Abstract
Introduction
Primary membranous nephropathy (MN) is characterized by the presence of antipodocyte antibodies, but studies describing phenotypic and functional abnormalities in circulating lymphocytes are limited.
Methods
We analyzed 68 different B- and T-cell subsets using flow cytometry in 30 MN patients (before initiating immunosuppression) compared with 31 patients with non–immune-mediated chronic kidney disease (CKD) and 12 healthy individuals. We also measured 19 serum cytokines in MN patients and in healthy controls. Lastly, we quantified the ex vivo production of phospholipase A2 receptor (PLA2R)-specific IgG by plasmablasts (measuring antibodies in culture supernatants and by the newly developed FluoroSpot assay [AutoImmun Diagnostika, Strasberg, Germany]) and assessed the circulating antibody repertoire by phage immunoprecipitation sequencing (PhIP-Seq).
Results
After adjusting for multiple testing, plasma cells and regulatory B cells (BREG) were significantly higher (P < 0.05) in MN patients compared with both control groups. The percentages of circulating plasma cells correlated with serum anti-PLA2R antibody levels (P = 0.042) and were associated with disease activity. Ex vivo–expanded PLA2R-specific IgG-producing plasmablasts generated from circulating PLA2R-specific memory B cells (mBCs) correlated with serum anti-PLA2R IgG antibodies (P < 0.001) in MN patients. Tumor necrosis factor-α (TNF-α) was the only significantly increased cytokine in MN patients (P < 0.05), whereas there was no significant difference across study groups in the autoantibody and antiviral antibody repertoire.
Conclusion
This extensive phenotypic and functional immune characterization shows that autoreactive plasma cells are present in the circulation of MN patients, providing a new therapeutic target and a candidate biomarker of disease activity.
Keywords: membranous nephropathy, phospholipase A2 receptor, plasma cells, regulatory B cells, regulatory T cells
Graphical abstract
Primary MN is an autoantibody-mediated glomerular disease that represents one of the leading causes of nephrotic syndrome in adults.1 MN autoantibodies are typically IgG that target podocyte antigens and deposit on the subepithelial layer of the glomerular capillary wall.2 MN kidneys show thickened glomerular basement membranes, complement activation,3, 4, 5 and glomerular capillary injury, leading to significant nephrotic-range proteinuria.6,7 About one-third of patients experience spontaneous remission, another third progress to end-stage renal failure, and the remaining third have persistent proteinuria with slower decline to end-stage renal failure.8, 9, 10, 11
Considerable advances have been made in the understanding of the pathophysiology of MN with the identification of autoantibodies targeting phospholipase A2 receptor in approximately 60% to 70% of MN patients.12, 13, 14, 15 In contrast with autoantibodies, circulating lymphocyte populations have been less thoroughly investigated in MN. Some investigators reported an increased CD4+/CD8+ T-cell ratio in MN patients with or without nephrotic proteinuria,16,17 which has been attributed to a reduction in CD8+ T cells.18 In 2017, Rosenzwajg et al.19 measured 33 lymphocyte subpopulations in 25 MN patients and 27 healthy subjects and found that MN patients had significantly lower regulatory T cells (TREG) compared with healthy controls.19 MN patients who responded to B-cell depleting therapy showed a significantly increased percentage of TREG than nonresponders. The same study19 showed that, consistent with previous reports on other autoimmune diseases,20,21 MN patients have an increased frequency of circulating naive B cells, whereas switched and nonswitched mBCs are decreased. This finding, together with the clinical efficacy of B-cell depleting therapies, has highlighted the central pathogenic role of B lymphocytes in MN. However, functional studies to assess the presence of circulating B cells reactive against PLA2R antigen have not yet been performed. Moreover, studies published so far are limited by the low number of B- and T-cell subsets analyzed, as well as by the lack of control subjects with CKD, which prevented the dissection of immune phenotypic abnormalities that may be common to all patients with renal insufficiency of any etiology.
To address these issues, we performed a detailed phenotypic and functional analysis of circulating B- and T-cell populations in patients with MN and in CKD and healthy controls to pinpoint the subsets that best identify the immune phenotypic abnormalities characteristic of MN.
Methods
Study Population
We included patients with new-onset MN enrolled at 2 centers: S. Orsola-Malpighi Hospital, University of Bologna, Bologna, Italy (n = 16), and Complejo Hospitalario de Navarra, Pamplona, Spain (Biobank Navarrabiomed, n = 14 [integrated in the Spanish National Biobanks Network]). Patients with MN were diagnosed using the standard criteria set by the World Health Organization.22 Exclusion criteria were secondary types of MN, such as lupus nephritis, Henoch-Schonlein purpura, hepatitis B virus– or hepatitis C virus–associated MN, neoplasia, pregnancy, concomitant other autoimmune diseases or other infections, and the use of immunosuppressive therapy. Primary (n = 7) and secondary (n = 2) MN patients used for the functional ex vivo assays were enrolled at Bellvitge University Hospital, Barcelona, Spain. All individuals were enrolled from November 2017 to June 2019. The definitions of clinical outcomes and the methods for autoantibodies targeting phospholipase A2 receptor measurement are reported in Supplementary Appendix.
As controls for immunophenotyping, we included patients with non–immune-mediated CKD (diabetic nephropathy, n = 9; chronic hypertensive nephropathy, n = 8; autosomal dominant polycystic kidney disease, n = 5; and unspecified CKD, n = 9). For PhIP-Seq, we used sera from the same patients included in the immune phenotyping studies and additional sera from 3 MN and 22 CKD patients. We also included sex- and age-matched healthy subjects with no known autoimmune or inflammatory diseases.
Samples and data from patients included in this study were processed following standard operating procedures with the appropriate approval of the ethics and scientific Committees of the 3 institutions.
Flow Cytometry Analysis
Peripheral blood mononuclear cells were isolated from peripheral blood by Ficoll gradient and stored in liquid nitrogen for batched analysis. We designed 7 multicolor flow cytometry panels to quantify 68 cell subsets and to calculate the CD4+/CD8+ T-cell ratio (Supplementary Table S1). The following fluorochrome-conjugated antihuman antibodies were used from BD Biosciences (Franklin Lakes, NJ): CD3-FITC, CD3-PerCP-Cy5.5, CD4-APC-Cy7, CD8-BV510, CD45RO-FITC, CD45RA-APC, CD45RA-APC-H7, CD71-PE, IgD-PerCP-Cy5.5, CD25-APC-Cy7, CD138-BV421, CCR4-PE, CD27-PE, CD95-BV421, CD28-BV421, CCR6-BV421, CXCR3-PE, IL-4-PE, and IFN-γ-PE-Cy7. From Biolegend (San Diego, CA), the following were used: CD19-BV510, CD56-FITC, CD27-APC, CD127-FITC, CD57-PerCp-Cy5.5, PD-1-APC-Cy7, and CXCR5-FITC, and from eBioscience (San Diego, CA), the following were used: CD4-PE-Cy7, CD21-PE, and CD24-APC-Cy7. From Miltenyi Biotec (Bergisch Gladbach, German), CD25-APC and KLRG1-PE were used; from Beckman Coulter (Brea, CA), CD38-PE-Cy7 was used; and from Invitrogen (Carlsbad, CA), IL-17-APC was used. Isotype controls IgG1,κ-FITC, IgG1,κ-PE, and IgG2b,κ-APC (BD Biosciences) were used for gate placement.
Intracellular staining for interleukin (IL)-17, IL-4, and interferon-γ was performed with extracellular markers for CD4+, CD8+, and CD19+. Cells were fixed and permeabilized using Intracellular Fixation and Permeabilization Buffer Set (eBioscience) according to the manufacturer’s instructions. Data were acquired (>1 × 106 events) on a 3-laser FACSLyric flow cytometer (BD Biosciences) and analyzed with FlowJo software (BD Life Sciences; Franklin Lakes, NJ).
Cytokine/Chemokine
We quantified plasma levels of CD40L, granulocyte colony-stimulating factor/colony-stimulating factor 3, granulocyte-macrophage colony-stimulating factor, interferon-γ, IL-1β, IL-10, IL-12p70, IL-13, IL-17A, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, monocyte chemoattractant protein-1, macrophage inflammatory protein-1β, TNF-α, and tumor necrosis factor-β in a subset of 19 MN patients and 8 healthy controls using xMAP Technology (Luminex, Austin, TX). Serum samples were not available for CKD controls. We created a multiplex panel combining 2 commercially available simplex kits: Human Custom Procarta Plex-19 plex (Cat. No. PPX-19-MXRWE2G; Invitrogen by Thermo Fisher Scientific, Waltham, MA) and Human High Sensitivity T Cell (Cat. No. HSTCMAG-28SK; Merck Millipore, Burlington, MA). The methodological details including assay protocol, standards, and sensitivity are available at the manufacturers’ websites (http://www.thermofisher.com and http://www.merckmillipore.com, respectively). All samples were measured undiluted and in duplicates. The chemo/cytokine standards were assayed in the same way as patient samples. The data were collected using xPONENT software (Luminex).
Functional Assessment of Circulating PLA2R-Specific IgG-Producing Plasmablasts
Quantification of PLA2R-Specific IgG by Polyclonally Expanded Plasmablasts
The induction and differentiation of circulating mBCs into antibody-secreting plasmablasts were performed as previously described.23, 24, 25 Briefly, peripheral blood mononuclear cells were resuspended at 1.5 × 106 cells/ml concentration in Iscove’s modified Dulbecco’s medium (Gibco Invitrogen, Paisley, UK) containing 10% fetal bovine serum (Hyclone, Gibco) supplemented with 2 mmol/l L-glutamine (Gibco Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco Invitrogen). Cells were activated for 6 days with 500 ng/ml α-CD40 mAb (R&D Systems, Minneapolis, MN), 2.5 μg/ml toll-like receptor-9 ligand oligodeoxynucleotides 2006 CpG (Hycult Biotechnology, Uden, the Netherlands), 600 IU/ml IL-2 (Sigma-Aldrich, St. Louis, MO), 25 ng/ml IL-10 (R&D Systems), and 100 ng/ml IL-21 (Gibco Invitrogen). Such polyclonal stimulation results in the appearance of an IgG antibody secreting cell–like population with a CD19+CD20lowCD27+CD38highIgD− phenotype that has been previously shown to arise from circulating mBCs preserving their original B-cell repertoire.24 Supernatants of these 6-day polyclonally stimulated mBCs were screened for anti–PLA2R-specific IgG antibodies using single-antigen flow bead assays on a customized Luminex platform (OriGene Technologies, Rockville, MD). The mean fluorescence intensity threshold for Luminex positivity was 500.
B-Cell FluoroSpot Assay to Track Circulating Frequencies of PLA2R-Specific IgG-Producing Plasmablasts
To test the frequencies of those PLA2R-specific mBCs capable of switching into a plasmablast-like phenotype producing PLA2R (IgG)-specific antibodies in vitro, we evaluated them in a new B-cell enzyme-linked immunosorbent FluoroSpot assay. Briefly, FluoroSpot plates were coated with a monoclonal antibody against human IgG and blocked to avoid unspecific interactions. Peripheral blood mononuclear cells were stimulated as specified earlier and seeded into FluoroSpot plates and incubated to release IgG. The total polyclonal IgG wells were used as positive controls to confirm cell viability for each subject per stimulation.
IgG antibody secreting cells were detected with anti-human IgG conjugated with alkaline phosphatase, and PLA2R-specific IgG antibody secreting cells were detected using fluorescent dye–labeled multimerized PLA2R monomers.
PhIP-Seq to Screen for Autoantibodies
PhIP-Seq was performed using a slightly modified version of previously published PhIP-Seq protocols.26,27 Briefly, we used a human proteome phage display library consisting of 259,345 overlapping 90-aa peptide sequences (human90 library28 and a human virome phage display library consisting of 93,904 overlapping 56-aa peptide sequences [VirScan library]).29 The IgG concentration of each plasma sample was determined using an in-house IgG enzyme-linked immunosorbent assay using capture and detection antibodies from Southern Biotech, Birmingham, AL (Cat. No. 2040-01 and Cat. No. 2042-05). Immunoprecipitation reactions occurred in 1-ml mixture containing plasma (2 μg IgG), 2.6 × 1010 plaque-forming units of the human peptidome library, and 0.94 × 1010 plaque-forming units of the virome library diluted in PBS. After rotating the reaction mix overnight at 4 °C, 20 μl each of magnetic protein A and protein G Dynabeads (Cat. No. 10002D and Cat. No.10004D, Invitrogen) were added to each reaction and rotated for 4 hours at 4 °C. The beads were washed 3 times using a 96-well magnetic stand, followed by resuspension of the beads in 20 μl of a polymerase chain reaction master mix containing Q5 polymerase (Cat. No. M0492L; New England BioLabs, Ipswich, MA). After 15 cycles of polymerase chain reaction, 2 μl of the polymerase chain reaction reaction was added to a second 20 cycle polymerase chain reaction for the addition of sample bar codes and P5/P7 Illumina adapters (Illumina, San Diego, CA). Sequencing was performed on an Illumina NextSeq 500 system (high output, single end, 75 bp) using custom sequencing primers. See Supplementary Appendix for information on data analyses.
Statistical Analysis
Of the original 68 cell subsets, we dropped 11 of them because they were missing in over 10% of the patients (in 10 of them, they were missing in over 50% of the subjects). Among the remaining 57 cell subsets, based on the degree of redundancy evaluated by statistical collinearity and correlation as well as by background knowledge, we eventually selected 30 cell subsets (Supplementary Table S1) for the main analyses and 52 cell subsets for sensitivity analyses (Supplementary Appendix and Supplementary Figure S1).
Among the 30 cell subsets, we used the Kruskal-Wallis joint test to identify those that were statistically significant across MN, CKD, and health controls. P values were adjusted for multiple testing using the Holm-Bonferroni procedure.30 Among the jointly significant subsets, we identified those that were also statistically significant in both pair-wise comparisons (i.e., between MN and CKD and between MN and healthy controls) using a Mann-Whitney 2-sample, 2-tailed test with a significant level of 0.025. For sensitivity analysis, we used random forest analysis,31 which consists in constructing a multitude of classification trees, each based on a random sample of the variables used for classification, and then summarizing which variables proved to be most useful in distinguishing between groups by ranking them according to variable importance. In contrast to pair-wise statistical testing (used previously), the random forest analysis accounts for the fact that different cell subsets may jointly help distinguishing MN from the other groups. Therefore, this approach accounts for possible interactions between different cell subsets (i.e., possible synergic effects of 2 different cell subsets in improving classification between MN and the other 2 groups). Further details of the additional statistical analyses (e.g., least absolute shrinkage and selection operator for variables from the 52 cell subsets) are reported in Supplementary Appendix. Random forest analysis and least absolute shrinkage and selection operator were not used for the purpose of building a prediction model but rather as a sensitivity analysis of cell subset selection because these methods may better handle nonlinear relations and interactions between cell subsets (random forest) and highly correlated covariates (least absolute shrinkage and selection operator).
In order to report findings that could easily be compared with future studies, we calculated nonparametric bootstrap 95% confidence intervals of the median and lower and upper quartiles of each significant cell subset for each of the 3 groups.32
As a final verification of our findings, we examined the linear relation between the selected cell subsets and anti-PLA2R antibody levels in MN patients in whom the titer was available and positive. To this purpose, we used gamma regression via generalized linear models due to the non-normal distribution with long right tails of anti-PLA2R antibody titer; the P value was estimated with the nonparametric Monte Carlo 2-sided permutation test.32 Gamma regression was also used to fit the relation between supernatant and serum mean fluorescence intensity.
We compared cytokine levels between patients with MN and healthy controls using the 2-sample Mann-Whitney test. A 2-tailed P value < 0.05 after accounting for multiple testing according to the Bonferroni method was regarded as statistically significant unless otherwise specified. All of the analyses were performed using Stata release 16.0 (StataCorp LLC, College Station, TX) and random forest using the R package randomForest (R version 3.6.2; R Core Team, Vienna, Austria).
Results
Patients and Control Characteristics
For flow cytometric analyses, we included 30 patients with MN, 31 patients with other non–immune-mediated CKDs, and 12 healthy controls (Table 1). MN patients had severe proteinuria and slightly impaired renal function. Consistent with the available literature,12,33 over 60% of them were positive for anti-PLA2R antibodies. Sex and age were similar across the 3 study groups (Table 1).
Table 1.
Characteristics of patients included in flow cytometric analyses
| MN (n = 30) | CKD (n = 31) | HCs (n = 12) | P Value | |
|---|---|---|---|---|
| Male (%) | 63.3 | 74.2 | 41.7 | 0.25 |
| Age (years) | 59.5 ± 15.9 | 58.3 ± 16.7 | 57.0 ± 8.4 | 0.57 |
| Serum creatinine (mg/dl) | 1.2 ± 0.9 | 3.3 ± 1.5 | NA | <0.001 |
| Serum albumin (g/dl) | 3.0 ± 0.8 | 4.1 ± 0.5 | NA | <0.001 |
| Proteinuria (g/24 h) | 4.9 ± 3.8 | 0.86 ± 0.9 | NA | <0.001 |
| Anti-PLA2R Ab pos (%)a | 60.0 | NA | NA |
Anti-PLA2R Ab, autoantibodies targeting phospholipase A2 receptor; CKD, chronic kidney disease; HCs, healthy controls; MN, membranous nephropathy; NA, not available.
Categoric variates were compared by the Fisher’s exact test and continuous variates by the Kruskal-Wallis or Mann-Whitney test.
Four patients had no anti-PLA2R Ab measured.
Circulating Plasma Cells and BREG Are Selectively Increased in MN Patients
We started by identifying the B- and T-cell subsets that, among the 68 that we tested, were significant in pair-wise comparisons between MN and both CKD and healthy controls (Supplementary Table S1).
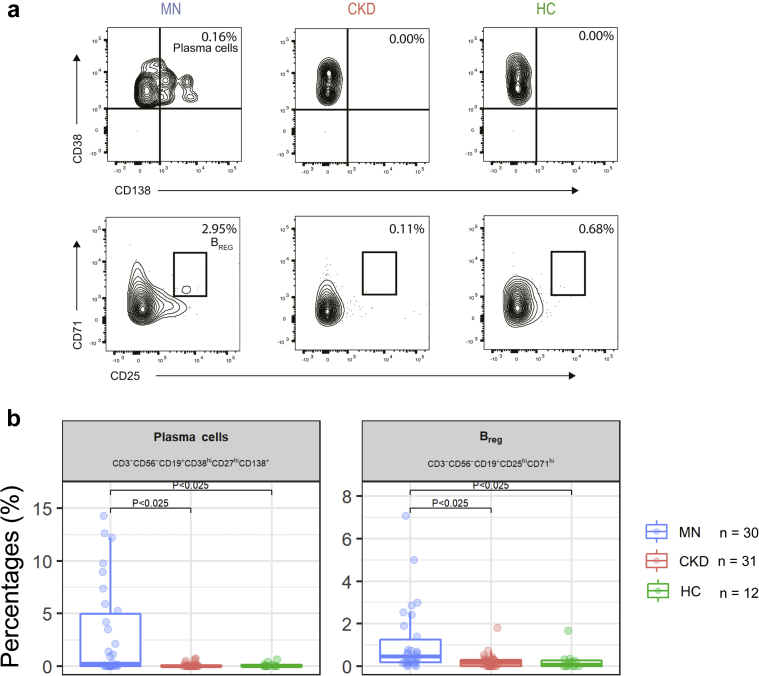
In the unadjusted analyses, we found that 17 cell subsets were significantly different between MN and HC, but, in most of the cases, MN and CKD patients had similar values, suggesting that such differences were not characteristic of MN patients. After adjusting for multiple testing, we found that only the percentages of plasma cells and BREG differed significantly in MN patients compared with both control groups (Figure 1a and b). There was no statistically significant difference between the 8 anti-PLA2R negative and the 18 positive MN patients concerning B and plasma cells (P = 0.20).
Figure 1.
Plasma cells and regulatory B cells (BREG) in membranous nephropathy (MN) and control subjects. (a) Representative plots for plasma cells (top) and BREG (bottom) in MN, chronic kidney disease (CKD), and healthy control (HC) individuals. (b) Boxplots showing the plasma cells and BREG percentages in MN, CKD patients, and HCs. P < 0.025 significant pair-wise differences (MN vs. CKD and MN vs. HC) after adjusting for multiple testing (Holm-Bonferroni procedure).
Next, we used random forest analysis to rank the cell subsets that best differed MN patients from control groups (see Statistical Analysis section and Supplementary Appendix). This analysis confirmed that plasma cells and, to a lower extent, BREG stood out in distinguishing patients with MN (Supplementary Figure S2). More in general, results from random forest analyses indicate that cell subsets of B-cell lineage overall distinguish MN patients from both control groups. Additional sensitivity analyses by least absolute shrinkage and selection operator (Supplementary Figure S3) converged to identify plasma cells as the most relevant subset to differentiate MN patients from the other groups.
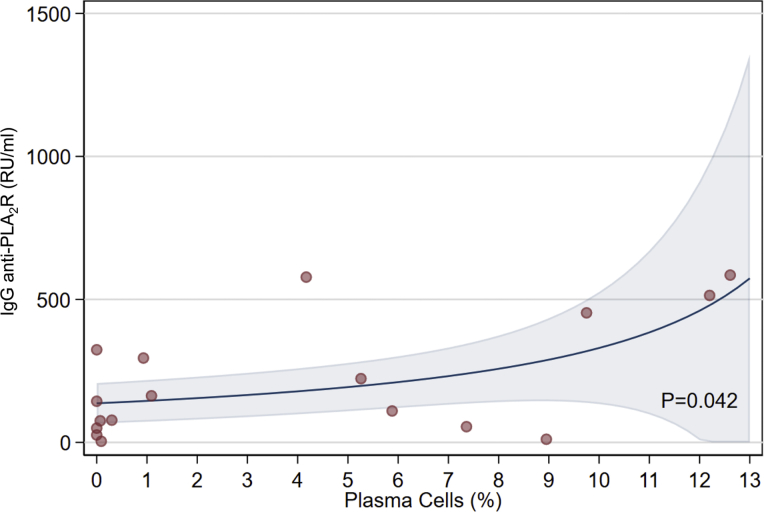
We also found a significant association between the percentage of circulating plasma cells and anti-PLA2R IgG levels in the subset of autoantibodies targeting phospholipase A2 receptor–positive patients (Figure 2).
Figure 2.
The relation between the percentage of plasma cells and serum anti–phospholipase A2 receptor (anti-PLA2R) IgG titer in membranous nephropathy patients with detectable anti-PLA2R antibodies. The line represents the fitted line via the gamma regression mode and the shaded area the 95% confidence interval of the line. The relation was statistically significant (P = 0.042) by the permutation test. Plasma cells are defined as CD3-CD56-CD19+CD38+highCD27+highCD138+ cells.
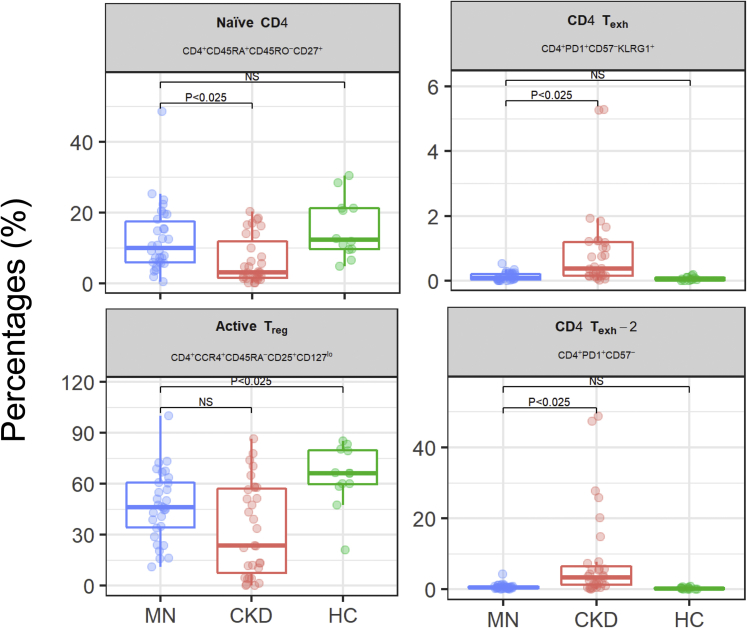
The percentages of exhausted CD4+ T cells and TREG were significantly lower in MN patients than in CKD patients and healthy controls, respectively, whereas naive CD4+ T cells were significantly higher than in CKD only (Figure 3). Follicular helper T cells did not significantly differ across the 3 groups. Similarly, intracellular expression of IFN-γ, IL-4, and IL-17 in CD4+ and CD8+ T cells was not significantly different across the 3 groups (Supplementary Figure S4). Overall, these data indicate that plasma cells are the lymphocyte subset that best characterizes MN patients.
Figure 3.
CD4+ T-cell subsets in membranous nephropathy (MN) patients and controls. Boxplots showing CD45RA+CD45RO−CD27+ naive CD4+ T cells, 2 exhausted PD1+CD57−CD4+ T-cell subsets, and CCR4+CD45RA-CD25+CD127low TREG in MN, chronic kidney disease (CKD) patients, and healthy controls (HCs). P < 0.025 significant pair-wise differences (MN vs. CKD and MN vs. HC) after adjusting for multiple testing (Holm-Bonferroni procedure).
MN Patients Have Increased Circulating Levels of TNF-α
We assayed a panel of 19 cytokines and chemokines in the sera of 19 MN patients and 8 healthy controls. Most cytokines and chemokines were not detectable in the circulation (Supplementary Table S2). Among the detectable ones, the only one that was significantly different between MN patients and healthy controls was TNF-α) (Supplementary Figure S5), which is implicated in plasma cell expansion and survival.34
MN Patients Have Autoreactive PLA2R-Specific IgG-Producing Plasmablasts in the Circulation
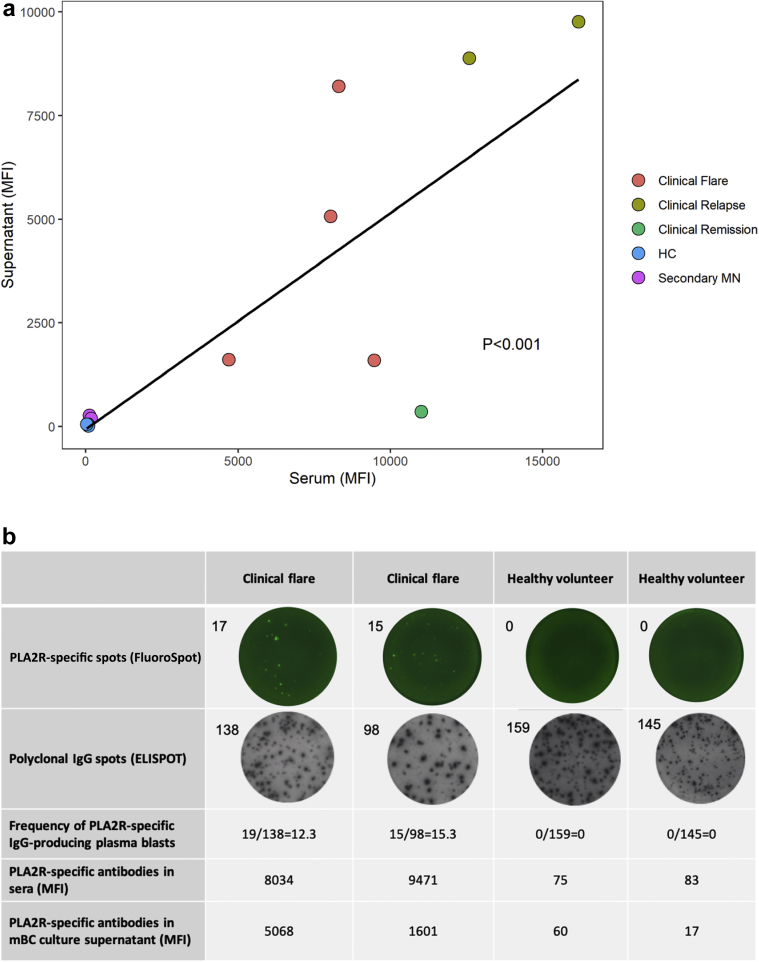
Based on these findings, we decided to focus our studies on plasma cells because they resulted as the cell subset that best characterizes patients with MN (Supplementary Figure S2). Although BREG were also increased in these patients, we reasoned that their increase was compensatory and not primarily pathogenic. Therefore, we asked whether circulating autoreactive PLA2R-specific mBCs from MN patients are capable of switching to PLA2R-specific IgG-producing plasma blasts (plasma cell precursors). To address this question, we measured PLA2R-specific IgG antibodies in the supernatants of polyclonally expanded memory B cells obtained from the circulation of 7 MN patients with serum autoantibodies targeting phospholipase A2 receptor, 2 patients with secondary MN, and 3 healthy controls.23, 24, 25 PLA2R-positive MN patients showed PLA2R-specific IgG antibody–producing plasmablasts in peripheral blood (Table 2). Interestingly, the levels of ex vivo–produced anti-PLA2R IgG were significantly correlated with the relative serum levels of autoantibodies (both measured by highly sensitive Luminex technology), suggesting that the autoreactive plasmablast pool is contributing to the overall production of autoantibodies (Figure 4a). One patient showing clinical remission after tacrolimus therapy displayed low levels of anti-PLA2R antibodies in the serum and no anti–PLA2R-specific IgG in the supernatants, possibly suggesting therapy-induced inhibition of autoreactive plasmablasts. None of the patients with secondary MN nor the healthy controls showed serum anti-PLA2R antibodies or anti–PLA2R-IgG–producing plasmablasts in the circulation (Figure 4a).
Table 2.
Clinical characteristics of patients described in Figure 4a
| MN onset (n = 4) |
MN relapse (n = 2) |
Clinical MN remission (n = 1) |
Secondary MN (n = 2) |
HC (n = 3) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 | #11 | #12 | |
| Age (years) | 37 | 38 | 68 | 28 | 69 | 48 | 63 | 76 | 77 | 25 | 52 | 32 |
| Gender | Male | Male | Male | Male | Male | Male | Male | Male | Male | Female | Male | Female |
| Months after diagnosis | 0 | 0 | 0 | 0 | 15 | 96 | 132 | 12 | 60 | 0 | 0 | 0 |
| Plasma albumin (g/dl) | 1.9 | 1.6 | 2.0 | 2.3 | 2.3 | 4.2 | 4.4 | 3.7 | 3.1 | 4.2 | 4.1 | 3.9 |
| Proteinuria (g/24 h) | 5.6 | 10 | 13.7 | 5.27 | 2.07 | 2.81 | 1 | 14.9 | 8.02 | 0 | 0 | 0 |
| Serum creatinine (mg/dl) | 1.34 | 2.18 | 1.35 | 1.11 | 6.58 | 1.64 | 1.09 | 2.01 | 2.11 | 1.02 | 0.90 | 0.97 |
| Immunosuppression | - | - | - | - | Tac | Tac | - | - | - | - | - | - |
| Serum anti-PLA2R Ab | ||||||||||||
| ELISA (semiquantitive) | + | + | ++ | + | ++ | ++ | ++ | - | - | - | - | - |
| Luminex (MFI) | 9471 | 8307 | 8034 | 4699 | 16,170 | 12588 | 11,005 | 125 | 180 | 75 | 83 | 32 |
| Supernatant anti-PLA22R Ab | ||||||||||||
| PLA2R-specific mBc (MFI) | 1601 | 8209 | 5068 | 1614 | 9765 | 8883 | 364 | 274 | 195 | 60 | 17 | 59 |
| Polyclonal mBc Ab production | 132 | 67 | 96 | 143 | 49 | 129 | 39 | 165 | 52 | 79 | 53 | 90 |
Anti-PLA2R Ab, autoantibodies targeting phospholipase A2 receptor; ELISA, enzyme-linked immunosorbent assay; HC, healthy control; mBC, memory B cell; MN, membranous nephropathy; Tac, tacrolimus.
ELISA results: -, +, and ++ correspond to <20 IU/l, 20 to 150 IU/l, and >150 IU/l, respectively.
All patients were negative for antithrombospondin antibodies.
Figure 4.
Ex vivo production of serum anti–phospholipase A2 receptor (anti-PLA2R) IgG by circulating plasmablasts from membranous nephropathy (MN) patients. (a) Association between anti–PLA2R-specific antibodies assessed in plasmablast cell culture supernatants and serum anti–PLA2R-specific IgG in patients with primary MN at different stages of disease activity, in patients with secondary MN, and in healthy controls. P < 0.001 by regression analysis. (b) PLA2R B-cell FluoroSpot, anti–PLA2R-specific antibodies in sera (mean fluorescent intensity [MFI]), and anti-PLA2R-specific antibodies in plasmablast cell culture supernatant (MFI) in 2 patients with MN and clinical flare and 2 healthy controls.
We next aimed to quantify in a functional manner the frequencies of circulating anti–PLA2R-specific IgG-producing plasmablasts in 2 representative MN patients with detectable autoantibodies targeting phospholipase A2 receptor to further demonstrate the capacity of this PLA2R-specific cell subset population to produce autoantibodies. To this purpose, we used a novel FluoroSpot platform24 that enables determination of the numbers of plasmablasts secreting PLA2R-targeted IgG over the global IgG-secreting plasma cell repertoire. We detected distinct PLA2R-specific IgG-producing plasma cells only in patients with anti–PLA2R-specific antibodies, whereas such cells were not detectable in the circulation of HCs (Figure 4b). In sum, increased circulating plasmablasts in MN patients are, at least in part, autoreactive.
No Difference in the Circulating Antibody Repertoires of MN, CKD, and Healthy Control Subjects
Because MN patients have increased levels of total circulating plasma cells, we tested whether there is any abnormality in the antibody repertoire overall. We performed PhIP-seq on serum IgG from an expanded cohort including 33 MN patients, 53 CKD patients, and 10 healthy controls. The PhIP-seq assay combines phage display of a library of peptides with next-generation sequencing to probe for responses against many possible epitopes simultaneously, with the key limitation that it can detect only responses against linear, rather than conformational, epitopes. We included a library of 259,345 overlapping 90-mer peptide sequences covering the human proteome (human90 library),26 as well as a library containing 93,904 overlapping 56-mer peptides from human-tropic viruses (VirScan library).29
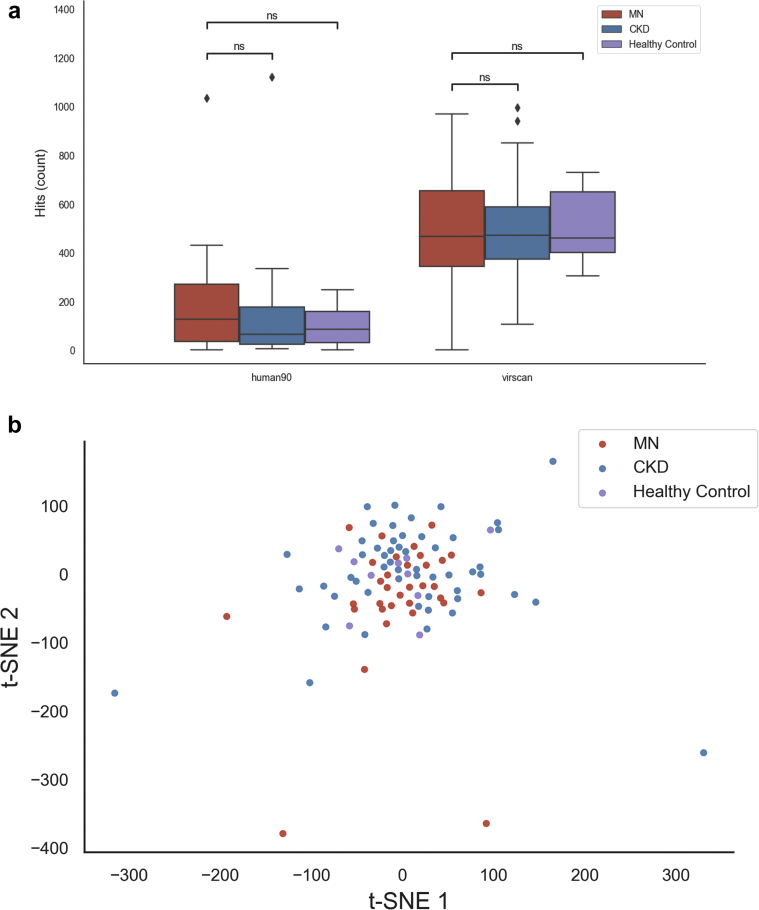
We did not find significant differences in the overall number of detected antibody specificities (hits) between MN patients, CKD patients, and healthy controls (Figure 5a). However, we noted a trend toward increased autoantibody hits for MN patients, with a median of 127 hits per MN patient compared with 64 for CKD patients and 85 for healthy controls (t test, P = 0.08). The number of viral hits was very similar between the groups (median of 468 for MN patients, 471 for CKD patients, and 461 for healthy controls).
Figure 5.
Phage immunoprecipitation sequencing (PhIP-Seq) analysis of circulating antibody repertoire. (a) Total detected antibody specificities (hits) per serum sample for PhIP-Seq libraries targeting the human proteome (human90 library) and human-tropic viruses (VirScan library). Differences between groups were assessed using a t test. (b) T-distributed stochastic neighbor embedding (t-SNE) visualization of the overlap in PhIP-Seq hits between samples. Samples with more hits in common are positioned closer together in this visualization.
No individual epitopes, genes, or viral taxa showed a significant enrichment in MN versus CKD or healthy control individuals after correction for multiple hypothesis testing (Supplementary Table S3). This included antibodies against PLA2R, which were not identified as PhIP-seq hits in any samples, likely due to the linear epitope limitation. To assess the overall clustering of the samples, we visualized the level of similarity between the hits for each pair of samples using a t-distributed stochastic neighbor embedding plot (Figure 5b). This did not indicate any clear clustering of MN, CKD, or healthy control individuals. Altogether, these data suggest that the self- and viral-directed serum antibody repertoires among MN, CKD, and healthy control individuals are broadly similar, but a note of caution is recommended in the consideration of the relatively small cohort size and PhIP-Seq limitations.
Discussion
The present study shows that MN patients display significantly higher percentages of circulating plasma cells and BREG than patients with non–immune-mediated CKD and healthy controls. Further analyses support the concept that a fraction of these circulating plasma cells are autoreactive because their percentages are associated with the concentration of serum anti-PLA2R antibodies and they actively produce anti-PLA2R antibodies when expanded ex vivo.
Despite increasing evidence that the depletion of circulating B cells with anti-CD20 antibodies reduces autoantibody levels,35 proteinuria,35,36 and histologic lesions,37 little evidence is available regarding the role of these cells in the pathogenesis of MN. The current paradigm postulates that B cells play a pathogenic role mainly as precursors of plasmablasts and plasma cells that possess the capacity of secreting antibodies.38 Most plasma cells may survive in the bone marrow for years in a quiescent form. However, peripheral antigen-specific mBCs recirculate in the bloodstream and may activate upon transient antigen reexposure38 and rapidly switch into antibody-producing plasmablasts,38 which have a much faster turnover.39 This could, at least in part, explain the efficacy of CD20 targeting therapies that have no direct effect on plasma cells.38,40,41 However, a fraction of MN patients do not respond to rituximab treatment. Our finding that MN patients have increased circulating plasma cells provides further rationale for testing the safety and efficacy of depleting plasma cells in MN patients with anti–PLA2R-specific antibodies (ClinicalTrials.gov identifier: NCT04145440).
Consistent with this hypothesis, others19 have shown abnormalities in the percentages of circulating B-cell subsets of patients with MN compared with health controls. Although our data support this finding, we showed that many of the immunologic differences between MN patients and healthy controls were not characteristic of MN, but they were shared between MN and individuals with nonimmunologic CKD. Therefore, changes in the overall B-cell pool may represent a feature of chronic renal disease regardless of the initial etiology.
In our study, circulating plasma cells were the subset that was most altered in MN patients. Increased percentages of circulating plasma cells might be caused by rapid generation of these cells or prolonged survival. In addition, disturbances in homing behavior or B-cell trafficking are possible causes of the observed expansion of this cell population in the peripheral blood of patients with active disease.42 The finding that the percentages of circulating plasma cells were significantly associated with the levels of serum anti-PLA2R autoantibodies strongly suggests that a fraction of these circulating cells are autoreactive. In order to gain further insight about the role of autoreactive B cells in MN patients, we used novel technologies to track circulating autoreactive B cells in an antigen-specific manner. Here, we document that MN patients with serum anti-PLA2R antibodies at the time of the clinical flare do have circulating mBCs capable of converting into PLA2R-specific IgG-producing plasmablasts. Interestingly, 1 patient with clinical remission but persistent anti–PLA2R-specific antibodies in the serum had no detectable circulating PLA2R-specific plasma cell frequencies, suggesting that the disappearance of these autoreactive cells from the circulation might anticipate clinical remission.
We observed increased levels of TNF-α in MN patients. The role of TNF-α in the pathogenesis of MN has been reported in previous studies,43, 44, 45 and there is a reported association between a polymorphism in the TNF-α gene and susceptibility to MN.46 Because activated B cells and plasma cells produce TNF-α,47,48 it is possible to speculate that increased levels of TNF-α are related to increased B-cell activation in MN patients.
To the best of our knowledge, our study is the first reporting increased BREG in patients with primary MN. Although we acknowledge that our analysis did not test the function of these cells, our data are consistent with those reported in kidney transplant recipients,49 where increased regulatory cells are thought to represent a counterregulatory mechanism to control alloreactive immunity. Increased regulatory cells may represent a reaction to control effector B- and T-cell responses and, consistent with this hypothesis, some of the B cells that are found in the renal interstitium of MN patients50 could have a regulatory function. The results presented here may form the basis for future studies aimed at understanding their pathogenic role.
Previous studies showed a reduction in total CD4+CD25+CD127low TREG in MN patients compared with healthy controls. Our study did not confirm this finding, but it showed that CCR4+CD45RA-TREG, a subset previously associated with increased regulatory function,51 was significantly lower in MN patients than in controls. Overall, this supports the idea that, similar to other autoimmune diseases,52,53 MN is associated with impaired TREG function, instead of TREG number.
PhIP-Seq uses oligonucleotide library synthesis to encode proteomic-scale peptide libraries for display on bacteriophage. These libraries are then immunoprecipitated using individual’s serum antibodies for subsequent analysis by high-throughput DNA sequencing. The methodology has been used to identify novel self-antigens associated with autoimmune disease and exposure to human viruses. Herein, PhIP-Seq analysis showed that no single autoantigen or antiviral specificity segregates MN from CKD or healthy controls. The fact that the technique does not account for the protein 3-dimensional structure may partially account for this negative result. However, it is reasonable to argue that abnormalities in the circulating B-cell and plasma cell compartments in MN patients are largely limited to the anti-PLA2R autoreactive clones.
Our article has numerous strengths. First, we performed an extensive immune phenotypic characterization of B- and T-cell subsets, including markers and cell populations that have not been previously considered. Second, the study design included a large number of samples from MN patients and both healthy and CKD controls, which allowed us to apply statistical tools adequate for multidimensional and highly correlated data in order to detect subsets that were likely unique to MN patients. Finally, we developed novel assays to evaluate the function of circulating autoreactive antibody-producing cells specific against the PLA2R antigen. The data obtained by using these new immune tools may significantly help in a better understanding of the pathogenic role of the autoimmune B cells and plasma cells in patients with MN as well as their value in predicting response to therapy and early detecting relapses, before an increase in autoantibodies.54 Importantly, the number of autoreactive B cells may also identify MN patients who would benefit the most from higher doses of rituximab.55
The main limitation of the present study is the lack of serial samples. Although the correlation between plasma cells and anti-PLA2R antibody levels supports a relationship between the percentages of these cells and disease activity, prospective studies are needed to better define the association between spontaneous or treatment-induced disease remission.
Altogether, our study newly documents that the main immunologic abnormalities in MN patients rely in increased circulating plasma cells and BREG. This pool of plasma cells contains autoantibody-producing cells. Our data provide the background for studies testing the hypothesis that targeting these cells ameliorates disease severity (ClinicalTrials.gov identifier: NCT04145440).56,57
Disclosure
UL is a scientific founder of an antibody therapeutics company. All the other authors declared no competing interests.
Acknowledgments
The study has been supported by an investigator-initiated grant from the Renal Research Institute. We thank all the study participants and the Biobank Navarrabiomed (PT13/0010/0051) integrated in the Spanish National Biobanks Network for their collaboration. PC is supported by the National Institutes of Health R01 grant number DK119431. This work was partially supported by the Instituto de Salud Carlos III (grant numbers PI16/01321 and PI19/01710) (cofunded by European Regional Development Fund, a way to build Europe). MJ received a research fellowship grant from the “Instituto de Salud Carlos III” [FI17/00233]. We thank Stephen Elledge for kindly providing the PhIP-Seq phage libraries.
Author Contributions
CC and SH performed most of the flow cytometry experiments with the help from LA, CF, and EC. MJ, JD, XF, JT, and OB performed the experiments with antibody-producing cells. AA and JM enrolled patients. TOD, EM, and UL performed the PhIP-Seq experiments and analyzed the data. CD performed the Luminex experiments. LP, GLM, LVR, and EF helped in data interpretation and critically revised the manuscript. UM performed all the statistical analyses. PC conceived and oversaw the project, designed the experiments, interpreted the results, and wrote the manuscript together with CC, UM, and OB.
Footnotes
Supplementary Appendix.
Table S1. A list of the analyzed cell subsets.
Table S2. Plasma cytokines levels in MN patients and healthy controls.
Supplementary References.
Figure S1. The correlation plot of the 52 cell subsets in 71 patients with complete data (30 with membranous nephropathy, 31 with chronic kidney disease, and 10 health controls).
Figure S2. Variable importance (VIMP) from random forest analysis. A large VIMP value for a cell subset indicates that misspecification of that variable decreases ability in the forest in distinguishing between subjects with membranous nephropathy and the other 2 conditions based on the cell subsets. VIMP close to 0 indicates that the cell subset contributes nothing to distinguishing between groups, and negative values indicate that the ability to distinguish between groups improves when the cell subset is misdefined. In the latter case, we assume noise is more informative than the true cell subset. As such, if we ignore cell subsets with negative and near zero values of VIMP, we are left mostly with B-cell subsets. Among them, plasma cells and regulatory B cells (Breg) had the largest VIMP.
Figure S3. Least absolute shrinkage and selection operator (LASSO) for variable selection. Standardized coefficient estimates as a function of the tuning parameter, which “shrinks" the coefficient toward 0 as its value (on x-axis) gets larger; by setting some coefficient to 0, the tuning parameter determines which variables the LASSO will eventually exclude. Plasma cells and regulatory B cells (BREG) had the largest standardized coefficients and would have been selected (along with B cells) as the only significant cell subsets had the tuning parameter been set to the level of the vertical dotted line.
Figure S4. Interferon (IFN)-g+, interleukin (IL)-4+, and IL-17+ CD4+ T-cell subsets in membranous nephropathy (MN), chronic kidney disease (CKD) patients, and healthy controls (HC). Data are presented as mean ± standard deviation. Each dot represents the value for a single patient.
Figure S5. Serum levels of tumor necrosis factor-α (TNF-α). Serum levels of TNF-α in patients with membranous nephropathy (MN) and healthy controls (HC). Data are presented as mean ± standard deviation. Each dot represents the value for a single patient. P < 0.05 by unpaired t test and Holm-Bonferroni adjustment for multiple comparisons.
Table S3. PhIP-Seq hits for autoantigens and viral antigens.
Supplementary Material
References
- 1.Glassock R.J. The pathogenesis of idiopathic membranous nephropathy: a 50-year odyssey. Am J Kidney Dis. 2010;56:157–167. doi: 10.1053/j.ajkd.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Heymann W., Hackel D.B., Harwood S. Production of nephrotic syndrome in rats by Freund's adjuvants and rat kidney suspensions. Proc Soc Exp Biol Med. 1959;100:660–664. doi: 10.3181/00379727-100-24736. [DOI] [PubMed] [Google Scholar]
- 3.Lateb M., Ouahmi H., Payre C. Anti-PLA2R1 antibodies containing sera induce in vitro cytotoxicity mediated by complement activation. J Immunol Res. 2019;2019:1324804. doi: 10.1155/2019/1324804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravindran A., Madden B., Charlesworth M.C. Proteomic analysis of complement proteins in membranous nephropathy. Kidney Int Rep. 2020;5:618–626. doi: 10.1016/j.ekir.2020.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brglez V., Boyer-Suavet S., Seitz-Polski B. Complement pathways in membranous nephropathy: complex and multifactorial. Kidney Int Rep. 2020;5:572–574. doi: 10.1016/j.ekir.2020.02.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W., Gao C., Dai H. Immunological pathogenesis of membranous nephropathy: focus on PLA2R1 and its role. Front Immunol. 2019;10:18091809. doi: 10.3389/fimmu.2019.01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma H., Sandor D.G., Beck L.H., Jr. The role of complement in membranous nephropathy. Semin Nephrol. 2013;33:531–542. doi: 10.1016/j.semnephrol.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erwin D.T., Donadio J.V., Jr., Holley K.E. The clinical course of idiopathic membranous nephropathy. Mayo Clin Proc. 1973;48:697–712. [PubMed] [Google Scholar]
- 9.Huh H., Lee H., Lee J.P. Factors affecting the long-term outcomes of idiopathic membranous nephropathy. BMC Nephrol. 2017;18:104. doi: 10.1186/s12882-017-0525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schieppati A., Mosconi L., Perna A. Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med. 1993;329:85–89. doi: 10.1056/NEJM199307083290203. [DOI] [PubMed] [Google Scholar]
- 11.Tran T.H., J. Hughes G, Greenfeld C. Overview of current and alternative therapies for idiopathic membranous nephropathy. Pharmacotherapy. 2015;35:396–411. doi: 10.1002/phar.1575. [DOI] [PubMed] [Google Scholar]
- 12.Bobart S.A., De Vriese A.S., Pawar A.S. Noninvasive diagnosis of primary membranous nephropathy using phospholipase A2 receptor antibodies. Kidney Int. 2019;95:429–438. doi: 10.1016/j.kint.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Beck L.H., Bonegio R.G., Lambeau G. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanigicherla D., Gummadova J., McKenzie E.A. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83:940–948. doi: 10.1038/ki.2012.486. [DOI] [PubMed] [Google Scholar]
- 15.Seitz-Polski B., Debiec H., Rousseau A. Phospholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J Am Soc Nephrol. 2018;29:401. doi: 10.1681/ASN.2017070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozaki T., Tomino Y., Nakayama S. Two-color analysis of lymphocyte subpopulations in patients with nephrotic syndrome due to membranous nephropathy. Clin Nephrol. 1992;38:75–80. [PubMed] [Google Scholar]
- 17.Wang B., Zuo K., Wu Y. Correlation between B lymphocyte abnormality and disease activity in patients with idiopathic membranous nephropathy. J Int Med Res. 2011;39:86–95. doi: 10.1177/147323001103900111. [DOI] [PubMed] [Google Scholar]
- 18.Cagnoli L., Tabacchi P., Pasquali S. T cell subset alterations in idiopathic glomerulonephritis. Clin Exp Immunol. 1982;50:70–76. [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenzwajg M., Languille E., Debiec H. B- and T-cell subpopulations in patients with severe idiopathic membranous nephropathy may predict an early response to rituximab. Kidney Int. 2017;92:227–237. doi: 10.1016/j.kint.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez–Bayona B., Ramos-Amaya A., Perez-Venegas J.J. Decreased frequency and activated phenotype of blood CD27 IgD IgM B lymphocytes is a permanent abnormality in systemic lupus erythematosus patients. Arthritis Res Ther. 2010;12:R108. doi: 10.1186/ar3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szabo K., Papp G., Szanto A. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjogren's syndrome and systemic lupus erythematosus. Clin Exp Immunol. 2016;183:76–89. doi: 10.1111/cei.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapter 7: idiopathic membranous nephropathy. Kidney Int Suppl (2011) 2012;2:186–197. doi: 10.1038/kisup.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luque S., Lucia M., Bestard O. Refinement of humoral immune monitoring in kidney transplantation: the role of "hidden" alloreactive memory B cells. Transpl Int. 2017;30:955–968. doi: 10.1111/tri.13014. [DOI] [PubMed] [Google Scholar]
- 24.Luque S., Lucia M., Crespo E. A multicolour HLA-specific B-cell FluoroSpot assay to functionally track circulating HLA-specific memory B cells. J Immunol Methods. 2018;462:23–33. doi: 10.1016/j.jim.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Luque S., Lucia M., Melilli E. Value of monitoring circulating donor-reactive memory B cells to characterize antibody-mediated rejection after kidney transplantation. Am J Transplant. 2019;19:368–380. doi: 10.1111/ajt.15055. [DOI] [PubMed] [Google Scholar]
- 26.Larman H.B., Zhao Z., Laserson U. Autoantigen discovery with a synthetic human peptidome. Nat Biotechnol. 2011;29:535–541. doi: 10.1038/nbt.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohan D., Wansley D.L., Sie B.M. PhIP-Seq characterization of serum antibodies using oligonucleotide-encoded peptidomes. Nat Protoc. 2018;13:1958–1978. doi: 10.1038/s41596-018-0025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu G.J., Shah A.A., Li M.Z. Systematic autoantigen analysis identifies a distinct subtype of scleroderma with coincident cancer. Proc Natl Acad Sci U S A. 2016;113:E7526–E7534. doi: 10.1073/pnas.1615990113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu G.J., Kula T., Xu Q. Viral immunology. Comprehensive serological profiling of human populations using a synthetic human virome. Science. 2015;348 doi: 10.1126/science.aaa0698. aaa0698-aaa0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright S.P. Adjusted P-values for simultaneous inference. Biometrics. 1992;48:1005–1013. [Google Scholar]
- 31.James G WD, Hastie T. Springer; New York, NY: 2013. An Introduction to Statistical Learning. [Google Scholar]
- 32.StataCorp . StataCorp; College Station, TX: 2019. Stata 16 Base Reference Manual. [Google Scholar]
- 33.Qin W., Beck L.H., Jr., Zeng C. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22:1137–1143. doi: 10.1681/ASN.2010090967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassese G., Arce S., Hauser A.E. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171:1684–1690. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 35.Ruggenenti P., Debiec H., Ruggiero B. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol. 2015;26:2545–2558. doi: 10.1681/ASN.2014070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fervenza F.C., Appel G.B., Barbour S.J. Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med. 2019;381:36–46. doi: 10.1056/NEJMoa1814427. [DOI] [PubMed] [Google Scholar]
- 37.Ruggenenti P., Cravedi P., Sghirlanzoni M.C. Effects of rituximab on morphofunctional abnormalities of membranous glomerulopathy. Clin J Am Soc Nephrol. 2008;3:1652–1659. doi: 10.2215/CJN.01730408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cambridge G., Leandro M.J., Edwards J.C. Serologic changes following B lymphocyte depletion therapy for rheumatoid arthritis. Arthritis Rheum. 2003;48:2146–2154. doi: 10.1002/art.11181. [DOI] [PubMed] [Google Scholar]
- 39.Salama A.D., Pusey C.D. Drug insight: rituximab in renal disease and transplantation. Nat Clin Pract Nephrol. 2006;2:221–230. doi: 10.1038/ncpneph0133. [DOI] [PubMed] [Google Scholar]
- 40.Ruggenenti P., Cravedi P., Remuzzi G. Latest treatment strategies for membranous nephropathy. Expert Opin Pharmacother. 2007;8:3159–3171. doi: 10.1517/14656566.8.18.3159. [DOI] [PubMed] [Google Scholar]
- 41.Cravedi P., Remuzzi G., Ruggenenti P. Rituximab in primary membranous nephropathy: first-line therapy, why not? Nephron Clin Pract. 2014;128:261–269. doi: 10.1159/000368589. [DOI] [PubMed] [Google Scholar]
- 42.Dörner T., Lipsky P.E. Correlation of circulating CD27high plasma cells and disease activity in systemic lupus erythematosus. Lupus. 2004;13:283–289. doi: 10.1191/0961203304lu1014oa. [DOI] [PubMed] [Google Scholar]
- 43.Bustos C., Gonzalez E., Muley R. Increase of tumour necrosis factor alpha synthesis and gene expression in peripheral blood mononuclear cells of children with idiopathic nephrotic syndrome. Eur J Clin Invest. 1994;24:799–805. doi: 10.1111/j.1365-2362.1994.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 44.Ihm C.G., Park J.K., Hong S.P. Circulating factors in sera or peripheral blood mononuclear cells in patients with membranous nephropathy or diabetic nephropathy. J Korean Med Sci. 1997;12:539–544. doi: 10.3346/jkms.1997.12.6.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suranyi M.G., Guasch A., Hall B.M. Elevated levels of tumor necrosis factor-alpha in the nephrotic syndrome in humans. Am J Kidney Dis. 1993;21:251–259. doi: 10.1016/s0272-6386(12)80742-6. [DOI] [PubMed] [Google Scholar]
- 46.Thibaudin D., Thibaudin L., Berthoux P. TNFA2 and d2 alleles of the tumor necrosis factor alpha gene polymorphism are associated with onset/occurrence of idiopathic membranous nephropathy. Kidney Int. 2007;71:431–437. doi: 10.1038/sj.ki.5002054. [DOI] [PubMed] [Google Scholar]
- 47.Di Girolamo N., Visvanathan K., Lloyd A. Expression of TNF-α by human plasma cells in chronic inflammation. J Leukoc Biol. 1997;61:667–678. doi: 10.1002/jlb.61.6.667. [DOI] [PubMed] [Google Scholar]
- 48.Sung S.S., Jung L.K., Walters J.A. Production of tumor necrosis factor/cachectin by human B cell lines and tonsillar B cells. J Exp Med. 1988;168:1539–1551. doi: 10.1084/jem.168.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muthukumar T., Dadhania D., Ding R. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353:2342–2351. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 50.Cohen C.D., Calvaresi N., Armelloni S. CD20-positive infiltrates in human membranous glomerulonephritis. J Nephrol. 2005;18:328–333. [PubMed] [Google Scholar]
- 51.Rosenblum M.D., Way S.S., Abbas A.K. Regulatory T cell memory. Nat Rev Immunol. 2016;16:90–101. doi: 10.1038/nri.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haas J., Hug A., Viehover A. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–3352. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 53.Long S.A., Buckner J.H. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol. 2011;187:2061–2066. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cravedi P., Jarque M., Angeletti A. Immune-monitoring disease activity in primary membranous nephropathy. Front Med (Lausanne) 2019;6:241. doi: 10.3389/fmed.2019.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seitz-Polski B., Dahan K., Debiec H. High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin J Am Soc Nephrol. 2019;14:1173. doi: 10.2215/CJN.11791018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartono C., Chung M., Kuo S.F. Bortezomib therapy for nephrotic syndrome due to idiopathic membranous nephropathy. J Nephrol. 2014;27:103–106. doi: 10.1007/s40620-013-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barbari A., Chehadi R., Kfoury Assouf H. Bortezomib as a novel approach to early recurrent membranous glomerulonephritis after kidney transplant refractory to combined conventional rituximab therapy. Exp Clin Transplant. 2017;15:350–354. doi: 10.6002/ect.2016.0350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.