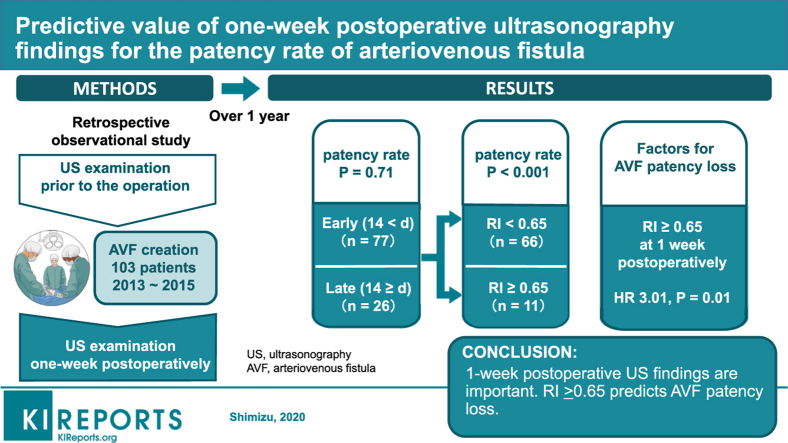
Abstract
Introduction
Most guidelines in different countries recommend waiting more than 2 weeks for the initial cannulation of an arteriovenous fistula (AVF) after its creation. Although an experienced examiner can subjectively determine if an AVF is ready for early cannulation, there is a lack of objective information to guide whether early cannulation is appropriate or how early cannulation may affect an AVF’s primary patency. The current study examined the relationship between the initial cannulation and the prognosis of AVF, considering ultrasonography (US) findings.
Methods
This retrospective observational study enrolled 103 patients with end-stage renal disease who had started hemodialysis therapy from 2013 to 2015 at the Juntendo University Hospital. All patients had been given a primary AVF before or after the initiation of dialysis, had undergone US examinations both before and 7 days after surgery, had initially cannulated the AVF at ≥7 days after surgery, and were observed for over 1 year.
Results
The factor associated with the loss of primary patency was a resistance index of brachial artery ≥0.65 on US examination at 7 days after surgery. There was no significant difference in patency rate between the early (within 14 days after surgery) and late initial cannulation groups (≥15 days after surgery).
Conclusion
Because a resistance index <0.65 on US findings at 7 days after surgery was a good indicator for predicting an excellent patency rate when we performed first cannulation of AVF located in the forearm within 2 weeks after its creation, 1-week postoperative US evaluation may provide crucial information.
Keywords: arteriovenous fistula, early cannulation, patency rate, resistance index, ultrasonography, vascular access
Graphical abstract
The number of patients with end-stage renal disease (ESRD) is increasing worldwide.1, 2, 3, 4 Thomas et al.2 reported that the global prevalence of maintenance dialysis has increased 1.7-fold from 165 in 1990 to 284 patients per million population in 2010. In Japan, the number of dialysis patients is increasing with each passing year. At the end of 2017, there were approximately 334,000 dialysis patients, an increase of more than 4800 relative to the previous year. Vascular access (VA) is necessary for patients on maintenance hemodialysis (HD), and maintenance of adequate VA for HD is a major issue for preserving the quality of life of ESRD patients. The native arteriovenous fistula (AVF) is the most common form of VA.5 The proportion of AVF in VA is approximately 90% in Japan, which is considerably higher than that in Europe and the United States.6 The Japanese Society for Dialysis Therapy guideline7 recommends that initial cannulation of a primary AVF should be performed at 2 weeks or more after surgery. The Kidney Disease Outcomes Quality Initiative guideline5 recommends that when the vein diameter is >0.4 cm, the vein has a flow rate of >500 ml/min, and at least 1 month has elapsed since fistula creation, a trial cannulation can be performed. In the Dialysis Outcomes and Practice Patterns Study,8 AVFs that were cannulated within 14 days of creation had a 2.27-fold increased risk for VA failure compared with those that were cannulated at 43–84 days after its creation.
However, on the basis of the data of the Dialysis Outcomes and Practice Patterns Study, Saran et al.9 observed that earlier cannulation of a newly placed VA for HD was not associated with an increased risk for VA failure. Ren et al.10 further reported that the patency rates after early use of autogenous forearm AVFs were not inferior to those previously reported in the literature. Therefore, some reports do not recommend avoiding early cannulation of AVF.
We previously reported the usefulness of preoperative vascular ultrasonography (US) examinations because the findings may be associated with the patency rate of AVF.11 Oprea et al.12 reported that preoperative US findings were related to a successful AVF maturation rate. According to a report by Gyori et al.,13 because patients who underwent preoperative duplex US showed higher patency rates and needed fewer revisions, standard preoperative US examination is a readily available tool that is used to improve the outcomes and cost-effectiveness in AVF surgery. Similarly, there have been some reports describing the usefulness of preoperative US examinations. On the other hand, the usefulness of postoperative US examinations has also been reported. Wong et al.14 revealed that most of the increase in fistula diameter and blood flow occurs within the first 2 weeks after surgery. According to the report by Robbin et al.,15 US measurements at 2 weeks after surgery might be useful for the early identification of fistulas that are unlikely to develop optimally. Thus, primary AVFs may mature at 2 weeks after surgery. Although these reports described the relationship between postoperative US examinations and maturation of AVF, there have been no reports about the usefulness of postoperative US to determine the timing of initial cannulation of AVF. Indeed, early cannulation within 2 weeks after surgery has been widely performed empirically. Because of the lack of specific evaluation methods for examining AVF maturity or allowing the use of AVF, a physical examination (palpation and auscultation) is currently employed to determine whether the AVF can be used. Accordingly, this study evaluated whether postoperative US findings could be used to guide the timing of the initial cannulation. We further investigated the relationship between the timing of initial cannulation and the patency rate of AVF.
Methods
We conducted a retrospective cohort study to examine the impact of early cannulation of AVFs on the patency rate and the usefulness of US examination in guiding the timing of cannulation. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study protocol was approved by the Ethics Committee of Juntendo University Hospital, Tokyo, Japan (approval no. 17-122). Informed consent was obtained in a manner approved by the ethics committee from all individual participants included in this study. This study is registered in the Japanese clinical trials registry of University hospital Medical Information Network (UMIN; no. UMIN000037871).
The primary end point of this study was the 1-year primary patency rate. In accordance with the method in a prior study,16 we defined primary patency as the state of no invention designed to maintain or reestablish patency and the absence of access thrombosis.
Patients
Inclusion criteria for our study were the initiation of HD therapy from September 2013 to August 2015 at the Juntendo University Hospital, Tokyo, Japan, and the creation of a primary AVF before or after HD initiation (n = 138 patients). Exclusion criteria were immediate patency loss of AVF or early thrombosis within 7 days (n = 21 patients); patients who were lost to follow-up (n = 13); and death within 1 year (n = 1 patient). A total of 103 Japanese patients with ESRD were enrolled in this study, with 103 forearm AVFs (98 forearm radiocephalic fistulae, 4 snuffbox fistulae, and 1 ulnobasilic fistula).
Preoperative Assessment
All patients enrolled in this study underwent physical and US examinations by nephrologists who performed the AVF surgery. The vessels were considered suitable for AVF creation if an arterial pulse was found in both the elbow and wrist upon physical and US examinations, and if the veins, including superficial veins, were widely patent using a tourniquet upon US examination. We performed US examination based on a standardized protocol using the LOGIQ e ultrasound system (GE Healthcare Japan, Tokyo, Japan) and a 5- to 13-MHz linear transducer (12L-RS, GE Healthcare) with patients in the recumbent position. After the application of a tourniquet, superficial veins were followed in cross-section with B-mode from the mid-upper arm to the wrist with intermittent vein compression. The cephalic vein (CV) was scanned from the mid-upper arm to the wrist, and the basilic vein (BV) was scanned from the elbow level to its drainage into the deep brachial veins. Internal diameters and vein depths were measured at the presumptive region of anastomosis. The arterial scan followed the vasculature from the brachial artery (BA) at the elbow to the radial artery (RA) or ulnar artery (UA) at the wrist in B-mode. The internal diameters and depths of the RA or UA were measured at the presumptive region of anastomosis, whereas they were measured at the elbow for the BA. Longitudinal color flow images were obtained from the BA at the elbow to the RA or UA at the wrist. Waveforms were recorded from a small sampling volume placed in the central flow stream at attempted angles of 60° relative to the vessel walls of the artery. Velocity sampling was performed at the RA or UA in the presumptive region of anastomosis and at the BA in the elbow by pulse Doppler mode.
Surgical Technique
The operation was performed under local anesthesia with 1% lidocaine containing 0.001% epinephrine. A shallow angle incision was made. Both the CV (or BV) and the RA (or UA) were isolated as distally as possible on the forearm or snuffbox. The CV (or BV) was dilated by injection of heparinized saline. The end of the CV (or BV) was ligated, and the sidewall of the CV (or BV) was anastomosed to the sidewall of the RA (or UA) using 6-0 polypropylene in a smooth loop configuration with a diameter of 6 mm. Suturing was initiated with posterior sutures, followed by anterior sutures. It was performed sufficiently without twist or flexion, which could cause an increased resistance index (RI). Upon confirming that the spasm had occurred, we injected heparin intravenously and massaged the anastomosis. The operators did not use a microscope during the surgery. No patient had an early artery angioplasty.
Postoperative Care and Assessment
No anticoagulation or antiplatelet was used to open the AVF after its creation. No specific exercise to encourage the maturity of AVF was uniformly instructed, although patients may have exercised arbitrarily; however, there is no information regarding this aspect.
At 7 days after surgery, the operator performed US examination using the same equipment as that used for the preoperative examination. The BA blood flow (BA-flow) was measured in the same manner as in the preoperative examination. We measured the RI in the BA by using the formula: RI = (A − B) / A, where A = peak systolic velocity and B = end diastolic velocity.
Cannulation
Initial cannulation of the AVF was performed by the nephrologists who performed the operation. The vein near the elbow was chosen for cannulation in patients who exhibited a well-dilated vein. If the vein was not dilated, we waited more than 2 weeks for AVF maturation. Plastic cannulae (17 gauge) were used in the HD cannulation. We encountered no severe cannulation injury that could require repair surgery.
Arteriovenous Fistula Follow-Up
We contacted the satellite dialysis clinics where the patients were attending once by telephone and mail. We obtained information regarding patency loss of AVF, including the date of first access thrombosis or any intervention to maintain or restore blood flow, as well as mortality or kidney transplant. The response rate for information was 88.9%.
Statistical Analysis
We estimated the patency rate using Kaplan–Meier analysis. The difference in patency rates between the 2 groups was examined using a log-rank test. Univariate and multivariate analyses of AVF survival were performed using the Cox proportional hazards model. Data are expressed as mean ± SD. P < 0.05 was considered statistically significant. All statistical analyses were performed using the Windows version of JMP software (version 12, SAS Institute, Inc., Cary, NC).
Results
Characteristics of Enrolled Patients
In total, 103 patients (including 29 women) were enrolled in this study. Patient characteristics are shown in Table 1. Average age at the time of AVF creation was 65.5 ± 15.1 years; 31% of patients had ESRD caused by diabetic nephropathy. Patients had been given AVF before (61%) and after (39%) dialysis initiation. The timing of initial cannulation was 7–336 days after surgery, including those who were observed for a while without dialysis after AVF creation. Average patency term was 293.1 ± 120.4 days.
Table 1.
Patient characteristicsa
| Characteristics | Total (n = 103) |
|---|---|
| Age at AVF surgery, yr | 65.5 ± 15.1 (20–93) |
| Sex (% male [ratio of male to female]) | 71.8 (74:29) |
| Etiology of end-stage renal disease (% DM [ratio of DM to non-DM]) | 31.1 (32:71) |
| AVF creation after dialysis initiation (% after [ratio of after to before]) | 38.8 (40:63) |
| Body height, m | 1.62 ± 0.1 (1.35–1.82) |
| Body weight, kg | 62.7 ± 14.4 (36.1–109.3) |
| Body surface area, m2 | 1.66 ± 0.21 (1.17–2.28) |
| Period of surgery to first cannulation, d | 25.2 ± 46.90 (7–336) |
| Patency time, d | 293.1 ± 120.4 (14–365) |
AVF, arteriovenous fistula; DM, diabetes mellitus.
Data are presented as mean ± SD (range) unless otherwise stated.
Ultrasonography Findings
The US findings are shown in Table 2, including average artery or vein internal diameter at the presumptive region of anastomosis, as well as average arterial blood flow at the presumptive region of anastomosis and average BA-flow.
Table 2.
Ultrasonography findingsa
| Measurement | Findings |
|---|---|
| Preoperative findings | |
| Diameter of cephalic vein or basilic vein, mm | 2.69 ± 0.70 (1.6–6.2) |
| Diameter of RA or UA, mm | 2.46 ± 0.46 (1.6–3.65) |
| Blood flow of RA or UA, ml/min | 21.22 ± 15.40 (2.42–100.41) |
| Blood flow of BA, ml/min | 77.75 ± 40.67 (13.45–219.77) |
| 7-d postoperative findings | |
| Blood flow of BA, ml/min | 645.1 ± 290.0 (214–1442.7) |
| Resistance index | 0.550 ± 0.107 (0.32–0.82) |
BA, brachial artery; RA, radial artery; UA, ulnar artery.
Data are presented as mean ± SD (range). One patient had a basilic vein, not a cephalic vein, and UA, not RA.
For the 7-day postoperative US findings, average BA-flow was 645.1 ± 290.0 ml/min (range, 214–1442.7 ml/min) and average RI was 0.550 ± 0.107 (range, 0.32–0.82).
Primary Patency Rate and Associated Factors
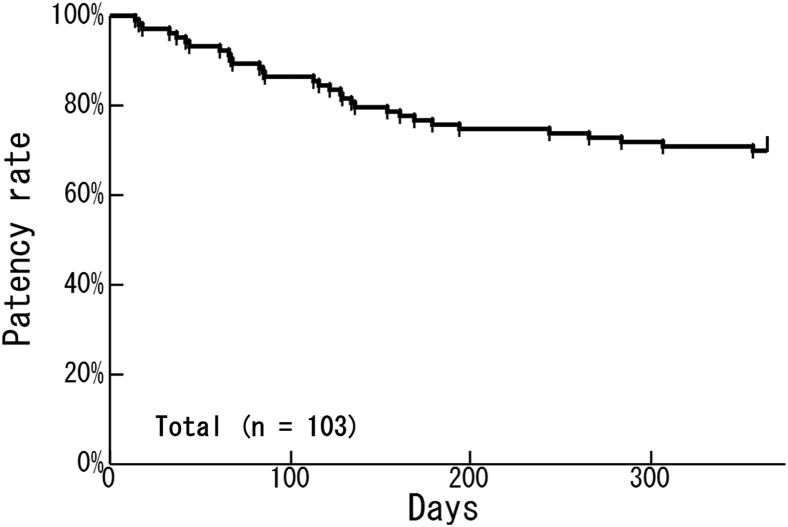
The primary patency rate at 1 year after surgery among all AVFs was 69.9% (Figure 1). Factors associated with the first patency loss of AVF within 1 year and each hazard ratio (HR) are shown in Table 3. Univariate analysis showed that patients aged ≥75 years had a higher risk than patients aged <75 years (HR = 2.15; 95% confidence interval [CI], 1.05–4.37; P = 0.04) and that the risk increased with age (HR = 1.03; 95% CI, 1.01–1.06; P = 0.02). The BA-flow and RI in US findings at 1 week after surgery were also associated with the risk for patency loss of AVF. A high RI (RI ≥ 0.65) was associated with a 3.17-fold higher risk for patency loss of AVF compared with a low RI (RI < 0.65) (HR = 3.17; 95% CI, 1.46–6.50; P = 0.005). The AVF created after the initiation of HD was not associated with the risk for patency loss of AVF compared with the AVF created before the initiation of HD (HR = 0.75; 95% CI, 0.34–1.56; P = 0.45). In multivariate analysis, a high RI (RI ≥ 0.65) had a 3.01-fold higher risk for patency loss of AVF compared with a low RI (RI < 0.65) (HR = 3.01; 95% CI, 1.28–6.97; P = 0.01).
Figure 1.
One-year primary patency rates of all included patients (69.9%; n = 103).
Table 3.
Factors associated with patency loss of arteriovenous fistulaa
| Parameters | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (per 1-yr increase) | 1.03 (1.01–1.06) | 0.02b | 1.02 (1.00–1.05) | 0.08 |
| Female (vs. male) | 1.27 (0.57–2.63) | 0.54 | 1.21 (0.51–2.65) | 0.65 |
| DM (vs. without DM) | 0.89 (0.39–1.88) | 0.77 | 0.75 (0.31–1.70) | 0.51 |
| Resistance index at 1 wk after surgery of ≥0.65 (vs. <0.65) | 3.17 (1.46–6.50) | 0.005b | 3.01 (1.28–6.97) | 0.01b |
| BA-flow at 1 wk after surgery of <400 ml/min (vs. ≥400 ml/min) | 2.02 (0.93–4.14) | 0.07 | 1.56 (0.63–3.74) | 0.33 |
| Period from surgery to initial cannulation of ≤14 d (vs. ≥15 d) | 0.86 (0.41–1.98) | 0.71 | 1.41 (0.59–3.71) | 0.45 |
| Body surface area (per 0.1-m2 increase) | 0.90 (0.76–1.07) | 0.23 | ||
| Diameter of RA < 2.0 mm (vs. ≥ 2.0 mm) | 0.51 (0.08–1.68) | 0.30 | ||
| Diameter of cephalic vein < 3.0 mm (vs. ≥ 3.0 mm) | 1.82 (0.80–4.91) | 0.16 | ||
| RA-flow before operation (per 1-ml/min increase) | 1.00 (0.97–1.02) | 0.69 | ||
| BA-flow before operation (per 1-ml/min increase) | 1.00 (0.99–1.01) | 0.45 | ||
| Arteriovenous fistula creation after dialysis initiation (vs. before) | 0.75 (0.34–1.56) | 0.45 | ||
CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; RA, radial artery; BA, brachial artery.
One patient had a basilic vein, not a cephalic vein, and an ulnar artery, not an RA.
P < 0.05.
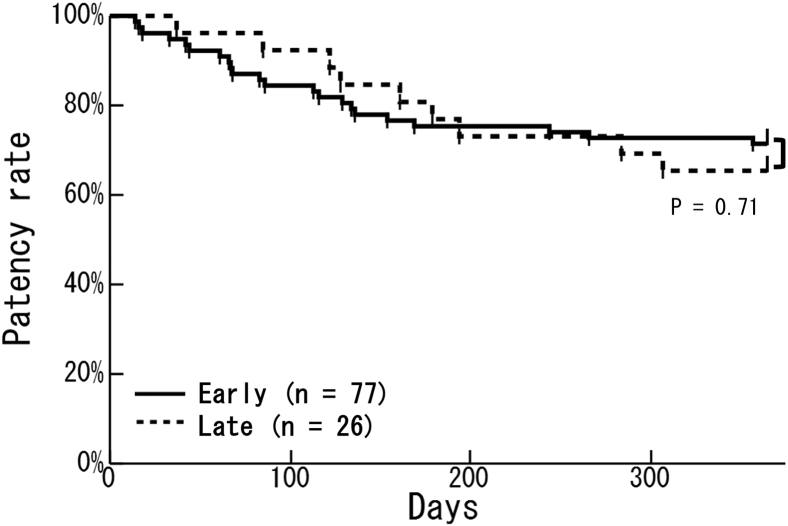
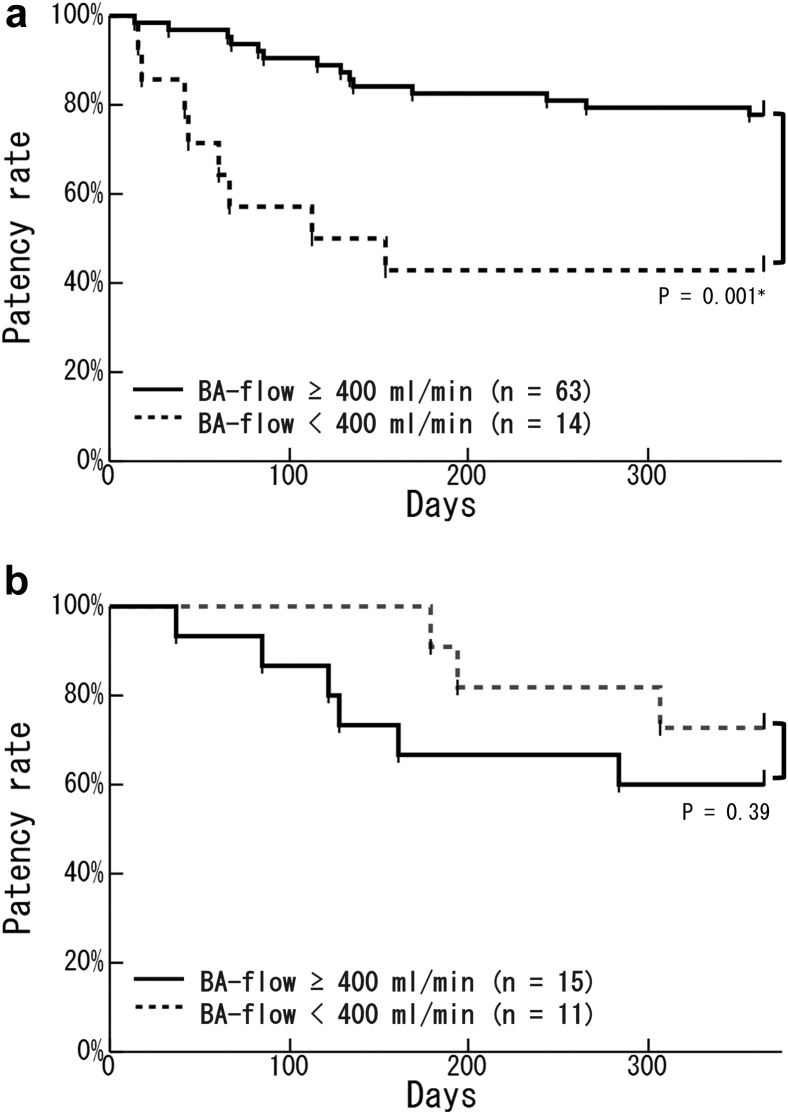
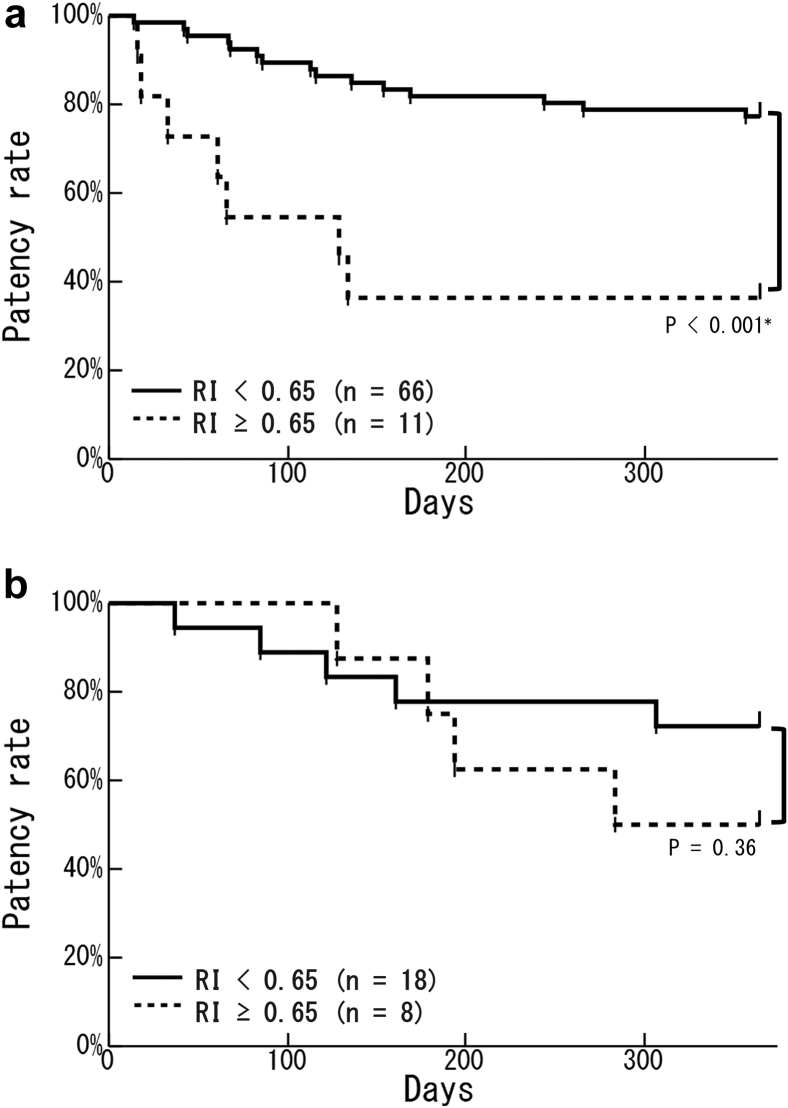
We divided all patients into 2 groups: early cannulation (77 patients who had initial cannulation within 14 days after surgery) and late cannulation (26 patients who had initial cannulation ≥15 days after surgery). Kaplan–Meier analysis showed no significant difference between groups in the primary patency rate (Figure 2). In the early cannulation group, the primary patency rate in patients with a high BA-flow (≥400 ml/min) was significantly higher than that in patients with a low BA-flow (<400 ml/min) (77.8% vs. 42.9%; P = 0.001) (Figure 3a). In the late cannulation group, there was no significant difference in the primary patency rate as a result of the BA-flow (Figure 3b). In the early cannulation group, the primary patency rate in patients with a low RI (RI < 0.65) was significantly higher than that in patients with a high RI (RI ≥ 0.65) (77.3% vs. 36.4%; P < 0.001) (Figure 4a). There was no significant difference in the primary patency rate as a result of RI in the late cannulation group (Figure 4b). Neither the early nor the late group had severe complications such as injury, hematoma, or infection.
Figure 2.
Comparison of primary patency rates between early and late cannulation groups. There was no significant difference in the primary patency rate between the early cannulation group (solid line; n = 77) and the late cannulation group (dotted line; n = 26) (P = 0.71). The early cannulation group underwent initial cannulation within 14 days after surgery and the late cannulation group underwent initial cannulation at ≥15 days after surgery.
Figure 3.
Kaplan–Meier curves of the relationship between the brachial artery blood flow (BA-flow) at 7 days after surgery and the patency rate in the early or late cannulation groups. (a) In the early cannulation group, patients with a BA-flow of ≥400 ml/min (solid line; n = 63) had a significantly higher patency rate than patients with a BA-flow of <400 ml/min (dotted line; n = 14) (P = 0.001∗). (b) In the late cannulation group, BA-flow did not affect the patency rate (patients with a BA-flow of ≥400 ml/min: solid line, n = 15; patients with a BA-flow of <400 ml/min: dotted line, n = 11) (P = 0.39).
Figure 4.
Kaplan–Meier curves of the relationship between the resistance index (RI) at 7 days after surgery and the patency rate in the early or late cannulation groups. (a) In the early cannulation group, patients with an RI of <0.65 (solid line; n = 66) had a significantly higher patency rate than did patients with an RI of ≥0.65 (dotted line; n = 11) (P < 0.001∗). (b) In the late cannulation group, RI did not affect the patency rate (patients with an RI of <0.65: solid line, n = 18; patients with an RI of ≥0.65: dotted line, n = 8) (P = 0.36).
Discussion
The 1-year primary patency rate of AVFs was 62% in a recent meta-analysis17 and 64% in our report.11 Therefore, the result of approximately 70% in the current study was considered high. The current study revealed that the factor associated with patency loss of AVF in the first year was the US findings at 1 week after surgery. To the best of our knowledge, this is the first report describing the effect of US findings at 1 week after AVF creation on both the primary patency rate and the timing of initial AVF. Our multivariate analysis showed that the patency rate of patients with an RI < 0.65 at 1 week after surgery is a good indicator for the future patency rate.
Several reports have shown that aging and diabetes mellitus (DM) may affect patency rates. In a meta-analysis by McGrogan et al.,18 primary AVF patency rates were 53.6% in 1889 AVFs among patients with a mean age of 76 years. Hwang et al.19 reported that in patients aged ≥70 years, the outcomes after AVF surgery were inferior to those of patients aged <70 years (i.e., primary success and primary patency rates were lower). We also reported that the patency rate was significantly lower in patients aged ≥75 years and in patients with DM.13 Indeed, a meta-analysis by Yan et al.20 revealed a significantly higher rate of AVF failure in diabetic patients compared with nondiabetic patients. Conversely, Okamuro et al.21 reported that DM was not significantly associated with increased radiocephalic AVF failure. Consistent with this report, we failed to find a relationship between DM and the AVF patency rate. Considering that 42% of ESRD patients who had initiated dialysis in Japan in 2016 had DM,22 the lower rate of DM patients (31%) in the current study may have influenced our findings.
The RI is typically a measure of vessel compliance. Its value can be influenced by the arterial structure, which is supposed to reflect the age or primary disease of patients, the condition of veins, such as twist or flexion, or the position along the vessel or the angle at which the RI is measured. It is obvious that the BA with a forearm AVF has an RI higher than that of the upper-arm AVF because, in general, the distal blood vessels have less blood flow and their diameter becomes narrower. In this study, all patients were given an AVF of anastomosis size of 6 mm located in the forearm. It was well-confirmed that there was no twist or flexion in surgery. Resistance index measurements were performed by the operator of the AVF, and at least 2 nephrologists attended to confirm the results. Therefore, in this study, RI can be assumed to have been measured under the same conditions. Because the patient’s age may influence RI, age was adjusted by multivariate analysis in this study.
There was no significant difference in patency rate between early initial cannulation (within 14 days after AVF creation) and late initial cannulation (≥15 days after AVF creation). However, early cannulation may not be suitable for all patients. Although the patency rate was good in patients with good US findings at 1 week after surgery (i.e., with a BA-flow ≥400 ml/min or an RI <0.65) in the early cannulation group, there was no relationship between US findings at 1 week after surgery and the patency rate in the late cannulation group. This group included patients from a variety of backgrounds, such as those whose AVF was judged as being immature in the US examination or for whom HD was not initiated immediately after AVF creation. There was no information about the condition of AVF at the time of initial cannulation. Therefore, US examination at 1 week after surgery is useful for predicting patency loss of AVF in the first year for patients who had been considered for early cannulation.
Japanese VA practice has some unique characteristics. Since before the onset of the Fistula First Initiative23 in Japan, 90% of HD patients had an AVF as the vascular access, 90% of which were located in the forearm. Upper-arm AVFs may reflect patients with poor vasculature. It has been reported that mean intervals until initial cannulation after AVF surgery are 25 days in Japan, 27 days in Italy, 42 days in Germany, 80 days in Spain, 86 days in France, 96 days in the United Kingdom, and 98 days in the United States.8 The period in Japan is considerably shorter than that in many other countries. Nevertheless, AVFs in Japan have a higher primary patency rate. We consider that a lower median blood flow rate (BFR) (at least 200 ml/min) and longer treatment time (at least 240 min) while maintaining Kt/tv (1.4 or higher), both of which are dialysis prescriptions recommended by the Japanese Society for Dialysis Therapy,24 and the surgical technique of Japanese physicians also may be the reasons of the higher successful use of AVF. Actually, the BFR at the time of initial HD is 160 ml/min in Japan, 200–265 ml/min in European countries, and 300 ml/min in the United States.8 The BFR prescribed for the first dialysis was the highest in the United States, which had the longest median time between creation and first cannulation. Therefore, the difference in initial BFR may suggest a difference in the period. Incidentally, it was reported that a higher machine BFR in the first week of dialysis is associated with reduced AVF survival.25
There were some limitations to this study. First, the study may have been influenced by the practice’s being unique to Japan. Because all patients had forearm AVF and the anastomosis diameter of AVF was the same (i.e., 6 mm), it was almost unnecessary to consider the difference in the conditions of RI measurement. However, whether this study’s findings hold true in other geographical areas or with AVF in the upper arm is unknown. Second, we could not perform sufficient follow-up for 13 patients. Third, this was a retrospective observational study with a small sample whose AVF was located in only the forearm. It was from a single-center in which rates of diabetic nephrology were lower than those among dialysis patients in Japan. Further long-term observational or prospective studies are needed to confirm our findings.
Ultrasound findings at 1 week after surgery have important information. An RI of <0.65 could be a good indicator for predicting an excellent patency rate if first cannulation of AVF is to be performed within 14 days. However, we acknowledge that further study is needed to confirm the findings of this study.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors declare no conflicts of interest associated with this work. This study has been registered in the Japanese clinical trials registry of UMIN (no. UMIN000037871). The authors thank all staff of the Dialysis Unit in Juntendo University Hospital and clinics who cooperated to follow-up.
References
- 1.Barsoum R.S. Chronic kidney disease in the developing world. N Engl J Med. 2006;354:997–999. doi: 10.1056/NEJMp058318. [DOI] [PubMed] [Google Scholar]
- 2.Thomas B., Wulf S., Bikbov B. Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol. 2015;26:2621–2633. doi: 10.1681/ASN.2014101017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arulkumaran N., Montero R.M., Singer M. Management of the dialysis patient in general intensive care. Br J Anaesth. 2012;108:183–192. doi: 10.1093/bja/aer461. [DOI] [PubMed] [Google Scholar]
- 4.Long B., Koyfman A., Lee C.M. Emergency medicine evaluation and management of the end stage renal disease patient. Am J Emerg Med. 2017;35:1946–1955. doi: 10.1016/j.ajem.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Vascular Access 2006 Work Group Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48(suppl 1):S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 6.Pisoni R.L., Zepel L., Fluck R. International differences in the location and use of arteriovenous accesses created for hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2018;71:469–478. doi: 10.1053/j.ajkd.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Kukita K., Ohira S., Amano I. 2011 update Japanese Society for Dialysis Therapy Guidelines of Vascular Access Construction and Repair for Chronic Hemodialysis. Ther Apher Dial. 2015;19(suppl 1):1–39. doi: 10.1111/1744-9987.12296. [DOI] [PubMed] [Google Scholar]
- 8.Rayner H.C., Pisoni R.L., Gillespie B.W. Creation, cannulation and survival of arteriovenous fistulae: data from the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2003;63:323–330. doi: 10.1046/j.1523-1755.2003.00724.x. [DOI] [PubMed] [Google Scholar]
- 9.Saran R., Dykstra D.M., Pisoni R.L. Timing of first cannulation and vascular access failure in haemodialysis: an analysis of practice patterns at dialysis facilities in the DOPPS. Nephrol Dial Transplant. 2004;19:2334–2340. doi: 10.1093/ndt/gfh363. [DOI] [PubMed] [Google Scholar]
- 10.Ren W., Jiang H., Du Y. Early use of autogenous arteriovenous fistula in patients with urgent hemodialysis. Int Urol Nephrol. 2017;49:1087–1093. doi: 10.1007/s11255-017-1557-3. [DOI] [PubMed] [Google Scholar]
- 11.Nakata J., Io H., Watanabe T. Impact of preoperative ultrasonography findings on the patency rate of vascular access in Japanese hemodialysis patients. Springerplus. 2016;5:462. doi: 10.1186/s40064-016-2082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oprea A., Molnar A., Vladutiu D. Correlation between preoperative vein and artery diameters and arteriovenous fistula outcome in patients with end-stage renal disease. Clujul Med. 2018;91:399–407. doi: 10.15386/cjmed-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gyori G.P., Eilenberg W., Dittrich L. Preoperative ultrasound improves patency and cost effectiveness in arteriovenous fistula surgery. J Vasc Surg. 2019;69:526–531. doi: 10.1016/j.jvs.2018.05.217. [DOI] [PubMed] [Google Scholar]
- 14.Wong V., Ward R., Taylor J. Factors associated with early failure of arteriovenous fistulae for haemodialysis access. Eur J Vasc Endovasc Surg. 1996;12:207–213. doi: 10.1016/s1078-5884(96)80108-0. [DOI] [PubMed] [Google Scholar]
- 15.Robbin M.L., Greene T., Cheung A.K. Arteriovenous fistula development in the first 6 weeks after creation. Radiology. 2016;279:620–629. doi: 10.1148/radiol.2015150385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidawy A.N., Gray R., Besarab A. Recommended standards for reports dealing with arteriovenous hemodialysis accesses. J Vasc Surg. 2002;35:603–610. doi: 10.1067/mva.2002.122025. [DOI] [PubMed] [Google Scholar]
- 17.Al-Jaishi A.A., Oliver M.J., Thomas S.M. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63:464–478. doi: 10.1053/j.ajkd.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 18.McGrogan D., Al Shakarchi J., Khawaja A. Arteriovenous fistula outcomes in the elderly. J Vasc Surg. 2015;62:1652–1657. doi: 10.1016/j.jvs.2015.07.067. [DOI] [PubMed] [Google Scholar]
- 19.Hwang D., Park S., Kim H.K. Comparative outcomes of vascular access in patients older than 70 years with end-stage renal disease. J Vasc Surg. 2019;69 doi: 10.1016/j.jvs.2018.07.061. 1196.e5–1206.e5. [DOI] [PubMed] [Google Scholar]
- 20.Yan Y., Ye D., Yang L. A meta-analysis of the association between diabetic patients and AVF failure in dialysis. Ren Fail. 2018;40:379–383. doi: 10.1080/0886022X.2018.1456464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamuro L., Gray K., Korn A. Careful patient selection achieves high radiocephalic arteriovenous fistula patency in diabetic and female patients. Ann Vasc Surg. 2019;57:16–21. doi: 10.1016/j.avsg.2018.12.057. [DOI] [PubMed] [Google Scholar]
- 22.Masakane I., Taniguchi M., Nakai S. Annual Dialysis Data Report 2016, JSDT Renal Data Registry. Ren Replace Ther. 2018;4:45. [Google Scholar]
- 23.Fistula First Initiative Questions and answers. https://www.cms.gov/Medicare/End-Stage-Renal-Disease/ESRDQualityImproveInit/downloads/fffaqs.pdf Available at:
- 24.Watanabe Y., Kawanishi H., Suzuki K. Japanese Society for Dialysis Therapy clinical guideline for “Maintenance hemodialysis: hemodialysis prescriptions.”. Ther Apher Dial. 2015;19(suppl 1):67–92. doi: 10.1111/1744-9987.12294. [DOI] [PubMed] [Google Scholar]
- 25.Wilmink T., Powers S., Hollingworth L. Effect of first cannulation time and dialysis machine blood flows on survival of arteriovenous fistulas. Nephrol Dial Transplant. 2018;33:841–846. doi: 10.1093/ndt/gfx278. [DOI] [PubMed] [Google Scholar]