Abstract
Introduction
Acute kidney injury (AKI) is a major cause of mortality worldwide, particularly in low-resource settings with limited diagnostic testing. Neutrophil gelatinase-associated lipocalin (NGAL) has shown promise in predicting AKI. Nested within a larger, prospective cohort study evaluating AKI incidence in admitted trauma patients, our objective was to evaluate a novel dipstick, NGALds, for the prediction of AKI in Malawi, Africa.
Methods
Participants were >6 months of age. Spearman rank correlation coefficients (R) assessed NGAL categories (negative [≤50 ng/ml], low risk [51−149 ng/ml], moderate risk [150−299 ng/ml], and high risk [≥300 ng/ml]) for the urine NGALds dipstick and laboratory-based NGAL Test.
Results
We enrolled 285 participants (one-third children). Thirteen percent developed AKI. The dipstick captured 45 of 52 participants (86.5%) with moderate- or high-risk NGAL values on laboratory-based testing (R = 0.74). The dipstick had sensitivity of 44.4%, specificity of 73.5%, positive predictive value of 19.5%, and negative predictive value of 90.2% for predicting AKI. Acute kidney injury was associated with an increased risk of mortality (relative risk [RR] = 3.9, 95% confidence interval [CI] = 1.9−8.2), but mortality risk greatly increased among children who first had a positive (≥150 ng/ml) NGALds result (RR = 12.0, 95% CI = 1.8−78.4).
Conclusions
The NGALds dipstick performed similarly to the NGAL Test in this low-resource setting and may be a useful tool to rule out AKI. It may be even more important in predicting high mortality risk among children.
Keywords: acute kidney injury, Africa, NGAL, point-of-care
Sub-Saharan African countries have been excluded from large surveillance studies of AKI.1,2 Difficulties with laboratory confirmation of AKI likely contribute to the paucity of studies on AKI in low-resource areas. The only diagnostic test for AKI (serum creatinine) continues to rely on laboratory infrastructure, which remains limited or nonexistent in low-resource settings. Currently, the only alternative, low-cost method for “diagnosing” AKI in such settings is anuria.
Extensive research has focused on discovery and validation of novel biomarkers for early detection of AKI, yet these biomarkers will do little to change the landscape of AKI recognition in low-resource settings if they cannot be adapted for areas with inadequate laboratory facilities. Urine NGAL is one such biomarker to consistently predict subsequent AKI diagnosis in both adults and children.3, 4, 5, 6, 7, 8, 9 Clinical research has shown the utility of real-time trending of urine NGAL to predict and to manage AKI, thereby improving patient care.3 In addition, a novel, point-of-care dipstick for urine NGAL (NGALds; Bioporto, Hellerup, Denmark) was recently developed. If it proves equally efficacious as the laboratory urine NGAL, it could be quite promising for improving AKI diagnostics in low-resource areas.
Malawi, a landlocked country in southeastern Africa, is regularly classified as 1 of the 5 poorest countries in the world (based on gross domestic product per capita).10 There are 2 large, central teaching hospitals in the country that provide the only nephrology care for a population of 18.6 million.11 Their laboratory services frequently experience shortages of reagents and power outages, limiting services at times quite severely. A novel diagnostic test for AKI that does not rely on reagents, electricity, or experienced laboratory technicians could be invaluable in this setting.
Nested within a larger prospective cohort study of trauma patients in Malawi, we aimed to validate the urine NGAL biomarker and new NGALds dipstick in a low-resource environment. We assessed both the laboratory-based NGALtest (NGAL Test) and the NGALds dipstick test characteristics for diagnosing AKI, as defined by creatinine-only Kidney Disease: Improving Global Outcomes (KDIGO) criteria.12
Methods
Study Design
This validation study of diagnostic accuracy was nested within a prospective cohort study to evaluate the incidence of AKI among admitted trauma patients.13 The study was conducted at Kamuzu Central Hospital (KCH) in Lilongwe, Malawi.
Study Population
The larger prospective cohort study included all admitted acute trauma patients at KCH (convenience sampling). The overall study inclusion criteria were age ≥6 months, weight >3 kg, and an expected admission >24 hours. Both the patient (if ≥7 years) and caregiver had to provide written informed consent/assent. Participants were excluded if trauma occurred >5 days before hospital arrival and if primary language was not English or Chichewa (the Malawian official and national languages, respectively). Enrollment (consent and laboratory diagnostics) had to occur within 18 hours of hospital arrival. The overall study screened 4547 patients (674 eligible) and enrolled 337 participants.13 For this nested validation study, an additional requirement for inclusion was the ability to provide a fresh urine sample on admission.
Outcome
The primary outcome of interest was the validity of the urine NGALds dipstick as compared to the urine NGAL laboratory test (NGAL Test) and the gold-standard AKI diagnosis as defined by creatinine-only KDIGO criteria.12 Serum creatinine, urine laboratory-based NGAL, and urine NGALds dipstick results were obtained simultaneously within 18 hours of arrival. A second serum creatinine value was obtained within 48 to 72 hours of admission. In Malawi, it is not routine for renal function tests or electrolytes to be obtained on any trauma patients. To maximize our accuracy for AKI by KDIGO, we chose to obtain 2 values 48 hours or more apart. Medical teams could obtain more laboratory tests as they deemed medically necessary. No participants had a baseline creatinine value prior to admission. For adult participants, we assumed a priori a baseline estimated glomerular filtration rate (eGFR) of 75 ml/min per 1.73 m2 and back-calculated a baseline creatinine using the Modification of Diet in Renal Disease (MDRD) Study equation, per KDIGO guidelines,12 and excluding the race variable as is preferred in African populations.14, 15, 16, 17 For pediatric participants, we assumed a priori a baseline eGFR of 120 ml/min per 1.73 m2 based on previous literature.18 We subsequently back-calculated a baseline creatinine using the new (bedside) Schwartz equation based on our previous work in Malawian children.13,19
As mentioned above, routine laboratory testing is quite unreliable at KCH’s main public laboratory. To ensure reliable and consistent laboratory results, all laboratory testing was conducted at the University of North Carolina (UNC) Project Malawi Laboratory, which is a state-of-the-art research laboratory on-site at KCH. This laboratory maintains a constant supply of reagents, quality control and assurance measurements, and back-up generators to ensure high-quality laboratory performance for multiple research projects. Creatinine values were obtained on fresh serum using the Jaffe method on Roche analyzer Cobas C311 (Basel, Switzerland). Urine laboratory-based NGAL tests were obtained on fresh urine samples using the same analyzer with daily control standards checked. The urine laboratory-based NGAL (NGAL Test) was reported as a continuous value in nanograms per milliliter (ng/ml). The novel urine NGALds dipstick was conducted at the bedside by trained study nurses who did not have prior knowledge of the laboratory-based NGAL and creatinine test results. On a fresh urine sample, 0.1 ml of urine was pipetted into a pre-prepared, freeze-dried reagent test tube. Buffer was added for 5 minutes. The dipstick was inserted and, after 10 minutes, if the control line was positive, the NGALds dipstick was compared to color-categorized NGAL values (0 if no color appeared, 25, 50, 100, 150, 300, and 600 ng/ml) for the closest comparison (Figure 1).
Figure 1.
Neutrophil gelatinase-associated lipocalin (NGALds) dipstick color category reference.
No adverse events were attributed to obtaining noninvasive urine specimens for the laboratory-based and dipstick NGALds tests.
Covariates
To determine whether the novel dipstick performed differently from other potential variables that may be more readily available in resource limited settings, we compared 2 other “tests” to the gold-standard AKI diagnosis. The participant or caregiver’s self-report of urine output was assessed on arrival; it was categorized as decreased or absent versus no change. In addition, a standard urine dipstick for assessing proteinuria was assessed on arrival and classified as negative/trace, 1+ (0.3 g/l), 2+ (1.0 g/l), 3+ (3.0 g/l), or 4+ (≥20 g/l).
Additional covariates obtained included gender, age (in years), mechanism of trauma, body location of injury, and laboratory-confirmed comorbidities (anemia, sickle cell disease, malaria).20 In addition, we assessed each participant’s final hospital outcome (discharged home, died, or left against medical advice) and length of hospitalization.
Sample Size
The overall study aimed to enroll a total of 480 trauma patients (50% children ≤18 years of age) who were admitted to KCH to optimally estimate the incidence of AKI in trauma patients. This nested study used all available trauma participants in the overall study who met inclusion/exclusion criteria.
Analysis
We assessed demographic and injury-related characteristics with descriptive statistics. Spearman rank correlation coefficients (R) assessed categorical groups of NGAL values for the urine NGALds dipstick and laboratory-based NGAL test. Categories included negative (≤50 ng/ml), low risk (51−149 ng/ml), moderate risk (150−299 ng/ml), and high risk (≥300 ng/ml). We used sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) to evaluate the validity of the urine NGAL Test (quantitative laboratory-based values) and urine NGALds dipstick (categorical values) to diagnose AKI. Subanalyses were conducted to assess whether the dipstick performed differently by age or gender. In addition, self-report of urine output and proteinuria by urine dipstick were individually compared to the gold-standard AKI by creatinine-only KDIGO criteria.
Data were double-entered into REDCap electronic data capture tools hosted at UNC.21 All statistical analyses were conducted in SAS, version 9.4 (SAS Institute, Inc., Cary, NC). The institutional review boards at UNC and Malawi’s National Health Science Research Committee approved this study.
Results
Of the 337 participants enrolled in the overall study, 285 (84.6%) had fresh urinary data on admission available for analysis. In all, 31.9% (n = 91) of eligible participants were children (Table 1). Trauma-related AKI was significantly associated with higher mortality risk compared to absence of AKI (22.2% vs. 5.6%, P < 0.001). No patient reported a history of chronic kidney disease or prior dialysis.
Table 1.
Demographics of hospitalized malawian trauma patients by development of acute kidney injury
| Demographic factors | Total |
AKI |
No AKI |
Missing data |
|---|---|---|---|---|
| N = 285 | 36 (12.6) | 249 (87.4) | ||
| Age, yr, median (IQR) | 25 (13, 38) | 30 (19,41) | 25 (13,38) | 2 |
| Children ≤18 yr | 91 (31.9) | 9 (25.0) | 82 (32.9) | 2 |
| Gender (male) | 225 (78.9) | 30 (83.3) | 195 (78.3) | 1 |
| Type/location of trauma | ||||
| Burns | 32 (11.2) | 8 (22.2) | 24 (9.6) | 5 |
| Motor vehicle related | 123 (43.2) | 16 (44.4) | 107 (43.0) | 5 |
| Assaults | 53 (18.6) | 6 (16.7) | 47 (18.9) | 5 |
| Truncal injury | 80 (28.1) | 15 (41.7) | 65 (26.1) | 2 |
| Multiple injuries | 149 (52.3) | 24 (66.7) | 125 (50.2) | 2 |
| Comorbidities | ||||
| Anemia | 137 (48.1) | 23 (63.9) | 114 (45.8) | 0 |
| Malaria | 22 (7.7) | 1 (2.8) | 21 (8.4) | 4 |
| Sickle cell traita | 20 (7.0) | 3 (8.3) | 17 (6.8) | 15 |
| Length of stay,b days, median (IQR) | 11 (6, 28) | 13 (8, 46) | 11 (6, 27) | 15c |
| Mortality | 22 (7.7) | 8 (22.2) | 14 (5.6) | 12d |
AKI, acute kidney injury; IQR, interquartile range.
All data are presented as n (%) unless otherwise specified.
No patients had sickle cell disease (HgSS), so only sickle cell trait (HgAS) is presented.
Length of stay is for those patients discharged alive (n = 250).
Six patients left against medical advice, 6 had lost files, and 3 discharge dates could not be confirmed.
Six patients left against medical advice and 6 had lost files.
Dipstick Versus Laboratory-Based NGAL
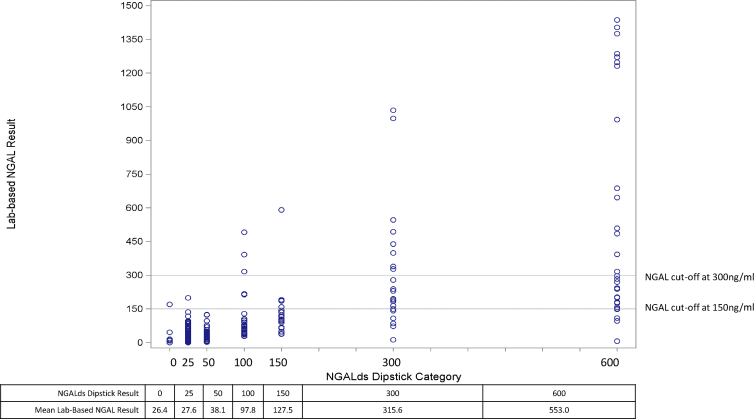
The NGALds dipstick categories correlated with laboratory-based NGAL test results (R = 0.74) on admission (Table 2). The dipstick captured 45 of 52 participants (86.5%) with moderate- or high-risk laboratory-based NGAL values. The plot presented in Figure 2 shows that, on average, the laboratory-based NGAL results matched the NGALds dipstick category (e.g., for all individuals with a dipstick result of 150, average laboratory-based NGAL result of 127.5). Subgroup analyses revealed similar results for adults and children.
Table 2.
Categorical comparisons of urine NGAL values on admission in Malawian trauma patients
| Risk category | Laboratory-based NGALa |
Spearman rank correlation | |||
|---|---|---|---|---|---|
| Negative (≤50) | Low risk (51−149) | Moderate risk (150−299) | High risk (≥300) | ||
| Negative (≤50) | 128 | 26 | 2 | 0 | 0.74 |
| Low risk (51−149) | 13 | 17 | 2 | 3 | |
| Moderate risk (150−299) | 4 | 15 | 4 | 1 | |
| High risk (≥300) | 2 | 8 | 18 | 22 | |
NGA, neutrophil gelatinase-associated lipocalin.
Twenty participants were missing laboratory-based test results.
Figure 2.
Laboratory-based neutrophil gelatinase-associated lipocalin (NGAL) results by NGALds dipstick categories. Twenty participants were missing laboratory-based NGAL values on admission. Individual points are depicted, with common thresholds indicated by the horizontal lines (at 150 ng/ml and 300 ng/ml). Actual NGALds dipstick results indicated below the plot with the corresponding average test result of the laboratory-based NGAL values for all individuals within the dipstick category.
Validity of NGAL to Diagnose AKI
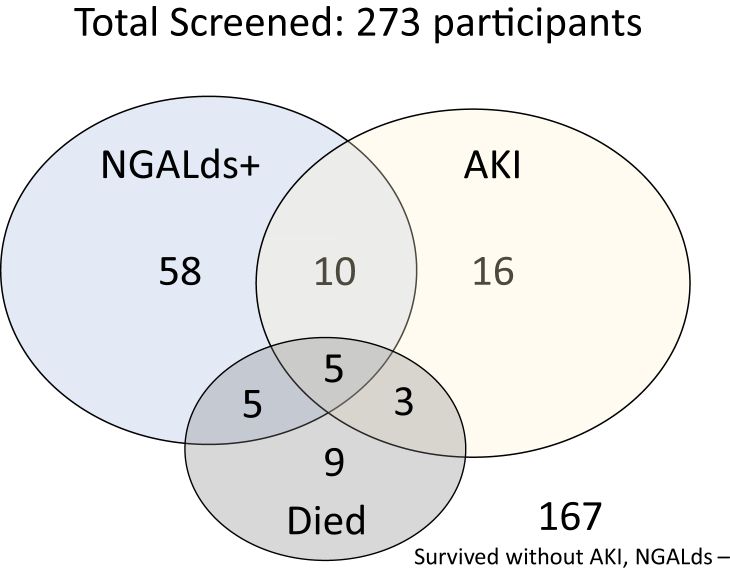
In all, 273 participants had both urine samples for dipstick testing and hospital outcome data (mortality) available, and 12.5% developed AKI. Figure 3 demonstrates that if we screened all trauma participants with dipsticks, subsequently tested creatinine for AKI confirmation among only those with positive dipsticks (≥150 ng/ml), then the dipstick would have captured 44% of AKI episodes (15 of 34). However, we would also drastically reduce the number that we screened with creatinine tests from 273 down to 78, an almost 75% reduction in testing. In addition, the NGALds dipstick was elevated (≥150 ng/ml) in 10 of 22 participants who died (45%). Table 3 provides the test characteristics of NGALds dipstick on admission urine samples compared to creatinine-only KDIGO-defined AKI diagnosis. Based on a threshold of 150 ng/ml, the dipstick had moderate specificity (73.5%) but poor sensitivity (44.4%). Subgroup analyses revealed consistent results in adults and children. However, among children, a threshold of 150 ng/ml had higher specificity (84.2%), with sensitivity of 22.2% for detecting AKI. Further analyses to assess the dipstick versus different stages of AKI were limited by the sample size. Only 12 patients had severe (stage 2 or 3) AKI. There was a strong association with a low NGALds dipstick level (<150 ng/ml) and no AKI (P < 0.0001). No association was found between dipstick result and individual AKI stages. A receiver operating characteristic (ROC) curve is provided in Supplementary Figure S1. The laboratory-based NGAL test performed similarly to the dipstick test for characterizing AKI (Supplementary Table S1).
Figure 3.
Admitted Malawian trauma patients screened with NGALds dipstick and interplay of positive dipstick, AKI, and mortality. Total screened participants with outcome data, n = 273; participants with NGALds+, n = 78; participants with AKI, n = 34; participants who died, n = 22; participants who survived without AKI and had NGALds− results, n = 167. AKI, acute kidney injury. NGALds+ refers to NGALds dipstick results ≥150 ng/ml. NGALds− refers to NGALds dipstick results <150 ng/ml.
Table 3.
Urine NGALds Dipstick Test Predictive Characteristics Using KDIGO-defined AKI (creatinine-onlya) in Malawian Trauma Patients
| Urine NGALds Dipstick category | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| 25 | 97.2 | 3.6 | 12.7 | 90.0 |
| 50 | 61.1 | 47.0 | 14.3 | 89.3 |
| 100 | 58.3 | 61.0 | 17.8 | 91.0 |
| 150 | 44.4 | 73.5 | 19.5 | 90.2 |
| 300 | 36.1 | 82.7 | 23.2 | 90.0 |
AKI, acute kidney injury; KDIGO, Kidney Disease: Improving Global Outcomes; NGAL, neutrophil gelatinase-associated lipocalin; NPV, negative predictive value; PPV, positive predictive value.
Using admission urine samples only (n = 285).
Validity of Proteinuria and Anuria to Diagnose AKI
The presence of proteinuria (≥0.3g/l) revealed test characteristics similar to those of NGAL for diagnosing AKI (sensitivity 46.0%, specificity 79.6%, PPV 24.6%, NPV 91.0%). As expected, higher proteinuria was linked to higher specificity, but sensitivity declined precipitously to <3% at ≥3.0 g/l. Proteinuria was present among 30% of participants who died, which is lower than the 45% captured by NGALds. A report of decreased or absent urine output was a very poor indicator for AKI (sensitivity 16.1%, specificity 86.4%) and was not available for 20.5% of participants.
Mortality
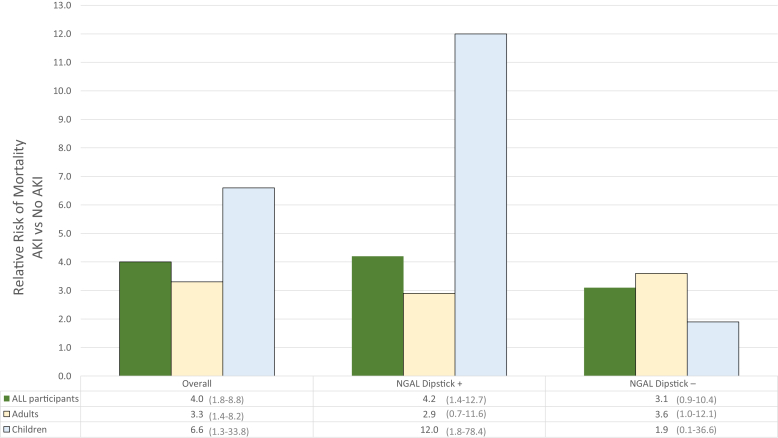
In our population, participants who developed AKI had 4 times higher mortality risk than those who did not develop AKI (RR = 4.0, 95% CI = 1.8−8.8) (Figure 4). When stratified by age, children had a slightly higher risk (RR = 6.6, 95% CI = 1.3−33.8). However, children with an elevated NGAL dipstick (≥150 ng/ml) who developed AKI had the highest mortality risk (RR = 12.0, 95% CI = 1.8−78.4). There were no differences by gender.
Figure 4.
Relative risk of mortality for Malawian trauma patients with acute kidney injury stratified by age and NGALds dipstick results. The figure presents the relative risk (RR) of mortality for those with and without AKI (n = 12 missing outcome data). Left section presents the RR of mortality among all participants (further stratified by age). Middle section presents the RR of mortality among only those individuals with first a positive NGAL dipstick on admission (further stratified by age). Right section presents the RR of mortality among only those individuals with first a negative NGAL dipstick on admission (further stratified by age). Actual RR is presented at the bottom, with 95% confidence interval in parentheses. AKI, acute kidney injury; NGAL, neutrophil gelatinase-associated lipocalin; Dipstick+, positive NGALds dipstick (≥150 ng/ml); Dipstick −, negative NGALds dipstick (<150 ng/ml).
Discussion
We present one of the first validation studies of the novel NGALds dipstick for AKI diagnosis. We found evidence to support the use of this point-of-care test to rule out AKI in trauma patients, particularly in low-resource settings. Moreover, children with an elevated dipstick level and AKI had an extremely high mortality rate, suggesting that this test may be useful for risk stratification of pediatric patients.
The dipstick was a useful tool to rule out AKI (specificity 73.5%, NPV 90.2%). For a diagnostic test, a high specificity to rule out disease is prioritized over sensitivity, which helps to rule in disease. In a country that currently uses subjective clinical signs on a routine basis for AKI diagnosis, determining which patients need closer monitoring is imperative and, at this stage, potentially more important than AKI confirmation. We do not believe that the NGALds dipstick will replace a serum creatinine test, but in an impoverished area, the dipstick might be a useful triage tool for district or rural hospitals that have no creatinine testing, as an objective marker to prioritize limited transportation for the sickest of patients. In addition, the dipstick could be incorporated into clinical algorithms for subsequent creatinine testing as a more cost-effective screening tool for AKI. Current estimates are for the test to be sold at $0.50 to $1 USD (85%−93% less expensive than the cost of serum creatinine, ∼$6.80 USD per test).
The dipstick seems to be more promising for predicting mortality after subsequent AKI diagnosis, particularly in children (RR = 12.0, 95% CI = 1.8−78.4) compared to adults (RR = 2.9, 95% CI = 0.7−11.6). This result is consistent with findings that laboratory-based NGAL is a better predictor of AKI in children compared to adults.22
Currently, the only point-of-care AKI diagnostic tests available clinically are expensive and require temperature-controlled units.23,24 We demonstrated that it was logistically feasible for clinical staff in a low-resource area to obtain urine (collected in >84% on admission, >90% overall) and to use a novel point-of-care dipstick. In addition, the dipstick has a shelf life at room temperature of >1 month. Given the limited laboratory infrastructure and unreliability of testing, clinicians in Malawi and many other low-resource areas often wait until a patient is anuric for 24+ hours before diagnosing AKI. This is often too late for preventive and management strategies to effectively change the AKI course and to prevent the need for dialysis, which is often a “death sentence” in areas with limited access to dialysis care. A robust point-of-care test could expand AKI screening/risk stratification to remote areas at district hospitals and health centers, expediting transfer of the most at-risk trauma patients.
This study has several limitations. Given resource limitations and costs, we were only able to obtain at most two creatinine values. Most AKI studies tend to obtain 4 to 7 daily creatinine values, so it is possible that we underdiagnosed AKI events. In addition, there is no standard consensus for defining baseline creatinine among children. We previously showed that the baseline creatinine definition for children has a strong impact on detected AKI incidence and that for this African setting, the new Schwartz equation may be optimal.13 We conducted a prospective study because retrospective and case-control studies are difficult in low-resource areas in which creatinine values are not routinely obtained. However, only 36 participants developed AKI, and only 12 developed severe AKI (stage 2 or 3), thereby limiting our inferences. Larger studies are needed. Our associations of AKI and NGALds dipstick results with mortality should not be viewed as causal. We are able to show an association only with mortality; these may both be indicating severity of trauma. Further studies should evaluate AKI and point-of-care test results in the context of trauma severity and other complications (such as sepsis) that could confound associations with patient mortality. Previous studies have indicated relationships between urinary tract infections and chronic kidney disease with creatinine and NGAL values. Although we did not assess for UTIs specifically in this study, all patients presented for trauma, so this should be minimal to nonexistent. No patients reported a history of chronic kidney disease, but this could have been missed. There is limited outpatient care for nephrology in Malawi, and baseline creatinine values were not known for any of our patients.
In conclusion, because AKI can be silent and asymptomatic until it is quite severe, the ability to detect AKI where reliable, routine laboratory testing is not available could improve care and outcomes. Our project is one of the first validation studies of the novel NGALds dipstick that holds such promise as an AKI point-of-care test. We showed that in a trauma population in Malawi, the dipstick was good at ruling out AKI, and may be useful for overall risk stratification of pediatric trauma patients. Further investigations are needed as the dipstick potentially has other applications beyond trauma in Malawi: other African countries, disaster settings, conflict zones, rural areas, and in the setting of other diseases with a high risk of AKI.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Funding was provided by the International Society of Nephrology, UNC Kidney Center, Malawi Surgical Initiative, and Bioporto. In addition, ECB was funded on the NIH/NIDDK T32-DK00775 Training Grant. Bioporto donated the NGALds dipsticks and provided a substantial discount for The NGAL Test. ZS was funded on NIH/Fogarty International Center & NHLBI D43-TW009340. The authors wish to thank the collaborative support of the Kamuzu Central Hospital and UNC Project research, clinical, and administrative staff.
Footnotes
Table S1. Urine laboratory-based NGAL test predictive characteristics using KDIGO-defined AKI (creatinine-only∗) in Malawian trauma patients.
Figure S1. ROC curve for NGALds Dipstick on AKI diagnosis.
STARD Checklist.
Supplementary Material
References
- 1.Susantitaphong P., Cruz D.N., Cerda J. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013;8:1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaddourah A., Basu R.K., Bagshaw S.M., Goldstein S.L. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376:11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varnell C.D., Goldstein S.L., Devarajan P., Basu R.K. Impact of near real-time urine neutrophil gelatinase–associated lipocalin assessment on clinical practice. Kidney Int Reports. 2017;2:1243–1249. doi: 10.1016/j.ekir.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra J., Dent C., Tarabishi R. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 5.Parikh C.R., Coca S.G., Thiessen-Philbrook H. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett M., Dent C.L., Ma Q. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zappitelli M., Washburn K.K., Arikan A.A. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: a prospective cohort study. Crit Care. 2007;11:R84. doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nickolas T.L., O’Rourke M.J., Yang J. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makris K., Markou N., Evodia E. Urinary neutrophil gelatinase-associated lipocalin (NGAL) as an early marker of acute kidney injury in critically ill multiple trauma patients. Clin Chem Lab Med. 2009;47:79–82. doi: 10.1515/CCLM.2009.004. [DOI] [PubMed] [Google Scholar]
- 10.The World Bank GDP per capita (current US$) https://data.worldbank.org/indicator/ny.gdp.pcap.cd?year_high_desc=false Available at:
- 11.The World Bank Population, Total. https://data.worldbank.org/indicator/sp.pop.totl Available at:
- 12.Kellum A.J. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2:1–138. [Google Scholar]
- 13.Bjornstad E.C., Muronya W., Smith Z.H. Incidence and epidemiology of acute kidney injury in a pediatric Malawian trauma cohort: a prospective observational study. BMC Nephrol. 2020;21:1–12. doi: 10.1186/s12882-020-01755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moodley N., Hariparshad S., Peer F., Gounden V. Evaluation of the CKD-EPI creatinine based glomerular filtration rate estimating equation in Black African and Indian adults in KwaZulu-Natal, South Africa. Clin Biochem. 2018;59:43–49. doi: 10.1016/j.clinbiochem.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Bukabau J.B., Sumaili E.K., Cavalier E. Performance of glomerular filtration rate estimation equations in Congolese healthy adults: the inopportunity of the ethnic correction. PLoS One. 2018;13:e0193384. doi: 10.1371/journal.pone.0193384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madala N.D., Nkwanyana N., Dubula T., Naiker I.P. Predictive performance of eGFR equations in South Africans of African and Indian ancestry compared with 99mTc-DTPA imaging. Int Urol Nephrol. 2012;44:847–855. doi: 10.1007/s11255-011-9928-7. [DOI] [PubMed] [Google Scholar]
- 17.Van Deventer H.E., George J.A., Paiker J.E. Estimating glomerular filtration rate in black South Africans by use of the modification of diet in renal disease and Cockcroft-Gault equations. Clin Chem. 2008;54:1197–1202. doi: 10.1373/clinchem.2007.099085. [DOI] [PubMed] [Google Scholar]
- 18.Zappitelli M., Parikh C.R., Akcan-Arikan A. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3:948–954. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz G.J., Munoz A., Schneider M.F. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobusingye O.C., Lett R.R. Hospital-based trauma registries in Uganda. J Trauma. 2000;48:498–502. doi: 10.1097/00005373-200003000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Harris P.A., Taylor R., Thielke R. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haase M., Bellomo R., Devarajan P. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Gibney R.T.N., Sever M.S., Vanholder R.C. Disaster nephrology: crush injury and beyond. Kidney Int. 2014;85:1049–1057. doi: 10.1038/ki.2013.392. [DOI] [PubMed] [Google Scholar]
- 24.Tran N.K., Godwin Z., Bockhold J., Kost G.J. Point-of-care testing at the disaster-emergency-critical care interface. Point Care. 2012;11:180–183. doi: 10.1097/POC.0b013e318265f7d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.