular (weekly) reassessment of target weight and fluid removal goals. In non-anuric patients, diuretics may be helpful to limit interdialytic weight gain and decrease ultrafiltration rates.45 The role of specific measures to prevent IDH, such as cooled dialysate or sodium modeling, has not been formally assessed in the AKI setting.
Access
Nearly all patients with AKI-D will have a central venous catheter as primary access. Furthermore, placement of arteriovenous access should be delayed (appropriately) while monitoring for kidney function recovery.46 These patients and their families will be at significant risk for infectious complications, and they should receive proper central venous catheter care and education before hospital discharge, which should continue as an outpatient. Although limited data exist regarding central venous catheter issues in those with AKI-D, much can be learned from investigations in other populations with central venous catheters.47,48 Patients with AKI-D should receive training about vein preservation. Delivery of these educational components is an excellent example of an important and easy quality measure for health systems and dialysis facilities to track.
Teaching
By definition, AKI-D is a potentially reversible condition, and studies suggest that upward of 40% of patients with AKI-D who are discharged on dialysis may recover to dialysis independence.44,49,50 Patients and their caregivers need to be educated to monitor for kidney function recovery. Many patients with AKI-D will not have had the benefit of nephrology care before the acute illness, so broader education regarding kidney health should be included when appropriate.14,46 In the dialysis facility, patients with AKI-D should be clearly identifiable separately from ESRD patients. Patients with dialysis-requiring AKI should undergo a regular care plan meeting (similar to the ESRD process) that articulates the individual's clinical trajectory and personalized expectations for kidney recovery.
Clearance
We believe dialysis facilities caring for patients with AKI-D need to have the appropriate processes to allow for more frequent blood and urine testing, assessing for kidney recovery.46 This can be done through blood work (e.g., trending predialysis SCr) and timed urine collections, and should be pursued at least weekly during the outpatient transition.51
Dialysis clearance should also be measured and used to guide prescription. Evidence from the large-scale, randomized controlled acute tubular necrosis trial suggests that for patients with AKI-D, a delivered Kt/tv urea of 1.2 thrice weekly can be considered adequate dialytic clearance.52 However, patients with AKI-D may have more individualized needs, such as higher catabolism requiring more dialysis for symptomatic control of uremia. Conversely, patients who begin to recover kidney function may tolerate a tapering of dialysis to less than 3 weekly treatments.
Hypotension
Intradialytic hypotension is associated with adverse outcomes and may decrease the likelihood of kidney function recovery in patients with AKI-D.43,44 Emphasizing the importance of limiting interdialytic weight gain is essential, as is the careful adjustment of antihypertensive medications.
Medications
As with any transition in health care settings, the move to outpatient AKI-D management should be accompanied by review and reconciliation of medications.53,54 The review and reconciliation process needs to be frequently repeated, especially when kidney function begins to recover, to ensure adequate and appropriate dosing of medications.20 Patients should be educated regarding the potential impact of kidney failure and dialysis on drug clearances, but they should also be instructed that as kidney function begins to recover, drug clearances may be improved and medication dosing may need to be increased or even discontinued. A specific review of common nephrotoxic medications and KENDS should also be performed.20
In addition to these potential measures that focus on processes of care, ideally, outcomes measures such as hospital readmission rates and dialysis independence recovery rates should be monitored. However, there are currently no established benchmarks, and these rates will vary based on patient characteristics. Given the dearth of data in this area, monitoring these rates can help to identify trends and potential quality improvement opportunities.
Conclusions
Quality measures are needed for the management of AKI and AKD patients after the index hospitalization to standardize care and improve patient outcomes. Relatively few patients receive follow-up laboratory testing of kidney function or post-AKI and AKD care. Table 3 provides a potential initial quality scorecard for a health care system to begin to monitor and improve AKI and AKD care. Although this tool has not been validated, it is based on the limited published data and expert opinion. It captures the basic numbers an institution will need to track in the short and long term to care for patients with AKI and AKD. Similarly, Supplementary Appendix A and Supplementary Figure S1 provide a hypothetical quality improvement project related to the care of patients with AKI and AKD.
Table 3.
Potential quality score card to track follow-up care of patients with AKI/AKD
| Patient metric | Current quarter n/rate | Prior quarter n/rate | Prior year event rate | Current year goal rate |
|---|---|---|---|---|
| Patients with dialysis-requiring AKI or AKD | ||||
| Of those with dialysis-requiring AKI or AKD, no. of unique hospitalized patients | ||||
| Of those with dialysis-requiring AKI or AKD no. of patients surviving to hospital discharge | ||||
| No. of patients with a measure of kidney function (serum creatinine, cystatin C, proteinuria, etc.) within 30 d of discharge | ||||
| No. of patients who no longer need RRT 90 d after discharge | ||||
| No. of patients who no longer need RRT by 90 d after discharge, who received nephrology follow-up within 6 mo |
AKD, acute kidney disease; AKI, acute kidney injury; RRT, renal replacement therapy.
In the future, outcomes such as 30-day readmission rates, catheter-associated infection rates, and short- and long-term mortality after an episode of AKI or AKD may be quality metrics that will be reported. For now, health care institutions should be aware of the number of patients who warrant post-AKI or AKD care as well as track those who go on to receive some form of follow-up. This focused follow-up care could be provided by either nephrology and non-nephrology providers, and this may be an important metric to track. Future quality improvement work and research could focus on the optimal management strategies and clinical effectiveness of the KAMPS and Weight Assessment, Access, Teaching, Clearance, Hypotension, and Medications bundle components and the potential development and validation of new bundles.
Footnotes
Acknowledgments: The ADQI XXII Participants.
Appendix A. Sample Quality Improvement Project
Figure S1. Pareto chart for analysis of the lack of appropriate medication list review at discharge and communication with primary physicians. AKI, acute kidney injury; Cum, Cumulative.
Contributor Information
Jay L. Koyner, Email: jkoyner@uchicago.edu.
Acute Disease Quality Initiative Investigators:
Jay L. Koyner, Kathleen D. Liu, Lui G. Forni, Kianoush Kashani, Michael Heung, Vin-Cent Wu, John A. Kellum, Michael Haase, Claudio Ronco, and Ravi Mehta
Disclosure
JLK is a consultant for Astute Medical, Sphingotec, and Baxter and receives research support from NxStage, Astute Medical, and Satellite Health Care. KDL is a consultant for Durect, Quark, and Portero; is on the advisory board for AstraZeneca; is a speaker for Baxter; and is a stockholder of Amgen. LGF receives research support from Baxter, La Jolla Pharmaceuticals, and Ortho-Clinical-Diagnostics and receives honoraria from Baxter, La Jolla Pharmaceuticals, Ortho-Clinical-Diagnostics, MediBeacon, and Fresenius. JAK is a consultant for Adrenomed, Astute Medical, Baxter, bioMérieux, Bioporto, Davita, Fresenius, Mallinckrodt, Novartis, NxStage, Potrero, RenalSense, and Sphingotech and receives grant support from Astute Medical, Baxter, bioMérieux, and RenalSense. MH is a consultant and lecturer for Abbott, Alere, Baxter, Novartis, Siemens, Roche, and FastBiomedical and receives grant support from Abbott Diagnostics. RLM is on the advisory board for Astute Medical, Baxter, Mallinckrodt, and Indalo Therapeutics and received research funds from Fresenius, Fresenius-Kabi, Grifols, and Relypsa. MHR reports honoraria from American Society of Nephrology and Baxter and serves on data safety monitoring boards for Retrophin and Reata Pharmaceuticals. All other authors declared no competing interests.
Supplementary Material
References
- 1.Chertow G.M., Burdick E., Honour M. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 2.Hobson C., Ozrazgat-Baslanti T., Kuxhausen A. Cost and mortality associated with postoperative acute kidney injury. Ann Surg. 2015;261:1207–1214. doi: 10.1097/SLA.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meersch M., Schmidt C., Hoffmeier A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gocze I., Jauch D., Gotz M. Biomarker-guided intervention to prevent acute kidney injury after major surgery: the prospective randomized BigpAK study. Ann Surg. 2018;267:1013–1020. doi: 10.1097/SLA.0000000000002485. [DOI] [PubMed] [Google Scholar]
- 5.Selby N.M., Casula A., Lamming L. An organizational-level program of intervention for AKI: a pragmatic stepped wedge cluster randomized trial. J Am Soc Nephrol. 2019;30:505–515. doi: 10.1681/ASN.2018090886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;(suppl):1–138. [Google Scholar]
- 7.Chawla L.S., Bellomo R., Bihorac A. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13:241–257. doi: 10.1038/nrneph.2017.2. [DOI] [PubMed] [Google Scholar]
- 8.Kashani K., Rosner M.H., Haase M. Quality improvement goals for acute kidney injury. Clin J Am Soc Nephrol. 2019;14:941–953. doi: 10.2215/CJN.01250119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawhney S., Marks A., Fluck N. Post-discharge kidney function is associated with subsequent ten-year renal progression risk among survivors of acute kidney injury. Kidney Int. 2017;92:440–452. doi: 10.1016/j.kint.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siew E.D., Peterson J.F., Eden S.K. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol. 2012;23:305–312. doi: 10.1681/ASN.2011030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karsanji D.J., Pannu N., Manns B.J. Disparity between nephrologists' opinions and contemporary practices for community follow-up after AKI hospitalization. Clin J Am Soc Nephrol. 2017;12:1753–1761. doi: 10.2215/CJN.01450217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishra P.K., Luckraz H., Nandi J. Long-term quality of life postacute kidney injury in cardiac surgery patients. Ann Card Anaesth. 2018;21:41–45. doi: 10.4103/aca.ACA_104_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James M.T., Pannu N., Hemmelgarn B.R. Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. JAMA. 2017;318:1787–1797. doi: 10.1001/jama.2017.16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harel Z., Wald R., Bargman J.M. Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int. 2013;83:901–908. doi: 10.1038/ki.2012.451. [DOI] [PubMed] [Google Scholar]
- 15.Sawhney S., Marks A., Fluck N. Acute kidney injury as an independent risk factor for unplanned 90-day hospital readmissions. BMC Nephrol. 2017;18:9. doi: 10.1186/s12882-016-0430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silver S.A., Harel Z., McArthur E. 30-day readmissions after an acute kidney injury hospitalization. Am J Med. 2017;130 doi: 10.1016/j.amjmed.2016.09.016. 163.e164–172.e164. [DOI] [PubMed] [Google Scholar]
- 17.Lee B.J., Hsu C.Y., Parikh R.V. Non-recovery from dialysis-requiring acute kidney injury and short-term mortality and cardiovascular risk: a cohort study. BMC Nephrol. 2018;19:134. doi: 10.1186/s12882-018-0924-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bansal N., Matheny M.E., Greevy R.A., Jr. Acute kidney injury and risk of incident heart failure among US veterans. Am J Kidney Dis. 2018;71:236–245. doi: 10.1053/j.ajkd.2017.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Hsu C.Y., Chinchilli V.M., Coca S. Post-acute kidney injury proteinuria and subsequent kidney disease progression: the Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) study. JAMA Intern Med. 2020;180:402–410. doi: 10.1001/jamainternmed.2019.6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostermann M., Chawla L.S., Forni L.G. Drug management in acute kidney disease – report of the Acute Disease Quality Initiative XVI meeting. Br J Clin Pharmacol. 2018;84:396–403. doi: 10.1111/bcp.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu V.C., Wu C.H., Huang T.M. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25:595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu V.C., Yang S.Y., Lin J.W. Kidney impairment in primary aldosteronism. Clin Chim Acta. 2011;412:1319–1325. doi: 10.1016/j.cca.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Lai C.F., Wu V.C., Huang T.M. Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit Care. 2012;16:R123. doi: 10.1186/cc11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu V.C., Shiao C.C., Chang C.H. Long-term outcomes after dialysis-requiring acute kidney injury. Biomed Res Int. 2014;2014:365186. doi: 10.1155/2014/365186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiao C.C., Wu P.C., Huang T.M. Long-term remote organ consequences following acute kidney injury. Crit Care. 2015;19:438. doi: 10.1186/s13054-015-1149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Renal Data System Acute kidney injury. https://www.usrds.org/2016/view/v1_05.aspx Available at:
- 27.Matheny M.E., Peterson J.F., Eden S.K. Laboratory test surveillance following acute kidney injury. PloS One. 2014;9:e103746. doi: 10.1371/journal.pone.0103746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng S.Y., Chou Y.H., Liao F.L. Losartan reduces ensuing chronic kidney disease and mortality after acute kidney injury. Sci Rep. 2016;6:34265. doi: 10.1038/srep34265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brar S., Ye F., James M.T. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with outcomes after acute kidney injury. JAMA Intern Med. 2018;178:1681–1690. doi: 10.1001/jamainternmed.2018.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Y.F., Chen L., Lin S.L. Potential target-organ protection of mineralocorticoid receptor antagonist in acute kidney disease. J Hypertens. 2019;37:125–134. doi: 10.1097/HJH.0000000000001876. [DOI] [PubMed] [Google Scholar]
- 31.Hsu C.Y., Hsu R.K., Yang J. Elevated BP after AKI. J Am Soc Nephrol. 2016;27:914–923. doi: 10.1681/ASN.2014111114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ftouh S., Thomas M. Acute kidney injury: summary of NICE guidance. BMJ. 2013;347:f4930. doi: 10.1136/bmj.f4930. [DOI] [PubMed] [Google Scholar]
- 33.Lapi F., Azoulay L., Yin H., Nessim S.J., Suissa S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ. 2013;346 doi: 10.1136/bmj.e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silver S.A., Goldstein S.L., Harel Z. Ambulatory care after acute kidney injury: an opportunity to improve patient outcomes. Can J Kidney Health Dis. 2015;2:36. doi: 10.1186/s40697-015-0071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C.L., Kor C.T., Chang C.C. Association of statin use with mortality after dialysis-requiring acute kidney injury: a population-based cohort study. Mayo Clinic Proc. 2018;93:1474–1483. doi: 10.1016/j.mayocp.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 36.Huang T.M., Wu V.C., Lin Y.F. Effects of statin use in advanced chronic kidney disease patients. J Clin Med. 2018;7:285. doi: 10.3390/jcm7090285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C.Y., Wu V.C., Lin C.J. Improvement in mortality and end-stage renal disease in patients with type 2 diabetes after acute kidney injury who are prescribed dipeptidyl peptidase-4 inhibitors. Mayo Clinic Proc. 2018;93:1760–1774. doi: 10.1016/j.mayocp.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai I.J., Lai T.S., Shiao C.C. Proton-pump inhibitors augment the risk of major adverse cardiovascular events and end-stage renal disease in patients with acute kidney injury after temporary dialysis. Clin Pharmacol Ther. 2020;107:1434–1445. doi: 10.1002/cpt.1762. [DOI] [PubMed] [Google Scholar]
- 39.Mehboob A., Zimmerman R., Abramson S., Parker M.G. Quality measures in acute kidney injury. Curr Opin Nephrol Hypertens. 2018;27:130–135. doi: 10.1097/MNH.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 40.Rewa O., Mottes T., Bagshaw S.M. Quality measures for acute kidney injury and continuous renal replacement therapy. Curr Opin Crit Care. 2015;21:490–499. doi: 10.1097/MCC.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 41.Heung M., Faubel S., Watnick S. Outpatient dialysis for patients with AKI: a policy approach to improving care. Clin J Am Soc Nephrol. 2015;10:1868–1874. doi: 10.2215/CJN.02290215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chou J.A., Kalantar-Zadeh K., Mathew A.T. A brief review of intradialytic hypotension with a focus on survival. Semin Dial. 2017;30:473–480. doi: 10.1111/sdi.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McAdams M., Ortiz-Soriano V., Jordan M. Kidney recovery in patients discharged to an acute rehabilitation facility with acute kidney injury requiring hemodialysis. Clin Nephrol. 2019;92:15–24. doi: 10.5414/CN109743. [DOI] [PubMed] [Google Scholar]
- 44.Pajewski R., Gipson P., Heung M. Predictors of post-hospitalization recovery of renal function among patients with acute kidney injury requiring dialysis. Hemodial Int. 2018;22:66–73. doi: 10.1111/hdi.12545. [DOI] [PubMed] [Google Scholar]
- 45.Sibbel S., Walker A.G., Colson C. Association of continuation of loop diuretics at hemodialysis initiation with clinical outcomes. Clin J Am Soc Nephrol. 2019;14:95–102. doi: 10.2215/CJN.05080418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cerdá J., Liu K.D., Cruz D.N. Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol. 2015;10:1859–1867. doi: 10.2215/CJN.01170215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan S.H., Lee K.C., Chao Y.F., Hsu L.F., Lin P.C. Effects of a family involvement program in patients with central-line insertion. Clin Nurs Res. 2015;24:253–268. doi: 10.1177/1054773813516789. [DOI] [PubMed] [Google Scholar]
- 48.DeLa Cruz R.F., Caillouet B., Guerrero S.S. Strategic patient education program to prevent catheter-related bloodstream infection. Clin J Oncol Nurs. 2012;16:E12–E17. doi: 10.1188/12.CJON.E12-E17. [DOI] [PubMed] [Google Scholar]
- 49.Hickson L.J., Chaudhary S., Williams A.W. Predictors of outpatient kidney function recovery among patients who initiate hemodialysis in the hospital. Am J Kidney Dis. 2015;65:592–602. doi: 10.1053/j.ajkd.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gautam S.C., Brooks C.H., Balogun R.A. Predictors and outcomes of post-hospitalization dialysis dependent acute kidney injury. Nephron. 2015;131:185–190. doi: 10.1159/000441607. [DOI] [PubMed] [Google Scholar]
- 51.Milutinovic J., Cutler R.E., Hoover P., Meijsen B., Scribner B.H. Measurement of residual glomerular filtration rate in the patient receiving repetitive hemodialysis. Kidney Int. 1975;8:185–190. doi: 10.1038/ki.1975.98. [DOI] [PubMed] [Google Scholar]
- 52.VA/NIH Acute Renal Failure Trial Network. Palevsky P.M., Zhang J.H. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coleman E.A., Smith J.D., Raha D., Min S.J. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165:1842–1847. doi: 10.1001/archinte.165.16.1842. [DOI] [PubMed] [Google Scholar]
- 54.Tam V.C., Knowles S.R., Cornish P.L. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ. 2005;173:510–515. doi: 10.1503/cmaj.045311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.