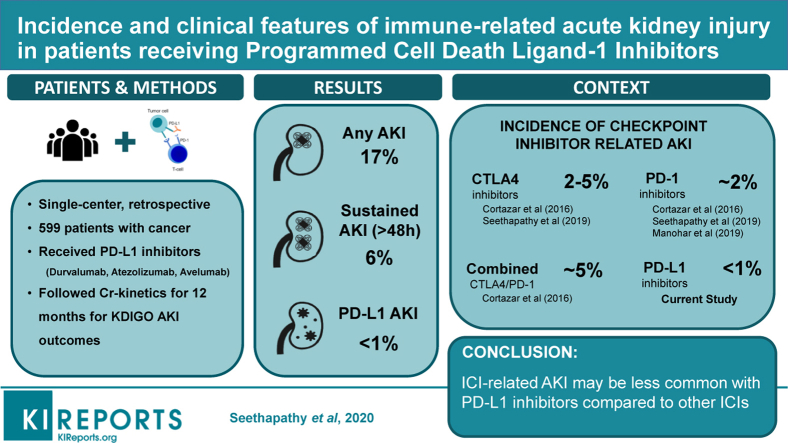
Abstract
Background
Programmed cell death receptor ligand 1 (PD-L1) inhibitors are immune checkpoint inhibitors (ICIs) with a side effect profile that may differ from other classes of ICIs such as those directed against cytotoxic T-lymphocyte−associated protein 4 (CTLA-4) and programmed cell death 1 receptor (PD-1). Being the more recently approved class of checkpoint inhibitors, there are no studies investigating the frequency, etiology and predictors of acute kidney injury (AKI) in patients receiving PD-L1 inhibitors.
Methods
This was a retrospective cohort study of patients who received PD-L1 inhibitors during 2017 to 2018 in our healthcare system. AKI was defined by a ≥1.5-fold rise in serum creatinine from baseline. The etiology of all cases of sustained AKI (lasting >48 hours) and clinical course were determined by review of electronic health records.
Results
The final analysis included 599 patients. Within 12 months of ICI initiation, 104 patients (17%) experienced AKI, and 36 (6%) experienced sustained AKI; however, only 5 (<1%) experienced suspected PD-L1–related AKI. The PD-L1–related AKI occurred a median of 99 days after starting therapy. All patients concurrently received another medication known to cause acute interstitial nephritis (proton pump inhibitors, nonsteroidal anti-inflammatory drugs, or antibiotics) at the time of the suspected PDL1-related AKI.
Conclusion
Although AKI is common in patients receiving PD-L1 therapy, the incidence of suspected PD-L1–related AKI is low (<1%) and may be less common when compared to other classes of ICIs. This cohort provides further validation that other drugs associated with acute interstitial nephritis may be involved in the pathogenesis of ICI-related AKI.
Keywords: acute interstitial nephritis, acute kidney injury, immune checkpoint inhibitor, immune-related adverse events, PD-L1 inhibitors
Graphical abstract
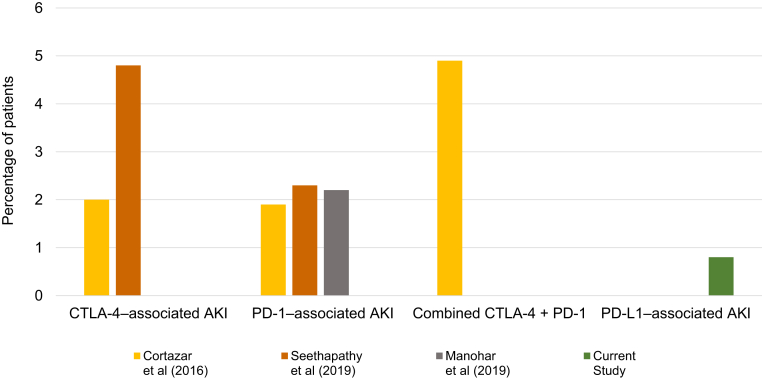
Acute interstitial nephritis (AIN) has been increasingly recognized as an important immune-related adverse event (irAE) that may complicate treatment with immune checkpoint inhibitor (ICI) therapy.1, 2, 3, 4 In a recently published study, we determined the incidence of sustained AKI to be 8% in patients receiving ICIs, with suspected ICI-related AKI occuring in 3% of patients.4 Other meta-analyses summarizing clinical trial data have estimated ICI-related AKI to occur in 1.4%–2.2% of patients receiving CTLA-4 or PD-1 monotherapy, and up to 4.9%% of patients receiving combination CTLA-4/PD-1 therapy.2,5
PD-L1 inhibitors, including atezolizumab, durvalumab, and avelumab, are a relatively new class of ICIs approved for multiple new cancer indications, and their use is rapidly increasing.6 Because they are a relatively new class of ICIs, clinical experience with these agents is limited. Prior studies evaluating AKI in patients on checkpoint inhibitors have included a very limited number (<40) of patients receiving PD-L1 inhibitors.1,2,4 Clinical trials have shown that the rate of hypothyroidism and pneumonitis are lower in patients receiving PD-L1 inhibitors than other ICI classes.7 It is not known whether this lower risk extends to nephrotoxicity as well. Hence, we sought to define the incidence, etiology, and clinical features of AKI in a large, unselected cohort of patients receiving PD-L1 inhibitors.
Methods
We performed a retrospective cohort study of all patients who received a PD-L1 inhibitor (durvalumab, atezolizumab, or avelumab) as first-line therapy for advanced malignancies from 2017 to 2018 at Mass General Brigham (Boston, MA) to evaluate changes in kidney function from baseline up to 1 year. The Research Patient Data Repository at Partners Healthcare System was used to collect data on baseline demographics, comorbidities, medications, and laboratory studies. Baseline use of medications known to be associated with acute interstitial nephritis (AIN), such as proton pump inhibitors (PPIs), nonsteroidal anti-inflammatory drugs (NSAIDs), and antibiotics, was defined by active prescriptions at the ICI start date. AKI was defined by a ≥1.5-fold rise in creatinine from baseline, and staged by Kidney Disease: Improving Global Outcomes (KDIGO) criteria.8 Sustained AKI was defined as AKI that lasted >48 hours; all cases were chart reviewed for etiology by 2 nephrologists (HS, MES), with a third (SG) available for disagreement. The etiology of sustained AKI was divided into the following: (i) suspected PD-L1–related AKI diagnosed by a kidney biopsy, or subspecialist determination with corticosteroid treatment, or unexplained sustained AKI occurring simultaneously with another irAE without an alternative cause for AKI identified; (ii) hemodynamic AKI (prerenal azotemia or acute tubular necrosis); (iii) obstruction; or (iv) AKI of undetermined cause if insufficient diagnostic workup was pursued. Detailed review of electronic medical records was used to obtain clinical information on all patients with sustained AKI. Death date was determined by manual chart review or was assumed at the time of last encounter for patients enrolled in hospice.
Baseline characteristics were described using means and SDs for continuous variables and counts and percentages for categorical variables. Descriptive analyses are reported for suspected PD-L1–related AKI, given the small number of events. The Institutional Review Board approved this study and waived the need for informed consent.
Results
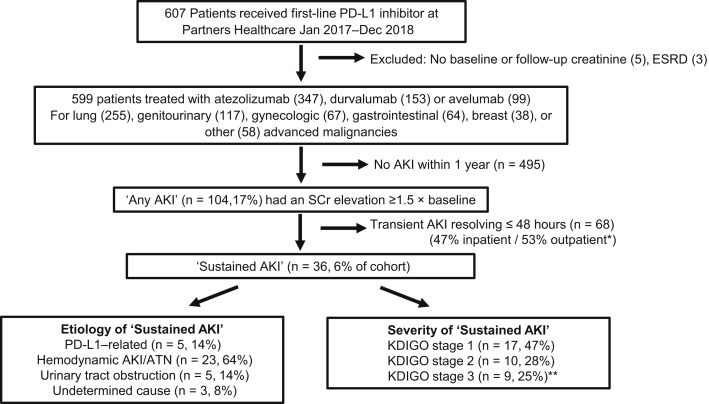
Between 1 January 2017 and 31 December 2018, a total of 607 patients were started on a PD-L1 inhibitor (Figure 1). Five patients were excluded because they did not have a baseline or a follow-up creatinine measurement within the 12-month follow-up period, and 3 patients were excluded because they were on hemodialysis at the time of PD-L1 initiation. In total, 599 patients had adequate creatinine measurements and were included in this study. The mean age was 65 (SD: 13) years, 50% were male, and 92% were white. The mean baseline creatinine value was 1.0 (SD: 0.3) mg/dl; 99 (17%) had an estimated glomerular filtration rate of <60 ml/min per 1.73 m2. Underlying malignancy types and individual PD-L1 inhibitors prescribed are shown in Table 1.
Figure 1.
Patient flow and acute kidney injury (AKI) outcomes among patients treated with programmed death ligand-1 (PD-L1) inhibitors. Overall, 17% of the included cohort developed AKI. ∗Among patients with transient AKI (lasting <48 hours), 36 (53%) occurred in outpatients; the majority did not have creatinine measurements immediately repeated, but it normalized by the following clinic visit (mean: 14 days, SD: 9 days until next check). Among those with AKI that was confirmed to have been sustained AKI, lasting for >48 hours, the etiology and Kidney Disease: Improving Global Outcomes (KDIGO) severity stage are shown. ∗∗Of the 9 patients with KDIGO grade 3 AKI, 4 patients had progressive kidney failure and died during the hospital stay, and none underwent renal replacement therapy. ATN, acute tubular necrosis; SCr, serum creatinine.
Table 1.
Baseline characteristics of patients receiving PD-L1 inhibitor therapy
| Characteristic | All patientsa |
No sustained AKI |
Sustained AKI |
PD-L1 inhibitor–related AKI |
|---|---|---|---|---|
| Mean ± SD or n (%) | ||||
| Patients, n | 599 | 563 | 36 | 5 |
| Age, yr | 65 ± 13 | 63 ± 14 | 63 ± 12 | 65 ± 12 |
| Baseline creatinine, mg/dl | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 |
| eGFR, ml/min | 88 ± 26 | 89 ± 26 | 82 ± 26 | 80 ± 20 |
| Male | 298 (50) | 281 (50) | 17 (47) | 2 (40) |
| Female | 301 (50) | 282 (50) | 19 (53) | 3 (60) |
| Race | ||||
| White | 554 (92) | 521 (92) | 33 (91) | 5 (100) |
| Black | 16 (3) | 15 (3) | 1 (3) | 0 |
| Asian | 11 (2) | 10 (2) | 1 (3) | 0 |
| Other/unknown | 18 (3) | 17 (3) | 1 (3) | 0 |
| Cirrhosis | 7 (1) | 7 (1) | 0 | 0 |
| Hypertension | 242 (40) | 229 (41) | 13 (36) | 2 (40) |
| Diabetes | 113 (19) | 103 (18) | 10 (28) | 0 |
| Baseline medications | ||||
| PPIb | 210 (35) | 192 (34) | 18 (50)b | 1 (20) |
| H2 blockers | 113 (19) | 104 (18) | 9 (25) | 0 |
| NSAIDs | 103 (17) | 95 (17) | 8 (22) | 1 (20) |
| Allopurinol | 12 (2) | 11 (2) | 1 (3) | 0 |
| ACEI/ARB | 135 (23) | 128 (23) | 7 (19) | 0 |
| Baseline kidney function, eGFR group | ||||
| <60 ml/min per 1.73 m2 | 99 (17) | 92 (16) | 7 (19) | 1 (20) |
| 60−90 ml/min per 1.73 m2 | 208 (35) | 192 (34) | 16 (44) | 3 (60) |
| >90 ml/min per 1.73 m2 | 292 (49) | 179 (50) | 13 (36) | 1 (20) |
| PD1 inhibitor type | ||||
| Atezolizumab | 347 (58) | 322 (57) | 25 (69) | 4 (80) |
| Avelumab | 99 (16) | 93 (17) | 6 (17) | 0 |
| Durvalumab | 153 (26) | 148 (26) | 5 (14) | 1 (20) |
| Malignancy | ||||
| Thoracic | 255 (43) | 244 (43) | 11 (31) | 2 (40) |
| Genitourinary | 117 (19) | 106 (19) | 11 (31) | 3 (60) |
| Gynecological | 67 (11) | 61 (11) | 6 (17) | 0 |
| Gastrointestinal | 64 (11) | 61 (11) | 3 (8) | 0 |
| Breast | 38 (6) | 36 (6) | 2 (5) | 0 |
| Other | 58 (10) | 55 (10) | 3 (8) | 0 |
ACEI, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate, NSAID, nonsteroidal anti-inflammatory drug; PD1, programmed cell death 1 receptor; PD-L1, programmed cell death ligand 1.
Baseline characteristics for ‘All patients’ are shown as a percentage of the overall cohort (N = 599). For the outcomes, sustained AKI and immune checkpoint inhibitor−related AKI, the percentage of events in each subgroup is presented. The first sustained AKI event was specified as the outcome for each patient. Comorbid conditions, including hypertension and cirrhosis, were determined by International Classification of Diseases Ninth or Tenth Revision codes appearing at least twice in the electronic medical record. Diagnosis of diabetes was determined by either a hemoglobin A1c ≥ 6.5% or by prescription of a glucose-lowering medication and a diagnosis code for diabetes. Other than race being unknown in a few patients, there were no missing demographic or comorbidities data.
In univariable models comparing demographic and clinical characteristics of patients who experienced “sustained AKI” vs. those who did not, only baseline proton pump inhibitor exposure (0.05) was significant at a P value of <0.10. This was included in the multivariable model for “sustained AKI” along with other clinically important variables that were determined a priori to be exposures of interest.
In total, 104 patients (17%) experienced AKI within the 1-year follow-up period, of whom 68 had transient AKI (lasting ≤48 hours) and the other 36 had sustained AKI (Figure 1). Baseline characteristics of patients with and without sustained AKI are shown in Table 1. Use of PPIs was more common among patients who developed sustained AKI.
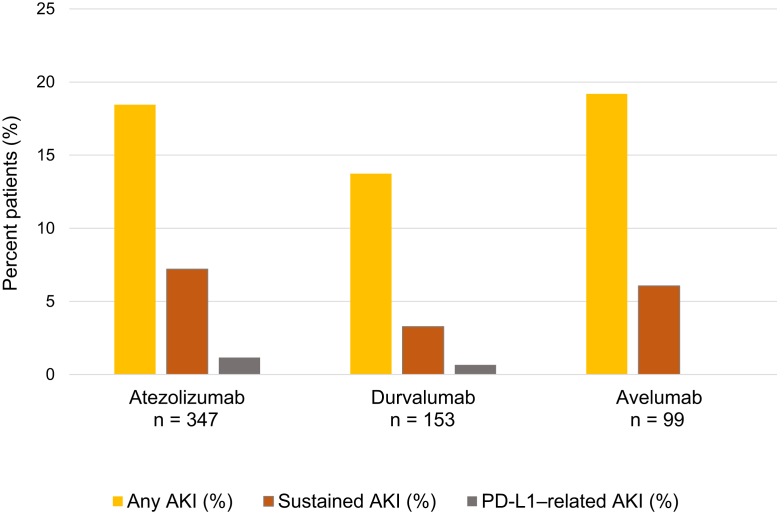
Hemodynamic AKI (including prerenal azotemia and acute tubular necrosis) was the most common cause of sustained AKI, occurring in 23 patients (64% of sustained AKI). Urinary tract obstruction occurred in 5 patients (14% of sustained AKI), suspected PD-L1 inhibitor−related AKI occurred in 5 (14% of sustained AKI), and the etiology of sustained AKI had an undetermined cause in 3 (8% of sustained AKI). De-identified case summaries of all 36 patients with sustained AKI are shown in Supplementary Table S1. The overall incidence and severity stages of sustained AKI for the individual PD-L1 inhibitor types are shown in Figure 2.
Figure 2.
Overall incidence of all acute kidney injury (AKI), sustained AKI, and programmed death ligand-1 (PD-L1)–related AKI in patients receiving atezolizumab, durvalumab, and avelumab. Among the 347 patients who received atezolizumab, 64 patients (18%) experienced any AKI, 25 (7%) had sustained AKI, and 4 (1%) had PD-L1–related AKI. Among the 153 patients who received durvalumab, 21 (14%) experienced any AKI, 5 (3%) had sustained AKI, and 1 (1%) experienced PD-L1–related AKI. Among the 99 patients who received avelumab, 19 (19%) experienced any AKI, 6 (6%) had sustained AKI, and none had PD-L1–related AKI.
The overall incidence of PD-L1–related AKI was <1% of the overall cohort; the rate per 100 person-years was 1.2 (95% confidence interval: 0.5–3.0). The presence of AIN was confirmed by biopsy in 1 of the 5 cases. The clinical features of the 5 patients are summarized in Table 2. Three of the 5 patients (60%) with suspected PD-L1–related AKI had a concurrent extrarenal irAE. Urinalysis was obtained in 3 patients with suspected PD-L1–related AKI; each had leukocyturia (>5 white blood cells per high-power field), including the patient with biopsy-proven AIN. None had hematuria or proteinuria (all ≤1+ on urinary dipstick). None had eosinophilia (<500 cells/μl). The median latency period from PD-L1 initiation to suspected PD-L1–related AKI was 99 days (interquartile range: 3–79 days). All patients were receiving a concomitant medication known to cause AIN (PPIs, NSAIDS, or antibiotics) at the time of suspected PD-L1–related AKI. The PD-L1 inhibitors were permanently discontinued in all 5 patients. Three patients received high-dose steroids (≥0.5 mg/kg per day in prednisone equivalents) tapered over 6 to 10 weeks; 2 of these patients experienced remission, whereas the third patient had persistently elevated creatinine. One patient transitioned to hospice without steroid treatment. The final patient had PD-L1 permanently discontinued with resolution of AKI. Only 1 patient was re-challenged with a different class of drug, a PD-1 inhibitor (nivolumab) 11 months after PD-L1–related AKI; that patient did not experience recurrent AKI.
Table 2.
Clinical characteristics of patients with suspected PD-L1–related AKI
| Age/race/sex | Cancer type | PD-L1 drug type | Time to AKI, mo | KDIGO AKI stage | Concurrent AIN-associated medications | Concurrent irAE | Kidney biopsy | Steroids | Outcome | Re-challenge |
|---|---|---|---|---|---|---|---|---|---|---|
| 65 WF | SCLC | Atezolizumab | 2 | 1 | PPI | Hypothyroidism | No | No | Transitioned to hospice and died | No |
| 65 WF | RCC | Atezolizumab | 6 | 2 | PPI, NSAIDs, amoxicillin | Colitis | No | Yes | Partial recovery but not to baseline | No |
| 50 WM | Bladder cancer | Atezolizumab | 8 | 1 | Ciprofloxacin | 0 | Yes (AIN) | Yes | No recovery | No |
| 65 WM | Bladder cancer | Atezolizumab | 4 | 3 | Levofloxacin | 0 | No | Yes | Full recovery to baseline | No |
| 55 WF | NSCLC | Durvalumab | 4 | 1 | SAIDs | Immune-related cytopenias | No | No | Full recovery to baseline | Yes Nivolumab 11 mo later without recurrence of AKI |
AIN, acute interstitial nephritis; AKI, acute kidney injury; irAE, immune-related adverse event; NSAIDs, nonsteroidal anti-inflammatory drugs; NSCLC, non–small cell lung cancer; PD-L1, programmed cell death receptor ligand 1; PPI, proton pump inhibitor; RCC, renal cell cancer; SCLC, small cell lung cancer; WF, white female; WM, white male.
Overall, the 1-year mortality in the cohort was high: 311 patients (52%) died. Of the patients with sustained AKI, mortality was 58%, and the median time to death after sustained AKI in those 21 patients was 10 days (interquartile range: 3–79 days). However, only 1 of the 5 patients (20%) with suspected PD-L1–related AKI died within 12 months.
Discussion
This is the largest cohort study evaluating kidney function in patients receiving PD-L1 inhibitors, the most recently approved class of ICIs used to treat advanced malignancies. Because durvalumab, avelumab, and atezolizumab were approved only in 2016 to 2017, prior studies describing AKI in patients receiving these drugs have been extremely limited. We found a lower incidence of suspected PD-L1–related AKI than in prior reports; those studies predominantly included patients on CTLA-4 inhibitors (ipilimumab) and PD-1 inhibitors (pembrolizumab and nivolumab; Figure 3).2,4,5 Our findings support recent pooled analyses of clinical trial data suggesting that irAEs may be lower in patients receiving PD-L1 inhibitors compared to other ICIs; this may be due to the selective blockade of the PD-L1 and not the PD-L2 ligand; sparing of the PD-1/PD-L2 interaction may be important for immune tolerance in certain organs.9, 10, 11 Although the number of cases of PD-L1–related AKI was small, the clinical features of suspected PD-L1–related AKI appear to be consistent with those in prior studies of patients with PD-1 and CTLA-4–related AKI; the majority had concomitant irAEs, and the mean onset was approximately 3 months after ICI initiation.2,4,12 In this study, all patients with suspected PD-L1–related AKI were on medications associated with AIN at the time of diagnosis, which strengthens the evidence that use of other concomitant medications associated with AIN may trigger nephritis in patients treated with ICIs. Drug-hapten–specific T-cell responses that drive type 4 hypersensitivity reactions affecting the skin and kidneys are regulated by PD-1/PDL-1 pathways; ICIs may inadvertently lead to activation of T cells that have previously been primed to a drug.13 Further research is needed to identify whether drug-specific T-cells are activated in patients with ICI-nephritis, and to determine whether minimizing exposure to potential AIN drugs that act as haptens can lower the rate of ICI-related AKI and other irAEs.14,15
Figure 3.
Incidence of programmed death ligand-1 (PD-L1)–related acute kidney injury (AKI) compared to historical estimates of cytotoxic T-lymphocyte−associated protein 4 (CTLA-4), programmed cell death 1 receptor (PD-1), and CTLA-4/PD-1 combination therapy–related AKI. The incidence of immune checkpoint inhibitor (ICI)–related AKI from clinical trial data was gathered in 2 meta-analyses (Cortazar et al.2 [2016] and Manohar et al.5 [2019]) and 1 observational real-world study (Seethapathy et al.4 [2019]). The incidence of CTLA-4–related AKI was 2% in Cortazar et al. and 4.8% in Seethapathy et al. The incidence of PD-1 related AKI was 1.9% in Cortazar et al., 2.2% in Manohar et al., and 2.3% in Seethapathy et al. The incidence of ICI-AKI with combination CTLA-4 and PD-1 therapy was 4.9% from Cortazar et al. In comparison, the incidence of PD-L1–related AKI was 0.8% in our study.
Similar to AKI in patients on other ICIs, the most likely etiology of sustained AKI in patients receiving PD-L1 inhibitors was hemodynamic AKI (prerenal azotemia or acute tubular necrosis). This cohort also had a substantial fraction of AKI due to urinary obstruction; this is likely explained by the higher prevalence of urothelial cancers in this cohort. This highlights the importance of full diagnostic workup, including imaging and kidney biopsy when needed to exclude alternative etiologies, in order to prevent a misdiagnosis of ICI-related AKI that could lead to unnecessary delays of future PD-L1 doses or corticosteroid treatment, both of which could compromise treatment of the underlying cancer.16,17
Our study has several limitations. The generalizability of this study may be reduced, given the limited racial diversity in this cohort. In addition, we may have underestimated the frequency of AKI if patients had AKI events managed at hospitals outside of our healthcare network. The retrospective nature of data collection and small number of cases limited our ability to phenotype all cases of sustained AKI; misdiagnosis of even a few cases could have significantly influence on the calculations of PD-L1–related AKI incidence. Finally, only 1 patient with PD-L1–related AKI underwent a kidney biopsy; thus, we were not able to exclude alternative pathologic lesions explaining AKI. However, other studies have shown that AIN is found in >90% of biopsied patients, and the absence of proteinuria in our patients argues against less common glomerular diseases leading to AKI.
Oncologists and nephrologists should be aware that PD-L1–related AKI may be less common than in patients treated with PD-1 or CTLA-4 inhibitors. Future multicenter studies are needed to precisely determine the incidence and predictors of PD-L1–related AKI, as well as to identify noninvasive biomarkers of ICI-AKI to enable more precise diagnosis.
Disclosure
KGB reports spouse employment at Devoted Health, royalties from UptoDate, and has served as a scientific consultant to Persistent Systems. DEL received research support from BioPorto Diagnostics. RS receives research support from Merck and Amgen. He serves as a consultant or on advisory boards of Merck, Amgen, Array, Novartis, Genentech, Replimmune, and Compugen. MES receives research support from Gilead and EMD-Serono and has served on advisory boards of Merck, Gilead, and Abbvie. LZ serves as a consultant to Merck. OR receives research support from Merck. He serves as a speaker for activities supported by educational grants from BMS and Merck and is a consultant for Merck, Celgene, Five Prime, GSK, GFK, Defined Health INC, Roche/Genentech, Puretech, Imvax, Leerink, and PRMA Consulting. In addition, he has a patent pending (“Methods of Using Pembrolizumab and Trebananib”). SG has served on an advisory board for AstraZeneca and is a Scientific Coordinator for the ASCEND trial sponsored by GlaxoSmithKline. SM reports salary from UpToDate. All the other authors declared no competing interests.
Footnotes
Table S1. De-identified case summaries of patients with sustained AKI.
Supplementary Material
References
- 1.Mamlouk O., Selamet U., Machado S. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer. 2019;7:2. doi: 10.1186/s40425-018-0478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortazar F.B., Marrone K.A., Troxell M.L. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90:638–647. doi: 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirali A.C., Perazella M.A., Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. 2016;68:287–291. doi: 10.1053/j.ajkd.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 4.Seethapathy H., Zhao S., Chute D.F. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. Clin J Am Soc Nephrol. 2019;14:1692–1700. doi: 10.2215/CJN.00990119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manohar S., Kompotiatis P., Thongprayoon C. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol Dial Transplant. 2019;34:108–117. doi: 10.1093/ndt/gfy105. [DOI] [PubMed] [Google Scholar]
- 6.Akinleye A., Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. 2019;12:92. doi: 10.1186/s13045-019-0779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxi S., Yang A., Gennarelli R.L. Immune-related adverse events for anti-PD-1 and anti-PD-L1 drugs: systematic review and meta-analysis. BMJ. 2018;360:2018–2019. doi: 10.1136/bmj.k793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2 [Google Scholar]
- 9.Khunger M., Rakshit S., Pasupuleti V. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152:271–281. doi: 10.1016/j.chest.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 10.Xiao Y., Yu S., Zhu B. RGMb is a novel binding partner for PD-l2 and its engagement with PD-l2 promotes respiratory tolerance. J Exp Med. 2014;211:943–959. doi: 10.1084/jem.20130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang P.F., Chen Y., Song S.Y. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: a meta-analysis. Front Pharmacol. 2017;18:730. doi: 10.3389/fphar.2017.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortazar F.B., Kibbelaar Z.A., Glezerman I.G. Clinical features and outcomes of immune checkpoint inhibitor-associated AKI: a multicenter study. J Am Soc Nephrol. 2020;31:435–446. doi: 10.1681/ASN.2019070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson A., Faulkner L., Lichtenfels M. the effect of inhibitory signals on the priming of drug hapten−specific t cells that express distinct V β receptors. J Immunol. 2017;199:1223–1237. doi: 10.4049/jimmunol.1602029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sibaud V. Dermatologic reactions to immune checkpoint inhibitors: skin toxicities and immunotherapy. Am J Clin Dermatol. 2018;19:345–361. doi: 10.1007/s40257-017-0336-3. [DOI] [PubMed] [Google Scholar]
- 15.Naidoo J., Page D.B., Li B.T. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faje A.T., Lawrence D., Flaherty K. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124:3706–3714. doi: 10.1002/cncr.31629. [DOI] [PubMed] [Google Scholar]
- 17.Hughes M.S., Molina G.E., Chen S.T. Budesonide treatment for microscopic colitis from immune checkpoint inhibitors. J Immunother Cancer. 2019;7:1–10. doi: 10.1186/s40425-019-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.