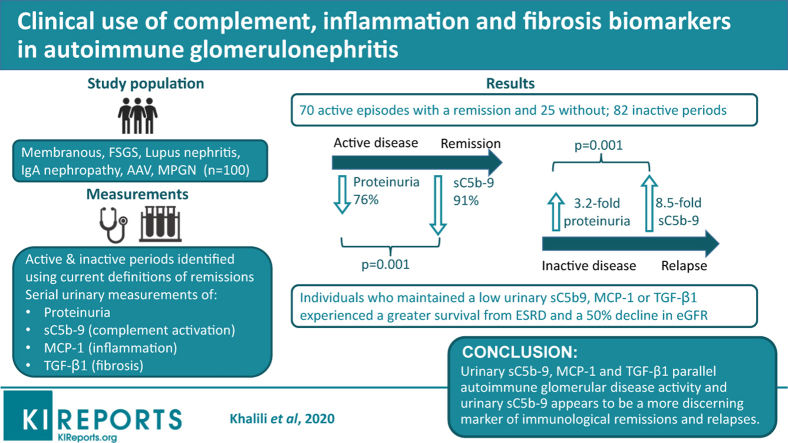
Abstract
Introduction
Complement activation, inflammation, and fibrosis play central roles in the mechanisms of injury in autoimmune glomerulonephritis (GN) but they are seldom assessed in epidemiologic studies. The measurement of urinary biomarkers of these pathways of injury could parallel disease activity and add clinical value beyond proteinuria.
Methods
We performed a prospective cohort study of 100 patients with focal and segmental glomerulosclerosis (FSGS), membranous nephropathy (MN), IgA nephropathy (IgAN), lupus nephritis (LN), anti-neutrophil cytoplasmic autoantibody–associated vasculitis (AAV), and membranoproliferative GN (MPGN) followed for 33 (18–54) months. Repeated urinary samples were collected throughout their follow-up to determine proteinuria, urinary sC5b-9, monocyte chemoattractant protein–1 (MCP-1), and transforming growth factor–beta 1 (TGF-β1), expressed as creatinine ratios. We identified 177 periods of active and inactive disease based on current remission definitions for each disease.
Results
Urinary sC5b-9, MCP-1, and TGF-β1 were present in each disease. In periods leading to a remission, the reduction of urinary sC5b-9 was 91%, greater than for proteinuria with 76%. During inactive periods, those who did not experience a relapse maintained lower levels of biomarkers compared with those who relapsed. At that time, the increase in urinary sC5b-9 was significantly greater than the rise in proteinuria (8.5-fold increase compared with 3.2-fold) and urinary MCP-1 and TGF-β1. Using current remission definitions for each disease, thresholds for each biomarker were determined using receiver operating characteristic curves. Individuals who averaged levels below these cutoffs during their follow-up had better renal outcomes.
Conclusion
In autoimmune glomerular diseases, urinary sC5b-9, MCP-1, and TGF-β1 are present and parallel disease activity and outcomes. Urinary sC5b-9 appears to be a more discerning marker of immunologic remissions and relapses.
Keywords: complement, sC5b-9, MCP-1, TGF-β1, urinary biomarkers, autoimmune glomerulonephritis
Graphical abstract
The assessment of patients with glomerular diseases during their follow-up focuses on monitoring proteinuria, blood pressure, and renal function. Definitions of active disease, remission, and relapse are based on these, as is the decision to administer immunosuppression. Unfortunately, these tools are imperfect, and some patients experience poor outcomes because of unrecognized ongoing immunologic damage whereas others with persistent proteinuria would not have progressed and are needlessly exposed to immunosuppressive therapy.1 Pathology findings are invaluable but are not often reassessed to guide patient care.
Complement activation plays a central role in the mechanisms of injury of autoimmune glomerular diseases. Classical, alternative or lectin activation pathways lead to the cascade of C3 convertase, C5 convertase, and the formation of C3a, C5a, and C5b-9 (membrane attack complex [MAC]), mediating most of the downstream effects.2, 3, 4, 5, 6, 7, 8 Immunologic triggers lead to inflammation and fibrosis through activation of multiple chemokines and growth factors.9,10 Many have been studied in the clinical setting including MCP-1, an inflammatory chemokine,11, 12, 13 and TGF-β1, a cytokine involved in the healing process after tissue injury.14,15 Urinary levels of soluble C5b-9 (sC5b-9), MCP-1, and TGF-β1 have shown variable correlations with outcomes.11,15, 16, 17, 18, 19, 20, 21, 22 Published studies thus far have not specifically addressed their serial measurement during a typical disease course marked by episodes of activity, remission, and relapse and whether they add a predictive value on top of proteinuria.
We prospectively evaluated the presence and variations in urinary levels of sC5b-9, MCP-1, and TGF-β1 as surrogate markers of complement activation, inflammation, and fibrosis in the course of autoimmune glomerular diseases to assess their clinical value. By being pathogenically linked to the mechanisms of injury, we hypothesize that higher urinary levels of these biomarkers will denote active disease and that their variations help predict remissions, relapses, and the loss of renal function.
Materials and Methods
Study Design
We performed a prospective observational study in subjects with autoimmune GN initiated in 2006 until 2020 in 2 hospitals affiliated with the University of Montreal, Canada. Each center’s ethics committee approved this study, and all participants gave informed consent prior to enrolment to participate and biobank urinary specimens to be used at a subsequent time date to test new hypotheses relevant to their disease. This work has been carried out in accordance with the declaration of Helsinki.
Patients and Samples
We included all individuals with biopsy-proven FSGS, MN, IgAN, LN, AAV, and MPGN who consented. Patients with FSGS, MN, and IgAN were considered primary. We collected from all available follow-ups the blood pressure, serum creatinine, proteinuria, and medication use including the number of antihypertensive drugs, the use of renin-angiotensin system blockade, and immunosuppressive treatments. Additional urinary samples were taken at the time of visits to simultaneously determine each biomarker. Proteinuria was determined from spot samples immediately after collection in each center’s laboratory and expressed in grams per gram (g/g) of creatinine. For all other biomarkers, urine samples were stored at 4 °C, centrifuged at 200g for 10 minutes, aliquoted in multiple 0.4-ml vials and stored at –80 °C until further processing.
Definitions
We identified periods of active and inactive disease according to proteinuria being above or below the remission threshold (Table 1).23, 24, 25, 26 For AAV, this was not based on a level of proteinuria but by the presence of a Birmingham vasculitis activity score of 0 at ≥6 months of follow-up.27 Active episodes were further divided into those leading to a remission and those that did not. Periods that lead to a remission were stopped at the first measurement that satisfied the definitions in Table 1. We recorded relapses, defined as a patient not meeting the remission criteria after it had been reached. Each episode required at least 2 measurements of all 4 biomarkers. To facilitate their comparison, we illustrate each period using 3 different time points, the first measurement (T1), the last (T3), and the average of all measurements in between (T2), if present (Supplementary Figure S1A). For active episodes leading to a remission, we assigned T1 to the highest proteinuria.
Table 1.
Remission definitions
| Disease | Remission definition |
| FSGS | 50% decrease in proteinuria to ≤3.5 g/d |
| MN | 50% decrease in proteinuria to ≤3.5 g/d |
| IgAN | 50% decrease in proteinuria to ≤1 g/d |
| LN | 50% decrease in proteinuria to ≤1 g/d |
| AAV | Normal renal BVAS score at ≥6 mo after induction |
| MPGN | 50% decrease in proteinuria to ≤1 g/d |
AAV, anti-neutrophil cytoplasmic autoantibody–associated vasculitis; BVAS, Birmingham vasculitis activity score; FSGS, focal and segmental glomerulosclerosis; IgAN, IgA nephropathy; LN, lupus nephritis; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis.
The estimated glomerular filtration rate (eGFR) was based on the CKD-EPI formula. We calculated the eGFR loss during each episode by the difference in eGFR between the first and last eGFR divided by the length of observation. We recorded whether subjects experienced a 50% decline in renal function or renal failure, defined as an eGFR ≤15 ml/min per 1.73 m2.
Finally, we estimated the average level of biomarkers (“exposure to”) by calculating the area under the curve (AUC) of measurements using rectangular integration, a simple method illustrated in Supplementary Figure S1B. An average by AUC for every biomarker was calculated for each period and also for the entire follow-up of a patient.
Urinary Biomarker Measurements
We used the human EIA Kits sC5b-9 (MicroVue, Quidel Corp., San Diego, CA). The soluble MAC, approximatively 1000 kDa, cannot pass through the glomerular barrier in normal conditions but appears in the urine in diseased states when locally expressed or when filtrated because of a higher glomerular permeability. Urine samples were diluted 1:3 in all but 27 of the 836 samples, which were diluted 1:15. The assay’s lower sensitivity threshold was 15 μg/L. Urinary MCP-1 and TGF- β1 were measured using 2 Human Cytokine/Chemokine plex kit (Millipore, St. Charles, MO) on a multiplex platform (Eve Technologies Corp., Calgary, Alberta, Canada). The assays’ lower sensitivity thresholds were 2 and 10 ng/L for MCP-1 and TGF-β1, respectively, and samples were not diluted. We report the values of urinary MAC in micrograms per millimole of creatinine and MCP-1, TGF-β1 in nanograms per millimole of creatinine.
Statistical Analyses
Normally distributed variables are presented as means ± SDs and nonparametric variables as medians (interquartile range). Correlations between biomarkers and the loss of eGFR loss were calculated using Spearman rho. Comparisons of the levels of biomarkers between different diseases were done using the Kruskal-Wallis, with subgroup analyses performed with Mann-Whitney U tests. We could not compare groups composed of different GN because the diagnosis influenced the level of each biomarker. However, it was possible to compare individuals to themselves at different time points using the Wilcoxon signed-rank test, regardless of their disease. We tested whether proteinuria and urinary sC5b-9, MCP-1, and TGF-β1 changed from T1 to T3 and if the proportion of change in each biomarker from T1 to T3 was statistically different from that of proteinuria. In those with a recurrent disease, we also assessed whether the increments in each biomarker at the time of a relapse differed in proportion from that of the proteinuria.
For every sample measured, we determined whether a patient was in remission or not and drew different receiver operating characteristic curves for each disease type with all the biomarkers’ values. The optimal cutoff for each urinary biomarker was obtained by identifying the point maximizing the sensitivity and specificity product. We propose remission thresholds for each new biomarker based on these cutoffs. This was not done for proteinuria, as it is included in the standard definitions of remission, with the exception of AAV.
Finally, using the average level of biomarkers for the entire follow-up, we categorized each individual as having levels above or below the threshold for remission. Based on these categories, we assessed the survival from a 50% decline in renal function or end-stage renal disease using Kaplan-Meier curves and log-rank tests. We curtailed the curves when only 10% of the cohort remained, which occurred at 6 years.28 Two-tailed P values less than 0.05 were considered statistically significant. Analyses were performed using SPSS software (version 24, SPSS Inc., Chicago, IL).
Results
Patient and Period Characteristics
One hundred patients participated in the study, including 37 women. There were 19 FSGS, 16 MN, 27 IgAN, 18 LN, 18 AAV, and 2 MPGN, which were considered autoimmune, noninfectious, and unrelated to a monoclonal gammopathy of renal significance. Two patients had diabetes but showed no signs of diabetic nephropathy at the biopsy. The initial age varied from 36 ± 11 years for LN to 64 ± 12 years for AAV. Subjects were followed for 33 (18–54) months, during which they had a median 7 (4–11) new biomarker measurements and twice as many proteinuria values. The disease course in each patient was divided in episodes of activity as depicted in Supplementary Figure S1A. These totaled 177 periods: 95 active events (70 with and 25 without a remission) and 82 inactive episodes (Table 2).
Table 2.
Patient characteristics (N = 100)
| Variable | FSGS (n = 19) | MN (n = 16) | IgAN (n = 27) | LN (n = 18) | AAV (n = 18) | MPGN (n = 2) |
|---|---|---|---|---|---|---|
| Age (yr) | 54 ± 19 | 55 ± 13 | 41 ± 15 | 36 ± 11 | 64 ± 12 | 51 ± 15 |
| Female sex, % | 37 | 31 | 33 | 61 | 22 | 50 |
| Total duration of follow-up (mo) | 22 (13–41) | 42 (19–63) | 22 (13–54) | 40 (20–71) | 36 (24–45) | 100 (95–106) |
| Active periods with remission (n) | 16 | 12 | 12 | 13 | 13 | 4 |
| Initial eGFR (ml/min per 1.73 m2) | 44 ± 28 | 61 ± 34 | 66 ± 20 | 74 ± 40 | 22 ± 14 | 29 ± 7 |
| Initial blood pressure (mm Hg) | 133/80 ± 19/10 | 141/82 ± 16/12 | 131/82 ± 15/10 | 131/82 ± 18/12 | 144/81 ± 20/14 | 110/71 ± 8/9 |
| Antihypertensive medication, n, % RASB | 3 (2–3), 94 | 2 (1–2), 83 | 1 (1–3), 91 | 2 (0–3), 36 | 2 (1–3), 60 | 3 (1–3), 75 |
| Initial proteinuria (g/g of creatinine) | 8.3 (4.2–10.1) | 9.4 (7.2–11.8) | 2.5 (1.2–4.1) | 6.8 (3.0–10.6) | 2.3 (1.2–5.8) | 2.2 (1.6–3.3) |
| Use of immunosuppression (%) | 81 | 75 | 92 | 100 | 100 | 100 |
| Duration (mo) | 10 (3–13) | 8 (6–16) | 9 (4–13) | 14 (10–20) | 5 (4–6) | 36 (17–60) |
| Rate of renal function changea | –12.6 ± 22.0 | –2.1 ± 21.2 | –8.7 ± 16.0 | +2.0 ± 10.5 | +38.4 ± 23.5 | –2.4 ± 1.3 |
| Active periods without remission (n) | 5 | 3 | 17 | 0 | 0 | 0 |
| Initial eGFR (ml/min per 1.73 m2) | 40 ± 7 | 74 ± 25 | 59 ± 37 | — | — | — |
| Initial blood pressure (mm Hg) | 146/81 ± 12/13 | 110/72 ± 14/2 | 129/82 ± 15/9 | — | — | — |
| Antihypertensive medication, n, % RASB | 3 (1–4), 80 | 2 (2–2), 100 | 2 (1–3), 100 | — | — | — |
| Initial proteinuria (g/g creatinine) | 5.5 (4.4–7.9) | 10.5 (7.3–12.0) | 1.4 (1.1–3.0) | — | — | — |
| Use of immunosuppression (%) | 40 | 67 | 53 | — | — | — |
| Duration (mo) | 16 (3–22) | 7 (6–18) | 17 (7–38) | — | — | — |
| Rate of renal function changea | –15.1 ± 17.9 | –7.8 ± 12.1 | –2.6 ± 5.7 | — | — | — |
| Inactive periods (n) | 17 | 13 | 12 | 18 | 18 | 4 |
| Initial eGFR (ml/min per 1.73 m2) | 33 ± 18 | 67 ± 36 | 66 ± 32 | 77 ± 38 | 36 ± 19 | 22 ± 5 |
| Initial blood pressure (mm Hg) | 130/74 ± 19/11 | 127/76 ± 20/11 | 136/81 ± 11/7 | 121/78 ± 12/11 | 137/80 ± 20/11 | 123/81 ±11/7 |
| Antihypertensive medication, n, % RASB | 2 (2–3), 100 | 2 (2–4), 92 | 2 (1–3), 82 | 2 (1–4), 65 | 2 (1–3), 50 | 3 (1–3), 75 |
| Initial proteinuria (g/g creatinine) | 1.8 (1.3–2.6) | 1.6 (0.4–2.4) | 0.6 (0.5–0.8) | 0.5 (0.2–1.0) | 0.9 (0.3–1.6) | 0.8 (0.5–0.9) |
| Use of immunosuppression (%) | 47 | 23 | 58 | 78 | 100 | 100 |
| Duration (mo) | 11 (6–22) | 27 (19–47) | 14 (5–26) | 24 (18–47) | 28 (19–37) | 11 (3–23) |
| Rate of renal function changea | +0.5 ± 5.3 | –0.3 ± 5.6 | +3.6 ± 14.0 | +0.6 ± 4.5 | +2.4 ± 5.4 | –5.3 ± 5.0 |
| Relapse (%) | 41 | 8 | 25 | 11 | 6 | 50 |
AAV, anti-neutrophil cytoplasmic autoantibody–associated vasculitis; eGFR, estimated glomerular filtration rate; FSGS, focal and segmental glomerulosclerosis; IgAN, IgA nephropathy; LN, lupus nephritis; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis; RASB, renin-angiotensin system blockade.
In milliliters per minute per 1.73 m2 per yr.
Baseline Levels of Urinary Biomarkers During All Active Periods (n = 95)
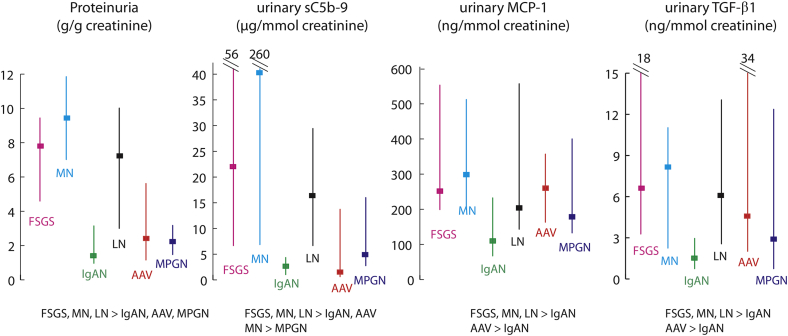
The initial eGFR varied from 22 ± 14 for AAV to 74 ± 40 ml/min per 1.73 m2 for LN (Table 2). The initial values of proteinuria, urinary sC5b-9, MCP-1, and TGF-β1 for all active periods are shown in Figure 1. There were 5 or fewer missing measurements for each biomarker out of the 836 samples collected. Each biomarker was measurable, and their level differed by disease (all Kruskal-Wallis P ≤ 0.001; see Figure 1 for post hoc analyses). The differences between diseases were more pronounced for proteinuria and urinary MAC compared with urinary MCP-1 and TGF-β1. Highest levels were observed in MN with medians of 42.2 (7.4–260.6) μg/mmol of creatinine for urinary sC5b-9 and 300 (201–516) and 8.2 (2.6–11.3) ng/mmol of creatinine for urinary MCP-1 and TGF-β1, respectively. By contrast, those with AAV and IgAN had initial levels of urinary MAC of only 1.4 (0.8–14.7) and 2.6 (0.9–4.8) μg/mmol creatinine, respectively. Initial levels of urinary MCP-1 and TGF-β1 were the lowest in patients with IgAN and MPGN. All initial measurement of biomarkers during active disease intercorrelated with each other (P ≤0.001, Spearman rho). In relation to proteinuria, urinary sC5b-9 had the strongest association (r = 0.73), followed by urinary TGF-β1 (r = 0.52) and MCP-1 (r = 0.37).
Figure 1.
Initial biomarker measurements during periods of active disease. Post hoc comparisons between groups were done using Mann-Whitney U test. AAV, anti-neutrophil cytoplasmic autoantibody–associated vasculitis; FSGS, focal and segmental glomerulosclerosis; IgAN, IgA nephropathy; LN, lupus nephritis; MCP-1, monocyte chemoattractant protein–1; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis; TGF-β1, transforming growth factor beta 1.
Active Periods Leading to a Remission (n = 70)
The highest proteinuria levels were seen in FSGS patients with 8.3 (4.2–10.1) g/g of creatinine, MN with 9.4 (7.2–11.8) and LN with 6.8 (3.0–10.6). It was of 2.5 (1.2–4.1) for IgAN, 2.3 (1.2–5.8) for AAV, and 2.2 (1.6–3.3) for MPGN (Table 2). The initial blood pressure was of 134/81 ± 19/11 mm Hg with 2 (1–3) antihypertensive medication. Periods had a median 9 (5–13) months’ duration. Overall, 90% received immunosuppression.
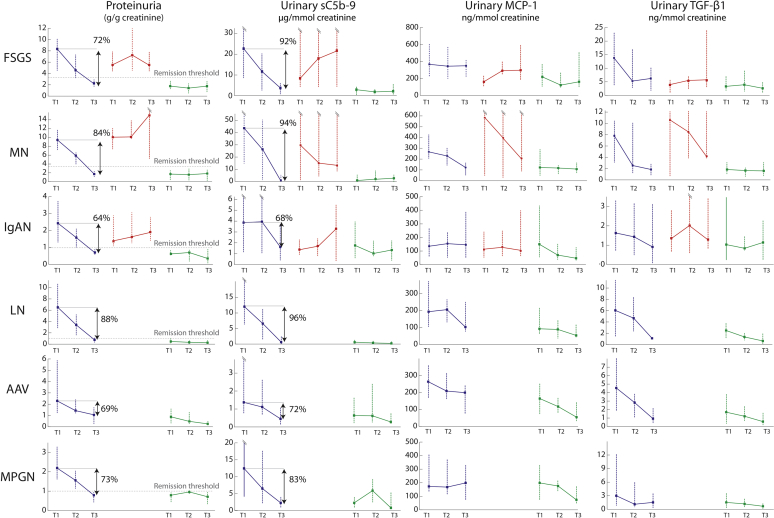
Urinary biomarkers all significantly decreased during remission (Figure 2). Overall, there was a reduction in urinary sC5b-9 of 91% (72%–97%) from the first (T1) to the last (T3) measurement, which was significantly greater than the 76% (64%–85%) decline in proteinuria (P = 0.001, Wilcoxon signed-rank test). This trend was observed for each disease, and the difference was statistically significant for FSGS, MN, and LN patients when tested individually. Overall, there was only a 34% and 66% reduction in urinary MCP-1 and TGF-β1, respectively, which was statistically less in comparison to proteinuria and urinary MAC (P < 0.001).
Figure 2.
Changes in biomarkers during active and inactive periods. Periods of active disease leading to a remission (blue lines) or without a remission (red lines) and inactive episodes (green lines) are illustrated at 3 different time points: T1, the first measurement; T3, the last measurement; and T2, the average of all measurements in between. We show the percentage reduction in proteinuria and sC5b-9. AAV, anti-neutrophil cytoplasmic autoantibody–associated vasculitis; FSGS, focal and segmental glomerulosclerosis; IgAN, IgA nephropathy; LN, lupus nephritis; MCP-1, monocyte chemoattractant protein–1; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis; TGF-β1, transforming growth factor beta 1.
Subjects with FSGS and IgAN experienced a greater decline in renal function with a loss of 12.6 ± 22.0 and 8.7 ± 16.0 ml/min per 1.73 m2 per year, respectively. By contrast, AAV patients gained 38.4 ± 23.5 and MN, LN, and MPGN experienced little change in eGFR. We found no association between biomarker levels assessed by the area under the curve (AUC) with the change of renal function, although there were few episodes per disease to analyze.
Active Periods Without a Remission (n = 25)
During these events, the proteinuria was 5.5 (4.4–7.9) g/g of creatinine for FSGS (n = 5), 10.5 (7.3- 12.0) for MN (n = 3), and 1.4 (1.1–3.0) for IgAN (n = 17) (Figure 2). No subjects with AAV, LN, and MPGN experienced active periods without a remission. The duration of follow-up was 16 (6–22) months and 52% received immunosuppression. Using Wilcoxon signed-rank tests, values did not significantly change from T1 to T3 for proteinuria (2.8 to 2.5 g/g creatinine) and for urinary TGF-β1 (1.4 to 2.2 ng/mmol of creatinine), whereas they tended to increase for urinary MAC (2.6 to 4.1 μg/mmol of creatinine, P = 0.049) and MCP-1 (126 to 206 ng/mmol of creatinine, P = 0.07). Overall, the loss of renal function was 5.7 ± 10.6 ml/min per 1.73 m2 per year. There were too few individuals in each disease group to test the association with the rate of renal function decline.
Inactive Periods (n = 82)
For these periods, the duration of follow-up was of 22 (11–32) months, and immunosuppression use varied from 23% of episodes for MN patients to 100% for AAV and MPGN patients (Table 2). For each disease type, levels of each urinary biomarker assessed by the AUC during inactive episodes were significantly lower compared with active episodes (Figure 2). There was little change in renal function.
These periods were followed by a relapse in 16 cases (20%). These occurred more frequently with MPGN, FSGS, and IgAN and more rarely with LN, AAV, and MN (Table 2). In inactive periods not followed by a relapse (n = 66), there was a significant reduction of 41%, 40%, 38%, and 40% for proteinuria, urinary MAC, MCP-1, and TGF-β1, respectively (all P ≤ 0.03). By contrast, these biomarkers remained unchanged in those who eventually relapsed (n = 16), except for a reduction in TGF-β1 (with a T1 to T3 reduction of 30%, P = 0.02).
Finally, we compared for each biomarker the last value during inactive episodes with the subsequent first value during a relapse. Although proteinuria increased 3.2-fold (1.9–8.3), it rose 8.5-fold (4.2–56.9) for urinary MAC (P = 0.001). Increases in urinary MCP-1 and TGF-β1 at the time of a relapse were significantly less pronounced, with 1.5-fold (0.8–2.1) and 1.8-fold (1.3–3.6) rise (P = 0.02 and 0.005 for urinary MCP-1 and TGF-β1, respectively, compared with changes in proteinuria).
Proposed Reference Values for Urinary Biomarkers
Because the levels of urinary biomarkers differed by disease type, we sought to determine optimal cutoffs using receiver operating characteristic curves. For each simultaneous measurement, we assigned a remission state based on the definitions in Table 1. The optimal cutoffs representing a state of remission for each biomarker are shown in Table 3, and the AUC of receiver operating characteristic curves are given in Supplementary Table S1.
Table 3.
Optimal cutoffs for urinary biomarkers based on clinical remission definitions
| Biomarker | FSGS | MN | IgAN | LN | AAV | MPGN |
|---|---|---|---|---|---|---|
| sC5b-9 (μg/mmol creatinine) | 7.7 | 8.7 | 1.2 | 0.8 | 0.5 | 1.9 |
| MCP-1 (ng/mmol creatinine) | 253 | 146 | 100 | 103 | 126 | 176 |
| TGF-β1 (ng/mmol creatinine) | 4.1 | 3.9 | 0.9 | 1.8 | 1.9 | 1.9 |
AAV, anti-neutrophil cytoplasmic autoantibody–associated vasculitis; FSGS, focal and segmental glomerulosclerosis; IgAN, IgA nephropathy; LN, lupus nephritis; MCP-1, monocyte chemoattractant protein–1; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis; TGF-β1, transforming growth factor beta 1.
The optimal cutoff for proteinuria determined by receiver operating characteristic curve for AAV was 1.0 g/g of creatinine. For other disease, the threshold for proteinuria was based on definitions in Table 2.
Urinary Biomarkers and Survival from a 50% Decline in Renal Function or End-Stage Renal Disease
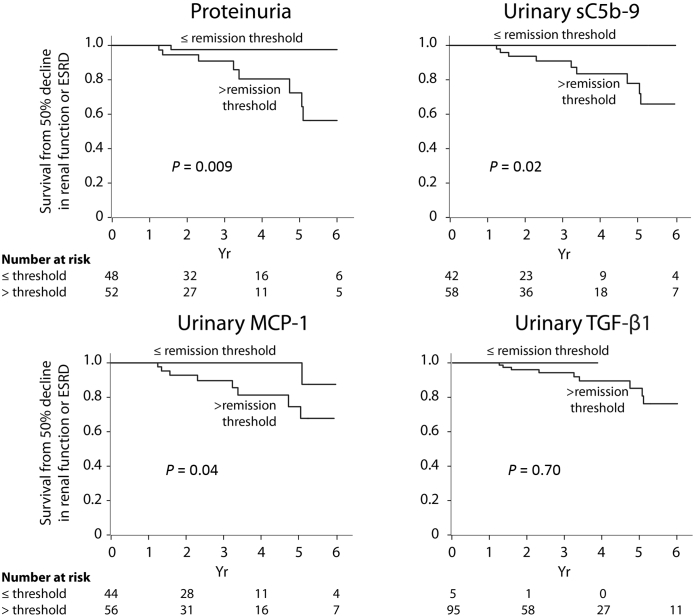
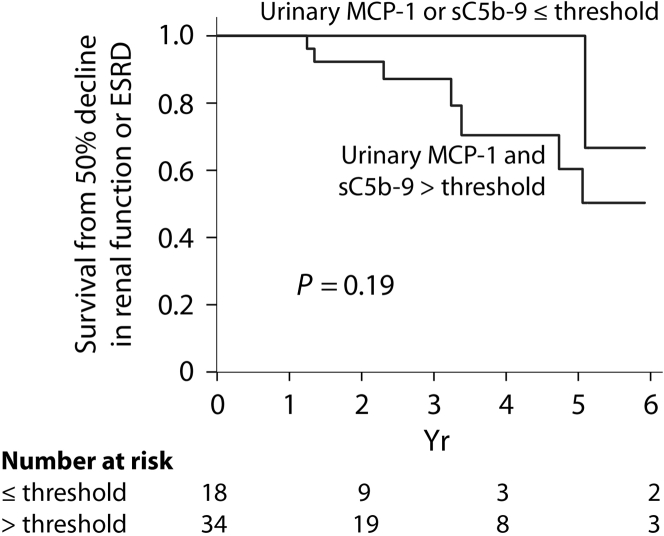
During the follow-up, 10 individuals experienced a 50% decline in renal function including 7 who developed end-stage renal disease. For each biomarker, we categorized individuals in 2 groups based on whether the average level assessed by the AUC for the entire follow-up was above or below the thresholds determined in Table 3. Figure 3 illustrates an almost complete renal survival when biomarkers’ levels are low. Among individuals with a proteinuria level above the remission threshold during their follow-up (n = 52), 5 had low sC5b-9, 10 had low MCP-1, and 3 had both biomarkers below the established remission thresholds. Only 1 of these 18 subjects experienced an event, which occurred after 5 years, although this was not statistically significant (Figure 4), suggesting that despite an elevated proteinuria, a low urinary MCP-1 or sC5b-9 portends a favorable outcome.
Figure 3.
Renal outcome according to levels of proteinuria, urinary sC5b-9, monocyte chemoattractant protein–1 (MCP-1), and transforming growth factor beta 1 (TGF-β1). ESRD, end-stage renal disease.
Figure 4.
Renal outcome in the subgroup with an elevated proteinuria during follow-up according to urinary levels of sC5b-9 and monocyte chemoattractant protein–1 (MCP-1). ESRD, end-stage renal disease.
Discussion
This prospective study explored the clinical value of urinary sC5b-9, MCP-1, and TGF-β1 in autoimmune GN. Levels of these biomarkers differed between diseases, were elevated in active disease, and declined with a remission. In comparison to proteinuria, the reduction in urinary MAC with a remission was significantly greater, and in turn when a relapse occurred, levels increased significantly more, supporting that urinary sC5b-9 is a more sensitive marker of immunologic activity compared to proteinuria. Urinary levels of MCP-1 and TGF-β1 differed less between GN compared with proteinuria, and they also varied significantly less during remissions and relapses. Nevertheless, they were still associated with renal outcomes. Interestingly, in patients who averaged a proteinuria above the remission threshold during their follow-up, we found that those who kept a low urinary MAC or MCP-1 had a favorable outcome. We could not demonstrate an added value using urinary TGF-β1, although the proposed clinical threshold was low and few in our cohort-maintained levels inferior to it.
There is an unmet need for biomarkers in autoimmune GN that could be used to monitor activity and guide immunosuppressive treatment. Renal physiopathology offers strong evidence of complement activation. Three pathways can initiate the cascade leading to signaling, inflammation and activation of innate and adaptive immunity.2, 3, 4, 5, 6, 7, 8,29 MCP-1, or CCL2, is an inducible chemokine that can be locally produced in the kidney in response to inflammatory stimuli and that acts as a recruiter for monocytes, participating in their transformation into macrophages.13 It also participates in recruitment of other cell types, notably T and B lymphocytes and natural killer cells.30,31 TGF-β1 is a cytokine with pleiotropic effects, notably immunoregulatory and antiproliferative actions. It participates in T-cell development and differentiation into regulatory and central memory T cells among others,32,33 and also participates in the healing process after tissue injury by acting in autocrine and paracrine signaling pathways. Without adequate negative feedback, its action can prove harmful owing to the accumulation of extracellular matrix.14,15,34 Experimental models of glomerular disease show improvement of kidney lesions with complement, MCP-1, or TGF-β1 inhibitions, making them ideal candidate biomarkers.35, 36, 37
We found that the levels of urinary biomarkers during active disease differed between GN. Compared with our published data in overt diabetic nephropathy (DN) using a similar methodology,38 urinary MAC was substantially higher in patients with FSGS, MN, and LN. By contrast, IgAN, AAV, and MPGN patients presented measurement levels of sC5b-9 similar to those with DN. Median levels of MCP-1 and TGF-β1 were up to 5 times higher than those seen in DN.
Whether urinary sC5b-9 equates to glomerular complement activation in autoimmune GN is debatable. Evidence supports that irrespective of the GN, the filtration of complementary components into the urinary space can lead to the intraluminal generation of sC5b-9 because of the lack of regulators on tubular epithelial cells, eventually causing tubulointerstitial injury.2,6,39, 40, 41 However, murine models of MN have demonstrated that urinary excretion of MAC ceases quickly once immune complexes are removed despite persistent proteinuria, analogous to the disappearance of PLA2R antibodies occurring prior to the decrease in proteinuria.42, 43, 44, 45 It could also be argued that the presence of urinary sC5b-9 stems from the passive filtration of circulating sC5b-9 with higher glomerular permeability, reflecting systemic rather than local formation. However, there is no strong evidence of systemic complement activation in primary MN and FSGS, where the highest levels of urinary MAC were found.6,46 In addition, studies have shown a poor correlation between urinary and plasma values, which should have been stronger if urinary measurements merely reflected the overflow of plasmatic sC5b-9.17,45 Other studies have addressed the clinical value of urinary complement biomarkers in glomerular diseases.16,17,47,48 In IgAN, a correlation exists between urinary MAC and proteinuria, interstitial fibrosis, and global glomerulosclerosis.42, 43, 44, 45 Evidence for higher urinary sC5b-9 or its failure to disappear in MN has been linked to a worse prognosis.42, 43, 44, 45,42, 43, 44, 45 Some of these studies were only cross-sectional, and when they did present follow-ups and outcomes, they considered the baseline value of urinary sC5b-9.
Previous studies have assessed urinary MCP-1 in AAV, revealing that levels were higher in active disease and associated with a worse prognosis.11,49, 50, 51 In the course of AAV, higher initial urinary TGF-β1 values correlated with the absence of improved renal function with immunosuppression.52 Tam et al. have shown that urinary MCP-1 decreases before kidney function improves49 and in the randomized trial with the C5a receptor inhibitor avacopan,53 urinary MCP-1 levels also declined markedly with remission, more so with avacopan than in the control group. In LN, there is also evidence of higher levels of MCP-1 in active disease, with levels declining following immunosuppression and rising several months before a relapse.12,20, 21, 22 In each of the GN we studied, previous studies have shown urinary TGF-β1 levels to be elevated during active disease and to correlate with histologic characteristics and proteinuria.15,54, 55, 56, 57 In patients with MN, higher initial urinary TGF-β1 levels were associated with a persistent nephrotic syndrome and kidney function decline at 12 months.57
The methodology employed in this study warrants comments. We carefully delineated different periods of activity for each individual as clinicians manage GN mostly based on activity, remission, and relapse. To our knowledge, this is the first study comparing multiple biomarkers in different autoimmune GNs simultaneously. Repeated observations for each patient along the course of their disease allowed us to perform repeated measures analysis irrespective of the type of GN, as individuals were compared to themselves. Yet, the number of subjects participating in this study was modest and the power insufficient to show beyond doubt an independent predictive value of these biomarkers. Therefore, results from this study will mandate further validation in larger cohorts. Nonetheless, our data clearly suggest an added value of urinary sC5b-9 and MCP-1.
To analyze outcomes using patients with different GN, we established thresholds for remission for each biomarker and disease. Although the relationship between urinary sC5b-9, MCP-1, and TGF-β1 and outcomes is perhaps overly simplified when biomarker levels are dichotomized, the use of thresholds helps illustrate their clinical applicability. For FSGS and MN, urinary sC5b-9 cutoffs neared 8 μg/mmol of creatinine as opposed to cutoffs below 2 μg/mmol of creatinine for IgAN, LN, AAV, and MPGN. It is possible that complement activation at the podocyte, typical for FSGS and MN, facilitates tubular excretion of sC5b-9 resulting in higher urinary levels of this very large protein.45 In addition, the smaller difference in thresholds for urinary MCP-1 and TGF-β1 between GN (Table 3) could reflect that these small peptides parallel inflammation and fibrosis in the entire renal parenchyma, rather than limited to the glomeruli. Interestingly, we previously proposed cutoffs for MCP-1 and TGF-β1 in DN to predict a more rapid loss of renal function of 48 and 1.3 ng/mmol, respectively.58
A possible limitation of our study is that for some active episodes, urgent immunosuppressive treatment was already initiated before the first available urinary sample, due to a delay in obtaining a histologic diagnosis before empiric therapy. This could in part explain why we did not observe a significantly greater decline in urinary MAC compared to proteinuria on remission in AAV, as the steepest decrease in biomarkers may not have been captured. We also did not show an advantage of urinary biomarkers beyond proteinuria in IgAN, perhaps because our patients did not have severe disease (2.5 g/g of creatinine at baseline during active episodes with a remission and 1.4 g/g for active periods without one). Furthermore, immunosuppressive treatments were not standardized, making it difficult to account for their effect on the proposed biomarkers. Another limitation is the absence of integration of histology findings, as this influences outcomes.59, 60, 61, 62, 63 We did not find a correlation between urinary biomarkers and change in kidney function during individual periods. This could be due to the short follow-up time of each episode and the limited number of episodes.
There is growing interest in therapies aimed at inhibiting complement activation, with several ongoing trials on complement-targeting drugs and new treatment opportunities underway.7,8,29,64 Reliably identifying patients with local complement activation who will benefit from these new drugs is essential. Our results suggest that urinary sC5b-9 is a sensitive marker of complement activation and is more discriminating than proteinuria in predicting remission and relapse. Urinary sC5b-9 and MCP-1 have a predictive value for renal outcomes, possibly beyond proteinuria. These biomarkers could be clinically relevant to assess and monitor more accurately immunologic activity and thus be useful clinical tools to guide treatment.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We thank nephrology teams of both hospital for their support. This study was partly supported by grants from the Fonds de la recherche du Québec–Santé (no. 14395) and from the Fondation de l’Hôpital du Sacré-Coeur de Montréal.
Footnotes
Figure S1. Period definitions and area under the curve calculation. (A) T1 was defined as the first measurement (or highest measurement in periods leading to a remission), T3 as the last measurement, and T2 as the average of all measurements in between T1 and T3. (B) The area under the curve was estimated using rectangular integration where the period between 2 measurements is divided into 2 rectangles of equal time (x-axis) and height equal to each measurement (y-axis). The summation of the surface of all rectangles divided by the total time approximates the level of a biomarker maintained during that period.
Table S1. Area under the ROC curve for different biomarkers and diseases.
Supplementary Material
References
- 1.Rauen T., Eitner F., Fitzner C. Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med. 2015;373:2225–2236. doi: 10.1056/NEJMoa1415463. [DOI] [PubMed] [Google Scholar]
- 2.Angeletti A., Reyes-Bahamonde J., Cravedi P. Complement in non-antibody-mediated kidney diseases. Front Med (Lausanne) 2017;4 doi: 10.3389/fmed.2017.00099. 99–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bomback A.S., Markowitz G.S., Appel G.B. Complement-mediated glomerular diseases: a tale of 3 pathways. Kidney Int Rep. 2016;1:148–155. doi: 10.1016/j.ekir.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathern D.R., Heeger P.S. Molecules great and small: the complement system. Clin J Am Soc Nephrol. 2015;10:1636–1650. doi: 10.2215/CJN.06230614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan Y., Zhao M.H. Complement in glomerular diseases. Nephrology (Carlton) 2018;23:11–15. doi: 10.1111/nep.13461. [DOI] [PubMed] [Google Scholar]
- 6.Thurman J.M. Complement in kidney disease: core curriculum 2015. Am J Kidney Dis. 2015;65:156–168. doi: 10.1053/j.ajkd.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zipfel P.F., Wiech T., Rudnick R. Complement inhibitors in clinical trials for glomerular diseases. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.02166. 2166–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurman J.M. Many drugs for many targets: novel treatments for complement-mediated glomerular disease. Nephrol Dial Transplant. 2017;32:i57–i64. doi: 10.1093/ndt/gfw228. [DOI] [PubMed] [Google Scholar]
- 9.Wada J., Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 2013;124:139–152. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- 10.Navarro-Gonzalez J.F., Mora-Fernandez C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 11.Moran S.M., Monach P.A., Zgaga L. Urinary soluble CD163 and monocyte chemoattractant protein-1 in the identification of subtle renal flare in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant. 2020;35:283–291. doi: 10.1093/ndt/gfy300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rovin B.H., Song H., Birmingham D.J. Urine chemokines as biomarkers of human systemic lupus erythematosus activity. J Am Soc Nephrol. 2005;16:467–473. doi: 10.1681/ASN.2004080658. [DOI] [PubMed] [Google Scholar]
- 13.Nadkarni G.N., Rao V., Ismail-Beigi F. Association of urinary biomarkers of inflammation, injury, and fibrosis with renal function decline: the ACCORD Trial. Clin J Am Soc Nephrol. 2016;11:1343–1352. doi: 10.2215/CJN.12051115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basile D.P. The transforming growth factor beta system in kidney disease and repair: recent progress and future directions. Curr Opin Nephrol Hypertens. 1999;8:21–30. doi: 10.1097/00041552-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Tsakas S., Goumenos D.S. Accurate measurement and clinical significance of urinary transforming growth factor-beta1. Am J Nephrol. 2006;26:186–193. doi: 10.1159/000093178. [DOI] [PubMed] [Google Scholar]
- 16.Thurman J.M., Wong M., Renner B. Complement activation in patients with focal segmental glomerulosclerosis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang M.F., Huang J., Zhang Y.M. Complement activation products in the circulation and urine of primary membranous nephropathy. BMC Nephrol. 2019;20:313. doi: 10.1186/s12882-019-1509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenchley P.E., Coupes B., Short C.D. Urinary C3dg and C5b-9 indicate active immune disease in human membranous nephropathy. Kidney Int. 1992;41:933–937. doi: 10.1038/ki.1992.143. [DOI] [PubMed] [Google Scholar]
- 19.Kon S.P., Coupes B., Short C.D. Urinary C5b-9 excretion and clinical course in idiopathic human membranous nephropathy. Kidney Int. 1995;48:1953–1958. doi: 10.1038/ki.1995.496. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y.H., Song G.G. Urinary MCP-1 as a biomarker for lupus nephritis: a meta-analysis. Z Rheumatol. 2017;76:357–363. doi: 10.1007/s00393-016-0109-z. [DOI] [PubMed] [Google Scholar]
- 21.Gupta R., Yadav A., Aggarwal A. Longitudinal assessment of monocyte chemoattractant protein-1 in lupus nephritis as a biomarker of disease activity. Clin Rheumatol. 2016;35:2707–2714. doi: 10.1007/s10067-016-3404-9. [DOI] [PubMed] [Google Scholar]
- 22.Alharazy S., Kong N.C.T., Mohd M. Urine monocyte chemoattractant protein-1 and lupus nephritis disease activity: preliminary report of a prospective longitudinal study. Autoimmune Dis. 2015;2015 doi: 10.1155/2015/962046. 962046–962046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reich H.N., Troyanov S., Scholey J.W. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol. 2007;18:3177–3183. doi: 10.1681/ASN.2007050526. [DOI] [PubMed] [Google Scholar]
- 24.Troyanov S., Wall C.A., Miller J.A. Focal and segmental glomerulosclerosis: definition and relevance of a partial remission. J Am Soc Nephrol. 2005;16:1061–1068. doi: 10.1681/ASN.2004070593. [DOI] [PubMed] [Google Scholar]
- 25.Troyanov S., Wall C.A., Miller J.A. Idiopathic membranous nephropathy: definition and relevance of a partial remission. Kidney Int. 2004;66:1199–1205. doi: 10.1111/j.1523-1755.2004.00873.x. [DOI] [PubMed] [Google Scholar]
- 26.Sprangers B., Monahan M., Appel G.B. Diagnosis and treatment of lupus nephritis flares—an update. Nat Rev Nephrol. 2012;8:709–717. doi: 10.1038/nrneph.2012.220. [DOI] [PubMed] [Google Scholar]
- 27.Miloslavsky E.M., Specks U., Merkel P.A. Clinical outcomes of remission induction therapy for severe antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2013;65:2441–2449. doi: 10.1002/art.38044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pocock S.J., Clayton T.C., Altman D.G. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359:1686–1689. doi: 10.1016/S0140-6736(02)08594-X. [DOI] [PubMed] [Google Scholar]
- 29.Andrighetto S., Leventhal J., Zaza G. Complement and complement targeting therapies in glomerular diseases. Int J Mol Sci. 2019;20:6336. doi: 10.3390/ijms20246336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshimura T. The chemokine MCP-1 (CCL2) in the host interaction with cancer: a foe or ally? Cell Mol Immunol. 2018;15:335–345. doi: 10.1038/cmi.2017.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gschwandtner M., Derler R., Midwood K.S. More than just attractive: how CCL2 influences myeloid cell behavior beyond chemotaxis. Front Immunol. 2019;10:2759. doi: 10.3389/fimmu.2019.02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M.O., Flavell R.A. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dahmani A., Janelle V., Carli C. TGFβ programs central-memory differentiation in ex vivo-stimulated human T cells. Cancer Immunol Res. 2019 doi: 10.1158/2326-6066.CIR-18-0691. canimm.0691.2018. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence D.A. Transforming growth factor-beta: an overview. Kidney Int Suppl. 1995;49:S19–S23. [PubMed] [Google Scholar]
- 35.Zhou A., Ueno H., Shimomura M. Blockade of TGF-beta action ameliorates renal dysfunction and histologic progression in anti-GBM nephritis. Kidney Int. 2003;64:92–101. doi: 10.1046/j.1523-1755.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 36.Huugen D., van Esch A., Xiao H. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int. 2007;71:646–654. doi: 10.1038/sj.ki.5002103. [DOI] [PubMed] [Google Scholar]
- 37.Schlöndorff D., Nelson P.J., Luckow B. Chemokines and renal disease. Kidney Int. 1997;51:610–621. doi: 10.1038/ki.1997.90. [DOI] [PubMed] [Google Scholar]
- 38.Pelletier K., Bonnefoy A., Chapdelaine H. Clinical value of complement activation biomarkers in overt diabetic nephropathy. Kidney Int Rep. 2019;4:797–805. doi: 10.1016/j.ekir.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nangaku M. Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. Intern Med. 2004;43:9–17. doi: 10.2169/internalmedicine.43.9. [DOI] [PubMed] [Google Scholar]
- 40.Hsu S.I., Couser W.G. Chronic progression of tubulointerstitial damage in proteinuric renal disease is mediated by complement activation: a therapeutic role for complement inhibitors? J Am Soc Nephrol. 2003;14:S186–S191. doi: 10.1097/01.asn.0000070032.58017.20. [DOI] [PubMed] [Google Scholar]
- 41.Morita Y., Ikeguchi H., Nakamura J. Complement activation products in the urine from proteinuric patients. J Am Soc Nephrol. 2000;11:700–707. doi: 10.1681/ASN.V114700. [DOI] [PubMed] [Google Scholar]
- 42.Pruchno C.J., Burns M.M., Schulze M. Urinary excretion of the C5b-9 membrane attack complex of complement is a marker of immune disease activity in autologous immune complex nephritis. Am J Pathol. 1991;138:203–211. [PMC free article] [PubMed] [Google Scholar]
- 43.Pruchno C.J., Burns M.W., Schulze M. Urinary excretion of C5b-9 reflects disease activity in passive Heymann nephritis. Kidney Int. 1989;36:65–71. doi: 10.1038/ki.1989.162. [DOI] [PubMed] [Google Scholar]
- 44.Beck L.H., Jr., Fervenza F.C., Beck D.M. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogrodowski J.L., Hebert L.A., Sedmak D. Measurement of SC5b-9 in urine in patients with the nephrotic syndrome. Kidney Int. 1991;40:1141–1147. doi: 10.1038/ki.1991.326. [DOI] [PubMed] [Google Scholar]
- 46.Thurman J.M., Nester C.M. All things complement. Clin J Am Soc Nephrol. 2016;11:1856–1866. doi: 10.2215/CJN.01710216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Onda K., Ohsawa I., Ohi H. Excretion of complement proteins and its activation marker C5b-9 in IgA nephropathy in relation to renal function. BMC Nephrol. 2011;12 doi: 10.1186/1471-2369-12-64. 64–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gou S.J., Yuan J., Wang C. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin J Am Soc Nephrol. 2013;8:1884–1891. doi: 10.2215/CJN.02790313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tam F.W., Sanders J.S., George A. Urinary monocyte chemoattractant protein-1 (MCP-1) is a marker of active renal vasculitis. Nephrol Dial Transplant. 2004;19:2761–2768. doi: 10.1093/ndt/gfh487. [DOI] [PubMed] [Google Scholar]
- 50.Jönsson N., Erlandsson E., Gunnarsson L. Monocyte chemoattractant protein-1 in antineutrophil cytoplasmic autoantibody-associated vasculitis: biomarker potential and association with polymorphisms in the MCP-1 and the CC chemokine receptor-2 gene. Mediators Inflamm. 2018;2018:6861257. doi: 10.1155/2018/6861257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lieberthal J.G., Cuthbertson D., Carette S. Urinary biomarkers in relapsing antineutrophil cytoplasmic antibody-associated vasculitis. J Rheumatol. 2013;40:674–683. doi: 10.3899/jrheum.120879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goumenos D.S., Kalliakmani P., Tsakas S. Urinary transforming growth factor-beta 1 as a marker of response to immunosuppressive treatment, in patients with crescentic nephritis. BMC Nephrol. 2005;6 doi: 10.1186/1471-2369-6-16. 16–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jayne D.R.W., Bruchfeld A.N., Harper L. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. 2017;28:2756–2767. doi: 10.1681/ASN.2016111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camilla R., Brachemi S., Pichette V. Urinary monocyte chemotactic protein 1: marker of renal function decline in diabetic and nondiabetic proteinuric renal disease. J Nephrol. 2011;24:60–67. doi: 10.5301/jn.2010.1458. [DOI] [PubMed] [Google Scholar]
- 55.De Muro P., Faedda R., Fresu P. Urinary transforming growth factor-beta 1 in various types of nephropathy. Pharmacol Res. 2004;49:293–298. doi: 10.1016/j.phrs.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Haramaki R., Tamaki K., Fujisawa M. Steroid therapy and urinary transforming growth factor-beta1 in IgA nephropathy. Am J Kidney Dis. 2001;38:1191–1198. doi: 10.1053/ajkd.2001.29209. [DOI] [PubMed] [Google Scholar]
- 57.Honkanen E., Teppo A.M., Tornroth T. Urinary transforming growth factor-beta 1 in membranous glomerulonephritis. Nephrol Dial Transplant. 1997;12:2562–2568. doi: 10.1093/ndt/12.12.2562. [DOI] [PubMed] [Google Scholar]
- 58.Verhave J.C., Bouchard J., Goupil R. Clinical value of inflammatory urinary biomarkers in overt diabetic nephropathy: a prospective study. Diabetes Res Clin Pract. 2013;101:333–340. doi: 10.1016/j.diabres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Trimarchi H., Barratt J., Cattran D.C. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Lepeytre F., Royal V., Lavoie P.L. Estimating the change in renal function during the first year of therapy in ANCA-associated vasculitis. Kidney Int Rep. 2019;4:594–602. doi: 10.1016/j.ekir.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bajema I.M., Wilhelmus S., Alpers C.E. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93:789–796. doi: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 62.Fogo A.B., Lusco M.A., Najafian B. AJKD atlas of renal pathology: membranous nephropathy. Am J Kidney Dis. 2015;66:e15–e17. doi: 10.1053/j.ajkd.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 63.Royal V., Zee J., Liu Q. Ultrastructural characterization of proteinuric patients predicts clinical outcomes. J Am Soc Nephrol. 2020;31:841–854. doi: 10.1681/ASN.2019080825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thurman J.M., Le Quintrec M. Targeting the complement cascade: novel treatments coming down the pike. Kidney Int. 2016;90:746–752. doi: 10.1016/j.kint.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.