Abstract
Introduction
It remains unclear whether an increased progression rate of chronic kidney disease (CKD) adds predictive information regarding cardiovascular disease (CVD) risk. The aim of this study was to evaluate the association between CKD progression, based on estimated glomerular filtration rate (eGFR) slope estimates and the risk for CVD.
Methods
We compared the updated eGFR slope calculated over multiple overlapping 2-year periods and the updated mean eGFR. Incident CKD subjects were selected from a prevalent population with diabetes (T2DM). Subjects from the UK Clinical Practice Research Data Link GOLD (CPRD) were followed from CKD diagnosis (n = 30,222) until heart failure (HF), myocardial infarction (MI), ischemic stroke (IS), or a composite end point including all 3 event types (MACE plus), mortality, database dropout, or end of study follow-up.
Results
Both the updated eGFR slope and updated mean eGFR were associated with MACE plus and HF. Updated eGFR slope decline of > –3 ml/min/1.73 m2 increased the risk for MACE plus (adjusted hazard ratio [HR] = 1.45; 95% confidence interval [CI], 1.26–1.67), HF (HR = 1.50; 95% CI, 1.27–1.76), and MI (HR = 1.39; 95% CI, 1.01–1.91).
Conclusions
This study strongly supports current evidence that CKD is an independent risk factor for CVD. From a clinical perspective, both rate of progression and cumulative status of CKD describe distinct aspects of the cardiorenal risk among persons with diabetes. This evidence is essential to enable more timely and improved use of treatments in this population.
Keywords: chronic kidney disease, diabetes, epidemiology, heart disease, hypertension, statistical
Graphical abstract
Chronic kidney disease (CKD) is an increasing global health problem.1 In 2016, the prevalence of chronic kidney disease was estimated to be 13.4% globally, increasing up to 35% in the elderly population.2 Data from Health Surveys from England estimated a population prevalence of CKD in the United Kingdom (UK) of 6% in men and 7% in women in 2010.3 Treatment options to slow CKD progression and address associated risks are limited, and in 2010, CKD ranked as the 18th most common cause of mortality.4 The National Health and Nutrition Examination Survey study from 2016 estimated that 6.9% of the US population had CKD stages 3 and 4 (glomerular filtration rate [GFR], 15–59 ml/min/1.73 m2) in 2011.5 In Western countries, type 2 diabetes mellitus (T2DM) is attributed as the leading cause of CKD, accounting for over 40% of the disease population.6, 7, 8, 9 The increase in patients with T2DM and associated microvascular disease is by inference driving the prevalence of diabetes-associated nephropathy.10, 11, 12
Morbidity and mortality in patients with diabetic kidney disease predominantly result from cardiovascular disease (CVD).13,14 Several studies have demonstrated a strong association between CKD and CVD; this is further pronounced in persons with T2DM.15,16 Less is known about the frequency and characteristics of CKD progression in relation to CVD outcomes and background factors such as hypertensive treatment and concomitant disease.17 Moreover, reported increased morbidity in patients with CKD may be limited by a priori assumptions that reductions in estimated GFR (eGFR) are stable over time.18 Matsushita et al.19 in 2009 was first to note that an annual decline in GFR using the Modification of Diet in Renal Disease study equation, measured at 2 points in time, was associated with coronary heart disease. A more recent meta-analysis by Greene et al.20 assessed eGFR slope as a surrogate end point for renal disease and found it to be correlated with multiple risk factors, treatment, and study design, but critical to CKD patient segmentation.
Today, it remains unclear whether the increased progression rate of CKD adds information regarding CVD risk outcomes; consequently, it remains difficult to time cardiovascular medicine prevention accurately in this patient population. Therefore, the main objective in this study was to evaluate the association between the rate of CKD progression, based on the eGFR and incidence of CVD types: heart failure (HF), myocardial infarction (MI), ischemic stroke (IS), and MACE plus (a composite variable including all CVD events) in a large contemporary population of CKD patients with T2DM newly provided a diagnosis in the UK.
Methods
Data were obtained from the Clinical Practice Research Data Link (CPRD), in which primary health care practitioners in the UK record patient information captured through Electronic Health Record information technology systems, and which are updated at regular intervals. The CPRD covers approximately 8% of the UK population and is representative of the general population in terms of age, sex, and ethnicity.21 The CPRD provides anonymous health care data that include demographic, laboratory, prescribed drug, and diagnosis information.22 Ethical approval was granted by the CPRD scientific committee and the National Information Governance Board of Ethics and Confidentiality Committee (approved by the Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory Agency database research ID no. ISAC 11_26).
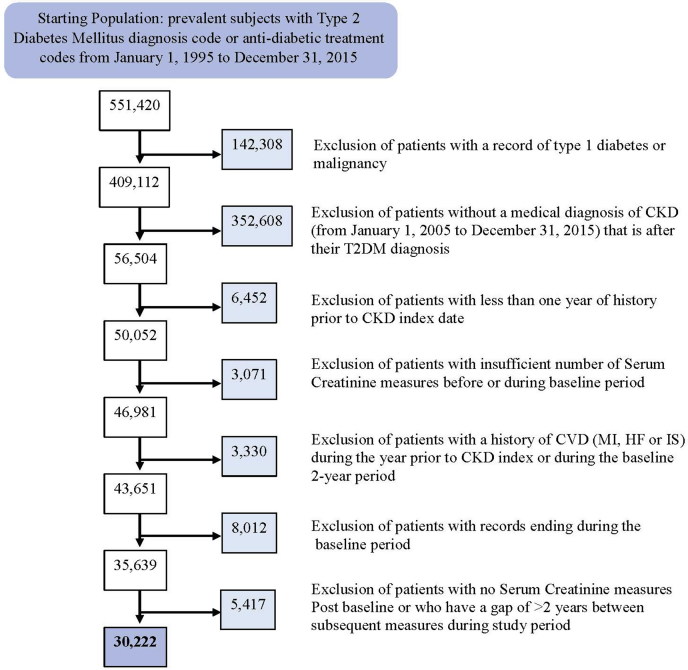
We identified 30,222 newly diagnosed CKD subjects from a prevalent population of T2DM, described in Figure 1. The original T2DM population included men and women with a medical code for diabetes mellitus or antidiabetic treatment codes from 1 January 1995 to 31 December 2015. Patients with a record of type 1 diabetes or malignancy were excluded. The T2DM population was then further restricted to those for whom a medical diagnosis based on Read codes for CKD was present from 1 January 2005 to 31 December 2015 and for whom the CKD selection date (index date) occurred after T2DM diagnosis. Patients were also required to have had at least 12 months of data before the CKD index date. All CKD patients with a history of CVD (HF, MI, IS, or MACE plus) during the year before CKD index or the 2 years after CKD index (baseline period) were excluded to ensure incident cases were captured during the follow-up period. Baseline CKD status was determined by taking the last eGFR in the year before CKD index or the first eGFR after the CKD index date to ensure the full CKD population was captured.
Figure 1.
Flowchart describing the final cohort of adults with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD). HF, hazard ratio; IS, ischemic stroke; MI, myocardial infarction.
All covariates were estimated and defined during an initial 2-year baseline period. This 2-year period allowed patients to stabilize in treatment for both CVD and T2DM after the CKD diagnosis and to estimate background characteristics, including the baseline slope. Patients with >2 serum creatinine values in this 2-year baseline period were excluded from the study. The following baseline covariates were derived by averaging values during the initial 2-year period: body mass index (kg/m2), systolic and diastolic blood pressure (mm Hg), triglycerides (mmol/l), low-density lipoprotein (mmol/l), potassium (mmol/l), and hyperkalemia (mean potassium > 5.5 mmol/l). Hypertensive medication for CVD subjects included baseline renin–angiotensin–aldosterone system inhibitors (RAASis) angiotensin II receptor blockers, and angiotensin-converting enzyme inhibitors and mineralocorticoid receptor antagonist use with at least 2 prescriptions with British National Formulary codes within the 2-year baseline period. Baseline smoking status was defined as the worst smoking category (nonsmoker, ex-smoker, or current smoker) recorded during the baseline period, and age (range, 19–103 years) was recorded on the subject’s CKD index date. Where missing values occurred, a category was created to describe missingness per variable.
The Chronic Kidney Disease Epi Equation23 was used to calculate eGFR; other formulas were previously assessed in CPRD by that group (Cabrera, C, Asiimwe A, Lee A. Estimating chronic kidney disease in a UK population with type II diabetes mellitus. [abstract]. Pharmacoepidemiol Drug Saf. 2012;21:S273). When calculating eGFR, any serum creatinine values that were >35 μmol/l or >1500 μmol/l were excluded from further eGFR calculations.24 Three approaches for computing eGFR change over time were tested to identify which was the most appropriate time-varying method to estimate CVD events in a statistical model. They included a baseline slope of eGFR (ml/min/1.73 m2/2-year period), the updated eGFR slope calculated over multiple overlapping 2-year periods, and the updated mean eGFR per 2-year slope period, all based on the Chronic Kidney Disease Epi Equation formula.23 The baseline slope and updated mean per slope period (in which mean values were included in categories ≥60 versus ≥30 to 60, and <30 ml/min/1.73 m2), along with the updated eGFR slope categories (reference: ≥3; ≥0 to <3, <0 to –3, and < –3 ml/min/1.73 m2) were the main variables of interest. The categorical updated eGFR slope was analyzed in relation to CVD morbidity for persons with T2DM in whom the most positive slope inclination was the reference category. The reference category ≥3 was chosen because it is the most beneficial category (least disease progression) for a patient, which facilitates trend assessments in hazard ratios (HRs) across categories ordered according to severity. A linear regression equation was used to determine the rate of progression (slope) over the baseline 2-year period; then, it was applied for subsequent overlapping 2-year periods.
The main time-dependent variable of interest was based on multiple updated slopes which were calculated throughout the follow-up from overlapping 2-year periods.
The eGFR slopes were calculated within each 2-year period beginning with the first eGFR measure recorded at least 6 months after the start of the previous slope. The first postbaseline slope for each patient began on day 0 (first day of the at-risk period). Standard practice in the UK and for CPRD since 2004, when the Quality and Outcomes Framework was initiated, has been to measure eGFR approximately twice a year for each patient.25,26 This allows the slope estimates to be based on multiple measures over time with at least 2 eGFR estimates for each 2-year period per individual, as further described in Table 1. Chronic kidney disease progression was estimated over time with an average of 5 overlapping slopes per subject.
Table 1.
Description of main time-dependent estimated glomerular filtration rate (eGFR) variable calculations
| General design | Definition |
|---|---|
| Slope linear regression equation | Regression equation |
| (y) = a + bx | |
| Slope (b) = (NΣXY – [ΣX] [ΣY]) / (NΣX2 – [ΣX]2) | |
| Intercept (a) = (ΣY – b[ΣX]) / N | |
| N = number of serum creatinine tests in a 2-yr period | |
| X = years from CKD index (date of the serum creatinine test in years from CKD index date) | |
| Y = eGFR value calculated from serum creatinine values (Chronic Kidney Disease Epi Equation formula) | |
| Baseline slope | Calculated from all eGFR values reported for the subject over the initial 2-yr baseline period using standard regression equation for slope |
| Baseline 2-yr period | All covariates were estimated and defined during an initial 2-yr baseline period starting from CKD index date |
| Follow-up period | Starts directly after the 2-yr baseline period. The end of the follow-up is defined as the first occurrence of a CVD event, subject lost to follow-up, or 31 December 2015, whichever comes first |
| Overlapping time windows | For each subject, the follow-up period is covered by overlapping time windows. The first time window covers the first 2 yr of the follow-up period. The second time window starts at the first eGFR value registered at least 6 mo after the start date of follow-up and stretches over the following 2 yr or to the end of follow-up. The next window starts at least 6 mo after the start of the previous time window, and so on until the whole follow-up is covered |
| Updated slope | The first slope is based on all eGFR values in the subject’s first time window (baseline 2-yr period), the second slope is based on all eGFR values in the second time window, and so on. In general, each subject will have multiple overlapping slopes, in which each slope represents the direction of change (or rate of progression) in eGFR during the previous 2 yr |
| Updated mean | The first mean is the mean of all eGFR values in the first time window, the second mean is based on all eGFR values in the second time window, and so forth. For each subject at each time point, in general, the updated mean eGFR represents the average level in eGFR over the last 2 yr |
CKD, chronic kidney disease.
For each CKD variable we created statistical models to assess CVD morbidity end points. Associations between CKD variables and CVD outcomes were estimated using Cox proportional hazards regression models. Each CKD progression estimate was analyzed in a model adjusted for confounders potentially relevant to CVD. Follow-up began directly after the initial 2-year baseline period. The time scale in the statistical model is based in years to estimate the annual decline in eGFR. Patients with no serum creatinine laboratory values after the index date or with a gap of more than 2 years between serum creatinine measures were excluded. We also conducted sensitivity analyses restricting slope inclinations (< –20 and >20) and found the model to be stable, (data not shown). In addition, baseline characteristics of the original prevalent T2DM cohort from 2005 to 2015 were also assessed by CVD outcomes. Supplemental Table S1 compares results obtained from our main analysis that assessed T2DM patients with CKD. Results from the proportional hazards models are presented as (relative risks [HRs]) and 95% confidence intervals (CIs).
Morbidity end points for the HRs were identified with READ codes and included HF, MI, IS, and a composite variable MACE plus, which included all CVD morbidity outcomes. The follow-up time used to estimate the time to an event was defined as the remaining study period after the initial 2-year baseline period. SAS software (version 9, SAS Institute, Cary, NC) was used for the analyses.
Results
The baseline characteristics in Table 2 illustrate a study population of 30,222 CKD subjects newly provided with a diagnosis from a cohort of prevalent T2DM subjects, stratified according to the eGFR slope categories per 2-year period (≥3, ≥0 to <3, <0 to –3, and ≤ –3 ml/min/1.73 m2) and cumulative incidence of morbidity end points (MACE plus, n = 2304; HF, n = 1691; MI, n = 459; and IS, n = 302).
Table 2.
Baseline characteristics in UK individuals with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD), by renal disease progression and cardiovascular disease outcomes
| Baseline variables | Total no. T2DM and CKD subjects | Baseline eGFR Slope |
Incident heart failure | Incident myocardial infarction | Incident ischemic stroke | Incident MACE plus | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Slope < –3 | Slope –3 to <0 | Slope 0–3 | Slope ≥3 | |||||||
| N (%)a | 30,222 | 8011 (27) | 7399 (24) | 6731 (22) | 8081 (27) | 1691 (5.6) | 459 (1.5) | 302 (1) | 2304 (7.6) | |
| Age, yr | Mean (SD) | 71 (11) | 71 (11) | 72 (10) | 71 (10) | 69 (11) | 74 (9) | 73 (10) | 74 (10) | 74 (9) |
| Male gender | n (%) | 13,942 (46) | 3858 (48) | 3522 (48) | 3037 (45) | 3525 (44) | 882 (52) | 273 (59) | 140 (46) | 1213 (53) |
| eGFR,b ml/min/1.73 m2 | Mean (SD) | 52 (16) | 55 (16) | 50 (17) | 50 (16) | 52 (15) | 47 (13) | 49 (16) | 47 (13) | 47 (14) |
| eGFR <60, ml/min/1.73 m2 | n (%) | 23,782 (79) | 5832 (73) | 6028 (82) | 5512 (82) | 6410 (79) | 1502 (89) | 382 (83) | 260 (86) | 2015 (88) |
| Smoker (yes)c | n (%) | 3387 (11) | 981 (12) | 740 (10) | 696 (10) | 970 (12) | 189 (11) | 64 (14) | 35 (12) | 269 (12) |
| Systolic blood pressure, mm Hg | Mean (SD) | 138 (13) | 139 (14) | 138 (13) | 138 (13) | 137 (13) | 139 (15) | 140 (14) | 139 (14) | 139 (15) |
| Diastolic blood pressure, mm Hg | Mean (SD) | 75 (7) | 75 (8) | 74 (7) | 75 (7) | 75 (7) | 73 (8) | 74 (8) | 74 (7) | 74 (8) |
| Body mass index, kg/m2 | Mean (SD) | 30.6 (6) | 30.8 (6) | 30.5 (6) | 30.4 (6) | 30.7 (6) | 31 (6) | 30 (6) | 30.1 (5) | 30.7 (6) |
| Low-density lipoprotein, mmol/l | Mean (SD) | 84 (29) | 83 (30) | 83(29) | 84 (29) | 85 (30) | 82 (29) | 86 (29) | 83 (29) | 83 (29) |
| Triglycerides, mmol/l | Mean (SD) | 162 (83) | 165(85) | 162 (83) | 159 (81) | 161 (83) | 166 (86) | 171 (92) | 168 (95) | 167 (88) |
| Hyperkalemia >5.5 mmol/l (yes) | n (%) | 731 (2.4) | 253 (3.2) | 215 (2.9) | 136 (2) | 127 (1.6) | 50 (3.0) | 15 (3.3) | 10 (3.3) | 70 (3) |
| Renin–angiotensin–aldosterone system inhibitor medication (yes)d | n (%) | 24,842 (82) | 6838 (85) | 6201 (84) | 5454 (81) | 6349 (79) | 1476 (87) | 382 (83) | 244 (81) | 1977 (86) |
| Mineralocorticoid receptor antagonist treatment (yes) | n (%) | 1297 (4) | 482 (6) | 266 (4) | 251 (4) | 298 (4) | 172 (10) | 29 (6) | 14 (5) | 204 (9) |
| T2DM duration ≥5 yr | n (%) | 15,267 (51) | 4342 (54) | 3824 (52) | 3363 (50) | 3738 (46) | 999 (59) | 258 (56) | 168 (56) | 1333 (58) |
eGFR, estimated glomerular filtration rate.
Cumulative incidence rates for cardiovascular disease outcomes.
eGFR was estimated using the Chronic Kidney Disease Epi Equation.
Approximately 40% were classified as ex-smokers across all categories.
Renin–angiotensin–aldosterone system inhibitor medication includes angiotensin II receptor blockers and angiotensin-converting enzyme inhibitor medication (yes).
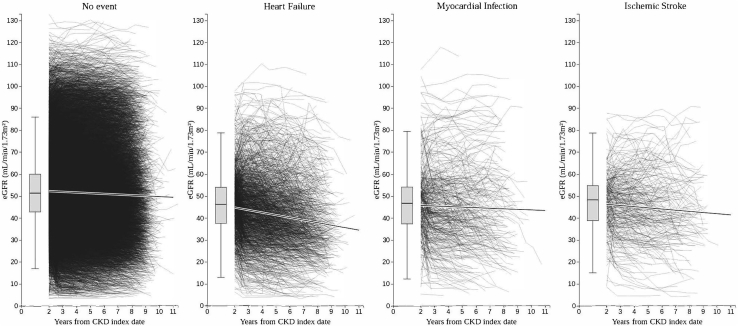
Median follow-up was 4.3 years (interquartile range, 2.4–6.4 years) during which a mean of 11 eGFR values/patient were captured. Women comprised 54% of the cohort. Mean age was 71 years at CKD diagnosis, mean systolic blood pressure was 138 mm Hg, and 82% of the population was categorized as overweight or obese (body mass index, ≥25 kg/m2). Baseline medication demonstrated that 82% were receiving angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker medication, 4% were receiving a mineralocorticoid receptor antagonist treatment, and approximately 2% indicated a possible hyperkalemic state during the baseline period. Median duration with T2DM was 5 years (interquartile range, 2.3–8.5 years), and 53% were smokers or ex-smokers. The cumulative incidence rate of CVD morbidity was MACE plus 7.6%, HF 5.6%, MI 1.5%, and IS 1%. This is illustrated in Figure 2, in which the updated mean eGFRs per slope period are plotted over the follow-up time and by incident CVD outcomes. Box plots indicate mean eGFR and range for all individuals during the 2-year baseline period, in which the predominant CVD diagnosis in this CKD population was HF.
Figure 2.
Updated mean estimated glomerular filtration rate (eGFR) in chronic kidney disease (CKD) subjects with type 2 diabetes mellitus illustrated over time and by incident cardiovascular disease outcomes.
Multivariable associations between covariables included in the models and CKD indicators are demonstrated in Table 3 for eGFR slope categories. Risk for CVD outcomes increased with age across end points by approximately 4%/y (MACE plus HR = 1.41; 95% CI, 1.27–1.56) and men were at greater risk compared with women. Other factors that had significant associations beyond CKD progression to CVD included angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker (HR = 1.29; 95% CI, 1.08–1.53) and mineralocorticoid receptor antagonist (HR = 2.96; 95%, CI 2.44–3.59) treatment among T2DM patients with CKD in relation to HF. Having T2DM for over 5 years at the time of CKD diagnosis also significantly increased CVD outcomes, except for IS, by approximately 35%. Smoking (HR = 1.35; 95% CI, 1.11–1.64) and being overweight (HR = 1.23; 95% CI, 1.04–1.45) were both significantly associated with HF events. Similar associations were noted for all adjusted multivariable model results. Specific outcomes for each eGFR indicator type are shown in Supplementary Table S2.
Table 3.
Multivariable association between categorical eGFR updated slope measure and cardiovascular disease outcomes in 30,222 adults with type 2 diabetes mellitus and CKDa
| Model covariates | Heart failure (n = 1185) | Myocardial infarction (n = 311) | Ischemic stroke (n = 195) | MACE plus (n = 1588) |
|---|---|---|---|---|
| Age at baseline, yr | 1.05 (1.04–1.05) | 1.02 (1.01–1.04) | 1.05 (1.04–1.07) | 1.04 (1.04–1.05) |
| Male gender | 1.34 (1.18–1.51) | 1.85 (1.46–2.35) | 1.19 (0.88–1.60) | 1.41 (1.27–1.56) |
| CKD updated slope categories¤ | ||||
| Updated eGFR slope ≥3 | Reference group | Reference group | Reference group | Reference group |
| Updated eGFR slope 0–3 | 1.02 (0.85–1.23) | 1.10 (0.78–1.57) | 1.19 (0.76–1.85) | 1.06 (0.90–1.24) |
| Updated eGFR slope –3 to <0 | 1.23 (1.03–1.47) | 1.16 (0.83–1.63) | 1.32 (0.86–2.01) | 1.22 (1.05–1.42) |
| Updated eGFR slope eGFR < –3 | 1.50 (1.27–1.76) | 1.39 (1.01–1.91) | 1.33 (0.88–2.01) | 1.45 (1.26–1.67) |
| Never-smoker | Reference group | Reference group | Reference group | Reference group |
| Ex-smoker | 1.14 (1.01–1.30) | 1.03 (0.80–1.31) | 0.89 (0.65–1.22) | 1.09 (0.98–1.21) |
| Current smoker | 1.35 (1.11–1.64) | 1.28 (0.89–1.83) | 1.43 (0.91–2.23) | 1.33 (1.13–1.57) |
| Systolic blood pressure, mm Hg | 1.01 (1.00–1.01) | 1.02 (1.01–1.03) | 1.00 (0.99–1.01) | 1.01 (1.00–1.01) |
| Diastolic blood pressure, mm Hg | 0.98 (0.97–0.99) | 0.98 (0.96–1.00) | 1.01 (0.98–1.03) | 0.99 (0.98–0.99) |
| Body mass index ≥25 kg/m2 | 1.23 (1.04–1.45) | 0.81 (0.61–1.08) | 1.10 (0.75–1.62) | 1.13 (0.98–1.30) |
| Low-density lipoprotein, mmol/lb | 1.00 (1.00–1.00) | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.00 (1.00–1.00) |
| Triglycerides, mmol/lc | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Hyperkalemia >5.5 mmol/l | 1.32 (0.97–1.81) | 1.29 (0.71–2.37) | 1.29 (0.57–2.92) | 1.32 (1.01–1.74) |
| Renin–angiotensin–aldosterone system inhibitors medication | 1.29 (1.08–1.53) | 0.94 (0.69–1.28) | 0.93 (0.64–1.35) | 1.17 (1.01–1.35) |
| Mineralocorticoid receptor antagonist treatment | 2.96 (2.44–3.59) | 1.29 (0.75–2.21) | 1.00 (0.47–2.14) | 2.50 (2.09–2.99) |
| Diabetes duration ≥5 yr | 1.36 (1.21–1.53) | 1.34 (1.07–1.68) | 1.26 (0.94–1.67) | 1.35 (1.22–1.49) |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
Bold text indicates statistically significant results.
Data are shown as hazard ratio (95% confidence interval). Estimated glomerular filtration rate slopes are based on the Chronic Kidney Disease Epi Equation by Levey et al.23 British National Formulary coding was used for mineralocorticoid receptor antagonist and renin–angiotensin–aldosterone system inhibitor medication angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. Exclusions were all prevalent cardiovascular disease events before follow-up.
Missing for low-density lipoprotein n = 7438 (24.6%);
Missing for triglycerides n = 4668 (15.5%). ¤
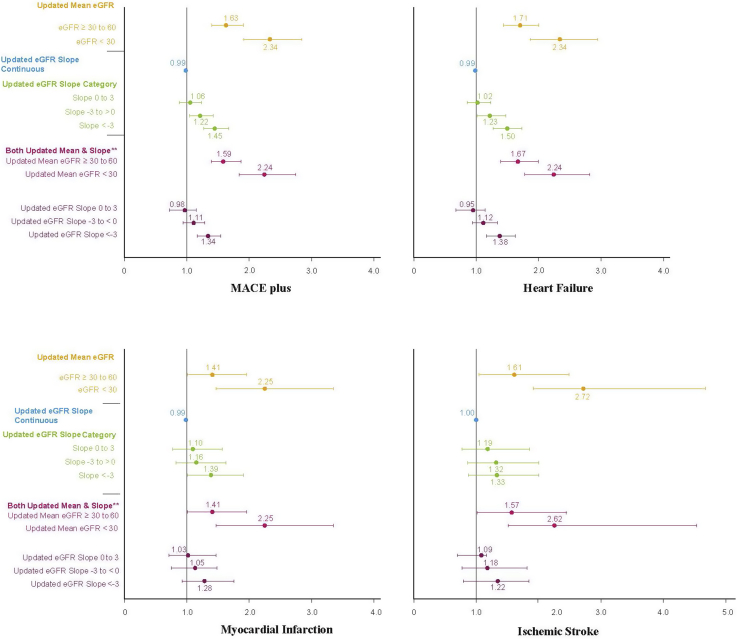
Regardless of T2DM status and generally accepted risk factors for CVD, renal function defined by eGFR remained an independent risk factor for heart disease. The updated eGFR slope (≥3 ml/min/1.73 m2 per 2-year period) was the reference category indicating no renal disease progression, which was compared with successively greater renal deterioration across the other slope categories. The fastest progressors of CKD, as assessed by their updated eGFR slopes over time of ≤ –3, were significantly associated with HF (HR = 1.50; 95% CI, 1.27–1.76), MI (HR = 1.39; 95% CI, 1.01–1.91), and MACE plus (HR = 1.45; 95% CI, 1.26–1.67). All other eGFR measures (eGFR updated mean, slopes as a continuous estimate, updated slope, and both the updated mean and slope entered in the same model) were included in similar models that demonstrated associations equivalent to those of CVD outcomes. Adjusted risk estimates are shown in Figure 3.
Figure 3.
Proportional hazards regression models in 30,222 patients with associated diabetic nephropathy estimating time to cardiovascular disease outcomes by updated mean and updated estimated glomerular filtration (eGFR) slope. Hazard ratios for chronic kidney disease variables and 95% confidence intervals are based on the linear effect model per mean or slope and categorized eGFR slope variables. Fully adjusted models include the baseline variables of age, sex, body mass index, systolic blood pressure, disatolic blood pressure, low-density lipoprotein, triglycerides, type 2 diabetes mellitus dutation, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, mineralocorticoid receptor antagonist treatment, and hyperkalemia. ∗eGFR updated mean category ≥60 ml and updated slope ≥3 are reference categories. ∗∗Updated mean and updated slope were assessed in the same model; interaction effects were nonsignificant for all events analyzed (results not shown).
Figure 3 presents HR estimates, including the main eGFR measures assessing current status and progression of CKD both alone as the main variable of interest and together in the same model. When assessed alone, in the model, the updated mean eGFR showed a gradient effect when the 2 more severe categories of CKD were compared with the reference category of ≥60 across all CVD outcomes. This association was most strongly noted in IS (HR = 2.72; 95% CI, 1.59–4.68), closely followed by HF (HR = 2.34; 95% CI, 1.86–2.94), MACE plus (HR = 2.33; 95% CI, 1.92–2.84), and MI (HR = 2.25; 95% CI, 1.47–3.45).
In the model in which the updated mean eGFR per 2-year slope intervals and updated eGFR slope were assessed simultaneously, the most current estimate of eGFR as measured by the updated mean demonstrated a stronger although slightly attenuated correlation compared with CVD outcomes over time, as illustrated by the MACE plus HRs for updated mean eGFR ≥ 30–60 ml/min/1.73 m2 (HR = 1.59; 95% CI, 1.37–1.86) and updated mean eGFR <30 ml/min/1.73 m2 (HR = 2.24; 95% CI, 1.84–2.74). The updated eGFR slope measures in the most rapidly progressing category, defined by a slope of < –3 ml/min/1.73 m2, were strongly associated with HF (HR = 1.38; 95% CI, 1.17–1.63) and MACE plus (HR = 1.34; 95% CI, 1.16–1.54) and less so with other CVD outcomes compared with the reference category (in which no progression in renal disease is assumed). Other categories of the updated eGFR slope were no longer statistically associated with CVD when the updated mean measure was included as a covariable in the model. Baseline eGFR slopes were also assessed and results were similar to those noted for the eGFR updated slope models. For instance, when baseline slope inclination category < –3 was compared with the referent level ≥3 in a fully adjusted model, the following results were noted: MACE plus (HR = 1.24; 95% CI, 1.08–1.43) and HF (HR = 1.20; 95% CI, 1.02–1.41).
Discussion
The association between renal disease progression and CVD risk was assessed among patients with T2DM and CKD in a UK primary health care setting. We found that progression of CKD appears to be associated with CVD risk, in which a monotonic increased risk of cardiovascular events was noted among those with the fastest rate of eGFR decline, most predominantly in HF. However, the most current CKD status (based on the mean estimate during the same 2-year slope periods and updated over time) was more consistently associated with all CVD event types independently of disease progression. Previous studies established an increasing prevalence of CKD2,19 and its importance to CVD risk, especially among subjects with both T2D and CKD.14,26,27 To the authors' knowledge, this study is the first to assess both progression of renal disease and current renal status in relation to the development of macrovascular outcomes, independantly of risk factors such as hypertension and cardiovascular medication, to better understand the underlying mechanisms of renal disease in this contemporary cohort of persons with T2DM.
Declining renal function over 10 years is a significant risk for CVD at any point in time throughout the trajectory of a T2DM patient’s disease history and beyond current renal status. We found that over one-fourth of subjects met our criteria for fast progressors at baseline with a decreasing slope of >3 ml/min/1.73 m2 (slope category < –3). A recent publication27 in a large contemporary population of US adults with 30–59 ml/min/1.73 m2 eGFR demonstrated that accelerated progression of kidney dysfunction within 2 years affected approximately 1 in 4 patients with diabetes and 1 in 7 without it. They noted, as also illustrated in this study, that the strongest independent correlates of fast CKD progression included elevated systolic blood pressure and HF.
When evaluating the statistical risk for current eGFR status, we found that the most current status of eGFR was more strongly associated with CVD risk across all event types. Lower updated mean measures of eGFR < 30 mL/min/1.73 m2 estimated the greatest risk for incident CVD compared with those with mean eGFR estimates > 60 mL/min/1.73 m2. Overall, measures of eGFR slope and mean appear to capture different aspects of the disease (rate of renal deterioration and current status). When analyzed in the same model, current eGFR status (updated mean eGFR) was more strongly associated with CVD outcomes compared with eGFR slope estimates. Among the fastest progressors, only eGFR slope category < –3 indicated a significant increase in risk for heart failure and the MACE plus composite events.
When modeling renal data, the analytical value of current status as estimated by the updated mean eGFR and the rate of decline over time as captured by slope estimates evaluate different effects on health outcomes. Although it is a weaker risk factor in the statistical models, the eGFR slope estimates added to the CVD risk profile beyond the patient’s last recorded mean eGFR value. This may affect clinical practice and clinical trial decision processes. By better describing the nonlinearity of renal disease progression and comparing this with a patient’s most current renal status, a more holistic picture may be obtained and more timely treatment provided.
As mentioned, additional analyses were conducted to assess the 2 main parameters (updated mean eGFR and eGFR updated slope) in the same model. The strong associations noted between both variables and CVD events persisted, but the updated mean eGFR was an overall stronger statistical risk factor. In addition, when assessed in a categorical manner, baseline slope became a significant factor of MACE plus and HF, indicating that the rate of decline possibly at any point throughout a subject’s history provides important clinical information. We also attempted to assess the potential interaction between the eGFR updated slope and updated mean eGFR to explore additional complex patient segmentations. However, we could not test statistical significance for this interaction owing to an insufficient dataset size. The interaction between the updated mean eGFR and age was tested to assess the natural deteriorating impact of age on the cardiorenal system, but we found it not to be significant in all models.
In a recent global meta-analysis, the prevalence of CKD in Europe was approximately 12% across stages 3–5 and a greater proportion were women.2 In the study be Go et al.28 from 2018, as well as our study, most T2DM subjects had advanced CKD, likely owing to the difficulty of identifying subjects during the more asymptomatic early stages of disease while in primary health care. Another important distinction among older T2DM subjects relates to hypertensive medication, for which a substantial gap exists between guideline recommendations and real-world prescribing patterns for RAASi among T2DM CKD patients with CVD comorbidities.29 The subjects in our study averaged age 64 years and the vast majority were receiving RAASi medication (82%). This descriptive pattern may change as the population becomes more severely diseased; less than a quarter had T2DM over 5 years.29 This study supports the growing body of evidence that CKD increases CVD risk independently of hypertension and T2DM26,30,31 and at any point in time throughout the trajectory of a T2DM patient’s disease history of declinining renal function. The strongest independent risk factors for CVD outcomes in addition to eGFR measures, both in our study and as previously demonstrated, were age, male gender, smoking status, hypertensive treatment, and duration of T2DM before CKD diagnosis. More difficult to disentangle in this study was the association between hyperkalemic status at baseline and MACE plus in addition to the high CVD risk noted among those receiving antihypertensive treatment. The association between hypertensive treatment and CVD outcomes may have been due to confounding by indication, reflecting a more diseased state, even during the baseline period. Therefore, metrics to estimate CKD progression more precisely are critical for the optimal timing of CVD interventions such as antihypertensive (RAASi or mineralocorticoid receptor antagonist) and antidiabetic medication such as sodium-glucose cotransporter-2 inhibitors. In our study, the impact of RAASi medication may have attenuated the association noted between renal disease progression and future CVD events. Hence, future retrospective and prospective studies are warranted in which the renal metrics studied here (updated mean eGFR and updated eGFR slope) are further analyzed in conjunction with different pharmacological CVD interventions to identify optimal time points for intervention as well as therapeutic target levels. The risk for cardiorenal outcomes may improve over time if renal health is maintained, and especially so among T2DM populations, as was recently demonstrated in a US study that demonstrated reductions in CVD outcomes.28
Along with unmeasured confounding, there were other relevant limitations to this study. Information on laboratory methods of measuring creatinine levels, which may influence eGFR levels to some extent, was not always available.31 However, similar creatinine levels were used to estimate both slope and updated mean values. In addition, almost a quarter of the subjects were missing low-density lipoprotein and/or triglyceride values at baseline. However, the proportion of subjects who experienced a CVD outcome was similar between those with measures for low-density lipoprotein and/or triglycerides (HF, 5.4%; MI, 1.4%; IS, 0.9%; and MACE, 7.3%) compared with those with missing values (HF, 6%; MI, 1.8%; IS, 1.2%; and MACE, 8.5%). Therefore, we believe the results were not skewed and the values were missing at random. From a statistical perspective, power was insufficient to assess interaction factors in the same model including both the eGFR updated mean and the updated slope. Finally, impaired kidney function and HF risk may occur in the prediabetic stages, making it difficult to assess risk factors in early asymptomatic stages of these diseases. Despite the limitations highlighted here, this remains a novel study assessing renal function decline over a long follow-up. It allowed the inclusion of multiple measures of eGFR, making it well-suited to study CKD progression rates adequately in subjects with T2DM and newly diagnosed CKD. Future studies may further assess these measures of eGFR using more complex marginal structural modeling techniques also to study the changing risk factor profile of CKD patients.
This study demonstrates that there is a strong association between CKD, based on eGFR, and the risk for CVD morbidity in a contemporary cohort of persons with T2DM. We found that the rate of progression and the cumulative status of CKD describe distinct aspects of the cardiorenal risk in persons with T2DM. More specifically, updated mean eGFR proved to be an important marker of current renal function independently of the rate of disease progression. The rate of renal deterioration remained highly correlated with incident HF events in T2DM subjects independently of most current renal health. This knowledge may be used in future clinical practice to provide more timely medical intervention to prevent CVD in patients with CKD.
Disclosure
All authors declare no support from any organization for the submitted work beyond AstraZeneca R&D; no financial relationships with any organizations that might have an interest in the submitted work; no other relationships or activities that could appear to have influenced the submitted work. PG, MO, VS, CC, AL, and SS are or were employed by AstraZeneca during the production of this manuscript. ML has been a consultant or received honoraria from AstraZeneca, Eli Lilly, MSD, and Novo Nordisk and received research grants from DexCom and Novo Nordisk. AstraZeneca funded access to the CPRD database.
Author Contributions
All authors read, revised, and approved the final manuscript. All authors confirm that the manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained. All authors took part in the design of the study and interpretation of results. AL and MO conducted the statistical analysis. CC wrote the manuscript. VS prepared the visualization of eGFR progression and reviewed and contributed to writing the manuscript. SS, PG, KW, and ML reviewed the statistical analyses and contributed to writing the manuscript. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. CSC is the guarantor of this work.
Footnotes
Table S1. Baseline characteristics among UK adults with type 2 diabetes mellitus (T2DM) from 1 January 2005 to 31 December 2015, by cardiovascular disease.
Table S2. Proportional hazards regression models in 30,222 adults with type 2 diabetes mellitus (T2DM) and CKD estimating time to cardiovascular disease outcomes by estimated GFR (eGFR) measures.
Translational Statement
This study demonstrates a strong association between chronic kidney disease and cardiovascular disease morbidity in a cohort of persons with type 2 diabetes mellitus. We found that the rate of progression and the cumulative status of chronic kidney disease describe distinct aspects of the cardiorenal risk in persons with type 2 diabetes mellitus. More specifically, updated mean estimated glomerular filtration rate proved to be an important marker of current renal function independently of the rate of disease progression, whereas the rate of renal deterioration remained highly correlated with the incident hazard ratio. This knowledge may be used in future clinical practice to provide more timely medical intervention to prevent cardiovascular disease in patients with chronic kidney disease.
Supplementary Material
References
- 1.Levey A.S., Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 2.Hill N.R., Fatoba S.T., Oke J.L. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PloS One. 2016;11 doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aitken G.R., Roderick P.J., Fraser S. Change in prevalence of chronic kidney disease in England over time: comparison of nationally representative cross-sectional surveys from 2003 to 2010. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-005480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jha V., Garcia-Garcia G., Iseki K. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 5.Murphy D., McCulloch C.E., Lin F. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165:473–481. doi: 10.7326/M16-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey R.A., Wang Y., Zhu V. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on kidney disease: improving Global Outcomes (KDIGO) staging. BMC Res Notes. 2014;7:415. doi: 10.1186/1756-0500-7-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parving H.H., Lewis J.B., Ravid M. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69:2057–2063. doi: 10.1038/sj.ki.5000377. [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation 2007 annual report. https://www.kidney.org/sites/default/files/docs/annual_report_2007.pdf Available at:
- 9.United States Renal Data System Annual data report 2006. https://www.usrds.org/atlas06.aspx Available at:
- 10.Alberti K.G., Eckel R.H., Grundy S.M. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd-Jones D.M. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation. 2010;121:1768–1777. doi: 10.1161/CIRCULATIONAHA.109.849166. [DOI] [PubMed] [Google Scholar]
- 12.Stevens R.J., Kothari V., Adler A.I. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56) Clin Sci (Lond) 2001;101:671–679. [PubMed] [Google Scholar]
- 13.Ronco C., Haapio M., House A.A. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 14.Afkarian M., Sachs M.C., Kestenbaum B. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol. 2013;24:302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gansevoort R.T., Correa-Rotter R., Hemmelgarn B.R. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 16.Holman R.R., Paul S.K., Bethel M.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 17.Holman R.R., Paul S.K., Bethel M.A. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359:1565–1576. doi: 10.1056/NEJMoa0806359. [DOI] [PubMed] [Google Scholar]
- 18.Zhong J., Yang H.C., Fogo A.B. A perspective on chronic kidney disease progression. Am J Physiol Renal Physiol. 2017;312:375–384. doi: 10.1152/ajprenal.00266.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsushita K., Selvin E., Bash L.D. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20:2617–2624. doi: 10.1681/ASN.2009010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greene T., Ying J., Vonesh E.F. Performance of GFR slope as a surrogate end point for kidney disease progression in clinical trials: a statistical simulation. J Am Soc Nephrol. 2019;30:1756–1769. doi: 10.1681/ASN.2019010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrett E., Gallagher A.M., Bhaskaran K. Data resource profile: Clinical Practice Research Datalink (CPRD) Int J Epidemiol. 2015;44:827–836. doi: 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical Practice Research Datalink. http://www.cprd.com Available at:
- 23.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couchoud C., Pozet N., Labeeuw M. Screening early renal failure: cut-off values for serum creatinine as an indicator of renal impairment. Kidney Int. 1999;55:1878–1884. doi: 10.1046/j.1523-1755.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- 25.National Institute for Health and Care Excellence NICE clinical guideline on chronic kidney disease (CKD) 2014. https://www.nice.org.uk/standards-and-indicators/qofindicators Available at: [PubMed]
- 26.National Institute for Health and Care Excellence Briefing paper: Quality and Outcomes Framework (QOF) indicator development programme 2014. https://www.nice.org.uk/get-involved/meetings-in-public/qof-advisory-committee Available at:
- 27.Go A.S., Chertow G.M., Fan D. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 28.Go A.S., Yang J., Tan T.C. Contemporary rates and predictors of fast progression of chronic kidney disease in adults with and without diabetes mellitus. BMC Nephrol. 2018;19:146. doi: 10.1186/s12882-018-0942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epstein M. Hyperkalemia constitutes a constraint for implementing renin-angiotensin-aldosterone inhibition: the widening gap between mandated treatment guidelines and the real-world clinical arena. Kidney Int Suppl. 2016;6:20–28. doi: 10.1016/j.kisu.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olsson M., Schnecke V., Cabrera C. Contemporary risk estimates of three HbA1c variables for myocardial infarction in 101,799 patients following diagnosis of type 2 diabetes. Diabetes Care. 2015;38:1481–1486. doi: 10.2337/dc14-2351. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama H., Kanno S., Takahashi S. Risks for glomerular filtration rate decline in association with progression of albuminuria in type 2 diabetes. Nephrol Dial Transplant. 2011;26:2924–2930. doi: 10.1093/ndt/gfq774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.